Abstract
Objectives
The aim of this study is to compare the effects of low-density pulsed ultrasound (LIPUS) treatment on growth factors/collagen production, histological, biomechanical, and function of rats with Achilles tendon injury.
Materials and methods
A total of 44 Wistar Albino rats were used in the study between April 2017 and June 2018. The rats were randomized to two treatment groups. Group 1 (n=6) received LIPUS treatment (0.3 Watt/cm2; 1 MHz, 1:5 pulse mode) and Group 2 (n=6)received sham ultrasound (US) treatment following Achilles tendon surgery. Transforming growth factor-beta 1 (TGF-β1) and collagen gene expression levels were evaluated using polymerase chain reaction. The histological evaluation was performed with the Bonar scoring system. The tensile strength was measured by biomechanical testing and the function was evaluated with the Achilles Functional Index (AFI).
Results
Although TGF-β1 expression and tensile strength evaluation showed a tendency to improve in favor of the LIPUS group, no statistically significant difference was found (p=0.065 and p=0.053, respectively). The COL3 gene expression in the LIPUS group and the COL1 expression in the sham US group were significantly higher. Bonar scores and AFI scores showed a statistically significant improvement in the LIPUS group, compared to the sham US group.
Conclusion
Our study results show that LIPUS yields positive effects on tendon histology and functional status in repaired Achilles tendon in rats.
Keywords: Achilles tendon, biomechanic, growth factor, histology, low-density therapeutic ultrasound, tendon injury
Introduction
During the tendon healing process, therapeutic ultrasound (TUS) has beneficial effects on reducing edema, increasing cellular metabolism, and tendon strength. It has been also reported that the synthesis of Type-1 and Type-3 collagen is enhanced and that collagen fibrils are better organized with TUS.[1-4] It produces these effects by heating the tissue. However, there is also the possibility of heat damage. Low-intensity pulsed TUS (LIPUS) enables the effects of TUS, without harmful heating effect. It has been also reported that LIPUS has more positive effects on tendon healing.[5-7]
Growth factors constitute the basic biology of tendon healing. As one of the growth factors family, the transforming growth factor beta (TGF-β) family plays an important role in the healing process by managing fibroblast migration and synthesis of extracellular matrix proteins in damaged tendons. In particular, TGF-β1 expression has been found to be effective as an indicator of molecular healing in tendon injuries.[8-11] It induces collagen production by fibroblast chemotaxis.[7] It also produces both Type-1 and Type-3 collagen products as a physiological response to trauma. Type-3 collagen plays a major role in the healing process of the tendon.[12]
One of the main determinants of tendon healing is the histological structure including collagen sequencing, matrix density, and vascularization adequacy. Besides, biomechanically sufficient strength and resistance of the tendon are among the good recovery criteria. Although the effects of these parameters have been demonstrated in different studies, their effect on function has not sufficiently studied, yet.[13]
In the present study, we aimed to investigate the effects of LIPUS treatment after Achilles tendon repair on TGF-β, collagen level, histology, biomechanical endurance, and function in a rat model.
Patients and Methods
In this experimental study, a total of 40 adult Wistar Albino rats (20 weeks, 250 g) were used between April 2017 and June 2018. The animals were kept under standard animal laboratory conditions with the appropriate amount of food and water required. The rats were randomized to either LIPUS treatment (n=6) or sham US (n=6). Since four rats died due to anesthesia complications (n=3) and infection (n=1) on the first postoperative day, additional four healthy rats were included in the study group by applying tendon surgery. The study protocol was approved by the Experimental Animals Ethics Committee of Pamukkale University Faculty of Medicine (PAUHADYEK-207/12).
