Abstract
The phytoecdysteroids (PEs) comprise a large group of biologically-active plant steroids, which have structures similar to those of insect-molting hormones. PEs are distributed in plants as secondary metabolites that offer protection against phytophagus (plant-eating) insects. When insects consume the plants containing these chemicals, they promptly molt and undergo metabolic destruction; the insects eventually die. Chemically, ecdysteroids are a group of polyhydroxylated ketosteroids that are structurally similar to androgens. The carbon skeleton of ecdysteroids is termed as cyclopentanoperhydro-phenanthrene with a β-side chain at carbon-17. The essential characteristics of ecdysteroids are a cis-(5β-H) junction of rings A and B, a 7-en-6-one chromophore, and a trans-(14α-OH) junction of rings C and D. Plants only synthesize PEs from mevalonic acid in the mevalonate pathway of the plant cell using acetyl-CoA as a precursor; the most common PE is 20-hydroxyecdysone. So far, over 400 PEs have been identified and reported, and a compilation of 166 PEs originating from 1998 has been previously reviewed. In the present review, we have summarized 212 new PEs reported between 1999 and 2019. We have also critically analyzed the biological, pharmacological, and medicinal properties of PEs to understand the full impact of these phytoconstituents in health and disease.
KEY WORDS: Phytoecdysteroids, Secondary metabolites, Antioxidant, Anti-inflammatory, Antimicrobial, Antidiabetic, Anticancer activity
Graphical abstract
Phytoecdysteroids (PEs) have shown a wide range of biological, pharmacological, and medicinal properties implicated in the prevention and therapy of acute and chronic diseases. PEs are effective against different types of cancer due to their anti-inflammatory and antioxidant mechanisms. It also has shown antidiabetic antimicrobial and hepatoprotective abilities.
1. Introduction
Phytoecdysteroids (PEs) are a class of biologically-active chemicals that plants synthesize for defense against phytophagous (plant-eating) insects. The name for ecdysteroids originate from the word “ecdysis”, which in turn is derived from the Greek word “ecdysis”, meaning shedded outer skin. Ecdysteroids were originally considered a class of steroid hormones that control the processes of insect moulting and metamorphosis1. The first ecdysteroid, named ecdysone, was isolated by Butenandt and Karlson2 in 1954 from the silkworm pupae; its structure was elucidated in 1965 by Huber and Hoppe3 using X-ray crystallography. Arthropod steroid hormones also known as zooecdysteroids were shown to regulate the development of arthropods and other invertebrates as well. The metabolism and pharmacological effects of such ecdysteroids has drawn considerable attention in mammalian systems4. Isolation of several members of this class from different plant sources indicate that these compounds not only control the moulting and metamorphoses of invertebrates, but also have control of other biological functions in plants and invertebrates5. The ecdysteroids derived from plants are called phytoecdysteroids; ecdysteroids derived from animals are known as zooecdysteroids. Several common ecdysteroids, such as ecdysone (E), 20-hydroxyecdysteroid (20-HE), makisterone A, and ajugasterone C, can be found in both plants as well as animals6. PEs are spread throughout the plant kingdom; however, a survey indicates that only ∼6% of all plants contain detectable levels of ecdysteroids6. A series of evidence suggests that PEs do not accumulate in the majority of plant species as the expression of the biosynthetic pathways in plants is downregulated6. In plants, they provide chemical defence against certain herbivorous insects through their allelochemical properties; one of such includes taste receptors which emithormonal disruptions and signal toxicity to insects and other invertebrates7, 8, 9. These chemicals are also able to alter and switch genes around in plants through DNA binding10,11. Even in mammals, these compounds have exhibited unique biological results, such as a stimulatory effect on growth and metabolism12, and an effect on anabolic and steroidal growth regulations13. A recent study reports that a mixture of PEs extracted from Serratula coronata enhances the productivity and vitality of ducklings, which suggests the existence of PEs’ growth and physiologically favoring properties14. For this reason, these compounds have been reported to be used by athletes and sports personnel as stimulants in moderately low dosages15. 20-HE was shown to have influences on the sexual activity of male rats16. Physiological and pharmacological studies indicate that ecdysteroids contribute to an increase in protein synthesis in body builders, patients with acquired immunodeficiency syndrome, and patients with cancer17. Ecdysteroids also have shown effects similar to those of antidepressants, shielding the body from stress and improving physical and sexual performances18. A recent study reported five PEs from ethyl acetate and n-butanol fractions of Sphenocentrum jollyanum that had shown urease inhibitory and antacid activities, suggesting their potential usage in ulcerative colitis19. Because of such claimed multifactorial activities20, dietary supplements containing ecdysteroids, especially 20-HE, are being marketed in the United States.
PEs, biosynthetically derived from cholesterol or other plant sterols, have multiple physiological roles in plants, and have a broad spectrum of pharmacological and medicinal properties in mammals. The major properties of PEs include hepatoprotective, hypoglycaemic, and anabolic effects on skeletal muscle21. PEs have been utilized by sportsmen and bodybuilders for improving physical performance, and for enhancing stress resistance by promoting vitality22. Different PEs could show binding interactions with human nuclear receptors22. The PE derivatives displayed hormonal effects on invertebrates and exerted several favorable, non-hormonal, biological effects on mammals23. The phytochemical properties of PEs and prospective bioactivities are novel in nature with respect to their abundance and application. Yet, a paucity of comprehensive records exists which nesecciates a need to summarize the current state of knowledge on biosynthesis, distribution, biological, and physiological properties of PEs and pharmacological impacts on human physiology and metabolism in normal and diseased conditions. However, the cumulative information remains limited to the review of 166 PEs reported by Baltaev in 199824. Thus, there is a need for updated and comprehensive information on ecdysteroids. In the past two decades, numerous research studies have reported diverse chemical and biosynthetic characteristics of PEs and their physiological roles in plants as well as their pharmacological and biomedicinal properties. In view of these advances, the present review summarizes the current knowledge on the biosynthesis, distribution, biological importance, and pharmacological applications of PEs. We have summarized 212 new phytoecdcysteroids reported from 18 plant families since 1999. These PE derivatives have been critically analyzed for their biological, pharmacological, and medicinal properties in order to understand the impact of these phytoconstituents in health and disease.
2. Methodology for literature search
The majority of the literature search was conducted electronically on multiple databases, such as SciFinder (http://cas.org/products/scifinder/index.html). Additional information was collected from reliable and authentic databases such as PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Science Direct, Scopus, Web of Science, Google Scholar, and the ecdysteroids electronic database (http://ecdybase.org). For this systematic review, we have followed the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines25. Appropriate and high-quality publications (1999‒2020) were collected. Letters from editors, book chapters, conference abstracts, and unpublished results were not incorporated. Only articles written in English were included in this review. Major keywords used include: phytoecdysteroids, phytoecdysones, ecdysteroids, ecdysones, secondary metabolites, phytoinsecticides, antioxidant, anti-inflammatory, antimicrobial, antidiabetic, anticancer properties, in vitro and in vivo studies.
3. Chemistry of phytoecdysteroids
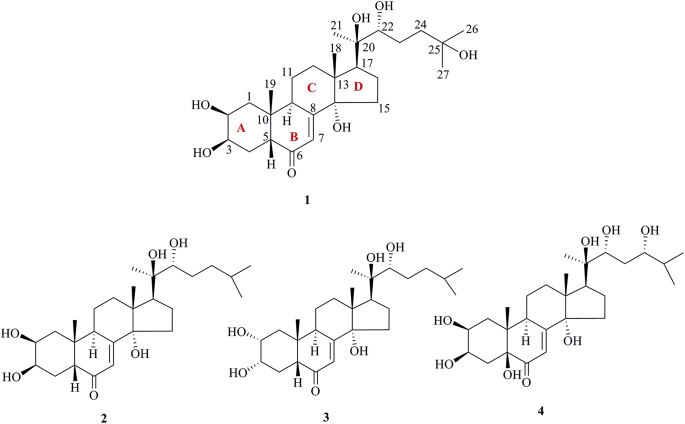
The carbon skeleton of ecdysteroids is termed cyclopentanoperhydro-phenanthrene and has a β-side chain at carbon-17. The essential characteristics of ecdysteroids are their composition of a cis-(5β-H) junction of rings A and B, a 7-en-6-one chromophore, and a trans-(14α-OH) junction of rings C and D. The sterol structure is modified to produce ecdysteroids; the trans A/B ring juncture in sterols is converted to a cis A/B ring juncture in the ecdysteroids. Chemically, these are C27, C28, or C29 polyhydroxy steroids that have a 14α-hydroxy-7-en-6-one chromophore and A/B-cis ring fusion. 20-HE (1) was identified from different varieties of arthropods and is now considered a major biologically-active ecdysteroid in arthropods. Its atom numbering is depicted in Fig. 1.
Figure 1.
Structures of 20-hydroxyecdysone (1), ponasterones A (2), B (3) and C (4).
Chemically, ecdysteroids are polar steroids in nature; their solubility is almost identical to that of a sugar molecule. As a result, they are soluble in aqueous mediums and are lipophilic26. The mammalian steroid hormones have more variable structures and they generally lack the polyhydroxylated side chain characteristic of ecdysteroids; accordingly, they are quite nonpolar26. Unlike invertebrates, which are incapable of synthesizing ecdysteroids and must consume dietary phytosterols that transform into ecdysteroids, plants can completely synthesize ecdysteroids from mevalonic acid and cholesterol27. So far, more than 200 PEs, mostly from genus Ajuga, Podocarpus, Polypodium, and Silene, have been reported6.
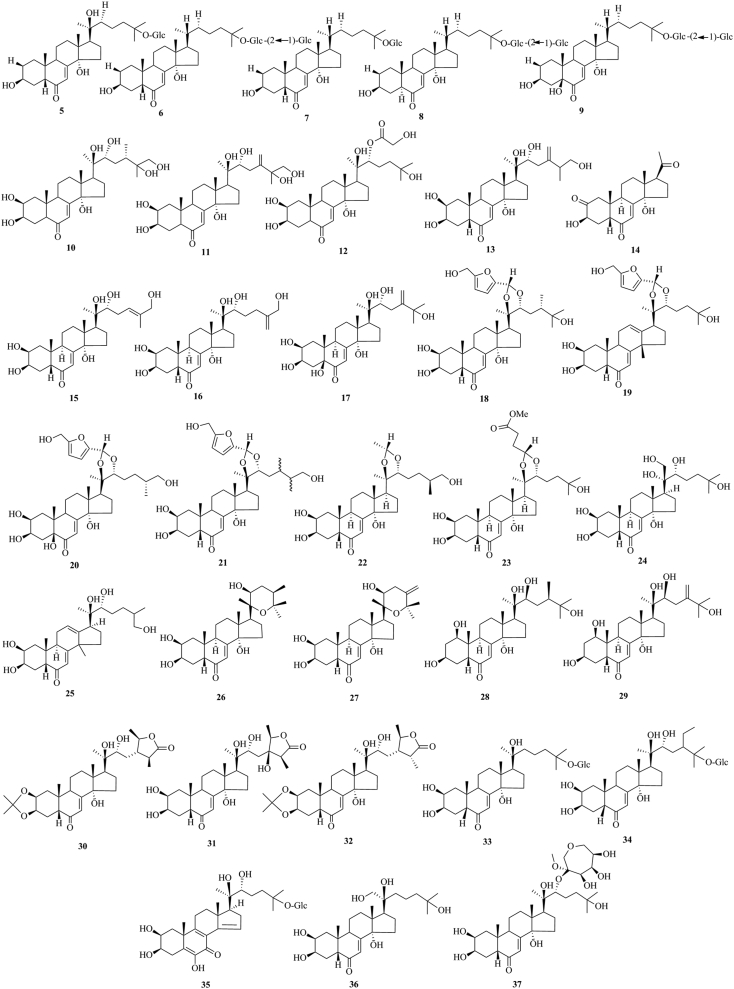
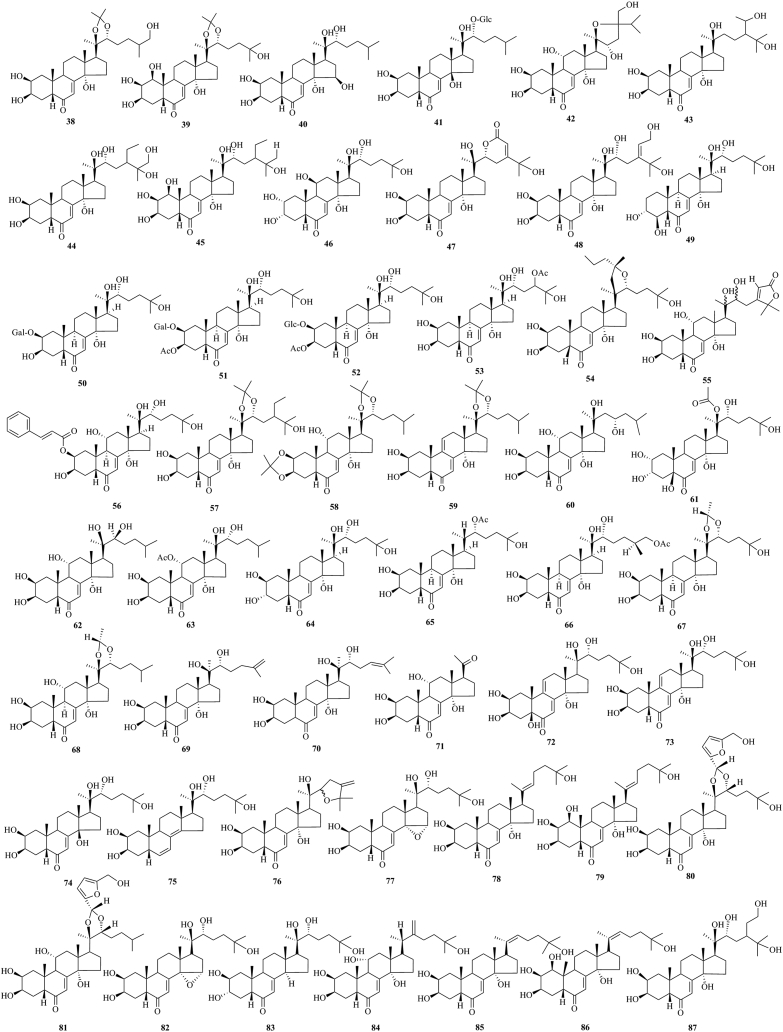
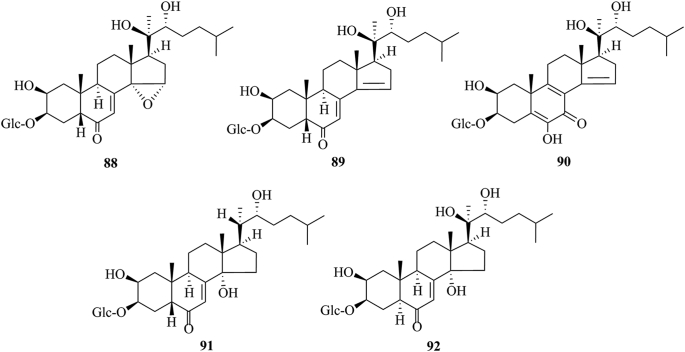
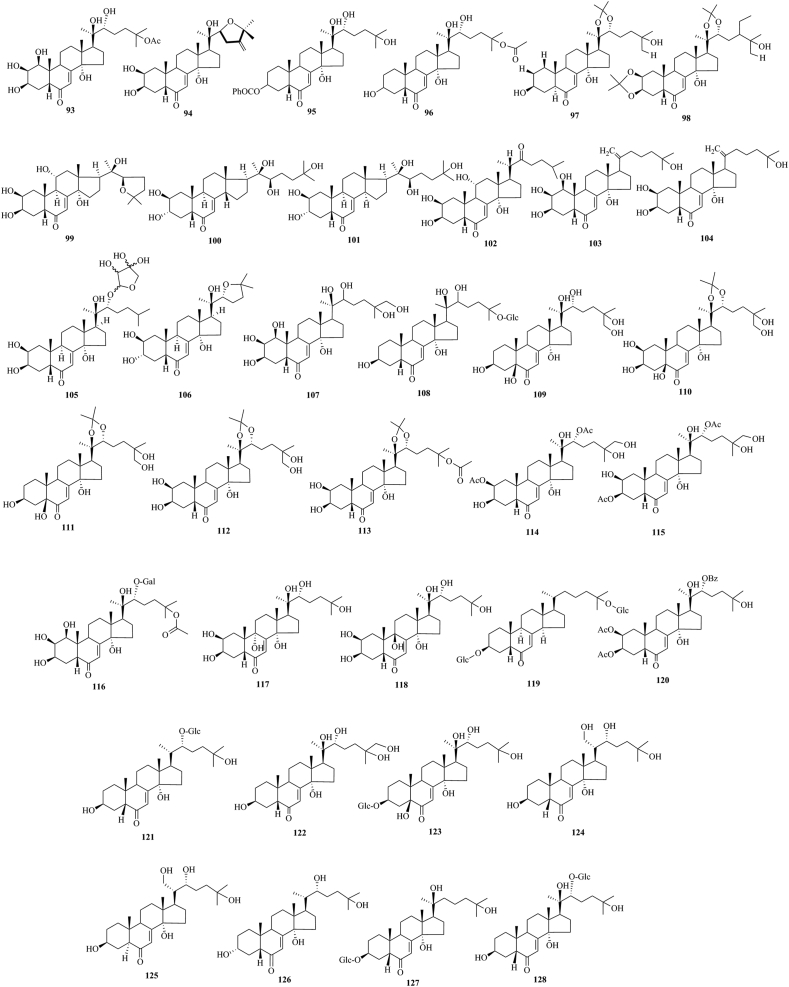
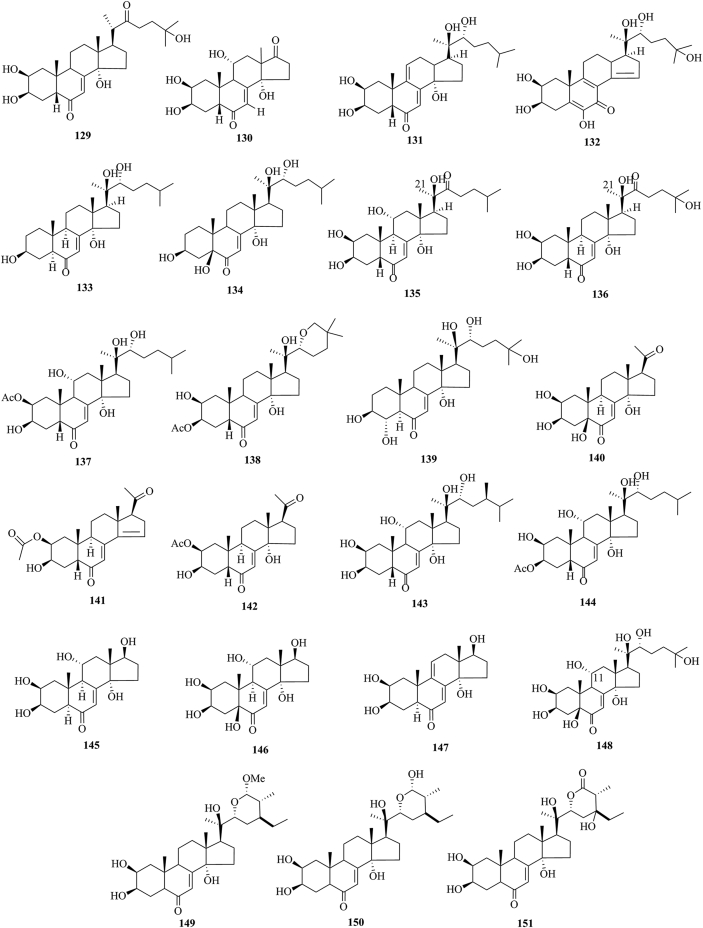
