We read with great interest the study by Way et al.1 published in this journal, which described and compared the acute changes in central arterial stiffness (AS), wave reflections, and central hemodynamics (CH) in response to a bout of high-intensity interval exercise (HIIE) and moderate-intensity continuous exercise (MICE) in adults with diabetes. The authors reported a significant reduction in augmentation index (AIx) corrected by a heart rate (HR) of 75 beats/min (AIx@75) and central systolic blood pressure after HIIE, without significant changes in the pulse wave velocity (PWV). In view of this important research topic and our recent contributions to it, we would like to raise some points of discussion that might help to better elucidate the findings observed in the current study, and also suggest pertinent directions to further studies.
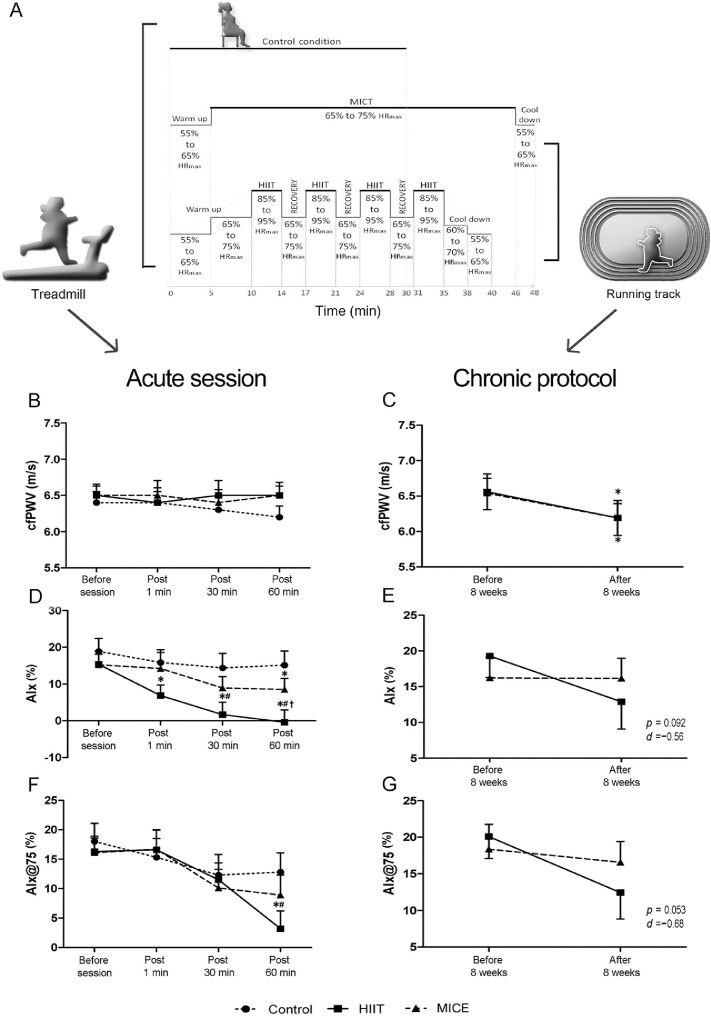
Although the acute and chronic responses of different modalities of exercise on AS and wave reflections have been extensively studied,2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 little is known about the effects of HIIE on these parameters.14 A recent review12 revealed that a single bout of aerobic exercise (AE) did not significantly change the PWV (mean difference (MD) = 0.00 m/s, 95% confidence interval (95%CI): –0.11 to 0.11, p = 0.96). We recently developed an akin study in young obese women, conducting an acute and chronic protocol (Fig. 1A). Similar results were reported in our study,15 in which we did not observe changes in PWV after either HIIE or MICE in obese women (Fig. 1B). The acute nature of the exercise seems to be insufficient stimulus to alter the structural proprieties of the arteries, such as elastin breakdown and collagen deposition in the media layers,16 which might justify the absence of change in PWV following AE observed by Way et al.1
Fig 1.
HIIE, MICE, and control condition protocols (A) of the acute bout of HIIE and MICE on cfPWV (B), AIx (D), and AIx@75 (F), and 8 weeks of HIIE and MICE on cfPWV (C), AIx (E), and AIx@75 (G) in young obese women. * Differences in relation to baseline values. # Differences in relation to control condition (interaction group vs. time). † Differences in relation to MICE (interaction group vs. time). AIx = augmentation index; AIx@75 = augmentation index corrected for 75 beats/min; cfPWV = carotid–femoral pulse wave velocity; HIIE = high-intensity interval exercise; HIIT = high-intensity interval training; HRmax = maximal heart rate; MICE = moderate-intensity continuous exercise; MICT = moderate-intensity continuous training. Adapted with permission from Hortmann et al.15 and de Oliveira et al.17
Differently from the acute effects, PWV has been shown to reduce chronically after AE intervention.3 In fact, we recently demonstrated that both HIIE and MICE 8-week protocols significantly reduced PWV in obese women, suggesting that PWV is an intensity-independent variable (Fig. 1C).17 Considering the similarity between the obese and the diabetic populations in terms of AS, it is expected that the same results can be found in individual with diabetes following AE chronically, for both HIIE and MICE.