Surgical technique
Under intraperitoneal anesthesia, ketamine hydrochloride and xylazine were used at a dose of 90 mg/kg+10 mg. The Achilles tendon was explored by a longitudinal incision from the starting point in the gastrocnemius muscle to the end point in the calcaneus. The tendon was cut 5 mm above the end point in the calcaneus and repaired with 5.0 absorbable sutures using the modified Kessler technique. The skin was closed using 3.0 polypropylene. Free activity of the rats was allowed in the postoperative period.[14]
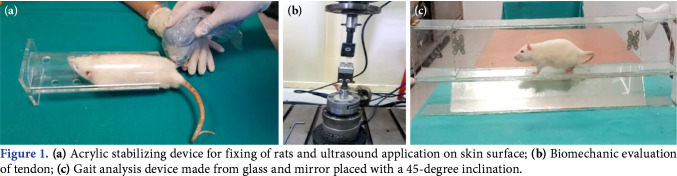
The LIPUS treatment was started 24 h after the operation. The treatment was performed with a 2-cm transducer and 15 consecutive days for 5 min/day.[15-19] After the rats were placed on a standard stabilizing device made of acrylic, a thin layer of acoustic gel was applied to the skin over the incision area and LIPUS was applied.[13] In one group, sham application was performed and LIPUS was applied to the other group. The dose of LIPUS was administered in a 1 MHz 1:5 pulse mode to 0.3 Watts/cm2 (Figure 1a).[15-19]
Figure 1. (a) Acrylic stabilizing device for fixing of rats and ultrasound application on skin surface; (b) Biomechanic evaluation of tendon; (c) Gait analysis device made from glass and mirror placed with a 45-degree inclination.
Evaluation parameters
The TGF-β1 and collagen messenger ribonucleic acid (mRNA) expression were evaluated by polymerase chain reaction (PCR). For ribonucleic acid (RNA) isolation, the tendons were stored at -20°C in RNAlater (QIAGEN GmbH, Hilden, Germany) solution, until total RNA was isolated. Then, it was homogenized to 1,000 rpm/90 sec with the help of tissue homogenizer (WiseTis HG-15D; Daihan Scientific Co, Seoul, Korea). Total RNA was isolated from homogenized tissue according to the protocol of gene extraction kit (Hibrig, Ankara, Turkey). The total RNAs obtained were converted to complementary deoxyribonucleic acid (cDNA) using the SensiFASTTM cDNA Synthesis Kit (BIO-65053, Bioline, London, UK), and isolation was executed according to isolation protocol of the kit. The quantitative reverse transcription-PCR (qRT-PCR) technique was used to determine the expression levels of the COL1A1, COL3A1, and TGF-β1 genes. The cDNAs were reacted using rat specific primers (COL1A1, COL3A1, TGF-β1, and β-actin) and Kilogreen MasterMix (ABM Company, Vancouver, Canada). The Rotor-GeneTM 6000 (Corbett Life Science Pty. Ltd., Sydney, Australia) was used for qRT-PCR reactions. The β-actin gene was used as a normalizer. The cycle threshold (Ct) values of all genes were calculated using 2 analysis Ct method for each sample.
For histological analysis, tissue samples were fixed and stored overnight with neutral buffered 10% formalin, then dried with alcohol and embedded in paraffin. The blocks were, then, cut longitudinally to a thickness of 5 μm. Hematoxylin & eosin staining was performed according to standard protocol and the results were evaluated by a blinded histologist. The Bonar scoring system was used to evaluate the vascularity, collagen structure, and tenocytes.[20] A low grade in Bonar scoring indicate normal histology, while a high grade indicate deterioration of histology.
For biomechanical evaluation, tendons were stored at -20°, until analysis. During the test, temperature was fixed at 20°C and the humidity was 40%. The frozen specimens were placed in a saline solution at room temperature to prevent moisture loss, until analysis. The proximal part of tendon was placed in the upper clamp and the distal part of the tendon was placed in the lower clamp. The upper clamp was connected to the load system and the lower clamp was connected to the ground of the machine. The system was applied with a displacement of 5 mm per min and operating at 250 N. Each tendon was recorded by loading, until it ruptured (Figure 1b).[20]
The Achilles Functional Index (AFI), a gait analysis described by Murrell et al.,[21] was applied to rats for functional analysis. As described previously,[22] a clear glass platform (80x6x112 cm) was prepared and a mirror with a 45° inclination placed under the platform (Figure 1c). A digital camera was placed one meter away from the platform. While the rats were walking, images were obtained simultaneously from the rats and the mirror under the platform in the sagittal plane. The rats were allowed to free activities at their walking speed. All parameters were obtained with both feet in contact with the ground. The print length (PL, the longitudinal length of the foot contact length) was measured from the sagittal view, toe- spread length (TS, distance between first and fifth toes), and intermediary toe-spread length (IT, distance between the second and fourth toes) were measured from the bottom view. Ideally, the AFI is approximately 0 in the normal rat tendon. As the AFI value becomes negative, it indicates worse functional impairment on the damaged side. All rats underwent gait analysis before surgery to determine normal values.