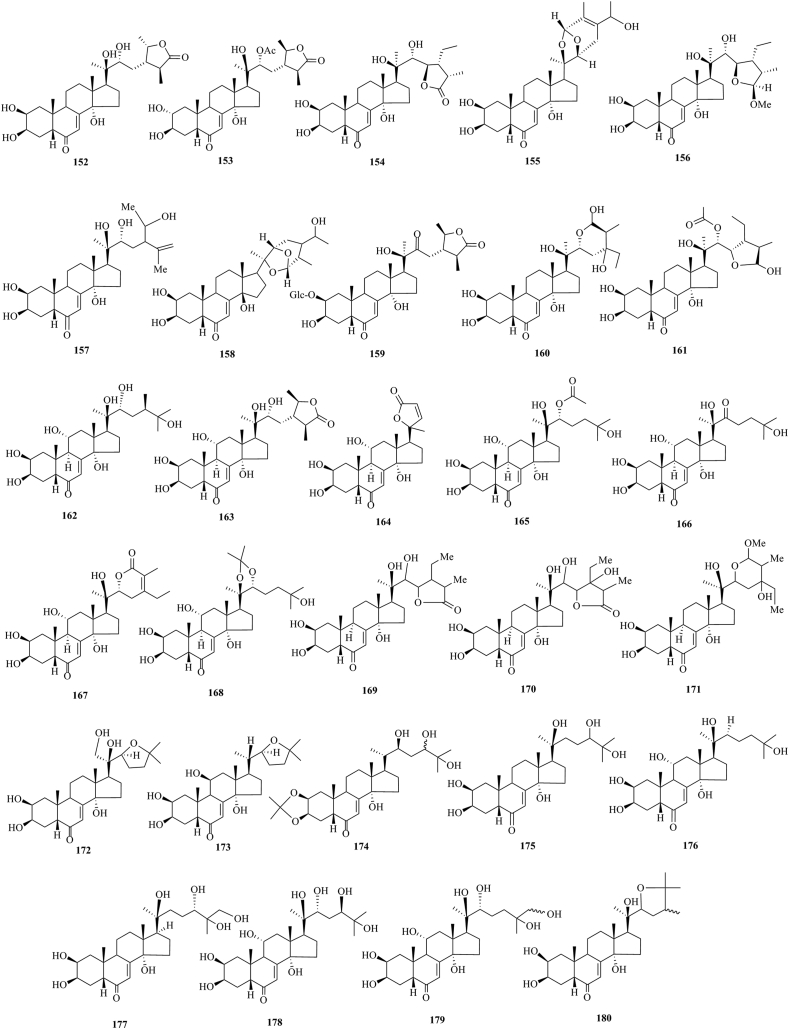
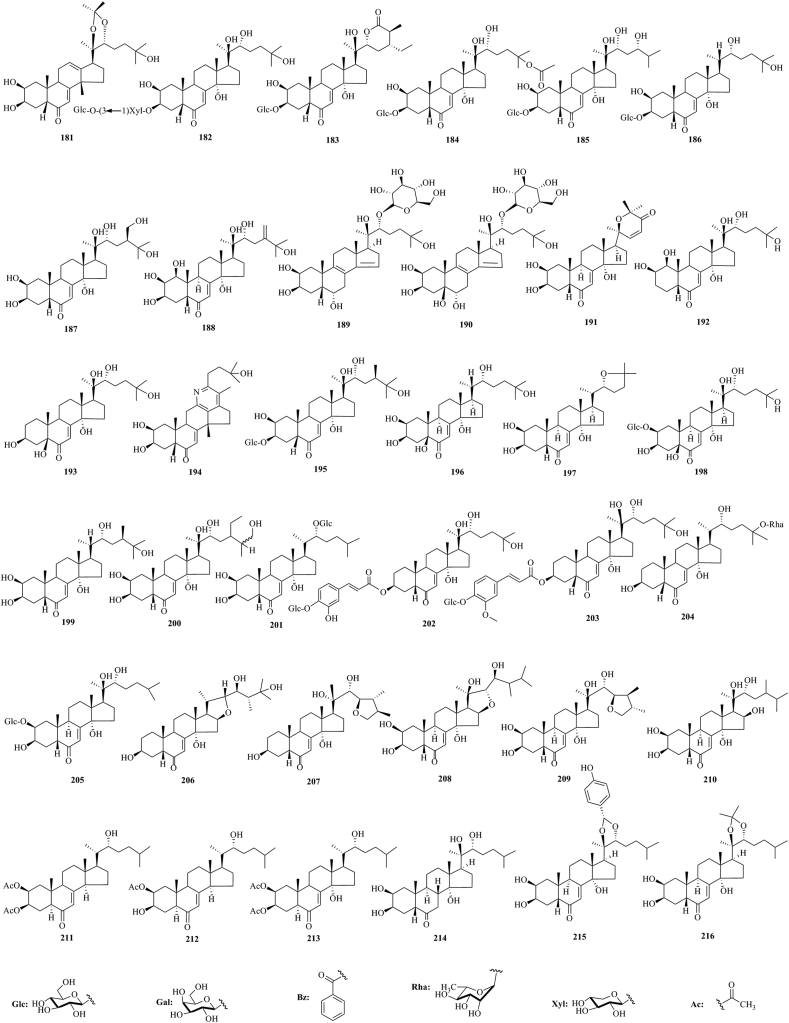
PEs have been reported from over 100 terrestrial plant families, representing ferns, gymnosperms, and angiosperms. The first isolation experiment of PE had a remarkable coincidence. Nakanishi et al.28,29 chemically investigated the leaves of Podocarpus nakaii (Podocarpaceae) and isolated three steroids, named ponasterones A (2), B (3) and C (4) (Fig. 1). Concurrently, an Australian group reported the isolation of 20-HE from the wood of Podocarpus elatus30. These reports stimulated further research on different plant species for the exploration of other members of this class. A comprehensive list of PEs containing 212 new phytoecdcysteroids (5–216) with their plant sources is presented in Supporting Information Table S1. The chemical structures of new PEs isolated from 18 plant families, such as Amaranthaceae, Asteraceae, Blechnaceae, Caryophyllaceae, Clavicipitaceae, Commelinaceae, Dioscoreaceae, Gleicheniaceae, Lamiaceae, Liliaceae, Limnanthaceae, Lygodiaceae, Malvaceae, Menispermaceae, Polypodiaceae, Polyporaceae, Rhodomelaceae, and Taxaceae, are illustrated in Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8.
Figure 2.
Structures of reported new phytoecdysteroids (5–37) from Amaranthaceae family.
Figure 3.
Structures of reported new phytoecdysteroids (38–87) from Asteraceae family.
Figure 4.
Structures of reported new phytoecdysteroids (88–92) from Blechnaceae family.
Figure 5.
Structures of reported new phytoecdysteroids (93–128) from Caryophyllaceae family.
Figure 6.
Structures of reported new phytoecdysteroids (129–151) from Clavicipitaceae, Commelinaceae, Dioscoreaceae, and Gleicheniaceae families.
Figure 7.
Structures of reported new phytoecdysteroids (152–180) from Lamiaceae family.
Figure 8.
Structures of reported new phytoecdysteroids (181–216) from Liliaceae, Limnanthaceae, Lygodiaceae, Malvaceae, Menispermaceae, Polypodiaceae, Polyporaceae, Rhodomelaceae, and Taxaceae families.
3.1. Isolation and separation of phytoecdysteroids
The isolation and purification of major and minor ecdysteroids from plant material involve a multi-step procedure. This includes extraction, separation, and finally purification through different chromatography methods, including thin-layer chromatography, column chromatography, and high-performance column chromatography (HPLC). The polar nature of ecdysteroids makes it a complex process to separate them from other polar plant constituents, including polyphenolic compounds, chlorophyll, lipids, steroids, triterpenoids, pigment materials, and amino acids5,31,32.
One of the common methods of the isolation of PEs is the solvent extraction of air-dried plant parts at room temperature, with a high excess of methanol or 95% ethanol. PEs were isolated from methanolic extract or 95% ethanol extract of the plant material followed by the removal of less polar organic compounds by partition with hexane and water. The residue of the aqueous portion was subjected to column chromatography through silica gel, alumina, Diaion HP-20, or Sephadex LH-20, and the fractions obtained from this chromatography were subjected to reverse phase HPLC using primarily silica gel 60G F254 or other silica gel columns as the stationary phase. Both aqueous methanol mixture and acetonitrile/trifluoroacetic acid mixture were used for the elution of the columns. In some cases, normal phase HPLC using dichloromethane/isopropyl alcohol/water mixture was effective for improved separation and isolation. Droplet countercurrent chromatography and rotation locular countercurrent chromatography techniques were also successful for separation33. The details of chromatographic procedures for separation of PEs were previously reviewed34.
3.2. Identification of phytoecdysteroids
The 14α-hydroxy-7-en-6-one chromophore of PEs showed characteristic ultra-violet absorption in methanol (MeOH) at λmax 240‒245 nm35. The proton nuclear magnetic resonance (1H-NMR) spectroscopy is limited in usefulness because it often gives spectra too complex to be analyzed. The 13C-NMR spectra with distortionless enhancement by polarization transfer experiments together with two-dimensional NMR (2D-NMR) experiments provide useful information regarding the skeletal structure. The key 2D-NMR includes correlation spectroscopy, heteronuclear single quantum coherence or correlation, heteronuclear multiple bond correlation, nuclear overhauser effect spectroscopy, and rotating frame nuclear overhauser effect spectroscopy. The carbon resonances of C-6 and C-20 appear to be the lowest at δC 201‒204 and 207‒209 ppm. The carbon resonances for C-2 and C-3 are near and δC 67‒69 and C-14’s carbon resonances are near δC 83‒85 ppm. Additionally, the carbon resonance values of C-7 and C-8 appear at δC 121‒123 and 162‒165 ppm. The 13C-NMR spectra of some PEs have already been reviewed36. However, it is necessary to include that therea method in analytical chemistry that would be appropriate for characterization of PEs does not exist.
3.3. Structural diversity and distribution of phytoecdysteroids in plant kingdom
In nature, PEs exist either in free-state or conjugated form, with sugars as glycosides or with organic acids as esters (such as acetate, benzoate, cinnamate, p-coumarate, and crotonate), sulphates, and isopropylidene or methyl ether formation. Among the sugars, glucose, galactose, and xylose are common. Although variations in the steroid ring structure are not substantial, the significant variations are found in the number, position, and orientation of hydroxyl groups and conjugating moieties. In a few cases, an extra oxo group may be located at the C-2, C-12, C-17, C-20, or C-22 position along with requisite C-6 position. Most of these structural modifications are found in different plant families, possibly due to their different uses of metabolites. The highlights of structural modifications of new PEs due to natural sources of variations for those reported after 1998 are mentioned in Supporting Information Table S1.
3.4. Biosynthesis of phytoecdysteroids
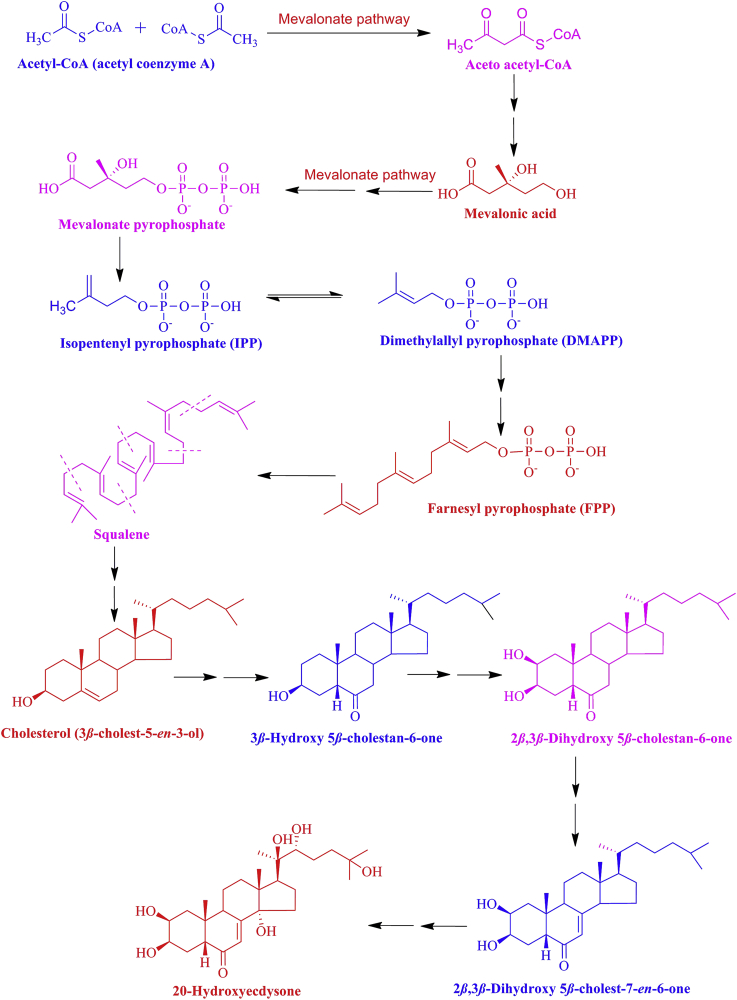
Plants synthesize PEs mainly from mevalonic acid via cholesterol in the mevalonate pathway of the plant cell, using acetyl-CoA as a precursor37. The important intermediates of the mevalonate pathway are isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). Higher plants are capable of producing IPP and DMAPP from pyruvate and glycerylaldehyde-3-phosphate also via the non-mevalonate pathway32. In the mevalonate pathway, the condensation reactions of six activated isoprene units form squalene and further undergo epoxidation and cyclization to form lanosterol. Additionally, the units undergo subsequent steps to form cholesterol32. Still, limited reports suggest the biosynthesis of PEs, yet with an unclear mechanism. The presence of PEs in various plant parts at different concentrations does not allow any conclusion to be drawn about the organ in which these compounds are primarily produced. Grebenok and Alder38 demonstrated that these compounds are possibly biosynthesized in developing tissues. The labelling experiments with 14C-mevalonic acid showed a prospective pathway involving the mevalonate pathway. Although the non-mevalonate pathway has not been reported, it is likely that 1-deoxy-d-xylulose could be used as an intermediate in mevalonate-independent deoxy-xylulose phosphate pathway (DOXP). Polypodium vulgare 20-HE might be synthesized from a Δ7 sterol with a reduced side chain at C-24; this was shown in spinach, yet the involvement of sterols was not explored38. Feeding experiments with labelled cholesterol in Taxas baccata and P. vulgare produced labelled ecdysone and 20-HE39. The location of the radiolabel in biosynthesized 20-HE indicated that hydrogen migration occurred from the 3α- and 4β-positions to C-4 and C-5 by concomitant 1,2-Wagner-Meerwein hydride migrations from the 4β-to the 5β- and from the 3α-to the 4α-position. The biosynthetic pathway study by Hyodo and Fujimoto40 in Ajaga reptans hairy roots indicated that 3β-hydroxy 5β-cholestan-6-one is converted into 2β,3β-dihydroxy 5β-cholestan-6-one and the latter is converted to 20-HE, suggesting that 7-ene can be introduced at a later stage in the biosynthetic pathway. Based on the biosynthetic studies by several groups, it can be concluded that 20-HE can be biosynthesized from cholesterol as well as mevalonic acid in plants following the pathway as illustrated in Fig. 9.
Figure 9.
Probable biosynthetic route of 20-hydroxyecdysone in plants.
4. New, naturally occurring phytoecdysteroids reported since 1999
This study comprehensively summarizes 212 new phytoecdcysteroids reported from 17 plant families from 1999 to 2019, along with various bioactivities of PEs. The new phytoecdcysteroids are numbered from 5 to 216 and the structures of those PEs are presented in Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8. These 18 plant families include Amaranthaceae, Asteraceae, Blechnaceae, Caryophyllaceae, Clavicipitaceae, Commelinaceae, Dioscoreaceae, Gleicheniaceae, Lamiaceae, Liliaceae, Limnanthaceae, Lygodiaceae, Malvaceae, Menispermaceae, Polypodiaceae, Polyporaceae, Rhodomelaceae, and Taxaceae. Out of the reported 212 new PEs, 33 are from the Amaranthaceae family (5–37, Fig. 2), 50 are from the Asteraceae family (38–87, Fig. 3), five are from the Blechnaceae family (88–92, Fig. 4), 36 are from the Caryophyllaceae family (93–128, Fig. 5), one is from the Clavicipitaceae family (129, Fig. 6), 18 are from the Commelinaceae family (130–147, Fig. 6), one is from the Dioscoreaceae family (148, Fig. 6), three are from the Gleicheniaceae family (149–151, Fig. 6), 29 are from the Lamiaceae family (152–180, Fig. 7), one is from the Liliaceae family (181, Fig. 8), one is from the Limnanthaceae family (182, Fig. 8), one is from the Lygodiaceae family (183, Fig. 8), five are from the Malvaceae family (184–188, Fig. 8), seven are from the Menispermaceae family (189–195, Fig. 8), ten are from the Polypodiaceae family (196–205, Fig. 8), five are from the Polyporaceae family (206–210, Fig. 8), three are from the Rhodomelaceae family (211–213, Fig. 8), and three are from the Taxaceae family (214–216, Fig. 8).
