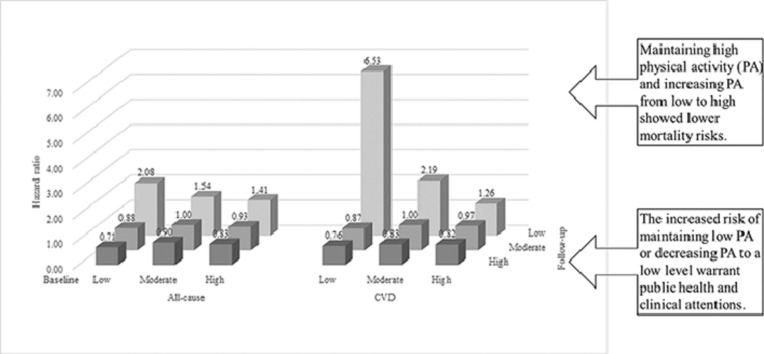
Highlights
-
•
Due to rapid urbanization and despite numerous physical activity (PA) promotion programs, PA levels in China have been continuously decreasing. However, it remains unclear whether increasing or decreasing PA is beneficial for middle-aged and older Chinese.
-
•
Maintaining high levels of PA and increasing PA from low levels to high levels lowers mortality risks for those at an older age, suggesting that substantial health benefits might be achieved by consistently maintaining or increasing engagement in PA.
-
•
The increased mortality risk brought on by maintaining PA at a low level or decreasing it to a low level warrants the attention of public health officials and clinicians.
Keywords: All-cause mortality, Cardiovascular disease mortality, Change in physical activity
Abstract
Background
Physical activity (PA) is generally encouraged. Studies from developed countries in the West have shown that maintenance of adequate PA or increasing PA are associated with lower mortality risk. It is unclear whether these associations apply to an older Chinese population. Hence, we examined the changes in PA prospectively among a middle-aged and older Chinese population over an average of 4 years and explored their subsequent mortality risks.
Methods
Metabolic equivalent scores of PA among participants in the Guangzhou Biobank Cohort Study were calculated. Participants were divided into 3 groups related to PA level, and changes in PA were classified into 9 categories. Information on vital status and causes of death from March 2008 to December 2012 (the first repeated examination) until December 31, 2017, was obtained via record linkage with the Death Registry.
Results
Of 18,104 participants aged 61.21 ± 6.85 years (mean ± SD), 1461 deaths occurred within 141,417 person-years. Compared to participants who maintained moderate PA, those who decreased PA from moderate or high levels to a low level had increased risks for all-cause mortality (hazard ratio (HR) = 1.47, 95% confidence interval (95%CI): 1.11–1.96). Participants who maintained a high level of PA (HR = 0.83, 95%CI: 0.70–0.98) or increased PA from low to high levels (HR = 0.71, 95%CI: 0.52–0.97) showed lower all-cause mortality risks. Those who maintained low PA levels showed a higher all-cause mortality risk, whereas those who increased their PA levels showed a non-significantly lower risk. Similar results were found for cardiovascular disease risk.
Conclusion
Even at an older age, maintaining a high PA level or increasing PA from low to high levels results in lower mortality risks, suggesting that substantial health benefits might be achieved by maintaining or increasing engagement in adequate levels of PA. The increased risk of maintaining a low PA level or decreasing PA to a low level warrants the attention of public health officials and clinicians.
Graphical abstract
1. Introduction
Physical inactivity is one of the leading risk factors for mortality.1 The 2017 Global Burden of Diseases report estimated that 1.26 million premature deaths and 23.7 million disability-adjusted life-years worldwide were attributable to low physical activity (PA).2 Current guidelines in the US,3 UK,4 and World Health Organization1 recommend at least 150 min of moderate-intensity aerobic PA, 75 min of vigorous-intensity aerobic PA, or an equivalent combination of the two each week. Prospective studies have shown that higher baseline PA was associated with lower risks of all-cause mortality,5, 6, 7, 8 cardiovascular disease (CVD),5,9,10 and cancer mortality.5,11,12 However, because PA might have changed during follow-up, effects of PA on long-term health using information assessed at a single point in time at baseline may be diluted or inflated due to misclassification of PA exposure. Assessment of changes in PA during follow-up might partly overcome this limitation.
Previous studies consistently have shown that maintenance of adequate PA or increasing PA was associated with a lower risk of mortality.13, 14, 15, 16, 17, 18, 19 However, most of these studies focused mainly on leisure time PA,13,16, 17, 18, 19 which constitutes a relatively small part of total PA. Moreover, these studies were mainly from developed countries in the West. Generalization of their results to contemporary populations or to populations in low- and middle-income countries (e.g., China) might be problematic because of differing social, cultural, and epidemiological patterns.
Hence, we examined the changes in PA from baseline to follow-up prospectively over an average of 4 years among participants in the Guangzhou Biobank Cohort Study (GBCS) and explored their subsequent mortality risks. Moreover, we explored interactions between their PA changes and potential effect modifiers, such as sex and baseline obesity status. We hypothesized that maintaining a high level of PA or increasing the PA level would be associated with a lower risk of mortality, whereas maintaining a low level of PA or decreasing PA from a moderate or high level to a low level would be associated with an increased risk of mortality.
2. Methods
2.1. Study subjects
The GBCS examined the baseline characteristics of 30,430 participants from 2003 to 2008. The Guangzhou Medical Ethics Committee of the Chinese Medical Association approved the study, and all participants gave written, informed consent before participation. All surviving participants were invited to the follow-up (the first repeated examination) from March 2008 to December 2012. Details of the GBCS baseline and some results from follow-up examinations have been reported previously.20, 21, 22, 23
Briefly, the GBCS is a 3-way collaboration among the Guangzhou 12th Hospital, the University of Hong Kong in China, and the University of Birmingham in the UK. Participants were recruited from The Guangzhou Health and Happiness Association for the Respectable Elders, a community social and welfare organization. The Guangzhou Health and Happiness Association for the Respectable Elders is unofficially aligned with the municipal government and has branches in all 10 districts of Guangzhou. Membership is open to permanent residents of Guangzhou aged 50 years or older for a nominal fee of 4 RMB (about 50 US cents) per month. The members of the Guangzhou Health and Happiness Association for the Respectable Elders make up about 7% of Guangzhou residents in this age group.
