Highlights
-
•
Interrupting prolonged sitting with physical activity breaks is effective in controlling postprandial glycemia and insulin responses.
-
•
Intermittently interrupting sitting with moderate-intensity physical activity is the optimal intervention strategy in reducing postprandial glycemia and insulin responses.
-
•
At least a 2-min activity break every 20–30 min to interrupt prolonged sitting reduces postprandial glycemia and insulin responses.
Keywords: Glucose, Insulin, Physical activity interruption, Prolonged sitting, Meta-analytic review
Abstract
Purpose
This study aimed to evaluate the effectiveness of physical activity (PA) interrupting prolonged sitting (PS) on postprandial glycemia and insulin responses among adults.
Methods
PubMed, EMBASE, Cochrane Library, Web of Science, CINAHL, PsycINFO, and the China National Knowledge Infrastructure databases were searched through September 30, 2020. Randomized controlled trials (RCTs) that examined the effect of all forms of PA interrupting PS on postprandial glycemia and/or insulin responses among adults without chronic diseases were included in this study. The risk of bias of included studies was evaluated based on the Cochrane tool. A network meta-analysis was performed to estimate the summary standardized mean differences (SMDs) with 95% confidence intervals (95%CIs) with random effects.
Results
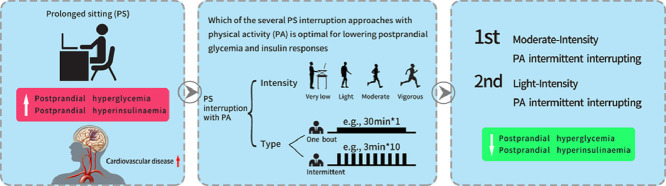
Thirty crossover RCTs were included in our review. These RCTs included 9 types of interventions that interrupted PS. When compared to PS by itself, light-intensity PA intermittent interrupting (LPA-INT) PS and moderate-intensity PA intermittent interrupting (MPA-INT) PS significantly lowered postprandial glycemia (SMD = –0.46, 95%CI: –0.70 to –0.21; SMD = –0.69, 95%CI: –1.00 to –0.37, respectively) and significantly reduced postprandial insulin response (SMD = –0.46, 95%CI: –0.66 to –0.26; SMD = –0.47, 95%CI: –0.77 to –0.17, respectively). Results of the clustered ranking plot indicated that MPA-INT was the most effective intervention in lowering postprandial glycemia and insulin responses.
Conclusion
Replacing PS with MPA-INT or LPA-INT has a positive effect in reducing postprandial glycemia and insulin responses, with MPA-INT being the optimal intervention strategy.
Graphical abstract
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide.1 CVD survivors often experience a low quality of life and high cost of medical treatment and rehabilitation,2 which may cause great psychological and financial burden on patients and their families. Therefore, effective early interventions in the modifiable risk factors for CVD is of great significance to clinical and public health practice. Postprandial hyperglycemia and hyperinsulinemia are considered as independent predictors for the development of future CVD,3, 4, 5 and thus effective interventions to lower postprandial hyperglycemia and hyperinsulinemia could be important for CVD prevention.
Excessive sitting time is widespread6,7 and has been shown to be independently associated with the risk of CVD.8 Since observational studies have shown that interrupting prolonged sitting (PS) is associated with better cardiovascular risk factor profile,9 many experimental studies have assessed the effects of interrupting PS with physical activity (PA) breaks on postprandial glycemia and insulin responses among various populations.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 Most, if not all, studies have suggested that interrupting PS with moderate-intensity PA (MPA) to vigorous-intensity PA (VPA) breaks—and even light-intensity PA (LPA) breaks—can lead to positive effects on postprandial glycemia and/or insulin responses. Three pairwise meta-analyses conducted in 2015, 2018, and 2019 synthesized the findings of 6,40 20,41 and 3742experimental studies, respectively, and concluded that interrupting PS had positive effects on postprandial glycemia and insulin responses. For the following reason, however, additional research, using a novel network meta-analysis (NMA), is needed to quantitatively review and summarize the latest literature: (1) the conclusions of previous meta-analyses should be revisited given the increased numbers of new publications issued since the meta-analyses were conducted; (2) the optimal strategy for reducing postprandial glycemia and insulin responses among the different interventions should be identified using NMA, which allows for comparing multiple treatments sharing one common comparator treatment that were not directly compared with one another in head-to-head studies;43 and (3) NMA can integrate evidence retrieved from direct and indirect comparisons and thus has potential to improve the precision of intervention effect estimates.43,44
Therefore, our review aims to perform an NMA to systematically evaluate the potential effects of various interventions that interrupt PS with PA on the postprandial glycemia and insulin responses among adults without chronic diseases. Identification of the effect of different characteristics of PA interventions that are optimally, or at least minimally, effective as strategies for postprandial glycemia and insulin control will help narrow the range of intervention strategies employed in future research and practice.
2. Methods
This study followed the criteria of Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Network Meta-Analyses (PRISMA-NMA).45 This NMA is registered in the International Prospective Register of Systematic Reviews (PROSPERO, registration number CRD42019121994).
2.1. Search strategy
The relevant randomized controlled trials (RCTs) published in Chinese or English that assessed the effects of interrupting PS on postprandial glycemia and insulin responses were identified by searching PubMed, EMBASE, Cochrane Library, Web of Science, CINAHL, PsycINFO, and the China National Knowledge Infrastructure through September 30, 2020. Keywords used in the search strategy included “exercise”, “standing”, “walking”, and “PA” cross-referenced to “prolonged sitting” and “sedentary” cross-referenced to “glucose” and “insulin” cross-referenced to “trial”, “intervention”, and “randomized”. In addition, the same terms were searched by using Google Scholar. Finally, the reference list of relevant studies and reviews from these searches were cross-checked for additional citations.
2.2. Eligibility criteria
All relevant studies were independently reviewed by three of the co-authors (MQ, PX, and HW). Disagreements were resolved through discussion by the group, with the addition of a 4th author (PC). A study was included if it met the following criteria.
-
(1)
Study participants were adults (≥18 years old) without diagnosis of any chronic mental or physical diseases. Participants with risk factors (e.g., elevated fasting glucose or overweight/obesity) for chronic diseases but without positive diagnosis for a chronic disease (e.g., diabetes or CVD) were eligible for inclusion (The rationale for this was to ensure that the studies were homogeneous for statistical comparisons).
-
(2)
The study performed interventions on interrupting PS time, such as standing or LPA, MPA, and VPA alone or in combination, and included a PS control group.
-
(3)
The study outcomes reported the effect of interrupting PS on at least 1 measure of postprandial glycemia or insulin response, such as the incremental area under the curve (iAUC).
-
(4)
If the study was conducted over consecutive days, experimental data could be extracted from the 1st day.
-
(5)
The study used an RCT design.
-
(6)
The study was published in Chinese or English.
2.3. Data extraction and risk-of-bias assessment
Using a shared data extraction sheet, 3 reviewers (MQ, PX, and HW) independently extracted data from the included studies. The sheet included a matrix for the following information for each study: author, year of publication, location, study design, participants’ ages and characteristics, detailed description of control and intervention groups, and outcomes of interest (postprandial glycemia and insulin responses).
Three reviewers (MQ, MC, and TZ) independently assessed the risk of bias for the included studies based on the Cochrane handbook for systematic reviews. The following items were used to evaluate the risk of bias for the study: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting, and (7) other biases. Each domain was graded as high risk of bias, low risk of bias, or unclear risk of bias. The review team (MQ, MC, TZ, and PC) resolved any discrepancies through group discussion.
2.4. Statistical analyses
Because some studies presented the results graphically,12,25,26,38 we extracted the data of means and standard deviations by using ImageJ (V.1.50i; https://imagej.nih.gov/ij/) to measure the length of the axes to calibrate and then the length of histogram.46 To ensure that the included studies were homogeneous for comparison, only the experimental data from the 1st day were included when the study was conducted over consecutive days.16,18,39 The iAUC for postprandial glycemia and insulin responses was used for data analyses in preference to total area under the curve, since the iAUC is recommended for evaluating differences in post-prandial responses.47 The standardized mean difference (SMD) with 95% confidence intervals (95%CIs) was calculated under the effect size for the outcomes of postprandial glycemia and insulin responses, respectively. SMD values <0.2, 0.2–<0.5, 0.5–<0.8, and ≥0.8 were categorized as trivial, small, moderate, and large effect sizes, respectively.
