Abstract
Chaya is an edible leaf popular in Mexico and Central America because of its high nutritional value. Studies in animal models have demonstrated the beneficial effects of Chaya, which include reduction of circulating lipids and increase in antioxidant activity. However, its hypolipidemic and antioxidant effects have not been demonstrated in humans. Thus, the aim of the present study was to evaluate the effect of Chaya on the lipid profile, lipid peroxidation, inflammation, and peripheral blood mononuclear cell gene expression in a population with dyslipidemia. We performed a single-arm trial in 30 participants with dyslipidemia who consumed 500 mL of Chaya beverage per day over a 6-week period. Interestingly, we observed a significant decrease in serum triglyceride concentration (P < 0.05) and an increase in plasma antioxidant activity and polyphenol concentration (P < 0.005) after 6 weeks of Chaya consumption. This was accompanied by a reduction in the oxidative stress marker MDA (P < 0.0001) and by an increase in the antioxidant enzyme CAT expression in peripheral blood mononuclear cells (P < 0.001). Altogether, our results demonstrate that consumption of Chaya has hypotriglyceridemic and antioxidant effects in subjects with dyslipidemia.
Keywords: Chaya, dyslipidemia, antioxidant activity, oxidative stress, triglycerides
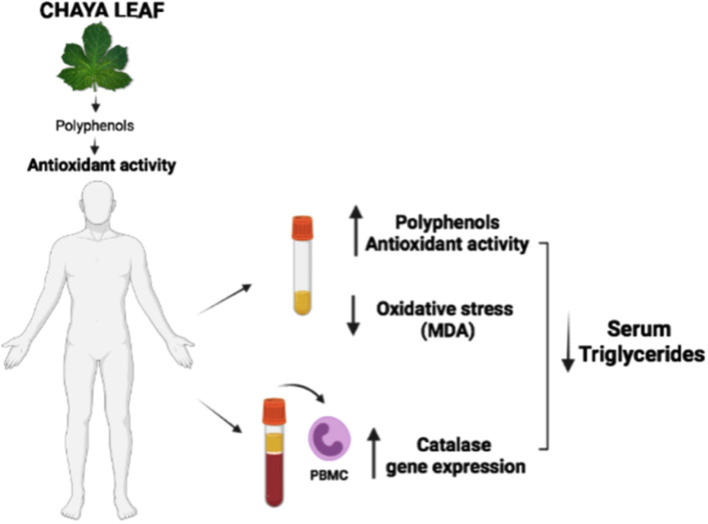
Graphical Abstract.
Effect of Chaya leaf on triglycerides, antioxidant activity and oxidative markers in subjects with dyslipidemia.
Introduction
Dyslipidemia is characterized by abnormal lipid profiles that include increased levels of triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and/or decreased levels of high-density lipoprotein cholesterol (HDL-C). Dyslipidemia is one of the most important risk factors in formation of atherogenic plaques and development of cardiovascular diseases (CVD). Furthermore, hyperlipidemia is related to other complications, such as insulin resistance, endothelial dysfunction, hypertension and oxidative stress (OS) (1). In the context of OS, the increase in reactive oxygen species (ROS) promotes the oxidation of different biomolecules such as proteins, DNA, and lipids (lipoperoxidation). Malondialdehyde (MDA) is an end product of lipoperoxidation, which increases the level of pro-inflammatory cytokines such as interleukins and C-reactive protein (CRP) to establish a pro-atherogenic environment that results in endothelial dysfunction (2). These complications occurring in the context of dyslipidemia are due in part to a decrease in the expression and activity of antioxidant enzymes such as catalase (CAT) and superoxide dismutase (SOD), which enhances OS (3).
One potential strategy to improve lipid profiles is the consumption of foods with a high content of potential bioactive compounds (4). Chaya (Cnidoscolus aconitifolius (Mill.) I.M. Johnst) is an edible leaf popular in Mexico and Central America for its potential use as a vegetable and/or as a medicinal plant. It is widely used as traditional remedy for the treatment of obesity, diabetes, gastrointestinal disorders, and kidney stones (5). Chaya is also an important component of the regular diet of indigenous communities because of its nutritional value; it contains dietary fiber, protein, minerals, vitamins A and C, flavonoids, and polyphenols. Kaempferol and quercetin are the most abundant phenolic compounds identified in Chaya (6). Studies in animal models have demonstrated the effects of Chaya, which included reduction of glucose intolerance (7), anti-inflammatory effects, antioxidant activity (8), and hypolipidemic effects (9). These effects are highly great relevant to the current worldwide epidemic of dyslipidemia; however, there are no human studies demonstrating hypolipidemic or antioxidant effects of Chaya. Thus, the aim of the present study was to evaluate the effect of Chaya consumption on the lipid profile, lipid peroxidation, inflammation, and gene expression in subjects with dyslipidemia.
Materials and Methods
Plant Collection
Chaya (Cnidoscolus aconitifolius (Mill.) I.M. Johnst) was collected in Mérida, Yucatán from March to June 2019. The qualified botanist José L. Tapia-Muñoz identified plant and a voucher specimen (72343) was deposited at the herbarium of the Centro de Investigación Científica de Yucatán.
Chemical Composition of the Beverage of Chaya and HPLC Analysis
The chemical composition of Chaya was analyzed with techniques recommended by the Association of Official Analytical Chemists (10). Antioxidant activity of Chaya was evaluated by oxygen radical absorbance capacity (ORAC) (11). The total polyphenol concentration was determined in Chaya using the Folin–Ciocalteu method (12). Additionally, we determined the phytochemical profile of a sample of Chaya with High-performance liquid chromatography (HPLC) system (Agilent 7 Technologies 1100) were done using a C18 column (Thermo Scientific ODS Hypersil) (250 × 4.6 mm, 5.0 μm). For HPLC analysis, a 2 g dry sample was obtained from beverage. Extraction was carried out using 2 g of dry sample with 30 mL of ethanol (85%) at 60 kHz in an ultrasonic extraction device, and repeated three times. The extract was stored at −4°C overnight. Then, 15 mL of methanol and 1.5 mL of hydrochloric acid were added and left to reflux for 2 h at 60°C. The solvent was removed on a rotary evaporator. The dried extract was dissolved in methanol; and approximately 1 mL of the extraction was filtered through a 0.45μm membrane and transferred into HPLC vials. The sample was eluted at a 1 mL/min flow rate with CH3OH: CH3CN: H2O (40:15:45) as a gradient mobile phase with 1% of acetic acid and detected by absorbance at 368 nm using a Waters 2998 photodiode array detector (13). The calibration curves of quercetin and kaempferol (Sigma, St. Louis, MO) were constructed using serial dilutions in ethanol of the standard solution (0.01, 0.05, 0.15, and 0.30 mg/mL). The sample was analyzed in triplicate.