4.1. Amaranthaceae
The conducted literature survey indicates that 33 new ecdysteroids have been reported from the Amaranthaceae family. The new ecdysteroids are 2,22-dideoxy-20-hydroxyecdysone 25-O-β-d-glucopyranoside (5), 2,22-dideoxyecdysone 25-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranoside (6), 2,22-deoxyecdysone 25-O-β-d-glucopyranoside (7), (5α)-2,22-dideoxyecdysone 25-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranoside (8), 2,22-dideoxy-5β-hydroxyecdysone 25-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranoside (9), isolated from the plant Froelichia floridana41, 20,26-dihydroxy 28-methyl ecdysone (10), 20,26-dihydroxy 24(28)-dehydro ecdysone (11), 20-hydroxyecdysone 22-glycolate (12), kancollosterone (13), isolated from the plant Chenopodium quinoa42,43, 3β,14α-dihydroxy-5β-pregn-7-ene-2,6,20-trione (14), 24,25-dehydroinokosterone (15), 25,27-dehydroinokosterone (16), 5β-hydroxy-24(28)-dehydromakisterone A (17), isolated from Chenopodium album Willd44,45, niuxixinsterone A [(20R,22R,24S)-20-O,22-O-(5′-hydroxymethyl)-furfurylidene-2β,3β,14α,25-tetrahydroxy-5β-ergost-7-en-6-one] (18), niuxixinsterone B [(20R,22R)-20-O,22-O-(5′-hydroxymethyl)-furfurylidene-2β,3β,25-trihydroxy-14β-methyl-18-nor-5β-cholesta-7,12-dien-6-one] (19), niuxixinsterone C [(20R,22R,25R)-20-O,22-O-(5′-hydroxymethyl)-furfurylidene-2β,3β,5β,14α,26-pentahydroxycholest-7-en-6-one] (20)46, niuxixinsterone D (21)47, (25S)-20,22-O-(R-ethylidene) inokosterone (22), 20,22-O-(R-3-methoxycarbonyl) propylidene-20-hydroxyecdysone (23)48, achyranthesterone A [2β,3β,14α,20S,21,22R,25-heptahydroxycholest-7-en-6-one] (24)49, (20R,22R)-2β,3β,20,22,26-pentahydroxy-cholestan-7,12-dien-6-one (25)50, isolated from the plant Achyranthes bidentata50, aervecdysteroid A [20,25-epoxy-2β,3β,14α,22β-tetrahydroxy-5β-ecdysteroid] (26), aervecdysteroid B [24,28-dehydro-20,25-epoxy-2β,3β,14α,22β-tetrahydroxy-5β-ecdysteroid] (27), aervecdysteroid C [1β,3β,14α,20β,22β,25-hexahydroxy-5β-ecdysteroid] (28), aervecdysteroid D [24,28-dehydro-1β,3β,14α,20β,22β,25-hexahydroxy-5β-ecdysteroid] (29), isolated from the plant Aerva javanica51, 2,3-isopropylidene cyasterone (30), 24-hydroxycyasterone (31), 2,3-isopropylidene isocyasterone (32), isolated from Cyathula officinalis Kuan52, pfaffiaglycosides C (33), pfaffiaglycosides D (34), pfaffiaglycosides E (35), isolated from Pfaffia glomerata53, (20R)-22-deoxy-20,21-dihydroxyecdysone (36), isolated from Rhagodia baccata (Labill.) Moq.54, and septanoecdysone (37), isolated from the plant Atriplex portulacoides L.55.
4.2. Asteraceae
The literature survey indicates that 50 new ecdysteroids have been reported from the Asteraceae family. These are inokosterone 20,22-acetonide (38), integristerone A-20,22-acetonide (39), 15-hydroxyponasterone A (40), 14-epi-ponasterone A 22-O-β-d-glucopyranoside (41), carthamoleusterone (42), 22-deoxy-28-hydroxymakisterone C (43), 26-hydroxymakisterone C (44) and 1β-hydroxymakisterone C (45), isolated from the plant Leuzea carthamoides56, lesterone [5β-cholest-7-en-2α,3α,11β,14α,20R,22R,25-heptahydroxy-6-one] (46), isolated from the plant L. carthamoides57, leuzeasterone (47) and (24Z)-29-hydroxy-24(28)-dehydromakisterone C (48) isolated from the plant L. carthamoides58, coronatasterone (2-deoxy-3-epi-4β,20-dihydroxyecdysone) [(20R,22R)-3α,4β,14α,20,22,25-hexahydroxy-5β-cholest-7-en-6-one] (49), isolated from Serratula coronate59, 20-hydroxyecdysone-2-O-β-d-galactopyranoside (50), 3-O-acetyl-20-hydroxyecdysone-2-O-β-d-galactopyranoside (51), 3-O-acetyl-20-hydroxyecdysone-2-O-β-d-glucopyranoside (52), 24-O-acetyl-epi-abutasterone (53), 20-hydroxyecdysone-20,22-butylidene acetal (54), isolated from the plant Serratula chinensis60,61, rhapontisterone R1 [2β,3β,11α,14α,20ɛ,22ɛ-hexahydroxy-stigma-7,24(28)-dien-6-oxo-28,25-carbolactone] (55), isolated from Rhaponticum uniflorum62, turkesterone-2-O-cinnamate (56), isolated from R. uniflorum63, makisterone C-20,22-acetonide (57), isolated from R. uniflorum64, ajugasterone C-2,3,20,22-diacetonide (58) and 5-deoxykaladasterone-20,22-monoacetonide (59), isolated from R. uniflorum65, uniflorsterone (60), isolated from R. uniflorum66, rapisterone D 20-acetate (61), isolated from L. carthamoides67, 22-epi-ajugasterone C (62), isolated from Serratula cichoracea68, ajugasterone 11-acetate (63)69, 3-epi-20-hydroxyecdysone (64)70, ecdysone 22-acetate (65), (25S)-inokosterone 26-acetate (66), 20,22-O-(R-ethylidene)-20-hydroxyecdysone (67) and 20,22-O-(R-ethylidene)-ajugasterone C (68), isolated from S. coronate L.71, 25,26-didehydroponasterone A (69) and stachysterone C (70), isolated from Klaseopsis chinensis72, 11α-hydroxypoststerone (71)73, herkesterone [5β,25-dihydroxydacryhainansterone] (72)73, 25-hydroxydacryhainansterone (73)74, 14-epi-20-hydroxyecdysone (74)74, 2β,3β,20R,22R,25-pentahydroxy-5β-cholest-6,8(14)-dien (75)75, 24-methylene-shidasterone (76)75, 14α,15α-epoxy-14,15-dihydrostachysterone B (77)75, 20,22-didehydro taxisterone (78)76, 1-hydroxy-20,22-didehydrotaxisterone (79)76, serfurosterone A (80)77, serfurosterone B (81)77,14,15α-epoxy-(20R,22R)-2β,3β,20,22,25-pentahydroxy-5β-cholesta-7,14-dien-6-one (82)78, (20R,22R)-2β,3α,20,22,25-pentahydroxy-5β-cholesta-7-en-6-one (83)78, 22-methylene-2β,3β,11α,14α,25-pentahydroxy-5β-cholesta-7-en-6-one (84)78, 2β,3β,14α,25-tetrahydroxy-5β-cholesta-7,20(22)-dien-6-one (85)78 and 1β,2β,3β,14α,25-pentahydroxy-5β-cholesta-7,20(22)-dien-6-one (86)78, isolated from Serratula wolffii, and (24R)-24-(2-hydroxyethyl)-20-hydroxyecdysone (87) isolated from the plant Serratula strangulate79.
4.3. Blechnaceae
The Literature survey indicates that five new ecdysteroids have been reported from the Blechnaceae family. These are brainesteroside A [14-deoxy-14α-15α-epoxyponasteroside A] (88), brainesteroside B [14-deoxy-14,15-didehydroponasteroside A] (89), brainesteroside C [25-deoxycalonysterone-3-O-β-d-glucopyranoside] (90), brainesteroside D [25-deoxyecdysone-3-O-β-d-glucopyranoside] (91), and brainesteroside E [5-epi-ponasteroside A] (92) isolated from the plant Brainea insignis80.
4.4. Caryophyllaceae
Thirty-six new ecdysteroids have been reported from the Caryophyllaceae family. The new ecdysteroids are integristerone A 25-acetate (93), isolated from Silene brahuica81, japonicone (22,25-epoxy-24-methylene-2,3,14,20-tetrahydroxycholest-7-en-6-one) (94), isolated from Sagina japonica82, 2-dehydroxyecdysterone-3-O-benzoate (95)83, 2-deoxyecdysterone-25-acetate (96)84, isolated from Silene wallichiana, 5α-2-deoxy-20-hydroxyecdysone 20,22-acetonide (97)85, makisterone C 2,3; 20,22-diacetonide (98)85 isolated from Silene viridiflora, (11α)-11-hydroxyshidasterone [(2β,3β,5β,11α,22R)-22,25-epoxy-2,3,11,14,20-pentahydroxycholest-7-en-6-one] (99), (2β,3β,5β,14β,22R)-2,3,20,22,25-pentahydroxycholest-7-en-6-one (100), (2β,3α,5β,14α,22R)-2,3,20,22,25-pentahydroxycholest-7-en-6-one (101), 22-dehydro-20-deoxy ajugasterone C (102), 1-hydroxy-22-deoxy-20,21-didehydro ecdysone (103), 22-deoxy-20,21-didehydro ecdysone (104), ponasterone A-22-apioside (2β,3β,14α,20R-tetrahydroxy-22-{[3,4-dihydroxy-4-(hydroxymethyl)-tetrahydrofuran-2-yl]oxy}-5β-cholest-7-en-6-one) (105), 3-epi-shidasterone [22,25-epoxy-2β,3α,14α,20R)-tetrahydroxy-5β-cholest-7-en-6-one] (106), isolated from S. wolffii86, 87, 88, 26-hydroxyintegristerone A (107), isolated from Silene frivaldszkyana89, 2-deoxy-20-hydroxyecdysone 25-glucoside (108), isolated from Silene gigantean89, 2-deoxy-5,20,26-trihydroxy ecdysone (109), 5,20,26-trihydroxyecdysone 20,22-acetonide (110), 2-deoxy-5,20,26-trihydroxyecdysone 20,22-acetonide (111), 20,26-dihydroxyecdysone 20,22-acetonide (112), 20-hydroxyecdysone 20,22-monoacetonide-25-acetate (113), 2,22-diacetate-20,26-dihydroxyecdysone (114), 3,22-diacetate-20,26-dihydroxyecdysone (115), isolated from S. viridiflora90, 91, 92, 22-O-α-d-galactosylintegristerone A 25-acetate (sileneoside H) (116), isolated from S. brahuica93, 9α,20-dihydroxyecdysone (117), 9β,20-dihydroxyecdysone (118), isolated from Silene italic ssp. nemoralis94,95, 3-O-β-d-glucopyranosyl-3β,25-dihydroxy-5β-cholest-7-en-6-one-25-O-β-d-glucopyranoside (119), isolated from Silene montbretiana96, 2,3-diacetate-22-benzoate-20-hydroxyecdysone (120), isolated from Silene guntensis B. Fedtsch97, 2-deoxyecdysone 22β-d-glucoside (121), 2-deoxy-20,26-dihydroxyecdysone (122) and 2-deoxypolypodine B 3β-d-glucoside (123), isolated from Silene pseudotites98, 2-deoxy-21-hydroxyecdysone (124) and 5α-2-deoxy-21-hydroxyecdysone (125), isolated from Silene otites99, 3α,14α,22R,25-tetrahydroxy-5β(H)-cholest-7-en-6-one (126), isolated from Acanthophyllum gypsophiloides100, and 2,22-dideoxy-20-hydroxyecdysone 3β-O-β-d-glucopyranoside (127) isolated from Cucubalus baccifer101 and 2-deoxy-20-hydroxyecdysone-22-O-β-d-glucopyranoside (128), isolated from the plant Silene italica ssp. nemoralis102.
4.5. Clavicipitaceae
One new ecdysteroid has been reported from the Clavicipitaceae family. The new ecdysteroid 22-dehydroecdysone (129) is isolated from Nomuraea rileyi (fungus)103.
4.6. Commelinaceae
Eighteen ecdysteroids have been isolated from the Commelinaceae family. These ecdysteroids are 11α-hydroxyrubrosterone (130)104, dacryhainansterone (131)105, calonysterone (132)105, cyanosterone A (133)106, cyanosterone B (134)107, 22-oxo-ajugasterone C (135)108, 22-oxo-20-hydroxyecdysone (136)108, ajugasterone C 2-acetate (137)109, shidasterone3-acetate (138)109, isolated from the plant Cyanotisar achnoidea, 3β,4α,14α,20R,22R,25-hexahydroxy-5α-cholest-7-en-6-one (139), isolated from the plant C. achnoidea C. B. Clarke110, 5β-hydroxypoststerone (140), 14,15-dehydro-poststerone 2-acetate (141), poststerone2-acetate (142), 24-epi-atrotosterone A (143), ajugasterone C 3-acetate (144), isolated from the plant Cyanotis longifolia111, callecdysterol A (145), callecdysterol B (146), and callecdysterol C (147), isolated from the plant Callisia fragrans112.
4.7. Dioscoreaceae
The conducted literature survey indicats that one new ecdysteroid, namely (20R)-5β-11α,20-trihydroxyecdysone (148), has been isolated from the plant Dioscorea dumetorum (Dioscoreaceae)113.
4.8. Gleicheniaceae
The literature survey indicates that three new ecdysteroids, (22R,24R,25S,26S)-2β,3β,14α,20R-tetrahydroxy-26α-methoxy-6-oxo-stigmast-7-ene-22,26-lactone (149), (22R,24R,25S)-2β,3β,14α,20R,26S-pentahydroxy-6-oxo-stigmast-7-ene-22,26-lactone (150), and (22R,25S)-2β,3β,14α,20R,24S-pentahydroxy-6,26-dioxo-stigmast-7-ene-22,26-lactone (151), have been isolated from the plant Diplopterygium rufopilosum (Gleicheniaceae)114.
4.9. Lamiaceae
A literature survey indicates that 29 new ecdysteroids have been reported from the Lamiaceae family. These are 28-epi-cyasterone [(22R,24S,25S,28S) 5β-stigmast-7-ene-26-oic acid, 2β,3β,14,20,22,28-hexahydroxy-6-oxo-γ-lactone] (152), isolated from Eriophyton wallchii115, ajugalide-E (153) from Ajuga taiwanensis116, breviflorasterone (154), ajugacetalsterone C (155), ajugacetalsterone D (156) from Ajuga macrosperma var. breviflora117, decumbesterone A (157)118 and ajugacetalsterone E (158)119 isolated from Ajuga decumbens, 22-dehydrocyasterone-2-glucoside (159), ajugacetalsterone A (160) and ajugacetalsterone B (161), isolated from Ajuga nipponensis120, 25-hydroxy-atrotosterone A (162), 11-hydroxy-cyasterone (163), 11-hydroxy-sidisterone (164), turkesterone 22-acetate (165), 22 oxo-turkesterone (166), 11-hydroxy-Δ24-capitasterone (167) and turkesterone 20,22-acetonide (168), isolated from Ajuga turkestanica121, reptanslactone A (169), reptanslactone B (170) and sendreisterone (171), isolated from Ajuga reptans var. reptans122, 21-hydroxyshidasterone (172), 11β-hydroxy-20-deoxyshidasterone (173) and 2,3-acetonide-24-hydroxyecdysone (174), isolated from Vitex doniana123, 24-epi-pinnatasterone (175)124 and scabrasterone (176)124, isolated from Vitex scabra, 26-hydroxypinnatasterone (177), isolated from Vitex cymosa125, (24R)-11α,20,24-trihydroxyecdysone (178)126, and 11α,20,26-trihydroxyecdysone (179)126 and 24-methylshidasterone (180)127, isolated from Vitex canescens.
4.10. Liliaceae
The literature survey indicates that one new ecdysteroid, stachysterone A-20,22-acetonide (181), has been isolated from Asparagus filicinus Buch.-Ham. (Liliaceae)128.
4.11. Limnanthaceae
The literature survey indicates that one new ecdysteroid, namely limnantheoside C (20-hydroxyecdysone 3-O-β-d-glucopyranosyl-[l→3]-β-d-xylopyranoside) (182), has been isolated from Limnanthes alba Hartw.(Limnanthaceae)129.
4.12. Lygodiaceae
The literature survey indicates that one new ecdysteroid, lygodiumsteroside A (183), has been isolated from Lygodium japonicum (Thunb.) Sw. (Lygodiaceae)130.
4.13. Malvaceae
Five new ecdysteroids have been reported from the Malvaceae family. These new ecdysteroids are 25-acetoxy-20-hydroxyecdysone-3-O-β-d-glucopyranoside {(2β,3β,5β,22R)-3-[(β-d-glucopyranosyl)oxy]-2,14,20,22-tetrahydroxy-6-oxo-cholest-7-en-25-yl acetate} (184), pterosterone-3-O-β-d-glucopyranoside [(2β,3β,5β,22R,24S)-2,14,20,22,24-pentahydroxy-6-oxo-cholest-7-en-3-yl β-d-glucopyranoside] (185), ecdysone-3-O-β-d-glucopyranoside [(2β,3β,5β,22R)-2,14,22-trihydroxy-6-oxo-cholest-7-en-3-yl β-d-glucopyranoside] (186), isolated from the plant Sida rhombifolia131, 20-hydroxy-24-hydroxymethyl ecdysone (187), isolated from the plant Sida spinosa132, and glutinosterone [(20R,22R)-1β,2β,3β,14α,20,22,25-heptahydroxy-5β-cholest-7,24 dien-6-one] (188), isolated from the plant Sida glutinosa133.
4.14. Menispermaceae
The literature survey indicates that seven new ecdysteroids have been reported from the Menispermaceae family. These are sphenocentroside A (189) and sphenocentroside B (190), isolated from Sphenocentrum jollyanum134, cycleasterone A (191), isolated from Cyclea barbata Miers135, 3-deoxy-1β,20-dihydroxyecdysone (192), 2-deoxy-5β,20-dihydroxyecdysone (193), and diploclidine (194) from Diploclisia glaucescens136, 137, 138 and fibraurecdyside A (195), isolated from Fibraurea tinctoria Lour139.
4.15. Polypodiaceae
The literature survey indicates that ten new ecdysteroids have been reported from Polypodiaceae family. The new ecdysteroids are 5-hydroxyecdysone (196), 20-deoxyshidasterone (197) and polypodine B 2-β-d-glucoside (198), isolated from the plant P. vulgare140, 20-deoxymakisterone A (199), 25-epimer of amarasterone A (200), 25-deoxyecdysone 22-β-d-glucoside (201) from Microsorum scolopendria141, E-2-deoxy-20-hydroxyecdysone 3-[4-(1-β-d-glucopyranosyl)]-caffeate (202), 2-deoxyecdysone 3-[4-(1-β-d-glucopyranosyl)]-ferulate (203), 2-deoxyecdysone 25-α-l-rhamnopyranoside (204), isolated from Microsorum membranifolium142,143 and ponasteroside B (205), isolated from Lepidogrammitis drymoglossoides144.
