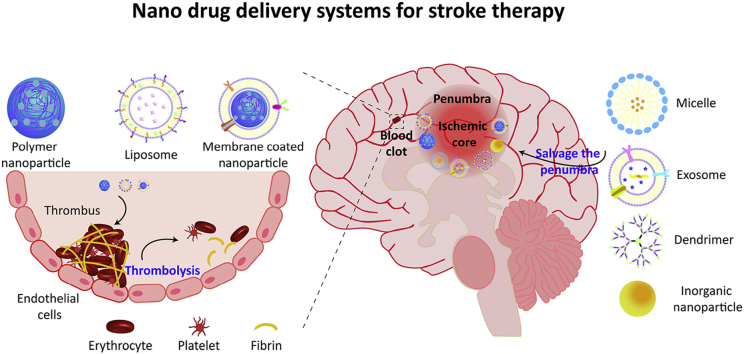
Abstract
Ischemic stroke is a cerebrovascular disease normally caused by interrupted blood supply to the brain. Ischemia would initiate the cascade reaction consisted of multiple biochemical events in the damaged areas of the brain, where the ischemic cascade eventually leads to cell death and brain infarction. Extensive researches focusing on different stages of the cascade reaction have been conducted with the aim of curing ischemic stroke. However, traditional treatment methods based on antithrombotic therapy and neuroprotective therapy are greatly limited for their poor safety and treatment efficacy. Nanomedicine provides new possibilities for treating stroke as they could improve the pharmacokinetic behavior of drugs in vivo, achieve effective drug accumulation at the target site, enhance the therapeutic effect and meanwhile reduce the side effect. In this review, we comprehensively describe the pathophysiology of stroke, traditional treatment strategies and emerging nanomedicines, summarize the barriers and methods for transporting nanomedicine to the lesions, and illustrate the latest progress of nanomedicine in treating ischemic stroke, with a view to providing a new feasible path for the treatment of cerebral ischemia.
KEY WORDS: Stroke, Ischemic cascade, Reperfusion, Neuroprotectant, Thrombolytics, Nanomedicine, Blood‒brain barrier
Abbreviations: AEPO, asialo-erythropoietin; APOE, apolipoprotein E; BBB, blood‒brain barrier; BCECs, brain capillary endothelial cells; CAT, catalase; Ce-NPs, ceria nanoparticles; COX-1, cyclooxygenase-1; cRGD, cyclic Arg-Gly-Asp; CsA, cyclosporine A; CXCR-4, C-X-C chemokine receptor type 4; DAMPs, damage-associated molecular patterns; e-PAM-R, arginine-poly-amidoamine ester; GFs, growth factors; GPIIb/IIIa, glycoprotein IIb/IIIa; Hb, hemoglobin; HMGB1, high mobility group protein B1; ICAM-1, intercellular adhesion molecule-1; IL-1β, interleukin-1β; IL-6, interleukin-6; iNOS, inducible nitric oxide synthase; LFA-1, lymphocyte function-associated antigen-1; LHb, liposomal Hb; MCAO, middle cerebral artery occlusion; miRNAs, microRNAs; MMPs, matrix metalloproteinases; MSC, mesenchymal stem cell; NF-κB, nuclear factor-κB; NGF, nerve growth factor; NMDAR, N-methyl-d-aspartate receptor; nNOS, neuron nitric oxide synthase; NOS, nitric oxide synthase; NPs, nanoparticles; NSCs, neural stem cells; PBCA, poly-butylcyanoacrylate; PCMS, poly (chloromethylstyrene); PEG, poly-ethylene-glycol; PEG-PLA, poly (ethylene-glycol)-b-poly (lactide); PLGA NPs, poly (l-lactide-co-glycolide) nanoparticles; PSD-95, postsynaptic density protein-95; PSGL-1, P-selectin glycoprotein ligand-1; RBCs, red blood cells; RES, reticuloendothelial system; RGD, Arg-Gly-Asp; ROS, reactive oxygen species; SDF-1, stromal cell-derived factor-1; SHp, stroke homing peptide; siRNA, small interfering RNA; SOD, superoxide dismutase; SUR1-TRPM4, sulfonylurea receptor 1-transient receptor potential melastatin-4; TEMPO, 2,2,6,6-tetramethylpiperidine-1-oxyl; TIA, transient ischemic attack; TNF-α, tumor necrosis factor-α
Graphical abstract
This review summarizes various nanomedicines, including liposomes, micelles, dendrimer, nanoparticles, and exosomes, which have been applied for the treatment of ischemic stroke. It especially focuses on their abilities to normalize various abnormalities at different phases of ischemic cascade, such as dissolving the clot and salvaging the penumbra.
1. Introduction
Stroke is the second leading cause of death and severely affects the daily life of survivals due to its high disability rate1. Ischemic stroke normally occurs when a vessel that supplies blood to the brain is blocked, and it makes up approximately 87% of all stroke cases2. Ischemia would initiate the ischemic cascade which involves a series of biochemical events, including energy failure3, ion imbalance and excitotoxicity4, oxidative stress5, 6, 7, cell death (apoptosis or necrosis), activation of the complement system8, 9, 10, 11, 12, initiation of the inflammation and immune response. The cascade would eventually lead to irreversible brain damage13. Many strategies have been adopted to reconstruct blood supply to the brain, aiming to address the aforementioned harmful effects of ischemia14. Thrombolysis with tissue plasminogen activator to realize recanalization to the brain is the gold standard for ischemic stroke treatment. However, the time window of thrombolysis is relatively narrow (≤4.5 h), due to the risk of hemorrhagic transformation (HT) caused by the low targeting ability to thrombus15,16. Thus, only a small number of patients could receive and benefit from the thrombolytic therapy in time. Moreover, restoring blood flow to the ischemic brain would cause secondary reperfusion injury. Reperfusion would exacerbate the production of reactive oxygen species (ROS) and the amplification of inflammation and immune response, which would cause undue neural death, impair the integrity of blood‒brain barrier (BBB) and eventually lead to brain edema17, 18, 19, 20. There are also numerous preclinical studies focusing on protecting neurons from the injury at different stages of ischemia and reperfusion. However, most neuroprotective agents have unneglectable defects including low solubility, short half-life and poor BBB permeability in vivo, which might contribute to the failure of many clinical trials21,22. It is difficult and costly to find safer and more effective new drugs to treat ischemic stroke. Thus, there is an increasing demand to endow them with pharmaceutical improvement to existing drugs to suit the clinical requirements.
Numerous nanomedicines have been developed to improve the efficacy and to reduce the side effects of traditional treatment methods, since well-designed nanomedicines possess many unique advantages. First, nanomedicine could increase the solubility of poorly soluble drugs, improve the stability and extend the half-lives of drugs in vivo23. Second, targeted modified nanomedicine could assist drugs to cross the BBB which is a main barrier for most drugs to reach the brain, and realize accumulation at the desired site to avoid non-specific distribution. Targeted modification could also help the nanomedicine to mainly taken up by specific cells, such as damaged neurons. Hence, nanomedicines with targeting ability could not only increase the therapeutic effect, but also reduce the injected dose and toxicity of drugs24. In addition, nanomedicines consisted of different functional materials could control the release of drugs, and the functionalized vehicle might be effective in treating ischemic stroke23. Moreover, nanomedicines provide possibilities for some emerging treatments, such as gene therapy, which is favor of recovering from injury at the chronic phase of stroke. Nanomedicines include liposomes, micelles, nanoparticles (NPs), emerging cell membrane coated NPs and exosomes, all of which have been applied in the preclinical studies for treating ischemic stroke. Apart from discussing the pathophysiology of ischemic stroke which might affect the design of nanomedicine, as well as traditional treatment strategies and their deficiencies, this review mainly focuses on investigating the barriers and methods to transporting nanomedicines to the targeting sites and summarizes preclinical studies of nanomedicines for the treatment of ischemic stroke according to various abnormalities at different phases of ischemic cascade.
2. The pathophysiology of ischemic stroke
Understanding the pathological mechanisms of ischemic stroke is helpful in finding therapeutic targets and developing more efficiently targeted nanomedicines. A series of molecular and cellular events are involved in the ischemic cascade, which occur at different time and interconnect with each other. In the ischemic core, hypoperfusion restricts the oxygen/glucose supply to the brain, which causes the energy failure of neurons3. Insufficient ATP causes the ion pumps to operate abnormally. As a result, the ion gradient across the cell membrane is out-of-balance13. Increased influx of Na+ and efflux of K+ leads to the depolarization of neurons and glial cells, as well as extracellular accumulation of glutamate that can cause excitotoxicity4. Intracellular accumulated Na+ and following increased Cl– will result in the swelling of cells, destruction of cell membrane and final necrosis. In the ischemic penumbra, extracellular accumulated glutamate over-activates the glutamate receptors, which leads to the influx of Ca2+ 13. Then numerous Ca2+-dependent enzymes are activated, including proteases which destroy the cell integrity, nitric oxide synthase (NOS) and enzymes producing ROS5,13. ROS not only directly reacts with macromolecules to cause lipid peroxidation, protein oxidation and DNA damage, but also causes indirect injury through multiple signaling pathways, such as caspase-mediated apoptosis6,7.
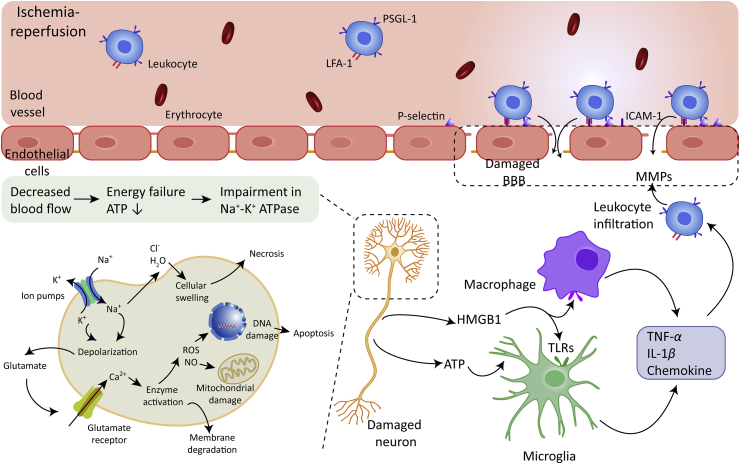
Reperfusion would exacerbate the production of ROS and subsequent activation of matrix metalloproteinases (MMPs), which would worsen the damage of neurovascular units17, 18, 19, 20,25,26. Neurons under stress and necrotic cells would release damage-associated molecular patterns (DAMPs), such as high mobility group protein B1 (HMGB1) and ATP, which would initiate the recruitment of intrinsic microglia and upregulate the expression of pro-inflammatory mediators27, 28, 29, 30. Pro-inflammatory mediators such as chemokines and adhesion molecules would drive and promote the infiltration of neutrophils and blood-borne macrophages into the ischemic brain31, 32, 33, 34, 35. Complement system can be activated in the blood and brain parenchyma as well, which leads to increased leukocytes infiltration8, 9, 10, 11, 12. Neutrophils and mononuclear phagocytes recruited to the ischemic brain will contribute to the production of inducible nitric oxide synthase (iNOS), ROS, MMPs and other inflammatory mediators such tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β), which eventually impairs the BBB permeability and leads to brain edema36,37. The multiple molecular and cellular events that involved in the ischemic cascade are illustrated in Fig. 1.
Figure 1.
Molecular and cellular events involved in the ischemic cascade, including energy failure, ion imbalance and excitotoxicity, oxidative stress, cell death (apoptosis or necrosis), initiation of the inflammation and immune response. The expression of P-selectin and intercellular adhesion molecule-1 (ICAM-1) on brain capillary endothelial cells (BCECs) are up-regulated during stroke. The interaction between P-selection and P-selectin glycoprotein ligand-1 (PSGL-1) which are expressed on leukocytes promotes the binding of leukocytes on BCECs. Leukocytes realized firmer adhesion on the vascular wall through the specific binding between the ICAM-1 and lymphocyte function associated antigen-1 (LFA-1), leukocytes subsequently infiltrate into the ischemic brain parenchyma through the damaged blood‒brain barrier (BBB). TLRs, Toll-like receptors.
3. Traditional treatment strategies
Conventional therapy for ischemic stroke mainly includes the employment of antithrombotic drugs to improve the brain blood circulation, and neuroprotectant to protect neurons from death induced by ischemia and reperfusion.
3.1. Antithrombotic drugs
Clinically used antithrombotic drugs are generally classified into antiplatelet and anticoagulant drugs, which are applied to prevent thrombosis, and thrombolytic drugs to dissolve the existed thrombus38. Platelets play a vital role in the formation of thrombus, and antiplatelet treatment not only prevents the formation of thrombus, but also reduces the growth of thrombus39. For instance, aspirin is the most widely used drug for antiplatelet treatment, as aspirin could selectively inhibit the cyclooxygenase-1 (COX-1) involved in the platelet adhesion at a low dosage without inhibiting the synthesis of prostacyclin40. Anticoagulants such as heparins, vitamin K antagonists and argatroban have also been applied for treating ischemic stroke, as they could reduce the formation of secondary blood clots to improve prognosis. In detail, heparins could bind to antithrombin to deactivate thrombin and inhibit clotting factors; vitamin K antagonists, such as warfarin would reduce the production of activated clotting factors; argatroban could directly inhibit thrombin to inhibit the formation of blood clot41.
Being different from antiplatelet and anticoagulant drugs, thrombolytic agents could activate plasminogen to form plasmin to degrade the fibrin involved in the thrombus, dissolve the formed clot and restore the blood supply to the ischemic brain. Urokinase and alteplase are inherent plasminogen activators in vivo. As alteplase could initiate the fibrin-specific fibrinolytic process, it is more widely applied clinically than urokinase. Many new plasminogen activators with improved fibrin specificity and prolonged half-life have been developed, such as desmoteplase and tenecteplase42, 43, 44.
3.2. Neuroprotective agents
Neuroprotective agents could act on different pathophysiological pathways involved in ischemic cascade, and thus exert neuroprotective effects at different stages of stroke. One main therapeutic goal is to prevent glutamate-mediated excitotoxicity. Memantine, an N-methyl-d-aspartate receptor (NMDAR) antagonist, is able to reduce the lesion volume without interfering with the normal physiological function of NMDAR45. Another method to reduce the deleterious effects of excitotoxicity is regulating downstream signaling pathways activated by glutamate, such as the influx of Ca2+. Nimodipine is a calcium channel blocker, and it could realize limited neuroprotective effects in preclinical models46. Eliminating oxidative stress is also a potential treatment strategy to rescue ischemic neurons. Edaravone is a free radical scavenger and able to inhibit lipid peroxidation, which has shown significant neuroprotective effect in clinical trials47,48.
Moreover, current research mainly focused on attenuating the immune-mediated inflammatory response during the acute phase of ischemic stroke. Neutrophils play an important role in the occurrence and development of inflammation, treatment with antibody against E-selectin could reduce the infiltration of neutrophils into the ischemic brain and realize reduction in the infarct volume49. Fingolimod is an immunosuppressant that restricts lymphocytes migration from lymph nodes and reduces their infiltration into the brain. Administration of fingolimod could significantly reduce the infarct volume and decrease the BBB permeability, and improve the neurological function of patients50. The application of anti-inflammation agents and immunosuppressant reveals a new strategy for neuroprotection, yet the clinical application of neuroprotectant is still not optimistic. Table 140, 41, 42,45, 46, 47, 48, 49, 50 summarizes the types of traditional treatment strategies, representative therapeutic agents and their molecular and cellular targets.
