Abstract
Stroke is considered a leading cause of mortality and neurological disability, which puts a huge burden on individuals and the community. To date, effective therapy for stroke has been limited by its complex pathological mechanisms. Autophagy refers to an intracellular degrading process with the involvement of lysosomes. Autophagy plays a critical role in maintaining the homeostasis and survival of cells by eliminating damaged or non-essential cellular constituents. Increasing evidence support that autophagy protects neuronal cells from ischemic injury. However, under certain circumstances, autophagy activation induces cell death and aggravates ischemic brain injury. Diverse naturally derived compounds have been found to modulate autophagy and exert neuroprotection against stroke. In the present work, we have reviewed recent advances in naturally derived compounds that regulate autophagy and discussed their potential application in stroke treatment.
KEY WORDS: Autophagy, Cerebral ischemia, Neuroprotection, Mitochondria, Lysosomal activation, Mitophagy, Natural compounds, Neurological disorders
Abbreviations: AD, Alzheimer's disease; ALS, amyotrophic lateral sclerosis; AMPK, 5′-adenosine monophosphate-activated protein kinase; ATF6, activating transcription factor 6; ATG, autophagy related genes; BCL-2, B-cell lymphoma 2; BNIP3L, BCL2/adenovirus; COPII, coat protein complex II; ER, endoplasmic reticulum; eIF2a, eukaryotic translation-initiation factor 2; FOXO, forkhead box O; FUNDC1, FUN14 domain containing 1; GPCR, G-protein coupled receptor; HD, Huntington's disease; IRE1, inositol-requiring enzyme 1; IPC, ischemic preconditioning; JNK, c-Jun N-terminal kinase; LAMP, lysosomal-associated membrane protein; LC3, light chain 3; LKB1, liver kinase B1; mTOR, mechanistic target of rapamycin; ΔΨm, mitochondrial membrane potential; TIGAR, TP53-induced glycolysis and apoptosis regulator; PD, Parkinson's disease; PERK, protein kinase R (PKR)-like endoplasmic reticulum kinase; PI3K, phosphatidylinositol 3-kinase; OGD/R, oxygen and glucose deprivation-reperfusion; ROS, reactive oxygen species; SQSTM1, sequestosome 1; TFEB, transcription factor EB; ULK, Unc-51- like kinase; Uro-A, urolithin A
Graphical abstract
This review summarizes the current knowledge on natural compounds, focusing on the potential implication of stroke, acting as modulators of autophagy.
1. Introduction
Ischemic stroke is characterized by the prompt loss of blood supply to brain regions, which leads to neuronal death, serious neurological deficiency, disability, and even mortality1,2. Ischemic stroke has been considered as one of the leading causes of neurological deficit and mortality worldwide3. To date, recombinant tissue plasminogen activator (rtPA) is the only therapeutically U.S. Food and Drug Administration (FDA)-approved drug for cerebral ischemic stroke. This strategy, however, is limited by a 3–4.5 hours' time window after ischemia onset and increases risk of intracerebral hemorrhage, making only a few patients (5%) benefit from it4, 5, 6. Besides thrombolysis, a variety of neuroprotectants that were effective in the pre-clinical stage, are found ineffective for human stroke7. This gap may partly attribute to the complex mechanisms underlying cerebral ischemia. Therefore, cutting-edge research for a better understanding of ischemic neuronal injury will provide opportunities to develop novel drugs protecting against stroke.
In ischemic brains, there is a deficiency of nutrients and oxygen, which potentially activates autophagy8, a term refers to the intracellular catabolic mechanism via lysosomes. Canonically, autophagy is activated by starvation situations like nutrient deprivation. Autophagy consequently leads to the removal of organelles and proteins to compensate for the starvation. The crucial function of autophagy has been documented in a broad spectrum of human diseases including cerebral ischemia. Either ischemia or the reperfusion process after ischemia has been associated with autophagy activation9,10. A variety of biomarkers of activated autophagy have been identified in the ischemic brains of mice11. It is still under debate, however, regarding the contribution of autophagy to cerebral ischemia. Autophagy has been reported to induce neuronal death in ischemic brains12,13 and blockage of autophagy protected brains from focal ischemic injury14. However, emerging evidence support the neuroprotective roles of autophagy by degrading neuronal organelles and proteins11,15,16. In addition, inhibition of autophagy either by deletion of Park 2 or Sirtuin 1 or by silencing of Atg 7 or Tsc1, further aggravated the ischemic neuronal injury11,17,18. Conversely, autophagy activation exerts the neuroprotective effect in ischemic brains and/or neuronal cells16,19,20. Accumulating evidence indicated the benefits of autophagy-activating drugs, including rapamycin, carbamazepine, and tyrosine kinase inhibitors in reducing ischemic brain injury21. Nevertheless, the prominent adverse effects of these drugs may impede their application in stroke therapy. Overall, autophagy was intimately linked to the pathology of stroke, and the contribution of autophagy in ischemic brains has been comprehensively reviewed recently8,20.
Natural products are derived from different natural sources. Increasing evidence emphasize a beneficial role of these naturally derived compounds for the prevention and therapy of human diseases including stroke22, 23, 24. Little is known about the role of natural products as modulators of autophagy for the treatment of ischemic stroke, although an epidemiological study suggested a direct correlation between a natural product-rich diet and neuroprotection, as well as a lower risk and severity of stroke25. The identification of compounds that can modulate autophagy is valuable for the development of novel therapy for ischemic stroke. In this review, we summarize the natural compounds that attenuate cerebral ischemia with properties of modulating the autophagy, and their application, pharmacological mechanisms, as well as the limitations. In addition, some natural compounds that showed potential application for neurological diseases by modulating autophagy, but have not been identified as potential anti-stroke drugs were also discussed.
2. Molecular machinery of autophagy
Because the detailed mechanisms underlying autophagy has been elegantly reviewed elsewhere26,27, here we only briefly overview the machinery of autophagy. Autophagy can be divided into several steps and finely controlled by distinct autophagy-linked genes (ATGs). Up to now, at least 32 ATG genes have been identified, and they play a critical role in regulating autophagy processes, including vesicle initiation, vesicle nucleation, elongation and completion, vesicle docking and fusion with lysosomes, and degradation of vesicle contents.
2.1. Formation of phagophore and vesicle initiation
The source of phagophore membranes is enigmatic. Multiple sources have been proposed, including the endoplasmic reticulum (ER)28, the endoplasmic reticulum–Golgi intermediate compartment29, the plasma membrane30, recycling endosomes31, the Golgi complex32, and lipid droplets33. The soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins allow the recruitment of proteins that enable the maturation of phagophores30,34.
The Unc-51-like kinase (ULK) complex, which is crucial for autophagy initiation, consists of ULK1, ULK2, a mammalian homolog of Atg 13 (mATG13), the scaffold protein FIP20035, 36, 37, and Atg 101 (an Atg13-binding protein)38. In steady-state, the mammalian target of rapamycin (mTOR) complex 1 (mTORC1) is bound to the ULK complex and phosphorylates ULK1 (or ULK2) and mATG13, resulting in autophagy inhibition. However, during nutrients deprivation, mTORC1 is disassociated from the ULK1 complex and thus triggers autophagy35, 36, 37,39.
2.2. Vesicle nucleation
In vesicle nucleation, phosphatidylinositol 3-kinase (PI3K) complexes serve as a signaling hub and regulate autophagy diversely. The class I PI3K complex suppresses while the class III PI3K complex promotes autophagy. In particular, beclin 1 regulates autophagy as a component of class III PI3K complex40,41.
2.3. Vesicle expansion and completion
Two ubiquitin-like conjugation systems are required to complete the vesicle expansion, namely the Atg12–Atg5–Atg16L1 complex and the Atg8s-phosphatidylethanolamine conjugation42,43. The Atg12–Atg5 conjugation further interacts with Atg16L to form the Atg16L complex43. On the other hand, the ATG8s (including LC3, GABARAP, GABARAPL1, etc.) are cleaved by ATG4 to generate cytosolic LC3-I which subsequently converts into LC3-II (the lipidated form of LC3-I) by conjugating to PE. The LC3-II is then recruited to the membrane of phagophore44,45.
2.4. Maturation (fusion with lysosomes) and autophagosome degradation
Autophagosomes maturation process refers to the autophagosomes fusion with endosomes/lysosomes, and the subsequent acidification of these fused autophagic vesicles. This process is regulated by the lysosomal-associated membrane protein 1 (LAMP-1), LAMP-2 and the small GTPase Rab 7, which participate in the recycling of lysosomal metabolites. The autophagic pathway depends on these steps for the autophagy flux46, 47, 48. Autophagosomes and their containing are degraded by lysosomal acid hydrolases including cathepsins B, D and L, and the degraded materials are diffused back into the cytosol from autophagolysosomes.
3. Regulation of autophagy
In mammalian cells, autophagy is precisely controlled by multiple signaling pathways, e.g., mTOR and serine/threonine kinase pathways, which can be regulated by a variety of small molecules that affect autophagy.
3.1. mTOR-dependent signaling pathways
mTOR involves in two protein complexes with diverse functions, mTORC1 that impedes autophagy and mTOR complex 2 (mTORC2) which is not closed with autophagy regulation49. mTORC1 senses amino acids, ATP and growth factors and suppresses autophagy in normal condition50. mTORC1 integrates upstream signals that inhibit autophagy via the class I PI3K-AKT/protein kinase B (PKB). The activated class I PI3K phosphorylates plasma membrane lipids which trigger the AKT by PDK151,52. Then the activated AKT further phosphorylates the tuberous sclerosis protein 2 (TSC2) and obstruct its interplay with TSC1, and ultimately results in mTORC1 activation53. AMP-activated protein kinase (AMPK) also regulates the mTORC1 and serves as an energy sensor41. AMPK is stimulated by a decline in ATP concentration during ischemia that increases the AMP/ATP ratio54,55. Consequently, TSC1/2 is phosphorylated by AMPK and inhibits mTORC1 activity through Rheb.