Statistical analysis
The study power was calculated as at least 80% with β=20 and α=0.05. For each parameter of the study (gene expression, histological analysis, biomechanical evaluation), six rats were required and 20 rats were planned for each group. Statistical analysis was performed using the PASW version 17.0 software (SPSS Inc., Chicago, IL, USA). Descriptive data were expressed in mean ± standard deviation (SD), median (min-max) or number and frequency. Non-parametric tests were used for the data. The Mann-Whitney U test was used to compare two independent groups. The Fisher’s exact test was used to compare categorical data between the groups. The Wilcoxon test was used to determine the difference between pre- and post- treatment in each group for the pre- and post-test AFI. The Spearman’s coefficient was used to analyze the correlations between variables. A p value of <0.05 was considered statistically significant.
Results
A total of 44 rats were used in the study and treatment was started at approximately 30 h postoperatively. In the gene expression analysis, there was a statistically significant increase in the COL3A1 expression in the LIPUS group, compared to sham US group. There was also a statistically significant increase in the expression of COL1A1 in the sham US group, compared to the LIPUS group. However, there was no statistically significant difference in the TGF-β1 levels between the groups (Table 1).
Table 1. Comparison of post-treatment COL1, COL3, TGF-β1 mRNA expression and tensile strength parameters of tendon between groups .
| LIPUS | Sham US | p | |||||||
| n | Mean±SD | Median | Min-Max | n | Mean±SD | Median | Min-Max | ||
| COL1 | 6 | 2.4±0.7 | 2.3 | 1.3-3.4 | 6 | 3.3±0.8 | 3.6 | 2.2-4.1 | 0.045* |
| COL3 | 6 | 47.8±1.2 | 48.6 | 0.0-58.3 | 6 | 24.6±1.4 | 17.9 | 13.8-47.8 | 0.028* |
| TGF-β1 | 6 | 0.4±0.5 | 0.19 | 0.01-1.3 | 6 | 0.03±0.03 | 0.010 | 0.002-0.08 | 0.065 |
| Tensile strength (Newton) | 8 | 61.8±11.1 | 59.6 | 50.1-76.5 | 6 | 49.1±10.6 | 50.9 | 25.3-58.3 | 0.053 |
| COL1: Collagen 1 mRNA expression; COL3: Collagen 3 mRNA expression; TGF-β1: Transforming growth factor-beta 1; LIPUS: Low-intensity pulsed ultrasound; US: Ultrasound; SD: Standard deviation; Mann-Whitney U test; * p<0.05 statistically significant. | |||||||||
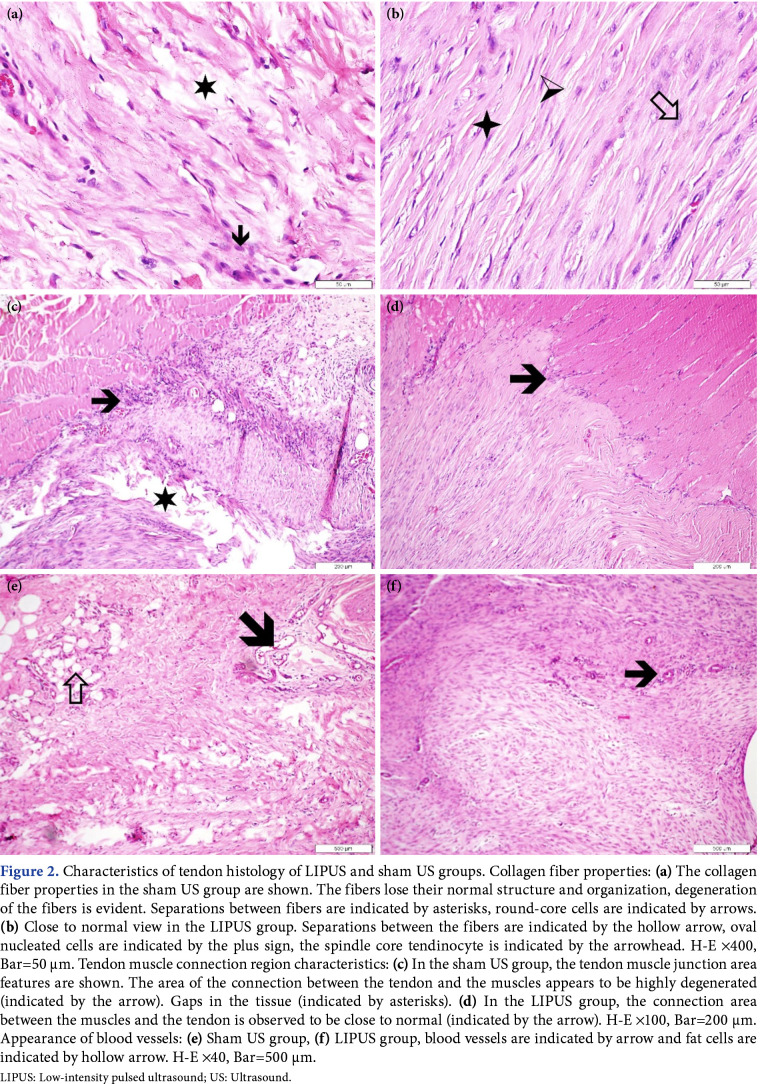
In the histological analysis, there was a statistically significant improvement in the LIPUS group compared to sham US group (Table 2). In the LIPUS group, one rat remained in the Bonar Stage 3, while one rat increased to the Bonar Stage 2 and four rats to the Bonar Stage 1. In the sham US group, all rats remained in Bonar Stage 3 (Figure 2).