2.2. Sociodemographic and health variables
The examinations of socio demographic and health variables were conducted in the Guangzhou 12th Hospital, including a face-to-face, computer-based interview by trained interviewers who assessed anthropomorphic and clinical parameters. Covariates included sex, age (years, as continuous), education (primary, middle school, or college), occupation (manual, non-manual, or others), personal income (<10,000 RMB/year, 10,000–<15,000 RMB/year, ≥15,000 RMB/year or not known; 1 US dollar ≈ 8 RMB), alcohol use (never, former, or current user), smoking (never, former, or current smoker), and self-rated health (very good, good, poor, or very poor) at baseline.
2.3. PA and change in PA
At the baseline and first follow-up examinations, PA was assessed by the Chinese Version of the International Physical Activity Questionnaire, which has been validated for Chinese adults.24 Information on the frequency and duration of walking; all vigorous and moderate activities lasting at least 10 min, including occupation or housework, transportation and leisure time; and time spent in sedentary activity (sitting and lying awake) were assessed. The reported minutes per week in each type of activity were weighted by a metabolic equivalent of the task (MET) based on its energy expenditure. Specifically, 1.0 MET was assigned for sitting, 3.3 METs for walking, 4.0 METs for moderate activity, and 8.0 METs for vigorous activity. The data were then converted to metabolic equivalent scores (MET-min/week). PA levels at baseline and follow-up were classified into 3 categories in ascending order (i.e., low, moderate, and high), and the changes in PA were classified into 9 categories (i.e., low–low, low–moderate, low–high, moderate–low, moderate–moderate, moderate–high, high–low, high–moderate, and high–high) within the 3 groups of baseline PA levels. Because of the small number of sub-categories in the 9 categories above, pooled analysis was conducted. Specifically, decreasing PA to a low level included moderate–low and high–low and increasing PA included low–moderate, low–high, and moderate–high.
The specific classification criteria for PA levels at baseline and follow-up are as follows. The low category was the lowest level of PA; individuals who did not meet the criteria for moderate or high categories were classified as having a low PA level. The moderate category was defined as weekly vigorous activity at least 3 days/week and achieving 480 MET-min/week, or any combination of walking, moderate or vigorous activities at least 5 days/week and achieving at least 600 MET-min/week. The high category was defined as weekly vigorous activity at least 3 days/week and achieving at least 1500 MET-min/week, or moderate activity 7 days/week and achieving at least 3000 MET-min/week.
2.4. Mortality
Information on the underlying causes of deaths up to December 2017 was obtained via record linkage with the Death Registry of the Guangzhou Centre for Disease Control and Prevention. Causes of death were coded by trained medical staff in each hospital according to the 10th revision of the International Classification of Diseases. When the death certificates were not issued by medical institutions (and hence might introduce a quality issue with the coding), the causes of death were verified by the Guangzhou Centre for Disease Control and Prevention as part of its quality assurance programmed by cross-checking past medical history and conducting a verbal autopsy. In addition, verbal autopsy meetings were conducted in the Guangzhou 12th Hospital to further clarify the deaths from unclear causes. A physician panel including 5 chief physicians from various disciplines reviewed all available medical records of the same individuals and assigned in a standard manner a cause of death, with assistance of an epidemiologist for unsettled cases.25 In this study, we analyzed mortality from all-cause and CVD.
2.5. Statistical analysis
The Pearson χ2 test and one-way analysis of variance were used to compare baseline characteristics of categories according to PA change. Cox regression was used to assess associations of PA change with all-cause and CVD mortality, with crude and adjusted hazard ratios (HRs) and 95% confidence intervals (95%CIs). The study period used in the calculation of person-years started from the first follow-up examination. To test the proportional hazards assumption, Schoenfeld residuals were used, and the assumption was met (p from 0.45 to 0.97). To reduce reverse causality or confounding bias due to underlying illnesses, we conducted sensitivity analyses by excluding participants who reported poor health status or had a history of hard CVD (self-reported stroke, myocardial infarction, or coronary heart disease)26 at baseline, and those who died within the first 3 years. Poor health status at baseline was defined by the presence of any of the following: (1) regular use of medication for chronic diseases, such as diabetes, hypercholesterolemia, or CVD; (2) any hospital admission in the past 6 months before baseline examination; (3) self-reported CVD history; or (4) self-reported cancer history.27 Participants who died of any other causes were regarded as censored at the date of death.28,29 To explore potential effect modification, we also checked for interactions between PA changes and potential effect modifiers including sex and baseline obesity status. We used the Chinese-specific body mass index (BMI) cut-offs recommended by World Health Organization. Underweight was defined as BMI < 18.5 kg/m2; normal, 18.5 kg/m2 ≤ BMI < 25.0 kg/m2; overweight, 25.0 kg/m2 ≤ BMI < 27.5 kg/m2; obese BMI ≥ 27.5 kg/m2.30 Because marginally significant interaction was found for PA changes and baseline obesity status (p = 0.05), we conducted stratification analyses by baseline obesity status. No evidence for sex interaction (p = 0.72) was found. All analyses were performed using STATA (Version 14.0; Stata Corp., College Station, TX, USA). All p values were 2-sided, and statistical significance was defined as p < 0.05.
3. Results
Of the 30,430 participants at baseline, 12,326 did not return for the first repeated examination including 384 were excluded because of loss to follow-up with unknown vital status, giving 18,104 participants (13,178 women and 4926 men) aged 61.21 ± 6.85 years (mean ± SD) in the present analysis. During the average follow-up of 7.8 ± 1.5 years, 1461 deaths (777 women, 53.2%; and 684 men, 46.8%) were recorded from the first repeated examination between 2008 and 2012 and December 2017.
Table 1 shows that, during baseline and the first repeated examination, most of participants (41.98%) maintained high PA, 29.78% increased PA from moderate to high, and 0.06% maintained a low level of PA. The moderate–moderate category was used as the reference in the subsequent analyses. Due to the small sample size of the low–low category (n = 11), we did not compare them with the others. Participants in the moderate–moderate and moderate–low categories were older, while those in the low–high category were younger. Moderate–moderate and low–moderate categories had more men. Those in the high–low category had lower socioeconomic position (indicated by low education, manual occupation, and low family and personal income). Those in the high–high category had a highest prevalence of current alcohol use, while the moderate–moderate category had a highest proportion of current smoking. The high–low category had a highest prevalence of good self-rated health, while the low–moderate category had more participants reporting good objective health (all p < 0.001).