Subjects
Subjects with dyslipidemia characterized by TG >150 mg/dL, TC >200 mg/dL and/or LDL-C >120 and who were aged 20–60 were recruited through advertisements at the Hospital Regional de Alta Especialidad de la Península de Yucatán. Participants did not receive pharmacological treatment for the control of lipids. Exclusion criteria for these participants included TG ≥ 350 mg/dL, diabetes, a history of cardiovascular events, and weight loss of 3 kg within the last 3 months, cancer, acquired immunodeficiency syndrome, kidney or liver disease, smoking, substance abuse, and alcohol consumption. The study was approved by the Ethics Committee of the Hospital Regional de Alta Especialidad de la Península de Yucatán (No. 2018-030), Instituto Nacional de Pediatría (No. 2019/03), and registered at www.clinicaltrials.gov (NCT04110392), and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Sample Size
The required sample size was estimated to be 21 participants based on an expected effect of a 10% difference with 90% power and an α error of 0.05 for detecting effects on serum lipids resulting from the consumption of Chaya. The effect size was estimated based on studies of lipid-lowering supplements in patients with dyslipidemia (14), and a loss of 30% was expected during the study follow up, resulting in selecting 30 participants for enrollment in the study.
Study Design
The study had a before-and-after design to evaluate the effect of 6 weeks of Chaya beverage consumption in patients with dyslipidemia (Figure 1). At the first visit, a screening evaluation was conducted to select the participants based on the inclusion criteria. Eligible subjects received seven bottles of Chaya beverage to last 1 week. The Chaya beverage was prepared per week as follows: 40 g of Chaya leaves, which were soaked, disinfected, and mixed with 1 L of purified water. Finally, 500 mL of the beverage was placed in bottles and stored at −4°C until consumption during 1 week. At each visit (weekly), seven bottles and a daily consumption form were given to participants, and the participants were instructed to drink one bottle all at once per day for 1 week and record it on the form. At the end of each visit, participants were asked to return the empty bottles and the daily consumption form. The participants were advised to maintain their normal lifestyle, activity level and diet, and were instructed to restrict the consumption of foods containing Chaya. At the baseline and end of the study, body weight, height, and waist circumference were determined by the Lohman method (15). The waist-to-height ratio (WHtR) was calculated by dividing the waist circumference (cm) by the height (m). Blood pressure was measured with a digital automatic blood pressure monitor (HEM-6122 LA, Omron Healthcare, Inc., Sunrise, FL, USA). Blood samples were collected after 8–10 h of fasting at the baseline and at the end of the study. Briefly, blood samples were centrifuged at 500xg for 5 min, and serum and plasma were maintained at −70°C until analysis. Serum TC, TG, LDL-C, HDL-C, and glucose were analyzed with a COBAS C11 (Roche Applied Science).
Figure 1.
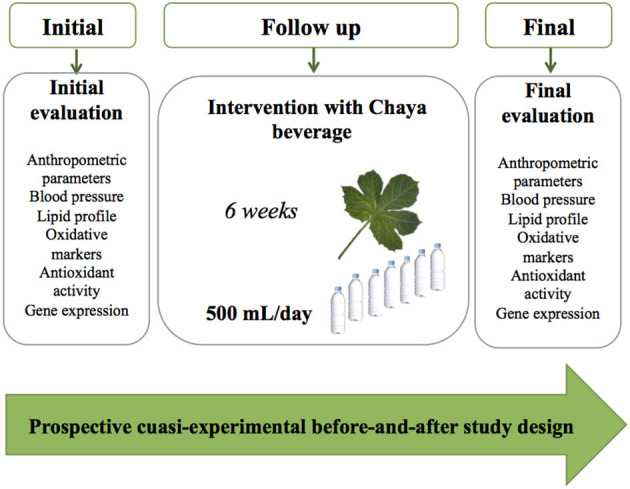
Study design.
Oxidative and Inflammation Markers
Serum MDA concentration was measured as a marker of lipid peroxidation by a spectrophotometric method as previously reported (16), and CRP was analyzed with the COBAS C11 (Roche Applied Science).
Determination of Antioxidant Activity and Total Polyphenols
Antioxidant activity of plasma was evaluated by ORAC (11), and the total polyphenol concentration was determined in plasma using the Folin–Ciocalteu method (12).
Peripheral Blood Mononuclear Cell Gene Expression by Quantitative Real-Time PCR
Total RNA was extracted from peripheral blood mononuclear cells (PBMC) using TRIzol according to the manufacturer's instructions. The mRNA abundance was measured by real-time quantitative PCR using SYBR®Premix, using actin as a reference for normalization. The primers are listed in Supplementary Table 1. The relative expression was calculated using the ΔΔCt method (17).
Statistical Analysis
Continuous variables were evaluated using the Kolmogorov-Smirnov test to analyze the type of distribution. Wilcoxon tests were performed for paired samples to compare the medians of the lipid profile, glucose, CRP, antioxidant activity, oxidative markers, and body weight at baseline and after the intervention. For gene expression, logarithmic transformation was performed before analysis with a dependent-samples t-test. A value of P < 0.05 was considered significant. Correlations between two variables were evaluated by Spearman's test. Data are presented as the mean ± standard deviation (SD) or standard error of the mean (SEM); or medians and 95% confidence intervals (95% CI). Data were analyzed using SPSS version 25 software for Macintosh (IBM Corp., Armonk, NY, USA).