Table 1.
Summary of traditional treatment strategies.
| Type of drugs | Therapeutic agent | Mechanism of action | Ref. |
|---|---|---|---|
| Antiplatelet drugs | Aspirin | Selectively inhibits the COX-1 involved in platelet adhesion | 40 |
| Anticoagulants | Heparins | Bind to antithrombin to deactivate thrombin and inhibit clotting factors | 41 |
| Warfarin | Vitamin K antagonist, reduces the production of activated clotting factors | 41 | |
| Argatroban | Directly Inhibits the thrombin | 41 | |
| Thrombolytics | Urokinase and alteplase | Activate the plasminogen to form plasmin | 42 |
| Neuroprotectant | Memantine | NMDAR antagonist | 45 |
| Nimodipine | Calcium channel blocker | 46 | |
| Edaravone | Free radical scavenger | 47,48 | |
| Antibody against E-selectin | Limits the infiltration of neutrophils into the ischemic brain | 49 | |
| Fingolimod | Reduces the infiltration of lymphocytes into the damaged brain | 50 |
3.3. Deficiencies of traditional treatment strategies
Although some antithrombotic drugs and neuroprotectant have been used clinically, there are still some problems needed to be resolved to obtain safer and more effective therapeutic agents. For most antithrombotic drugs, the non-specific distribution and low thrombus targeting efficiency would increase the risk of bleeding in patients. Anticoagulant therapy could reduce the recurrence rate of ischemia during acute phage of stroke, but the benefit is often offset by the increase in symptomatic intracranial hemorrhage41. The effect of thrombolysis with plasminogen activator is also limited, since the circulation time of thrombolytic proteins is relatively short in vivo due to the clearance of proteinase, and the use of thrombolytics would increase the risk of bleeding. For instance, alteplase might amplify the role of MMPs while dissolving the thrombus, and thereby destroys the intercellular matrix of the BBB and increases the probability of intracerebral hemorrhage51.
There are also some deficiencies in neuroprotective treatment. Most neuroprotectant showed limited effects in treating ischemic stroke for their short half-lives, potential toxicity, poor specificity of distribution, as well as low targeting efficiency to cross the BBB to achieve effective drug accumulation at the aimed lesions.52 For instance, the half-life of edaravone in vivo is as short as 5.4 min, which seriously hinders the effectiveness of the agent. In addition, biological macromolecules such as superoxide dismutase (SOD) which could efficiently eliminate ROS, and cyclosporine A (CsA) which could inhibit the inflammatory response, have exhibited fine protective effect on damaged neurons. However, these protein or peptide drugs also possess poor stability and short half-lives in vivo, and extremely low capability in crossing the BBB53,54. Moreover, gene medicines could also be applied for treating stroke, however, it would also encounter the plight of protein or peptide drugs55,56. If these neuroprotectant could be successfully delivered to the ischemic brain, these therapeutic molecules are hopeful to play a more effective and lasting protective role.
Currently, in the absence of more effective drugs in treating ischemic stroke, it is necessary to devote pharmaceutical improvements to existing drugs to prolong their half-life in vivo, meanwhile, to decrease their non-specific distribution in non-targeting tissues, and to improve their targeting ability to brain lesions to obtain better results. Thus, there is a great demand in developing more efficient and safer delivery strategy of antithrombotic drugs and neuroprotectant for ischemic stroke treatment.
4. Barriers in targeted drug delivery to the damaged brain
The ideal targeted nanomedicine for cerebral ischemia treatment is regarded to be able to deliver drugs to thrombus site or the damaged brain parenchyma effectively, and further to specific cells, such as neurons or glial cells. For therapy with neuroprotectant, although intracranial or intrathecal injection could obtain greater benefits sometimes, their unsustainable drug concentration and invasiveness limits their long-term application, and systemic administration is a more practical method in most cases. Once the nanomedicines enter the blood, they tend to protect the loaded drug from the enzyme degradation; the relatively particle with higher size of nanomedicines could also prevent the drug from being quickly eliminated by the kidney. However, nanomedicines with larger size might be opsonized by plasma proteins and subsequently phagocytosed by the reticuloendothelial system (RES), resulting in non-targeted accumulation in liver and spleen. PEGylation could help the nanomedicines to evade the phagocytosis, achieve longer circulation period in the blood, and reduce its non-specific distribution57. PEG would generate hydrophilic sheath on the surface of NPs to shield the opsonization of opsonin and reduce the phagocytosis of macrophages. Longer circulation time is required for nanomedicine delivery, which can increase the chance of nanomedicine of leaking into the brain through the damaged BBB, as well as provide more opportunities for targeting ligands to combine with receptors or transporters. In this section, we will mainly discuss the obstacles and strategies that the neuroprotective nanomedicines encounter before they penetrate into the brain parenchyma across the BBB. Thrombus targeting strategies for thrombolytics-loaded nanomedicine are discussed in Section 5.1.
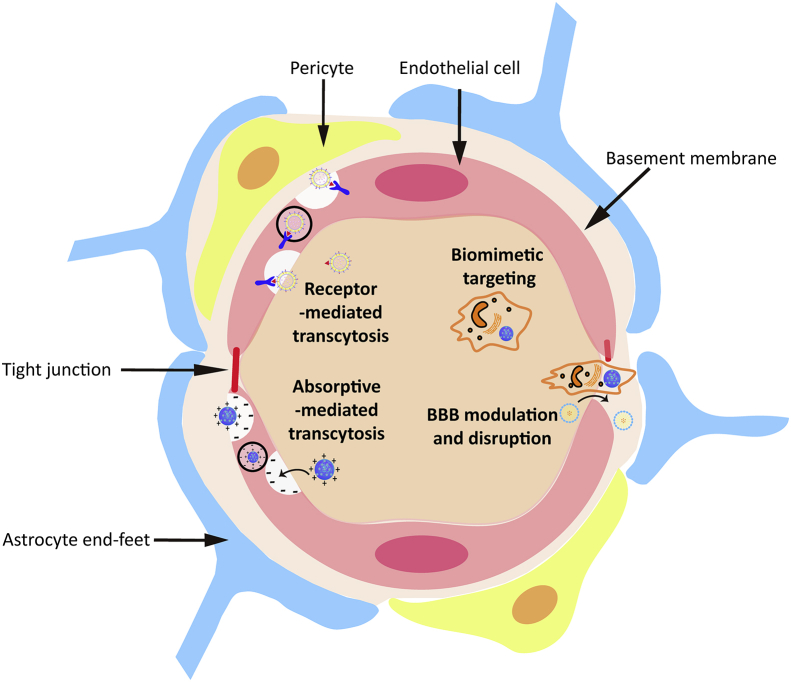
4.1. BBB
BBB is a special and inherent structure between the brain tissue and blood. It is mainly composed of brain capillary endothelial cells and their tight junctions (TJs), pericytes embedded in capillary basement membrane, and astrocytes end-feet around the capillary (Fig. 2). While restricting the entry of toxic substances into the central nervous system, the BBB also prevents almost all macromolecular drugs and nearly 98% of small molecule drugs from entering the brain tissue. It is difficult for nanomedicines to cross the tight BBB; fortunately, there are strategies that could be adopted to help nanomedicine go cross the BBB and diffuse into the brain parenchyma.
Figure 2.
Schematic illustration of the BBB and the strategies for nanomedicines to cross the BBB.
4.2. Strategies for nanomedicines to cross the BBB
4.2.1. BBB disruption and modulation
It is reported that the structure of BBB is partially destroyed during the occurrence of stroke58,59. Subsequently, the permeability of BBB to molecules or particles increases, which provides more possibilities for nanomedicine without targeted modification to enter the brain parenchyma. In the early stage, the upregulated expression of caveolin in endothelial cells would enhance the transcellular transport of nanomedicine; the following breakdown of TJs will promote the particles to cross the BBB via the paracellular route59. There were many unmodified nanomedicines which achieved passive accumulation in brain through the disrupted BBB after stroke53,55,60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70.
However, the disruption of the BBB is not obvious in the first few hours after the ischemic attack, and this period coincides with the treatment window58. Moreover, the crossing of compromised BBB in stroke is often not sufficient for nanomedicines to achieve therapeutically drug concentration. Modifying vehicles with targeting ligand could assist them to cross the intact BBB through receptor-mediated transcytosis as discussed in section 4.2.2.
Recently, some studies adopted BBB modulators to boost the crossing efficiency. The more researched modulators are adenosine 2 A receptor (A2AR) agonist, which bind to the A2AR over-expressed on the luminal and abluminal side of cerebral capillaries during stroke58,71,72, such as lexiscan, purine nucleotide derivative CGS21680. Activating A2AR could temporally improve the BBB permeability by actively opening the TJs, subsequently helps nanomedicine achieve effective accumulation in the ischemic brain. For instance, edaravone-loaded PEG-PLA micelles were anchored with CGS21680, which acted as both a targeting and signaling molecule, and this strategy simultaneously increases the paracellular transport and receptor-mediated transcellular transport of the nanomedicines71. In another study, Lexiscan and an apoptosis inhibitor were co-encapsulated in PEG-PLGA-NPs modified with targeting ligand chlorotoxin. NPs released lexiscan after entering the ischemic site via the damaged BBB, which in turn momently improve the BBB permeability to promote the entry of more NPs58.
4.2.2. Ligand-based targeting
Numerous receptors and transporters are highly expressed on brain capillary endothelial cells to transport glucose, amino acids, proteins, and other nutriments to maintain brain homeostasis73. Modifying nanomedicines with aforementioned endogenous ligands or exogenous derivatives could help them cross the BBB actively via receptor- or transporter-mediated transcytosis. The studied receptors and transporters mainly include transferrin receptor (TfR), low-density lipoprotein receptor (LDLR) and nicotinic acetylcholine receptor (nAChR). There are also some receptors highly expressed after ischemia and reperfusion, which provides more opportunities for targeted delivery of nanomedicines. In addition to covalently conjugating ligands to nanomedicine, NPs can also achieve active targeting by adsorbing endogenous ligands in the blood. For instance, puerarin-loaded PBCA NPs were coated with polysorbate 80 which could adsorb apolipoprotein E (APOE) in blood. This method enhanced the accumulation of NPs in brain via the interaction between APOE and LDLR-related protein (LRP)74. The ligands corresponding to the receptors and their applications in targeted delivery by nanomedicines are summarized in Table 258,71,74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88.
Table 2.
Ligands corresponding to receptors and their applications in targeted delivery of nanomedicines.
| Receptor | Nanomedicine modified with corresponding ligand | Ref. |
|---|---|---|
| SDF-1 | PLGA-NPs coated with NSCs membrane that highly expressed CXCR4 | 75 |
| CXCR4 | Thrombin responsive size-shrinkable NPs modified with AMD3100 | 76 |
| CA 1 | Glyburide-loaded betulinic acid NPs | 77 |
| TfR and NMDAR | ZL006-loaded liposomes modified with T7 peptide and SHp | 78,79 |
| NMDAR | NR2B9c-loaded dextran NPs coated with RBCs membrane which modified with SHp | 80 |
| Integrin αvβ3 | Curcumin-loaded exosomes modified with cRGD | 81 |
| LRP | Puerarin-loaded PBCA NPs coated with polysorbate 80 which could adsorb apolipoprotein E in the blood | 74 |
| A2AR | Edaravone-loaded PEG-PLA micelles modified with CGS21680 | 71 |
| NMDAR1 | SOD-loaded nanomedicine modified with anti-NMDAR1 antibody | 82 |
| Integrin αvβ3 | PEGylated Ce-NPs conjugated with biotinylated-LXW7 | 83 |
| LRP | Edaravone-loaded Ce-NPs modified with angiopep-2 | 84 |
| EPO receptor | AEPO PEGylated liposomes conjugated with AEPO | 85,86 |
| MMP-2 | Lexiscan-loaded PLGA-NPs modified with targeting ligand chlorotoxin | 58 |
| LRP1 | NGF-loaded albumin NPs modified with APOE | 87 |
| nAChR | miR-124-loaded exosomes modified with rabies virus glycoprotein which could bind the nAChR on BBB | 88 |
4.2.3. Biomimetic targeting
Targeted nanomedicines have shown broad applying prospects in treating stroke, though due to the characteristics of exogenous nanomaterials, there is still a risk of being recognized and cleared by the RES. Recently, the bionic nanomedicine delivery strategies based on live cells, cell membrane vesicles and exosomes have been widely researched. Encapsulating nanomedicine onto live cells or coating nanomedicines with endogenous cell membrane as a functional material not only reduces the immunogenicity of nanomedicine, prolongs their circulation time, but also confers an accurate targeting capability on them89,90.
Neutrophils and macrophages are main targeted cells with inflammatory tropism, as they could respond to chemokine and migrate to damaged brain. The ligand on the cell membrane such as LFA-1 could bind to ICAM-1 highly expressed on capillary endothelial cells, and promote the retention of loaded nanomedicine to enter the brain parenchyma. For instance, targeting ligand PGP-decorated PEG-DGL-CAT NPs hitchhiked the circulating neutrophils in blood via the binding between chemoattractant PGP and specific receptor on neutrophils. Neutrophils carrying NPs infiltrated and migrated to the ischemic area where they transfer NPs to damaged neurons through transient intercellular connections91.
Exosomes are cell membrane vesicles with nanoscale, which can also be used for targeted drug delivery since they retain the membrane protein of the original cells92,93. Further targeted modification of cell membrane vesicles and exosomes could improve their targeting ability60,75,80,81,88. For instance, cRGD-modified mesenchymal stromal cell (MSC)-derived exosomes loading with curcumin accumulated more at the lesion area, compared to unmodified exosomes, as surface modification improved the active targeting ability of exosomes81.
4.2.4. Adsorptive-mediated transcytosis
Electrostatic interactions between cationic protein/cell penetrating peptide (CPP) with positive charge and brain capillary endothelial cells with negative charge could assist nanomedicine to cross the BBB through adsorptive-mediated transcytosis (AMT). For instance, modification with TAT (belonging to the CPP family) improved the ability of nanoplatelets crossing the BBB to deliver loaded drug94. In another research, cationic bovine serum albumin conjugated tanshinone PEG-PLA NPs possessed the ability to attach to the negatively charged brain microvascular lumen sides, and increased the amount of tanshinone in the ischemic brain through AMT95.
4.2.5. Bypass the BBB
In addition to highly invasive intracranial and intrathecal injections, intranasal administration provides a new and feasible route to bypass the BBB. Intranasal administration can mainly deliver nanomedicine to the olfactory bulb or other brain regions through the olfactory nerve. For instance, curcumin-loaded embryonic stem cell exosomes delivered drug into brain intra-nasally, which decreased the level of ROS and infarct volume96. In addition, the positively charged vehicle could increase the penetration of nanomedicine into nasal epithelial cells to enhance the transport of drugs to the brain. Arginine-poly-amidoamine ester (e-PAM-R), a biodegradable polycationic ester, was selected to carry anti-HMGB1 siRNA intranasally to improve the delivery efficiency of siRNA97.
5. Nanomedicines for stroke treatment
5.1. Nanomedicines improving the efficacy and safety of thrombolytics
There will be more serious and rapid damage in the ischemic core where the blood flow is lowest within the ischemic regions98, the cells in ischemic core are doomed to die regardless of any treatments, unless blood supply recovers rapidly. In the ischemic penumbra which is at the edge of the ischemic core, neuronal injury develops slowly due to the existence of microcirculation which is an adjacent capillary network that maintains brain perfusion above the threshold of immediate cell death13. Early reperfusion is the main goal of most interventions to ensure cells in penumbra resistant to the ischemia-induced death. However, the effect of thrombolysis with plasminogen activator is limited, for their short half-life and low targeting efficiency. As expected, nanomedicines have been developed to deliver thrombolytics efficiently and precisely via physical targeting, ligand-mediated active targeting and biomimetic targeting. Moreover, the well-designed response release further improves the effectiveness and safety of delivering thrombolytics with nanocarriers.