Table 1.
Baseline characteristics of 18,104 participants aged 50+ in the Guangzhou Biobank Cohort Study first examined in 2003–2008 and followed up to December 2017.
| Low |
Moderate |
High |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Moderate | High | Low | Moderate | High | Low | Moderate | High | All | p | |
| n (row %) | 11 (0.06) | 319 (1.76) | 1147 (6.34) | 165 (0.91) | 1644 (9.08) | 5392 (29.78) | 198 (1.09) | 1628 (8.99) | 7600 (41.98) | 18,104 (100.00) | — |
| Age (year)a | 61.64 ± 7.41 | 60.25 ± 7.43 | 58.97 ± 6.49 | 63.35 ± 6.81 | 63.12 ± 6.96 | 61.08 ± 6.84 | 62.86 ± 6.55 | 62.52 ± 6.50 | 60.89 ± 6.78 | 61.21 ± 6.85 | <0.001 |
| Sex (% men) | 27.27 | 32.92 | 27.29 | 25.45 | 34.37 | 27.93 | 21.21 | 28.62 | 24.79 | 27.21 | <0.001 |
| Education (% primary or below) | 54.55 | 43.26 | 36.36 | 39.39 | 40.02 | 39.24 | 48.99 | 43.49 | 37.64 | 39.03 | <0.001 |
| Occupation (manual, %) | 45.45 | 52.98 | 60.24 | 57.58 | 55.66 | 58.29 | 69.19 | 59.89 | 61.89 | 59.84 | <0.001 |
| Family income (<30,000 RMB/year, %) | 45.45 | 35.42 | 37.23 | 38.18 | 40.02 | 37.26 | 42.93 | 36.98 | 35.79 | 36.91 | <0.001 |
| Personal income (<10,000 RMB/year, %) | 27.27 | 32.92 | 36.70 | 36.97 | 33.27 | 31.03 | 37.88 | 34.46 | 30.75 | 31.94 | <0.001 |
| Current alcohol user (%) | 27.27 | 13.48 | 12.38 | 12.73 | 18.43 | 21.48 | 18.69 | 22.97 | 32.47 | 25.13 | <0.001 |
| Current smoker (%) | 27.27 | 11.91 | 11.51 | 12.73 | 13.38 | 9.66 | 9.60 | 9.21 | 7.75 | 9.35 | <0.001 |
| Self-rated health (good, %) | 45.45 | 80.25 | 78.38 | 80.00 | 80.17 | 80.64 | 83.84 | 82.92 | 81.88 | 81.18 | <0.001 |
| Objective health status (good, %) | 81.82 | 88.09 | 85.00 | 73.94 | 77.55 | 79.21 | 87.37 | 80.47 | 82.74 | 81.22 | <0.001 |
| Baseline metabolic equivalent score (MET-min/week)a | 281.95 ± 164.93 | 629.72 ± 756.28 | 655.27 ± 747.16 | 1868.49 ± 762.03 | 1797.10 ± 738.69 | 1807.46 ± 725.59 | 5697.57 ± 2308.72 | 5601.50 ± 2675.96 | 6028.27 ± 2982.34 | 3999.59 ± 3071.46 | <0.001 |
| Follow-up metabolic equivalent score (MET-min/week)a | 478.90 ± 227.42 | 2073.55 ± 765.66 | 7351.02 ± 3249.04 | 623.93 ± 1116.91 | 2026.76 ± 759.40 | 6911.43 ±3113.57 | 556.29 ± 833.82 | 2062.40 ± 718.45 | 7218.95 ± 3035.37 | 6031.39 ± 3471.50 | <0.001 |
Notes: 1 RMB ≈ 12.5 US cents, 1 US dollar ≈ 8 RMB. The percentages are rounded to retain 2 decimal places, so the sum may not equal to 100%.
Data are presented as mean ± SD.
Abbreviations: MET = metabolic equivalent of the task; PA = physical activity.
3.1. Change in PA and mortality
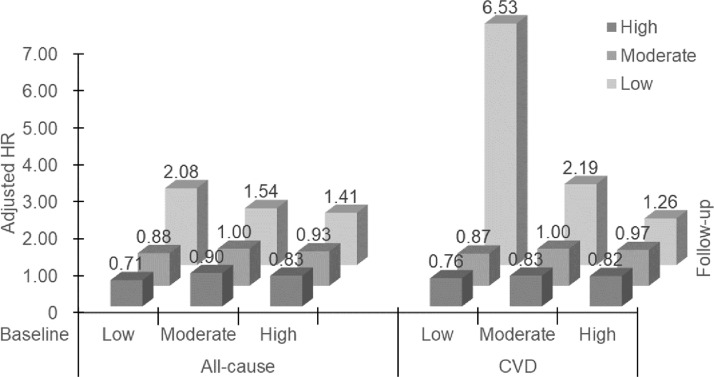
Table 2 and Fig. 1 show mortality risks in the 9 categories under the 3 groups of baseline PA level. For all-cause mortality, compared to the moderate–moderate category, after adjusting for sex, age, education, occupation, personal income, alcohol use, smoking status, and self-rated health, those who decreased PA to a low level showed a higher risk (HR = 1.47, 95%CI: 1.11–1.96). Specifically, the moderate–low (HR = 1.54, 95%CI: 1.05–2.26) and the high–low (HR = 1.41, 95%CI: 0.97–2.06) categories showed increased risks. However, the high–high category had a lower risk (HR = 0.83, 95%CI: 0.70–0.98). Moreover, the low–low category showed the highest mortality risk (HR = 2.08, 95%CI: 0.51–8.40). Increasing PA showed a lower risk (HR = 0.87, 95%CI: 0.74–1.03), which was similar to the high–high category. Specifically, the low–moderate, low–high, and moderate–high categories consistently showed lower mortality risks (HR = 0.88, 95%CI: 0.59–1.33; HR = 0.71, 95%CI: 0.52–0.97; HR = 0.90, 95%CI: 0.76–1.07; respectively), although some of the adjusted HRs were not statistically significant. As expected, the associations in the low–high (i.e., increasing low PA to a high level) category were more pronounced than in the low–moderate category (i.e., increasing low PA to a moderate level).
Table 2.