3.2. mTOR-independent signaling pathways
Autophagy is also regulated independently of the mTOR pathway. In nutrient-rich environments, beclin 1 binds to B-cell lymphoma 2 (BCL-2), which is an antiapoptotic BCL-2 family protein. Upon nutrient deprivation, BCL-2 is phosphorylated by Jun N-terminal kinase 1 (JNK1) and thus separate from beclin 1, which promotes autophagosome initiation56. Noteworthy, beclin 1 may also play a part in autophagosomes maturation57,58. Besides, two downstream cascades of RAS, namely RAS–PtdIns3K and RAS–RAF-1–ERK1/2 pathways, serve as effectors in regulating autophagy oppositely59. These signaling pathways provide an alternative manner to sense growth factor or amino acids absence in mTOR-independent way.
3.3. Transcription factor EB (TFEB)
Autophagy–lysosome pathway is controlled by the transcriptional factor TFEB. TFEB modulates cellular anabolism and catabolism by coordinating lysosomal and nucleus function. In steady state, TFEB locates in the cytosol and translocates into the nucleus during starvation. The translocated TFEB promotes the transcription of target genes that encode critical proteins for autophagosomes and lysosomes biogenesis60,61.
4. Autophagy activation in ischemic stroke
Once a stroke episode occurs, a variety of stress factors may participate in autophagy activation in ischemic neurons. These factors may include, but not limited to, the production of reactive oxygen species (ROS), the aggregation of misfolded proteins, the intracellular calcium overload, bioenergetic crisis, and dramatic loss of amino acids62. Unfolded proteins induce ER stress, which triggers autophagy via several signaling pathways63, 64, 65. Among the response to the protein to ER stress, protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE1) and activating transcription factor 6 (ATF6) serve as sensor proteins that are bound and suppressed by the ER chaperone glucose-regulated proteins (GRP-78) under normal conditions66. In the occurrence of ER stress, GRP-78 is detached from these sensor proteins and interacts with misfolded proteins, consequently activating the sensors67. Specifically, in the case of ischemia, PERK phosphorylates eukaryotic translation-initiation factor 2 (eIF2α) and upregulates the autophagy-related proteins like ATG1268. Additionally, ischemia also triggers the pathways downstream of TRAF2 and IRE169. After being translocated and cleaved in Golgi apparatus, ATF6 is activated and further induces the transcription of ER chaperones and other components to degrade proteins that are essential to the ER70.
As a result of calcium overload and ATP exhaustion in ischemic neurons, CaMKK and LKB1 are activated and phosphorylate AMPK15. AMPK phosphorylates Raptor or TSC2 and thus inhibits the mTOR pathway to induce autophagy71,72. Additionally, β-arrestin 1, a scaffold protein that enables Vps34 and beclin 1 interaction, is upregulated in the context of cerebral ischemia. Moreover, β-arrestin 1 knockout enhanced the vulnerability of mice to ischemic brain injury, which may be resulted from the autophagy deficiency73.
Oxidative stress caused by accumulated ROS is an inevitable consequence of ischemic brains. Increasing ROS upregulates TP53, which functions as an autophagy inducer. The TP53-induced glycolysis and apoptosis regulator (TIGAR) and DNA damage-regulated autophagy modulator (DRAM), both of which mediate the transcription of TP53, play crucial roles in autophagy regulation74. Besides, as a result of reduced oxygen supply, the hypoxia sensor HIF-1α triggers the transcription of mitophagy receptors including FUNDC1, BNIP3 and NIX75,76. Moreover, ROS activates NRF2, which further upregulates the expression of sequestosome 1 (SQSTM1), the adaptor protein involved in selective autophagy77. ROS accumulation also activates FOXO3, which increases the abundance of LC3 to promote autophagosomes generation75,78. In addition, ROS accumulation causes PERK pathway activation79. All these above-mentioned pathways link oxidative stress with autophagy induction in ischemic neurons.
5. The role of autophagy in cerebral ischemia
Autophagy activation has been proved in several animal models of brain ischemia although the role of autophagy remains controversial8,80. The contribution of autophagy to ischemic stroke is likely to rely on the activity of autophagy whilst overactivated autophagy promotes neuronal cell demise81. Autophagy has also been identified in brains subjected to ischemia/reperfusion (I/R) injury. As revealed in focal brain ischemia models, autophagy activation was observed at the lesion boundary and treatment of 3-methyladenine, an autophagy inhibitor, significantly decreases the volumes of brain infarction even when it was administrated as late as 3 h after ischemia12,82. In addition, in a chronic phase after ischemic insult, autophagy inhibition was shown to improve motor function of mice83. These data emphasized the detrimental role of autophagy activation in ischemic brains. However, as a double-edge-sword in brain ischemia8,20,83, autophagy has also been proved to confer neuroprotection. For example, beclin 1 is triggered following neonatal hypoxia-ischemia (HI)84 and focal cerebral ischemia85 and is associated with a rise in LC3-II. In neonatal cerebral HI, there is also an increase in lysosomal activity in impaired cortical and hippocampal CA3 neurons. In these models, the increased turnover of LC3-II suggested an enhancement in the autophagic flux86. Autophagy removes damaged mitochondria (mitophagy) during the reperfusion phase, which attenuates mitochondria-induced neuronal apoptosis and ischemic damage11. In addition, moderate ER stress secured against ischemic brain injury during reperfusion by improving mitophagy16. Further researches demonstrated that both PARK2 and NIX involve in the mitophagy induction in a mutually independent manner19,87. Most recently the intracellular process of mitophagy in ischemic neurons has been revealed88. Thus, autophagy has a neuroprotective role in ischemic models, both in vitro and in vivo. Additionally, it comes to a consensus that autophagosome does not simply pick its cargo “randomly”. Rather, several types of selective autophagy have been identified in ischemic brains. Besides the afore mentioned mitophagy, current evidence implies the involvement of ER-phagy, pexophagy and ribophagy in the pathology of cerebral ischemia. However, the underlying mechanisms and contribution of these selective autophagy processes to ischemia were not fully understood89, 90, 91, 92.
6. Natural compounds modulating autophagy with potential implication for cerebral ischemia
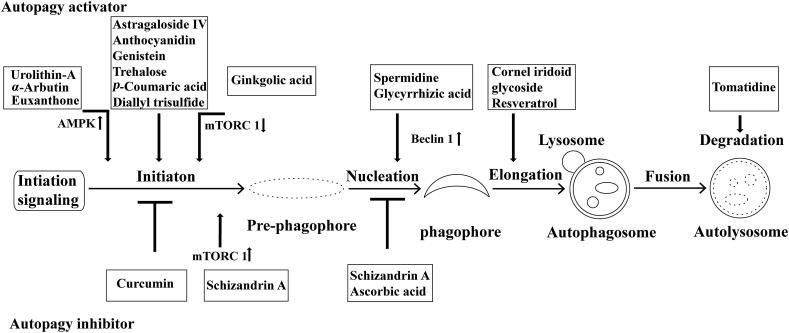
A variety of natural compounds are reported to modulate autophagy in neuronal cells. Interestingly, most of the literature described the efficacy of natural compounds in autophagy induction, but only a few researches documented autophagy inhibition by natural compounds. Here we collected the current evidence indicating the neuroprotective effect of these natural compounds in experimental stroke models. Besides, the autophagy-modulating compounds showing beneficial effects in other neurological disorders except for ischemia were also introduced (Table 193, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151).
Table 1.
Effect of natural compounds on the modulation of autophagy in stroke or other neurological disorders.
| Natural compound | Autophagy regulation | Potential implication | Ref. |
|---|---|---|---|
Urolithin A 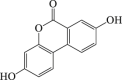
|
Autophagy flux↑LC3-II ↑ SQSTM1 ↓ |
Parkinson's disease94 Cerebral stroke96 |
93, 94, 95, 96 |
Tomatidine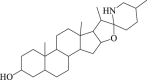
|
Autophagy flux↑ Lysosomal activation ↑ |
Alzheimer's disease97 Cerebral stroke100 |
97, 98, 99, 100 |
Spermidine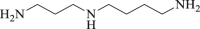
|
Autophagy flux ↑ Beclin 1 ↑ SQSTM1 ↓ |
Cerebral stroke103,104 | 101, 102, 103, 104 |
Anthocyanidin 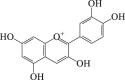
|
LC3-II ↑ ROS ↓ |
Cerebral stroke105 | 105 |
Astragaloside IV 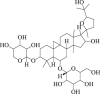
|
LC3-II ↑ SQSTM1 ↓ |
Parkinson's disease106 Depression107 Cerebral ischemia108 |
106, 107, 108 |
Curcumin 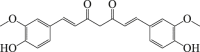
|
Autophagy flux ↓ LC3-II ↓ SQSTM1 ↑ |
Cerebral stroke111,112 | 109, 110, 111, 112 |
Schizandrin A 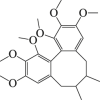
|
Beclin 1↓ LC3-II ↓ mTORC 1 ↑ AMPK↓ | Cerebral stroke113, 114, 115 | 113, 114, 115 |
Ascorbic acid 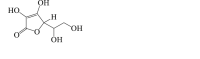
|
Beclin 1↓ LC3-II ↓ |
Neurotoxicity116 Seizure117 |
116,117 |
Ginkgolic acid 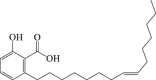
|
mTORC 1 ↓ LC3-II ↑ Beclin 1 ↑ ATG-5 ↑ |
Parkinson's disease120 | 118, 119, 120 |
α-Arbutin 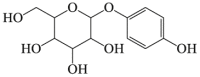
|
ROS ↓ AMPK-p62 autophagy pathway |
Parkinson's disease122,123 | 121, 122, 123 |
Glycyrrhizic acid 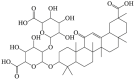
|
LC3-II ↑ Beclin 1 ↑ |
Parkinson's disease125,126 | 124, 125, 126 |
Euxanthone 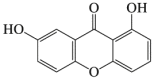
|
LC3-II ↑ Beclin 1 ↑ SQSTM1 ↓ |
Neurite outgrowth126 Alzheimer's disease129 |
127, 128, 129 |
Morroniside 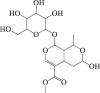 Loganin 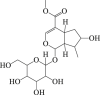
|
LC3-II ↑ Beclin-1 ↑ ATG7 ↑ ATG12 ↑ |
Chronic cerebral hypoperfusion130 Traumatic brain injury131 Alzheimer's disease132 |
130, 131, 132 |
Resveratrol 
|
LC3-II ↑ ATG 4 ↑ SQSTM1 ↓ |
Spinal cord injury133 Alzheimer's disease134 Huntington's disease135,136 |
133, 134, 135, 136 |
Genistein 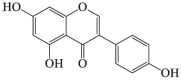
|
LC3-II ↑ TFEB ↑ |
Alzheimer's disease139 Parkinson's disease140 Huntington's disease141 |
137, 138, 139, 140, 141 |
Trehalose 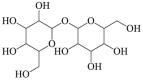
|
TFEB ↑ LC3-II ↑ |
Parkinson's disease143 Alzheimer's disease144 Amyotrophic lateral sclerosis145 |
142, 143, 144, 145 |
p-Coumaric acid 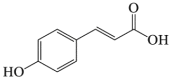
|
LC3-II ↑ SQSTM1 ↓ |
Amyotrophic lateral sclerosis148 | 146, 147, 148 |
Diallyl trisulfide 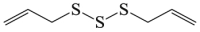
|
LC3-II ↑ ROS ↓ |
Amyotrophic lateral sclerosis150,151 | 149, 150, 151 |
6.1. Urolithin A (Uro-A)
Uro-A is a natural polyphenol that is rendered with ellagitannins and ellagic acid by human gut microbiota152. Several types of research indicated that Uro-A induced autophagy in human colorectal cancer cells and macrophages, and protects against kidney injury though TFEB activation93,153. Emerging data have showed that Uro-A protects against Parkinson's disease by enhancement of neuronal survival94. Uro-A induces mitophagy in Caenorhabditis elegans and murine cells95. In addition, our previous study showed that in neuronal ischemic models, pre-treatment with Uro-A triggers autophagy in both cultured cells and mice brains. Uro-A-induced autophagy attenuated ER stress and thus prevented neuronal cell demise. Autophagy induction by Uro-A was required for its neuroprotective effect96. Interestingly, Uro-A failed to induce mitophagy in ischemic neurons as it does in C. elegans. It is possible due to that Uro-A failed to reinforce the loss of mitochondrial membrane potential (ΔΨm) in ischemic neurons, whose ΔΨm has extensively lost. As a comparison, Uro-A caused the loss of ΔΨm in intact cells, which might be sufficient for mitophagy induction95.