5.1.1. Physical targeting
Physical targeting refers to guiding NPs to the target site with the assistance of external manipulation, or enabling the response release of drug from nanomedicine at the target site under the action of heat or mechanical force such as ultrasound. PEG crossed glycol chitosan soft NPs with good ultrasound response ability were synthesized to deliver urokinase99,100. NPs achieved better effect in degrading clots and protecting the BBB, though NPs did not decrease the risk of HT than free protein.
5.1.2. Ligand-mediated active targeting
Modifying vehicle with specific ligand could also confer the targeting ability on thrombolytics. As the fibrin is a major component of thrombus, anti-fibrin antibody-modified polystyrene NPs were applied to deliver alteplase through conjugating proteins on the surface of NPs. Alteplase NPs showed much less activity than free enzyme in the absence of embolus, which would reduce the unspecific cleavage of plasminogen and further decrease the risk of bleeding101. Glycoprotein IIb/IIIa (GPIIb/IIIa) receptors on platelet membrane may play a key role in the aggregation of platelets and the formation of thrombus, cyclic Arg-Gly-Asp (cRGD) peptide which could specifically bind the GPIIb/IIIa receptors is employed to mediate targeting thrombolysis, such as cRGD-modified target sensitive streptokinase (SK) liposomes102, 103, 104. After the interactions between cRGD with activated platelets liposomes consisted of cRGD-palmitic acid underwent destabilization and released encapsulated SK around the clot102.
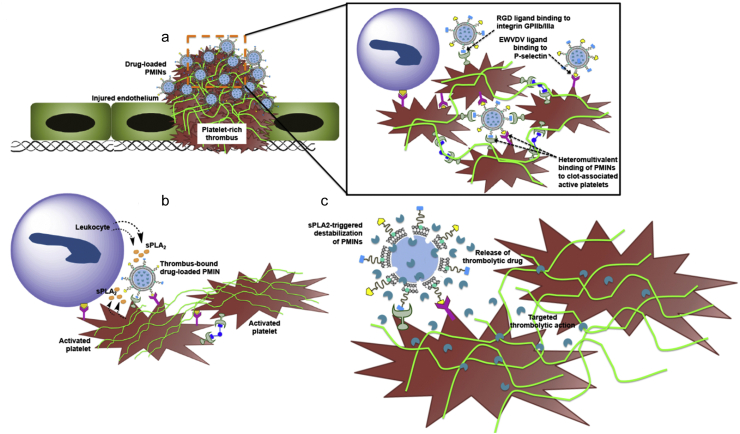
Apart from single-targeted ligand modification, there are some studies on dual ligand-modified nanomedicine with higher targeting efficiency105. The overexpressed P-selectin on activated platelets mediate the interaction between thrombocytes. Inspired from this mechanism, dual-targeted nanovesicles modified with cRGD and P-selectin targeting peptide were developed to deliver SK105. Nanovesicles composed of glycerophospholipids achieved stimuli-responsive release of drugs after locating at the target site, as glycerophospholipids could be cleaved by phospholipase A2 which is overproduced by activated platelets and leukocytes in thrombus105 (Fig. 3). Nanovesicles showed better therapeutic effects, and the risk of bleeding was reduced compared to free drug.
Figure 3.
Schematic diagram of targeted thrombolysis using nanovesicles inspired from activated platelets. (a) Nanovesicles bind the activated platelets in thrombus through the interaction between RGD and GPIIb-IIIa, as well as P-selectin targeting peptide and P-selectin. (b) Nanovesicles composed of glycerophospholipids achieve responsive release of drugs after locating at the target site, as the glycerophospholipids are cleaved by phospholipase A2 overproduced by activated platelets and leukocytes in thrombus. (c) Drug released from degraded nanovesicle realizes targeted fibrinolysis. PMINs: platelet microparticle-inspired nanovesicles. Reprinted with the permission from Ref. 105. Copyright © 2017, Elsevier Ltd.
5.1.3. Biomimetic targeting
Considering the role of platelets in thrombus formation, bioengineered nanoplatelets were developed for treating ischemic stroke. The polymeric NP core was coated with platelets membrane, the membrane was conjugated with TAT-peptide-coupled alteplase linked with amino acid sequences which could be cleaved by thrombin94. The nanoplatelets can avoid the recognition and phagocytosis of the RES, and adhere to the thrombus site to prolong the retention of NPs and achieve responsive release of alteplase. Biomimetic targeting nanomedicines based on cell membrane have become an emerging effective strategy in treating ischemic stroke.
In conclusion, targeted delivery of plasminogen activator via nanomedicine could increase the accumulation of drugs at the thrombus site, thereby reducing the dosage and minimizing the side effects of thrombolytics. The strategies, including achievement and limitation in targeted delivery of thrombolytics are summarized in Table 394,99, 100, 101, 102, 103, 104, 105.
Table 3.
Summary of targeted delivery of thrombolytics with nanomedicines.
| Strategy | Target | Ligand | Nanomedicine | Achievement | Limitation | Ref. |
|---|---|---|---|---|---|---|
| Physical targeting | Urokinase-loaded NPs | Enhanced clots degradation and BBB protection | No reduction in the risk of HT | 99,100 | ||
| Ligand-mediated active targeting | Fibrin | Anti-fibrin antibody | Alteplase-loaded NPs | Showed less activity in the absence of embolus | Lack of experimental data in vivo | 101 |
| GPIIb/IIIa receptors | cRGD peptide | Target sensitive SK-loaded liposomes | Target sensitive release of SK; enhanced clot dissolution in vivo | No data about the risk of HT | 102, 103, 104 | |
| GPIIb/IIIa receptors P-selectin | cRGD peptide P-selectin targeting peptide | Phospholipase A2 responsive SK-loaded nanovesicles | Reduced the risk of bleeding | No data about the risk of cerebral hemorrhage | 105 | |
| Biomimetic targeting | Thrombus | Membrane proteins on platelets | Alteplase-loaded NPs coated with platelets membrane | Achieved responsive response of alteplase in thrombus | No data about the risk of cerebral hemorrhage | 94 |
5.2. Nanomedicines delivering oxygen to the ischemic brain
For patients who are not suitable for thrombolysis or have shown microcirculation impairment due to the formation of microthrombus, delivering sufficient O2 to the ischemic penumbra is a potential method. Hemoglobin (Hb) is a natural O2 carrier, but short half-life and hypertension response limit its clinical application106,107. Liposomal Hb (LHb) could not only prolong the circulation time of Hb in vivo, but also deliver sufficient O2 to cross the obstacles in the microcirculation and get to the penumbra where red blood cells (RBCs) can seldom reach108,109. In a rat thrombosis model induced by photochemistry, fluorescein-labeled RBCs were mainly observed in larger vessels, whereas fluorescent liposomes filled all vessels including capillaries in a manner similar to plasma108. LHb significantly reduced the infarct volume of ischemic penumbra, compared to free Hb and homologous blood which contains lots of RBCs carrying Hb109. Moreover, LHb can also significantly attenuate the infarction size in transient ischemic model when infused immediately after the middle cerebral artery occlusion (MCAO)110,111. Though delivering O2 could rescue dying neurons by relieving hypoxia in the penumbra, too much O2 after reperfusion may cause the significant increase in ROS, the timing and dosage of administration need further investigation.
5.3. Nanomedicines regulating ion imbalance and excitotoxicity
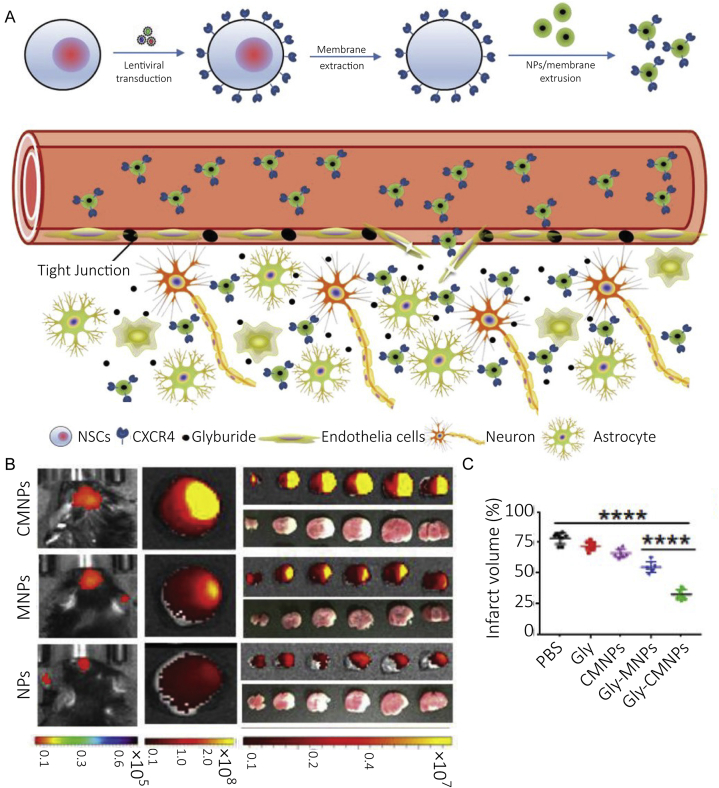
Ion imbalance and excitotoxicity occur upstream during the ischemic cascade, and regulating the progression of these events is expected to inhibit the amplification of damage in the early stage of stroke as well as produce more effective intervention outcomes. The sulfonylurea receptor 1-transient receptor potential melastatin-4 (SUR1–TRPM4) complex is a nonselective cation channel which is sensitive to ATP, and the expression of the channel is upregulated in ischemic neurons, astrocytes and capillaries112. Depletion of ATP in the ischemic brain leads to the continued opening of SUR1-TRPM4 channel which increases the influx of Na+, causes the depolarization of cells and cytotoxic edema, and finally leads to the necrocytosiss and brain swelling113. Glyburide is an inhibitor of SUR1–TRPM4 complex, and has shown treating effect in reducing edema and infract volume in animal and human114. However, glyburide is a substrate for P-glycoprotein, which limits its ability in penetrating the BBB to realize the effective therapeutic concentration115. As expected, several nanomedicines have been developed to deliver glyburide, such as poly (l-lactide-co-glycolide) nanoparticles (PLGA-NPs)75, thrombin-responsive size-shrinkable brain targeting NPs76, glyburide-loaded betulinic acid NPs77. It has been reported that neural stem cells (NSCs) could migrate to the injured site due to the interaction between C-X-C chemokine receptor type 4 (CXCR-4) and ligand stromal cell-derived factor-1 (SDF-1) which is overexpressed in the ischemic brain. PLGA-NPs coated with membrane derived from NSCs overexpressed CXCR4 demonstrated higher effects in enhancing brain targeting delivery of glyburide, which significantly reduced the infract volume75 (Fig. 4). Thrombin-responsive NPs could shrink their size after crossing the damaged BBB and arriving at the ischemic site where thrombin is highly expressed, which enhanced the penetration of NPs in the brain parenchyma76. Betulinic acid NPs realized penetrating the ischemic brain through the interaction between betulinic acid and cannabinoid receptor one overexpressed on the damaged BBB, which can improve the delivery efficacy of glyburide to the brain77.
Figure 4.
(A) Scheme of targeted delivery of PLGA NPs coated with membrane derived from NSCs which overexpressed CXCR4 to the ischemic brain. Lentiviral transduction was applied to transform NSCs to overexpress CXCR4. PLGA NPs accumulate in the ischemic area after intravenous injection, and glyburide entrapped in NPs are released to rescue neurons. (B) The accumulation of IR-780-loaded NPs in ischemic brain (n = 3), CMNPs showed significantly higher accumulation than MNPs or NPs. (C) Infarct volume (n = 5), of MCAO mice treated with different formulations. CMNPs: CXCR4-overexpressing membrane-coated NPs; Gly: glyburide; MNPs: membrane-coated NPs. Reprinted with the permission from Ref. 75. Copyright © 2019, WILEY-VCH.
Postsynaptic density protein-95 (PSD-95) participates in the activation of neuron nitric oxide synthase (nNOS) mediated by NMDAR via the formation of NMDAR/PSD-95/nNOS complex, disrupting the association between nNOS and PSD-95 provides a method to ameliorate the damage caused by excitotoxicity116. ZL006 could block the nNOS-PSD-95 interaction selectively, however, the drug has obvious drawbacks in the ability to accumulate in the ischemic brain78. Dual targeted liposomes conjugated with T7 peptide and stroke-homing peptide (SHp) were developed to deliver ZL006 accurately, and T7 peptide could help the liposomes transport across the BBB through TfR-mediated transcytosis24,117, SHp preferentially homed to the ischemic territory and mediated the endocytosis of liposomes by damaged neurons via glutamate receptors79. Dual targeted liposome could significantly reduce the infarct volume, and improve neurological outcomes in stroke rats, compared with T7 peptide or SHp-modified liposomes. In another research, dextran NPs coated with RBCs membrane modified with SHp were developed to deliver NR2B9c peptide which could prevent the binding of PSD-95 to NMDAR to reduce the excitotoxicity induced by glutamate80.
Tacrolimus could inhibit the activation of Ca2+-dependent calcineurin through binding the tacrolimus-binding protein in neurons. However, frequent use of high-dose tacrolimus to achieve good therapeutic effect has shown side effects118. Liposomal tacrolimus can improve the solubility of drug, and reduce the administration dosage without affecting the neuroprotective efficacy, as liposomal drug diffused more into the ischemic brain than free drugs through the damaged BBB60. Table 4 summarizes the strategies in targeted delivery of nanomedicines which could deliver oxygen, regulate ion imbalance and excitotoxicity.
Table 4.
The strategies, including achievements and limitations in targeted delivery of nanomedicines which could deliver oxygen, regulate ion imbalance and excitotoxicity.
| Phase of cascade | Target | Nanomedicine and intervention strategy | Achievement | Limitation | Ref. |
|---|---|---|---|---|---|
| Oxygen deficiency | Passively | Hb-loaded liposomes which carry oxygen | Delivered sufficient O2 to the penumbra where Hb-containing RBCs rarely reach | Too much O2 after reperfusion might cause the significant increase of ROS | 108, 109, 110, 111 |
| Ion imbalance | |||||
| SUR1-TRPM4 complex | SDF-1 | PLGA-NPs coated with NSCs membrane that highly expressed CXCR4 | Reduced the infract volume | Long term administration might increase the risk of hypoglycemia | 75 |
| Inhibitor: glyburide | CXCR4 | Thrombin responsive size-shrinkable NPs modified with AMD3100 | Enhanced the penetration of NPs in the brain | 76 | |
| CA 1 | Glyburide-loaded betulinic acid NPs | Reduced the oxidative stress | 77 | ||
| Excitotoxicity | |||||
| NMDAR/PSD-95/nNOS complex | TfR and NMDAR | ZL006-loaded liposomes modified with T7 peptide and SHp | Reduced the infarct volume and improved neurological outcomes | 78,79 | |
| NMDAR | NR2B9c-loaded dextran NPs coated with RBCs membrane modified with SHp | Ameliorated neurological deficit | 80 | ||
| Ca2+-dependent calcineurin | Passively | Tacrolimus-loaded liposomes | Suppressed the expansion of brain damage without side effects | NPs mainly presented in the ischemic core rather penumbra | 60,118 |
CA 1, cannabinoid receptor 1.