Results
Analysis of Chemical Composition and Identification of Bioactive Compounds in Chaya Beverage
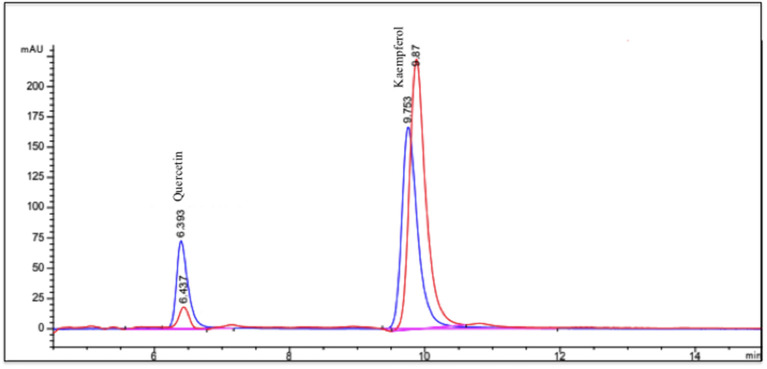
The Chaya beverage mainly contained protein, fiber, and minerals (Table 1). Its antioxidant activity was 807 ± 10.53 Trolox equivalents (μmoles/L), and the concentration of polyphenols was 1,359 ± 47.2 mg of gallic acid equivalents (GAE)/L. The HPLC profile obtained showed a pattern with two peaks that represented the quercetin and kaempferol present in Chaya (Figure 2). The retention time of quercetin was determined at 6.39 min and kaempferol at 9.75 min. The concentration of quercetin in the Chaya beverage was 0.21 ± 0.008 mg/mL and kaempferol was 1.72 ± 0.015 mg/mL.
Table 1.
Chemical composition of the beverage Chaya. Data are expressed as a portion of daily intake (20 g/500 mL). Values represent the mean ± SEM.
| Content (g/500 mL) | |
|---|---|
| Protein | 1.02 ± 0.02 |
| Carbohydrates | 0.59 ± 0.05 |
| Fiber | 0.58 ± 0.03 |
| Minerals | 0.38 ± 0.01 |
| Lipids | 0.18 ± 0.02 |
Figure 2.
HPLC profile of Chaya. Principal compounds in Chaya are quercetin (6.43 min) and kaempferol (9.87 min) (red line).
Anthropometric Parameters, Blood Pressure, and Glucose Concentration in Subjects With Dyslipidemia
A total of 112 participants were screened, and 30 subjects were enrolled. The study population had a mean age of 42.5 ± 10.8 years, and 46.7% (n = 14) were male. At baseline, subjects had a body mass index (BMI) of 29.2 ± 4.41 kg/m2, and blood glucose of 89.1 (95% CI, 74.2–127) mg/dL. was 115 (95% CI, 98–180) and 74 (95% CI, 59–180) mmHg, respectively. After 6 weeks of the dietary strategy, the subjects with dyslipidemia who consumed the Chaya beverage exhibited a decrease in systolic BP (−3.5%) (P < 0.05) (Table 2). However, diastolic BP, glucose concentration, body weight, and BMI did not show differences after intervention. At the end of the study, a percentage of 94 ± 5.1 of adherence to the intervention was reported. The consumption of Chaya did not have adverse effects in the participants.
Table 2.
Anthropometric characteristics, blood pressure, and biochemical parameters at baseline and after intervention with Chaya.
| Characteristic | Baseline | Week 6 | P-value |
|---|---|---|---|
| Weight (kg) | 72.2 (50.4–115) | 72.3 (50.1–117) | 0.050 |
| BMI (kg/m2) | 29.2 ± 4.41 | 29.1 ± 4.41 | 0.160 |
| Circumference of waist (cm) | 93 (74.1–120) | 93 (72.8–118) | 0.292 |
| WHtR | 0.58 (0.46–0.69) | 0.59 (0.48–0.68) | 0.791 |
| Systolic BP (mmHg) | 115 (98–180) | 112 (93.4–166) | 0.032 |
| Diastolic BP (mmHg) | 74 (59–180) | 73 (61.7–106) | 0.992 |
| Glucose (mg/dL) | 89.1 (74.2–127) | 91.9 (80.1–107) | 0.052 |
| Triglycerides (mg/dL) | 208 (114–301) | 166 (90–328) | 0.049 |
| TC (mg/dL) | 188 (143–264) | 186 (149–260) | 0.133 |
| HDL-C (mg/dL) | 39.7 (27.6–70.3) | 41.5 (28.9–58.7) | 0.425 |
| LDL-C (mg/dL) | 121 (72.7–200) | 120 (95.1–185) | 0.632 |
Values represent the mean ± SD or median (95% confidence interval). Body mass index, (BMI); Waist-to-height ratio (WHtR); Blood pressure (BP); Total cholesterol (TC); Highdensity lipoprotein cholesterol (HDL-C); Low-density lipoprotein cholesterol (LDL-C). Statistical analysis was determined by the Wilcoxon test or dependent-samples t-test.
Lipid Profiles in Subjects Before and After Chaya Beverage Intervention
The subjects with dyslipidemia included in this study showed lipid alterations in TG (208 mg/dL; 95% CI, 114–301) and LDL-C (121 mg/dL; 95% CI, 72.7–200). After consuming Chaya for 6 weeks, there was a significant reduction of 20.6 % in the concentration of TG (P < 0.05) (Table 2). In contrast, circulating levels of TC, LDL-C, and HDL-C did not show differences after Chaya consumption.
Chaya Consumption Enhanced Antioxidant Activity in Subjects With Dyslipidemia
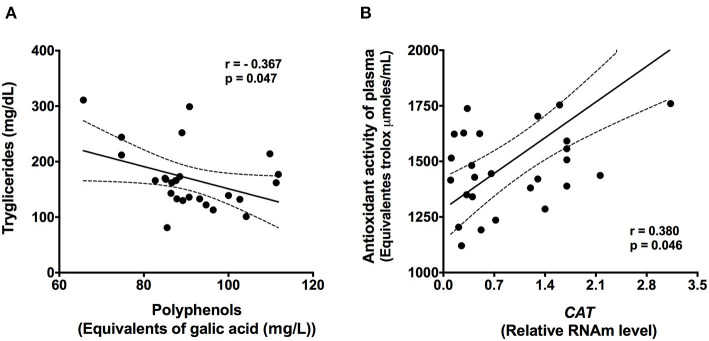
Interestingly, the antioxidant activity increased in plasma from 1324 Trolox equivalents (μmoles/mL) (95% CI, 963–2078) to 1485 (95% CI, 1160–2416) (P < 0.05) (Figure 3A). This was accompanied by a significant increase of 4% in the plasma polyphenol concentration (P < 0.005) (Figure 3B). Furthermore, subjects with dyslipidemia showed an inverse correlation between plasma polyphenols and serum TG (r = −0.367, P = 0.047; Figure 4A).