6.2. Tomatidine
Tomatidine is a steroidal alkaloid derived from the Solanaceae family. It has documented that in SH-SY5Y cells, tomatidine abolished oxidative stress by increasing the antioxidant enzyme activity as well as reducing apoptosis97. Emerging studies have indicated that tomatidine activates autophagic degradation in a variety of species98,99. Furthermore, our recent data indicated that the treatment of N2a cells with tomatidine enhanced autophagic flux mainly through facilitating lysosomal degradation rather than autophagosome generation in the context of ischemia. We also found that tomatidine enhanced the expression of TFEB in the model of OGD/R injury, indicating increased transcriptional activity of TFEB100.
6.3. Spermidine
Spermidine can be found in a variety of food154. Several clinical trials have revealed the benefits of spermidine in improving cognition and memory, but its neuroprotective effect is undetermined in the patients experienced cerebral ischemia155,156. Previous research suggested that spermidine improved cardiac dysfunction following myocardial infarction through enhanced autophagic flux via AMPK/mTOR signaling pathway101. Furthermore, spermidine increases the many species’ lifespan by autophagy induction102,157. It has been revealed that spermidine also increased the neuron number following the ischemia in chicks103. Moreover, spermidine was found to effectively reduce the neuronal injury by autophagy induction either in cultured cells or in animals. Mechanically, spermidine attenuated the staurosporine-induced caspase 3 activation and prevented beclin 1 from cleavage and thus retained beclin 1-mediated autophagy104.
6.4. Anthocyanidin
Anthocyanidins are common plant pigments. Previous research indicated that anthocyanidin switched autophagy to apoptosis in cancer cells158. Mounting evidence from epidemiological studies has suggested that anthocyanins improved cognitive, memory and motor performance of patients with neurodegeneration159,160. Anthocyanins activated autophagy, decreased oxidative stress and protected glial cells that subjected to OGD. Depletion of Atg 5, an essential regulator of autophagy, abolished the neuroprotection conferred by anthocyanin, indicating autophagy is indispensable in the neuroprotective effect of anthocyanin105. Thus, these data provided a rationale for the use of anthocyanin as a preventive agent for ischemic brain dysfunction.
6.5. Astragaloside IV
Astragaloside IV is a saponin isolated from the Astragalus membranaceus161. Studies have shown that astragaloside IV protects dopaminergic neuron from neuroinflammation and oxidative stress in a Parkinson's disease mouse model106 and showed the anti-depression effect107. A recent study revealed that astragaloside IV plays a neuroprotective role against cerebral I/R injury in rats by down-regulating apoptosis. Astragaloside IV promoted autophagy flux by downregulating SQSTM1 in these models, which is required to counteracting ischemia-induced neuronal cell apoptosis108. Intriguingly, SQSTM1-dependent autophagy triggered the intracellular anti-oxidation defenses by releasing the NRF2 from KEAP1, which is a master factor for controlling the transcription of several anti-oxidative enzymes162. Overall, the current data suggested that astragaloside IV could be a potential implication for stroke therapy by induction of autophagy.
6.6. Curcumin
Curcumin is extracted from Curcuma longa Linn163. Several reports have documented that curcumin has antioxidant and anti-inflammatory properties109,164. Curcumin protected against ischemia–reperfusion injury in cardiomyocytes by inhibiting autophagy and apoptosis110. Curcumin can penetrate the blood–brain barrier and emerging data showed that curcumin has a neuroprotective effect against ischemic brain injury via anti-apoptotic effect111,165. Furthermore, curcumin significantly reversed the MCAO-induced increase in the level of LC3-II/I ratio and decrease in SQSTM1 protein expression112. Thus, curcumin is a known bioactive agent capable of protecting against cerebral ischemia through suppressing overactivated autophagy112. Given the multiple bioactivities of curcumin, it was not clear how curcumin modulate autophagy in ischemic neurons, and whether this is a direct effect of curcumin on autophagy regulation.
6.7. Schizandrin A
Schizandrin A is a bioactive lignin compound that is refined from Schisandra chinensis166. Previous research demonstrated that schizandrin A attenuated cerebral ischemia–reperfusion injury by inhibiting the caspase 3 activity of neuronal cells induced by OGD/R113. Furthermore, SMXZF, a combination of Rb1, Rg1, schizandrin, and DT-13 demonstrated the neuroprotection against I/R injury by suppression of autophagy through the AMPK/mTOR and JNK pathways114. Notably, schizandrin A alone was sufficient to inhibit autophagy through the AMPK/mTOR pathway in OGD/R model115. These data suggest the autophagy modulating effect of schizandrin A and highlight its potential implication for stroke therapy.
6.8. Ascorbic acid
Ascorbic acid, also known as vitamin C and ascorbate, is a vitamin found in various food167. Ascorbic acid plays an essential role as an antioxidant, enzyme cofactor, and neuromodulator in brain168,169. The current evidence from bench work supports a neuroprotective role of ascorbic acid against cerebral ischemia170. Nevertheless, ascorbic acid supplementary has little impact on the final outcome of stroke patients, regardless the significantly increased the total antioxidative capacity in the serum from stroke patients171. The antioxidative property of ascorbic acid is critical for its efficacy against methamphetamine-induced neurotoxicity116 and pilocarpine-induced seizure in rats117. Interestingly, it seems that ascorbic acid may either induce or inhibit autophagy in different models117,172. Overall, the apparent discrimination from bench work and clinical trials of ascorbic acid on stroke need further explanation.
6.9. Ginkgolic acid
Ginkgolic acid is a natural compound that is extracted from Ginkgo biloba leaves173. Previous research showed that ginkgolic acid has an anti-cancer effect in human colon cancer by triggering intrinsic apoptosis and autophagy through ROS generation118. The cytotoxicity of ginkgolic acid against HepG2 cells was reversed by 3-MA or beclin 1-specific siRNAs, suggesting the critical role of autophagy119. Recently, the neuroprotective effect of ginkgolic acid was revealed. GA decreased intracytoplasmic aggregates of α-synuclein, a model related to Parkinson's disease (PD) that characterized by impaired mitophagy. The efficacy of ginkgolic acid was accompanied by increased autophagosomes, and autophagy inhibitors blocked ginkgolic acid-dependent clearance of α-synuclein120. It remained unexplored whether ginkgolic acid can serve as a neuroprotectant against ischemic neuronal injury by autophagy activation.
6.10. α-Arbutin
α-Arbutin is a natural polyphenol that is derived from Ericaceae species174. Interestingly, α-arbutin was used as a cosmetic whitening agent due to its antioxidant effects121,175. The α-arbutin was thus assumed to have potential implications for PD122. Indeed, recent research confirmed the effect of α-arbutin on PD models. In addition, α-arbutin was found to activate AMPK and induction of autophagy pathway123. Given the AMPK also played a key role in regulating autophagy in ischemic neurons176, whether these molecular mechanisms of α-arbutin can be recapitulated in stroke models is worth further verification.
6.11. Glycyrrhizic acid
Glycyrrhizic acid is the main bioactive component of Glycyrrhizae Radix177. Previous study revealed that glycyrrhizic acid induced autophagy by PI3K/AKT/mTOR pathway to reduce inflammatory lung injury124. Glycyrrhizic acid impeded rotenone-induced dopaminergic neurodegeneration125. Furthermore, the study also showed that glycyrrhizic acid upregulated LC3B II/I conversion, beclin 1 expression, and further autophagy in SH-SY5Y neuroblastoma cells, which were reversed by the 3-MA autophagy inhibitor126. Further investigation needs to explore the efficacy of glycyrrhizic acid in ischemic neuronal injury and the potential involvement of autophagy modulation.