4.16. Polyporaceae
From the Polyporaceae family, the conducted literature survey indicates that five new ecdysteroids, (20S,20R,24R)-16,22-epoxy-3β,14α,23β,25-tetrahydroxyergost-7-en-6-one (206), (23R,24R,25R)-23,26-epoxy-3β,14α,20α,22α-tetrahydroxyergost-7-en-6-one (207), polyporoid A (208), polyporoid B (209), and polyporoid C (210) are all isolated from the plant Polyporus umbellatus145,146.
4.17. Rhodomelaceae
A literature survey indicates that three new ecdysteroids have been reported from the Rhodomelaceae family. These are alfredensterol (211), 3-deacetoxy alfredensterol (212), and 14α-hydroxy alfredensterol (213), isolated from Laurencia alfredensis (red algae)147.
4.18. Taxaceae
From the Taxaceae family, a conducted literature survey indicates that three new ecdysteroids have been reported. The new ecdysteroids are 7,8β-dihydroponasterone A (214), isolated from the plant Taxus cuspidate148, ponasterone A 20,22-p-hydroxybenzylidene acetal (215), and ponasterone A 20,22-acetonide (216), isolated from the plant Taxus canadensis Marsh149.
5. Biological and pharmacological activities of phytoecdysteroids
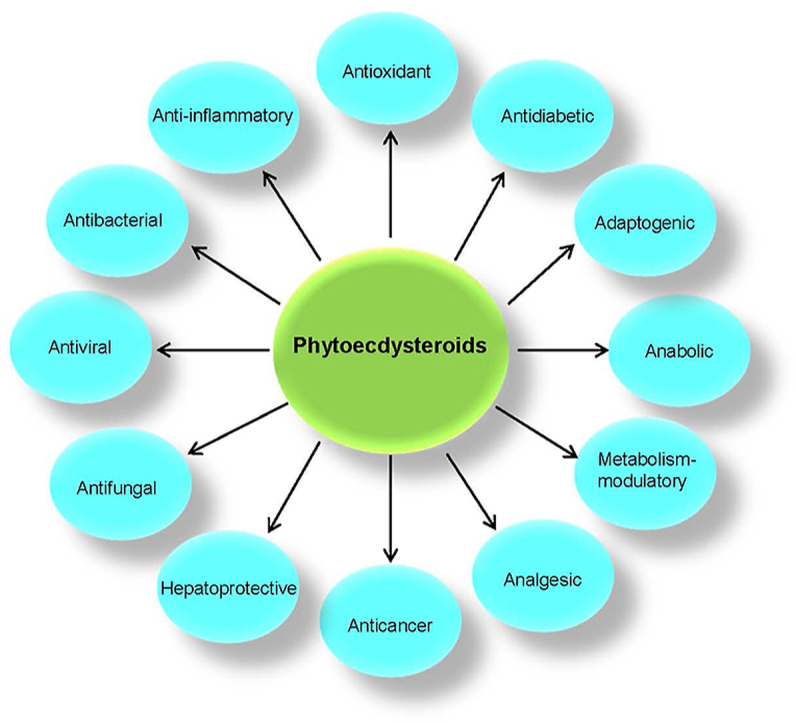
PEs play prominent roles in growth regulation and in the protection of plant species; they may have potential therapeutic applications. Various pharmacological effects of new PEs based on in vitro and in vivo studies are presented in Table 1. PEs have been known for their numerous bioactivities relating to their broad-spectrum general health-promoting effects. They are known for their numerous beneficial effects on mammals, which include as anabolic, adaptogenic, antidiabetic, hypolipidemic, and hepatoprotective effects150, 151, 152, 153. The prospective results demonstrate that PEs can be considered efficacious against several acute and chronic pathophysiological conditions as discussed further.
Table 1.
Pharmacological effects of new phytoecdysteroids (since 1999) based on in vitro and in vivo studies.
| Name of the ecdysteroids | Pharmacological activity | Study model | Cell line used | Conc./dose | Effect | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| 2,22-Dideoxy-20-hydroxyecdysone 25-O-β-d-glucopyranoside (5), 2,22-dideoxyecdysone 25-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranoside (6) | Evaluated human Topo I inhibition and no significant effect have been observed. | In vitro | Escherichia coli | 30–312 μmol/L | Growth inhibition | Topoisomerase I inhibition | 41 |
| 20,26-Dihydroxy 28-methyl ecdysone (10), 20,26-dihydroxy 24(28)-dehydro ecdysone (11), 20-hydroxyecdysone 22-glycolate (12) | Evaluated inhibitory effect on calf skin collagenase and scavenging DPPH free radicals, as well as in chelating the iron metal ions and significant effect have been observed. | In vitro | Clostridium histolyticum | DPPH assay: 5–30 μg/mL; metal ion chelation: 10–70 μg/mL; collagenase assay: 5–100 μg/mL | Antioxidant | Free-radical-scavenging; collagenase inhibition | 43 |
| 3β,14α-Dihydroxy-5β-pregn-7-ene-2,6,20-trione (14) | Evaluated effects on germination and growth of Lactuca sativa L. and have shown little influence on germination as well as on shoot length. | Ex situ | Lactuca sativa | 10–1000 μmol/L | Seed germination inhibition | Phytotoxicity | 44 |
| Niuxixinsterone D (21) | Evaluated inhibitory effects against LPS-induced NO production in RAW 264.7 macrophagesand exhibited anti-neuroinflammatory activity with inhibited 29.7 and 26.0% NO production. | In vitro | RAW 264.7 macrophages | 50 μmol/L | Anti-neuroinflammatory activity | Inhibition of nitric oxide production | 47 |
| Aervecdysteroid A (20,25-epoxy-2β,3β,14α,22β-tetrahydroxy-5b-ecdysteroid) (26), aervecdysteroid B (24,28-dehydro-20,25-epoxy-2β,3β, 14α,22β-tetrahydroxy-5β-ecdysteroid) (27), aervecdysteroid C (1β,3β,14α,20β,22β,25-hexahydroxy-5β-ecdysteroid) (28), aervecdysteroid D (24,28-dehydro-1β,3β,14α,20β,22β,25-hexahydroxy-5β-ecdysteroid) (29) | Evaluated enzyme inhibitory activities against AChE, BChE and LOX and no significant effect have been observed. | In vitro | Ovine seminal vesicles microsomes | 100 μmol/L | Anti-inflammatory | Inhibition of AChE, BChE and LOX enzymes | 51 |
| (20R)-22-Deoxy-20,21-dihydroxyecdysone (36) | Evaluated agonistic activity in the Drosophila melanogaster BII cell bioassay and shown good activity. | In vitro | Drosophila melanogaster BII cell bioassay | ED50 20 μmol/L | Agonistic activity | Growth inhibition | 54 |
| Septanoecdysone (37) | Evaluated antioxidant activity by using DPPH, ABTS+, Fe3+ and catalase assays. Also evaluated antibacterial and anticholinesterase activities and shown significant activities. | In vitro |
E. coli; Human plasma |
DPPH: 0.062–1.0 mg/mL; ABTS: 2.45 mmol/L; others: 0.062–1.0 mg/mL | Antioxidant, antibacterial, anti-inflammatory | Free-radical-scavenging; ChE inhibition; growth inhibitory | 55 |
| 22-epi-Ajugasterone C (62) | Weak DPPH radical-scavenging and antimicrobial activity against multi resistant strains Gram positive bacteria (Staphylococcus aureus 118), Gram negative bacteria such as (Escherichia coli 113, Klebsiella pneumoniae 112, Proteus mirabilis 23, Serratia sp. 2028, Pseudomonas aurogenosa43) and yeasts (Candida albicans). | In vitro | E. coli | 1–300 μg/mL | Antioxidant, anti-microbial | Free-radical-scavenging; growth inhibitory | 68 |
| 3-epi-20-Hydroxyecdysone (64), ecdysone 22-acetate (65), (25S)-inokosterone 26-acetate (66), 20,22-O-(R-ethylidene)-20-hydroxyecdysone (67), 20,22-O-(R-ethylidene)-ajugasterone C (68) | Evaluated biological activity in the agonist version of the Drosophila melanogaster BII cell bioassay. | In vitro | Drosophila melanogaster BII cell line | EC50 16, 1.1, 0.6, 43, and 62 μmol/L | Agonistic activity | Growth inhibition | 70,71 |
| 20,22-Didehydro taxisterone (78), 1-hydroxy-20,22-didehydrotaxisterone (79) | Evaluated toxicity in the oral aphid Acyrthosiphonpisum (Harris) test and exhibited low toxicity. | In vitro | (Acyrthosiphonpisum Harris) | LC50> 100 ppm; LC50 = 48.5 ppm | Oral aphid test | Cytotoxic activity | 76 |
| (24R)-24-(2-Hydroxyethyl)-20-hydroxyecdysone (87) | Evaluated antioxidant activity on AAPH. -induced hemolysis of human RBC and Fe2+ + cysteine-induced lipid peroxidation of liver microsome and showed significant activity. |
In vitro | E. coli; liver microsome | 10 μL | Antioxidant activity; lipid peroxidation inhibition | Free-radical-scavenging; iron chelation; blood hemolysis prevention | 79 |
| 3-O-β-d-Glucopyranosyl-3β,25-dihydroxy-5β-cholest-7-en-6-one-25-O-β-d-glucopyranoside (119) | Evaluated cytotoxic activity against two cancer cell lines of A549 (human lung adenocarcinoma) and HeLa cells (human epitheloidcervix carcinoma) by using the MTT assay and no significant activity have been observed. | In vitro | Lung cancer cell line (A549); leukemiacell line (HeLa) | 12.5–100 μmol/L | Cytotoxic activity | Growth inhibitory | 96 |
| 2,3-Diacetate-22-benzoate-20-hydroxyecdysone (120) | Evaluated cytotoxicity, antiproliferative activities in HeLa, HepG-2, and MCF-7 cell linesand antioxidant activities and showed moderate activities. | In vitro | Human cervix adenocarcinoma (HeLa), hepatocellular carcinoma (HepG-2), breast adenocarcinoma (MCF-7) cells | 1–500 μg/mL | Cytotoxic activity; antiproliferative; antioxidant | Cell growth inhibition; free-radical-scavenging | 97 |
| 3α,14α,22R,25-Tetrahydroxy-5β(H)-cholest-7-en-6-one (126) | Evaluated anti-inflammatory and analgesic activities. | In vivo | Acetic acid induced inflammation in rat | 50 mg/kg | Anti-inflammatory, analgesic activities | Inhibition of peritoneal exudation and irritation | 100 |
| (20R)-5β-11α,20-Trihydroxyecdysone (148) | Evaluated antifungal activity against three Candida species viz. Candida albicans, Candida glabrata and Candida tropicalis and no significant effect have been observed. | In vitro | Candida albicans, Candida glabrata and Candida tropicalis | MIC: >200 μg/mL | Antifungal activity | Growth inhibition | 113 |
| (22R,24R,25S,26S)-2β,3β,14α,20R-Tetrahydroxy-26α-methoxy-6-oxo-stigmast-7-ene-22,26-lactone (149), (22R,24R,25S)-2β,3β,14α,20R,26S-pentahydroxy-6-oxo-stigmast-7-ene-22,26-lactone (150),(22R,25S)-2β,3β,14α,20R,24S-pentahydroxy-6,26-dioxo-stigmast-7-ene-22,26-lactone (151) | Evaluated antifungal and antibacterial properties by disk diffusion method and exhibited moderate antibacterial activity against oral pathogens. | In vitro | Candida albicans, C. tropicalis, C. glabrata; Streptococcus mutans, and S. viridians cultures | MIC: 48–72 mg/mL | Antifungal and antibacterial activities | Growth inhibitory | 114 |
| Decumbesterone A (157) | Evaluated inhibitory effects on EBV-EA induction by the tumorpromoter (TPA) as a primary screening test for antitumor-promoters and showed very strong inhibitory effects. | In vivo | DMBA/TPA-induced skin tumor in mice | 1–32 nmol | Inhibitory effects | Inhibition of Epstein-Barr virus early antigen | 118 |
| Ajugacetalsterone E (158) | Evaluated cytotoxic activity against MCF-7, GepG2, A549, and B16 cell lines using MTT method and the inhibition of superoxide anion generation and elastase release and moderate activity have been observed. | In vitro | MCF-7, HepG2, A549, B16 cell lines | 10–48 μmol/L | Cytotoxic and antioxidant activity | Growth inhibition, inhibition of SOD generation and elastase release | 119 |
| Ajugacetalsterone A (160) | Evaluated cytotoxicity against human lung cancer cell A-549, human hepatoma cell HepG2, human breast cancer cell MCF-7 and proliferating epidermal carcinoma cell HeLa using MTT method and significant activity have been observed. | In vitro | A-549, HepG2, MCF-7 cell lines | IC50 26.5 ± 1.3, 33.3 ± 1.4, 25.4 ± 1.8 and 28.1 ± 2.9 μmol/L | Cytotoxic activity; antiproliferative | Cell growth inhibition | 170 |
| 21-Hydroxyshidasterone (172), 11β-hydroxy-20-deoxyshidasterone (173), 2,3-acetonide-24-hydroxyecdysone (174) | Evaluated anti-inflammatory activity on rat and significant activity have been observed. | In vivo | Carrageenan-induced inflammation in Sprague‒Dawley Rats | 100 mg/kg | Anti-inflammatory and analgesic activities | Inhibition of paw oedema inflammation, inhibition of histamine and serotonin | 123 |
| 24-epi-Pinnatasterone (175), scabrasterone (176) | Evaluated Musca assay for moulting hormone activity and exhibited very low activity. | In vivo | Musca bioassay | EC50: 0.5, 1 μmol/L | Susceptibility activity | Moulting hormone activity | 124 |
| Stachysterone A-20,22-acetonide (181) | Evaluated cytotoxicity against human breast adenocarcinoma MDA-MB-231 cell line and no significant activity have been observed | In vitro | Human breast adenocarcinoma MDA-MB-231 cell line | 2–50 μmol/L | Cytotoxicity activity | Growth inhibitory, inhibition of cell proliferation | 128 |
| Glutinosterone (188) | Evaluated liver function in terms of serum enzyme activity, lipid metabolic enzyme and antibacterial activity and exhibited significant activity. | In vitro | Human blood cells | 5–25 μg/mL | Liver function improvemen, antibacterial activity | Inhibition of hepatotoxicity markers SGOT, SGPT and ALP | 133 |
| Sphenocentroside A (189), sphenocentroside B (190) | Screened antimicrobial activity and moderate activity have been observed. | In vitro | E. coli | 20 μg/mL | Antimicrobial activity | Growth inhibition | 134 |
| Cycleasterone A (191) | Evaluated hepatoprotective activity against APAP-induced toxicity in HepG2 cells and moderate activity have been observed. | In vitro | N-acetyl-p-aminophenol (APAP)-induced toxicity in HepG2 cells | 10 μmol/L | Hepatoprotective activity | Improving cell survival | 135 |
| Fibraurecdyside A (195) | Evaluated inhibitory effects against cytochrome P450 3A4 (CYP3A4) and no significant activity have been observed. | In vitro | E. coli | 1–2 μmol/L | Growth inhibitory, antioxidant activity | Inhibition of cytochrome P450 3A4 (CYP3A4) | 139 |
| Polyporoid A (208), polyporoid B (209), polyporoid C (210) | Evaluated anti-inflammatory activity against TPA-induced inflammation in mice and exhibited significant inhibitory activity. | In vivo | TPA-induced inflammation in mice | ID50 0.1–1 μmol/L | Anti-inflammatory activity | Inhibition of topical skin inflammation | 146 |
| Alfredensterol (211), 3-deacetoxy alfredensterol (212), 14α-hydroxy alfredensterol (213) | Evaluated antiproliferative activity against MDA-MB-231 breast and HeLa human cervical cancer cell lines and exhibited moderate activity. | In vivo | MDA-MB-231 and HeLa cell lines | MDA-MB-231, IC50 = 27.6, 21.6 and 15.8 μmol/L; HeLa IC50 = 25.6, 43.5 and 48.2 μmol/L | Anti-proliferative activity | Inhibition of cell growth and proliferation | 147 |
AAPH, 2,2′-azobis(2-amidinopropane) dihydrochloride; ABTS+, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate); AChE, acetylcholinesterase; APAP, N-acetyl-p-aminophenol; BChE, butyrylcholinesterase; CYP3A4, cytochrome P450 3A4; EBV-EA, Epstein-Barr virus early antigen; EC50, half maximal effective concentration; ID50, median infectious dose; LC50, lethal concentration 50; LOX, lipoxygenase; MIC, minimum inhibitory concentration; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; RBC, red blood cell; TPA, 12-O-tetradecanoylphorbol-13-acetate.
5.1. Antioxidant activities
The antioxidant properties of PEs have been reported to display beneficial effects in various chronic conditions. A novel phytoecdysterone ajugacetalsterone E (158) exhibited in vitro cytotoxic activity, superoxide anion generation, and elastase release in N-formyl-methionyl-leucyl-phenylalanine/cytochalasin B (FMLP/CB)-induced human neutrophils119. 20,26-dihydroxy-28-methyl ecdysone (10), 20,26-dihydroxy-24(28)-dehydro ecdysone (11), and 20-hydroxyecdysone 22-glycolate (12), isolated from C. quinoa seeds, have exhibited inhibitory effects on calf skin collagenase and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity. Hence, these ecdysteroids may be considered as natural chemicals to prevent and delay both collagenase-related skin damages and oxidative stress43. Another PE derivative cyasterone along with chikusetsusaponin IV could significantly inhibit inflammatory and oxidative enzyme levels, such as thromboxane A2 (TXA2), endothelin (ET), malondialdehyde (MDA), and cyclooxygenase-2 (COX-2) as well as promote the activities of endothelial nitric oxide synthase (eNOS) and superoxide dismutase (SOD)154. PE 22-epi-ajugasterone C (62) also showed in vitro antioxidant activity by DPPH radical scanging assay68.