HRs for all-cause and CVD mortality by changes in PA from 2003 to 2008 (baseline) to 2012 (first repeated examination), and followed up for mortality until December 2017 in 18,104 participants of the Guangzhou Biobank Cohort Study.
| Change in PA | Deaths/person-years (n) | Mortality rate (per 1000 person-years) | Crude HR (95%CI) | AHRa (95%CI) |
|---|---|---|---|---|
| All-cause | ||||
| Low–low | 2/83.48 | 23.96 | 1.66 (0.41–6.70) | 2.08 (0.51–8.40) |
| Low–moderate | 26/2512.70 | 10.35 | 0.70 (0.46–1.05) | 0.88 (0.59–1.33) |
| Low–high | 54/8697.42 | 6.21 | 0.44 (0.33–0.59)⁎⁎⁎ | 0.71 (0.52–0.97)* |
| Moderate–low | 30/1368.80 | 21.92 | 1.36 (0.92–1.99) | 1.54 (1.05–2.26)* |
| Moderate–moderate | 203/13,220.90 | 15.35 | Ref. | Ref. |
| Moderate–high | 430/41,821.73 | 10.28 | 0.71 (0.60–0.83)⁎⁎⁎ | 0.90 (0.76–1.07) |
| High–low | 32/1669.26 | 19.17 | 1.18 (0.81–1.71) | 1.41 (0.97–2.06) |
| High–moderate | 168/13,293.67 | 12.64 | 0.81 (0.66–0.99)* | 0.93 (0.76–1.15) |
| High–high | 516/58,748.58 | 8.78 | 0.60 (0.51–0.71)⁎⁎⁎ | 0.83 (0.70–0.98)* |
| Increaseb | 510/53,031.85 | 9.62 | 0.66 (0.56–0.78)⁎⁎⁎ | 0.87 (0.74–1.03) |
| Decrease to lowc | 62/3038.06 | 20.41 | 1.26 (0.95–1.67) | 1.47 (1.11–1.96)⁎⁎ |
| CVD | ||||
| Low–low | 2/83.48 | 23.96 | 4.79 (1.17–19.51)* | 6.53 (1.58–26.91)⁎⁎ |
| Low–moderate | 9/2512.70 | 3.58 | 0.69 (0.35–1.38) | 0.87 (0.43–1.73) |
| Low–high | 18/8697.42 | 2.07 | 0.42 (0.25–0.71)⁎⁎ | 0.76 (0.45–1.28) |
| Moderate–low | 16/1368.80 | 11.69 | 1.95 (1.13–3.35)* | 2.19 (1.28–3.78)⁎⁎ |
| Moderate–moderate | 73/13,220.90 | 5.52 | Ref. | Ref. |
| Moderate–high | 139/41,821.73 | 3.32 | 0.65 (0.49–0.86)⁎⁎ | 0.83 (0.62–1.11) |
| High–low | 11/1669.26 | 6.59 | 1.08 (0.57–2.04) | 1.26 (0.67–2.39) |
| High–moderate | 64/13,293.67 | 4.81 | 0.84 (0.60–1.18) | 0.97 (0.69–1.36) |
| High–high | 178/58,748.58 | 3.03 | 0.59 (0.45–0.77)⁎⁎⁎ | 0.82 (0.62–1.08) |
| Increaseb | 166/53,031.85 | 3.13 | 0.62 (0.47–0.81)⁎⁎ | 0.83 (0.62–1.09) |
| Decrease to lowc | 27/3038.06 | 8.89 | 1.47 (0.94–2.29) | 1.69 (1.08–2.64)* |
Adjusted for sex, age, occupation, personal income, education, alcohol use, smoking, and self-rated health.
Increase: low–moderate, low–high, and moderate–high.
Decrease to low: moderate–low and high–low.
p < 0.05,
p < 0.01,
p < 0.001, compared with the moderate-moderate category.
Abbreviations: 95%CI = 95% confidence interval; AHR = adjusted hazard ratio; CVD = cardiovascular disease; HR = hazard ratio; PA = physical activity; Ref. = reference.
Fig. 1.
Adjusted HRs for all-cause and CVD mortality by changes in PA from 2003 to 2008 (baseline) to 2012 (first repeated examination), and followed up for mortality until December 2017 in 18,104 participants of the Guangzhou Biobank Cohort Study. HRs are adjusted for sex, age, occupation, personal income, education, alcohol use, smoking, and self-rated health. CVD = cardiovascular disease; HR = hazard ratio; PA = physical activity.
For CVD mortality, the results were similar. Compared to the moderate–moderate category, those who decreased PA to a low level showed higher risk (HR = 1.69, 95%CI: 1.08–2.64). The risks in the low–low, moderate–low, and high–low categories also showed higher mortality risks (HR = 6.53, 95%CI: 1.58–26.91; HR = 2.19, 95%CI: 1.28–3.78; HR = 1.26, 95%CI: 0.67–2.39). Moreover, the high–high category showed a non-significantly lower risk (HR = 0.82, 95%CI: 0.62–1.08). Increasing PA level (low–moderate, low–high, and moderate–high) consistently showed lower mortality risks, although the results were not statistically significant (HR = 0.87, 95%CI: 0.43–1.73; HR = 0.76, 95%CI: 0.45–1.28; HR= 0.83, 95%CI: 0.62–1.11) (pooled HR = 0.83, 95%CI: 0.62–1.09).
Supplementary Tables 1 and 2 show that, after excluding those with poor health or with a hard CVD history at baseline, the HRs of all-cause and CVD mortality in those who decreased PA (moderate–low and high–low) were attenuated to non-significant, although the trends did not vary substantially. Furthermore, the results remained the same after excluding deaths within the first 3 years (Supplementary Table 3).