Figure 2. Characteristics of tendon histology of LIPUS and sham US groups. Collagen fiber properties: (a) The collagen fiber properties in the sham US group are shown. The fibers lose their normal structure and organization, degeneration of the fibers is evident. Separations between fibers are indicated by asterisks, round-core cells are indicated by arrows. (b) Close to normal view in the LIPUS group. Separations between the fibers are indicated by the hollow arrow, oval nucleated cells are indicated by the plus sign, the spindle core tendinocyte is indicated by the arrowhead. H-E ¥400, Bar=50 μm. Tendon muscle connection region characteristics: (c) In the sham US group, the tendon muscle junction area features are shown. The area of the connection between the tendon and the muscles appears to be highly degenerated (indicated by the arrow). Gaps in the tissue (indicated by asterisks). (d) In the LIPUS group, the connection area between the muscles and the tendon is observed to be close to normal (indicated by the arrow). H-E ¥100, Bar=200 μm. Appearance of blood vessels: (e) Sham US group, (f) LIPUS group, blood vessels are indicated by arrow and fat cells are indicated by hollow arrow. H-E ¥40, Bar=500 μm. LIPUS: Low-intensity pulsed ultrasound; US: Ultrasound.
Table 2. Inter-group evaluation of Bonar scoring.
| LIPUS (n=6) | Sham US (n=6) | ||||
| n | % | n | % | p | |
| Bonar Grade 1 | 4 | 66.7 | 0 | 0 | 0.015* |
| Bonar Grade 2-3 2 | 33.3 | 6 | 100 | ||
| LIPUS: Low-intensity pulsed ultrasound; US: Ultrasound; Fisher’s exact test; * p<0.05 statistically significant. | |||||
For biomechanical evaluation, eight tendons were evaluated in biomechanical laboratory. In the sham US group, one tendon was excluded from the evaluation, as the calcaneus was small and could not be attached to the device clamp. Another tendon was removed from the evaluation, as the muscle part attached to the upper clamp was not compatible with the device. Evaluation was performed with eight rat tendons in the LIPUS group and six rat tendons in the sham US group. There was no statistically significant difference in the comparison of tensile strength of the biomechanical evaluation parameters after treatment between the groups (Table 1).
There was no significant difference between the groups in the preoperative AFI evaluation (Table 3). Pre- and post-treatment intra-group comparisons showed a statistically significant decrease in both groups (p<0.05) (Table 3). Post-treatment inter-group evaluation was significantly better in the LIPUS group. The AFI scores were significantly lower in the LIPUS group, compared to the sham US group (p<0.05) (Table 3).