A series of pairwise meta-analyses was conducted by using the random effects model. Heterogeneity of between-study comparisons was assessed by the I2 statistic; values of I2 <25%, 25%–<50%, 50%–<75%, and ≥75% were defined as very low, low, moderate, and high degrees of heterogeneity, respectively. p ≤ 0.1 was considered significant in the I2 statistic. Publication bias was examined by using Egger's test, if ≥3 studies were available.
Random effects NMA was performed using network family commands in STATA (Version 14.0; STATA Corp., College Station, TX, USA). Network geometry was first created to visualize the relative magnitude of available evidence on interrupting PS and outcomes.48 The size of each node and the thickness of each line were proportional to the number of participants. SMD (with 95%CI) was the main summary outcome from the NMA. Global and local tests were carried out for the presence of inconsistency, while allowing for heterogeneity. The global inconsistency test compares the fit and parsimony of consistency and inconsistency models.49 The local inconsistency test evaluates the difference between direct and indirect estimates in all closed loops in the network.49 Node splitting was used to evaluate the inconsistency of the model. Furthermore, probability ranking for each intervention was carried out using surface under cumulative ranking curve (SUCRA) percentage values as one of the final predictions. SUCRAs range from 0% to 100%; larger SUCRAs indicate more effective intervention methods. A clustered ranking plot was performed to evaluate the optimal strategy, based on the SUCRA values of intervention methods for postprandial glycemia and insulin response. Last, comparison-adjusted funnel plots were used to assess potential publication bias.
To test the robustness of the results and examine potential moderator variables for the primary outcomes, subgroup analyses were performed according to the following variables: sex ratio (male/female <1 or ≥1), age group (18–<45 years old, 45–<60 years old, or ≥60 years old), weight status (normal weight or overweight/obese), geographic area (Europe, America, or Asia/Oceania), interval time (≤20 min, 30 min, 45 min, or 60 min), intervention duration (≤2 min, 2.5‒5 min, 6‒20 min, or ≥30 min), indicator type (iAUC or total area under the curve), postprandial responses assessment methods (oral glucose tolerance test, mixed meal or multiple meal) and experimental period length (<450 min or ≥450 min).
All analyses were performed with STATA statistical software (STATA Corp.). A two-tailed p value of ≤0.05 was considered statistically significant if not otherwise specified.
3. Results
3.1. Study selection
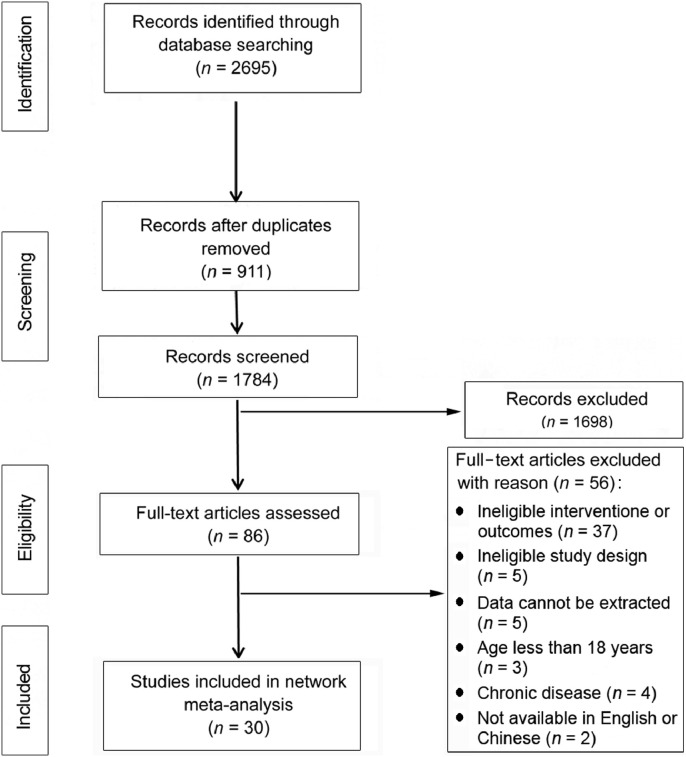
Of the 2695 studies searched from 7 databases, 2665 were excluded for one or more of the following reasons: (1) the intervention did not interrupt PS time, (2) the outcomes of interest did not include postprandial glycemia or insulin response, (3) the study did not have an RCT design, (4) the means and standard deviations of related outcome variables were not reported or could not be derived, (5) participants were under 18 years old or were diagnosed with a chronic disease, and (6) the article was not published in Chinese or English. Finally, a total of 30 crossover RCT studies met the inclusion criteria (Fig. 1).
Fig. 1.
Study selection flow chart.
3.2. Study characteristics
Among the 30 RCT studies included in the analyses, 18 RCTs had 3 arms,10, 11, 12, 13,15,17, 18, 19, 20,22,25,26,31, 32, 33,35,38,39 10 studies had 2 arms,14,16,18,23,27, 28, 29, 30,34,37 and 2 studies had 4 arms.24,36 Sixteen studies were conducted in the Europe (53.3%),11,15,17,18,21, 22, 23,25,27, 28, 29, 30,32,33,37,38 6 studies in the US (20.0%),12,20,24,26,35,39 5 studies in Oceania (16.7%),10,13,14,16,31 and 3 studies in Asia (10.0%).19,34,36 The participant sample size ranged from 1015,22,24,26 to 70,13 and 365 (55.9%) of the 653 total participants were males. The mean age was 39.1 years (range: 19.2‒70.0 years). All studies were written in English. In addition, 29 of the 30 studies reported results on the postprandial glycemia response, and 23 of 30 studies reported results on the postprandial insulin response. Table 1 shows the characteristics of the included studies.
Table 1.
Characteristics of 30 included studies.