Figure 3.
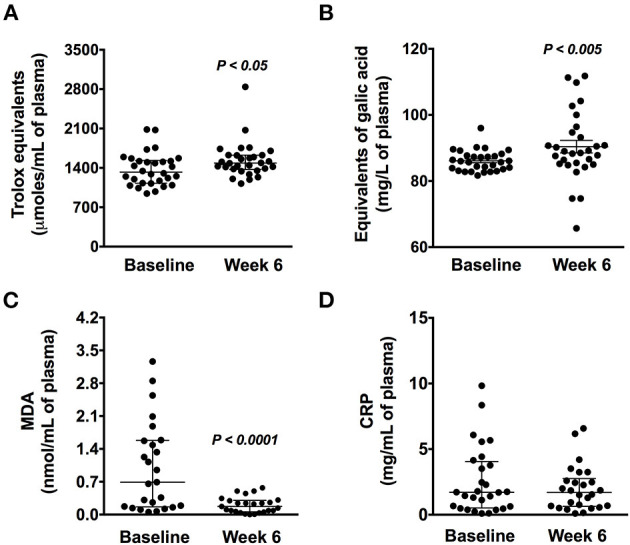
Oxidative and inflammation markers in subjects with dyslipidemia at baseline and before Chaya intervention. (A) Antioxidant activity. (B) Plasma polyphenol concentration. (C) Plasma malondialdehyde. (D) C-reactive protein concentration. Data are expressed as the median (95% confidence interval) (n = 30). Statistical analysis was performed using Wilcoxon tests.
Figure 4.
Correlations between serum triglycerides and plasma polyphenols (A) and between plasma antioxidant activity and CAT gene expression (B) (n = 30). Statistical analysis was performed by Spearman's test (95% confidence interval).
Chaya Intake Decreased in Oxidative Marker MDA
Notably, the 6-week Chaya intervention reduced the MDA concentration by 80.4% (P < 0.0001) (Figure 3C) with no changes in the inflammatory marker CRP (Figure 3D). There is evidence of a relationship between OS and inflammation and the development of CVD and mortality (18, 19).
Differential Expression in Peripheral Blood Mononuclear Cells of Antioxidant and Lipid Metabolism Genes Resulting From Chaya Consumption in Subjects With Dyslipidemia
As expected, the antioxidant enzyme CAT showed a significant increase after Chaya intake (P < 0.001) without changes in the expression of SOD1 (Figures 5A,B) in PBMC. In fact, there was a positive correlation between the gene expression of CAT and plasma antioxidant activity (r = 0.380, P = 0.046; Figure 4B). In addition, the expression of lipid-related genes was evaluated. After the consumption of Chaya, there was a significant decrease in the gene expression of HMGCR (hydroxy-methyl-glutaryl coenzyme A reductase) in PBMC, which is involved in hepatic cholesterol synthesis (Figure 5C), and this decrease was accompanied by an increase in the gene expression of ABCA1 (ATP-binding cassette transporter A1) (Figure 5D), which mediates the efflux of cholesterol to regulate lipid levels.
Figure 5.
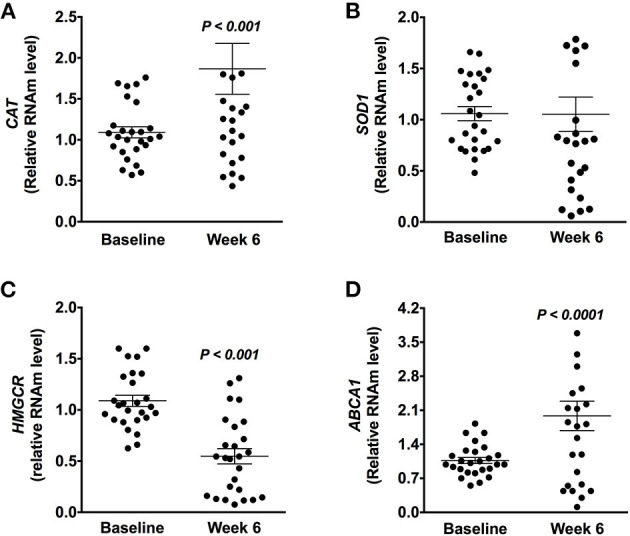
Differential expression in peripheral blood mononuclear cell of genes involved in antioxidant activity and cholesterol flux modulated by Chaya intake. (A) Relative gene expression of CAT (catalase). (B) Relative gene expression of SOD (Superoxide dismutase 1). (C) Relative gene expression of HMGCR (hydroxy-methyl-glutaryl coenzyme A reductase). (D) Relative gene expression of ABCA1 (ATP-binding cassette transporter A1). Data are expressed as the mean ± SD. Statistical analysis was performed using a paired t-test after logarithmic transformation.
Discussion
Our results demonstrated that a nutritional strategy that includes the consumption of a Chaya beverage for 6 weeks reduced plasma triglyceride concentration and lipoperoxidation, increased plasma antioxidant activity, and modulated the expression of antioxidant and lipid metabolism genes in PBMC in subjects with dyslipidemia.