Table 3. Intra- and inter-group comparisons of AFI scores.
| LIPUS | Sham US | p | |||||
| Mean±SD | Median | Min-Max | Mean±SD | Median | Min-Max | ||
| AFI PrT | -8.4±3.5 | -9.1 | -1.3 - -14.5 | -7.3±4.1 | -6.8 | -1.3 - -15.04 | 0.3 |
| AFI PoT | -34.8±8.5 | -32.4 | -25.1 - -50.5 | -42.0±8.6 | -41.6 | -28.6 - -60.7 | 0.009* |
| p** | 0.001* | 0.001* | |||||
| AFI ( PrT-PoT) | -26.4±6.3 | -24.5 | -16.7 - -40.2 | -34.8±6.1 | -33.9 | -25.8 - -49.5 | 0.001* |
| AFI: Achilles Functional Index; LIPUS: Low-intensity pulsed ultrasound; US: Ultrasound; SD: Standard deviation; PrT: Pre-test; PoT: Post-test; Mann-Whitney U test; * p<0.05 statistically significant; Wilcoxon test; ** p<0.05 statistically significant. | |||||||
According to the relationship of change in AFI to the parameters affecting the improvement, the only significant correlation was found using the histological evaluation as determined by the Bonar scoring (r=0.678; p=0.015) (Table 4).
Table 4. Correlation analysis results.
| p | r | |
| Tensile strength-AFI change | 0.2 | -0.4 |
| COL1-AFI change | 50.46 | 0.2 |
| C COL3-AFI change | 0.3 | -0.4 |
| TGF-β1-AFI change | 0.5 | -0.24 |
| Bonar score-AFI change | 0.015* | 0.7 |
| AFI: Achilles Functional Index; COL1: Collagen 1 mRNA; COL3: Collagen 3 mRNA; TGF-β1: Transforming growth factor-beta 1. | ||
Discussion
According to the results of our study, 15 sessions of LIPUS application after Achilles tendon repair, COL3A1 mRNA expression level, histological improvement, and functional status were found to be significantly better than sham US. The TGF-β1 and tendon tensile strength were better in the LIPUS group, although it did not reach statistical significance. Functional status was positively correlated with the histological improvement.
The TGF-β1 is a growth factor which plays an important role in increasing the tendon tensile strength with an intrinsic healing capability.[23-25] It induces fibroblast and collagen production and peaks on Day 14 of wound healing and remains high until Day 28.[15] Therapuetic ultrasound has been reported to induce TGF-β1 secretion in tendon cells, while high intensity or prolonged application leads to thermal damage.[13,15,26] It has been shown that TGF-β1 and collagen Type-1 and Type-3 levels increase with LIPUS.[26] Although TGF-β1 mRNA expression was higher in the LIPUS group in our study, it was not statistically significant. According to our results, COL3A1 mRNA expression level was significantly higher in the LIPUS group, compared to sham US group, while COL1A1 mRNA expression level was significantly higher in the sham US group, compared to the LIPUS group. However, the studies following the physiological wound healing process have shown that the level of COL3 increases in the first 24 h.[26] The level of COL1 begins to increase a few days after injury.[27] The significant excess of COL3 seems to be compatible with the healing physiology in the LIPUS group.
Collagen distribution and organization were mostly examined in the histological analysis.[3,15,18,27] In a randomized-controlled study, 15 sessions of LIPUS and continuous US treatment were applied after Achilles tendon surgery and compared with the sham US group.[18] The organization of histological collagen bundles was significantly better in the LIPUS group. Based on the results of this study and our study, we can speculate that LIPUS treatment, which is applied in the early phase of tendon healing, has positive contributions to the histological healing process.
According to the histological analysis of rat tendons in the second and fourth weeks, the fibroblast-collagen matrix ratio was found to be lower in the US group at the second postoperative week, compared to the control group; however, collagen was more mature in the US group. Histological evaluation at four weeks postoperatively revealed that the fibroblast-collagen matrix ratio was similar in the two groups, while the scar tissue was more mature in the US group.[27]
The LIPUS and sham US were compared in another randomized-controlled trial which was designed to determine when postoperative LIPUS therapy would be initiated.[15] The LIPUS treatment was administered in four groups, starting on the postoperative first day, first week, second week, and fourth week. Histological evaluations were performed at six weeks. The group which was operated and the treatment was not started was followed as the control group. As a result, a better collagen fibril organization was obtained in the LIPUS-treated groups starting from the first day, compared to the control group. However, when the LIPUS treatment was started at the second week after surgery, there was no significant difference between the LIPUS and control groups in the organization of the collagen fibrils. The group in which the LIPUS treatment was started at four weeks, the organization of collagen fibers was significantly worse than control group. The authors attributed this to late US disruption of collagen remodeling leading to poor collagen fiber distribution. In the present study, under the guidance of these studies, LIPUS treatment was started in the early period and better results were obtained histologically.