3.2. Stratification analyses by baseline BMI status
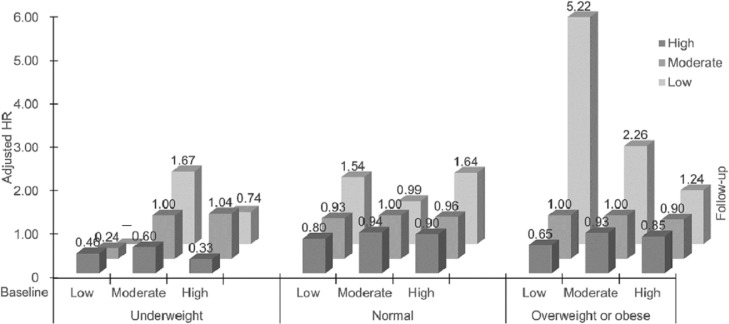
Table 3 and Fig. 2 show that among participants who were underweight, compared to the moderate–moderate category, the high–high category had a 67% lower risk of all-cause mortality (HR = 0.33, 95%CI: 0.15–0.69). In participants who had normal weight, the high–low category showed a 64% higher risk of all-cause mortality (HR = 1.64, 95% CI: 1.01–2.64). In the overweight or obesity category, the moderate–low category was associated with more than a 2-fold risk of all-cause mortality (HR = 2.26, 95%CI: 1.27–4.02). Although the magnitude of the risks for the 2 pooled categories (i.e., the “increase” and the “decrease to low” categories) varied by different baseline obesity status, the directions of the associations were consistent, showing that those who decreased PA to a low level showed an increased all-cause mortality risk, whereas those who increased PA showed a lower all-cause mortality risk.
Table 3.
HRs for all-cause mortality by changes in PA from 2003 to 2008 (baseline) to 2012 (first repeated examination), and followed up for mortality until December 2017 in 18,104 participants of the Guangzhou Biobank Cohort Study, stratified by BMI.
| Change in PA | Deaths/person-years (n) | Mortality rate (per 1000 person-years) | Crude HR (95%CI) | AHRa (95%CI) |
|---|---|---|---|---|
| Underweight | ||||
| Low–low | 0/8.57 | 0 | — | — |
| Low–moderate | 1/145.63 | 6.87 | 0.25 (0.03–1.91) | 0.24 (0.03–1.89) |
| Low–high | 3/341.70 | 8.78 | 0.34 (0.10–1.15) | 0.46 (0.13–1.61) |
| Moderate–low | 5/44.40 | 112.61 | 3.79 (1.39–10.31)⁎⁎ | 1.67 (0.53–5.25) |
| Moderate–moderate | 17/579.93 | 29.31 | Ref. | Ref. |
| Moderate–high | 22/1668.46 | 13.19 | 0.48 (0.25–0.90)* | 0.60 (0.30–1.18) |
| High–low | 2/102.99 | 19.42 | 0.62 (0.14–2.70) | 0.74 (0.16–3.41) |
| High–moderate | 16/621.58 | 25.74 | 0.86 (0.43–1.71) | 1.04 (0.51–2.15) |
| High–high | 15/2281.62 | 6.57 | 0.23 (0.12–0.47)⁎⁎⁎ | 0.33 (0.15–0.69)⁎⁎ |
| Increaseb | 26/2155.79 | 12.06 | 0.44 (0.24–0.82)⁎⁎ | 0.54 (0.28–1.05) |
| Decrease to lowc | 7/147.39 | 47.62 | 1.54 (0.64–3.75) | 1.18 (0.46–3.08) |
| Normal | ||||
| Low–low | 1/54.82 | 18.24 | 1.27 (0.18–9.08) | 1.54 (0.21–11.08) |
| Low–moderate | 15/1522.94 | 9.85 | 0.69 (0.40–1.17) | 0.93 (0.54–1.60) |
| Low–high | 34/5296.14 | 6.42 | 0.47 (0.32–0.69)⁎⁎ | 0.80 (0.54–1.19) |
| Moderate–low | 11/779.99 | 14.10 | 0.90 (0.48–1.66) | 0.99 (0.53–1.84) |
| Moderate–moderate | 116/7806.85 | 14.86 | Ref. | Ref. |
| Moderate–high | 263/26,111.29 | 10.07 | 0.72 (0.57–0.89)⁎⁎ | 0.94 (0.75–1.17) |
| High–low | 20/986.39 | 20.28 | 1.29 (0.80–2.07) | 1.64 (1.01–2.64)* |
| High–moderate | 97/8095.07 | 11.98 | 0.79 (0.60–1.04) | 0.96 (0.73–1.27) |
| High–high | 327/37,305.36 | 8.77 | 0.62 (0.50–0.77)⁎⁎⁎ | 0.90 (0.72–1.12) |
| Increaseb | 312/32,930.37 | 9.47 | 0.68 (0.55–0.84)⁎⁎⁎ | 0.92 (0.74–1.14) |
| Decrease to lowc | 31/1766.38 | 17.55 | 1.11 (0.75–1.66) | 1.33 (0.89–1.98) |
| Overweight or obese | ||||
| Low–low | 1/13.98 | 71.53 | 5.65 (0.78–40.68) | 5.22 (0.71–38.26) |
| Low–moderate | 10/828.19 | 12.07 | 0.85 (0.44–1.66) | 1.00 (0.51–1.94) |
| Low–high | 17/3028.74 | 5.61 | 0.41 (0.24–0.70)⁎⁎ | 0.65 (0.38–1.12) |
| Moderate–low | 14/544.41 | 25.72 | 1.69 (0.95–2.99) | 2.26 (1.27–4.02)⁎⁎ |
| Moderate–moderate | 70/4794.59 | 14.60 | Ref. | Ref. |
| Moderate–high | 144/13,942.58 | 10.33 | 0.74 (0.56–0.99) | 0.93 (0.70–1.24) |
| High–low | 10/579.88 | 17.24 | 1.13 (0.58–2.19) | 1.24 (0.64–2.41) |
| High–moderate | 55/4558.13 | 12.07 | 0.82 (0.57–1.16) | 0.90 (0.63–1.28) |
| High–high | 174/19,088.07 | 9.12 | 0.65 (0.49–0.86)⁎⁎ | 0.85 (0.64–1.12) |
| Increaseb | 171/17,799.51 | 9.61 | 0.69 (0.53–0.92)* | 0.90 (0.68–1.19) |
| Decrease to lowc | 24/1124.29 | 21.35 | 1.40 (0.88–2.22) | 1.68 (1.05–2.68)* |
Notes: underweight, BMI <18.5 kg/m2; normal, 18.5 kg/m2 ≤ BMI < 25.0 kg/m2; overweight, 25.0 kg/m2 ≤ BMI < 27.5 kg/m2; obese, BMI ≥ 27.5 kg/m2.
Adjusted for sex, age, occupation, personal income, education, drinking, smoking, and self-rated health.
Increase: low–moderate, low–high, and moderate–high.
Decrease to low: moderate–low and high–low.
p < 0.05,
p < 0.01,
p < 0.001, compared with the moderate-moderate category.