| Study | Study Age (year) a | Participants | Design | Arms | Outcomesb |
|---|---|---|---|---|---|
| Dunstan et al. (2012)10 | 53.8 ± 4.9 | 19 overweight/obese adults, 11 males | Randomized crossover trial, ≥6 days washout period |
PS: 420 min LPA-INT: sitting interrupted with 2-min bouts of light-intensity walking every 20 min MPA-INT: sitting interrupted with 2-min bouts of moderate-intensity walking every 20 min |
1, 2 |
| Duvivier et al. (2013)11 | 21.0 ± 2.0 | 18 healthy adults, 2 males | Randomized crossover trial, ≥10 days washout period |
PS: 840 min/day LPA-INT: sitting 480 min/day and 360 min activity breaks with 240 min of walking and 120 min of standing VPA-INT: sitting 780 min/day and 60 min of vigorous exercise |
1, 2 |
| Newsom et al. (2013)12 | 28.0 ± 2.0 | 11 obese adults, 3 males | Randomized crossover trial, ≥7 days washout period |
PS: 480 min LPA-CON: sitting 390 min and a single bout of exercise (∼70 min, 50%VO2peak) MPA-CON: sitting 375 min and a single bout of exercise (∼55 min, 65%VO2peak) |
1, 2 |
| Peddie et al. (2013)13 | 25.9 ± 5.3 | 70 normal-weight adults, 28 males | Randomized crossover trial, ≥6 days (6–13) washout period |
PS: 540 min MPA-CON: sitting 510 min and a single 30-min bout of brisk walking (60%VO2max) MPA-INT: sitting 510 min and 100-s bouts of brisk walking (60%VO2max) every 30 min |
1, 2 |
| Holmstrup et al. (2014)20 | 25 (18–35) | 11 young obese participants with impaired glucose tolerance, 8 males | Randomized crossover trial |
PS: 720 min MPA-CON: sitting interrupted with a single 60-min bout of moderate-intensity exercise (60%–65%VO2max) after breakfast MPA-INT: sitting interrupted with 5-min bouts of moderate-intensity exercise every 60 min |
1, 2 |
| Thorp et al. (2014)14 | 48.2 ± 7.9 | 23 overweight/obese adults, 17 males | Randomized crossover trial, 7‒35 days washout period |
PS: 480 min STA-INT: sitting interrupted with 30 min of standing every 30 min |
1, 2 |
| Bailey et al. (2015)15 | 24.0 ± 3.0 | 10 non-obese adults, 7 males | Randomized crossover trial, ≥6 days washout period |
PS: 300 min STA-INT: sitting interrupted with 2-min bouts of standing every 20 min LPA-INT: sitting interrupted with 2-min bouts of light-intensity walking (3.2 km/h) every 20 min |
1 |
| Larsen et al. (2015)16 | 56.7 ± 1.5 | 19 overweight/obese adults, 11 males | Randomized crossover trial ≥12 days washout period |
PS: 420 min LPA-INT: sitting interrupted with 2-min bouts of walking every 20 min (3.2 km/h) |
1, 2 |
| Bailey et al. (2016)17 | 26.6 ± 8.5 | 13 healthy adults, 6 males | Randomized crossover trial, ≥7 days washout period |
PS: 300 min LPA-INT: sitting interrupted with 2-min bouts of light-intensity walking (3.2 km/h) every 20 min MPA-INT: sitting interrupted with 2-min bouts of moderate-intensity walking (5.8‒7.9 km/h) every 20 min |
1, 2 |
| Hansen et al. (2016)21 | 22 (20–23) | 14 healthy, young, normal-weight adults, 6 males | Randomized crossover trial, ≥4 days washout period |
PS: 150 min LPA-INT: sitting interrupted with 2-min bouts of light-intensity walking (3.5‒4.5 km/h) every 20 min |
1 |
| Hawari et al. (2016)22 | 33.0 ± 13.0 | 10 normoglycemic, overweight/obese males | Randomized crossover trial, 7‒14 days washout period |
PS: 480 min STA-CON: sitting interrupted with 15-min bouts of standing every 30 min STA-INT: sitting interrupted with 10 × 1.5-min bouts of standing every 30 min |
1, 2 |
| Henson et al. (2016)18 | 66.6 ± 4.7 | 22 overweight/obese, dysglycemic, postmenopausal women | Randomized crossover trial, 7‒22 days washout period |
PS: 450 min STA-INT: sitting interrupted with 5-min bouts of standing every 30 min LPA-INT: sitting interrupted with 5-min bouts of light-intensity walking every 30 min |
1, 2 |
| Miyashita et al. (2016)19 | 68.8 ± 3.2 | 15 postmenopausal women | Randomized crossover trial, ≥7 days washout period |
PS: 480 min LPA-CON: sitting interrupted with a single 30-min bout of light-intensity walking after breakfast LPA-INT: sitting interrupted with 1.5-min bouts of light-intensity walking every 15 min after 2 meals (20 breaks) |
1, 2 |
| Wennberg et al. (2016)23 | 59.7 ± 8.1 | 19 overweight/obese adults, 10 males | Randomized crossover trial, 6 days washout period |
PS: 420 min LPA-INT: sitting interrupted with 3-min bouts of light-intensity walking (3.2km/h) every 30 min |
2 |
| Benatti et al. (2017)39 | 30.1 ± 8.8 | 14 physically inactive, healthy adult males | Randomized crossover trial, 5‒15 days washout period |
PS: 540 min STA-INT: sitting interrupted by 15 min of standing every 30 min during the 9-h sitting period MPA-CON: sitting interrupted with a single 30-min bout of moderate-intensity exercise (50%–55%VO2max) on treadmill |
1, 2 |
| Bhammar et al. (2017)24 | 32.0 ± 5.0 | 10 overweight/obese adults, 5 males | Randomized crossover trial, 6‒14 days washout period |
PS: 540 min MPA-CON: sitting interrupted with a single 60-min bout of moderate-intensity walking (71% ± 4% HRmax) MPA-INT: sitting interrupted with 2-min bouts of moderate-intensity walking (53% ± 5% HRmax) every 20 min; a total of 21 breaks VPA-INT: sitting interrupted with 2-min bouts of vigorous-intensity walking (79% ± 4% HRmax) every 60 min; a total of 8 breaks |
1 |
| Brocklebank et al. (2017)25 | 52.4 ± 5.1 | 17 middle-aged office workers, 8 males | Randomized crossover trial, 24-h washout period |
PS: 300 min STA-INT: sitting interrupted by 2 min of standing every 20 min LPA-INT: sitting interrupted by 2 min of light-intensity walking every 20 min |
1 |
| Kerr et al. (2017)26 | 66.0 ± 9.0 | 10 sedentary, overweight or obese postmenopausal women | Randomized crossover trial, ≥7 days washout period |
Prolonged sitting: 300 min STA-INT: sitting interrupted with 2 min of standing every 20 min, or sitting interrupted with 10 min of standing every hour LPA-INT: sitting interrupted by 2 min of light-intensity walking every 60 min |
1, 2 |
| McCarthy et al. (2017)27 | 66.0 ± 6.0 | 13 obese adults, 6 males | Randomized crossover trial, ≥7 days washout period |
PS: 450 min LPA-INT: sitting interrupted with 5 min of seated arm ergometry (∼3 km/h light-intensity walking) every 30 min |
1, 2 |
| Pulsford et al. (2017)38 | 40.2 ± 12.2 | 25 inactive males | Randomized crossover trial, ≥6 days washout period |
PS: 420 min STA-INT: sitting interrupted with 2-min bouts of standing every 20 min LPA-INT: sitting interrupted with 2-min bouts of light-intensity walking (3.2 km/h) every 20 min |
1, 2 |
| Champion et al. (2018)28 | 35.8 ± 10.9 | 24 inactive adults, 12 males |
Randomized crossover trial, ≥6 days washout period |
PS: 390 min LPA-INT: sitting interrupted with 20 min of light-intensity walking every 60 min |
1, 2 |
| Maylor et al. (2018)31 | 29.0 ± 9.0 | 14 sedentary and inactive adults, 7 males | Randomized crossover trial, 6–35 days washout period |
PS: 480 min MPA-CON: sitting interrupted with 30 min of continuous moderate-intensity PA (60%VO2reserve) followed by PS for the remainder of the condition VPA-INT: sitting interrupted with 2 min 32 s bouts of high-intensity PA (85%VO2reserve) every 60 min |
1, 2 |
| Sperlich et al. (2018)29 | 22.0 ± 2.0 | 12 normal-weight students, 5 males | Randomized crossover trial, ≥3 days washout period |
PS: 180 min VPA-CON: sitting interrupted with a single 6-min bout of high-intensity exercise (e.g., squats, lunge, and running) |
1 |
| Altenburg et al. (2019)33 | 19.2 ± 0.6 | 20 healthy-weight males | Randomized crossover trial, ≥6 days washout period |
PS: 300 min STA-INT: sitting with hourly 10-min standing interruptions SitActive: sitting on a stability ball |
1, 2 |
| Chrismas et al. (2019)34 | 27 (21‒44) | 11 sedentary obese females | Randomized crossover trial, 6‒8 days washout period |
PS: 300 min MPA-INT: sitting interrupted with 3 min of moderate-intensity walking every 30 min at a speed corresponding to a rating of perceived exertion (RPE) of 12‒14 identified during the familiarization session |
1, 2 |
| Freire et al. (2019)35 | 24.4 ± 3.8 | 25 excess body fat adults, 10 males | Randomized crossover trial, 7 days washout period |
PS: 600 min MPA-INT: sitting interrupted with 5 min of moderate-intensity walking at habitual gait speed (reaching 10,000 steps) every 20 min VPA-CON: sitting interrupted with a single 10-min bout of high-intensity exercise (running) |
1 |
| Hawari et al. (2019)30 | 37.0 ± 16.0 | 14 overweight/obese adults, 11 males | Randomized crossover trial, 1‒2 weeks washout period |
PS: 390 min LPA-INT: sitting interrupted with 30 s of 10 chair squats every 20 min |
1, 2 |
| Maylor et al. (2019)37 | 33.8 ± 13.4 | 14 sedentary and inactive females | Randomized crossover trial, ≥7 days washout period |
PS: 450 min MPA-INT: sitting interrupted with 2 min of moderate-intensity treadmill physical activity every 30 min |
1, 2 |
| Ma et al. (2020)36 | 24.0 ± 3.0 | 16 non-obese, inactive, healthy adults, 7 males | Randomized crossover trial, ≥7 days washout period |
PS: 540 min MPA-INT - Group 1: sitting interrupted with 3-min bouts of moderate-intensity walking (60%VO2max) every 30 min, a total of 36-min breaks MPA-INT - Group 2: sitting interrupted with 5-min bouts of moderate-intensity walking (60%VO2max) every 45 min, a total of 45-min breaks MPA-INT - Group 3: sitting interrupted with 8-min bouts of moderate-intensity walking (60%VO2max) every 60 min, a total of 56-min breaks |
1 |
| Yates et al. (2020)32 | 70 (67‒75) | 60 overweight adults (30 South Asian and 30 white European), 31 males | Randomized crossover trial, ≥7 days washout period |
PS: 450 min STA-INT: sitting interrupted with 5-min standing breaks every 30 min LPA-INT: sitting interrupted with 5-min self-paced walking breaks every 30 min |
1, 2 |
Abbreviations: HR = heart rate; HRmax = maximal heart rate; LPA-CON = light-intensity physical activity one bout of continuous interrupting; LPA-INT = light-intensity physical activity intermittent interrupting; MPA-CON = moderate-intensity physical activity one bout of continuous interrupting; MPA-INT = moderate-intensity physical activity intermittent interrupting; PS = prolonged sitting; SitActive = active sitting interrupting (e.g., sitting on a stability ball); STA-CON = standing one bout of continuous interrupting; STA-INT = standing intermittent interrupting; VO2max = maximal oxygen uptake; VO2peak = peak oxygen uptake; VO2reserve = reserve oxygen uptake; VPA-CON = vigorous-intensity physical activity one bout of continuous interrupting; VPA-INT = vigorous-intensity physical activity intermittent interrupting.