5.4. Nanomedicines reducing oxidative stress
Therapy employing antioxidants to scavenge excessive ROS, not only attenuates the oxidative stress injury, but also reduces downstream apoptosis and inflammatory responses. Therefore, antioxidant therapy now is a major method to treat injury caused by the ischemia and reperfusion. Antioxidants in preclinical research mainly include small molecule compounds, antioxidase and inorganics. Nanomedicines could improve the pharmacokinetic performance of antioxidants, and assist them to cross the BBB to accumulate at the damaged brain.
Many small molecule antioxidants are plant-derived phenolic and keto compounds, which could react with ROS, such as baicalin119, luteolin120, curcumin121, resveratrol61, tanshinone95, and puerarin74. However, the poor solubility, short half-livest and limited BBB penetrability restrict their application. Nanotechnology-based delivery strategies have been developed to solve these problems, such as resveratrol albumin NPs61, cationic BSA conjugated tanshinone PEG-PLA NPs95, curcumin-loaded NPs62,121,122, curcumin-loaded exosomes96, baicalin-loaded liposome63, luteolin-loaded PEG-PLGA micelles64,123, and puerarin-loaded poly-butylcyanoacrylate (PBCA) NPs74. Taken curcumin as an example, hydrophobic curcumin is easy to be metabolized and eliminated in vivo, and it is also poorly absorbed by cells. PLGA-NPs-containing curcumin showed higher uptake in neurons than free drug, as cells internalized NPs through endocytosis rather than passive diffusion, and NPs exerted more efficient anti-oxidative activity on neurons treated with H2O262,121,122. In addition, curcumin entrapped in PEG-PLA NPs realized higher accumulation in the ischemic penumbra through the damaged BBB, compared with free curcumin62. The NPs can extend the half-life of curcumin in vivo, which increased the chance of the drug penetrating into the damaged brain during the opening window of BBB. In another study, curcumin-loaded embryonic stem cell exosomes not only improved the stability and solubility of drug, but also solved the problem of poor absorption. Moreover, exosomes, which possess the ability to bypass the BBB upon administrated intra-nasally, could transport more drugs into the ischemic brain. Therefore, treatment with embryonic stem cell exosomes loaded with curcumin can significantly decrease the ROS level in the damaged brain, reduce the area of infarct, and attenuate the breakdown of neurovascular units96.
In addition to natural antioxidants derived from plant, there are also many synthetic small molecule antioxidants, such as edaravone, nitroxyl radicals 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO). PEG-PLA micelles modified with CGS21680 were developed to prolong the circulation time of edaravone71, and the BBB modulation strategy could help the edaravone micelles realize more effective accumulation in the ischemic brain and rescue more neurons. As a nitroxyl radical, TEMPO is found to be quickly reduced by antioxidase in vivo, and administration with TEMPO might induce hypotension65. pH-Sensitive PEG-poly-chloromethylstyrene (PEG-b-PCMS) micelles were designed to prolong the half-life of TEMPO, as well as to reduce the dosage and toxicity124. The chloromethyl groups of PEG-b-PCMS were converted to TEMPOs via the amination with 4-amino-TEMPO, and the micelles would decompose and expose the TEMPO upon the protonation of amino groups in intracellular acidic compartments124,125. TEMPO-contained micelles significantly reduced the production of ROS in the ischemic area, and limited the BBB damage66.
In some cases, the polymers that construct the nanomedicines could also scavenge the ROS and preserve the damaged neurons, such as ROS-sensitive phenylboronic ester-contained polymers in micelles and NPs23,80, nanomedicines would collapse and simultaneously release the encapsulated drug when eliminating the high-level ROS. In an interesting study, melanin, a heterogeneous biological polymer, itself can form highly water-dispersible NPs which have the capability against multiple ROS67. Coincidentally, betulinic acid NPs also exhibited antioxidant capacity to reduce the oxidative stress and infarct volume in a dose-dependent manner77.
SOD can convert superoxide anion to less toxic H2O2, catalase (CAT) can convert H2O2 to H2O and O2, and MCAO mice that overexpresses SOD or CAT showed less injury after reperfusion126,127. However, the short half-life and low BBB penetrability greatly limit the application of exogenous antioxidase for stroke treatment54,128. Liposomes, PBCA NPs, and PLGA-NPs extended the half-life of SOD, and the biphase opening of the BBB after reperfusion helped them achieve enhanced accumulation of SOD in the ischemic brain82. Modification with anti-NMDAR1 antibody helped liposomes and NPs realize enhanced uptake by neurons, which showed significant improvement in reducing the level of oxidative stress and infract volume, compared to nontargeted nanomedicines and free SOD82. In addition, rats receiving PLGA-NPs loaded with CAT and SOD immediately after reperfusion showed less infiltrated neutrophils and caspase-3 positive cells in the brain than animals receiving alteplase alone, as reducing oxidative stress is beneficial to inhibit the downstream cascade reaction68. Moreover, targeting inflammatory cells circulating in blood mentioned above provides new ideas for targeted delivery of antioxidase91.
Ceria nanoparticles (Ce-NPs) which possess the ability to scavenge multiple ROS were applied to treat ischemic stroke69. Though the size of Ce-NPs was about 20 nm, the concentration of ceria in non-ischemic brain was low. However, there was significant improvement in the concentration of Ce-NPs in the ischemic brain, as the BBB was damaged after ischemia. In order to realize better treatment, PEGylated Ce-NPs was conjugated with biotinylated-LXW7, targeted modified Ce-NPs could cross the BBB through the interactions between LXW7 and αvβ3 which is upregulated on BBB after stroke, Ce-NPs modified with LXW7 exhibited better effects in reducing infarction size and the degree of BBB breakdown than Ce-NPs83. In another study, PEGylated Ce-NPs modified with angiopep-2 were used to load edaravone through van der Waals forces, modified Ce-NPs accumulated in the brain tissue via the LRP1-mediated transcytosis. Edaravone-loaded Ce-NPs exerted synergistic antioxidant effect to rescue damaged neurons, and protected BBB from further damage84. Inorganic NPs which have good uniformity are easy to prepare and modify, and have been gradually applied in the field of treatment and drug delivery, though their biosafety still needs further serious investigation. Table 5 summarizes the strategies in targeted delivery of nanomedicines in reducing oxidative stress.
Table 5.
The strategies, including achievements and limitations in targeted delivery of nanomedicines in reducing oxidative stress.
| Phase of cascade | Target | Nanomedicine and intervention strategy | Achievement | Limitation | Ref. |
|---|---|---|---|---|---|
| Oxidative stress | |||||
| Small molecule antioxidants | Passively | Curcumin-loaded PLGA NPs | Prevented neurons from oxidative damage | No experimental data in vivo | 121,122 |
| Passively | Curcumin-loaded PEG-PLA NPs | Protected the BBB against oxidative stress; ameliorated neuronal apoptosis and the release of inflammatory mediators. | Conjugating with targeting ligand might further improve the treatment effect | 62 | |
| Bypass the BBB | Curcumin-loaded embryonic stem cell exosomes | Delivered drug into brain intra-nasally, decreased the level of ROS | The safety of nasal administration has not been investigated | 96 | |
| Integrin αvβ3 | Curcumin-loaded exosomes modified with cRGD | Downregulated activated microglia and the expression of inflammatory cytokines | 81 | ||
| Passively | Resveratrol-loaded albumin NPs | Reduced oxidative stress and the apoptosis of neurons | Passive targeting might not be suitable for long-term administration | 61 | |
| AMT | Tanshinone-loaded PEG-PLA NPs conjugated with cationic BSA | Inhibited the inflammatory cascades | There is risks of being phagocytized by the RES | 95 | |
| Passively | Baicalin-loaded liposomes | Improved the accumulation of drug in the ischemic brain | Non-PEGylated liposomes were mainly phagocytized by the RES | 63 | |
| Passively | Luteolin-loaded PEG-PLGA micelles | Inhibited oxidative stress and decreased the release of inflammatory mediators | Targeting efficiency might need further improvement | 64 | |
| LRP | Puerarin-loaded PBCA NPs coated with polysorbate 80 | Reduced the infarct volume | 74 | ||
| A2AR BBB modulation | Edaravone-loaded PEG-PLA micelles modified with CGS21680 | Exerted a more persistent ROS eliminating effect and rescued more neurons | The safety of BBB modulator needs further study | 71 | |
| Passively | PEG-b-PCMS-TEMPO micelles | Suppressed oxidative stress and apoptosis, protected neurovascular units | The study did not reveal the effect and toxicity of NPs in subacute phase | 65,66 | |
| Passively | Melanin-loaded NPs conjugated with PEG | Showed antioxidative effects against multiple RONS, suppressed the expression of inflammatory cytokines | The potential toxicity of the degradation byproducts of PEG-MeNPs needs further investigation | 67 | |
| Antioxidase | NMDAR1 | SOD-loaded liposomes, PBCA NPs, and PLGA-NPs; nanomedicines were modified with anti-NMDAR1 antibody | Reduced the oxidative stress and apoptosis, and inhibited the release of inflammatory mediators | The effect of administration in subacute phase is not satisfactory | 82 |
| Passively | CAT/SOD-loaded PLGA-NPs | Mitigated apoptosis and inflammatory response; promoted neurogenesis | Carotid injection is not conducive to long-term administration | 68 | |
| Biomimetic targeting | PEG-DGL-CAT NPs decorated with PGP, which could hitchhike the circulating neutrophils | Reduced the infarct volume via anti-oxidative effect | The fate of NPs in neutrophils needs further investigation | 91 | |
| Inorganics | Passively | PEGylated ceria NPs | Scavenged ROS and reduced apoptosis | Targeting ability need further improvement | 69 |
| Integrin αvβ3 | PEGylated Ce-NPs conjugated with biotinylated-LXW7 | Showed better effects in mitigating the oxidative stress and apoptosis | There was no improvement in neurological function | 83 | |
| LRP | Edaravone-loaded Ce-NPs modified with angiopep-2 | Exerted synergistic ROS scavenging ability | 84 | ||
A2AR, adenosine 2 A receptor; BSA, bovine serum albumin; LRP, lipoprotein receptor-related protein; RONS, reactive oxygen and nitrogen species.
5.5. Nanomedicine reducing apoptosis
Apoptosis is an intermediate event of the ischemic cascade. Reducing apoptosis could not only rescue the stressed neurons, but also weaken the downstream inflammation and immune response, which is conducive to inhibit the deterioration of stroke. Most of the antioxidants mentioned above also have anti-apoptotic ability, as oxidative stress is a main cause of apoptosis61,62,66,68,81,82. In addition, the more researched anti-apoptotic drugs are mainly bio-macromolecules, and ligand-modified NPs and exosomes have been developed to deliver these drugs58,85,86,129. Asialo-erythropoietin (AEPO) could bind the erythropoietin receptor expressed on neurons, and protect the neurons from apoptosis via the activation of protein kinase Akt-1/protein kinase B and extracellular signal-regulated kinases130,131. PEGylated liposomes could prolong the circulation period of AEPO, and the interactions between AEPO and erythropoietin receptor conferred the neuron targeting ability of liposomes upon crossing the damaged BBB. There was more accumulation and retention of 125I-labeled AEPO-liposome in the ischemic hemisphere, in rats which were injected with free AEPO or liposomal AEPO. A single injection of AEPO-liposomes significantly inhibited neuronal apoptosis and improved neurological outcomes, compared to administration of free AEPO85,86. Moreover, anti-apoptosis protein BCL-2 could prevent the caspase apoptosis cascade, pigment epithelium-derived factor is believed to protect neurons from apoptosis through activation of ERK1/2 and induction of BCL-2132. For instance, exosomes origin from adipose-derived mesenchymal stem cells which can overexpress pigment epithelium-derived factor could reduce brain damage by inhibiting neuronal apoptosis129. In another study, Nogo-66 receptor antagonist Nogo extracellular peptide 1–40 could reduce the increased caspase-3 activity in ischemic brain133, and the peptide encapsulated in PLGA-NPs modified with targeting ligand chlorotoxin significantly reduced the infarct volume and enhanced the survival of ischemic mice, compared with free drug58. Table 6 summarizes the strategies in targeted delivery of nanomedicines in reducing apoptosis.
Table 6.
The strategies, including achievements and limitations in targeted delivery of nanomedicines in reducing apoptosis.
| Phase of cascade | Target | Nanomedicine and intervention strategy | Achievement | Limitation | Ref. |
|---|---|---|---|---|---|
| Apoptosis | EPO receptor | AEPO conjugated PEGylated liposomes | Achieved strengthened accumulation in neurons, inhibited neuronal apoptosis | It might increase the risk of hematopoietic effect | 85,86 |
| MMP-2 and BBB modulation | Nogo extracellular peptide encapsulated in lexiscan-loaded PLGA-NPs modified with targeting ligand chlorotoxin | Reduced the infarct volume and enhanced the survival of ischemic mice | The safety of lexiscan needs further investigation | 58 | |
| Biomimetic targeting | Exosomes derived from ADSCs that overexpress PEDF | Ameliorated neuronal apoptosis | 129 |
PEDF, pigment epithelium-derived factor.
5.6. Nanomedicines regulating inflammation and immune response
In the ischemic brain, injured cells can release DAMPs which would activate the innate immunity and initiate the process of inflammatory response134. For instance, HMGB1 activates pattern recognition receptors on microglia, which will upregulate the expression of pro-inflammatory gene through multiple signaling pathways, such as the transcription factor nuclear factor-κB (NF-κB)29,30. Numerous inflammatory mediators are NF-κB-dependent, such as ICAM-1, IL-2,6, TNF-α and chemokines. NF-κB could also be activated by ROS or cytokines like TNF-α, IL-2, and IL-630. In addition, pattern recognition receptors are also found in the cerebral endothelium, astrocytes and neurons29. The inflammatory mediators released from the brain cells might spread to the blood vessels to amplify the expression of inflammatory mediators, which drives the infiltration of leukocytes into the ischemic brain and aggravate the progress of inflammatory response13,31, 32, 33, 34, 35, 36, 37. The inflammatory and immune responses would dramatically expand the brain damage, and curbing the occurrence and progression of inflammation is particularly important for protecting the damaged brain. In addition to inhibiting the upstream events of the ischemic cascade, inhibiting vital components in the inflammatory response, such as pro-inflammatory mediators and cells, could also exert anti-inflammatory effects.
5.6.1. Nanomedicines inhibiting pro-inflammatory mediators
As HMGB1 plays an important role in inflammatory response, inhibiting the production of HMGB1 might be an effective way to reduce the injury caused by inflammation29,30. Gene silencing mediated by RNA interference technology could regulate the specific protein functions, yet the delivery of small interfering RNA (siRNA) in vivo limits the therapeutic application of this technology. Arginine-poly-amidoamine ester (e-PAM-R) was chosen as the carrier to improve the delivery and transfection efficiency of anti-HMGB1 siRNA56. The transfection efficiency of e-PAM-R/siRNA complex was thus improved, as reverse transcription-polymerase chain reaction revealed that there was significant downregulation of HMGB1 mRNA levels in primary cortical cultures treated with NMDA or H2O2, and treatment with e-PAM-R/siRNA reduced the neuronal death. E-PAM-R can successfully deliver siRNA into the brain cells, since there was notable reduction in HMGB1 level and infarct volume after intranasal delivery of e-PAM-R/siRNA in stroke rats135.