The tensile strength of the tendon is the most commonly used parameter for biomechanical evaluation. In our study, although the tensile strength was higher in the LIPUS group, no statistically significant difference was obtained. The reason for the difference in borderline biomechanical evaluation may be related to the US application time in our study. In a randomized-controlled trial with the same dose and duration of treatment, the duration of treatment was 28 days and, at the end of this period, a statistically significant improvement in the tensile strength and final load was observed in the LIPUS group, compared to the sham US group.[17]
In another randomized-controlled trial, 60 rat patellar tendons were examined to determine whether the treatment had an effect on biomechanical strength of the tendon for more than two or four weeks.[15] The LIPUS treatment was applied for 20 min daily for two weeks. As a result, tendon strength was found to be better at two, four, and six weeks, compared to the sham US group. In the same study, in the groups treated with LIPUS for four and six weeks starting from the first postoperative day, the strength of the tendon did not increase more than the two-week application at six weeks of evaluation. Although two weeks of LIPUS application seems to be sufficient, unlike the aforementioned study, found no significant difference between the two groups in the tensile strength which can be attributed to the shorter application period in our study. However, considering that 5-min application is also effective,[5,28] it can be suggested that the duration of LIPUS application is still worth researching.
In a study researching which dose of LIPUS was effective for tendon endurance, 1 Watt/cm2 and 2 Watt/cm2 US therapy and exercises were applied daily on postoperative Day 5 until Day 30.[29] At the end of the treatment, there was a statistically significant improvement in the tensile strength in the group with high-dose pulsed US (2 Watts/cm2) and running exercise group, compared to the control group. In our study, the US dose was lower, which may indicate that the dose may be higher with pulse application. There is still a need for further studies in this field. Another reason for this difference may be the difference in the time to start treatment.
In our study, AFI was used for functional evaluation. The AFI scores worsened in both groups, as expected after surgery. However, this deterioration was significantly lower in the LIPUS group. Although the positive effects of US therapy on tendon healing have been shown in many studies previously,[13-19] the functional effect of US on gait has been relatively less studied. Our study is the first to show that gait after Achilles tendon injury with LIPUS treatment is more physiological.
In the literature, there are two randomized- controlled trials evaluating the functionality by using US treatment after Achilles surgery.[29,30] In the study of Ng et al.,[30] the rats who received low- and high-dose continuous TUS, which were started on postoperative Day 6 and lasted for six sessions per week, were compared with the control group. The AFI was measured at three, 10, and 30 days postoperatively and there was no statistically significant difference in the AFI scores between the groups. In the study of Ng et al.,[29] low-dose and high-dose pulse-US therapy, which was applied from the 5th to 30th postoperative day, was compared with walking and swimming exercise groups. At the end of the study, no statistically significant difference was found between the groups in the measurement of AFI. The reason for the lack of improvement in gait analysis in these studies may be the fact that US treatment was started relatively late after surgery, and the dose was high for both continuous and pulsed US.
In our study, the relationship between AFI and evaluation parameters was examined and a significant positive correlation was found only between the AFI difference and Bonar scoring. This finding suggests that a structurally well-organized tissue may contribute positively to function. In the literature, there is no comparison between functional evaluation and tendon biology, histology and biomechanical healing parameters. Our study is, therefore, the first to evaluate the relationships between function and other parameters.
One of the limitations of our study is the lack of evaluation of the long-term efficacy of treatment given to rats. Although there is no activity restriction in our study, the lack of addition of exercise therapy supported by studies, which is one of the effective factors in tendon healing, can be considered another limitation. In addition, sham US was applied as the control group, and the absence of a control group without any application can be deemed as a limitation. The other limitations of the study was the underpowered study design for COL1, TGF-β1, and tensile strength due to the small number of rats in each group. Therefore, generalization of some results of this study is limited.