6.12. Euxanthone
Euxanthone is a xanthone derivative extracted from plant Polygala caudate127. Euxanthone suppressed ovarian cancer by inducing apoptosis and autophagy128. Furthermore, euxanthone also stimulates neurite outgrowth127, suggesting its therapeutic potential for neurological disorders. Recently research proved that euxanthone displayed the protective effects against neurotoxicity triggered by Aβ in vivo and in vitro. Euxanthone reversed Aβ1–42-reduced neuronal autophagy in the hippocampus129. Furthermore, euxanthone also showed the protection against Aβ1–42-induced oxidative stress and apoptosis by autophagy induction in the neuroblastic PC12 cells129. Its efficacy on ischemic brains needs identification.
6.13. Cornel iridoid glycoside
Cornel iridoid glycoside is the main ingredient of traditional medicinal plant Cornus officinalis178. Cornel iridoid glycoside treatment improved the learning and memory in rats suffered from chronic cerebral hypoperfusion130 and it also protected against traumatic brain injury131. Emerging data indicate that cornel iridoid glycoside promotes the clearance of neurotoxic tau oligomers via autophagy and thus counteracted memory deficits. Mechanically, it upregulated the expressions of ATG7, ATG12, beclin 1, and LC3II both in vivo and in vitro132. Given the autophagy-regulating and neuroprotection properties of cornel iridoid glycoside, its potential implications for cerebral stroke are worth further investigation.
6.14. Resveratrol
Resveratrol is a common dietary polyphenol179. Accumulating evidence shows that resveratrol has neuroprotective properties against neurological disorder133,134. Resveratrol extended the therapeutic time window of r-tPA and improved the outcome of stroke patients180, although it remained unknown whether these effects were attributed to autophagy modulation. Resveratrol showed the protection against spinal cord injury through the activation of the SIRT1/AMPK autophagy signaling pathway. Previous studies showed that it is useful for the treatment of Huntington's disease135. Recently research demonstrated that resveratrol degraded polyQ-Htt aggregates by restoring the ATG4-mediated autophagosome formation in dopaminergic neuroblastoma SH-SY5Y cells136. However, the potential implications of resveratrol against cerebral stroke remained undetermined.
6.15. Genistein
Genistein is an isoflavone isolated from Dyer's Broom (Genista tinctorial)181. Previous research showed that genistein induced autophagy by inactivating mTOR signaling against podocyte injury137. It has been previously proved that genistein enhanced the lysosomal activities via TFEB against GAGs accumulation in CNS138 and neuroprotective effects of genistein against Alzheimer's disease139 and overexpressing A53T mutant α-synuclein140. Recently research proved that genistein significantly decreased both mutated huntingtin level and a number of aggregates through stimulating autophagy on an HD cellular model but at doses as high as 150 mg/kg/day141. Genistein is a potent neuroprotectant, thus, it would be necessary to further investigate its protection against stroke.
6.16. Trehalose
Trehalose is an essential natural disaccharide that is found in sunflower seeds, moonwort, Selaginella plants and sea algae. Trehalose promoted the clearance of A53T mutant α-synuclein in PC12 cells, which was reversed by 3-methyladenine142,143, indicating the involvement of autophagy in trehalose-induced neuroprotection. Besides, trehalose reverted protein processing abnormalities by lowering the ROS generation, caspase 3 levels and elevating LC3 levels in Huntington's disease142,144. Recently research demonstrated that trehalose regulated autophagy by PPP3- and TFEB-dependent manner in mouse motoneuron-like hybrid cell line145. However, its potential protective effect against cerebral ischemia needs to be further examined in future studies.
6.17. p-Coumaric acid
p-Coumaric acid is a phenolic compound found in Gnetum cleistostachyum182. Previous research confirmed that p-coumaric acid induced growth arrest by activating the autophagy and ROS production146. Furthermore, p-coumaric acid protects against doxorubicin induced cardiotoxicity in H9c2 cells by activating autophagy147. Recently research showed that p-coumaric acid raised the LC3-II level and reduced the SQSTM1 protein level in ALS cell models148. Given the autophagy-regulating and neuroprotection properties of p-coumaric acid, its potential implications for cerebral stroke need to be determined.
6.18. Diallyl trisulfide
Diallyl trisulfide is a major organosulfur compound that found in garlic oil183. Diallyl trisulfide inhibits apoptosis in macrophages by inhibiting mTOR phosphorylation and further autophagy activation149. Diallyl trisulfide also has a neuroprotective effect against SOD1-G93A transgenic mice150. Recently, it has been confirmed that diallyl trisulfide is a promising neuroprotective agent by inducing autophagy and suppressing the increase levels of ROS in ALS151. However, the potential implications of diallyl trisulfide against cerebral stroke remained undetermined.
7. Conclusions
The role of autophagy in cerebral ischemia remains controversial and there are no clinical trials related to modulation of autophagy in treating stroke due to the paucity of knowledge in this field. Nevertheless, autophagy is considered as an endogenous strategy to protect neurons in response to ischemia. Remarkably, some natural compounds serve as neuroprotectants, at least in part, via autophagy modulation (Fig. 1). It should be noted that it cannot be excluded that other mechanisms, e.g., antioxidation and anti-apoptosis, may also contribute greatly to the potential neuroprotection of these natural compounds. The use of natural compounds may lay the foundation for a new pharmacological approach to stroke treatment. Such natural compounds can have the advantage of reducing ischemic brain injury by modulating autophagic processes through multiple targets. Nevertheless, there are a variety of natural compounds known with autophagy-modulating properties that have not been identified as potential anti-stroke drugs. Given the documented neuroprotection of these compounds for the other neurological disorders, their potential implication for stroke would be promising and surely need further investigation.
Figure 1.
Autophagic processes amenable to therapeutic modulation. Several natural compounds are available to activate or inhibit autophagy at the indicated phases of autophagy.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (81822044, 81773703 and 81630098), the Fundamental Research Funds for the Central Universities (2019XZZX004-17, China).
Author contributions
Xiangnan Zhang and Zhong Chen were responsible for the conception and design of the review. Anil Ahsan analyzed the literatures, summarized the results and drafted the manuscript. Mengru Liu, Yanrong Zheng, Wenping Yan, Lin Pan, Yue Li, Shijia Ma, Xingxian Zhang, Ming Cao, Zhanxun Wu, and Weiwei Hu provided critical discussion on the literatures and revised the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Zhong Chen, Email: chenzhong@zju.edu.cn.
Xiangnan Zhang, Email: xiangnan_zhang@zju.edu.cn.
References
- 1.Guo Z.H., Li F., Wang W.Z. The mechanisms of brain ischemic insult and potential protective interventions. Neurosci Bull. 2009;25:139–152. doi: 10.1007/s12264-009-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyle K.P., Simon R.P., Stenzel-Poore M.P. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feigin V.L., Norrving B., Mensah G.A. Global burden of stroke. Circ Res. 2017;120:439–448. doi: 10.1161/CIRCRESAHA.116.308413. [DOI] [PubMed] [Google Scholar]
- 4.Donnan G.A., Fisher M., Macleod M., Davis S.M. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 5.Gladstone D.J., Black S.E., Hakim A.M. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 6.California Acute Stroke Pilot Registry (CASPR) Investigators. Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology. 2005;64:654–659. doi: 10.1212/01.WNL.0000151850.39648.51. [DOI] [PubMed] [Google Scholar]
- 7.Galluzzi L., Bravo-San Pedro J.M., Levine B., Green D.R., Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16:487–511. doi: 10.1038/nrd.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galluzzi L., Bravo-San Pedro J.M., Blomgren K., Kroemer G. Autophagy in acute brain injury. Nat Rev Neurosci. 2016;17:467–484. doi: 10.1038/nrn.2016.51. [DOI] [PubMed] [Google Scholar]
- 9.Sheng R., Zhang L.S., Han R., Liu X.Q., Gao B., Qin Z.H. Autophagy activation is associated with neuroprotection in a rat model of focal cerebral ischemic preconditioning. Autophagy. 2010;6:482–494. doi: 10.4161/auto.6.4.11737. [DOI] [PubMed] [Google Scholar]
- 10.Wang P., Guan Y.F., Du H., Zhai Q.W., Su D.F., Miao C.Y. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy. 2012;8:77–87. doi: 10.4161/auto.8.1.18274. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X., Yan H., Yuan Y., Gao J., Shen Z., Cheng Y. Cerebral ischemia–reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy. 2013;9:1321–1333. doi: 10.4161/auto.25132. [DOI] [PubMed] [Google Scholar]
- 12.Wen Y.D., Sheng R., Zhang L.S., Han R., Zhang X., Zhang X.D. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 2008;4:762–769. doi: 10.4161/auto.6412. [DOI] [PubMed] [Google Scholar]
- 13.Adhami F., Liao G., Morozov Y.M., Schloemer A., Schmithorst V.J., Lorenz J.N. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol. 2006;169:566–583. doi: 10.2353/ajpath.2006.051066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing S., Zhang Y., Li J., Zhang J., Li Y., Dang C. Beclin 1 knockdown inhibits autophagic activation and prevents the secondary neurodegenerative damage in the ipsilateral thalamus following focal cerebral infarction. Autophagy. 2012;8:63–76. doi: 10.4161/auto.8.1.18217. [DOI] [PubMed] [Google Scholar]