In addition to HMGB1, TNF-α is also an important inflammatory mediator. CsA is a hydrophobic peptide consisting of 11 amino acid residues, which could inhibit the release of TNF-α and IL-1, and exert anti-inflammatory and neuroprotective activities. However, CsA is effective only at high-dose administration, which results in severe side effects such as seizure. Liposomal CsA showed significant suppression of inflammation and reduction of infarct size, and animals receiving liposomal CsA did not show obvious side effects53.
As one part of the innate immunity, complement system is also activated in the ischemic brain. After the ischemia and reperfusion, injured neurons expressed complement component C1q, C3 and C5, microglia and astrocytes can also produce C1q and C310, 11, 12. C3 induces the phagocytosis of neurons by microglia through the interactions between C3b deposited on the neuronal surface and complement receptor three on microglia12. Mice deficient in C3 were protected from reperfusion injury, which showed that inhibiting complement activation is a potential treatment method for stroke11. To improve the efficiency of RNA interference, PEG-PLA NPs were applied to carry anti-complement C3 siRNA (C3-siRNA) with the assistance of cationic lipid, which is believed based on the electrostatic interactions. NPs that can deliver siRNA into the damaged brain and subsequently engulf by the microglia, and reduced the level of C3 in microglia and microglial neurotoxicity. Moreover, gene therapy with C3-siRNA-PEG-PLA-NPs decreased the infiltration of inflammatory cells and the level of pro-inflammatory mediators, and improved neurological outcomes55.
5.6.2. Nanomedicines regulating inflammatory cells
As the resident immune cells in the brain, microglia is rapidly activated and then migrate to the damaged site under the action of DAMPs and chemokines136. Although activated microglia are generally believed to exacerbate the damage, some studies have shown that unlike the classically activated M1-phenotype microglia with pro-inflammatory activity, alternatively activated M2-phenotype microglia could exert anti-inflammatory effects137. In the peri-infarct region, newly recruited microglia are mainly M2-phenotype, but M1-phenotype gradually occupy main position to cause brain damage138. Therefore, promoting the microglial polarization from M1 to M2 in the acute phase of stroke might be beneficial to reduce the inflammatory response at the lesion site.
The main pro-inflammatory cytokines secreted by M1 microglia are NF-κB-dependent, such as IL-1, IL-6, TNF-α, and regulating the NF-κB signaling pathway may promote the phenotypic transition from M1 to M2. Since NF-κB could also be activated by ROS, eliminating ROS might help achieve the phenotypic shift62,95. For instance, PEGylated Ce-NPs can drive microglial polarization from M1 to M2 via scavenging multiple ROS, which is in favor of the resolution of inflammation and reduction of cell death139.
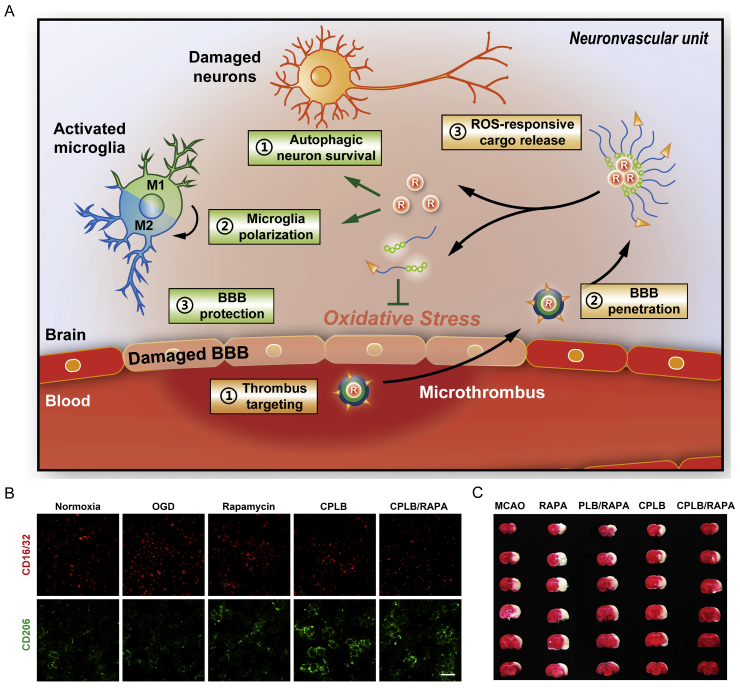
Nanomedicines have also been used to deliver small molecules to promote the phenotypic transition of microglia. Rapamycin could promote the microglia polarization from M1 to M2, yet the poor BBB permeability limits its application. Fibrin-binding peptide-modified micelles that contain ROS-sensitive phenylboronic ester units were applied to deliver rapamycin into the damaged brain. The modified micelles could realize higher retention in the thrombus site and subsequently accumulated in the ischemic brain. Micelles would collapse and release entrapped rapamycin response to the high-level ROS in the ischemic brain, which could protect neurons through eliminating ROS, and the liberated rapamycin would promote the phenotypic transition of microglia from M1 to M2, thereby inhibiting the damage of neurons and reducing the inflammation response23 (Fig. 5). The double-sided role of microglia reveals that the treatment of stroke should shift from simply inhibiting the activity of microglia to regulating the balance of cell phenotype.
Figure 5.
(A) Schematic diagram of CPLB/RAPA micelle in modulating the damaged brain suffered from ischemia: 1) micelle bind the microthrombus through the interaction between fibrin and fibrin-binding peptide; 2) micelle accumulate in the ischemic brain via crossing the damaged BBB; 3) ROS initiate the release of rapamycin, rapamycin promote the phenotypic transition of microglia from M1 to M2, thereby inhibiting the damage of neurons and reducing the inflammation response. (B) Immunostaining of M1 (red) and M2 (green) microglia treatment with different formulations (scale bar = 50 μm). (C) Representative TTC staining images of ischemic brain from rats with different treatment. RAPA: rapamycin; PLB: PEG-phenylboronic ester modified polylysine; CPLB: fibrin-binding peptide CREKA modified PLB. Reprinted with the permission from Ref. 23. Copyright © 2019, WILEY-VCH.
5.6.3. Nanomedicines for pretreatment with TNF-α
Previous research has shown that patients with transient ischemic attack (TIA) preceding ischemic stroke had better neurological outcome, and this ischemic tolerance was related to the overexpression of TNF-α induced by TIA135. Although TNF is generally considered to be a proinflammatory factor, pretreatment with TNF-α could also induce the production of anti-inflammatory mediators such as manganous superoxide dismutase through activating the NF-κB signaling pathway140, 141, 142. Pretreatment with TNF-α significantly reduced the brain injury caused by following ischemia in mice, yet the half-life of TNF-α is short, which limit its application143. PEG-b-(poly (ethylenediamine l-glutamate)-g-poly (l-lysine)) was applied to load negatively charged TNF-α via the electrostatic interactions, and the circulation time of TNF-α in vivo was prolonged. Preconditioned with TNF-α-loaded NPs to induce the ischemic tolerance would attenuate the oxidative stress damage and the inflammatory activity, and reduce the neuronal apoptosis level in the ischemic brain after reperfusion70. Pretreating to induce ischemic tolerance provides a new idea for the treatment of ischemic stroke, as for patients who have experienced TIA, pretreatment with effective nanomedicine might reduce the severity of following stroke.
5.7. Nanomedicines promoting tissue repair
Post-ischemic inflammation would subside gradually, and the damaged brain will undergo a structural and functional recovery process to return to homeostasis. Emerging evidences have shown that the resolution of inflammation is an active process benefiting from the action of anti-inflammatory mediators and immune cells144. This process comprises the elimination of dead cells, the establishment of an anti-inflammatory microenvironment, and the production of growth factors (GFs) that promote the repair of brain13. Resident microglia and infiltrated macrophages are main phagocytes responsible for the clearing of dead cells after stroke145,146. TGF-β and IL-10, two of the most important anti-inflammatory mediators, which are secreted in concert with the phagocytosis of dead cells, play a vital role in the establishment of anti-inflammatory microenvironment which is in favor of tissue repair147. As M2 microglia are responsible for the phagocytosis to remove cell debris and the secretion of anti-inflammatory factors to restrict brain damage, the nanomedicines that regulate the microglial phenotype transition also helps brain tissue recovery after stroke23,62.
GFs play a vital role in the development and survival of endothelial cells, glial cells and neurons148. Ischemia would downregulate the level of GFs during the acute phase of stroke149, which leads to neuron death, and treatment with GFs could protect brain against ischemic injury150, 151, 152. Moreover, GFs also promoted the compensatory activation and differentiation of NSCs into specific brain cells, and the migration of NSCs to infarcted area, which is in favor of accelerating the recovery of cerebral function at the chronic phase of stroke153,154. For instance, administration of nerve growth factor (NGF) promoted the proliferation of NSCs155, and consecutive treatment enhanced striatal neurogenesis after stroke156,157. APOE-modified human serum albumin NPs carrying NGF could assist the peptide penetrate into the damaged brain via the interaction between APOE and LRP1, and significantly promoted the brain recovery on two week post-stroke87. Superparamagnetic iron oxide encapsulated in APOE-modified albumin NPs could enhance the outcome of magnetic resonance imaging, which could be applied to monitor the structural recovery of brain in real-time.
Recent studies have also shown that multiple microRNAs (miRNAs) are involved in the process of neuronal remodeling, and targeting delivery of miRNAs to mediate neurogenesis is expected to promote the prognosis after stroke158. A study has indicated that viral vectors carrying miR-124 could promote angioneurogenesis in mice post-ischemia159. However, viral vectors might induce the immune response in vivo. Exosomes hold great promise as a gene delivery vector given their unique properties, including low immunogenicity, high delivery efficiency, and inherent ability to cross the BBB81. Rabies virus glycoprotein-modified exosomes containing miR-124 could effectively transported miRNA into the ischemic area, and reduced the brain injury by promoting neural precursor cells in the infarcted area to differentiate into neurons88. In another study, exosomes containing miR-17-92, obtained from the mesenchymal stromal cells transfected with miR-17-92-contained plasmids could cross the BBB, promote neurogenesis and axon growth, as well as functional recovery after stroke92.
Furthermore, exosomes separated from stem cell have been studied for their inherent powerful regenerative ability, as exosomes carry multiple proteins and genes that are related with tissue repair160. A large number of evidences have shown that the MSC-derived exosomes possess numerous advantages in the treatment of stroke by enhancing neuroprotection and nerve regeneration, improving neurovascular plasticity, and promoting functional recovery93. Therefore, exosomes originating from stem cells could not only deliver drugs to treat stroke, but also enhance the natural process of cerebral repair after injury induced by ischemia. Table 7 summarizes the strategies in targeted delivery of nanomedicines which could regulate inflammation response and promote tissue repair.
Table 7.
The strategies, including achievements and limitations in targeted delivery of nanomedicines which could regulate inflammation response and promote tissue repair.
| Phase of cascade | Target | Nanomedicine and intervention strategy | Achievement | Limitation | Ref. |
|---|---|---|---|---|---|
| Inflammation | |||||
| Inhibiting pro-inflammatory mediators | Bypass the BBB | Intranasal delivery of e-PAM-R/anti-HMGB1 siRNA complex | Reduced the level of HMGB1 and suppressed the infarct volume | The safety of nasal administration needs further investigation | 97 |
| Passively | CsA-loaded liposomes | Suppressed inflammation and infarct volume | The targeting efficiency need further improvement | 53 | |
| Passively | Anti-complement C3 siRNA-loaded PEG-PLA NPs | Decreased the infiltration of inflammatory cells and the level of pro-inflammatory mediators | Conjugating with targeting ligands might improve the treatment effect | 55 | |
| Regulating inflammatory cells | PEGylated Ce-NPs | Drove microglial polarization from M1 to M2 | No experimental data in vivo | 139 | |
| Fibrin | Rapamycin-loaded micelles modified with fibrin-binding peptide | Promoted the phenotypic transition of microglia from M1 to M2 | 23 | ||
| Pretreatment | Passively | TNF-α-loaded PEG-b-(PELG-g-PLL) NPs | Attenuated the inflammatory activity | The side effects need further study | 70 |
| Tissue repair | LRP1 | NGF-loaded albumin NPs modified with APOE | Promoted the brain recovery on two weeks post-stroke | The effect of NPs in improving neurological deficits need further evaluation | 87 |
| Biomimetic targeting | miR-124-loaded exosomes modified with rabies virus glycoprotein which could bind the nAChR on BBB | Promoted neural precursor cells in the infarcted area to differentiate into neurons | Neuron-specific targeting ability needs further improvement | 88 | |
| Biomimetic targeting | miR-17-92 contained exosomes derived from MSCs | Promoted neurogenesis, axon growth and functional recovery | 92 | ||
| Biomimetic targeting | Exosomes derived from MSCs | Enhanced neuroprotection and nerve regeneration, improved neurovascular plasticity | 162 | ||
MSCs, mesenchymal stromal cells; nAChR, nicotinic acetylcholine receptor.
5.8. Nanomedicine regulating multiple abnormalities
It is worth noting that various events of the ischemic cascade interact with each other. Upstream events will trigger downstream events, and downstream events will in turn amplify upstream events. Therefore, synergistic suppression of multiple abnormalities is expected to achieve better outcomes. Some drugs, such as curcumin, possess anti-oxidation, anti-apoptosis, and anti-inflammatory effects, and thus could simultaneously inhibit multiple abnormalities. In addition, there are many studies that integrated multiple agents with different functions into nanomedicine to realize combined therapy23,77,80,94,161.
For instance, nanoplatelets were developed to deliver alteplase and neuroprotectant ZL006. In detail, ZL006 was loaded in polymeric NP core, TAT-peptide-linked alteplase was conjugated on the surface of platelets membrane, and the linker could be cleaved by thrombin. NPs coated with platelets membrane retained the thrombus targeting ability of platelets, and released alteplase response to the highly expressed thrombin in clots. Then, the broken of the linker exposed the blocked TAT peptide, which promoted the penetration of NPs across the BBB to deliver ZL006. The combination of thrombolytics and neuroprotectant significantly reduced ischemia-related damage in MCAO rats94.
In addition to combined treatment with thrombolytics and neuroprotective agents, inhibiting multiple stages of the ischemic cascade is also a feasible method. Before reperfusion, hypoxia not only causes neuronal energy depletion, but also leads to the production of ROS; then reperfusion brings a boost of oxygen and leads to a large amount of ROS. T7-peptide-modified erythrocyte vesicles which contain Hb and manganous tetroxide NPs were developed to resolve this problem. The multifunctional vesicles showed superior O2 release capacity in the damaged brain before thrombolysis, and efficiently inhibited the boost of O2 after reperfusion, as Hb releases O2 under hypoxia and stores O2 in an oxygen-rich environment. In addition, manganous tetroxide NPs exhibited long-lasting scavenging ability against ROS. Regulating the level of O2 and ROS rescued ischemic brain before thrombolysis and after reperfusion161.
Moreover, polymer NPs with ROS-sensitive property are used to deliver drugs, which achieved antioxidant therapy through eliminating ROS, and realized combined therapy via carrying different drugs. For instance, glyburide-encapsulated betulinic acid NPs77, NR2B9c peptide-loaded boronic ester-modified dextran NPs80, and rapamycin-loaded boronic ester-modified polymer micelles23. The strategies, including achievement and limitation in targeted delivery of nanomedicine which could regulate multiple abnormalities are summarized in Table 8.
Table 8.