In conclusion, our study results indicate that LIPUS treatment in the early period after Achilles tendon injury can yield positive results in tendon healing. Nonetheless, clinical studies are needed to evaluate this effect in human tendon injuries.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The study was realized with supporting of "specialization thesis support project" fund of university "Scientific Research Project" (2019TIPF022).
References
- 1.Birch HL. Tendon matrix composition and turnover in relation to functional requirements. Int J Exp Pathol. 2007;88:241–248. doi: 10.1111/j.1365-2613.2007.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- 3.Quigley AS, Bancelin S, Deska-Gauthier D, Légaré F, Kreplak L, Veres SP. In tendons, differing physiological requirements lead to functionally distinct nanostructures. Sci Rep. 2018;8:4409–4409. doi: 10.1038/s41598-018-22741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meier Bürgisser G, Calcagni M, Bachmann E, Fessel G, Snedeker JG, Giovanoli P, et al. Rabbit Achilles tendon full transection model - wound healing, adhesion formation and biomechanics at 3, 6 and 12 weeks post-surgery. Biol Open. 2016;5:1324–1333. doi: 10.1242/bio.020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herod TW, Chambers NC, Veres SP. Collagen fibrils in functionally distinct tendons have differing structural responses to tendon rupture and fatigue loading. Acta Biomater. 2016;42:296–307. doi: 10.1016/j.actbio.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Sammer DM, Chung KC. Advances in the healing of flexor tendon injuries. Wound Repair Regen. 2014;22 Suppl 1:25–29. doi: 10.1111/wrr.12161. [DOI] [PubMed] [Google Scholar]
- 7.Yang G, Rothrauff BB, Tuan RS. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today. 2013;99:203–222. doi: 10.1002/bdrc.21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heisterbach PE, Todorov A, Flückiger R, Evans CH, Majewski M. Effect of BMP-12, TGF-β1 and autologous conditioned serum on growth factor expression in Achilles tendon healing. Knee Surg Sports Traumatol Arthrosc. 2012;20:1907–1914. doi: 10.1007/s00167-011-1772-x. [DOI] [PubMed] [Google Scholar]
- 9.Farhat YM, Al-Maliki AA, Chen T, Juneja SC, Schwarz EM, O'Keefe RJ, et al. Gene expression analysis of the pleiotropic effects of TGF-β1 in an in vitro model of flexor tendon healing. e51411PLoS One. 2012;7 doi: 10.1371/journal.pone.0051411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q, Lu H, Yang H. Chitosan inhibits fibroblasts growth in Achilles tendon via TGF-β1/Smad3 pathway by miR-29b. Int J Clin Exp Pathol. 2014;7:8462–8470. [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C, Liu YJ. Biomechanic and histologic analysis of fibroblastic effects of tendon-to-bone healing by transforming growth factor β1 (TGF-β1) in rotator cuff tears. Acta Cir Bras. 2017;32:1045–1055. doi: 10.1590/s0102-865020170120000006. [DOI] [PubMed] [Google Scholar]
- 12.Maffulli N, Kader D. Tendinopathy of tendo achillis. J Bone Joint Surg [Br] 2002;84:1–8. doi: 10.1302/0301-620x.84b1.12792. [DOI] [PubMed] [Google Scholar]
- 13.Farcic TS, Baldan CS, Cattapan CG, Parizotto NA, João SM, Casarotto RA. Treatment time of ultrasound therapy interferes with the organization of collagen fibers in rat tendons. Braz J Phys Ther. 2013;17:263–271. doi: 10.1590/s1413-35552012005000090. [DOI] [PubMed] [Google Scholar]
- 14.Dabak TK, Sertkaya O, Acar N, Donmez BO, Ustunel I. The effect of phospholipids (surfactant) on adhesion and biomechanical properties of tendon: A rat achilles tendon repair model. Biomed Res Int. 2015;2015:689314–689314. doi: 10.1155/2015/689314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu SC, Shum WT, Hung LK, Wong MW, Qin L, Chan KM. Low-intensity pulsed ultrasound on tendon healing: a study of the effect of treatment duration and treatment initiation. Am J Sports Med. 2008;36:1742–1749. doi: 10.1177/0363546508318193. [DOI] [PubMed] [Google Scholar]