- 15.Gabryel B., Kost A., Kasprowska D. Neuronal autophagy in cerebral ischemia—a potential target for neuroprotective strategies?. Pharmacol Rep. 2012;64:1–15. doi: 10.1016/s1734-1140(12)70725-9. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X., Yuan Y., Jiang L., Zhang J., Gao J., Shen Z. Endoplasmic reticulum stress induced by tunicamycin and thapsigargin protects against transient ischemic brain injury: involvement of PARK2-dependent mitophagy. Autophagy. 2014;10:1801–1813. doi: 10.4161/auto.32136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papadakis M., Hadley G., Xilouri M., Hoyte L.C., Nagel S., McMenamin M.M. Tsc1 (hamartin) confers neuroprotection against ischemia by inducing autophagy. Nat Med. 2013;19:351–357. doi: 10.1038/nm.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang P., Xu T.Y., Guan Y.F., Tian W.W., Viollet B., Rui Y.C. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Ann Neurol. 2011;69:360–374. doi: 10.1002/ana.22236. [DOI] [PubMed] [Google Scholar]
- 19.Shen Z., Zheng Y., Wu J., Chen Y., Wu X., Zhou Y. PARK2-dependent mitophagy induced by acidic postconditioning protects against focal cerebral ischemia and extends the reperfusion window. Autophagy. 2017;13:473–485. doi: 10.1080/15548627.2016.1274596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P., Shao B.Z., Deng Z., Chen S., Yue Z., Miao C.Y. Autophagy in ischemic stroke. Prog Neurobiol. 2018;163–164:98–117. doi: 10.1016/j.pneurobio.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Levine B., Packer M., Codogno P. Development of autophagy inducers in clinical medicine. J Clin Invest. 2015;125:14–24. doi: 10.1172/JCI73938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suntar I., Sureda A., Belwal T., Sanches Silva A., Vacca R.A., Tewari D. Natural products, PGC-1α, and Duchenne muscular dystrophy. Acta Pharm Sin B. 2020;10:734–745. doi: 10.1016/j.apsb.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasri H., Baradaran A., Shirzad H., Rafieian-Kopaei M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int J Prev Med. 2014;5:1487–1499. [PMC free article] [PubMed] [Google Scholar]
- 24.Sewell R.D.E., Rafieian-Kopaei M. The history and ups and downs of herbal medicines usage. J HerbMed Pharmacol. 2014;3:1–3. [Google Scholar]
- 25.Ashafaq M., Raza S.S., Khan M.M., Ahmad A., Javed H., Ahmad M.E. Catechin hydrate ameliorates redox imbalance and limits inflammatory response in focal cerebral ischemia. Neurochem Res. 2012;37:1747–1760. doi: 10.1007/s11064-012-0786-1. [DOI] [PubMed] [Google Scholar]
- 26.Dikic I., Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 27.Yu L., Chen Y., Tooze S.A. Autophagy pathway: cellular and molecular mechanisms. Autophagy. 2018;14:207–215. doi: 10.1080/15548627.2017.1378838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamasaki M., Furuta N., Matsuda A., Nezu A., Yamamoto A., Fujita N. Autophagosomes form at ER–mitochondria contact sites. Nature. 2013;495:389–393. doi: 10.1038/nature11910. [DOI] [PubMed] [Google Scholar]
- 29.Ge L., Zhang M., Schekman R. Phosphatidylinositol 3-kinase and COPII generate LC3 lipidation vesicles from the ER–Golgi intermediate compartment. Elife. 2014;3 doi: 10.7554/eLife.04135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreau K., Ravikumar B., Renna M., Puri C., Rubinsztein D.C. Autophagosome precursor maturation requires homotypic fusion. Cell. 2011;146:303–317. doi: 10.1016/j.cell.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pozueta J., Lefort R., Ribe E.M., Troy C.M., Arancio O., Shelanski M. Caspase-2 is required for dendritic spine and behavioural alterations in J20 APP transgenic mice. Nat Commun. 2013;4:1939. doi: 10.1038/ncomms2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohashi Y., Munro S. Membrane delivery to the yeast autophagosome from the Golgi–endosomal system. Mol Biol Cell. 2010;21:3998–4008. doi: 10.1091/mbc.E10-05-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shpilka T., Welter E., Borovsky N., Amar N., Mari M., Reggiori F. Lipid droplets and their component triglycerides and steryl esters regulate autophagosome biogenesis. EMBO J. 2015;34:2117–2131. doi: 10.15252/embj.201490315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nair U., Jotwani A., Geng J., Gammoh N., Richerson D., Yen W.L. SNARE proteins are required for macroautophagy. Cell. 2011;146:290–302. doi: 10.1016/j.cell.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung C.H., Jun C.B., Ro S.H., Kim Y.M., Otto N.M., Cao J. ULK−Atg 13−FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganley I.G., Lam du H., Wang J., Ding X., Chen S., Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosokawa N., Hara T., Kaizuka T., Kishi C., Takamura A., Miura Y. Nutrient-dependent mTORC1 association with the ULK1–Atg 13–FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mercer C.A., Kaliappan A., Dennis P.B. A novel, human Atg 13 binding protein, Atg 101, interacts with ULK1 and is essential for macroautophagy. Autophagy. 2009;5:649–662. doi: 10.4161/auto.5.5.8249. [DOI] [PubMed] [Google Scholar]
- 39.Xiang H., Zhang J., Lin C., Zhang L., Liu B., Ouyang L. Targeting autophagy-related protein kinases for potential therapeutic purpose. Acta Pharm Sin B. 2020;10:569–581. doi: 10.1016/j.apsb.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funderburk S.F., Wang Q.J., Yue Z. The Beclin 1-VPS34 complex—at the crossroads of autophagy and beyond. Trends Cell Biol. 2010;20:355–362. doi: 10.1016/j.tcb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Z., Klionsky D.J. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol. 2001;2:211–216. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- 43.Geng J., Klionsky D.J. The Atg 8 and Atg 12 ubiquitin-like conjugation systems in macroautophagy. ‘Protein modifications: beyond the usual suspects’ review series. EMBO Rep. 2008;9:859–864. doi: 10.1038/embor.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanada T., Noda N.N., Satomi Y., Ichimura Y., Fujioka Y., Takao T. The Atg 12–Atg 5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem. 2007;282:37298–37302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- 45.Fujita N., Itoh T., Omori H., Fukuda M., Noda T., Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell. 2008;19:2092–2100. doi: 10.1091/mbc.E07-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jager S., Bucci C., Tanida I., Ueno T., Kominami E., Saftig P. Role for Rab 7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117:4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka Y., Guhde G., Suter A., Eskelinen E.L., Hartmann D., Lullmann-Rauch R. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406:902–906. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- 48.Codogno P., Meijer A.J. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12(Suppl 2):1509–1518. doi: 10.1038/sj.cdd.4401751. [DOI] [PubMed] [Google Scholar]
- 49.Guertin D.A., Sabatini D.M. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 50.Schmelzle T., Hall M.N. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 51.Alessi D.R., James S.R., Downes C.P., Holmes A.B., Gaffney P.R., Reese C.B. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 52.Stokoe D., Stephens L.R., Copeland T., Gaffney P.R., Reese C.B., Painter G.F. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 53.Long X., Lin Y., Ortiz-Vega S., Yonezawa K., Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 54.Hoyer-Hansen M., Bastholm L., Szyniarowski P., Campanella M., Szabadkai G., Farkas T. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Liang J., Shao S.H., Xu Z.X., Hennessy B., Ding Z., Larrea M. The energy sensing LKB1–AMPK pathway regulates p27(kip 1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 56.Wei Y., Pattingre S., Sinha S., Bassik M., Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang C., Lee J.S., Inn K.S., Gack M.U., Li Q., Roberts E.A. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat Cell Biol. 2008;10:776–787. doi: 10.1038/ncb1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao Y., Klionsky D.J. Physiological functions of Atg 6/Beclin 1: a unique autophagy-related protein. Cell Res. 2007;17:839–849. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- 59.He C., Klionsky D.J. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sardiello M., Palmieri M., di Ronza A., Medina D.L., Valenza M., Gennarino V.A. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 61.Settembre C., Di Malta C., Polito V.A., Garcia Arencibia M., Vetrini F., Erdin S. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Golstein P., Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Wang J., Davis S., Menon S., Zhang J., Ding J., Cervantes S. Ypt1/Rab 1 regulates Hrr25/CK1delta kinase activity in ER–Golgi traffic and macroautophagy. J Cell Biol. 2015;210:273–285. doi: 10.1083/jcb.201408075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoyer-Hansen M., Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 65.Yin Y., Sun G., Li E., Kiselyov K., Sun D. ER stress and impaired autophagy flux in neuronal degeneration and brain injury. Ageing Res Rev. 2017;34:3–14. doi: 10.1016/j.arr.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim I., Xu W., Reed J.C. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 67.Rao R.V., Bredesen D.E. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol. 2004;16:653–662. doi: 10.1016/j.ceb.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu L., Cash T.P., Jones R.G., Keith B., Thompson C.B., Simon M.C. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell. 2006;21:521–531. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Todd D.J., Lee A.H., Glimcher L.H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 70.Flamment M., Hajduch E., Ferre P., Foufelle F. New insights into ER stress-induced insulin resistance. Trends Endocrinol Metab. 2012;23:381–390. doi: 10.1016/j.tem.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Gabryel B., Kost A., Kasprowska D., Liber S., Machnik G., Wiaderkiewicz R. AMP-activated protein kinase is involved in induction of protective autophagy in astrocytes exposed to oxygen-glucose deprivation. Cell Biol Int. 2014;38:1086–1097. doi: 10.1002/cbin.10299. [DOI] [PubMed] [Google Scholar]