The strategies, including achievements and limitations in targeted delivery of nanomedicines which could regulate multiple abnormalities.
| Phase of cascade | Target | Nanomedicine and intervention strategy | Achievement | Limitation | Ref. |
|---|---|---|---|---|---|
| Combined therapy | Biomimetic targeting and AMT | Nanoplatelets delivering alteplase and neuroprotectant ZL006, TAT-peptide-linked alteplase was conjugated with platelets membrane | Reduced ischemia-related damage in MCAO rats | 94 | |
| TfR | Hb and manganous tetroxide NPs-loaded erythrocyte vesicles modified with T7-peptide | Rescued ischemic brain before thrombolysis and after reperfusion | The safety of inorganic NPs needs long-term investigation | 161 | |
| CA 1 | Glyburide-loaded betulinic acid NPs | Improved the delivery of glyburide to the brain and reduced the oxidative stress | Long term administration might increase the risk of hypoglycemia | 77 | |
| NMDAR | NR2B9c-loaded boronic ester dextran NPs coated with RBCs membrane and modified with SHp | Ameliorated oxidative stress and neurological deficit | 80 | ||
| Anti-firbin antibody | Rapamycin-loaded polymer micelles contained boronic ester group | Ameliorated oxidative stress and promoted the phenotypic transition of microglia from M1 to M2 | 23 |
AMT, adsorptive-mediated transport.
5.9. The response release of drug in the pathological site
For targeted delivery of nanomedicines, the release of the drug in response to the microenvironment of lesion could reduce the leakage of the drug in blood, increase the concentration of the drug at the targeting site, and reduce the administrated dosage and side effects. In the treatment of stroke, enzymes that are highly expressed in thrombus, such as thrombin, phospholipase A2, and ROS in ischemic brain tissue are often employed as stimulators of response release. Table 9 summarizes the nanomedicines which possess response release ability and the corresponding stimulators reviewed in this article.
Table 9.
Nanomedicines possessing response release ability and the corresponding stimulator.
| Stimulator | Nanomedicine | Ref. |
|---|---|---|
| Ultrasound | Urokinase-loaded PEG crossed glycol chitosan soft NPs | 99,100 |
| Target sensitive | Target sensitive SK-loaded liposomes modified with cRGD | 102, 103, 104 |
| Phospholipase A2 | SK-loaded nanovesicles composed of glycerophospholipids | 105 |
| Thrombin | Nanoplatelets conjugated with TAT-peptide-coupled alteplase | 94 |
| Thrombin | Thrombin responsive size-shrinkable NPs | 76 |
| ROS | Glyburide-loaded betulinic acid NPs | 77 |
| ROS | Rapamycin-loaded polymer micelles contained boronic ester group | 23 |
| ROS | NR2B9c peptide-loaded NPs contained boronic ester group | 80 |
6. Summary and prospect
Due to the lack of effective treatment method, the therapy and long-term recovery of ischemic stroke remains a main challenge for doctors and patients. Recently, nanomedicines have been widely used in the treatment of stroke, as it improved the therapeutic effect of traditional drugs and provided possibilities for the realization of emerging therapies, such as gene therapy52. Nanomedicines for targeted delivery of thrombolytics and neuroprotectant have also exhibited good results in preclinical research.
It is worth mentioning that the ischemic cascade events occur at different intervals and interacts with each other163. Therefore, a great number of nanomedicines have shown functions against multiple pathological events at different stages of the cascade reaction62,82. In theory, intervening the upstream events of ischemic cascade should be suitable for the treatment of stroke, yet some upstream events occur very quickly, even if the interventions were carried out within one or 2 h after ischemic attack, the damage caused by aforementioned events is still difficult to reverse completely46. Thus, nanomedicines targeting downstream events of ischemic cascade such as oxidative stress, inflammation response, and tissue repair might be more promising in the treatment of stroke23,88.
The properties of nanocarrier, including particle size, shape, hydrophilic characteristics and surface charge would affect the fate of nanomedicines in vivo. For instance, normally, the half-life of negatively charged NPs in vivo is longer, since positively charged NPs tend to absorb proteins such as opsonin in blood. PEGylation would enhance the hydrophilicity of NPs and reduce the opsonizing effect of opsonin, which further reduces the phagocytosis of RES and extends the circulation time of nanomedicine.
In addition, the unique pathophysiological environment of the body will also affect the fate of particles. After ischemia and reperfusion, the integrity of the BBB is destroyed and the permeability increases, nanomedicines could accumulate in the brain through the damaged BBB. Non-targeted nanomedicine might mainly accumulate in the ischemic core where the BBB is disturbed more seriously60,118, which is not conducive to the rescue of ischemic penumbra. In order to improve the permeability of the BBB, BBB modulators were adopted to temporarily open the tight junction to allow more NPs to penetrate into the brain. The long-term safety of this method still needs further investigation, as excessive opening of the BBB will increase the risk of hemorrhagic transformation.
Modifying nanomedicines with specific ligands through covalent conjugation or electrostatic interaction would further increase the accumulation of NPs in the brain through receptor-mediated transcytosis. After stroke, multiple receptors are highly expressed on BBB, which provides new opportunities for the targeted delivery of nanomedicine78,80,84. It is worth noting that targeted nanomedicine modified with endogenous ligand may encounter competition with endogenous ligand in the blood when passing through the BBB, which reduces the targeting efficiency. Fortunately, antibodies with stronger specificity to receptors have been developed, and the interactions are not affected by endogenous ligands.
Among numerous nanomedicines, liposomes, micelles, and polymeric NPs are mainly investigated due to their mature preparation technology, with the hope of translation into clinical application. Although inorganic NPs have favorable uniformity and are easy to be industrialized, the safety needs further serious investigation if used in human. In recent years, biomimetic nanomedicines, based on living cells or cell membrane vesicles/exosomes, have provided new opportunities for drugtargeting delivery. The unique biocompatibility and safety, and inherent targeting properties promote them as a research hotspot of nanomedicines for targeted treatment of stroke, with the expectation of bringing new breakthroughs in stroke treatment81. As the development of new carrier and deeper understanding of pathological mechanisms, nanomedicines are casting new lights in stroke treatment.
Acknowledgments
We acknowledge the support from the International Cooperative project of the National Key R&D Program of China (Grant No. 2017YFE0126900), National Natural Science Foundation of China (Grant No. 81872808), Program of Shanghai Academic Research Leader (18XD1400500,China) and Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01,China) and ZJ Lab.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Tao Sun, Email: sunt@fudan.edu.cn.
Chen Jiang, Email: jiangchen@shmu.edu.cn.
Author contributions
Chao Li composed the article under the guidance of Tao Sun and Chen Jiang.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P. Heart disease and stroke statistics-‒2020 update: a report from the American heart association. Circulation. 2020;141:e139–e196. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Sacco R.L., Kasner S.E., Broderick J.P., Caplan L.R., Connors J.J., Culebras A. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karaszewski B., Wardlaw J.M., Marshall I., Cvoro V., Wartolowska K., Haga K. Early brain temperature elevation and anaerobic metabolism in human acute ischaemic stroke. Brain. 2009;132:955–964. doi: 10.1093/brain/awp010. [DOI] [PubMed] [Google Scholar]
- 4.Castillo J., Loza M.I., Mirelman D., Brea J., Blanco M., Sobrino T. A novel mechanism of neuroprotection: blood glutamate grabber. J Cerebr Blood Flow Metabol. 2016;36:292–301. doi: 10.1177/0271678X15606721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewén A., Matz P., Chan P.H. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 6.Chan P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cerebr Blood Flow Metabol. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Gürsoy-Ozdemir Y., Bolay H., Saribaş O., Dalkara T. Role of endothelial nitric oxide generation and peroxynitrite formation in reperfusion injury after focal cerebral ischemia. Stroke. 2000;31:1974–1980. doi: 10.1161/01.str.31.8.1974. [DOI] [PubMed] [Google Scholar]
- 8.Peerschke E.I., Yin W., Ghebrehiwet B. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol. 2010;47:2170–2175. doi: 10.1016/j.molimm.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peerschke E.I., Reid K.B., Ghebrehiwet B. Platelet activation by C1q results in the induction of alpha IIb/beta 3 integrins (GPIIb-IIIa) and the expression of P-selectin and procoagulant activity. J Exp Med. 1993;178:579–587. doi: 10.1084/jem.178.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J., Kim L.J., Mealey R., Marsh H.C., Jr., Zhang Y., Tenner A.J. Neuronal protection in stroke by an sLex-glycosylated complement inhibitory protein. Science. 1999;285:595–599. doi: 10.1126/science.285.5427.595. [DOI] [PubMed] [Google Scholar]
- 11.Mocco J., Mack W.J., Ducruet A.F., Sosunov S.A., Sughrue M.E., Hassid B.G. Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circ Res. 2006;99:209–217. doi: 10.1161/01.RES.0000232544.90675.42. [DOI] [PubMed] [Google Scholar]
- 12.Brown G.C., Neher J.J. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15:209–216. doi: 10.1038/nrn3710. [DOI] [PubMed] [Google Scholar]
- 13.Iadecola C., Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powers W.J., Rabinstein A.A., Ackerson T., Adeoye O.M., Bambakidis N.C., Becker K. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 15.Hacke W., Donnan G., Fieschi C., Kaste M., Kummer R., Broderick J.P. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 16.Frank B., Grotta J.C., Alexandrov A.V., Bluhmki E., Lyden P., Meretoja A. Thrombolysis in stroke despite contraindications or warnings?. Stroke. 2013;44:727–733. doi: 10.1161/STROKEAHA.112.674622. [DOI] [PubMed] [Google Scholar]
- 17.Peters O., Back T., Lindauer U., Busch C., Megow D., Dreier J. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J Cerebr Blood Flow Metabol. 1998;18:196–205. doi: 10.1097/00004647-199802000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Allen C.L., Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 19.Kevil C.G., Oshima T., Alexander B., Coe L.L., Alexander J.S. H2O2-mediated permeability: role of MAPK and occludin. Am J Physiol Cell Physiol. 2000;279:C21–C30. doi: 10.1152/ajpcell.2000.279.1.C21. [DOI] [PubMed] [Google Scholar]
- 20.Kelly P.J., Morrow J.D., Ning M.M., Koroshetz W., Lo E.H., Terry E. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: the biomarker evaluation for antioxidant therapies in stroke (BEAT-Stroke) study. Stroke. 2008;39:100–104. doi: 10.1161/STROKEAHA.107.488189. [DOI] [PubMed] [Google Scholar]
- 21.Chamorro Á., Dirnagl U., Urra X., Planas A.M. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 22.O'Collins V.E., Macleod M.R., Donnan G.A., Horky L.L., Worp B.H., Howells D.W. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y.F., Li C., Chen Q.J., Liu P.X., Guo Q., Zhang Y. Microthrombus-targeting micelles for neurovascular remodeling and enhanced microcirculatory perfusion in acute ischemic stroke. Adv Mater. 2019;31 doi: 10.1002/adma.201808361. [DOI] [PubMed] [Google Scholar]
- 24.Han L., Li J.F., Huang S.X., Huang R.Q., Liu S.H., Hu X. Peptide-conjugated polyamidoamine dendrimer as a nanoscale tumor-targeted T1 magnetic resonance imaging contrast agent. Biomaterials. 2011;32:2989–2998. doi: 10.1016/j.biomaterials.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Fabian R.H., DeWitt D.S., Kent T.A. In vivo detection of superoxide anion production by the brain using a cytochrome c electrode. J Cerebr Blood Flow Metabol. 1995;15:242–247. doi: 10.1038/jcbfm.1995.30. [DOI] [PubMed] [Google Scholar]
- 26.Yamato M., Egashira T., Utsumi H. Application of in vivo ESR spectroscopy to measurement of cerebrovascular ROS generation in stroke. Free Radic Biol Med. 2003;35:1619–1631. doi: 10.1016/j.freeradbiomed.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Melani A., Turchi D., Vannucchi M.G., Cipriani S., Gianfriddo M., Pedata F. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int. 2005;47:442–448. doi: 10.1016/j.neuint.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Bours M.J., Swennen E.L., Virgilio F.D., Cronstein B.N., Dagnelie P.C. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Marsh B.J., Williams-Karnesky R.L., Stenzel-Poore M.P. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience. 2009;158:1007–1020. doi: 10.1016/j.neuroscience.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harari O.A., Liao J.K. NF-κB and innate immunity in ischemic stroke. Ann N Y Acad Sci. 2010;1207:32–40. doi: 10.1111/j.1749-6632.2010.05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandoval K.E., Witt K.A. Blood‒brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Huang J., Upadhyay U.M., Tamargo R.J. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 33.Yamasaki Y., Matsuo Y., Matsuura N., Onodera H., Itoyama Y., Kogure K. Transient increase of cytokine-induced neutrophil chemoattractant, a member of the interleukin-8 family, in ischemic brain areas after focal ischemia in rats. Stroke. 1995;26:318–322. doi: 10.1161/01.str.26.2.318. [DOI] [PubMed] [Google Scholar]
- 34.Minami M., Satoh M. Chemokines and their receptors in the brain: pathophysiological roles in ischemic brain injury. Life Sci. 2003;74:321–327. doi: 10.1016/j.lfs.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Wang X., Yue T.L., Barone F.C., Feuerstein G.Z. Monocyte chemoattractant protein-1 messenger RNA expression in rat ischemic cortex. Stroke. 1995;26:661–665. doi: 10.1161/01.str.26.4.661. [DOI] [PubMed] [Google Scholar]
- 36.Heo J.H., Han S.W., Lee S.K. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005;39:51–70. doi: 10.1016/j.freeradbiomed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 37.Rosell A., Cuadrado E., Ortega-Aznar A., Hernández-Guillamon M., Lo E.H., Montaner J. MMP-9-positive neutrophil infiltration is associated to blood‒brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- 38.Huang T., Li N., Gao J.Q. Recent strategies on targeted delivery of thrombolytics. Asian J Pharm Sci. 2019;14:233–247. doi: 10.1016/j.ajps.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mega J.L., Simon T. Pharmacology of antithrombotic drugs: an assessment of oral antiplatelet and anticoagulant treatments. Lancet. 2015;386:281–291. doi: 10.1016/S0140-6736(15)60243-4. [DOI] [PubMed] [Google Scholar]