The mean ± SD or the mean with range of age in years was reported.
Outcome 1 stands for postprandial glycemia; Outcome 2 stands for postprandial insulin response.
3.3. Risk-of-bias assessment
The risk-of-bias assessment for the included studies is summarized in Supplementary Table 1, with most of the studies (n = 18, 60.0%) rated as having a low risk of bias in the domain of random sequence generation. For the domain of allocation concealment, 8 (26.7%) studies were at low risk of bias. Unsurprisingly, all studies (n = 30, 100%) were graded as having a high risk of bias for blinding of participants and personnel because it was impossible to blind the different interrupting interventions. In terms of blinding of the outcome assessment, only 2 studies (6.7%) masked their outcome assessors to the treatment allocations. A low risk of bias for incomplete outcome data was reported for 26 studies (86.7%), whereas a low risk of bias for selective reporting was reported for 12 studies (40.0%). Finally, all studies (n = 30, 100%) were classified as having an unclear risk of bias for other bias domains.
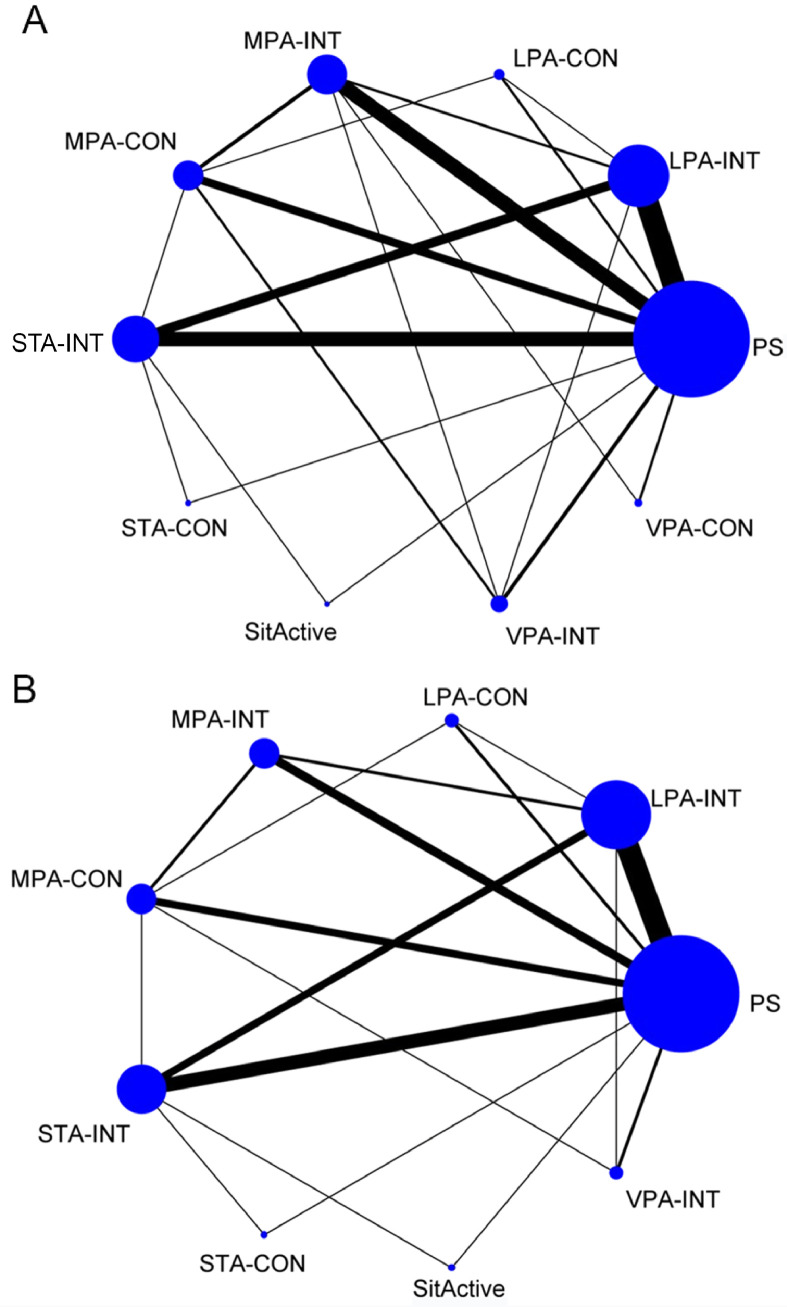
3.4. Network plots
The network plot of eligible comparisons on postprandial glycemia and insulin responses are shown in Fig. 2. A total of 9 types of specific interrupting PS interventions were identified:
-
(1)
active sitting interrupting (SitActive) (e.g., sitting on a stability ball);
-
(2)
standing intermittent interrupting (STA-INT);
-
(3)
standing one bout of continuous interrupting (STA-CON);
-
(4)
LPA intermittent interrupting (LPA-INT);
-
(5)
LPA one bout of continuous interrupting (LPA-CON);
-
(6)
MPA intermittent interrupting (MPA-INT);
-
(7)
MPA one bout of continuous interrupting (MPA-CON);
-
(8)
VPA intermittent interrupting (VPA-INT);
-
(9)
VPA one bout of continuous interrupting (VPA-CON).
Fig. 2.
Network plots for comparisons of outcomes. Comparisons of different interrupting prolonged sitting interventions on postprandial (A) glycemia and (B) insulin response. LPA-CON = light-intensity physical activity one bout of continuous interrupting; LPA-INT = light-intensity physical activity intermittent interrupting; MPA-CON = moderate-intensity physical activity one bout of continuous interrupting; MPA-INT = moderate-intensity physical activity intermittent interrupting; PS = prolonged sitting; SitActive = active sitting interrupting (e.g., sitting on a stability ball); STA-CON = standing one bout of continuous interrupting; STA-INT = standing intermittent interrupting; VPA-CON = vigorous-intensity physical activity one bout of continuous interrupting; VPA-INT = vigorous-intensity physical activity intermittent interrupting.