5.2. Anti-inflammatory effects
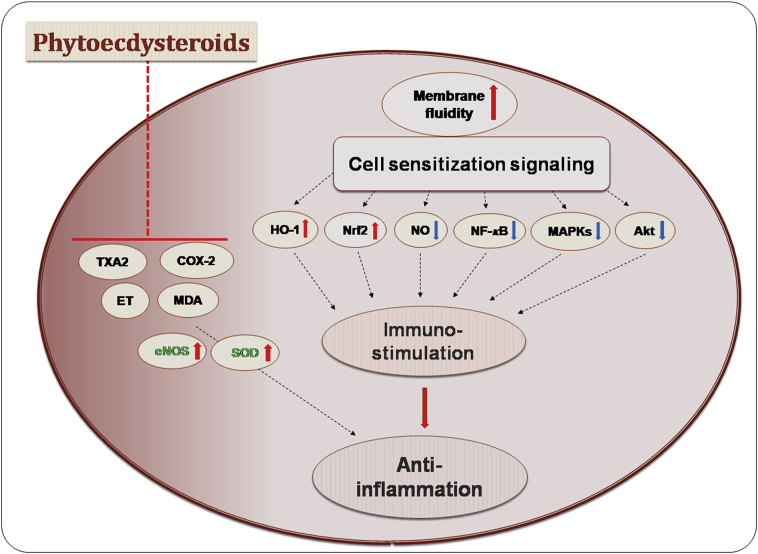
Antioxidant and antimicrobial effects are often associated with anti-inflammatory responses, and such a combination of bioactivities was also reported for several pytoecdysteroids. The methanolic extract of stem bark of V. doniana led to purification of three new PEs: (21-hydroxyshidasterone, 11β-hydroxy-20-deoxyshidasterone, and 2,3-acetonide-24-hydroxyecdysone) as well as several known PEs (ecdysteroids shidasterone, ajugasterone C, 24-hydroxyecdysone and 11β,24-hydroxyecdysone). The PEs 21-hydroxyshidasterone (172), 11β-hydroxy-20-deoxyshidasterone (173), and 2,3-acetonide-24-hydroxyecdysone (174) displayed significant inflammation-inhibitory effects on rat paw edema development induced by carrageenan in Sprague–Dawley rats123. PE niuxixinsterone D (21) was tested for inhibitory effects against lipopolyschharide (LPS)-induced nitric oxide (NO) production in RAW 264.7 macrophages. Niuxixinsterone D and serfurosterone A showed anti-neuroinflammatory activity by inhibiting NO production by 29.7% and 26.0%, respectively47. Another PE 3α,14α,22R,25-tetrahydroxy-5β(H)-cholest-7-en-6-one (126), isolated from A. gypsophiloides, was shown to possess anti-inflammatory and analgesic activities100. A recent report also showed various biological activities of PEs which were isolated from Silenegenus. α-Ecdysone, in particular, was investigated for possible immunomodulatory and anti-inflammatory effects on LPS-treated RAW 264.7 macrophage cells and in a zebrafish model155. The compound showed potent immunostimulatory effects by enhancing membrane fluidity and lysosomal enzyme activity. α-Ecdysone simultaneously inhibited the levels of NO and suppressed the levels of pro-inflammatory mediators and cytokines. An intriguing mechanism of the action involved increased heme oxygenase-1 (HO-1) and nuclear factor erythroid 2-related factor 2 (Nrf-2) production and mitigation of nuclear factor-kappa-light-chain-enhancer of activated B (NF-κB) activity, as well as a reduction in mitogen-activated protein kinases (MAPKs) and protein kinase B (Akt) activation155. Some of the known PEs, such as paristerone, ecdysterone, and capitasterone isolated from the stems of Diploclisia glaucescens, have shown significant anti-inflammatory activity with half maximal inhibitory concentration (IC50) values ranging from 1.5 to 11.6 μmol/L, respectively; this was discovered by measuring the inhibitory ratios of β-glucuronidase release in rat polymorphonuclear leukocytes (PMNs) induced by platelet-activating factors156. These observations overall conclude that diverse PE constituents exhibit notable anti-inflammatory effects (Fig. 10).
Figure 10.
Overview of anti-inflammatory effects of phytoecdysteroids. Phytoecdysteroids tend to interfere with the membrane fluidity which leads to cell sensitization and modulate various cellular signaling processes in immunomodulation and inflammation. This especially involves the inhibition of NF-κB, COX-2, MDA, MAPKs and Akt, and activation of HO-1, Nrf2, eNOS and SOD. Akt, protein kinase B; COX-2, cyclooxygenase-2; eNOS, endothelial nitric oxide synthase; ET, endothelin; HO-1, hemaoxygenase-1; MAPKs, mitogen-activated protein kinases; MDA, malondialdehyde; NF-κB, nuclear factor-κB; NO, nitric oxide; Nrf2, nuclear factor erythroid 2-related factor 2; SOD, superoxide dismutase; TXA2, thromboxane A2.
5.3. Tissue differentiation activities
Metabolic pathways related to anabolic activity associated with the biosynthetic processes forming molecules from smaller units. This mainly relates to the energy requirement as an endergonic process, and cell and tissue repair and regeneration. PEs have shown anabolic activity in several in vitro and in vivo studies with a characteristic increase in growth and skeletal muscle mass as well as an increase in fiber size through muscle-specific effects in different animals157. 20-HE has been shown to improve dermal thickness151,158 and promote wound-healing in vivo13,150. Additionally, 20-HE was also found to enhance keratinocyte differentiation153, increase protein synthesis via Akt activation159, and inhibit collagenase activity in vitro152. Furthermore, 20-HE has also been shown to reduce production of reactive oxygen species (ROS) induced by cobalt chloride and hydrogen peroxide in PC12 rat pheochromocytoma cells and B35 rat neuroblastoma cells, respectively160. Complex phytochemical mixtures can exert diverse biochemical effects on cellular signaling pathways, protein synthesis, and enzymatic activities as presented in Fig. 11. The positive interactions between molecules, termed potentiation, can lead to additive or synergistic effects that enhance the individual bioactivity of a particular molecule161. PEs also possess a promising future in skin care due to their perception as being a safe material, their abundance, and their sustainability162. The three main significant biochemical targets in skin care include matrix metalloproteinases, the endopeptidases responsible for collagen breakdown, and other targets, including tyrosinase. Amongst these targets, tyrosinase is the key enzyme involved in skin pigmentation and ROS-mediated oxidative stress, exacerbating the aging process. Additionally, it has been discovered that PEs present in cosmetic preparation protect skin from pigmentation and aging163. The probable mechanisms of such dermal effects need elucidation.
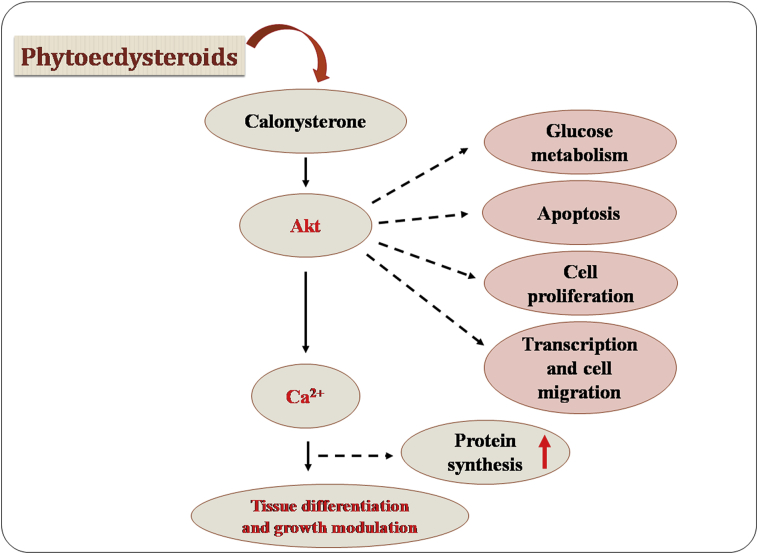
Figure 11.
Tissue differentiation and growth modulatory activities of phytoecdysteroids. Calonysterone specifically alteres tissue differentiation and growth modulation, and affects cell proliferation and survival signalling mechanisms. Akt, protein kinase B.
5.4. Metabolism-modulatory effects
PEs exert biomedical effects in different clinico-pathophysiological conditions through a variety of mechanisms. Recently, PE derivatives have shown their non-hormonal anabolic and adaptogenic effects in mammals, including humans13, through a probable mechanism involving the activation of protein kinase B (also known as Akt)105. Calonysterone (132) has exerted a stronger effect on the Akt phosphorylation in mammalian skeletal muscle cells than the more abundant 20-HE105. Akt is a serine/threonine-kinase which plays multiple key roles in cellular signaling and metabolic processes, such as glucose metabolism, apoptosis, cell proliferation, transcription, and cell migration. Akt activation followed by elevation in intracellular calcium levels led to an increased protein synthesis in a mouse skeletal muscle mytotube cell line C2C12164. Although the mechanism behind PE-mediated activation of Akt is not clear, its downstream effectors represent a major signaling pathway that controls protein turnover and may regulate skeletal muscle activity, which also has been reported for maintaining the aging of muscles163. The metabolism-modulatory and tissue differentiation activities of calonysterone indicate the interconnecting mechanisms of tissue differentiation and growth modulation (Fig. 11).
5.5. Antidiabetic potential
The anabolic modulatory activities of PEs suggest their potential use in diabetic conditions for their blood glucose-lowering properties through the direct stimulation of pancreatic β-cells165. PEs extracted from the plant Ajuga iva (Lamiaceae) have exhibited in vivo potential therapeutic effects against experimental diabetes induced by alloxan166. The extracted PE caused reduction in blood glucose levels in the diabetic rats; it also reduced the levels of blood urea nitrogen, creatinine, triglycerides, cholesterol, and lipid peroxidation. These effects were mediated by increasing the levels of antioxidant enzymes, such as catalase, SOD, and glutathione peroxidase activity. Regeneration of islets and reduction of the acinar cells atrophy was also reported166. Another study reported that the administration of PEs in the form of phytoestrogen inhibited glucogenic and lipogenic enzymes and were associated with the regulation of the antioxidant enzymes activity, especially catalase, glutathione peroxidase, and SOD. The decreases of these enzyme levels and activity reductions are known to be essential for the reduced secretion of pancreatic insulin167. These reports indicate that PEs may be used for reducing the ROS levels and thus oxidative stress and glucose oxidation, which is a common characteristic of diabetes.
Diabetes is further associated with reduced wound-healing and slow regeneration as a sequential mechanism associated with tissue growth factor. A PE-rich plant, Stachys hissarica, has exhibited in vivo wound-healing activity in rats. The wound-healing activity of this plant is more effective than the known drug methyluracil (2,4-dioxo-6-methyl-1,2,3,4-tetrahydropyrimidine), especially in the case of alloxan-induced diabetic animals168. These reports are indicative of antidiabetic as well as wound-healing properties of PEs and project their possible applications in pathophysiological conditions of oxidative stress.
5.6. Antimicrobial activities
Antioxidant effects were further reported in association with antimicrobial responses of PEs, such as 22-epi-ajugasterone C (62), which has shown DPPH radical scanging activity followed by antimicrobial properties. It showed a weak scavenging effect as compared with myricetin and exhibited antimicrobial properties against multi-resistant strains, such as Staphylococcus aureus, Escherichia coli, Serratia sp., Klebsiella pneumoniae, and Candida albicans68. (22R,24R,25S,26S)-2β,3β,14α,20R-Tetrahydroxy-26α-methoxy-6-oxo-stigmast-7-ene-22,26-lactone (149), (22R,24R,25S)-2β,3β,14α,20R,26S-pentahydroxy-6-oxo-stigmast-7-ene-22,26-lactone (150), and (22R,25S)-2β,3β,14α,20R,24S-pentahydroxy-6,26-dioxo-stigmast-7-ene-22,26-lactone (151) also exhibited moderate antibacterial activities against tested oral pathogens114.
5.7. Anticancer activities
Some of the bioactivities of PEs are related to cell proliferation and growth. A known PE Z-24(28)-dehydroamarasterone B, isolated from the seeds of L. carthamoides, has show potent growth-inhibitory activity against Drosophila melanogaster B11 cells with median effective dose (ED50) of 0.52 μmol/L169. Ajugacetalsterone E and four other neoclerodane diterpenoids from A. decumbens inhibited cell proliferation of the MCF-7, GepG2, A549, and B16 cell lines as an anticancer prospect119. Two PEs extracted from Ajuga forrestii and evaluated for their cytotoxic effects against the A-549 human lung cancer cell, HepG2 human hepatoma cell, MCF-7 human breast cancer cell, and HeLa human cervical carcinoma cell showed no significant inhibitory activities. However, they might interact when used in combination with other extracted PEs. An aqueous extract of A. forrestii containing iridoid compounds exhibited potential cytotoxicity with IC50 values ranging between 10 and 19 μmol/L, while selected iridoid glycosides exhibited more significant cytotoxicities with IC50 values ranging between 7 and 14 μmol/L170. A lipophilic Leuzea root extract containing the major PE 20-HE demonstrated potent cytotoxic effects on MCF-7 cells. The extract exhibitied cell proliferation with IC50 30 μg/mL, yet 20-HE alone was not as effective171. Genome-wide expression analysis for differential transcriptional expression of 241 genes showed that Leuzea extract treatment was comparatively more effective that treatment with tamoxifen, a commonly used medication for preventing breast cancer in women171. The cytotoxic potentials of n-butanolic fraction of Ajuga chamaecistus, which mainly contains melilotoside, phenylethyl glycosides, and PEs, were assessed against human cancer cell lines (T47D, HT-29, and Caco-2) and the normal cell line (NIH 3T3) showed no cytotoxicity even up to 400 μg/mL concentration of the extract172. Additionally, a previous study using three major compounds (20-HE, cyasterone, and 8-acetylharpagide) from the diethyl ether fraction of A. chamaecistus showed inactivity in the cytotoxicity evaluation172.
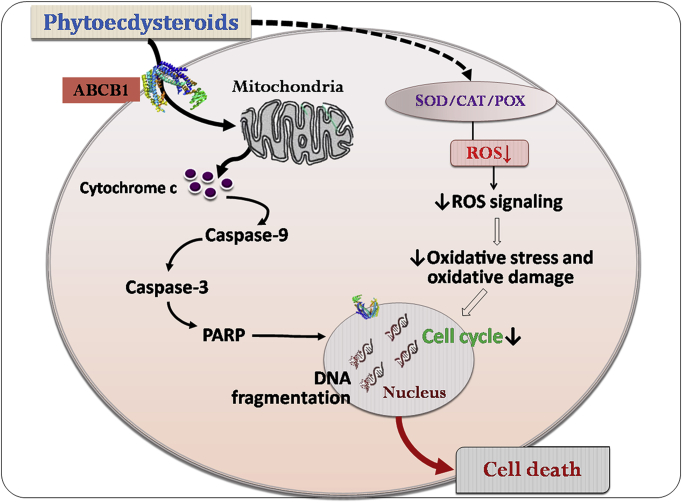
The ABCB1 transporter is a type of efflux pump that plays a key role in the chemo-resistance of various tumors and particularly of cancer stem cells. Fifty-eight ecdysteroids were tested for their activity against L5178 mouse T-cell lymphoma cells and their subcell line transfected with pHa MDR1/A retrovirus overexpressing the human ABCB1 efflux pump173. The tested PEs showed slight inhibition of cell proliferation, but notable modulation of the efflux of rhodamine 123 mediated by the ABCB1 transporter173. Screened PEs showed mild to strong synergism or antagonism when tested in combination with doxorubicin, which suggests that ecdysteroids could be potentially used for enhancing the specific structure‒activity relationships for cancer chemotherapeutics. Another study analysed the cytotoxic effects of a preparation of three fluorinated derivatives of PEs on the ABCB1 efflux transporter expressing four human breast cancer cell lines (MCF-7, MDA-MB-231, MDA-MB-361 and T47D), a neuroblastoma cell line (SH-SY5Y), and a mouse lymphoma cell line (L5178) by MTT assay174. Fluorinated PE derivatives increased the ABCB1 inhibitory effect and caused inhibition of cell proliferation. Also, the derivative compound 5 (14,25-difluoro analog) exerted higher chemo-sensitizing activity to doxorubicin as compared to its parental compound174. Seeing the importance of ABCB1 efflux pump transporters in cancer chemo-resistance and stem cell properties, PE derivatives could be potentially used in sensitizing ABCB1 over-expressing cancer cells for enhanced chemotherapeutic potentials.
An interesting study tested the combinatorial effects of 20-HE with half doses of cisplatin and adriamycin combination on the development of subcutaneously and intraperitoneally transplanted P388 and L1210 leukemia and metastasizing B16 melanoma175. 20-HE significantly stimulated the chemotherapeutic effects at low doses via a mechanism involving of cytostatic effects, resulting in tumor growth and an increase in the survival rate and lifespans of mice. Also, the 20-HE showed a comparably improved antimetastatic activity index at high doses of the antitumor drugs in mice. Mechanistically, it was demonstrated that the low doses of cisplatin and ecdysterone affected the biochemical dynamics of cells as well as altering the protein and DNA biosynthesis in the liver, pancreas, thymus, spleen, and adrenals of tumor-bearing mice. The combination of cisplatin with higher doses of 20-HE could affect the metastatic response175. These observations lead to the hypothesis that these doses of PEs can influence the therapeutic index, which would warrant considerable attention in the processes of drug development and disease management. The plausible interconnecting pathways involving oxidative stress and free radicals, apoptosis, and cell cycle regulation are depicted in Fig. 12. These observations additionally suggest that PEs can influence the therapeutic index of chemotherapeutic agents by modulating cell proliferation mechanism and inducing apoptosis, which fetches considerable attention in the process of drug development and disease management.
Figure 12.
Proposed mechanism of the anticancer effects of phytoecdysteroids through induction of cell death. Phytoecdysteroids interact through the ABCB1 transmembrane protein and activates mitochondrial apoptosis pathway as well as it suppresses oxidative stress and causes cell cycle arrest. These collectively lead to inhibition of cell proliferation and cell death. ABCB1, transmembrane efflux transporter; CAT, catalase; PARP, poly(ADP-ribose) polymerase; POX, peroxidase; ROS, reactive oxygen species; SOD, superoxide dismutase.
6. Molecular mechanisms of action of phytoecdysteroids
The first characterized ecdysteroid, ecdysone, was isolated from the silkworm Bombyx mori2. In Spinacia oleracea L. (spinach), the major ecdysteroid is ecdysterone, which can occur in concentrations between 50 and 800 μg per g fresh weight tissue, depending on growth rate of the plant176. Numerous studies have described a variety of pharmacological effects of ecdysteroids, such as an increase in carbohydrate and fatty acid metabolism, stimulation of immune response, and an increase in protein synthesis and general health-promoting activity177.