- 41.Sandercock P.A., Counsell C., Kane E.J. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2015;2015 doi: 10.1002/14651858.CD000024.pub4. Cd000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanswell P., Modi N., Combs D., Danays T. Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet. 2002;41:1229–1245. doi: 10.2165/00003088-200241150-00001. [DOI] [PubMed] [Google Scholar]
- 43.Campbell B.C.V., Mitchell P.J., Churilov L., Yassi N., Kleinig T.J., Dowling R.J. Tenecteplase versus alteplase before thrombectomy forischemic stroke. N Engl J Med. 2018;378:1573–1582. doi: 10.1056/NEJMoa1716405. [DOI] [PubMed] [Google Scholar]
- 44.Medcalf R.L. Desmoteplase: discovery, insights and opportunities for ischaemic stroke. Br J Pharmacol. 2012;165:75–89. doi: 10.1111/j.1476-5381.2011.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trotman M., Vermehren P., Gibson C.L., Fern R. The dichotomy of memantine treatment for ischemic stroke: dose-dependent protective and detrimental effects. J Cerebr Blood Flow Metabol. 2015;35:230–239. doi: 10.1038/jcbfm.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horn J., Haan R.J., Vermeulen M., Luiten P.G., Limburg M. Nimodipine in animal model experiments of focal cerebral ischemia: a systematic review. Stroke. 2001;32:2433–2438. doi: 10.1161/hs1001.096009. [DOI] [PubMed] [Google Scholar]
- 47.Amemiya S., Kamiya T., Nito C., Inaba T., Kato K., Ueda M. Anti-apoptotic and neuroprotective effects of edaravone following transient focal ischemia in rats. Eur J Pharmacol. 2005;516:125–130. doi: 10.1016/j.ejphar.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 48.Edaravone Acute Infarction Study Group Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15:222–229. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- 49.Huang J., Choudhri T.F., Winfree C.J., McTaggart R.A., Kiss S., Mocco J. Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke. 2000;31:3047–3053. [PubMed] [Google Scholar]
- 50.Fu Y., Zhang N.N., Ren L., Yan Y.P., Sun N., Li Y.J. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci U S A. 2014;111:18315–18320. doi: 10.1073/pnas.1416166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X.Y., Tsuji K., Lee S.R., Ning M.M., Furie K.L., Buchan A.M. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- 52.Kaviarasi S., Yuba E., Harada A., Krishnan U.M. Emerging paradigms in nanotechnology for imaging and treatment of cerebral ischemia. J Control Release. 2019;300:22–45. doi: 10.1016/j.jconrel.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 53.Partoazar A., Nasoohi S., Rezayat S.M., Gilani K., Mehr S.E., Amani A. Nanoliposome containing cyclosporine A reduced neuroinflammation responses and improved neurological activities in cerebral ischemia/reperfusion in rat. Fundam Clin Pharmacol. 2017;31:185–193. doi: 10.1111/fcp.12244. [DOI] [PubMed] [Google Scholar]
- 54.Warner D.S., Sheng H.X., Batinić-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207:3221–3231. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y., Li S.Y., Shen S., Wang J. Protecting neurons from cerebral ischemia/reperfusion injury via nanoparticle-mediated delivery of an siRNA to inhibit microglial neurotoxicity. Biomaterials. 2018;161:95–105. doi: 10.1016/j.biomaterials.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 56.Kim I.D., Lim C.M., Kim J.B., Nam H.Y., Nam K., Kim S.W. Neuroprotection by biodegradable PAMAM ester (e-PAM-R)-mediated HMGB1 siRNA delivery in primary cortical cultures and in the postischemic brain. J Control Release. 2010;142:422–430. doi: 10.1016/j.jconrel.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 57.Zahednezhad F., Saadat M., Valizadeh H., Zakeri-Milani P., Baradaran B. Liposome and immune system interplay: challenges and potentials. J Control Release. 2019;305:194–209. doi: 10.1016/j.jconrel.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 58.Han L., Cai Q., Tian D.F., Kong D.K., Gou X.C., Chen Z.M. Targeted drug delivery to ischemic stroke via chlorotoxin-anchored, lexiscan-loaded nanoparticles. Nanomedicine. 2016;12:1833–1842. doi: 10.1016/j.nano.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Ahmady Z.S., Jasim D., Ahmad S.S., Wong R., Haley M., Coutts G. Selective liposomal transport through blood‒brain barrier disruption in ischemic stroke reveals two distinct therapeutic opportunities. ACS Nano. 2019;13:12470–12486. doi: 10.1021/acsnano.9b01808. [DOI] [PubMed] [Google Scholar]
- 60.Fukuta T., Ishii T., Asai T., Sato A., Kikuchi T., Shimizu K. Treatment of stroke with liposomal neuroprotective agents under cerebral ischemia conditions. Eur J Pharm Biopharm. 2015;97:1–7. doi: 10.1016/j.ejpb.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 61.Xu H.E., Hua Y., Zhong J., Li X.L., Xu W., Cai Y.Y. Resveratrol delivery by albumin nanoparticles improved neurological function and neuronal damage in transient middle cerebral artery occlusion rats. Front Pharmacol. 2018;9:1403. doi: 10.3389/fphar.2018.01403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y., Luo J., Li S.Y. Nano-curcumin simultaneously protects the blood‒brain barrier and reduces M1 microglial activation during cerebral ischemia‒reperfusion injury. ACS Appl Mater Interfaces. 2019;11:3763–3770. doi: 10.1021/acsami.8b20594. [DOI] [PubMed] [Google Scholar]
- 63.Li N., Feng L.L., Tan Y.J., Xiang Y., Zhang R.Q., Yang M. Preparation, characterization, pharmacokinetics and biodistribution of baicalin-loaded liposome on cerebral ischemia‒reperfusion after i.v. administration in rats. Molecules. 2018;23:1747–1751. doi: 10.3390/molecules23071747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan L.W., Liang C., Wang Y.Y., Jiang Y., Zeng S.Q., Tan R. Pharmacodynamic effect of luteolin micelles on alleviating cerebral ischemia reperfusion injury. Pharmaceutics. 2018;10:248. doi: 10.3390/pharmaceutics10040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marushima A., Suzuki K., Nagasaki Y., Yoshitomi T., Toh K., Tsurushima H. Newly synthesized radical-containing nanoparticles enhance neuroprotection after cerebral ischemia‒reperfusion injury. Neurosurgery. 2011;68:1418–1425. doi: 10.1227/NEU.0b013e31820c02d9. [DOI] [PubMed] [Google Scholar]
- 66.Hosoo H., Marushima A., Nagasaki Y., Hirayama A., Ito H., Puentes S. Neurovascular unit protection from cerebral ischemia‒reperfusion injury by radical-containing nanoparticles in mice. Stroke. 2017;48:2238–2247. doi: 10.1161/STROKEAHA.116.016356. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y.L., Ai K.L., Ji X.Y., Askhatova D., Du R., Lu L.H. Comprehensive insights into the multi-antioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemicstroke. J Am Chem Soc. 2017;139:856–862. doi: 10.1021/jacs.6b11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petro M., Jaffer H., Yang J., Kabu S., Morris V.B., Labhasetwar V. Tissue plasminogen activator followed by antioxidant-loaded nanoparticle delivery promotes activation/mobilization of progenitor cells in infarcted rat brain. Biomaterials. 2016;81:169–180. doi: 10.1016/j.biomaterials.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim C.K., Kim T., Choi I.Y., Soh M., Kim D., Kim Y.J. Ceria nanoparticles that can protect against ischemic stroke. Angew Chem Int Ed Engl. 2012;51:11039–11043. doi: 10.1002/anie.201203780. [DOI] [PubMed] [Google Scholar]
- 70.Xu G.T., Gu H., Hu B., Tong F., Liu D.J., Yu X.J. PEG-b-(PELG-g-PLL) nanoparticles as TNF-α nanocarriers: potential cerebral ischemia/reperfusion injury therapeutic applications. Int J Nanomed. 2017;12:2243–2254. doi: 10.2147/IJN.S130842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin Q., Cai Y., Li S.H., Liu H.R., Zhou X.Y., Lu C.Q. Edaravone-encapsulated agonistic micelles rescue ischemic brain tissue by tuning blood‒brain barrier permeability. Theranostics. 2017;7:884–898. doi: 10.7150/thno.18219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han L., Kong D.K., Zheng M.Q., Murikinati S., Ma C., Yuan P. Increased nanoparticle delivery to brain tumors by autocatalytic priming for improved treatment and imaging. ACS Nano. 2016;10:4209–4218. doi: 10.1021/acsnano.5b07573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu Y.F., Jiang C. Brain-targeted polymers for gene delivery in the treatment of brain diseases. Top Curr Chem. 2017;375:48. doi: 10.1007/s41061-017-0138-3. (Cham) [DOI] [PubMed] [Google Scholar]
- 74.Zhao L.X., Liu A.C., Yu S.W., Wang Z.X., Lin X.Q., Zhai G.X. The permeability of puerarin-loaded poly(butylcyanoacrylate) nanoparticles coated with polysorbate 80 on the blood‒brain barrier and its protective effect against cerebral ischemia/reperfusion injury. Biol Pharm Bull. 2013;36:1263–1270. doi: 10.1248/bpb.b12-00769. [DOI] [PubMed] [Google Scholar]
- 75.Ma J.N., Zhang S.Q., Liu J., Liu F.Y., Du F.Y., Li M. Targeted drug delivery to stroke via chemotactic recruitment of nanoparticles coated with membrane of engineered neural stem cells. Small. 2019;15 doi: 10.1002/smll.201902011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo X., Deng G., Liu J., Zou P., Du F.Y., Liu F.Y. Thrombin-responsive, brain-targeting nanoparticles for improved stroke therapy. ACS Nano. 2018;12:8723–8732. doi: 10.1021/acsnano.8b04787. [DOI] [PubMed] [Google Scholar]
- 77.Deng G., Ma C., Zhao H.T., Zhang S.Q., Liu J., Liu F.Y. Anti-edema and antioxidant combination therapy for ischemic stroke via glyburide-loaded betulinic acid nanoparticles. Theranostics. 2019;9:6991–7002. doi: 10.7150/thno.35791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Z.Y., Zhao Y., Jiang Y., Lv W., Wu L., Wang B.Y. Enhanced anti-ischemic stroke of ZL006 by T7-conjugated PEGylated liposomes drug delivery system. Sci Rep. 2015;5:12651. doi: 10.1038/srep12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao Y., Jiang Y., Lv W., Wang Z.Y., Lv L.Y., Wang B.Y. Dual targeted nanocarrier for brain ischemic stroke treatment. J Control Release. 2016;233:64–71. doi: 10.1016/j.jconrel.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 80.Lv W., Xu J.P., Wang X.Q., Li X.R., Xu Q.W., Xin H.L. Bioengineered boronic ester modified dextran polymer nanoparticles as reactive oxygen species responsive nanocarrier for ischemic stroke treatment. ACS Nano. 2018;12:5417–5426. doi: 10.1021/acsnano.8b00477. [DOI] [PubMed] [Google Scholar]
- 81.Tian T., Zhang H.X., He C.P., Fan S., Zhu Y.L., Qi C. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–149. doi: 10.1016/j.biomaterials.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 82.Yun X., Maximov V.D., Yu J., Zhu H., Vertegel A.A., Kindy M.S. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J Cerebr Blood Flow Metabol. 2013;33:583–592. doi: 10.1038/jcbfm.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang T., Li C.Y., Jia J.J., Chi J.S., Zhou D., Li J.Z. Combination therapy with LXW7 and ceria nanoparticles protects against acute cerebral ischemia/reperfusion injury in rats. Curr Med Sci. 2018;38:144–152. doi: 10.1007/s11596-018-1858-5. [DOI] [PubMed] [Google Scholar]
- 84.Bao Q.Q., Hu P., Xu Y.Y., Cheng T.S., Wei C.Y., Pan L.M. Simultaneous blood‒brain barrier crossing and protection for stroke treatment based on edaravone-loaded ceria nanoparticles. ACS Nano. 2018;12:6794–6805. doi: 10.1021/acsnano.8b01994. [DOI] [PubMed] [Google Scholar]
- 85.Ishii T., Asai T., Oyama D., Fukuta T., Yasuda N., Shimizu K. Amelioration of cerebral ischemia‒reperfusion injury based on liposomal drug delivery system with asialo-erythropoietin. J Control Release. 2012;160:81–87. doi: 10.1016/j.jconrel.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 86.Ishii T., Asai T., Fukuta T., Oyama D., Yasuda N., Agato Y. A single injection of liposomal asialo-erythropoietin improves motor function deficit caused by cerebral ischemia/reperfusion. Int J Pharm. 2012;439:269–274. doi: 10.1016/j.ijpharm.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 87.Feczkó T., Piiper A., Ansar S., Blixt F.W., Ashtikar M., Schiffmann S. Stimulating brain recovery after stroke using theranostic albumin nanocarriers loaded with nerve growth factor in combination therapy. J Control Release. 2019;293:63–72. doi: 10.1016/j.jconrel.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 88.Yang J.L., Zhang X.F., Chen X.J., Wang L., Yang G.D. Exosome-mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu C.M., Zhang L., Aryal S., Cheung C., Fang R.H., Zhang L.F. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hu C.M., Fang R.H., Wang K.C., Luk B.T., Thamphiwatana S., Dehaini D. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang C., Ling C.L., Pang L., Wang Q., Liu J.X., Wang B.S. Direct macromolecular drug delivery to cerebral ischemia area using neutrophil-mediated nanoparticles. Theranostics. 2017;7:3260–3275. doi: 10.7150/thno.19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xin H.Q., Katakowski M., Wang F.J., Qian J.Y., Liu X.S., Ali M.M. MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. 2017;48:747–753. doi: 10.1161/STROKEAHA.116.015204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xin H.Q., Li Y., Cui Y.S., Yang J.J., Zhang Z.G., Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cerebr Blood Flow Metabol. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu J.P., Wang X.Q., Yin H.Y., Cao X., Hu Q.Y., Lv W. Sequentially site-specific delivery of thrombolytics and neuroprotectant for enhanced treatment of ischemic stroke. ACS Nano. 2019;13:8577–8588. doi: 10.1021/acsnano.9b01798. [DOI] [PubMed] [Google Scholar]
- 95.Liu X., An C.Y., Jin P., Liu X.S., Wang L.H. Protective effects of cationic bovine serum albumin-conjugated PEGylated tanshinone IIA nanoparticles on cerebral ischemia. Biomaterials. 2013;34:817–830. doi: 10.1016/j.biomaterials.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 96.Kalani A., Chaturvedi P., Kamat P.K., Maldonado C., Bauer P., Joshua I.G. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia‒reperfusion injury. Int J Biochem Cell Biol. 2016;79:360–369. doi: 10.1016/j.biocel.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim I.D., Shin J.H., Kim S.W., Choi S., Ahn J., Han P.L. Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Mol Ther. 2012;20:829–839. doi: 10.1038/mt.2011.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harston G.W., Okell T.W., Sheerin F., Schulz U., Mathieson P., Reckless I. Quantification of serial cerebral blood flow in acute stroke using arterial spin labeling. Stroke. 2017;48:123–130. doi: 10.1161/STROKEAHA.116.014707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jin H.Q., Tan H., Zhao L.L., Sun W.P., Zhu L.J., Sun Y.A. Ultrasound-triggered thrombolysis using urokinase-loaded nanogels. Int J Pharm. 2012;434:384–390. doi: 10.1016/j.ijpharm.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 100.Teng Y.M., Jin H.Q., Nan D., Li M.N., Fan C.H., Liu Y.Y. In vivo evaluation of urokinase-loaded hollow nanogels for sonothrombolysis on suture embolization-induced acute ischemic stroke rat model. Bioact Mater. 2018;3:102–109. doi: 10.1016/j.bioactmat.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yurko Y., Maximov V., Andreozzi E., Thompson G.L., Vertegel A.A. Design of biomedical nanodevices for dissolution of blood clots. Mater Sci Eng C. 2009;29:737–741. [Google Scholar]