Residents in middle-income countries experience health disparities and less healthy eating patterns; thus, it is very common for inhabitants to use traditional foods or herbs to control different types of diseases. Chaya leaves are used in Mayan traditional medicine to treat kidney stones, diabetes, and high BP; infusions or teas make its administration from Chaya leaves. However, communities use native food components to control several diseases mainly based on empirical knowledge of their effects on health (20). The relevance of our work resides in the evaluation of a dietary strategy that included the use of Chaya, an inexpensive and native regional food, to decrease lipid alterations. A few studies in vitro and in animal models have demonstrated antioxidant, anti-inflammatory, hypoglycemic, and hypolipidemic effects of Chaya (8, 21). In fact, a study in rats showed that doses of 0.5–1.5 mg/kg of Chaya extracts administered intragastrically decreased the concentrations of TG and TC (9). Interestingly, in the present study, we observed an inverse correlation between plasma polyphenols and serum TG, supporting the hypothesis that the hypolipidemic effects of Chaya consumption may be attributed to its effect on polyphenols. This lipid-lowering effect promoted by polyphenols could be attributed to the activation of AMP-activated protein kinase (AMPK) and inactivation of acetyl-CoA carboxylase, which in turn promotes reduced fatty acid synthesis and increased fatty acid oxidation (22). In vitro and in vivo studies have shown that polyphenols, such as quercetin and kaempferol, reduce plasma TG by regulating fatty acid mobilization and triglyceride synthesis (23–25). In addition, AMPK activation also involves an increase in energy expenditure, reduction in lipogenesis, and reduction in fat mass and OS through activation of its energetic sensors (26–28). However, the effects of polyphenols in our study were not associated with changes in body weight, which could be attributed to the dose used or to the time of intervention. The lack of effect on BMI has been previously observed with an intervention with 300 mL of blackberry juice drunk daily for 8 weeks (29). Conversely, our results suggest that the hypolipidemic effects of Chaya may also be related to the antioxidant effects of polyphenols. Studies have shown that persistent ROS generation is involved in lipid dysregulation caused by decreased fatty acid uptake, lipogenesis, and β-oxidation of fatty acids (30, 31). In fact, one study showed a statistically significant correlation between the oxidative stress index and serum cholesterol (p < 0.001; r = 0.596) and triglycerides (p < 0.001; r = 0.476) (32). Thus, the antioxidant activity of polyphenols reduces ROS production and also the increase of endogenous antioxidants ameliorates hyperlipidemia and the alterations of many cell or tissue types caused by OS in hyperlipidemia (33).
Our work is the first study conducted in humans to show the clinical hypotriglyceridemic and antioxidant effect of Chaya consumption. Notably, consumption of the Chaya beverage (40 g/L) showed antioxidant activity. These results are similar to the antioxidant effect resulting from the consumption of most antioxidant-rich foods, which are reference sources of polyphenols and widely known for their health-promoting properties (34–38). For example, one study demonstrated that intervention with Mate tea (1L/day) for 90 days promoted a significant increase in serum antioxidant activity compared to baseline levels in the population with dyslipidemia (P < 0.05) (35). Another study demonstrated a significant increase in total antioxidant capacity in subjects with hypercholesterolemia by daily consumption of red wine (125 mL for women or 250 mL for men) for 1 month (36). Another popular beverage that showed a similar antioxidant effect is coffee; its consumption (600 mL/day) for 8 weeks increased plasma antioxidant activity in subjects with hypercholesterolemia (37). In addition, orange juice consumption (750 mL/day) for 8 weeks increased antioxidant activity and decreased TC in overweight subjects (38). This is important as it has been shown that not all interventions involving antioxidant foods in subjects with dyslipidemia generated an effect on circulating antioxidant activity (39). This types of foods have received considerable attention because of their potential antioxidant activity by hydroxyl groups and double bonds in chemical structure of polyphenols (40). In addition, the consumption of different foods with high concentrations of polyphenols, such as beverages made from fruits and vegetables (41), blackberry juice (29) and red grapes (42), showed a significant reduction in the lipid profile of subjects with dyslipidemia. Despite the beneficial effects of these foods on dyslipidemia, most of them are not always accessible to different communities in accordance with their annual availability or to the lifestyle and traditions of the population of those communities. Therefore, based on our results, Chaya represent a low-cost nutritional strategy against dyslipidemias in subjects with access to this plant such as families in the state of Yucatan and also throughout Central America (43).
Interestingly, we observed that consumption of Chaya not only increased antioxidant activity but also decreased the levels of MDA in subjects with dyslipidemia, suggesting a protective effect against OS. Other foods that have been extensively studied for their antioxidant activity and reduction of the oxidative damage in lipids are orange juice (−55%) and coffee (−10.3%) (37, 38). The antioxidant activity of polyphenols can suppress the oxidative modification of plasma lipids and lipoproteins, which are major targets of many antiatherogenic agents and the preferable strategy to prevent the development of CVD. Furthermore, polyphenols, such as quercetin and kaempferol, which are also present in Chaya, can act by activating the transcription factor Nrf2, which travels to the nucleus to bind to the antioxidant response element sequence and induce the expression of genes of the endogenous antioxidant system such as CAT and SOD (44). Our results demonstrated that Chaya consumption induced an increase in CAT gene expression in PBMC, which is in agreement with previous evidence that dietary intervention with food rich in antioxidants can modulate antioxidant enzymes in subjects with dyslipidemia; for example, the consumption of the traditional Chinese herb Graptopetalum paraguayense significantly increased the enzymatic activity of glutathione peroxidase and CAT (45), and aqueous wolfberry fruit intake enhanced the activity of CAT (46). These results suggest that polyphenols present in Chaya contribute to maintaining the redox homeostasis of cells through the activation of antioxidant enzymes. Although an increase of polyphenols in plasma induced the antioxidant machinery, it was not enough to generate a decrease in inflammatory marker CRP, which could be attributed to the doses of the bioactive compound in Chaya. The anti-inflammatory effect is directly dependent of the doses of polyphenols. In fact, a systematic review and meta-analysis showed a tendency toward lower serum concentrations of inflammatory biomarkers only at dose of >200 mg/day of polyphenols from grapes (31) and only 90 mg in plasma (47).
PBMC gene expression could be used as a tool to assess potential markers of the mechanism of action of the intervention. Indeed, one study demonstrated that statins can regulate gene expression in PBMCs from patients with dyslipidemia before detectable changes in lipid profile occur (48). The intervention with Chaya significantly regulated HMGCR and ABCA1 gene expression in PBMC, despite no differences observed in serum total cholesterol in our participants, such as was found for TG. In this sense, recent studies have suggested a function for ABCA1, especially macrophage ABCA1, in lipid metabolism (49). Evidence showed that gene expression of ABCA1 is induced during differentiation of monocytes into macrophages and up-regulated by cholesterol influx. The proposed molecular mechanism is the induction of ABCA1 gene expression by peroxisome proliferator-activated receptors -α and -γ in human macrophages and macrophage-derived foam cells, which promotes increased cholesterol flow from macrophages to the liver (50). Thus, this process could be control susceptibility to macrophage recruitment into different tissues and development of atherosclerosis (51).