Intermittent interrupting means that PS was intermittently interrupted by multiple PA bouts, and one bout of continuous interrupting means that PS was interrupted by a continuous non-stop bout of PA. All 9 interventions were observed for the postprandial glycemia outcome; Interventions 1‒8 were observed for the postprandial insulin outcome.
For postprandial glycemia, all 9 interrupting PS interventions were compared directly with PS and at least one other intervention. Similar comparisons were made for postprandial insulin response; all 8 interrupting PS interventions were compared with PS and at least one other intervention. Detailed results of pairwise meta-analyses were indicated in the supplementary materials. Postprandial glycemia was remarkably lower for the LPA-INT and MPA-INT interventions compared with PS (Supplementary Table 2). Similarly, LPA-INT and MPA-INT marginally reduced the postprandial insulin response compared with PS (Supplementary Table 3).
3.5. The results of NMA and subgroup analyses
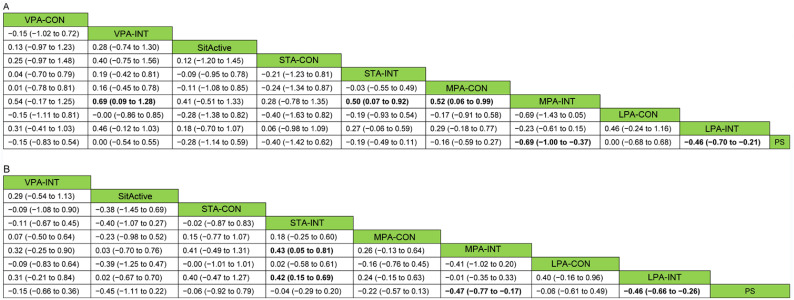
The results for the outcomes (using NMA for the analysis) are presented in Fig. 3. Both LPA-INT and MPA-INT yielded better outcomes in reducing postprandial glycemia compared with PS (SMD = ‒0.46, 95%CI: ‒0.70 to ‒0.21; SMD = ‒0.69, 95%CI: ‒1.00 to ‒0.37, respectively), while other types of interruption (active sitting, standing, and VPA interruption) did not yield significantly lower postprandial glycemia. Furthermore, MPA-INT was significantly more effective than MPA-CON, STA-INT, and VPA-INT in reducing postprandial glycemia. Similar results were observed for insulin response. LPA-INT and MPA-INT showed significantly better effects in reducing postprandial insulin concentration compared to PS (SMD = ‒0.46, 95%CI: ‒0.66 to ‒0.26; SMD = ‒0.47, 95%CI: ‒0.77 to ‒0.17, respectively). In addition, LPA-INT and MPA-INT were more effective than STA-INT in reducing postprandial insulin.
Fig. 3.
Comparisons of the effects (SMD (95%CI)) of different prolonged sitting interruption methods on postprandial (A) glucose and (B) insulin response using network meta-analysis. Significant differences are highlighted by bold type. 95%CI = 95% confidence interval; LPA-CON = light physical activity one bout of continuous interrupting; LPA-INT = light-intensity physical activity intermittent interrupting; MPA-CON = moderate-intensity physical activity one bout of continuous interrupting; MPA-INT = moderate-intensity physical activity intermittent interrupting; PS = prolonged sitting; SitActive = active sitting interrupting; SMD = standardized mean difference; STA-CON = standing one bout of continuous interrupting; STA-INT = standing intermittent interrupting; VPA-CON = vigorous-intensity physical activity one bout of continuous interrupting; VPA-INT = vigorous-intensity physical activity intermittent interrupting.
The test of global inconsistency did not show any significant differences between the consistency and inconsistency models for postprandial glycemia (p = 0.98) or for insulin response (p = 0.78). The test for local inconsistency also revealed no significant differences between direct and indirect estimates in all closed loops for postprandial glycemia and insulin responses (Supplementary Figs. 1 and 2). Similarly, the test for inconsistency in the node-splitting model indicated that all comparisons among direct and indirect estimates were consistent for postprandial glycemia and insulin responses (Supplementary Tables 4 and 5). Furthermore, publication bias was not evident based on the comparison-adjusted funnel plot for the outcomes (Supplementary Figs. 3 and 4).
The results of subgroup NMA for postprandial glycemia and insulin responses were not marginally modified for most of the comparisons (Supplementary Tables 6 and 7). It is worth noting that LPA-INT achieved the benefit of reduced postprandial glycemia for relatively older participants (≥45 years of age) compared with relatively young participants (<45 years of age). Similarly, LPA-INT was effective in reducing postprandial glycemia and insulin responses among participants who were overweight/obese than among those whose weight status was normal. Moreover, the results also indicated that the 2-min LPA-INT intervention strategy applied every 20–30 min significantly reduced postprandial glycemia and insulin responses compared to the PS condition.
3.6. Ranking probability
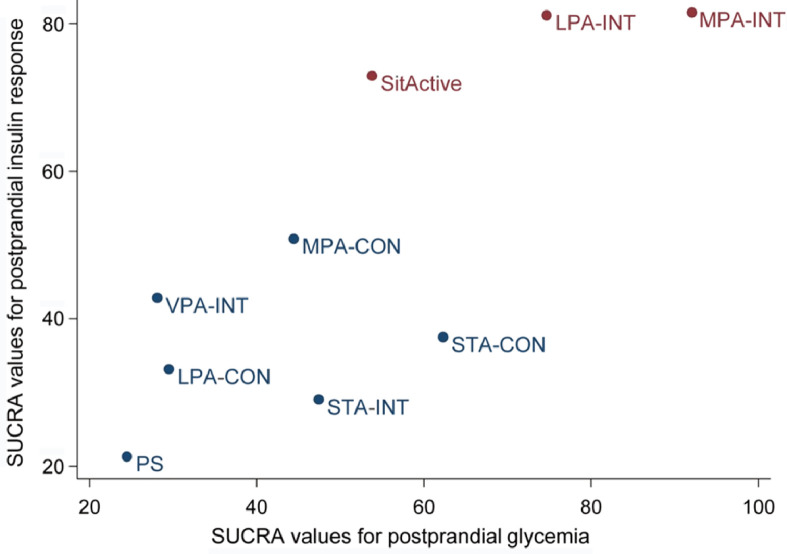
The ranking of intervention effectiveness based on the cumulative probability and SUCRAs are shown in Supplementary Figs. 5 and 6. For postprandial glycemia, the most effective intervention was MPA-INT (91.8%) and the least effective intervention was VPA-INT (28.4%). For the postprandial insulin response, the most effective intervention was also MPA-INT (81.5%) and the least effective intervention was STA-INT (29.0%). In the clustered ranking plot based on the SUCRA values of intervention methods for postprandial glycemia and insulin response, LPA-INT was comparable to MPA-INT and MPA-INT appeared to be the optimal intervention method (Fig. 4).
Fig. 4.
Clustered ranking plot based on the SUCRA values of intervention methods for postprandial glycemia and insulin response. Intervention methods appearing in the upper right corner are more effective than the other interventions. LPA-CON = light-intensity physical activity one bout of continuous interrupting; LPA-INT = light-intensity physical activity intermittent interrupting; MPA-CON = moderate-intensity physical activity one bout of continuous interrupting; MPA-INT = moderate-intensity physical activity intermittent interrupting; PS = prolonged sitting; SitActive = active sitting interrupting (e.g., sitting on a stability ball); STA-CON = standing one bout of continuous interrupting; STA-INT = standing intermittent interrupting; SUCRA = surface under cumulative ranking curve; VPA-INT = vigorous-intensity physical activity intermittent interrupting.
4. Discussion
4.1. Summary of evidence
The purpose of this review was to evaluate, by quantitatively synthesizing evidence from RCTs, how different characteristics of PA breaks during PS may lower postprandial glycemia and insulin responses. In general, our findings suggest that interrupting PS with MPA-INT and LPA-INT had at least a small effect on reducing postprandial glycemia and insulin responses, with MPA-INT being the optimal intervention strategy.