- 72.Jiang T., Yu J.T., Zhu X.C., Wang H.F., Tan M.S., Cao L. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014;171:3146–3157. doi: 10.1111/bph.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang P., Xu T.Y., Wei K., Guan Y.F., Wang X., Xu H. ARRB1/beta-arrestin-1 mediates neuroprotection through coordination of BECN1-dependent autophagy in cerebral ischemia. Autophagy. 2014;10:1535–1548. doi: 10.4161/auto.29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheung E.C., Ludwig R.L., Vousden K.H. Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc Natl Acad Sci U S A. 2012;109:20491–20496. doi: 10.1073/pnas.1206530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mahalingaiah P.K., Singh K.P. Chronic oxidative stress increases growth and tumorigenic potential of MCF-7 breast cancer cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu L., Feng D., Chen G., Chen M., Zheng Q., Song P. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14:177–185. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- 77.Puissant A., Fenouille N., Auberger P. When autophagy meets cancer through p62/SQSTM1. Am J Cancer Res. 2012;2:397–413. [PMC free article] [PubMed] [Google Scholar]
- 78.Salcher S., Hermann M., Kiechl-Kohlendorfer U., Ausserlechner M.J., Obexer P. C10ORF10/DEPP-mediated ROS accumulation is a critical modulator of FOXO3-induced autophagy. Mol Cancer. 2017;16:95. doi: 10.1186/s12943-017-0661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim H.J., Joe Y., Kim S.K., Park S.U., Park J., Chen Y. Carbon monoxide protects against hepatic steatosis in mice by inducing sestrin-2 via the PERK–eIF2alpha–ATF4 pathway. Free Radic Biol Med. 2017;110:81–91. doi: 10.1016/j.freeradbiomed.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 80.Wei K., Wang P., Miao C.Y. A double-edged sword with therapeutic potential: an updated role of autophagy in ischemic cerebral injury. CNS Neurosci Ther. 2012;18:879–886. doi: 10.1111/cns.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Acevedo Arozena A. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J.Y., Xia Q., Chu K.T., Pan J., Sun L.N., Zeng B. Severe global cerebral ischemia-induced programmed necrosis of hippocampal CA1 neurons in rat is prevented by 3-methyladenine: a widely used inhibitor of autophagy. J Neuropathol Exp Neurol. 2011;70:314–322. doi: 10.1097/NEN.0b013e31821352bd. [DOI] [PubMed] [Google Scholar]
- 83.Wu X.L., Lu S.S., Liu M.R., Tang W.D., Chen J.Z., Zheng Y.R. Melatonin receptor agonist ramelteon attenuates mouse acute and chronic ischemic brain injury. Acta Pharmacol Sin. 2020;41:1016–1024. doi: 10.1038/s41401-020-0361-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carloni S., Buonocore G., Balduini W. Protective role of autophagy in neonatal hypoxia–ischemia induced brain injury. Neurobiol Dis. 2008;32:329–339. doi: 10.1016/j.nbd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 85.Rami A., Langhagen A., Steiger S. Focal cerebral ischemia induces upregulation of Beclin 1 and autophagy-like cell death. Neurobiol Dis. 2008;29:132–141. doi: 10.1016/j.nbd.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 86.Ginet V., Puyal J., Clarke P.G., Truttmann A.C. Enhancement of autophagic flux after neonatal cerebral hypoxia–ischemia and its region-specific relationship to apoptotic mechanisms. Am J Pathol. 2009;175:1962–1974. doi: 10.2353/ajpath.2009.090463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuan Y., Zheng Y., Zhang X., Chen Y., Wu X., Wu J. BNIP3L/NIX-mediated mitophagy protects against ischemic brain injury independent of PARK2. Autophagy. 2017;13:1754–1766. doi: 10.1080/15548627.2017.1357792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng Y., Zhang X., Wu X., Jiang L., Ahsan A., Ma S. Somatic autophagy of axonal mitochondria in ischemic neurons. J Cell Biol. 2019;218:1891–1907. doi: 10.1083/jcb.201804101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Su Y., Li F. Endoplasmic reticulum stress in brain ischemia. Int J Neurosci. 2016;126:681–691. doi: 10.3109/00207454.2015.1059836. [DOI] [PubMed] [Google Scholar]
- 90.Conway O., Akpinar H.A., Rogov V.V., Kirkin V. Selective autophagy receptors in neuronal health and disease. J Mol Biol. 2020;432:2483–2509. doi: 10.1016/j.jmb.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 91.Kim K.A., Shin D., Kim J.H., Shin Y.J., Rajanikant G.K., Majid A. Role of autophagy in endothelial damage and blood–brain barrier disruption in ischemic stroke. Stroke. 2018;49:1571–1579. doi: 10.1161/STROKEAHA.117.017287. [DOI] [PubMed] [Google Scholar]
- 92.Carloni S., Albertini M.C., Galluzzi L., Buonocore G., Proietti F., Balduini W. Increased autophagy reduces endoplasmic reticulum stress after neonatal hypoxia-ischemia: role of protein synthesis and autophagic pathways. Exp Neurol. 2014;255:103–112. doi: 10.1016/j.expneurol.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y., Huang H., Jin Y., Shen K., Chen X., Xu Z. Role of TFEB in autophagic modulation of ischemia reperfusion injury in mice kidney and protection by urolithin A. Food Chem Toxicol. 2019;131:110591. doi: 10.1016/j.fct.2019.110591. [DOI] [PubMed] [Google Scholar]
- 94.Kujawska M., Jourdes M., Kurpik M., Szulc M., Szaefer H., Chmielarz P. Neuroprotective effects of pomegranate juice against Parkinson's disease and presence of ellagitannins-derived metabolite-urolithin A-in the brain. Int J Mol Sci. 2019;21:202. doi: 10.3390/ijms21010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ryu D., Mouchiroud L., Andreux P.A., Katsyuba E., Moullan N., Nicolet-Dit-Felix A.A. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22:879–888. doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
- 96.Ahsan A., Zheng Y.R., Wu X.L., Tang W.D., Liu M.R., Ma S.J. Urolithin A-activated autophagy but not mitophagy protects against ischemic neuronal injury by inhibiting ER stress in vitro and in vivo. CNS Neurosci Ther. 2019;25:976–986. doi: 10.1111/cns.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang S.L., He H.B., Zou K., Bai C.H., Xue Y.H., Wang J.Z. Protective effect of tomatine against hydrogen peroxide-induced neurotoxicity in neuroblastoma (SH-SY5Y) cells. J Pharm Pharmacol. 2014;66:844–854. doi: 10.1111/jphp.12205. [DOI] [PubMed] [Google Scholar]
- 98.Fang E.F., Waltz T.B., Kassahun H., Lu Q., Kerr J.S., Morevati M. Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN-1/Nrf 2 pathway. Sci Rep. 2017;7:46208. doi: 10.1038/srep46208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Waltz T.B., Fivenson E.M., Morevati M., Li C., Becker K.G., Bohr V.A. Sarcopenia, aging and prospective interventional strategies. Curr Med Chem. 2018;25:5588–5596. doi: 10.2174/0929867324666170801095850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahsan A., Zheng Y., Ma S., Liu M., Cao M., Li Y. Tomatidine protects against ischemic neuronal injury by improving lysosomal function. Eur J Pharmacol. 2020;882:173280. doi: 10.1016/j.ejphar.2020.173280. [DOI] [PubMed] [Google Scholar]
- 101.Yan J., Yan J.Y., Wang Y.X., Ling Y.N., Song X.D., Wang S.Y. Spermidine-enhanced autophagic flux improves cardiac dysfunction following myocardial infarction by targeting the AMPK/mTOR signalling pathway. Br J Pharmacol. 2019;176:3126–3142. doi: 10.1111/bph.14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eisenberg T., Abdellatif M., Schroeder S., Primessnig U., Stekovic S., Pendl T. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22:1428–1438. doi: 10.1038/nm.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaplan S., Onger M.E., Altunkaynak B.Z., Elibol E., Deniz O.G., Karayigit M.O. Effects of spermine and the passive avoidance learning (PAL) following cerebral ischemia in chicks: association with neuroprotection of pyramidal cells. J Chem Neuroanat. 2018;88:41–45. doi: 10.1016/j.jchemneu.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 104.Yang Y., Chen S., Zhang Y., Lin X., Song Y., Xue Z. Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated Beclin 1 cleavage. Cell Death Dis. 2017;8:e2738. doi: 10.1038/cddis.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim Y.K., Yoon H.H., Lee Y.D., Youn D.Y., Ha T.J., Kim H.S. Anthocyanin extracts from black soybean (Glycine max L.) protect human glial cells against oxygen–glucose deprivation by promoting autophagy. Biomol Ther (Seoul) 2012;20:68–74. doi: 10.4062/biomolther.2012.20.1.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang C., Mo Y., Xu E., Wen H., Wei R., Li S. Astragaloside IV ameliorates motor deficits and dopaminergic neuron degeneration via inhibiting neuroinflammation and oxidative stress in a Parkinson's disease mouse model. Int Immunopharm. 2019;75:105651. doi: 10.1016/j.intimp.2019.05.036. [DOI] [PubMed] [Google Scholar]