- 16.Enwemeka CS, Rodriguez O, Mendosa S. The biomechanical effects of low-intensity ultrasound on healing tendons. Ultrasound Med Biol. 1990;16:801–807. doi: 10.1016/0301-5629(90)90044-d. [DOI] [PubMed] [Google Scholar]
- 17.Jeremias Júnior SL, Camanho GL, Bassit AC, Forgas A, Ingham SJ, Abdalla RJ. Low-intensity pulsed ultrasound accelerates healing in rat calcaneus tendon injuries. J Orthop Sports Phys Ther. 2011;41:526–531. doi: 10.2519/jospt.2011.3468. [DOI] [PubMed] [Google Scholar]
- 18.da Cunha A, Parizotto NA, Vidal Bde C. The effect of therapeutic ultrasound on repair of the achilles tendon (tendo calcaneus) of the rat. Ultrasound Med Biol. 2001;27:1691–1696. doi: 10.1016/s0301-5629(01)00477-x. [DOI] [PubMed] [Google Scholar]
- 19.Ng GY, Fung DT. The effect of therapeutic ultrasound intensity on the ultrastructural morphology of tendon repair. Ultrasound Med Biol. 2007;33:1750–1754. doi: 10.1016/j.ultrasmedbio.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Çınar BM, Çirci E, Balçık C, Güven G, Akpınar S, Derincek A. The effects of extracorporeal shock waves on carrageenan-induced Achilles tendinitis in rats: a biomechanical and histological analysis. Acta Orthop Traumatol Turc. 2013;47:266–272. doi: 10.3944/aott.2013.2784. [DOI] [PubMed] [Google Scholar]
- 21.Murrell GA, Lilly EG, Davies H, Best TM, Goldner RD, Seaber AV. The achilles functional index. J Orthop Res. 1992;10:398–404. doi: 10.1002/jor.1100100313. [DOI] [PubMed] [Google Scholar]
- 22.Liang JI, Chen MY, Hsieh TH, Liu CY, Lam CF, Chen JJ, et al. Video-based gait analysis for functional evaluation of healing achilles tendon in rats. Ann Biomed Eng. 2012;40:2532–2540. doi: 10.1007/s10439-012-0619-z. [DOI] [PubMed] [Google Scholar]
- 23.Loiselle AE, Yukata K, Geary MB, Kondabolu S, Shi S, Jonason JH, et al. Development of antisense oligonucleotide (ASO) technology against Tgf-β signaling to prevent scarring during flexor tendon repair. J Orthop Res. 2015;33:859–866. doi: 10.1002/jor.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia C, Ding C, Yang X, Sun K, Tian S. Effects of antisense transforming growth factor-beta1 gene transfer on the biological activities of tendon sheath fibroblasts. Orthopedics. 2010;33 doi: 10.3928/01477447-20100625-06. [DOI] [PubMed] [Google Scholar]
- 25.Branford OA, Klass BR, Grobbelaar AO, Rolfe KJ. The growth factors involved in flexor tendon repair and adhesion formation. J Hand Surg Eur Vol. 2014;39:60–70. doi: 10.1177/1753193413509231. [DOI] [PubMed] [Google Scholar]
- 26.Tsai WC, Pang JH, Hsu CC, Chu NK, Lin MS, Hu CF. Ultrasound stimulation of types I and III collagen expression of tendon cell and upregulation of transforming growth factor beta. J Orthop Res. 2006;24:1310–1316. doi: 10.1002/jor.20130. [DOI] [PubMed] [Google Scholar]
- 27.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6:181–190. [PubMed] [Google Scholar]
- 28.Yeung CK, Guo X, Ng YF. Pulsed ultrasound treatment accelerates the repair of Achilles tendon rupture in rats. J Orthop Res. 2006;24:193–201. doi: 10.1002/jor.20020. [DOI] [PubMed] [Google Scholar]
- 29.Ng GY, Ng CO, See EK. Comparison of therapeutic ultrasound and exercises for augmenting tendon healing in rats. Ultrasound Med Biol. 2004;30:1539–1543. doi: 10.1016/j.ultrasmedbio.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 30.Ng CO, Ng GY, See EK, Leung MC. Therapeutic ultrasound improves strength of achilles tendon repair in rats. Ultrasound Med Biol. 2003;29:1501–1506. doi: 10.1016/s0301-5629(03)01018-4. [DOI] [PubMed] [Google Scholar]