Abbreviations: 95%CI = 95% confidence interval; AHR = adjusted hazard ratio; BMI = body mass index; HR = hazard ratio; PA = physical activity; Ref. = reference.
Fig. 2.
Adjusted hazard ratios (HRs) for all-cause mortality by changes in PA from 2003 to 2008 (baseline) to 2012 (first repeated examination), and followed up for mortality until December 2017 in 18,104 participants of the Guangzhou Biobank Cohort Study, stratified by BMI. Underweight: BMI <18.5 kg/m2; normal: 18.5 kg/m2 ≤ BMI < 25.0 kg/m2; overweight: 25.0 kg/m2 ≤ BMI < 27.5 kg/m2; obese: BMI ≥ 27.5 kg/m2. HRs are adjusted for sex, age, occupation, personal income, education, alcohol use, smoking, and self-rated health. BMI = body mass index; PA = physical activity.
4. Discussion
To our knowledge, this study is the first to examine the association between PA change and mortality in Asia. We found that compared to participants maintaining moderate PA, those who maintained high PA or increased PA (especially from low to high) showed lower risks of all-cause and CVD mortality. Although the adjusted HRs for participants with increasing PA were not statistically significant, the trend was toward a lower mortality risk. By contrast, those who maintained low PA or decreased PA to a low level showed increased risks for all-cause and CVD mortality. Our results indicate that in middle-aged and older people regardless of baseline obesity status, consistent engagement in adequate PA or increasing PA would be beneficial, whereas individuals maintaining low PA or decreasing PA to a low level would have higher risks of all-cause and CVD mortality.
Maintaining high PA was associated with a lower mortality risk, while, interestingly, the benefit of increasing PA was similar. These findings are in line with some previous cohort studies in Western countries.13, 14, 15, 16, 17,19,31, 32, 33, 34, 35, 36 For example, a study of 315,059 participants aged 50–71 years from the National Institutes of Health-AARP (formerly the American Association of Retired Persons) Diet and Health Study showed that maintaining higher leisure time PA levels and increasing leisure time PA in later adulthood were associated with a low risk of all-cause mortality.13 A population-based study in the UK involving 14,599 participants aged 40–79 years showed that increasing PA during middle and late life was associated with substantial longevity gains based on a follow-up of 12.5 years.14 Moreover, the benefits were sustained in all categories with different baseline obesity status;13,18 the results were sustained especially for participants who were overweight or obese, indicating that most middle-aged and older people could benefit from a more active lifestyle, regardless of obesity status.
In our study, the findings that maintaining low PA or decreasing PA to a low level was associated with increased mortality risks were also consistent with the findings in other previous studies.37,38 The Evergreen Project, a study of 357 participants aged 80–85 years, found that maintaining inactivity and decreasing PA level to inactive during the 5 years after baseline were associated with a higher mortality risk during a follow-up 18 years later.37 The Helsinki Health Study analyzed PA change in 5475 participants 40–60 years old and found that decreasing PA among aging people was associated with poorer physical health functioning.38
Our study extends these findings to a Chinese population ≥50 years old. Moreover, in low- to middle-income countries, including China, leisure time PA constitutes a relatively small part of total PA, and the benefits of sufficient PA in other domains (e.g., occupation or housework) might have counteracted the adverse effects of the lack of leisure time PA or accelerated the benefits of leisure time PA. Note that most of the studies previously cited accounted for leisure time PA only.13,16,17,19,38 Hence, our study, which examined an overall pattern of PA, accounted for occupation or housework, transportation and leisure time, as well as sedentary activity (sitting and lying awake). Thus, it enabled us to examine the association of total PA changes with mortality. Moreover, most of the previous studies cited assessed the associations of PA changes with all-cause mortality only.15,16,18,19,37 However, our study extends this evidence to CVD mortality as well. We identified only a limited number of studies that examined the association between PA change and CVD mortality,13,14,34,39 but the results of these studies were generally consistent with ours, showing a potential beneficial effect of maintaining adequate PA on CVD mortality. Our results showed that even at an older age consistent adequate PA or increasing engagement in adequate PA had significant health benefits, whereas maintaining low PA or decreasing PA to a low level warrants closer attention.
Some reasons for the protective effect of adequate PA in old age have been discussed previously. For example, PA has been shown to be beneficial for cardiorespiratory fitness and mental health40 by inducing chronic vascular functional adaptation and structural arterial remodeling,41 as well as increasing brain volume and improving memory, attention, anxiety, and depression.42 In addition, maintenance of adequate PA may also reduce the risks of obesity, falls, osteoporosis, and muscular weakness,43 which all contribute to lower risks of mortality. Moreover, PA may modulate gene expression through epigenetic alterations to reduce adverse effects due to aging.44 However, our results showing that the HRs for mortality risks due to decreased PA were attenuated in healthier participants indicated that reverse causality might have played a role. Decreasing PA could be a warning signal for underlying diseases, a situation that needs urgent attention and clinical interventions.
Our study has several strengths, including (1) a large sample size, (2) the use of the same questionnaire at both examinations, which were, on average, 4 years apart, (3) the use of computer-assisted face-to-face interviews, and (4) consideration of a wide range of potential confounding factors.
Our study also has some limitations. First, because information about PA was collected by self-report using a Chinese version of the International Physical Activity Questionnaire, measurement errors might have affected the precision of the estimates. Moreover, such measurement errors might lead to a misclassification of the PA categories; thus, the changes in PA categories might not correctly reflect the actual changes. However, because the same questionnaire was used in both baseline and the repeated examinations, this concern may be partly alleviated. Furthermore, the questionnaire has been validated and has been shown to have satisfactory validity and reliability.24 Future studies using objective measures of PA, such as wearable devices or energy expenditures monitored by app-based smartphones, may provide more accurate estimates. Second, the duration of the follow-up may not have been long enough, so the number of deaths in some categories was small, especially in the underweight group. Third, because our participants were ≥50 years old at baseline, those with very low PA levels might not have participated in the cohort because of illnesses or because they were less conscious of their health. Hence, survivor bias might have biased the results toward the null. Moreover, due to the strong advocacy for national fitness since the 2008 Olympic Games in Beijing, participants in our cohort might have had a higher overall level of PA during the first follow-up examination (2008–2012). But our results would be most relevant to populations with rapid aging and a higher overall level of PA. Fourth, women were oversampled in this study, as they have been in other population-based elderly cohorts. However, within sex and age group, the participants had fairly similar levels of chronic diseases compared to nationally representative samples of urban Chinese.23 Moreover, we found no evidence for effect modification due to sex (p = 0.72, for sex interaction), indicating that the associations between changes in PA and the risks of mortality did not vary by sex. In addition, the results were also adjusted for sex to minimize its potential confounding effect. Thus, the unbalanced sex ratio might not be a major concern in the current study. Finally, although our results were adjusted for a wide range of potential confounding factors, a residual confounding effect cannot be fully ruled out.