- 107.Song M.T., Ruan J., Zhang R.Y., Deng J., Ma Z.Q., Ma S.P. Astragaloside IV ameliorates neuroinflammation-induced depressive-like behaviors in mice via the PPARγ/NF-κB/NLRP3 inflammasome axis. Acta Pharmacol Sin. 2018;39:1559–1570. doi: 10.1038/aps.2017.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Y., Zhang Y., Jin X.F., Zhou X.H., Dong X.H., Yu W.T. The Role of Astragaloside IV against cerebral ischemia/reperfusion injury: suppression of apoptosis via promotion of P62-LC3-autophagy. Molecules. 2019;24:1838. doi: 10.3390/molecules24091838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lim C.S., Jin D.Q., Mok H., Oh S.J., Lee J.U., Hwang J.K. Antioxidant and antiinflammatory activities of xanthorrhizol in hippocampal neurons and primary cultured microglia. J Neurosci Res. 2005;82:831–838. doi: 10.1002/jnr.20692. [DOI] [PubMed] [Google Scholar]
- 110.Huang Z., Ye B., Dai Z., Wu X., Lu Z., Shan P. Curcumin inhibits autophagy and apoptosis in hypoxia/reoxygenation-induced myocytes. Mol Med Rep. 2015;11:4678–4684. doi: 10.3892/mmr.2015.3322. [DOI] [PubMed] [Google Scholar]
- 111.Miao Y., Zhao S., Gao Y., Wang R., Wu Q., Wu H. Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: the possible role of Sirt1 signaling. Brain Res Bull. 2016;121:9–15. doi: 10.1016/j.brainresbull.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Y., Fang M., Sun Y., Zhang T., Shi N., Li J. Curcumin attenuates cerebral ischemia injury in Sprague–Dawley rats and PC12 cells by suppressing overactivated autophagy. J Photochem Photobiol B. 2018;184:1–6. doi: 10.1016/j.jphotobiol.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 113.Wang C.P., Li G.C., Shi Y.W., Zhang X.C., Li J.L., Wang Z.W. Neuroprotective effect of schizandrin A on oxygen and glucose deprivation/reperfusion-induced cell injury in primary culture of rat cortical neurons. J Physiol Biochem. 2014;70:735–747. doi: 10.1007/s13105-014-0342-3. [DOI] [PubMed] [Google Scholar]
- 114.Guo Z., Cao G., Yang H., Zhou H., Li L., Cao Z. A combination of four active compounds alleviates cerebral ischemia–reperfusion injury in correlation with inhibition of autophagy and modulation of AMPK/mTOR and JNK pathways. J Neurosci Res. 2014;92:1295–1306. doi: 10.1002/jnr.23400. [DOI] [PubMed] [Google Scholar]
- 115.Wang G., Wang T., Zhang Y., Li F., Yu B., Kou J. Schizandrin protects against OGD/R-induced neuronal injury by suppressing autophagy: involvement of the AMPK/mTOR Pathway. Molecules. 2019;24:3624. doi: 10.3390/molecules24193624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang Y.N., Yang L.Y., Wang J.Y., Lai C.C., Chiu C.T., Wang J.Y. l-Ascorbate protects against methamphetamine-induced neurotoxicity of cortical cells via inhibiting oxidative stress, autophagy, and apoptosis. Mol Neurobiol. 2017;54:125–136. doi: 10.1007/s12035-015-9561-z. [DOI] [PubMed] [Google Scholar]
- 117.Dong Y., Wang S., Zhang T., Zhao X., Liu X., Cao L. Ascorbic acid ameliorates seizures and brain damage in rats through inhibiting autophagy. Brain Res. 2013;1535:115–123. doi: 10.1016/j.brainres.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 118.Liu Y., Yang B., Zhang L., Cong X., Liu Z., Hu Y. Ginkgolic acid induces interplay between apoptosis and autophagy regulated by ROS generation in colon cancer. Biochem Biophys Res Commun. 2018;498:246–253. doi: 10.1016/j.bbrc.2018.01.091. [DOI] [PubMed] [Google Scholar]
- 119.Qi Q.M., Xue Y.C., Lv J., Sun D., Du J.X., Cai S.Q. Ginkgolic acids induce HepG2 cell death via a combination of apoptosis, autophagy and the mitochondrial pathway. Oncol Lett. 2018;15:6400–6408. doi: 10.3892/ol.2018.8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vijayakumaran S., Nakamura Y., Henley J.M., Pountney D.L. Ginkgolic acid promotes autophagy-dependent clearance of intracellular α-synuclein aggregates. Mol Cell Neurosci. 2019;101:103416. doi: 10.1016/j.mcn.2019.103416. [DOI] [PubMed] [Google Scholar]
- 121.Garcia-Jimenez A., Teruel-Puche J.A., Berna J., Rodriguez-Lopez J.N., Tudela J., Garcia-Canovas F. Action of tyrosinase on alpha and beta-arbutin: a kinetic study. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tessari I., Bisaglia M., Valle F., Samori B., Bergantino E., Mammi S. The reaction of alpha-synuclein with tyrosinase: possible implications for Parkinson disease. J Biol Chem. 2008;283:16808–16817. doi: 10.1074/jbc.M709014200. [DOI] [PubMed] [Google Scholar]
- 123.Ding Y., Kong D., Zhou T., Yang N.D., Xin C., Xu J. α-Arbutin protects against Parkinson's disease-associated mitochondrial dysfunction in vitro and in vivo. NeuroMolecular Med. 2020;22:56–67. doi: 10.1007/s12017-019-08562-6. [DOI] [PubMed] [Google Scholar]
- 124.Qu L., Chen C., He W., Chen Y., Li Y., Wen Y. Glycyrrhizic acid ameliorates LPS-induced acute lung injury by regulating autophagy through the PI3K/AKT/mTOR pathway. Am J Transl Res. 2019;11:2042–2055. [PMC free article] [PubMed] [Google Scholar]
- 125.Ojha S., Javed H., Azimullah S., Abul Khair S.B., Haque M.E. Glycyrrhizic acid attenuates neuroinflammation and oxidative stress in rotenone model of Parkinson's disease. Neurotox Res. 2016;29:275–287. doi: 10.1007/s12640-015-9579-z. [DOI] [PubMed] [Google Scholar]
- 126.Yang G., Li J., Cai Y., Yang Z., Li R., Fu W. Glycyrrhizic acid alleviates 6-hydroxydopamine and corticosterone-induced neurotoxicity in SH-SY5Y cells through modulating autophagy. Neurochem Res. 2018;43:1914–1926. doi: 10.1007/s11064-018-2609-5. [DOI] [PubMed] [Google Scholar]
- 127.Naidu M., Kuan C.Y., Lo W.L., Raza M., Tolkovsky A., Mak N.K. Analysis of the action of euxanthone, a plant-derived compound that stimulates neurite outgrowth. Neuroscience. 2007;148:915–924. doi: 10.1016/j.neuroscience.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 128.Zhu L., Liu X., Li D., Sun S., Wang Y., Sun X. Autophagy is a pro-survival mechanism in ovarian cancer against the apoptotic effects of euxanthone. Biomed Pharmacother. 2018;103:708–718. doi: 10.1016/j.biopha.2018.04.090. [DOI] [PubMed] [Google Scholar]
- 129.Yuan H., Jiang C., Zhao J., Zhao Y., Zhang Y., Xu Y. Euxanthone Attenuates Aβ1–42-induced oxidative stress and apoptosis by triggering autophagy. J Mol Neurosci. 2018;66:512–523. doi: 10.1007/s12031-018-1175-2. [DOI] [PubMed] [Google Scholar]
- 130.Wang M.Y., Meng M., Yang C.C., Zhang L., Li Y.L., Zhang L. Cornel iridoid glycoside improves cognitive impairment induced by chronic cerebral hypoperfusion via activating PI3K/Akt/GSK-3β/CREB pathway in rats. Behav Brain Res. 2020;379:112319. doi: 10.1016/j.bbr.2019.112319. [DOI] [PubMed] [Google Scholar]
- 131.Ma D., Wang N., Fan X., Zhang L., Luo Y., Huang R. Protective effects of cornel iridoid glycoside in rats after traumatic brain injury. Neurochem Res. 2018;43:959–971. doi: 10.1007/s11064-018-2501-3. [DOI] [PubMed] [Google Scholar]
- 132.Yang C., Li X., Zhang L., Li Y., Li L., Zhang L. Cornel iridoid glycoside induces autophagy to protect against tau oligomer neurotoxicity induced by the activation of glycogen synthase kinase-3 beta. J Nat Med. 2019;73:717–726. doi: 10.1007/s11418-019-01318-3. [DOI] [PubMed] [Google Scholar]
- 133.Zhao H., Chen S., Gao K., Zhou Z., Wang C., Shen Z. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience. 2017;348:241–251. doi: 10.1016/j.neuroscience.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 134.Kou X., Chen N. Resveratrol as a natural autophagy regulator for prevention and treatment of Alzheimer's disease. Nutrients. 2017;9:927. doi: 10.3390/nu9090927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maher P., Dargusch R., Bodai L., Gerard P.E., Purcell J.M., Marsh J.L. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington's disease. Hum Mol Genet. 2011;20:261–270. doi: 10.1093/hmg/ddq460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vidoni C., Secomandi E., Castiglioni A., Melone M.A.B., Isidoro C. Resveratrol protects neuronal-like cells expressing mutant Huntingtin from dopamine toxicity by rescuing ATG4-mediated autophagosome formation. Neurochem Int. 2018;117:174–187. doi: 10.1016/j.neuint.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 137.Wang Y., Li Y., Zhang T., Chi Y., Liu M., Liu Y. Genistein and Myd88 activate autophagy in high glucose-induced renal podocytes in vitro. Med Sci Mon Int Med J Exp Clin Res. 2018;24:4823–4831. doi: 10.12659/MSM.910868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moskot M., Montefusco S., Jakobkiewicz-Banecka J., Mozolewski P., Wegrzyn A., Di Bernardo D. The phytoestrogen genistein modulates lysosomal metabolism and transcription factor EB (TFEB) activation. J Biol Chem. 2014;289:17054–17069. doi: 10.1074/jbc.M114.555300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pierzynowska K., Podlacha M., Gaffke L., Majkutewicz I., Mantej J., Wegrzyn A. Autophagy-dependent mechanism of genistein-mediated elimination of behavioral and biochemical defects in the rat model of sporadic Alzheimer's disease. Neuropharmacology. 2019;148:332–346. doi: 10.1016/j.neuropharm.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 140.Wu H.C., Hu Q.L., Zhang S.J., Wang Y.M., Jin Z.K., Lv L.F. Neuroprotective effects of genistein on SH-SY5Y cells overexpressing A53T mutant α-synuclein. Neural Regen Res. 2018;13:1375–1383. doi: 10.4103/1673-5374.235250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pierzynowska K., Gaffke L., Hac A., Mantej J., Niedzialek N., Brokowska J. Correction of Huntington's disease phenotype by genistein-induced autophagy in the cellular model. NeuroMolecular Med. 2018;20:112–123. doi: 10.1007/s12017-018-8482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hosseinpour-Moghaddam K., Caraglia M., Sahebkar A. Autophagy induction by trehalose: molecular mechanisms and therapeutic impacts. J Cell Physiol. 2018;233:6524–6543. doi: 10.1002/jcp.26583. [DOI] [PubMed] [Google Scholar]