Our results on the antioxidant and hypotriglyceridemic effects of Chaya consumption are very interesting. However, this study has some limitations, such as the study design and the small sample size. This type of study did not include a control group and age of participants was highly variable, but they are useful as a first approximation to evaluate the feasibility and acceptability of interventions. In addition, some factors that may affect the outcome measurements, such as dietary caloric restriction, were not examined in the present study. However, the intention of the study was to evaluate the effect of this intervention without modifying the lifestyle of the participants, which contributes to know the effect of Chaya and not of the other modifications that could have been suggested to the population. Thus it remains to be explored whether the findings of this study can be applied to other groups.
Conclusion
Altogether, our results demonstrate that consumption of Chaya has hypotriglyceridemic and antioxidant effects in subjects with dyslipidemia.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
This study involving human participants was reviewed and approved by the Ethics Committee of the Hospital Regional de Alta Especialidad de la Península de Yucatán (No. 2018-030) and Instituto Nacional de Pediatría (No. 2019/03). The participants provided their written informed consent to participate in this study.
Author Contributions
MG-C, IM-V, and AA-N designed the study. TC-C, YC-C, CM-M, and JT-G performed the experiments. CP-M, RL, and AG-S analyzed the data. AA-N, IM-V, NT, and AT wrote the paper. All authors contributed to the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank José L. Tapia-Muñoz, from the Natural Resources Unit at the Centro de Investigación Científica de Yucatán, for the identification and authentication of Chaya leaves. We thank Priscila Villanueva Luna for her support with the participants, as well as Victor Fernando Villanueva Abuxapqui for allowing us the use of areas to carry out the project, and Lilia G. Noriega for critical reading of our manuscript. A special acknowledgment to my husband Evander Ponce Vargas for his support in this project.
Glossary
Abbreviations
- ABCA1
ATP-binding cassette transporter A1 gene
- AMPK
AMP-activated protein kinase
- BMI
body mass index
- BP
blood pressure
- CAT
catalase
- CRP
C-reactive protein
- CVD
cardiovascular diseases
- HDL-C
high-density lipoprotein cholesterol
- HMGCR
hydroxy-methyl-glutaryl coenzyme A reductase
- HPLC
High-performance liquid chromatography
- LDL-C
low-density lipoprotein cholesterol
- MDA
Malondialdehyde
- ORAC
oxygen radical absorbance capacity
- OS
oxidative stress
- PBMC
peripheral blood mononuclear cells
- ROS
reactive oxygen species
- SD
standard deviation
- SEM
standard error of the mean
- SOD
superoxide dismutase
- TC
Total cholesterol
- TG
triglycerides
- WHtR
waist-to-height ratio
- 95% CI
95% confidence intervals.
Footnotes
Funding. Monetary sponsorship was received from Hospital Regional de Alta Especialidad de la Península de Yucatán, Mérida, Yucatán. The edition style of this article was support by Universidad Modelo, Mérida, Yucatán.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.666243/full#supplementary-material
References
- 1.Park K, Gross M, Lee DH, Holvoet P, Himes JH, Shikany JM, et al. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care. (2009) 32:1302–7. 10.2337/dc09-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viigimaa M, Abina J, Zemtsovskaya G, Tikhaze A, Konovalova G, Kumskova E, et al. Malondialdehyde-modified low-density lipoproteins as biomarker for atherosclerosis. Blood Press. (2010) 19:164–8. 10.3109/08037051.2010.484158 [DOI] [PubMed] [Google Scholar]
- 3.Pedro T, Martinez-Hervas S, Tormo C, Garcia-Garcia AB, Saez-Tormo G, Ascaso JF, et al. Oxidative stress and antioxidant enzyme values in lymphomonocytes after an oral unsaturated fat load test in familial hypercholesterolemic subjects. Transl Res. (2013) 161:50–6. 10.1016/j.trsl.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 4.Scognamiglio M, Costa D, Sorriento A, Napoli C. Current drugs and nutraceuticals for the treatment of patients with dyslipidemias. Curr Pharm Des. (2019) 25:85–95. 10.2174/1381612825666190130101108 [DOI] [PubMed] [Google Scholar]
- 5.Kuti JO, Torres ES. Potential nutritional and health benefits of tree spinach. Progress New Crops. (1996) 13:516–20. [Google Scholar]
- 6.Perez-Gonzalez MZ, Macias-Rubalcava ML, Hernandez-Ortega S, Siordia-Reyes AG, Jimenez-Arellanes MA. Additional compounds and the therapeutic potential of Cnidoscolus chayamansa (McVaugh) against hepatotoxicity induced by antitubercular drugs. Biomed Pharmacother. (2019) 117:109140. 10.1016/j.biopha.2019.109140 [DOI] [PubMed] [Google Scholar]
- 7.Pillai KK, Chidambaranathan N, Halith MM, Jayaprakash S, Narayanan N. Anti-hyperglycemic effect of alcoholic extracts of Cnidoscolus chayamansa in experimental diabetes and their effects on key metabolic enzymes involved in carbohydrate metabolism. Int J Res Pharm Chem. (2012) 2:179–87. [Google Scholar]
- 8.Garcia-Rodriguez RV, Gutierrez-Rebolledo GA, Mendez-Bolaina E, Sanchez-Medina A, Maldonado-Saavedra O, Dominguez-Ortiz MA, et al. Cnidoscolus chayamansa Mc Vaugh, an important antioxidant, anti-inflammatory and cardioprotective plant used in Mexico. J Ethnopharmacol. (2014) 151:937–43. 10.1016/j.jep.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 9.Miranda-Velasquez L, Oranday-Cardenas A, Lozano-Garza H, Rivas-Morales C, Chamorro-Cevallos G, Cruz-Vega DE. Hypocholesterolemic activity from the leaf extracts of Cnidoscolus chayamansa. Plant Foods Hum Nutr. (2010) 65:392–5. 10.1007/s11130-010-0202-4 [DOI] [PubMed] [Google Scholar]