4.2. Comparisons with previous studies
Findings from our NMA echoed the results of 4 previous reviews. The 1st review on the similar topic was conducted by Benatti and Ried-Larsen,50 whose results suggested that LPA and standing may lead to favorable changes in the postprandial metabolic parameters (e.g., postprandial glycemia) in physically inactive and type 2 diabetes participants, whereas a higher intensity or volume of PA seemed to present positive outcomes in young, physically active participants. However, because the review by Benatti and Ried-Larsen50 had a qualitative design, the magnitude of the effect size on postprandial glycemia and insulin responses brought about by interrupting PS remained unknown.
Three other quantitative systematic reviews have filled some of this knowledge gap.40, 41, 42 In a pairwise meta-analyses combining the results of 6 experimental studies, Chastin and his colleagues40 found that both LPA and MPA interrupting PS had beneficial effects on glycemia and insulin responses. Their results were similar to the findings of Saunders41 and Loh,42 who conducted systematic reviews and meta-analyses of 20 and 37 experimental studies, respectively. Notably, there remained a large knowledge gap with respect to what is the optimal, or at least the minimal, strategy for obtaining positive outcomes from various interrupting PS interventions (i.e., multiple combinations by type and intensity). Therefore, the NMA performed in our study makes robust contributions to the literature beyond the previous findings by quantitatively estimating the effects from 30 RCTs. It provides details on which of the several PS interruption approaches is optimal for lowering postprandial glycemia and insulin responses, thus providing additional information and valuable contributions to this field of research.
4.3. Optimal strategies among different types of interventions for postprandial glycemic and insulin responses
Empirical evidence suggests that PS interruption is associated with more favorable changes in glycemia and insulin metabolism than the changes occurring with uninterrupted PS.40, 41, 42,50 However, the most effective approach regarding the intensity and frequency of PA breaks for reducing postprandial glycemia and insulin responses has remained largely unexplored.
A number of studies have shown that increased PA has a significantly positive effect in lowering postprandial glycemia and insulin responses compared to PS.40, 41, 42,50 Also, the characteristics of PA interventions, such as the intensity of PA interruptions, have potential to moderate the effects of postprandial glycemia and insulin responses. For example, when the dose of PA is identical, a higher-intensity intervention is more likely to yield better results in reducing postprandial glycemia15,17,38 and insulin responses38 compared to PS. However, since energy expenditure was not comparable in most of the cited studies, it was difficult to draw a clear conclusion about whether the positive effects of interrupting PS with different intensities of PA are due to reduced sitting time or to increased energy expenditure. This question prompted researchers to study the comparative energy expenditures among the different interventions.
When energy expenditures were matched, the reduction in sitting time caused by standing and light-intensity walking interventions improved insulin sensitivity in healthy sedentary participants to a greater extent than did structured MPA to VPA.11 This finding was replicated with participants with type 2 diabetes.51 Collectively, these results suggest that the magnitude of glycemia control and insulin sensitivity brought about by reducing sitting time may be more pronounced than the magnitude achieved by increasing exercise during interruptions in sitting.
Furthermore, intermittent interruptions in PS appeared to have more favorable outcomes in reducing postprandial glycemia and insulin responses compared to one bout of continuous interrupting. When the exercise intensity and energy expenditures were comparable, MPA-INT was more effective than MPA-CON.13,20 This indicates that interventions with multiple activity breaks are more effective in lowering postprandial glycemia and insulin responses than an equivalent amount of continuous PA. Therefore, based on the present evidence, reducing the amount of PS through multiple PA breaks may be key to postprandial hyperglycemia and hyperinsulinemia control.
The above notion is also supported by findings in our study, which indicate that interventions with multiple PA breaks, including MPA-INT and LPA-INT, yield significant effects in reducing postprandial glycemia and insulin responses when compared to PS. Also, in our review MPA-INT was more effective than MPA-CON in postprandial glycemia responses, which was consistent with the results when energy expenditures were matched in Loh et al.’s42 systematic review and meta-analysis. Moreover, in our study, although MPA-INT was the optimal strategy based on the SUCRAs, the effect sizes between MPA-INT and LPA-INT on postprandial glycemia and insulin responses were not significantly different. Furthermore, our findings from the subgroup analyses showed that even interrupting PS with 2 min of LPA-INT every 20‒30 min had a significant effect on postprandial glycemia and insulin responses. This finding has important clinical relevance for those in need of postprandial hyperglycemia and hyperinsulinemia control, including those who are overweight/obese or older persons who may be less inclined to engage in structured and more intense PA.
Also, we were surprised that beneficial results were not observed for VPA-INT, which has a higher intensity than MPA-INT and LPA-INT. This finding may have 2 explanations. First, only 3 studies involving VPA-INT were included in our review, while 17 interventions employed LPA-INT and 11 interventions employed MPA-INT. This suggests that more research is needed to identify the effects of VPA-INT on postprandial glycemia and insulin responses. Second, a longer time interval is needed for VPA-INT than for lower-intensity interrupting exercises. Interventions in most of the studies with beneficial findings involved PS interrupted with 2‒3 min of MPA or LPA every 20‒30 min, while the interval for each 2–3-min bout of VPA interrupting PS was 60 min. This short duration of VPA following a longer sitting interval may not be sufficiently frequent to upregulate the physiological mechanisms responsible for glucose utilization.52
4.4. Potential mechanisms
Several potential mechanisms for lowering postprandial glycemia have been proposed: (1) activity breaks activate the signaling pathway caused by muscle contraction, which leads to an increase in the expression of the muscle glucose transporter 4 (GLUT4) protein and mRNA, thereby enhancing glucose uptake (which is independent of the insulin effect),53 (2) activity breaks increase the activity of glycogen synthase and hexokinase, which are related to improvements in insulin sensitivity,54 (3) activity breaks improve the capacity of fat oxidation55 and reduce the concentration of intracellular diacylglycerol and ceramide, which may enhance insulin sensitivity,56,57 and (4) activity breaks change the fatty acid composition of phospholipids in skeletal muscle, which can influence insulin sensitivity.58
4.5. Strengths and limitations
Our review has several strengths. First, to the best of our knowledge, our review is the first to use NMA to quantitatively compare the effectiveness of different PA interventions on postprandial glycemia and insulin responses. This design can provide indirect comparisons of interventions and can suggest the optimal intervention among multiple interventions tested. Second, all studies included in our review were RCTs, which provide more convincing evidence that causal relationships can be drawn between PS interruption and postprandial glycemia and insulin responses. Third, our findings are strengthened by both pairwise analyses and NMAs, and further strengthened by subgroup analyses.
Several limitations, however, should be noted. First, the methods for assessing postprandial responses and the characteristics of interventions (e.g., duration, interval, and length of the experimental period) varied across the included studies. These differences might have generated heterogeneity and lowered the precision of the overall effect estimation. However, subgroup analyses indicated that the findings in our study were not materially modified by the methods used for assessing postprandial responses and the characteristics of interventions. Second, some comparisons are limited by the number of included studies, which may lead to difficulty in identifying the true effect of interventions on study outcomes.59 Also, there were not enough studies to enable us to compare the effects of interrupting PS with different modalities of PA breaks on postprandial glycemia and insulin responses. However, our review has incorporated the findings from the latest and most comprehensive literature. Third, publication bias caused by our exclusion of studies published in languages other than English and Chinese cannot be completely eliminated, although comparison-adjusted funnel plots did not indicate publication bias.
4.6. Implications and future research
The findings in our review provide robust evidence that interrupting PS time plays an important role in reducing postprandial glycemia and insulin responses, which sheds new light on postprandial glycemia and insulin control. The findings indicate that individuals should interrupt PS with at least a 2-min activity break every 20‒30 min, with the goal of reducing the risk of chronic diseases (e.g., CVD and type 2 diabetes) associated with elevated postprandial glycemia and insulin response. Furthermore, we advocate for future studies that address several important issues. First, the findings of our study should be confirmed among CVD and diabetes patients. These patients need postprandial glycemia control; but thus far, published studies are limited in these populations. Second, in addition to postprandial glycemia and insulin responses, other cardiometabolic health outcomes (e.g., blood pressure) should be examined in order to provide clinical settings with empirical evidence on postprandial glycemia control that supports the interruption of PS with activity breaks.