- 143.Lan D.M., Liu F.T., Zhao J., Chen Y., Wu J.J., Ding Z.T. Effect of trehalose on PC12 cells overexpressing wild-type or A53T mutant α-synuclein. Neurochem Res. 2012;37:2025–2032. doi: 10.1007/s11064-012-0823-0. [DOI] [PubMed] [Google Scholar]
- 144.Fernandez-Estevez M.A., Casarejos M.J., Lopez Sendon J., Garcia Caldentey J., Ruiz C., Gomez A. Trehalose reverses cell malfunction in fibroblasts from normal and Huntington's disease patients caused by proteosome inhibition. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rusmini P., Cortese K., Crippa V., Cristofani R., Cicardi M.E., Ferrari V. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy. 2019;15:631–651. doi: 10.1080/15548627.2018.1535292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shailasree S., Venkataramana M., Niranjana S.R., Prakash H.S. Cytotoxic effect of p-coumaric acid on neuroblastoma, N2a cell via generation of reactive oxygen species leading to dysfunction of mitochondria inducing apoptosis and autophagy. Mol Neurobiol. 2015;51:119–130. doi: 10.1007/s12035-014-8700-2. [DOI] [PubMed] [Google Scholar]
- 147.Sunitha M.C., Dhanyakrishnan R., PrakashKumar B., Nevin K.G. p-Coumaric acid mediated protection of H9c2 cells from doxorubicin-induced cardiotoxicity: involvement of augmented Nrf 2 and autophagy. Biomed Pharmacother. 2018;102:823–832. doi: 10.1016/j.biopha.2018.03.089. [DOI] [PubMed] [Google Scholar]
- 148.Ueda T., Ito T., Kurita H., Inden M., Hozumi I. p-Coumaric acid has protective effects against mutant copper-zinc superoxide dismutase 1 via the activation of autophagy in N2a cells. Int J Mol Sci. 2019;20:2942. doi: 10.3390/ijms20122942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wu Y., Hu Y., Zhou H., Zhu J., Tong Z., Qin S. Organosulfur compounds induce cytoprotective autophagy against apoptosis by inhibiting mTOR phosphorylation activity in macrophages. Acta Biochim Biophys Sin. 2018;50:1085–1093. doi: 10.1093/abbs/gmy114. [DOI] [PubMed] [Google Scholar]
- 150.Guo Y., Zhang K., Wang Q., Li Z., Yin Y., Xu Q. Neuroprotective effects of diallyl trisulfide in SOD1-G93A transgenic mouse model of amyotrophic lateral sclerosis. Brain Res. 2011;1374:110–115. doi: 10.1016/j.brainres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 151.Liu C., Leng B., Li Y., Jiang H., Duan W., Guo Y. Diallyl trisulfide protects motor neurons from the neurotoxic protein TDP-43 via activating lysosomal degradation and the antioxidant response. Neurochem Res. 2018;43:2304–2312. doi: 10.1007/s11064-018-2651-3. [DOI] [PubMed] [Google Scholar]
- 152.Cerda B., Periago P., Espin J.C., Tomas-Barberan F.A. Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds. J Agric Food Chem. 2005;53:5571–5576. doi: 10.1021/jf050384i. [DOI] [PubMed] [Google Scholar]
- 153.Boakye Y.D., Groyer L., Heiss E.H. An increased autophagic flux contributes to the anti-inflammatory potential of urolithin A in macrophages. Biochim Biophys Acta. 2018;1862:61–70. doi: 10.1016/j.bbagen.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Atiya Ali M., Poortvliet E., Strömberg R., Yngve A. Polyamines in foods: development of a food database. Food Nutr Res. 2011;55:5572. doi: 10.3402/fnr.v55i0.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wirth M., Schwarz C., Benson G., Horn N., Buchert R., Lange C. Effects of spermidine supplementation on cognition and biomarkers in older adults with subjective cognitive decline (SmartAge)-study protocol for a randomized controlled trial. Alzheimer's Res Ther. 2019;11:36. doi: 10.1186/s13195-019-0484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wirth M., Benson G., Schwarz C., Kobe T., Grittner U., Schmitz D. The effect of spermidine on memory performance in older adults at risk for dementia: a randomized controlled trial. Cortex. 2018;109:181–188. doi: 10.1016/j.cortex.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 157.Gupta V.K., Scheunemann L., Eisenberg T., Mertel S., Bhukel A., Koemans T.S. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci. 2013;16:1453–1460. doi: 10.1038/nn.3512. [DOI] [PubMed] [Google Scholar]
- 158.Lin B.W., Gong C.C., Song H.F., Cui Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br J Pharmacol. 2017;174:1226–1243. doi: 10.1111/bph.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Youdim K.A., Shukitt-Hale B., Joseph J.A. Flavonoids and the brain: interactions at the blood–brain barrier and their physiological effects on the central nervous system. Free Radic Biol Med. 2004;37:1683–1693. doi: 10.1016/j.freeradbiomed.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 160.Hung T.C., Chang T.T., Fan M.J., Lee C.C., Chen C.Y. In silico insight into potent of anthocyanin regulation of FKBP52 to prevent Alzheimer's disease. Evid Based Complement Alternat Med. 2014;2014:450592. doi: 10.1155/2014/450592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ren S., Zhang H., Mu Y., Sun M., Liu P. Pharmacological effects of astragaloside IV: a literature review. J Tradit Chin Med. 2013;33:413–416. doi: 10.1016/s0254-6272(13)60189-2. [DOI] [PubMed] [Google Scholar]
- 162.Shah S.Z.A., Zhao D., Hussain T., Sabir N., Mangi M.H., Yang L. p62-Keap1-NRF2-ARE pathway: a contentious player for selective targeting of autophagy, oxidative stress and mitochondrial dysfunction in prion diseases. Front Mol Neurosci. 2018;11:310. doi: 10.3389/fnmol.2018.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ammon H.P., Wahl M.A. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 164.Dutta S., Padhye S., Priyadarsini K.I., Newton C. Antioxidant and antiproliferative activity of curcumin semicarbazone. Bioorg Med Chem Lett. 2005;15:2738–2744. doi: 10.1016/j.bmcl.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 165.Zhao J., Zhao Y., Zheng W., Lu Y., Feng G., Yu S. Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain Res. 2008;1229:224–232. doi: 10.1016/j.brainres.2008.06.117. [DOI] [PubMed] [Google Scholar]
- 166.Sowndhararajan K., Deepa P., Kim M., Park S.J., Kim S. An overview of neuroprotective and cognitive enhancement properties of lignans from Schisandra chinensis. Biomed Pharmacother. 2018;97:958–968. doi: 10.1016/j.biopha.2017.10.145. [DOI] [PubMed] [Google Scholar]
- 167.Li Y., Schellhorn H.E. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137:2171–2184. doi: 10.1093/jn/137.10.2171. [DOI] [PubMed] [Google Scholar]
- 168.Rice M.E. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 169.Hediger M.A. New view at C. Nat Med. 2002;8:445–446. doi: 10.1038/nm0502-445. [DOI] [PubMed] [Google Scholar]
- 170.Chang C.Y., Chen J.Y., Wu M.H., Hu M.L. Therapeutic treatment with vitamin C reduces focal cerebral ischemia-induced brain infarction in rats by attenuating disruptions of blood brain barrier and cerebral neuronal apoptosis. Free Radic Biol Med. 2020;155:29–36. doi: 10.1016/j.freeradbiomed.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 171.Lagowska-Lenard M., Stelmasiak Z., Bartosik-Psujek H. Influence of vitamin C on markers of oxidative stress in the earliest period of ischemic stroke. Pharmacol Rep. 2010;62:751–756. doi: 10.1016/s1734-1140(10)70334-0. [DOI] [PubMed] [Google Scholar]
- 172.Choi Y.K., Kang J.I., Han S., Kim Y.R., Jo J., Kang Y.W. l-Ascorbic acid inhibits breast cancer growth by inducing IRE/JNK/CHOP-related endoplasmic reticulum stress-mediated p62/SQSTM1 accumulation in the nucleus. Nutrients. 2020;12:1351. doi: 10.3390/nu12051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fuzzati N., Pace R., Villa F. A simple HPLC–UV method for the assay of ginkgolic acids in Ginkgo biloba extracts. Fitoterapia. 2003;74:247–256. doi: 10.1016/s0367-326x(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 174.Carmen P., Vlase L., Tamas M. Natural resources containing arbutin. Determination of arbutin in the leaves of Bergenia crassifolia (L.) Fritsch. acclimated in Romania. Not Bot Hort Agrobot Cluj. 2009;37:129–132. [Google Scholar]
- 175.Liu C.Q., Deng L., Zhang P., Zhang S.R., Liu L., Xu T. Screening of high alpha-arbutin producing strains and production of alpha-arbutin by fermentation. World J Microbiol Biotechnol. 2013;29:1391–1398. doi: 10.1007/s11274-013-1302-8. [DOI] [PubMed] [Google Scholar]
- 176.Jiang S., Li T., Ji T., Yi W., Yang Z., Wang S. AMPK: potential therapeutic target for ischemic stroke. Theranostics. 2018;8:4535–4551. doi: 10.7150/thno.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen J., Xu P., Zhu S., Hu Y. Advance in research for pharmacokinetics and drug interactions of licorice. Chin J Clin Pharmacol. 2010;15:1174–1182. [Google Scholar]
- 178.Song S., Zhang X.J. Experience analysis of using cornus officinalis. Jilin J Tradit Chin Med. 2006;26:3–5. [Google Scholar]
- 179.Richard T., Pawlus A.D., Iglésias M.L., Pedrot E., Waffo-Teguo P., Mérillon J.M. Neuroprotective properties of resveratrol and derivatives. Ann N Y Acad Sci. 2011;1215:103–108. doi: 10.1111/j.1749-6632.2010.05865.x. [DOI] [PubMed] [Google Scholar]
- 180.Chen J., Bai Q., Zhao Z., Sui H., Xie X. Resveratrol improves delayed r-tPA treatment outcome by reducing MMPs. Acta Neurol Scand. 2016;134:54–60. doi: 10.1111/ane.12511. [DOI] [PubMed] [Google Scholar]
- 181.Walter E. Genistin (an isoflavone glucoside) and its aglucone, genistein, from soybeans. J Am Chem Soc. 1941;63:3273–3276. [Google Scholar]
- 182.Yao C.-S., Lin M., Liu X., Wang Y. Stilbene derivatives from Gnetum cleistostachyum. J Asian Nat Prod Res. 2005;7:131–137. doi: 10.1080/10286020310001625102. [DOI] [PubMed] [Google Scholar]
- 183.Block E. The Royal Society of Chemistry; Cambridge: 2010. Garlic and other alliums: the lore and the sciences; pp. 1–32. [Google Scholar]