- 10.Helrich K, Horwitz W, Williams S. Official Methods of Analysis of the Association of Official Analytical Chemists: Changes in Official Methods of Analysis of AOAC International. Suppl. to the 15. Ed. 5: AOAC. Arlington (1994). [Google Scholar]
- 11.Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Prior RL. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J Agric Food Chem. (2002) 50:4437–44. 10.1021/jf0201529 [DOI] [PubMed] [Google Scholar]
- 12.Koren E, Kohen R, Ginsburg I. Polyphenols enhance total oxidant-scavenging capacities of human blood by binding to red blood cells. Exp Biol Med (Maywood). (2010) 235:689–99. 10.1258/ebm.2010.009370 [DOI] [PubMed] [Google Scholar]
- 13.Zu Y, Li C, Fu Y, Zhao C. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J Pharm Biomed Anal. (2006) 41:714–9. 10.1016/j.jpba.2005.04.052 [DOI] [PubMed] [Google Scholar]
- 14.Messina D, Soto C, Mendez A, Corte C, Kemnitz M, Avena V, et al. Lipid - lowering effect of mate tea intake in dyslipidemic subjects. Nutr Hosp. (2015) 31:2131–9. 10.3305/nh.2015.31.5.8386 [DOI] [PubMed] [Google Scholar]
- 15.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books Champaign; (1988). [Google Scholar]
- 16.Gerard-Monnier D, Erdelmeier I, Regnard K, Moze-Henry N, Yadan JC, Chaudiere J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem Res Toxicol. (1998) 11:1176–83. 10.1021/tx9701790 [DOI] [PubMed] [Google Scholar]
- 17.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. (2001) 29:e45. 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xuan Y, Bobak M, Anusruti A, Jansen EH, Pajak A, Tamosiunas A, et al. Association of serum markers of oxidative stress with myocardial infarction and stroke: pooled results from four large European cohort studies. Eur J Epidemiol. (2019) 34:471–81. 10.1007/s10654-018-0457-x [DOI] [PubMed] [Google Scholar]
- 19.Xuan Y, Gào X, Holleczek B, Brenner H, Schöttker B. Prediction of myocardial infarction, stroke and cardiovascular mortality with urinary biomarkers of oxidative stress: results from a large cohort study. Int J Cardiol. (2018) 273:223–9. 10.1016/j.ijcard.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Romero P, Pichardo-Ontiveros E, Avila-Nava A, Vazquez-Manjarrez N, Tovar AR, Pedraza-Chaverri J, et al. The effect of nopal (Opuntia ficus indica) on postprandial blood glucose, incretins, and antioxidant activity in Mexican patients with type 2 diabetes after consumption of two different composition breakfasts. J Acad Nutr Diet. (2014) 114:1811–8. 10.1016/j.jand.2014.06.352 [DOI] [PubMed] [Google Scholar]
- 21.Loarca-Pina G, Mendoza S, Ramos-Gomez M, Reynoso R. Antioxidant, antimutagenic, and antidiabetic activities of edible leaves from Cnidoscolus chayamansa Mc Vaugh. J Food Sci. (2010) 75:H68–72. 10.1111/j.1750-3841.2009.01505.x [DOI] [PubMed] [Google Scholar]
- 22.Gasparrini M, Giampieri FM, Alvarez Suarez J, Mazzoni LY, Forbes Hernandez TL, Quiles J, et al. AMPK as a new attractive therapeutic target for disease prevention: the role of dietary compounds AMPK and disease prevention. Curr Drug Targets. (2016) 17:865–89. 10.2174/1573399811666150615150235 [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Zorita S, Lasa A, Abendaño N, Fernandez-Quintela A, Mosqueda-Solís A, Garcia-Sobreviela MP, et al. Phenolic compounds apigenin, hesperidin and kaempferol reduce in vitro lipid accumulation in human adipocytes. J Transl Med. (2017) 15:1–10. 10.1186/s12967-017-1343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuipers EN, Dam ADv, Held NM, Mol IM, Houtkooper RH, Rensen PC, et al. Quercetin lowers plasma triglycerides accompanied by white adipose tissue browning in diet-induced obese mice. Int J Mol Sci. (2018) 19:1786. 10.3390/ijms19061786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo M-J, Lee Y-J, Hwang J-H, Kim K-J, Lee B-Y. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J Nutr Biochem. (2015) 26:1308–16. 10.1016/j.jnutbio.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 26.Eo H, Jeon Y-j, Lee M, Lim Y. Brown Alga Ecklonia cava polyphenol extract ameliorates hepatic lipogenesis, oxidative stress, and inflammation by activation of AMPK and SIRT1 in high-fat diet-induced obese mice. J Agric Food Chem. (2015) 63:349–59. 10.1021/jf502830b [DOI] [PubMed] [Google Scholar]
- 27.Luna-Vital D, Luzardo-Ocampo I, Cuellar-Nuñez ML, Loarca-Piña G, de Mejia EG. Maize extract rich in ferulic acid and anthocyanins prevents high-fat-induced obesity in mice by modulating SIRT1, AMPK and IL-6 associated metabolic and inflammatory pathways. J Nutr Biochem. (2020) 79:108343. 10.1016/j.jnutbio.2020.108343 [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Yasen M, Tang D, Ye J, Aisa HA, Xin X. Polyphenol-enriched extract of Rosa rugosa Thunb regulates lipid metabolism in diabetic rats by activation of AMPK pathway. Biomed Pharmacother. (2018) 100:29–35. 10.1016/j.biopha.2018.01.143 [DOI] [PubMed] [Google Scholar]
- 29.Aghababaee SK, Vafa M, Shidfar F, Tahavorgar A, Gohari M, Katebi D, et al. Effects of blackberry (Morus nigra L.) consumption on serum concentration of lipoproteins, apo AI, apo B, and high-sensitivity-C-reactive protein and blood pressure in dyslipidemic patients. J Res Med Sci. (2015) 20:684. 10.4103/1735-1995.166227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassan W, Rongyin G, Daoud A, Ding L, Wang L, Liu J, et al. Reduced oxidative stress contributes to the lipid lowering effects of isoquercitrin in free fatty acids induced hepatocytes. Oxid Med Cell Longev. (2014) 2014:313602. 10.1155/2014/313602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huc L, Lemarié A, Guéraud F, Héliès-Toussaint C. Low concentrations of bisphenol A induce lipid accumulation mediated by the production of reactive oxygen species in the mitochondria of HepG2 cells. Toxicol In Vitro. (2012) 26:709–17. 10.1016/j.tiv.2012.03.017 [DOI] [PubMed] [Google Scholar]