5. Conclusion
The findings of this comprehensive review suggest that interrupting PS with a PA break is an effective intervention for postprandial glycemia and insulin responses. Furthermore, LPA-INT may be considered as a preferred option for those who are overweight/obese or who are ≥45 years of age. Additionally, MPA-INT should be considered as an optimal intervention during PS for individuals with normal weight or who are <45 years of age.
Acknowledgments
Acknowledgments
The authors thank Dr Shi Qu, from the Institute of Urology, West China Hospital of Sichuan University, for his advice on data analysis. This work was supported by grants to MQ from the National Key Research and Development Program of China (2020YFC2003301 and 2020YFC2007005), National Natural Science Foundation of China (81703252), and Shanghai Committee of Science and Technology of China (19080503000); and to HW from the Public Welfare Technology Project of the Zhejiang Science Department (LGF18H170006).
Authors’ contributions
MQ performed the literature search, conceived and designed the study, analyzed the data, and drafted the manuscript; PX analyzed the data, advised on interpretation of the data, and critically revised the manuscript; HW and JW performed the literature search; MC and TZ performed the literature search and assessed the risk of bias of included studies; WC, TH, and ZG critically revised the manuscript; PC advised on the analysis and interpretation of the data and critically revised the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2020.12.006.
Contributor Information
Minghui Quan, Email: quanminghui@163.com.
Peijie Chen, Email: chenpeijie@sus.edu.cn.
Appendix. Supplementary materials
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kern DM, Mellstrom C, Hunt PR. Long-term cardiovascular risk and costs for myocardial infarction survivors in a US commercially insured population. Curr Med Res Opin. 2016;32:703–711. doi: 10.1185/03007995.2015.1136607. [DOI] [PubMed] [Google Scholar]
- 3.Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164:2147–2155. doi: 10.1001/archinte.164.19.2147. [DOI] [PubMed] [Google Scholar]
- 4.Cavalot F, Pagliarino A, Valle M. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: Lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care. 2011;34:2237–2243. doi: 10.2337/dc10-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stout RW. Hyperinsulinaemia–A possible risk factor for cardiovascular disease in diabetes mellitus. Horm Metab Res Suppl. 1985;15:37–41. [PubMed] [Google Scholar]
- 6.Bennie JA, Chau JY, van der Ploeg HP, Stamatakis E, Do A, Bauman A. The prevalence and correlates of sitting in European adults–a comparison of 32 Eurobarometer-participating countries. Int J Behav Nutr Phys Act. 2013;10:107. doi: 10.1186/1479-5868-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Cao C, Kantor ED. Trends in sedentary behavior among the US population, 2001–2016. JAMA. 2019;321:1587–1597. doi: 10.1001/jama.2019.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas A, Oh PI, Faulkner GE. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann Intern Med. 2015;162:123–132. doi: 10.7326/M14-1651. [DOI] [PubMed] [Google Scholar]
- 9.Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur Heart J. 2011;32:590–597. doi: 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunstan DW, Kingwell BA, Larsen R. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35:976–983. doi: 10.2337/dc11-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duvivier BM, Schaper NC, Bremers MA. Minimal intensity physical activity (standing and walking) of longer duration improves insulin action and plasma lipids more than shorter periods of moderate to vigorous exercise (cycling) in sedentary subjects when energy expenditure is comparable. PLoS One. 2013;8:e55542. doi: 10.1371/journal.pone.0055542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newsom SA, Everett AC, Hinko A, Horowitz JF. A single session of low-intensity exercise is sufficient to enhance insulin sensitivity into the next day in obese adults. Diabetes Care. 2013;36:2516–2522. doi: 10.2337/dc12-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peddie MC, Bone JL, Rehrer NJ, Skeaff CM, Gray AR, Perry TL. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: A randomized crossover trial. Am J Clin Nutr. 2013;98:358–366. doi: 10.3945/ajcn.112.051763. [DOI] [PubMed] [Google Scholar]
- 14.Thorp AA, Kingwell BA, Sethi P, Hammond L, Owen N, Dunstan DW. Alternating bouts of sitting and standing attenuate postprandial glucose responses. Med Sci Sports Exerc. 2014;46:2053–2061. doi: 10.1249/MSS.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 15.Bailey DP, Locke CD. Breaking up prolonged sitting with light-intensity walking improves postprandial glycemia, but breaking up sitting with standing does not. J Sci Med Sport. 2015;18:294–298. doi: 10.1016/j.jsams.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Larsen RN, Kingwell BA, Robinson C. Breaking up of prolonged sitting over three days sustains, but does not enhance, lowering of postprandial plasma glucose and insulin in overweight and obese adults. Clin Sci (Lond) 2015;129:117–127. doi: 10.1042/CS20140790. [DOI] [PubMed] [Google Scholar]
- 17.Bailey DP, Broom DR, Chrismas BC, Taylor L, Flynn E, Hough J. Breaking up prolonged sitting time with walking does not affect appetite or gut hormone concentrations but does induce an energy deficit and suppresses postprandial glycaemia in sedentary adults. Appl Physiol Nutr Metab. 2016;41:324–331. doi: 10.1139/apnm-2015-0462. [DOI] [PubMed] [Google Scholar]
- 18.Henson J, Davies MJ, Bodicoat DH. Breaking up prolonged sitting with standing or walking attenuates the postprandial metabolic response in postmenopausal women: A randomized acute study. Diabetes Care. 2016;39:130–138. doi: 10.2337/dc15-1240. [DOI] [PubMed] [Google Scholar]
- 19.Miyashita M, Edamoto K, Kidokoro T. Interrupting sitting time with regular walks attenuates postprandial triglycerides. Int J Sports Med. 2016;37:97–103. doi: 10.1055/s-0035-1559791. [DOI] [PubMed] [Google Scholar]
- 20.Holmstrup M, Fairchild T, Keslacy S, Weinstock R, Kanaley J. Multiple short bouts of exercise over 12-h period reduce glucose excursions more than an energy-matched single bout of exercise. Metabolism. 2014;63:510–519. doi: 10.1016/j.metabol.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen RK, Andersen JB, Vinther AS, Pielmeier U, Larsen RG. Breaking up prolonged sitting does not alter postprandial glycemia in young, normal-weight men and women. Int J Sports Med. 2016;37:1097–1102. doi: 10.1055/s-0042-113466. [DOI] [PubMed] [Google Scholar]
- 22.Hawari NS, Al-Shayji I, Wilson J, Gill JM. Frequency of breaks in sedentary time and postprandial metabolic responses. Med Sci Sports Exerc. 2016;48:2495–2502. doi: 10.1249/MSS.0000000000001034. [DOI] [PubMed] [Google Scholar]
- 23.Wennberg P, Boraxbekk CJ, Wheeler M. Acute effects of breaking up prolonged sitting on fatigue and cognition: A pilot study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-009630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhammar DM, Sawyer BJ, Tucker WJ, Gaesser GA. Breaks in sitting time: Effects on continuously monitored glucose and blood pressure. Med Sci Sports Exerc. 2017;49:2119–2130. doi: 10.1249/MSS.0000000000001315. [DOI] [PubMed] [Google Scholar]
- 25.Brocklebank LA, Andrews RC, Page A, Falconer CL, Leary S, Cooper A. The acute effects of breaking up seated office work with standing or light-intensity walking on interstitial glucose concentration: A randomized crossover trial. J Phys Act Health. 2017;14:617–625. doi: 10.1123/jpah.2016-0366. [DOI] [PubMed] [Google Scholar]