- 102.Vaidya B., Nayak M.K., Dash D., Agrawal G.P., Vyas S.P. Development and characterization of site specific target sensitive liposomes for the delivery of thrombolytic agents. Int J Pharm. 2011;403:254–261. doi: 10.1016/j.ijpharm.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 103.Vaidya B., Agrawal G.P., Vyas S.P. Platelets directed liposomes for the delivery of streptokinase: development and characterization. Eur J Pharm Sci. 2011;44:589–594. doi: 10.1016/j.ejps.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 104.Vaidya B., Nayak M.K., Dash D., Agrawal G.P., Vyas S.P. Development and characterization of highly selective target-sensitive liposomes for the delivery of streptokinase: in vitro/in vivo studies. Drug Deliv. 2016;23:801–807. doi: 10.3109/10717544.2014.916770. [DOI] [PubMed] [Google Scholar]
- 105.Pawlowski C.L., Li W., Sun M., Ravichandran K., Hickman D., Kos C. Platelet microparticle-inspired clot-responsive nanomedicine for targeted fibrinolysis. Biomaterials. 2017;128:94–108. doi: 10.1016/j.biomaterials.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saxena R., Wijnhoud A.D., Carton H., Hacke W., Kaste M., Przybelski R.J. Controlled safety study of a hemoglobin-based oxygen carrier, DCLHb, in acute ischemic stroke. Stroke. 1999;30:993–996. doi: 10.1161/01.str.30.5.993. [DOI] [PubMed] [Google Scholar]
- 107.Bobofchak K.M., Mito T., Texel S.J., Bellelli A., Nemoto M., Traystman R.J. A recombinant polymeric hemoglobin with conformational, functional, and physiological characteristics of an in vivo O2 transporter. Am J Physiol Heart Circ Physiol. 2003;285:H549–H561. doi: 10.1152/ajpheart.00037.2003. [DOI] [PubMed] [Google Scholar]
- 108.Kawaguchi A.T., Fukumoto D., Haida M., Ogata Y., Yamano M., Tsukada H. Liposome-encapsulated hemoglobin reduces the size of cerebral infarction in the rat: evaluation with photochemically induced thrombosis of the middle cerebral artery. Stroke. 2007;38:1626–1632. doi: 10.1161/STROKEAHA.106.467290. [DOI] [PubMed] [Google Scholar]
- 109.Fukumoto D., Kawaguchi A.T., Haida M., Yamano M., Ogata Y., Tsukada H. Liposome-encapsulated hemoglobin reduces the size of cerebral infarction in rats: effect of oxygen affinity. Artif Organs. 2009;33:159–163. doi: 10.1111/j.1525-1594.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 110.Hamadate N., Yamaguchi T., Sugawara A., Togashi H., Izumi T., Yoshida T. Liposome-encapsulated hemoglobin ameliorates impairment of fear memory and hippocampal dysfunction after cerebral ischemia in rats. J Pharmacol Sci. 2010;114:409–419. doi: 10.1254/jphs.10207fp. [DOI] [PubMed] [Google Scholar]
- 111.Komatsu H., Furuya T., Sato N., Ohta K., Matsuura A., Ohmura T. Effect of hemoglobin vesicle, a cellular-type artificial oxygen carrier, on middle cerebral artery occlusion- and arachidonic acid-induced stroke models in rats. Neurosci Lett. 2007;421:121–125. doi: 10.1016/j.neulet.2007.04.080. [DOI] [PubMed] [Google Scholar]
- 112.Simard J.M., Chen M.K., Tarasov K.V., Bhatta S., Ivanova S., Melnitchenko L. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12:433–440. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Simard J.M., Sheth K.N., Kimberly W.T., Stern B.J., Zoppo G.J., Jacobson S. Glibenclamide in cerebral ischemia and stroke. Neurocrit Care. 2014;20:319–333. doi: 10.1007/s12028-013-9923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pergakis M., Badjatia N., Chaturvedi S., Cronin C.A., Kimberly W.T., Sheth K.N. BIIB093 (IV glibenclamide): an investigational compound for the prevention and treatment of severe cerebral edema. Expet Opin Invest Drugs. 2019;28:1031–1040. doi: 10.1080/13543784.2019.1681967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tournier N., Saba W., Cisternino S., Peyronneau M.A., Damont A., Goutal S. Effects of selected OATP and/or ABC transporter inhibitors on the brain and whole-body distribution of glyburide. AAPS J. 2013;15:1082–1090. doi: 10.1208/s12248-013-9514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou L., Li F., Xu H.B., Luo C.X., Wu H.Y., Zhu M.M. Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat Med. 2010;16:1439–1443. doi: 10.1038/nm.2245. [DOI] [PubMed] [Google Scholar]
- 117.Liu S.H., Guo Y.B., Huang R.Q., Li J.F., Huang S.X., Kuang Y.Y. Gene and doxorubicin co-delivery system for targeting therapy of glioma. Biomaterials. 2012;33:4907–4916. doi: 10.1016/j.biomaterials.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 118.Ishii T., Asai T., Oyama D., Agato Y., Yasuda N., Fukuta T. Treatment of cerebral ischemia‒reperfusion injury with PEGylated liposomes encapsulating FK506. FASEB J. 2013;27:1362–1370. doi: 10.1096/fj.12-221325. [DOI] [PubMed] [Google Scholar]
- 119.Cao Y.G., Mao X.Y., Sun C.Y., Zheng P., Gao J.Q., Wang X.R. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Res Bull. 2011;85:396–402. doi: 10.1016/j.brainresbull.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 120.Qiao H.M., Dong L.P., Zhang X.J., Zhu C.H., Zhang X.L., Wang L.N. Protective effect of luteolin in experimental ischemic stroke: upregulated SOD1, CAT, BCL-2 and claudin-5, down-regulated MDA and Bax expression. Neurochem Res. 2012;37:2014–2024. doi: 10.1007/s11064-012-0822-1. [DOI] [PubMed] [Google Scholar]
- 121.Doggui S., Sahni J.K., Arseneault M., Dao L., Ramassamy C. Neuronal uptake and neuroprotective effect of curcumin-loaded PLGA nanoparticles on the human SK-N-SH cell line. J Alzheimers Dis. 2012;30:377–392. doi: 10.3233/JAD-2012-112141. [DOI] [PubMed] [Google Scholar]
- 122.Djiokeng Paka G., Doggui S., Zaghmi A., Safar R., Dao L., Reisch A. Neuronal uptake and neuroprotective properties of curcumin-loaded nanoparticles on SK-N-SH cell line: role of poly(lactide-co-glycolide) polymeric matrix composition. Mol Pharm. 2016;13:391–403. doi: 10.1021/acs.molpharmaceut.5b00611. [DOI] [PubMed] [Google Scholar]
- 123.Qiu J.F., Gao X., Wang B.L., Wei X.W., Gou M.L., Men K. Preparation and characterization of monomethoxy poly(ethylene glycol)-poly(ε-caprolactone) micelles for the solubilization and in vivo delivery of luteolin. Int J Nanomed. 2013;8:3061–3069. doi: 10.2147/IJN.S45062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoshitomi T., Suzuki R., Mamiya T., Matsui H., Hirayama A., Nagasaki Y. pH-sensitive radical-containing-nanoparticle (RNP) for the L-band-EPR imaging of low pH circumstances. Bioconjugate Chem. 2009;20:1792–1798. doi: 10.1021/bc900214f. [DOI] [PubMed] [Google Scholar]
- 125.Yoshitomi T., Miyamoto D., Nagasaki Y. Design of core-shell-type nanoparticles carrying stable radicals in the core. Biomacromolecules. 2009;10:596–601. doi: 10.1021/bm801278n. [DOI] [PubMed] [Google Scholar]
- 126.Kinouchi H., Epstein C.J., Mizui T., Carlson E., Chen S.F., Chan P.H. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci U S A. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Armogida M., Spalloni A., Amantea D., Nutini M., Petrelli F., Longone P. The protective role of catalase against cerebral ischemia in vitro and in vivo. Int J Immunopathol Pharmacol. 2011;24:735–747. doi: 10.1177/039463201102400320. [DOI] [PubMed] [Google Scholar]
- 128.Reddy M.K., Labhasetwar V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: an effective strategy to reduce ischemia‒reperfusion injury. FASEB J. 2009;23:1384–1395. doi: 10.1096/fj.08-116947. [DOI] [PubMed] [Google Scholar]
- 129.Huang X., Ding J., Li Y.F., Liu W.J., Ji J.L., Wang H. Exosomes derived from PEDF modified adipose-derived mesenchymal stem cells ameliorate cerebral ischemia‒reperfusion injury by regulation of autophagy and apoptosis. Exp Cell Res. 2018;371:269–277. doi: 10.1016/j.yexcr.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 130.Sirén A.L., Fratelli M., Brines M., Goemans C., Casagrande S., Lewczuk P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Erbayraktar S., Grasso G., Sfacteria A., Xie Q.W., Coleman T., Kreilgaard M. Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity in vivo. Proc Natl Acad Sci U S A. 2003;100:6741–6746. doi: 10.1073/pnas.1031753100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sanchez A., Tripathy D., Yin X., Luo J., Martinez J., Grammas P. Pigment epithelium-derived factor (PEDF) protects cortical neurons in vitro from oxidant injury by activation of extracellular signal-regulated kinase (ERK) 1/2 and induction of BCL-2. Neurosci Res. 2012;72:1–8. doi: 10.1016/j.neures.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Q., Gou X.C., Xiong L.Z., Jin W.L., Chen S.Y., Hou L.C. Trans-activator of transcription-mediated delivery of NEP1-40 protein into brain has a neuroprotective effect against focal cerebral ischemic injury via inhibition of neuronal apoptosis. Anesthesiology. 2008;108:1071–1080. doi: 10.1097/ALN.0b013e318173f66b. [DOI] [PubMed] [Google Scholar]
- 134.Chamorro Á., Meisel A., Planas A.M., Urra X., Beek D.V., Veltkamp R. The immunology of acute stroke. Nat Rev Neurol. 2012;8:401–410. doi: 10.1038/nrneurol.2012.98. [DOI] [PubMed] [Google Scholar]
- 135.Castillo J., Moro M.A., Blanco M., Leira R., Serena J., Lizasoain I. The release of tumor necrosis factor-alpha is associated with ischemic tolerance in human stroke. Ann Neurol. 2003;54:811–819. doi: 10.1002/ana.10765. [DOI] [PubMed] [Google Scholar]
- 136.Zhao S.C., Ma L.S., Chu Z.H., Xu H., Wu W.Q., Liu F.D. Regulation of microglial activation in stroke. Acta Pharmacol Sin. 2017;38:445–458. doi: 10.1038/aps.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xia C.Y., Zhang S., Gao Y., Wang Z.Z., Chen N.H. Selective modulation of microglia polarization to M2 phenotype for stroke treatment. Int Immunopharm. 2015;25:377–382. doi: 10.1016/j.intimp.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 138.Hu X.M., Li P.Y., Guo Y.L., Wang H.Y., Leak R.K., Chen S. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 139.Zeng F., Wu Y.W., Li X.W., Ge X.J., Guo Q.H., Lou X.B. Custom-made ceria nanoparticles show a neuroprotective effect by modulating phenotypic polarization of the microglia. Angew Chem Int Ed Engl. 2018;57:5808–5812. doi: 10.1002/anie.201802309. [DOI] [PubMed] [Google Scholar]
- 140.Wong G.H., Goeddel D.V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988;242:941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- 141.Hallenbeck J.M. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- 142.Ginis I., Jaiswal R., Klimanis D., Liu J., Greenspon J., Hallenbeck J.M. TNF-alpha-induced tolerance to ischemic injury involves differential control of NF-kappaB transactivation: the role of NF-kappaB association with p300 adaptor. J Cerebr Blood Flow Metabol. 2002;22:142–152. doi: 10.1097/00004647-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 143.Nawashiro H., Tasaki K., Ruetzler C.A., Hallenbeck J.M. TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. J Cerebr Blood Flow Metabol. 1997;17:483–490. doi: 10.1097/00004647-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 144.Spite M., Serhan C.N. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107:1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schilling M., Besselmann M., Müller M., Strecker J.K., Ringelstein E.B., Kiefer R. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2005;196:290–297. doi: 10.1016/j.expneurol.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 146.Davalos D., Grutzendler J., Yang G., Kim J.V., Zuo Y., Jung S. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 147.Nathan C., Ding A.H. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 148.Abe K. Therapeutic potential of neurotrophic factors and neural stem cells against ischemic brain injury. J Cerebr Blood Flow Metabol. 2000;20:1393–1408. doi: 10.1097/00004647-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 149.Lee T.H., Kato H., Chen S.T., Kogure K., Itoyama Y. Expression of nerve growth factor and trkA after transient focal cerebral ischemia in rats. Stroke. 1998;29:1687–1696. doi: 10.1161/01.str.29.8.1687. [DOI] [PubMed] [Google Scholar]
- 150.Holtzman D.M., Sheldon R.A., Jaffe W., Cheng Y., Ferriero D.M. Nerve growth factor protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol. 1996;39:114–122. doi: 10.1002/ana.410390117. [DOI] [PubMed] [Google Scholar]
- 151.Shigeno T., Mima T., Takakura K., Graham D.I., Kato G., Hashimoto Y. Amelioration of delayed neuronal death in the hippocampus by nerve growth factor. J Neurosci. 1991;11:2914–2919. doi: 10.1523/JNEUROSCI.11-09-02914.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kitagawa H., Hayashi T., Mitsumoto Y., Koga N., Itoyama Y., Abe K. Reduction of ischemic brain injury by topical application of glial cell line-derived neurotrophic factor after permanent middle cerebral artery occlusion in rats. Stroke. 1998;29:1417–1422. doi: 10.1161/01.str.29.7.1417. [DOI] [PubMed] [Google Scholar]
- 153.Takagi Y., Nozaki K., Takahashi J., Yodoi J., Ishikawa M., Hashimoto N. Proliferation of neuronal precursor cells in the dentate gyrus is accelerated after transient forebrain ischemia in mice. Brain Res. 1999;831:283–287. doi: 10.1016/s0006-8993(99)01411-0. [DOI] [PubMed] [Google Scholar]
- 154.Craig C.G., Tropepe V., Morshead C.M., Reynolds B.A., Weiss S., Kooy D.V. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhao Y., Xie P., Zhu X.F., Cai Z.Y. Neural stem cell transplantation and nerve growth factor promote neurological recovery in rats with ischemic stroke. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:1123–1126. [PubMed] [Google Scholar]
- 156.Zhu W.S., Cheng S.M., Xu G.L., Ma M.M., Zhou Z.M., Liu D.Z. Intranasal nerve growth factor enhances striatal neurogenesis in adult rats with focal cerebral ischemia. Drug Deliv. 2011;18:338–343. doi: 10.3109/10717544.2011.557785. [DOI] [PubMed] [Google Scholar]
- 157.Rhim T., Lee M. Targeted delivery of growth factors in ischemic stroke animal models. Expet Opin Drug Deliv. 2016;13:709–723. doi: 10.1517/17425247.2016.1144588. [DOI] [PubMed] [Google Scholar]
- 158.Liu X.S., Chopp M., Zhang R.L., Zhang Z.G. MicroRNAs in cerebral ischemia-induced neurogenesis. J Neuropathol Exp Neurol. 2013;72:718–722. doi: 10.1097/NEN.0b013e31829e4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Doeppner T.R., Doehring M., Bretschneider E., Zechariah A., Kaltwasser B., Müller B. MicroRNA-124 protects against focal cerebral ischemia via mechanisms involving Usp14-dependent REST degradation. Acta Neuropathol. 2013;126:251–265. doi: 10.1007/s00401-013-1142-5. [DOI] [PubMed] [Google Scholar]
- 160.Xin H.Q., Li Y., Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci. 2014;8:377. doi: 10.3389/fncel.2014.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shi J.J., Yu W.Y., Xu L.H., Yin N., Liu W., Zhang K.X. Bioinspired nanosponge for salvaging ischemic stroke via free radical scavenging and self-adapted oxygen regulating. Nano Lett. 2020;20:780–789. doi: 10.1021/acs.nanolett.9b04974. [DOI] [PubMed] [Google Scholar]
- 162.Otero-Ortega L., Gómez de Frutos M.C., Laso-García F., Rodríguez-Frutos B., Medina-Gutiérrez E., López J.A. Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J Cerebr Blood Flow Metabol. 2018;38:767–779. doi: 10.1177/0271678X17708917. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 163.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]