The bioactivity of PE and molecular mechanisms are not well characterized. In arthropods, the effects of PEs are mediated through nuclear receptor complexes consisting of two proteins, which are ecdy receptor and ultraspiracle protein. In mammals, PEs execute their effects through human steroid hormone receptors as the nuclear receptor complex is not expressed in mammals. Besides that, no significant binding affinity of PEs to the androgen receptors (ARs) has been observed13,157. Alternatively, it was hypothesized that PEs interact with receptors located in the cell membrane of mammalian cells. The antioxidant effects of 20-HE on rat liver mitochondrial fractions were analyzed, and showed that it intervenes with the liposomal membrane oxidation178. The rate of free-radical formation was reduced by 20-HE in cholesterol-dependent manner, while α-tocopherol showed lower antioxidant effect in the membrane. 20-HE showed potent antioxidant action in combination with α-tocopherol in membranes that contain excess cholesterol, which indicates the free radical scavenging mechanism178. The treatment of mouse skeletal muscle cell line C2C12 (murine myotubes and human primary myotubes) with PE demonstrated an increase in protein synthesis activity by up to 20%157,164. Furthermore, it strengthened the griping capacity in rats, which is a health promoting activity. These effects were observed to be mediated via inhibition of the phosphoinositide kinase-3 (PI3K) in skeletal muscle cells; the PI3K pathway activates Akt which was shown to increase protein synthesis in skeletal muscles C2C12 myotubes when treated with 20-HE164.
Growing evidence suggests that besides androgens and insulin-like growth factor 1, estrogens are also involved in the regulation of skeletal muscle homeostasis. Estrogen receptors (ERs) are ligand-activated transcription factors belonging to the family of nuclear receptors179. With respect to molecular mechanisms mediating the anabolic effects of PEs, it has been demonstrated that selective activation of ERs strongly induces regeneration and most likely induces de novo fibre formation in toxin-damaged muscles of female rats180. These observations indicate a major role of ERs in skeletal muscle homeostasis in both genders. Studies on animal models also have a focus of identifying the potential role of PEs on ERs.
In addition to classical nuclear receptor responses, mammalian steroid hormones that are structurally related to PEs have been known to elicit rapid non-genomic signaling events that mediate cell proliferation and survival. These responses may involve alterations in secondary signals, such as Ca2+ or cyclic adenosine monophosphate181. Mammalian steroid hormones increase Ca2+ influx in a variety of cell types, including cardiac myocytes and skeletal muscle182,183. Several of these effects have been attributed to a new role for already identified nuclear receptors, such as the ERs and ARs13. Thus, PEs may be considered as putative target molecules of ER and AR for their respective signaling processes of anabolic function, protective effects, and anti-inflammatory effects.
The anticancer effects of a lipophilic Leuzea root extract containing 20-HE was found to be medated via gene regulation in an antiproliferative manner through downregulation of cyclin E2 (CCNE2), forkhead box M1 (FOXM1), G-2 and S-phase expressed 1 (GTSE1), proliferating cell nuclear antigen (PCNA), induced expression of cyclin G2 (CCNG2), growth arrest and DNA-damage-inducible, alpha (GADD45α) and tumor protein p53 inducible nuclear protein 1 (TP53INP1) pointed to cell cycle arrest at the G1/S-transition checkpoint in human bread adenocarcinoma cells MCF-7171. In addition, approximately thirty genes associated with DNA replication and synthesis appeared to be downregulated by the extract, which indicated that reduced replication rate and cell cycle arrest at the G1/S-transition checkpoint is more specific than the molecule mechanism in MCF-7 cells171. The comprehensive miscellaneous biological effects of PEs corroborate with the intereconnective mechanisms of tissue differentiation and the growth modulation, apoptosis, cell proliferation, and modulation of glycolytic metabolism (Fig. 11). These intercorrelated mechanisms affect the acute and chronic pathophysiological conditions of oxidative stress and inflammation, especially diabetes and cancer.
7. Conclusions and future directions
In this review, we have summarized the isolation and characteristics of 212 new PEs from different plant species, reported between 1999 and 2019. The diverse bioactivities that were reported for these PEs include antioxidant, anti-inflammatory, antimicrobial, analgesic, anabolic, adaptogenic, hepatoprotective, antidiabetic, and anticancer properties (Fig. 13). In addition to this, we have discussed various mechanisms by which several PEs exert their biological actions. However, despite the copious amount of PE research that has been performed over the past two decades, most of the results cited in the current review are based on in vitro studies; data associated with in vivo and clinical studies are very limited. The versatile and potent pharmacological properties of this group of phytochemicals allow them to be of considerable interest, especially for their antioxidant, anti-inflammatory, and growth-regulatory activities. This further adds to their scientific characterization and specific bioactivity. The function of ABCB1 efflux pump transporters was limited by the usage of PE derivatives, which then leads to the inhibition of the proliferation of cancer cells. As ABCB1 are essentially involved in cancer chemo-resistance and the stemness of cancer cells, PE derivatives appear to be potential target compounds in the chemotherapeutic drug development process. Also, PEs derivatives have the potential to enhance the therapeutic efficacy of chemotherapeutics agents, especially tamoxifen, cisplatin and doxorubicine, in a synergistic or agonistic manner in several types of cancer cells. PE calonysterone appears to modulate Akt signaling, which further correlates with various essential cell growth signaling processes, such as glycolytic metabolism, cell proliferation, apoptosis, cell migration, and tissue differentiation. The antioxidant, anti-inflammatory, and growth-modulatory properties of PEs represent a considerable benefit of using them in therapy and in the prevention of human diseases. As the two conditions are essentially associated with carcinogenesis, PEs can also be utilized to manage not only oxidative stress and inflammation, but also to limit the carcinogenic process through their usage. This review discusses the promising biological and pharmacological properties of various PE-rich plant extracts, which may be utilized in the development of nutraceutical and pharmaceutical products after further confirmatory research on their efficacy and safety.
Figure 13.
Summary of various biological and pharmacological effects of ecdysteroids.
Acknowledgments
Niranjan Das wishes to thank Dr. Dipannita Chakraborty, former Principal of Netaji Subhas Mahavidyalaya, Udaipur, Tripura, India, for her support, cooperation, and encouragement during the preparation of the manuscript.
Author contributions
Niranjan Das conceived the study and prepared the manuscript draft. Siddhartha Kumar Mishra and Eunüs S. Ali analysed the contents and assisted in the finalization of the manuscript draft. Anusha Bishayee improved the language of the manuscript. Anupam Bishayee supervised the entire project and edited the manuscript with suggestions for improvements. All the authors made conceptual contributions to the content and have read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2020.10.012.
Contributor Information
Niranjan Das, Email: ndnsmu@gmail.com.
Anupam Bishayee, Email: abishayee@lecom.edu, abishayee@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Nakanishi K. The ecdysones. Pure Appl Chem. 1971;25:167–195. doi: 10.1351/pac197125010167. [DOI] [PubMed] [Google Scholar]
- 2.Butenandt A., Karlson P. About the isolation of a metamorphosis hormone of insects in crystallized form. Z Naturforsch. 1954;9B:389. [Google Scholar]
- 3.Huber R., Hoppe W. On the chemistry of ecdysone. VII. Analysis of the crystal and molecular structure of the molting hormone in insects, ecdysone, using the automized folding molecule method. Chem Ber. 1965;98:2403–2424. doi: 10.1002/cber.19650980744. [DOI] [PubMed] [Google Scholar]
- 4.Dinan L., Lafont R. Effects and applications of arthropod steroid hormones (ecdysteroids) in mammals. J Endocrinol. 2006;191:1–8. doi: 10.1677/joe.1.06900. [DOI] [PubMed] [Google Scholar]
- 5.Bathori M., Pongracz Z. Phytoecdysteroids—from isolation to their effects on humans. Curr Med Chem. 2005;12:153–172. doi: 10.2174/0929867053363450. [DOI] [PubMed] [Google Scholar]
- 6.Dinan L. Phytoecdysteroids: biological aspects. Phytochemistry. 2001;57:325–339. doi: 10.1016/s0031-9422(01)00078-4. [DOI] [PubMed] [Google Scholar]
- 7.Ma W.C. Some properties of gustation in the larva of Pieris brassicae. Entomol Exp Appl. 1969;12:584–590. [Google Scholar]
- 8.Robbins W.E., Kaplanis J.N., Thompson M.J., Shortino T.J., Joyner S.C. Ecdysones and synthetic analogs: molting hormone activity and inhibitive effects on insect growth, metamorphosis and reproduction. Steroids. 1970;16:105–125. doi: 10.1016/s0039-128x(70)80100-3. [DOI] [PubMed] [Google Scholar]
- 9.Kubo I., Klocke J.A., Asano S. Effects of ingested phytoecdysteroids on the growth and development of two Lepidopterous larvae. J Insect Physiol. 1983;29:307–316. [Google Scholar]
- 10.Jepson I., Martinez A., Sweetman J.P. Chemical-inducible gene expression systems for plants—a review. Pestic Sci. 1998;54:360–367. [Google Scholar]
- 11.Martinez A., Sparks C., Drayton P., Thompson J., Greenland A., Jepson I. Creation of ecdysone receptor chimeras in plants for controlled regulation of gene expression. Mol Gen Genet. 1999;261:546–552. doi: 10.1007/s004380050999. [DOI] [PubMed] [Google Scholar]
- 12.Bajguz A., Koronka A. Effect of ecdysone application on the growth and biochemical changes in Chlorella vulgaris cells. Plant Physiol Biochem. 2001;39:707–715. [Google Scholar]
- 13.Bathori M., Toth N., Hunyadi A., Marki A., Zador E. Phytoecdysteroids and anabolic-androgenic steroids—structure and effects on humans. Curr Med Chem. 2008;15:75–91. doi: 10.2174/092986708783330674. [DOI] [PubMed] [Google Scholar]
- 14.Khaziev D., Galina C., Gadiev R., Valitov F., Gumarova G., Galyautdinov I. Phytoecdisteroids from Serratula coronata when growing ducklings. Res Vet Sci. 2020;128:170–176. doi: 10.1016/j.rvsc.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Syrov V.N. Mechanism of the anabolic action of phytoecdisteroids in mammals. Nauchnye Doki Vyss Shkoly Biol Nauki. 1984;11:16–20. [PubMed] [Google Scholar]
- 16.YuR M., Syrov V. Effect of phytoecdysteroids on the sexual activity of male rats. Dokl Akad Nauk Resp Uzb. 1992:347–349. 1992. [Google Scholar]
- 17.Chiang H.C., Wang J.J., Wu R.T. Immunomodulating effects of the hydrolysis products of formosanin C and beta-ecdysone from Paris formosana Hayata. Anticancer Res. 1992;12:1475–1478. [PubMed] [Google Scholar]
- 18.Mirzaev Iu R., Syrov V.N., Khrushev S.A., Iskanderova S.D. Effect of ecdystene on parameters of the sexual function under experimental and clinical conditions. Eksp Klin Farmakol. 2000;63:35–37. [PubMed] [Google Scholar]
- 19.Akinwumi I.A., Sonibare M.A., Yeye E.O., Khan M. Bioassay-guided isolation and identification of anti-ulcer ecdysteroids from the seeds of Sphenocentrum jollyanum Pierre (Menispermaceae) Steroids. 2020;159:108636. doi: 10.1016/j.steroids.2020.108636. [DOI] [PubMed] [Google Scholar]
- 20.Lafont R., Dinan L. Practical uses for ecdysteroids in mammals including humans: an update. J Insect Sci. 2003;3:7. doi: 10.1093/jis/3.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarkowska D., Strnad M. Plant ecdysteroids: plant sterols with intriguing distributions, biological effects and relations to plant hormones. Planta. 2016;244:545–555. doi: 10.1007/s00425-016-2561-z. [DOI] [PubMed] [Google Scholar]
- 22.Lapenna S., Gemen R., Wollgast J., Worth A., Maragkoudakis P., Caldeira S. Assessing herbal products with health claims. Crit Rev Food Sci Nutr. 2015;55:1918–1928. doi: 10.1080/10408398.2012.726661. [DOI] [PubMed] [Google Scholar]
- 23.Cahlikova L., Macakova K., Chlebek J., Host'alkova A., Kulhankova A., Opletal L. Ecdysterone and its activity on some degenerative diseases. Nat Prod Commun. 2011;6:707–718. [PubMed] [Google Scholar]
- 24.Baltaev U.A. Phytoecdysteroids: structure, sources and biosynthetic pathways in plants. Bioorg Khim. 2000;26:892–925. doi: 10.1023/a:1026662505403. [DOI] [PubMed] [Google Scholar]
- 25.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis-John P.A. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert L.I., Rybczynski R., Warren J.T. Control and biochemical nature of the ecdysteroidogenic pathway. Annu Rev Entomol. 2002;47:883–916. doi: 10.1146/annurev.ento.47.091201.145302. [DOI] [PubMed] [Google Scholar]
- 27.Grebenok R.J., Venkatachari S., Adler J.H. Biosynthesis of ecdysone and ecdysone phosphates in spinach. Phytochemistry. 1994;36:1399–1408. [Google Scholar]
- 28.Nakanishi K., Koreeda M., Sasaki S., Chang M.L., Hsu H.Y. Insect hormones. The structure of ponasterone A, insect-moulting hormone from the leaves of Podocarpus nakaii Hay. Chem Commun. 1966;24:915–917. (London) [Google Scholar]
- 29.Nakanishi K., Koreeda M., Chang M.L., Hsu H.Y. Insect hormones V. The structures of ponasterones B and C. Tetrahedron Lett. 1968;9:1105–1110. [Google Scholar]
- 30.Galbraith M.N., Horn D.H.S. An insect-moulting hormone from a plant. Chem Commun (London) 1966;24:905–906. [Google Scholar]
- 31.Ghosh D., Laddha K.S. Extraction and monitoring of phytoecdysteroids through HPLC. J Chromatogr Sci. 2006;44:22–26. doi: 10.1093/chromsci/44.1.22. [DOI] [PubMed] [Google Scholar]
- 32.Thiem B., Kikowska M., Malinski M.P., Kruszka D., Napierala M., Florek E. Ecdysteroids: production in plant in vitro cultures. Phytochem Rev. 2017;16:603–622. doi: 10.1007/s11101-016-9483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minli Z., Stout M.J., Kubo I. Isolation of ecdysteroids from Vitex strickeri using RLCC and recycling HPLC. Phytochemistry. 1992;31:247–250. [Google Scholar]
- 34.Lafont R., Morgan E.D., Wilson I.D. Chromatographic procedures for phytoecdysteroids. J Chromatogr A. 1994;658:31–53. [Google Scholar]
- 35.Ikekawa N., Ikeda T., Mizuno T., Ohnishi E., Sakurai S. Isolation of a new ecdysteroid, 2,22-dideoxy-20-hydroxyecdysone, from the ovaries of the silkworm Bombyx mori. J Chem Soc, Chem Commun. 1980;10:448–449. [Google Scholar]
- 36.Hikino H., Okuyama T., Konno C., Takemoto T. Carbon-13 nuclear magnetic resonance spectra of phytoecdysones. Chem Pharm Bull. 1975;23:125–132. [Google Scholar]
- 37.Chaubey M.K. Role of phytoecdysteroids in insect pest management: a review. J Agron. 2018;17:1. [Google Scholar]
- 38.Grebenok R.J., Adler J.H. Ecdysteroid biosynthesis during the ontogeny of spinach leaves. Phytochemistry. 1993;33:341–347. [Google Scholar]
- 39.Cook I.F., Lloyd-Jones J.G., Rees H.H., Goodwin T.W. The stereochemistry of hydrogen elimination from C-7 during biosynthesis of ecdysones in insects and plants. Biochem J. 1973;136:135–145. doi: 10.1042/bj1360135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyodo R., Fujimoto Y. Biosynthesis of 20-hydroxyecdysone in Ajuga hairy roots: the possibility of 7-ene introduction at a late stage. Phytochemistry. 2000;53:733–737. doi: 10.1016/s0031-9422(00)00018-2. [DOI] [PubMed] [Google Scholar]
- 41.Wang P., Li S., Ownby S., Zhang Z., Yuan W., Zhang W. Ecdysteroids and a sucrose phenylpropanoid ester from Froelichia floridana. Phytochemistry. 2009;70:430–436. doi: 10.1016/j.phytochem.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 42.Dini I., Tenore G.C., Dini A. Nutritional and antinutritional composition of Kancolla seeds: an interesting and underexploited andine food plant. Food Chem. 2005;92:125–132. [Google Scholar]