5. Conclusion
Compared to participants maintaining a moderate PA level, maintenance of high PA and increasing PA from low to high lowered mortality risks, while maintaining low PA or decreasing PA to a low level increased mortality risks. Our results show that even at an older age, consistent or increasing engagement in adequate PA is beneficial, while maintaining low PA or decreasing PA to a low level might warrant the attention of public health officials and clinicians.
Acknowledgments
Acknowledgments
The Guangzhou Biobank Cohort Study investigators included the following staff members at the Guangzhou 12th Hospital: Weisen Zhang, Ming Cao, Tong Zhu, Bin Liu, and Chaoqiang Jiang (co-Principal Investigator). The study investigators also included the following staff members at the University of Hong Kong— C Mary Schooling, Sarah McGhee, Gabriel M Leung, Richard Fielding, and Tai Hing Lam (co-Principal Investigator)—and at the University of Birmingham: Peymane Adab, G Neil Thomas, and Kar Keung Cheng (co-Principal Investigator). This work was supported by the National Natural Science Foundation (No. 81941019); the National Key R&D Program of China (No. 2017YFC0907100); the Natural Science Foundation of Guangdong (No. 2018A030313140); the Guangzhou Science and Technology Bureau, Guangzhou, China (No. 201704030132); the Major Infectious Disease Prevention and Control of the National Science and Technique Major Project (No. 2018ZX10715004); and the University of Birmingham, UK.
Authors’ contributions
YH helped conceive the design of the study, analyzed the data, and draft the manuscript; LX helped conceive the design of the study, analyzed the data, and revised the article for critically important intellectual content; THL helped conceive the design of the study and revised the article for critically important intellectual content; WZ helped conceive the design of the study and revised the article; FZ and YJ helped conceive the design of the study and performed data collection; CJ helped conceive the design of the study and helped draft the manuscript; KKC helped conceive the design of the study and supervised data analyses. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jshs.2020.08.007.
Contributor Information
Lin Xu, Email: xulin27@mail.sysu.edu.cn.
Weisen Zhang, Email: zwsgzcn@163.com.
Appendix. Supplementary materials
References
- 1.World Health Organization. Global recommendations on physical activity for health. Available at:https://www.who.int/dietphysicalactivity/factsheet_recommendations/en/. [accessed 03.03.2020]. [PubMed]
- 2.GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. Physical activity guidelines for Americans (2nd ed). Available at :https://health.gov/our-work/physical-activity/current-guidelines. [accessed 13.08.2020].
- 4.U.K. Department of Health and Social Care. UK Chief Medical Officers' physical activity guidelines. Available at: https://www.gov.uk/government/publications/physical-activity-guidelines-uk-chief-medical-officers-report. [accessed 13.08.2020].
- 5.Arem H, Moore SC, Patel A. Leisure time physical activity and mortality: A detailed pooled analysis of the dose–response relationship. JAMA Intern Med. 2015;175:959–967. doi: 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samitz G, Egger M, Zwahlen M. Domains of physical activity and all-cause mortality: Systematic review and dose–response meta-analysis of cohort studies. Int J Epidemiol. 2011;40:1382–1400. doi: 10.1093/ije/dyr112. [DOI] [PubMed] [Google Scholar]
- 7.Kim J. Association between meeting physical activity guidelines and mortality in Korean adults: An 8-year prospective study. J Exerc Nutrition Biochem. 2017;21:23–29. doi: 10.20463/jenb.2016.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y, Zhang R, Liu Y. Association of regular physical activity with total and cause-specific mortality among middle-aged and older Chinese: A prospective cohort study. Sci Rep. 2017;7:39939. doi: 10.1038/srep39939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett DA, Du H, Clarke R. Association of physical activity with risk of major cardiovascular diseases in Chinese men and women. JAMA Cardiol. 2017;2:1349–1358. doi: 10.1001/jamacardio.2017.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wahid A, Manek N, Nichols M. Quantifying the association between physical activity and cardiovascular disease and diabetes: A systematic review and meta-analysis. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rezende LFMD, de Sá TH, Markozannes G. Physical activity and cancer: An umbrella review of the literature including 22 major anatomical sites and 770,000 cancer cases. Br J Sports Med. 2018;52:826–833. doi: 10.1136/bjsports-2017-098391. [DOI] [PubMed] [Google Scholar]
- 12.Li T, Wei S, Shi Y. The dose–response effect of physical activity on cancer mortality: Findings from 71 prospective cohort studies. Br J Sports Med. 2016;50:339–345. doi: 10.1136/bjsports-2015-094927. [DOI] [PubMed] [Google Scholar]
- 13.Saint-Maurice PF, Coughlan D, Kelly SP. Association of leisure-time physical activity across the adult life course with all-cause and cause-specific mortality. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mok A, Khaw KT, Luben R, Wareham N, Brage S. Physical activity trajectories and mortality: Population based cohort study. BMJ. 2019;365:l2323. doi: 10.1136/bmj.l2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis ZH, Markides KS, Ottenbacher KJ, Al Snih S. The impact of 10-year physical activity changes on 7-year mortality in older Mexican Americans. J Phys Act Health. 2018;15:30–39. doi: 10.1123/jpah.2016-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talbot LA, Morrell CH, Fleg JL, Metter EJ. Changes in leisure time physical activity and risk of all-cause mortality in men and women: The Baltimore Longitudinal Study of Aging. Prev Med. 2007;45:169–176. doi: 10.1016/j.ypmed.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Petersen CB, Grønbæk M, Helge JW, Thygesen LC, Schnohr P, Tolstrup JS. Changes in physical activity in leisure time and the risk of myocardial infarction, ischemic heart disease, and all-cause mortality. Eur J Epidemiol. 2012;27:91–99. doi: 10.1007/s10654-012-9656-z. [DOI] [PubMed] [Google Scholar]