Regarding AE changes in AIx, the up-to-date meta-analysis identified a reduction in AIx acutely (MD = –4.54%, 95%CI: –7.05 to –2.04, p = 0.0004).12 Nevertheless, previous evidence reported that the AIx is highly dependent on HR,18 whereas AIx@75 has been proposed to mitigate the influence of HR on AIx, better representing wave reflection in different conditions, such as exercise. Interestingly, the same study observed a significant increase in AIx@75 (MD = 3.58%, 95%CI: 0.56–6.61) after AE.12 These findings suggest that the changes observed in AIx were being driven by an increase in HR. Of note, a high heterogeneity was found in this review, and its results should be interpreted with caution. Contrariwise, Way et al.1 found a significant reduction in AIx@75 after HIIE in diabetic subjects. It means that the effects of HIIE on AIx were independent of changes in HR. Our study found a similar result in young obese women, in which a single bout of HIIE promoted significant reductions in AIx acutely (Fig. 1D), wherein no statistically significant effects were verified after 8 weeks of HIIE (Fig. 1E). The same results were observed for AIx@75 (Figs. 1F and 1G).15 Like Way et al.,1 we also found a significant reduction in central systolic blood pressure after a single bout of HIIE.15 It suggests that AIx seems to be more responsive to AE than PWV acutely.5 AIx represents the reflected wave, having a major role in the cross-talk between the macrocirculation and the microcirculation.16 Therefore, it might be assumed that AIx represents the capacity of the peripheral vessels to assimilate the forward pressure wave that travels along the arterial tree toward the periphery. Surprisingly, although not significant, our study showed an acute decrease in AIx for the control group (Fig. 1D).15 The same reduction was reported by Way et al.1 Although it might likely have happened due to the rest period to which the subjects were exposed, it also raises awareness of the oscillatory and unstable nature of this variable.
In terms of chronic effects of AE on AIx, similar to observed by PWV, it is expected a reduction of AIx after AE intervention.3 In accord with that finding, we found a tendency toward reductions in AIx and AIx@75 after 8 weeks of HIIE in obese women (Figs. 1E and 1G).17 This tendency to decrease observed only after HIIE could suggest that AIx is an intensity-dependent variable. Thus, the clinical relevance of the exercise-induce changes in AIx and AIx@75 still needs to be properly addressed.
Another point of discussion is the sex differences regarding acute AIx responses to AE. It appears that AIx in women is more susceptible to reduce after a single bout of AE than in men. Women have greater β-adrenergic receptor sensitivity, leading to a greater vasodilation for a given amount of sympathetic nervous activity and consequent reduction of AIx.19 Way et al.1 have merged men and women in a single sample, and this sex-combined analysis may have attenuated the AIx responses to AE. Moreover, the authors included individuals with a wide range of age (29–59 years). Having in mind that aging is a determining factor of the stiffening process,16 these individuals could have been in different stages of vascular aging, which might also have influenced the results. Yet, they included individuals with type 1 and type 2 diabetes. Even though it seems that there is no significant difference between these conditions on the progressive development of AS and impairments on CH,20 baseline differences between these groups were reported. Baseline differences in age, sex, body mass index, waist circumference, glycosylated hemoglobin, and duration of diabetes could represent a potential risk of bias.
In summary, AE can promote acute and chronic benefits to mitigate AS and the deterioration of CH. Moreover, despite a tendency for HIIE in eliciting greater outcomes in these parameters, the differences between HIIE and MICE are yet to become further investigated. Studies with different populations and different clinical settings (e.g., prehypertension to established hypertension) are mandatory to better establish the role of the intensity as well as of the intermittent nature of different AE modalities on AS and CH.