- 43.Nsimba R.Y., Kikuzaki H., Konishi Y. Ecdysteroids act as inhibitors of calf skin collagenase and oxidative stress. J Biochem Mol Toxicol. 2008;4:240–250. doi: 10.1002/jbt.20234. [DOI] [PubMed] [Google Scholar]
- 44.DellaGreca M., D'Abrosca B., Fiorentino A., Previtera L., Zarrelli A. Structure elucidation and phytotoxicity of ecdysteroids from Chenopodium album. Chem Biodivers. 2005;2:457–462. doi: 10.1002/cbdv.200590025. [DOI] [PubMed] [Google Scholar]
- 45.Kumpun S., Maria A., Crouzet S., Evrard-Todeschi N., Girault J.P., Lafont R. Ecdysteroids from Chenopodium quinoa Willd., an ancient Andean crop of high nutritional value. Food Chem. 2011;125:1226–1234. [Google Scholar]
- 46.Wang Q.H., Yang L., Jiang H., Wang Z.B., Yang B.Y., Kuang H.X. Three new phytoecdysteroids containing a furan ring from the roots of Achyranthes bidentata Bl. Molecules. 2011;16:5989–5997. doi: 10.3390/molecules16075989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang L., Jiang H., Yan M.L., Xing X.D., Zhang Y.Y., Wei N. A new phytoecdysteroid from the roots of Achyranthes bidentata Bl. Nat Prod Res. 2017;31:1073–1079. doi: 10.1080/14786419.2016.1272114. [DOI] [PubMed] [Google Scholar]
- 48.Zhang M., Zhou Z.Y., Wang J., Cao Y., Chen X.X., Zhang W.M. Phytoecdysteroids from the roots of Achyranthes bidentata Blume. Molecules. 2012;17:3324–3332. doi: 10.3390/molecules17033324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meng D.L., Li X., Wang J.H., Li W. A new phytosterone from Achyranthes bidentata B1. J Asian Nat Prod Res. 2005;7:181–184. doi: 10.1080/102860203100001625094. [DOI] [PubMed] [Google Scholar]
- 50.Li X., Zhao W., Meng D., Qiao A. A new phytosterone from the roots of Achyranthes bidentata. Fitoterapia. 2007;78:607–608. doi: 10.1016/j.fitote.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Saleem M., Musaddiq S., Riaz N. Ecdysteroids from the flowers of Aerva javanica. Steroids. 2013;78:1098–1102. doi: 10.1016/j.steroids.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 52.Zhou R., Li B.G., Zhang G.L. Chemical study on Cyathula officinalis Kuan. J Asian Nat Prod Res. 2005;7:245–252. doi: 10.1080/10286020410001721159. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura S., Chen G., Nakashima S., Matsuda H., Pei Y., Yoshikawa M. Brazilian natural medicines. IV. New noroleanane-type triterpene and ecdysterone-type sterol glycosides and melanogenesis inhibitors from the roots of Pfaffia glomerata. Chem Pharm Bull (Tokyo) 2010;58:690–695. doi: 10.1248/cpb.58.690. [DOI] [PubMed] [Google Scholar]
- 54.Dinan L., Sarker S.D., Bourne P., Whiting P., Sik V., Rees H.H. Phytoecdysteroids in seeds and plants of Rhagodia baccata (Labill.) Moq. (Chenopodiaceae) Arch Insect Biochem Physiol. 1999;41:18–23. doi: 10.1002/(SICI)1520-6327(1999)41:1<18::AID-ARCH4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 55.Ben Nejma A., Nguir A., Ben Jannet H., Hamza M.A., Daïch A., Othman M. New septanoside and 20-hydroxyecdysone septanoside derivative from Atriplex portulacoides roots with preliminary biological activities. Bioorg Med Chem Lett. 2015;25:1665–1670. doi: 10.1016/j.bmcl.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 56.Budesinsky M., Vokac K., Harmatha J., Cvacka J. Additional minor ecdysteroid components of Leuzea carthamoides. Steroids. 2008;73:502–514. doi: 10.1016/j.steroids.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 57.Borovikova E.B., Baltaev U.A. Lesterone, a new phytoecdysteroid from the seeds of Leuzea carthamoides. Chem Nat Compd. 1999;35:182–183. [Google Scholar]
- 58.Vokáč K., Budesinsky M., Harmatha J. Minor ecdysteroid components of Leuzea carthamoides. Collect Czechoslov Chem Commun. 2002;67:124–139. [Google Scholar]
- 59.Odinokov V.N., Galyautdinov I.V., Fatykhov A.A., Khalilov L.M. A new phytoecdysteroid. Russ Chem Bull. 2000;49:1923–1924. [Google Scholar]
- 60.Zhang Z.Y., Yang W.Q., Fan C.L., Zhao H.N., Huang X.J., Wang Y. New ecdysteroid and ecdysteroid glycosides from the roots of Serratula chinensis. J Asian Nat Prod Res. 2017;19:208–214. doi: 10.1080/10286020.2016.1209492. [DOI] [PubMed] [Google Scholar]
- 61.Ling T., Ma W.Z., Wei X.Y. Ecdysteroids from the roots of Serratula chinensis. J Trop Subtrop Bot. 2003;11:143–147. [Google Scholar]
- 62.Li X.Q., Wang J.H., Wang S.X., Li X. A new phytoecdysone from the roots of Rhaponticum uniflorum. J Asian Nat Prod Res. 2000;2:225–229. doi: 10.1080/10286020008039915. [DOI] [PubMed] [Google Scholar]
- 63.Olennikov D.N., Kashchenko N.I. New flavonoids and turkesterone-2-O-cinnamate from leaves of Rhaponticum uniflorum. Chem Nat Compd. 2019;55:256–264. [Google Scholar]
- 64.Olennikov D.N. Makisterone C-20,22-acetonide from Rhaponticum uniflorum. Chem Nat Compd. 2018;54:930–933. [Google Scholar]
- 65.Zhang Y.H., Wang H.Q. Ecdysteroids from Rhaponticum uniflorum. Pharmazie. 2001;56:828–829. [PubMed] [Google Scholar]
- 66.Cheng J., Huang M., Zhang Z., Cheng D., Zhang G. A new ecdysterone from Rhaponticum uniflorum. Acta Bot Boreali Occident Sin. 2002;22:1457–1459. [Google Scholar]
- 67.Borovikova E.B., Shangaraeva G.S., Baltaev U.A. Rhapisterone D 20-acetate from the seeds of Leuzea carthamoides. Chem Nat Compd. 1999;35:184–185. [Google Scholar]
- 68.Lamia A., Habiba L., Amel A., Francisco L., Ignacio B., Samir B. Isolation, antioxidant and antimicrobial activities of ecdysteroids from Serratula cichoracea. Curr Bioact Compd. 2018;14:60–66. [Google Scholar]
- 69.Galyautdinov I.V., Sadretdinova Z.R., Muslimov Z.S., Gareev V.F., Khalilov L.M., Odinokov V.N. New minor phytoecdysteroids from the juice of Serratula coronata L. (Asteraceae) J Med Plants Study. 2016;4:30–34. [Google Scholar]
- 70.Odinokov V.N., Galyautdinov I.V., Nedopekin D.V., Khalilov L.M., Shashkov A.S., Kachala V.V. Phytoecdysteroids from the juice of Serratula coronata L. (Asteraceae) Insect Biochem Mol Biol. 2002;32:161–165. doi: 10.1016/s0965-1748(01)00106-0. [DOI] [PubMed] [Google Scholar]
- 71.Odinokov V.N., Kumpun S., Galyautdinov I.V., Evrard-Todeschi N., Veskina N.A., Khalilov L.M. Low-polarity phytoecdysteroids from the juice of Serratula coronata L. (Asteraceae) Collect Czech Chem Commun. 2005;70:2038–2052. [Google Scholar]
- 72.Ling T., Zhang Z., Xia T., Ling W., Wan X. Phytoecdysteroids and other constituents from the roots of Klaseopsis chinensis. Biochem Syst Ecol. 2009;37:49–51. [Google Scholar]
- 73.Hunyadi A., Toth G., Simon A., Mák M., Kele Z., Máthé I. Two new ecdysteroids from Serratula wolffii. J Nat Prod. 2004;67:1070–1072. doi: 10.1021/np030544p. [DOI] [PubMed] [Google Scholar]
- 74.Hunyadi A., Gergely A., Simon A., Tóth G., Veress G., Báthori M. Preparative-scale chromatography of ecdysteroids of Serratula wolffii Andrae. J Chromatogr Sci. 2007;45:76–86. doi: 10.1093/chromsci/45.2.76. [DOI] [PubMed] [Google Scholar]
- 75.Simon A., Tóth G., Liktor-Busa E., Kele Z., Takác M., Gergely A. Three new steroids from the roots of Serratula wolffii. Steroids. 2007;72:751–755. doi: 10.1016/j.steroids.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 76.Liktor-Busa E., Simon A., Tóth G., Fekete G., Kele Z., Báthori M. Ecdysteroids from Serratula wolffii roots. J Nat Prod. 2007;70:884–886. doi: 10.1021/np070037y. [DOI] [PubMed] [Google Scholar]
- 77.Liktor-Busa E., Simon A., Tóth G., Báthori M. The first two ecdysteroids containing a furan ring from Serratula wolffii. Tetrahedron Lett. 2008;49:1738–1740. [Google Scholar]
- 78.Liktor-Busa E., Simon A., Báthori M. Serratula wolffii, as a source of new ecdysteroids. Planta Med. 2007;73:364. [Google Scholar]
- 79.Dai J.Q., Cai Y.J., Shi Y.P., Zhang Y.H., Liu Z.L., Yang L. Antioxidant activity of ecdysteroids from Serratula strangulata. Chin J Chem. 2002;20:497–501. [Google Scholar]
- 80.Wu P., Xie H., Tao W., Miao S., Wei X. Phytoecdysteroids from the rhizomes of Brainea insignis. Phytochemistry. 2010;71:975–981. doi: 10.1016/j.phytochem.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 81.Sadikov Z.T., Saatov Z. Phytoecdysteroids of plants of the genus Silene XX. Integristerone A 25-acetate from Silene brahuica. Chem Nat Compd. 1999;35:440–441. [Google Scholar]
- 82.Jia A., Li Y., Zhou J., Gao J. Three phytoecdysteroids from Sagina japonica and potential biotransforming pathways of japonicone. Chem Nat Compd. 2010;46:738–741. [Google Scholar]
- 83.Mamadalieva N.Z., Ramazanov N.S., Dinan L.N., Saatov Z. Phytoecdysteroids of plants of the Silene genus. 2-Dehydroxyecdysterone-3-O-benzoate from Silene wallichiana. Chem Nat Compd. 2000;36:513–515. [Google Scholar]
- 84.Mamadalieva N.Z., Saatov Z., Kachala V.V., Shashkov A.S. Phytoecdysteroids of plants of the Silene genus. 2-Deoxyecdysterone-25-acetate from Silene wallichiana. Chem Nat Compd. 2002;38:179–181. [Google Scholar]
- 85.Simon A., Tóth N., Tóth G., Kele Z., Groska J., Báthori M. Ecdysteroids from Silene viridiflora. Helv Chim Acta. 2009;92:753–761. [Google Scholar]
- 86.Simon A., Liktor-Busa E., Tóth G., Kele Z., Groska J., Báthori M. Additional minor phytoecdysteroids of Serratula wolffii. Helv Chim Acta. 2008;91:1640–1645. [Google Scholar]
- 87.Takacs M., Simon A., Liktor-Busa E., Báthori M., Zsila F., Bikádi Z. Structure and stereochemistry of novel ecdysteroids from the roots of Serratula wolffii. Magn Reson Chem. 2010;48:386–391. doi: 10.1002/mrc.2581. [DOI] [PubMed] [Google Scholar]
- 88.Ványolós A., Béni Z., Dékány M., Simon A., Báthori M. Novel ecdysteroids from Serratula wolffii. Sci World J. 2012;2012:651275. doi: 10.1100/2012/651275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zibareva L., Yeriomina V.I., Munkhjargal N., Girault J.P., Dinan L., Lafont R. The phytoecdysteroid profiles of 7 species of Silene (Caryophyllaceae) Arch Insect Biochem Physiol. 2009;72:234–248. doi: 10.1002/arch.20331. [DOI] [PubMed] [Google Scholar]
- 90.Mamadalieva N.Z., Janibekov A.A., Girault J.P., Lafont R. Two minor phytoecdysteroids of the plant Silene viridiflora. Nat Prod Commun. 2010;5:1579–1582. [PubMed] [Google Scholar]
- 91.Mamadalieva N.Z., Zibareva L.N., Evrard-Todeschi N., Girault J.P., Maria A., Ramazanov N.S. New minor ecdysteroids from Silene viridiflora. Collect Czech Chem Commun. 2004;69:1675–1680. [Google Scholar]
- 92.Toth N., Simon A., Toth G., Kele Z., Hunyadi A., Bathori M. 26-Hydroxylated ecdysteroids from Silene viridiflora. J Nat Prod. 2008;71:1461–1463. doi: 10.1021/np800139q. [DOI] [PubMed] [Google Scholar]
- 93.Sadikov Z.T., Saatov Z., Girault J.P., Lafont R., Sileneoside H. A new phytoecdysteroid from Silene brahuica. J Nat Prod. 2000;63:987–988. doi: 10.1021/np990609h. [DOI] [PubMed] [Google Scholar]
- 94.Pongracz Z., Bathori M., Toth G., Simon A., Mak M., Mathe I. 9alpha,20-Dihydroxyecdysone, a new natural ecdysteroid from Silene italica ssp. nemoralis. J Nat Prod. 2003;66:450–451. doi: 10.1021/np0205194. [DOI] [PubMed] [Google Scholar]
- 95.Simon A., Pongrácz Z., Tóth G., Mák M., Máthé I., Báthori M. A new ecdysteroid with unique 9β-OH and four other ecdysteroids from Silene italica ssp. nemoralis. Steroids. 2004;69:389–394. doi: 10.1016/j.steroids.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 96.Kılınç H., Masullo M., Bottone A., Karayıldırım T., Alankuş Ö., Piacente S. Chemical constituents of Silene montbretiana. Nat Prod Res. 2019;33:335–339. doi: 10.1080/14786419.2018.1451998. [DOI] [PubMed] [Google Scholar]
- 97.Mamadalieva N.Z., El-Readi M.Z., Janibekov A.A., Tahrani A., Wink M. Phytoecdysteroids of Silene guntensis and their in vitro cytotoxic and antioxidant activity. Zeitschrift fur Naturforschung C, J biosci. 2011;66:215–224. doi: 10.1515/znc-2011-5-604. [DOI] [PubMed] [Google Scholar]
- 98.Meng Y., Whiting P., Zibareva L., Bertho G., Girault J.P., Lafont R. Identification and quantitative analysis of the phytoecdysteroids in Silene species (Caryophyllaceae) by high-performance liquid chromatography. Novel ecdysteroids from S. pseudotites. J Chromatogr A. 2001;935:309–319. doi: 10.1016/s0021-9673(01)00893-7. [DOI] [PubMed] [Google Scholar]
- 99.Báthori M., Girault J.P., Kalasz H., Mathé I., Dinan L.N., Lafont R. Complex phytoecdysteroid cocktail of Silene otites (Caryophyllaceae) Arch Insect Biochem Physiol. 1999;41:1–8. [Google Scholar]
- 100.Tuleuov B.I., Zavarzin I.V., Shashkov A.S., Chernoburova E.I., Adekenov S.M. 3α,14α,22R,25-Tetrahydroxy-5β(H)-cholest-7-en-6-one, a phytoecdysteroid from Acanthophyllum gypsophiloides possessing anti-inflammatory and analgesic activities. Russ Chem Bull. 2018;67:663–666. [Google Scholar]
- 101.Cheng Y.X., Zhou J., Tan N.H., Ding Z.T. Phytoecdysterones from Cucubalus baccifer (Caryophyllaceae) Acta Bot Sin. 2001;43:316–318. [Google Scholar]
- 102.Bathori M., Pongracz Z., Toth G., Simon A., Kandra L., Kele Z. Isolation of a new member of the ecdysteroid glycoside family: 2-deoxy-20-hydroxyecdysone 22-O-beta-d-glucopyranoside. J Chromatogr Sci. 2002;40:409–415. doi: 10.1093/chromsci/40.7.409. [DOI] [PubMed] [Google Scholar]
- 103.Kiuchi M., Yasui H., Hayasaka S., Kamimura M. Entomogenous fungus Nomuraea rileyi inhibits host insect molting by C22-oxidizing inactivation of hemolymph ecdysteroids. Arch Insect Biochem Physiol. 2003;52:35–44. doi: 10.1002/arch.10060. [DOI] [PubMed] [Google Scholar]
- 104.Tan C.Y., Wang J.H., Li X. Phytoecdysteroid constituents from Cyanotis arachnoidea. J Asian Nat Prod Res. 2003;5:237–240. doi: 10.1080/1028602031000105830. [DOI] [PubMed] [Google Scholar]
- 105.Issaadi H.M., Tsai Y.C., Chang F.R., Hunyadi A. Centrifugal partition chromatography in the isolation of minor ecdysteroids from Cyanotis arachnoidea. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1054:44–49. doi: 10.1016/j.jchromb.2017.03.043. [DOI] [PubMed] [Google Scholar]
- 106.Tan C.Y., Wang J.H., Zhang H., Li X. A new phytosterone from Cyanotis arachnoidea. J Asian Nat Prod Res. 2002;4:7–10. doi: 10.1080/10286020290019631. [DOI] [PubMed] [Google Scholar]
- 107.Tan C.Y., Wang J.H., Xiao W., Li X. A new phytoecdysterone from Cyanotis arachnoidea. Chin Chem Lett. 2002;13:245–246. [Google Scholar]
- 108.Tan C.Y., Wang J.H., Li X., Du Y.G., Bai X.F. Chemical constituents of Cyanotis arachnoidea. Yao Xue Xue Bao. 2003;38:760–762. [PubMed] [Google Scholar]
- 109.Hunyadi A., Herke I., Lengyel K., Báthori M., Kele Z., Simon A. Ecdysteroid-containing food supplements from Cyanotis arachnoidea on the European market: evidence for spinach product counterfeiting. Sci Rep. 2016;6:37322. doi: 10.1038/srep37322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tan C., Kong L., Li X., Li W., Li N. Isolation and analysis of a new phytoecdysteroid from Cyanotis arachnoidea C. B. Clarke. Se Pu. 2011;29:937–941. [PubMed] [Google Scholar]
- 111.Crouzet S., Maria A., Dinan L., Lafont R., Girault J.P. Ecdysteroids from Cyanotis longifolia Benth. (Commelinaceae) Arch Insect Biochem Physiol. 2009;72:194–209. doi: 10.1002/arch.20329. [DOI] [PubMed] [Google Scholar]
- 112.Hang D.T., Hang N.T., Anh Hle T., Nhiem N.X., Hue C.T., Binh P.T. 1H and 13C NMR assignments of new ecdysteroids from Callisia fragrans. Magn Reson Chem. 2015;53:379–382. doi: 10.1002/mrc.4214. [DOI] [PubMed] [Google Scholar]
- 113.Sautour M., Canon F., Miyamoto T., Dongmo A., Lacaille-Dubois M.A. A new ecdysteroid and other constituents from two Dioscorea species. Biochem Syst Ecol. 2008;36:559–563. [Google Scholar]
- 114.Hu J., Shi X., Mao X., Li H., Chen J., Shi J. Ecdysteroids from the ethanol extract of Diplopterygium rufopilosum. Phytochem Lett. 2014;8:73–76. [Google Scholar]
- 115.Yi J., Luo Y., Li B., Zhang G. Phytoecdysteroids and glycoceramides from Eriophyton wallchii. Steroids. 2004;69:809–815. doi: 10.1016/j.steroids.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 116.Chan Y.Y., Wu T.S., Kuoh C.S., Damu A.G. A new phytoecdysteroid from Ajuga taiwanensis. Chem Pharm Bull (Tokyo) 2005;53:836–838. doi: 10.1248/cpb.53.836. [DOI] [PubMed] [Google Scholar]