- 32.Turkdogan KA, Akpinar O, Karabacak M, Akpinar H, Turkdogan FT, Karahan O. Association between oxidative stress index and serum lipid levels in healthy young adults. J Pak Med Assoc. (2014) 64:379–81. [PubMed] [Google Scholar]
- 33.Meydani M, Hasan ST. Dietary polyphenols and obesity. Nutrients. (2010) 2:737–51. 10.3390/nu2070737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ninfali P, Chiarabini A, Angelino D. The ORAC/kcal ratio qualifies nutritional and functional properties of fruit juices, nectars, and fruit drinks. Int J Food Sci Nutr. (2014) 65:708–12. 10.3109/09637486.2014.918591 [DOI] [PubMed] [Google Scholar]
- 35.Boaventura BCB, Di Pietro PF, Stefanuto A, Klein GA, de Morais EC, de Andrade F, et al. Association of mate tea (Ilex paraguariensis) intake and dietary intervention and effects on oxidative stress biomarkers of dyslipidemic subjects. Nutrition. (2012) 28:657–64. 10.1016/j.nut.2011.10.017 [DOI] [PubMed] [Google Scholar]
- 36.Apostolidou C, Adamopoulos K, Lymperaki E, Iliadis S, Papapreponis P, Kourtidou-Papadeli C. Cardiovascular risk and benefits from antioxidant dietary intervention with red wine in asymptomatic hypercholesterolemics. Clin Nutr ESPEN. (2015) 10:e224–33. 10.1016/j.clnesp.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Lopez S, Sarria B, Mateos R, Bravo-Clemente L. Moderate consumption of a soluble green/roasted coffee rich in caffeoylquinic acids reduces cardiovascular risk markers: results from a randomized, cross-over, controlled trial in healthy and hypercholesterolemic subjects. Eur J Nutr. (2019) 58:865–78. 10.1007/s00394-018-1726-x [DOI] [PubMed] [Google Scholar]
- 38.Dourado GK, Cesar TB. Investigation of cytokines, oxidative stress, metabolic, and inflammatory biomarkers after orange juice consumption by normal and overweight subjects. Food Nutr Res. (2015) 59:28147. 10.3402/fnr.v59.28147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medina-Vera I, Gómez-de-Regil L, Gutiérrez-Solis AL, Lugo R, Guevara-Cruz M, Pedraza-Chaverri J, et al. Dietary strategies by foods with antioxidant effect on nutritional management of dyslipidemias: a systematic review. Antioxidants. (2021) 10:225. 10.3390/antiox10020225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L, et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. (2010) 9:3. 10.1186/1475-2891-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiu H-F, Chen Y-J, Lu Y-Y, Han Y-C, Shen Y-C, Venkatakrishnan K, et al. Regulatory efficacy of fermented plant extract on the intestinal microflora and lipid profile in mildly hypercholesterolemic individuals. J Food Drug Anal. (2017) 25:819–27. 10.1016/j.jfda.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahbar AR, Mahmoudabadi MMS, Islam MS. Comparative effects of red and white grapes on oxidative markers and lipidemic parameters in adult hypercholesterolemic humans. Food Funct. (2015) 6:1992–8. 10.1039/C5FO00100E [DOI] [PubMed] [Google Scholar]
- 43.Ross-Ibarra J, Molina-Cruz A. The ethnobotany of Chaya (Cnidoscolus aconitifoliusSSP. Aconitifoliusbreckon) La Etnobotanica de Chaya (Cnidoscolus aconitifoliusssp. aconitifoliusBreckon): a nutritious Maya Vegetable Una Verdura Nutritiva Maya. Econ Bot. (2002) 56:350–65. 10.1663/0013-0001(2002)056[0350:TEOCCA]2.0.CO;2 [DOI] [Google Scholar]
- 44.Khurana S, Venkataraman K, Hollingsworth A, Piche M, Tai TC. Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients. (2013) 5:3779–827. 10.3390/nu5103779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu-Ling L, Hsin-Yi P, Hui-Min H, Ching-Hsiu L, Su-Tze C. Effects of Graptopetalum paraguayense consumption on serum lipid profiles and antioxidative status in hypercholesteremic subjects. J Sci Food Agric. (2011) 91:1230–5. 10.1002/jsfa.4304 [DOI] [PubMed] [Google Scholar]
- 46.Lee YJ, Ahn Y, Kwon O, Lee MY, Lee CH, Lee S, et al. Dietary wolfberry extract modifies oxidative stress by controlling the expression of inflammatory mRNAs in overweight and hypercholesterolemic subjects: a randomized, double-blind, placebo-controlled trial. J Agric Food Chem. (2017) 65:309–16. 10.1021/acs.jafc.6b04701 [DOI] [PubMed] [Google Scholar]
- 47.Haghighatdoost F, Gholami A, Hariri M. Effect of grape polyphenols on selected inflammatory mediators: a systematic review and meta-analysis randomized clinical trials. EXCLI J. (2020) 19:251–67. 10.17179/excli2020-1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wibaut-Berlaimont V, Randi A, Mandryko V, Lunnon M, Haskard D, Naoumova R. Atorvastatin affects leukocyte gene expression in dyslipidemia patients: in vivo regulation of hemostasis, inflammation and apoptosis. J Thromb Haemost. (2005) 3:677–85. 10.1111/j.1538-7836.2005.01211.x [DOI] [PubMed] [Google Scholar]
- 49.Schmitz G, Kaminski W, Porsch-Özcürümez M, Klucken J, Orso E, Bodzioch M, et al. ATP-binding cassette transporter A1 (ABCA1) in macrophages: a dual function in inflammation and lipid metabolism? Pathobiology. (1999) 67:236–40. 10.1159/000028100 [DOI] [PubMed] [Google Scholar]
- 50.Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, et al. PPAR-α and PPAR-γ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. (2001) 7:53–8. 10.1038/83348 [DOI] [PubMed] [Google Scholar]
- 51.Van Eck M, Bos IST, Kaminski WE, Orsó E, Rothe G, Twisk J, et al. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc Natl Acad Sci U S A. (2002) 99:6298–303. 10.1073/pnas.092327399 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.