- 26.Kerr J, Crist K, Vital DG. Acute glucoregulatory and vascular outcomes of three strategies for interrupting prolonged sitting time in postmenopausal women: A pilot, laboratory-based, randomized, controlled, 4-condition, 4-period crossover trial. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarthy M, Edwardson CL, Davies MJ. Breaking up sedentary time with seated upper body activity can regulate metabolic health in obese high-risk adults: A randomized crossover trial. Diabetes Obes Metab. 2017;19:1732–1739. doi: 10.1111/dom.13016. [DOI] [PubMed] [Google Scholar]
- 28.Champion RB, Smith LR, Smith J. Reducing prolonged sedentary time using a treadmill desk acutely improves cardiometabolic risk markers in male and female adults. J Sports Sci. 2018;36:2484–2491. doi: 10.1080/02640414.2018.1464744. [DOI] [PubMed] [Google Scholar]
- 29.Sperlich B, De Clerck I, Zinner C, Holmberg HC, Wallmann-Sperlich B. Prolonged sitting interrupted by 6-min of high-intensity exercise: Circulatory, metabolic, hormonal, thermal, cognitive, and perceptual responses. Front Physiol. 2018;9:1279. doi: 10.3389/fphys.2018.01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawari NSA, Wilson J, Gill JMR. Effects of breaking up sedentary time with “chair squats” on postprandial metabolism. J Sports Sci. 2019;37:331–338. doi: 10.1080/02640414.2018.1500856. [DOI] [PubMed] [Google Scholar]
- 31.Maylor BD, Zakrzewski-Fruer JK, Orton CJ, Bailey DP. Beneficial postprandial lipaemic effects of interrupting sedentary time with high-intensity physical activity versus a continuous moderate-intensity physical activity bout: A randomised crossover trial. J Sci Med Sport. 2018;21:1250–1255. doi: 10.1016/j.jsams.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 32.Yates T, Edwardson CL, Celis-Morales C. Metabolic effects of breaking prolonged sitting with standing or light walking in older South Asians and White Europeans: A randomized acute study. J Gerontol A Biol Sci Med Sci. 2020;75:139–146. doi: 10.1093/gerona/gly252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altenburg TM, Rotteveel J, Serne EH, Chinapaw MJM. Standing is not enough: A randomized crossover study on the acute cardiometabolic effects of variations in sitting in healthy young men. J Sci Med Sport. 2019;22:790–796. doi: 10.1016/j.jsams.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Chrismas BCR, Taylor L, Cherif A. Postprandial insulin and triglyceride concentrations are suppressed in response to breaking up prolonged sitting in Qatari females. Front Physiol. 2019;10:706. doi: 10.3389/fphys.2019.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freire YA, Macedo GAD, Browne RAV. Effect of breaks in prolonged sitting or low-volume high-intensity interval exercise on markers of metabolic syndrome in adults with excess body fat: A crossover trial. J Phys Act Health. 2019;16:727–735. doi: 10.1123/jpah.2018-0492. [DOI] [PubMed] [Google Scholar]
- 36.Ma SX, Zhu Z, Zhang L, Liu XM, Lin YY, Cao ZB. Metabolic effects of three different activity bouts during sitting in inactive adults. Med Sci Sports Exerc. 2020;52:851–858. doi: 10.1249/MSS.0000000000002212. [DOI] [PubMed] [Google Scholar]
- 37.Maylor BD, Zakrzewski-Fruer JK, Stensel DJ, Orton CJ, Bailey DP. Effects of frequency and duration of interrupting sitting on cardiometabolic risk markers. Int J Sports Med. 2019;40:818–824. doi: 10.1055/a-0997-6650. [DOI] [PubMed] [Google Scholar]
- 38.Pulsford RM, Blackwell J, Hillsdon M, Kos K. Intermittent walking, but not standing, improves postprandial insulin and glucose relative to sustained sitting: A randomised cross-over study in inactive middle-aged men. J Sci Med Sport. 2017;20:278–283. doi: 10.1016/j.jsams.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Benatti FB, Larsen SA, Kofoed K. Intermittent standing but not a moderate exercise bout reduces postprandial glycemia. Med Sci Sports Exerc. 2017;49:2305–2314. doi: 10.1249/MSS.0000000000001354. [DOI] [PubMed] [Google Scholar]
- 40.Chastin SF, Egerton T, Leask C, Stamatakis E. Meta-analysis of the relationship between breaks in sedentary behavior and cardiometabolic health. Obesity (Silver Spring) 2015;23:1800–1810. doi: 10.1002/oby.21180. [DOI] [PubMed] [Google Scholar]
- 41.Saunders TJ, Atkinson HF, Burr J, MacEwen B, Skeaff CM, Peddie MC. The acute metabolic and vascular impact of interrupting prolonged sitting: A systematic review and meta-analysis. Sports Med. 2018;48:2347–2366. doi: 10.1007/s40279-018-0963-8. [DOI] [PubMed] [Google Scholar]
- 42.Loh R, Stamatakis E, Folkerts D, Allgrove JE, Moir HJ. Effects of interrupting prolonged sitting with physical activity breaks on blood glucose, insulin and triacylglycerol measures: A systematic review and meta-analysis. Sports Med. 2020;50:295–330. doi: 10.1007/s40279-019-01183-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ. 2013;346:f2914. doi: 10.1136/bmj.f2914. [DOI] [PubMed] [Google Scholar]
- 44.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 45.Hutton B, Salanti G, Caldwell DM. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 46.Vucic K, Jelicic Kadic A, Puljak L. Survey of Cochrane protocols found methods for data extraction from figures not mentioned or unclear. J Clin Epidemiol. 2015;68:1161–1164. doi: 10.1016/j.jclinepi.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 47.Wolever TM. Effect of blood sampling schedule and method of calculating the area under the curve on validity and precision of glycaemic index values. Br J Nutr. 2004;91:295–301. doi: 10.1079/bjn20031054. [DOI] [PubMed] [Google Scholar]
- 48.Salanti G, Kavvoura FK, Ioannidis JP. Exploring the geometry of treatment networks. Ann Intern Med. 2008;148:544–553. doi: 10.7326/0003-4819-148-7-200804010-00011. [DOI] [PubMed] [Google Scholar]
- 49.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benatti FB, Ried-Larsen M. The effects of breaking up prolonged sitting time: A review of experimental studies. Med Sci Sports Exerc. 2015;47:2053–2061. doi: 10.1249/MSS.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 51.Duvivier BM, Schaper NC, Hesselink MK. Breaking sitting with light activities vs. structured exercise: A randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes. Diabetologia. 2017;60:490–498. doi: 10.1007/s00125-016-4161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Latouche C, Jowett JB, Carey AL. Effects of breaking up prolonged sitting on skeletal muscle gene expression. J Appl Physiol (1985) 2013;114:453–460. doi: 10.1152/japplphysiol.00978.2012. [DOI] [PubMed] [Google Scholar]
- 53.Dela F, Ploug T, Handberg A. Physical training increases muscle GLUT4 protein and mRNA in patients with NIDDM. Diabetes. 1994;43:862–865. doi: 10.2337/diab.43.7.862. [DOI] [PubMed] [Google Scholar]
- 54.Prats C, Helge JW, Nordby P. Dual regulation of muscle glycogen synthase during exercise by activation and compartmentalization. J Biol Chem. 2009;284:15692–15700. doi: 10.1074/jbc.M900845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinson SL, Hattersley J, Frost GS, Chambers ES, Wallis GA. Maximal fat oxidation during exercise is positively associated with 24-hour fat oxidation and insulin sensitivity in young, healthy men. J Appl Physiol (1985) 2015;118:1415–1422. doi: 10.1152/japplphysiol.00058.2015. [DOI] [PubMed] [Google Scholar]
- 56.Bergman BC, Brozinick JT, Strauss A. Muscle sphingolipids during rest and exercise: A C18:0 signature for insulin resistance in humans. Diabetologia. 2016;59:785–798. doi: 10.1007/s00125-015-3850-y. [DOI] [PubMed] [Google Scholar]
- 57.Dube JJ, Amati F, Toledo FG. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia. 2011;54:1147–1156. doi: 10.1007/s00125-011-2065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andersson A, Sjodin A, Olsson R, Vessby B. Effects of physical exercise on phospholipid fatty acid composition in skeletal muscle. Am J Physiol. 1998;274:E432–E438. doi: 10.1152/ajpendo.1998.274.3.E432. [DOI] [PubMed] [Google Scholar]
- 59.Su X, McDonough DJ, Chu H. Application of network meta-analysis in the field of physical activity and health promotion. J Sport Health Sci. 2020;9:511–520. doi: 10.1016/j.jshs.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.