- 117.Castro A., Coll J., Tandron Y.A., Pant A.K., Mathela C.S. Phytoecdysteroids from Ajuga macrosperma var. breviflora roots. J Nat Prod. 2008;71:1294–1296. doi: 10.1021/np800131f. [DOI] [PubMed] [Google Scholar]
- 118.Takasaki M., Tokuda H., Nishino H., Konoshima T. Cancer chemopreventive agents (antitumor-promoters) from Ajuga decumbens. J Nat Prod. 1999;62:972–975. doi: 10.1021/np990033w. [DOI] [PubMed] [Google Scholar]
- 119.Chen H., Tang B.Q., Chen L., Liang J.Y., Sun J.B. Neo-clerodane diterpenes and phytoecdysteroids from Ajuga decumbens Thunb. and evaluation of their effects on cytotoxic, superoxide anion generation and elastase release in vitro. Fitoterapia. 2018;129:7–12. doi: 10.1016/j.fitote.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 120.Coll J., Tandron Y.A., Zeng X. New phytoecdysteroids from cultured plants of Ajuga nipponensis Makino. Steroids. 2007;72:270–277. doi: 10.1016/j.steroids.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 121.Guibout L., Mamadalieva N., Balducci C., Girault J.P., Lafont R. The minor ecdysteroids from Ajuga turkestanica. Phytochem Anal. 2015;26:293–300. doi: 10.1002/pca.2563. [DOI] [PubMed] [Google Scholar]
- 122.Vanyolos A., Simon A., Toth G., Polgár L., Kele Z., Ilku A. C-29 ecdysteroids from Ajuga reptans var. reptans. J Nat Prod. 2009;72:929–932. doi: 10.1021/np800708g. [DOI] [PubMed] [Google Scholar]
- 123.Ochieng C.O., Ishola I.O., Opiyo S.A., Manguro L.A., Owuor P.O., Wong K.C. Phytoecdysteroids from the stem bark of Vitex doniana and their anti-inflammatory effects. Planta Med. 2013;79:52–59. doi: 10.1055/s-0032-1327880. [DOI] [PubMed] [Google Scholar]
- 124.Suksamrarn A., Kumpun S., Yingyongnarongkul B.E. Ecdysteroids of Vitex scabra stem bark. J Nat Prod. 2002;65:1690–1692. doi: 10.1021/np020199o. [DOI] [PubMed] [Google Scholar]
- 125.dos Santos T.C., Delle Monache F., Leitao S.G. Ecdysteroids from two Brazilian Vitex species. Fitoterapia. 2001;72:215–220. doi: 10.1016/s0367-326x(00)00304-x. [DOI] [PubMed] [Google Scholar]
- 126.Suksamrarn A., Promrangsan N., Jintasirikul A. Highly oxygenated ecdysteroids from Vitex canescens root bark. Phytochemistry. 2000;53:921–924. doi: 10.1016/s0031-9422(99)00458-6. [DOI] [PubMed] [Google Scholar]
- 127.Laosooksathit S., Preecha P., Suksamrarn A. Ecdysteroids as insect control agents : a new ecdysteroid from stem bark of Vitex canescens. J KMITNB. 2003;13:1–13. [Google Scholar]
- 128.Wu J.J., Cheng K.W., Zuo X.F., Wang M.F., Li P., Zhang L.Y. Steroidal saponins and ecdysterone from Asparagus filicinus and their cytotoxic activities. Steroids. 2010;75:734–739. doi: 10.1016/j.steroids.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 129.Meng Y., Whiting P., Sik V., Rees H.H., Dinan L. Limnantheoside C (20-hydroxyecdysone 3-O-beta-d-glucopyranosyl-[1→3]-beta-d-xylopyranoside), a phytoecdysteroid from seeds of Limnanthes alba (limnanthaceae) Zeitschrift fur Naturforschung C, J biosci. 2001;56:988–994. doi: 10.1515/znc-2001-11-1214. [DOI] [PubMed] [Google Scholar]
- 130.Zhu L., Zhang G., Chen L., Wang S., Li P., Li L. A new ecdysteroside from Lygodium japonicum (Thunb.) Sw. J Nat Med. 2009;63:215–219. doi: 10.1007/s11418-008-0310-8. [DOI] [PubMed] [Google Scholar]
- 131.Jadhav A.N., Pawar R.S., Avula B., Khan I.A. Ecdysteroid glycosides from Sida rhombifolia L. Chem Biodivers. 2007;4:2225–2230. doi: 10.1002/cbdv.200790180. [DOI] [PubMed] [Google Scholar]
- 132.Darwish F.M., Reinecke M.G. Ecdysteroids and other constituents from Sida spinosa L. Phytochemistry. 2003;62:1179–1184. doi: 10.1016/s0031-9422(03)00021-9. [DOI] [PubMed] [Google Scholar]
- 133.Das N., Saha T., Bhattacharjee S.J. A new biologically active ecdysteroid from the aerial parts of Sida glutinosa. Pharmacogn Phytochem. 2014;3:73–78. [Google Scholar]
- 134.Ajayi T.O., Srivedavyasasri R., Nyong E.E., Odeniyi M.A., Moody J.O., Ross S.A. Two new phytoecdysteroids from Sphenocentrum jollyanum Pierre root. Steroids. 2019;150:108456. doi: 10.1016/j.steroids.2019.108456. [DOI] [PubMed] [Google Scholar]
- 135.Wang X.J., Zhang Q., Peng Y.R., Li L., Qu J., Liu Y.B. Two azafluoranthene alkaloids and a phytoecdysone from the stems of Cyclea barbata. J Asian Nat Prod Res. 2019;21:217–226. doi: 10.1080/10286020.2018.1564137. [DOI] [PubMed] [Google Scholar]
- 136.Jayasinghe L., Jayasooriya C.P., Hara N., Fujimoto Y. A pyridine ring-containing ecdysteroid from Diploclisia glaucescens. Tetrahedron Lett. 2003;44:8769–8771. [Google Scholar]
- 137.Jayasinghe L., Jayasooriya C.P., Oyama K., Fujimoto Y. 3-Deoxy-1β,20-dihydroxyecdysone from the leaves of Diploclisia glaucescens. Steroids. 2002;67:555–558. doi: 10.1016/s0039-128x(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 138.Jayasinghe L., Mallika Kumarihamy B.M., Suranga Arundathie B.G., Dissanayake L., Hara N., Fujimoto Y. A new ecdysteroid, 2-deoxy-5β,20-dihydroxyecdysone from the fruits of Diploclisia glaucescens. Steroids. 2003;68:447–450. doi: 10.1016/s0039-128x(03)00046-1. [DOI] [PubMed] [Google Scholar]
- 139.Su C.R., Ueng Y.F., Dung N.X., Vijaya Bhaskar Reddy M., Wu T.S. Cytochrome P3A4 inhibitors and other constituents of Fibraurea tinctoria. J Nat Prod. 2007;70:1930–1933. doi: 10.1021/np0704248. [DOI] [PubMed] [Google Scholar]
- 140.Simon A., Vanyolos A., Beni Z., Dekany M., Toth G., Bathori M. Ecdysteroids from Polypodium vulgare L. Steroids. 2011;76:1419–1424. doi: 10.1016/j.steroids.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 141.Snogan E., Vahirua-Lechat I., Ho R., Bertho G., Girault J.P., Ortiga S. Ecdysteroids from the medicinal fern Microsorum scolopendria (Burm. f.) Phytochem Anal. 2007;18:441–450. doi: 10.1002/pca.1000. [DOI] [PubMed] [Google Scholar]
- 142.Ho R., Girault J.P., Raharivelomanana P., Lafont R. E- and Z-isomers of new phytoecdysteroid conjugates from French Polynesian Microsorum membranifolium (Polypodiaceae) fronds. Molecules. 2012;17:11598–11606. doi: 10.3390/molecules171011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ho R., Girault J.P., Cousteau P.Y., Bianchini J.P., Raharivelomanana P., Lafont R. Isolation of a new class of ecdysteroid conjugates (glucosyl-ferulates) using a combination of liquid chromatographic methods. J Chromatogr Sci. 2008;46:102–110. doi: 10.1093/chromsci/46.2.102. [DOI] [PubMed] [Google Scholar]
- 144.Yao J.N., Li Z.F., Lou H.Y., Huang L., Liang G.Y., Cao P.X. A new ecdysteroidal glycoside from Lepidogrammitis drymoglossoides (Bak.) Ching. J Carbohydr Chem. 2014;33:206–211. [Google Scholar]
- 145.Sun Y., Yasukawa K. New anti-inflammatory ergostane-type ecdysteroids from the sclerotium of Polyporus umbellatus. Bioorg Med Chem Lett. 2008;18:3417–3420. doi: 10.1016/j.bmcl.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 146.Zhou W.W., Lin W.H., Guo S.X. Two new polyporusterones isolated from the sclerotia of Polyporus umbellatus. Chem Pharm Bull (Tokyo) 2007;55:1148–1150. doi: 10.1248/cpb.55.1148. [DOI] [PubMed] [Google Scholar]
- 147.Dziwornu G.A., Caira M.R., Mare J.A. Isolation, characterization and antiproliferative activity of new metabolites from the South African endemic red algal species Laurencia alfredensis. Molecules. 2017;22:1–4. doi: 10.3390/molecules22040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shi Q.W., Dong M., Huo C.H., Su X.H., Li X., Yamada T. 7,8β-Dihydroponasterone A, a new phytoecdysteroid from the needles of the Japanese Yew, Taxus cuspidata. J Braz Chem Soc. 2007;18:1081–1084. [Google Scholar]
- 149.Fang Y., Ni Z.Y., Yamada T., Wang Y.F., Zhang M.L., Dong M. Two new phytoecdysteroids from the needles of Taxus canadensis. Z Naturforsch B Chem Sci. 2010;65:1401–1405. [Google Scholar]
- 150.Chen Q., Xia Y., Qiu Z. Effect of ecdysterone on glucose metabolism in vitro. Life Sci. 2006;78:1108–1113. doi: 10.1016/j.lfs.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 151.Dinan L. The Karlson Lecture. Phytoecdysteroids: what use are they? Arch Insect Biochem. 2009;72:126–141. doi: 10.1002/arch.20334. [DOI] [PubMed] [Google Scholar]
- 152.Hamden K., Ayadi F., Jamoussi K., Masmoudi H., Elfeki A. Therapeutic effect of phytoecdysteroids rich extract from Ajuga iva on alloxan induced diabetic rats liver, kidney and pancreas. Biofactors. 2008;33:165–175. doi: 10.1002/biof.5520330302. [DOI] [PubMed] [Google Scholar]
- 153.Kizelsztein P., Govorko D., Komarnytsky S., Evans A., Wang Z., Cefalu W.T. 20-Hydroxyecdysone decreases weight and hyperglycemia in a diet-induced obesity mice model. Am J Physiol Endocrinol Metab. 2009;296:E433–E439. doi: 10.1152/ajpendo.90772.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cao Y., Gu C., Zhao F., Tang Y., Cui X., Shi L. Therapeutic effects of Cyathula officinalis Kuan and its active fraction on acute blood stasis rat model and identification constituents by HPLC-QTOF/MS/MS. Pharmacogn mag. 2017;13:693–701. doi: 10.4103/pm.pm_560_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bhardwaj M., Mamadalieva N.Z., Chauhan A.K., Kang S.C. α-Ecdysone suppresses inflammatory responses via the Nrf2 pathway in lipopolysaccharide-stimulated RAW 264.7 cells. Int Immunopharmacol. 2019;73:405–413. doi: 10.1016/j.intimp.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 156.Fang L., Li J., Zhou J., Wang X., Guo L. Isolation and purification of three ecdysteroids from the stems of Diploclisia glaucescens by high-speed countercurrent chromatography and their anti-inflammatory activities in vitro. Molecules. 2017;22:1–8. doi: 10.3390/molecules22081310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gorelick-Feldman J., Maclean D., Ilic N., Poulev A., Lila M.A., Cheng D. Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J Agric Food Chem. 2008;56:3532–3537. doi: 10.1021/jf073059z. [DOI] [PubMed] [Google Scholar]
- 158.Laekeman G., Vlietinck A. Phytoecdysteroids: phytochemistry and pharmacological activity. In: Ramawat K.G., Mérillon J.M., editors. Natural products: phytochemistry, botany and metabolism of alkaloids, phenolics and terpenes. Springer Berlin Heidelberg; Berlin, Heidelberg: 2013. pp. 3827–3849. [Google Scholar]
- 159.Kutepova T.A., Syrov V.N., Khushbaktova Z.A., Saatov Z. Hypoglycemic activity of the total ecdysteroid extract from Ajuga turkestanica. Phar Chem J. 2001;35:608–609. [Google Scholar]
- 160.Todorov I.N., Mitrokhin Y.I., Efremova O.I., Sidorenko L.I. The effect of ecdysterone on the biosynthesis of proteins and nucleic acids in mice. Phar Chem J. 2000;34:455–458. [Google Scholar]
- 161.Petersen Q., Cambie R., Russell G. Jones oxidation of 20-hydroxyecdysone (Crustecdysone) Aust J Chem. 1993;46:1961–1964. [Google Scholar]
- 162.Hunyadi A., Csábi J., Martins A., Molnár J., Balázs A., Tóth G. Backstabbing P-gp: side-chain cleaved ecdysteroid 2,3-dioxolanes hyper-sensitize MDR cancer cells to doxorubicin without efflux inhibition. Molecules. 2017;22:1–8. doi: 10.3390/molecules22020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schiaffino S., Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle. 2011;1:1–4. doi: 10.1186/2044-5040-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gorelick-Feldman J., Cohick W., Raskin I. Ecdysteroids elicit a rapid Ca2+ flux leading to Akt activation and increased protein synthesis in skeletal muscle cells. Steroids. 2010;75:632–637. doi: 10.1016/j.steroids.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Islam M.S., Choi H. Green tea, anti-diabetic or diabetogenic: a dose response study. Biofactors. 2007;29:45–53. doi: 10.1002/biof.5520290105. [DOI] [PubMed] [Google Scholar]
- 166.Wang J.J., Jin H., Zheng S.L., Xia P., Cai Y., Ni X.J. Phytoecdysteroids from Ajugaiva act as potential antidiabetic agent against alloxan-induced diabetic male albino rats. Biomed Pharmacother. 2017;96:480–488. doi: 10.1016/j.biopha.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 167.Vina J., Borras C., Gomez-Cabrera M.C., Orr W.C. Part of the series: from dietary antioxidants to regulators in cellular signalling and gene expression role of reactive oxygen species and (phyto)oestrogens in the modulation of adaptive response to stress. Free Radic Res. 2006;40:111–119. doi: 10.1080/10715760500405778. [DOI] [PubMed] [Google Scholar]
- 168.Ramazanov N.S., Bobayev I.D., Yusupova U.Y., Aliyeva N.K., Egamova F.R., Yuldasheva N.K. Phytoecdysteroids-containing extract from Stachys hissarica plant and its wound-healing activity. Nat Prod Res. 2017;31:593–597. doi: 10.1080/14786419.2016.1205058. [DOI] [PubMed] [Google Scholar]
- 169.Baltayev U.A., Dinan L., Girault J.P., Lafont R. 24(241)[Z]-dehydroamarasterone B, a phytoecdysteroid from seeds of Leuzea carthamoides. Phytochemistry. 1997;46:103–105. doi: 10.1016/s0031-9422(97)00218-5. [DOI] [PubMed] [Google Scholar]
- 170.Chen T., Diao Q.Y., Yu H.Z., Jiao C.L., Ruan J. Phytochemical, cytotoxic and chemotaxonomic study on Ajuga forrestii Diels (Labiatae) Nat Prod Res. 2018;32:977–981. doi: 10.1080/14786419.2017.1371161. [DOI] [PubMed] [Google Scholar]
- 171.Gaube F., Wolfl S., Pusch L., Werner U., Kroll T.C., Schrenk D. Effects of Leuzea carthamoides on human breast adenocarcinoma MCF-7 cells determined by gene expression profiling and functional assays. Planta Med. 2008;74:1701–1708. doi: 10.1055/s-0028-1088316. [DOI] [PubMed] [Google Scholar]
- 172.Sadati N., Ostad S., Karimian Z., Ardekani M.R.S., Akbarzadeh T., Hadjiakhoondi A. Phytochemical study and in vitro cytotoxic effect of Ajuga chamaecistus ssp. tomentella. Asian J Chem. 2012;24:2871–2874. doi: 10.1515/znc-2012-5-606. [DOI] [PubMed] [Google Scholar]
- 173.Martins A., Toth N., Vanyolos A., Beni Z., Zupko I., Molnar J. Significant activity of ecdysteroids on the resistance to doxorubicin in mammalian cancer cells expressing the human ABCB1 transporter. J Med Chem. 2012;55:5034–5043. doi: 10.1021/jm300424n. [DOI] [PubMed] [Google Scholar]
- 174.Csábi J., Martins A., Sinka I., Csorba A., Molnar J., Zupko I. Synthesis and in vitro evaluation of the antitumor potential and chemo-sensitizing activity of fluorinated ecdysteroid derivatives. Med Chem Comm. 2016;7:2282–2289. [Google Scholar]
- 175.Konovalova N.P., Mitrokhin Iu I., Volkova L.M., Sidorenko L.I., Todorov I.N. Ecdysterone modulates antitumor activity of cytostatics and biosynthesis of macromolecules in tumor-bearing animals. Izv Akad Nauk Ser Biol. 2002;6:650–658. [PubMed] [Google Scholar]
- 176.Grebenok R.J., Ripa P.V., Adler J.H. Occurrence and levels of ecdysteroids in spinach. Lipids. 1991;26:666–668. [Google Scholar]
- 177.Slama K., Lafont R. Insect hormones-ecdysteroids: their presence and actions in vertebrates. Eur J Entomol. 1995;92:355–377. [Google Scholar]
- 178.Kuzmenko A., Niki E., Noguchi N. New functions of 20-hydroxyecdysone in lipid peroxidation. J Oleo Sci. 2001;50:497–506. [Google Scholar]
- 179.Katzenellenbogen B.S., Choi I., Delage-Mourroux R., Ediger T.R., Martini P.G., Montano M. Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology. J Steroid Biochem Mol Biol. 2000;74:279–285. doi: 10.1016/s0960-0760(00)00104-7. [DOI] [PubMed] [Google Scholar]
- 180.Velders M., Schleipen B., Fritzemeier K.H., Zierau O., Diel P. Selective estrogen receptor-beta activation stimulates skeletal muscle growth and regeneration. FASEB J. 2012;26:1909–1920. doi: 10.1096/fj.11-194779. [DOI] [PubMed] [Google Scholar]
- 181.Losel R.M., Falkenstein E., Feuring M., Schultz A., Tillmann H.C., Rossol-Haseroth K. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- 182.Estrada M., Espinosa A., Muller M., Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144:3586–3597. doi: 10.1210/en.2002-0164. [DOI] [PubMed] [Google Scholar]
- 183.Vicencio J.M., Ibarra C., Estrada M., Chiong M., Soto D., Parra V. Testosterone induces an intracellular calcium increase by a nongenomic mechanism in cultured rat cardiac myocytes. Endocrinology. 2006;147:1386–1395. doi: 10.1210/en.2005-1139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.