- 18.Balboa-Castillo T, Guallar-Castillón P, León-Muñoz LM, Graciani A, López-García E, Rodríguez-Artalejo F. Physical activity and mortality related to obesity and functional status in older adults in Spain. Am J Prev Med. 2011;40:39–46. doi: 10.1016/j.amepre.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Byberg L, Melhus H, Gedeborg R. Total mortality after changes in leisure time physical activity in 50 year old men: 35 year follow-up of population based cohort. Br J Sports Med. 2009;338:b688. doi: 10.1136/bmj.b688. [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Lam TH, Jiang CQ. Adiposity and incident diabetes within 4 years of follow-up: The Guangzhou Biobank Cohort Study. Diabet Med. 2017;34:1400–1406. doi: 10.1111/dme.13378. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Jiang CQ, Schooling CM, Zhang WS, Cheng KK, Lam TH. Liver enzymes and incident diabetes in China: A prospective analysis of 10,764 participants in the Guangzhou Biobank Cohort Study. J Epidemiol Community Health. 2015;69:1040–1044. doi: 10.1136/jech-2015-205518. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, Jiang CQ, Lam TH. Sleep duration and memory in the elderly Chinese: Longitudinal analysis of the Guangzhou Biobank Cohort Study. Sleep. 2014;37:1737–1744. doi: 10.5665/sleep.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang C, Thomas GN, Lam TH. Cohort profile: The Guangzhou Biobank Cohort Study, a Guangzhou-Hong Kong-Birmingham collaboration. Int J Epidemiol. 2006;35:844–852. doi: 10.1093/ije/dyl131. [DOI] [PubMed] [Google Scholar]
- 24.Deng HB, Macfarlane DJ, Thomas GN. Reliability and validity of the IPAQ-Chinese: The Guangzhou Biobank Cohort study. Med Sci Sports Exerc. Feb 2008;40:303–307. doi: 10.1249/mss.0b013e31815b0db5. [DOI] [PubMed] [Google Scholar]
- 25.Wang T, Jiang CQ, Xu L. White blood cell count and all-cause and cause-specific mortality in the Guangzhou biobank cohort study. BMC Public Health. 2018;18:1232. doi: 10.1186/s12889-018-6073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pencina MJ, D'Agostino RB, Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: The Framingham Heart Study. Circulation. 2009;119:3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu L, Lam TH, Jiang CQ. Changes in adiposity in an older Chinese population in rapid economic transition. Obesity (Silver Spring) 2016;24:2217–2223. doi: 10.1002/oby.21599. [DOI] [PubMed] [Google Scholar]
- 28.Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer. 2004;91:1229–1235. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen PK, Abildstrom SZ, Rosthoj S. Competing risks as a multi-state model. Stat Methods Med Res. 2002;11:203–215. doi: 10.1191/0962280202sm281ra. [DOI] [PubMed] [Google Scholar]
- 30.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. The Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 31.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 32.Steffen-Batey L, Nichaman MZ, Goff DC., Jr Change in level of physical activity and risk of all-cause mortality or reinfarction – The Corpus Christi Heart Project. Circulation. 2000;102:2204–2209. doi: 10.1161/01.cir.102.18.2204. [DOI] [PubMed] [Google Scholar]
- 33.Wannamethee SG, Shaper AG, Walker M. Changes in physical activity, mortality, and incidence of coronary heart disease in older men. The Lancet. 1998;351:1603–1608. doi: 10.1016/S0140-6736(97)12355-8. [DOI] [PubMed] [Google Scholar]
- 34.Nordstoga AL, Zotcheva E, Svedahl ER, Nilsen TIL, Skarpsno ES. Long-term changes in body weight and physical activity in relation to all-cause and cardiovascular mortality: The HUNT study. Int J Behav Nutr Phys Act. 2019;16:45. doi: 10.1186/s12966-019-0809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregg EW, Cauley JA, Stone K. Relationship of changes in physical activity and mortality among older women. JAMA. 2003;289:2379–2386. doi: 10.1001/jama.289.18.2379. [DOI] [PubMed] [Google Scholar]
- 36.Kieffer SK, Croci I, Wisloff U, Nauman J. Temporal changes in a novel metric of physical activity tracking (personal activity intelligence) and mortality: The HUNT Study, Norway. Prog Cardiovasc Dis. 2019;62:186–192. doi: 10.1016/j.pcad.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Aijo M, Kauppinen M, Kujala UM, Parkatti T. Physical activity, fitness, and all-cause mortality: An 18-year follow-up among old people. J Sport Health Sci. 2016;5:437–442. doi: 10.1016/j.jshs.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holstila A, Manty M, Rahkonen O, Lahelma E, Lahti J. Changes in leisure-time physical activity and physical and mental health functioning: A follow-up study. Scand J Med Sci Sports. 2017;27:1785–1792. doi: 10.1111/sms.12758. [DOI] [PubMed] [Google Scholar]
- 39.Higueras-Fresnillo S, Guallar-Castillon P, Cabanas-Sanchez V, Banegas JR, Rodriguez-Artalejo F, Martinez-Gomez D. Changes in physical activity and cardiovascular mortality in older adults. J Geriatr Cardiol. Apr 2017;14:280–281. doi: 10.11909/j.issn.1671-5411.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruegsegger GN, Booth FW. Health benefits of exercise. Cold Spring Harb Perspect Med. 2018;8 doi: 10.1101/cshperspect.a029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green DJ, Smith KJ. Effects of exercise on vascular function, structure, and health in humans. Cold Spring Harb Perspect Med. 2018;8 doi: 10.1101/cshperspect.a029819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galloza J, Castillo B, Micheo W. Benefits of exercise in the older population. Phys Med Rehabil Clin N Am. 2017;28:659–669. doi: 10.1016/j.pmr.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 43.McPhee JS, French DP, Jackson D, Nazroo J, Pendleton N, Degens H. Physical activity in older age: Perspectives for healthy ageing and frailty. Biogerontology. 2016;17:567–580. doi: 10.1007/s10522-016-9641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grazioli E, Dimauro I, Mercatelli N. Physical activity in the prevention of human diseases: Role of epigenetic modifications. BMC Genomics. 2017;18(Suppl. 8):802. doi: 10.1186/s12864-017-4193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.