Authors’ contributions
WAL conceived the study and drafted the paper; JCL and CFS drafted the paper; RTPO conceived the study and critically reviewed the paper. All authors contributed to the concept of the commentary. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Way KL, Lee AS, Twigg SM, Johnson NA. The effect of acute aerobic exercise on central arterial stiffness, wave reflections, and hemodynamics in adults with diabetes: A randomized cross-over design. J Sport Health Sci. 2021;10:499–506. doi: 10.1016/j.jshs.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyachi M. Effects of resistance training on arterial stiffness: A meta-analysis. Br J Sports Med. 2013;47:393–396. doi: 10.1136/bjsports-2012-090488. [DOI] [PubMed] [Google Scholar]
- 3.Ashor AW, Lara J, Siervo M, Celis-Morales C, Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;15 doi: 10.1371/journal.pone.0110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montero D, Roberts CK, Vinet A. Effect of aerobic exercise training on arterial stiffness in obese populations: A systematic review and meta-analysis. Sports Med. 2014;44:833–843. doi: 10.1007/s40279-014-0165-y. [DOI] [PubMed] [Google Scholar]
- 5.Montero D, Roche E, Martinez-Rodriguez A. The impact of aerobic exercise training on arterial stiffness in pre- and hypertensive subjects: A systematic review and meta-analysis. Int J Cardiol. 2014;173:361–368. doi: 10.1016/j.ijcard.2014.03.072. [DOI] [PubMed] [Google Scholar]
- 6.Montero D, Vinet A, Roberts CK. Effect of combined aerobic and resistance training versus aerobic training on arterial stiffness. Int J Cardiol. 2015;178:69–76. doi: 10.1016/j.ijcard.2014.10.147. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Hanssen H, Cordes M, Rossmeissl A, Endes S, Schmidt-Trucksäss A. Aerobic, resistance and combined exercise training on arterial stiffness in normotensive and hypertensive adults: A review. Eur J Sport Sci. 2015;15:443–457. doi: 10.1080/17461391.2014.955129. [DOI] [PubMed] [Google Scholar]
- 8.Huang C, Wang J, Deng S, She Q, Wu L. The effects of aerobic endurance exercise on pulse wave velocity and intima media thickness in adults: A systematic review and meta-analysis. Scand J Med Sci Sports. 2016;26:478–487. doi: 10.1111/sms.12495. [DOI] [PubMed] [Google Scholar]
- 9.Mutter AF, Cooke AB, Saleh O, Gomez YH, Daskalopoulou SS. A systematic review on the effect of acute aerobic exercise on arterial stiffness reveals a differential response in the upper and lower arterial segments. Hypertens Res. 2017;40:146–172. doi: 10.1038/hr.2016.111. [DOI] [PubMed] [Google Scholar]
- 10.Sardeli AV, Gaspari AF, Chacon-mikahil MP. Acute, short-, and long-term effects of different types of exercise in central arterial stiffness: A systematic review and meta-analysis. J Sports Med Phys Fitness. 2018;58:923–932. doi: 10.23736/S0022-4707.17.07486-2. [DOI] [PubMed] [Google Scholar]
- 11.Figueroa A, Okamoto T, Jaime SJ, Fahs CA. Impact of high-and low-intensity resistance training on arterial stiffness and blood pressure in adults across the lifespan: A review. Eur J Appl Physiol. 2018;471:467–478. doi: 10.1007/s00424-018-2235-8. [DOI] [PubMed] [Google Scholar]
- 12.Pierce DR, Doma K, Leicht AS. Acute effects of exercise mode on arterial stiffness and wave reflection in healthy young adults: A systematic review and meta-analysis. Front Physiol. 2018;9:73. doi: 10.3389/fphys.2018.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Way KL, Sultana RN, Sabag A, Baker MK, Johnson NA. The effect of high Intensity interval training versus moderate intensity continuous training on arterial stiffness and 24 h blood pressure responses: A systematic review and meta-analysis. J Sci Med Sport. 2018;22:385–391. doi: 10.1016/j.jsams.2018.09.228. [DOI] [PubMed] [Google Scholar]
- 14.Lopes WA, Hortmann K, Oliveira GH, Okawa RTP. Does 6 weeks of HIIT alter structural and functional cardiac and arterial stiffness in young adults? Eur J Appl Physiol. 2019;119:1041–1042. doi: 10.1007/s00421-019-04093-x. [DOI] [PubMed] [Google Scholar]
- 15.Hortmann K, Boutouyrie P, Locatelli JC. Acute effects of high-intensity interval training and moderate-intensity continuous training on arterial stiffness in young obese women. Eur J Prev Cardiol. 2020 doi: 10.1177/2047487320909302. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Boutouyrie P, Bruno RM. The clinical significance and application of vascular stiffness measurements. Am J Hypertens. 2019;32:4–11. doi: 10.1093/ajh/hpy145. [DOI] [PubMed] [Google Scholar]
- 17.de Oliveira GH, Boutouyrie P, Simões CF. The impact of high-intensity interval training (HIIT) and moderate-intensity continuous training (MICT) on arterial stiffness and blood pressure in young obese women: A randomized controlled trial. Hypertens Res. 2020;43:1315–1318. doi: 10.1038/s41440-020-0477-2. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525:263–270. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan H, Ranadive SM, Heffernan KS. Hemodynamic and arterial stiffness differences between African-Americans and Caucasians after maximal exercise. Am J Physiol Circ Physiol. 2014;306:H60–H68. doi: 10.1152/ajpheart.00710.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brooks BA, Molyneaux LM, Yue DK. Augmentation of central arterial pressure in type 2 diabetes. Diabet Med. 2001;18:374–380. doi: 10.1046/j.1464-5491.2001.00479.x. [DOI] [PubMed] [Google Scholar]