Abstract
Background
The disease course of inflammatory bowel disease (IBD) following treatment with glucagon-like peptide (GLP)-1 based therapies is unclear. The aim of this study was to examine the disease course of IBD in patients treated with GLP-1 based therapies compared with treatment with other antidiabetics.
Methods
Using nationwide Danish registries, we identified patients with IBD and type 2 diabetes who received antidiabetic treatment between 1 January 2007 and 31 March 2019. The primary outcome was a composite of the need for oral corticosteroids, tumour necrosis factor-α inhibitors, IBD-related hospitalisation, or IBD-related surgery. In the setting of a new-user active comparator design, we used Poisson regression to estimate incidence rate ratios (IRR) comparing treatment with GLP-1 receptor agonists and dipeptidyl peptidase (DPP)-4 inhibitors with other antidiabetic therapies. The analyses were adjusted for age, sex, calendar year, IBD severity, and metformin use.
Findings
We identified 3751 patients with a diagnosis of IBD and type 2 diabetes and with a prescription of an antidiabetic drug (GLP-1 receptor agonists/DPP-4 inhibitors: 982 patients; other antidiabetic treatment: 2769 patients). The adjusted IRR of the composite outcome was 0·52 (95% CI: 0·42–0·65) for patients exposed to GLP-1 receptor agonists/DPP-4 inhibitors compared with patients exposed to other antidiabetics.
Interpretation
In patients with IBD and type 2 diabetes, we observed a lower risk of adverse clinical events amongst patients treated with GLP-1 based therapies compared with treatment with other antidiabetics. These findings suggest that treatment with GLP-1 based therapies may improve the disease course of IBD.
Keywords: Colitis ulcerative, Crohn's disease, Glucagon-like-peptide 1 receptor agonists, Dipeptidyl peptidase-4 inhibitors, Pharmacoepidemiology, Prognosis
Abbreviations: ATC, Anatomical Therapeutic Chemical; CD, Crohn's disease; DPP, dipeptidyl peptidase; GLP, glucagon-like-peptide; IBD, inflammatory bowel disease; IMID, immune-mediated inflammatory disease; ICD, International Classification of Diseases; IR, incidence rate; IRR, incidence rate ratios; PY, person-years; SGLT2, Sodium-glucose Cotransporter-2; TNF, tumour necrosis factor; UC, ulcerative colitis
Research in context.
Evidence before this study
DPP-4 inhibitors and GLP-1 receptor agonists alleviate gut inflammation in mice.
Added value of this study
Compared with other antidiabetic drugs, treatment with GLP-1 based therapies was associated with a decreased risk of adverse clinical events defined as a composite of the need for oral corticosteroid treatment, need for TNF-α-inhibitor treatment, IBD-related hospitalisation, or IBD-related major surgery.
Implications of all the available evidence
GLP-1 based therapy may represent a new treatment option for inflammatory bowel disease.
Alt-text: Unlabelled box
1. Introduction
Inflammatory bowel disease (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), are chronic intestinal diseases affecting millions of individuals worldwide [1]. The diseases often occur during early adulthood, and many patients experience a life-long disease course with relapsing and remitting inflammation of their gut [1], [2], [3]. Although the aetiology is not fully understood, the development of both CD and UC is believed to be caused by uncontrolled inflammation and immune dysregulation in individuals who are genetically predisposed [4].
The prevalence of multimorbidity is increasing [5], and patients with IBD may experience coexisting conditions, such as diabetes. Management of multimorbidity is challenging on many levels [5]. Regarding co-medication, treatment of one condition may be contraindicated for another condition (e.g., non-steroidal anti-inflammatory drug use in patients with severe renal disease) [6]. However, it is also possible that treatment of one chronic condition will have a positive impact on a concurrent chronic condition (e.g., metoprolol treatment for hypertension and essential tremor) [7].
GLP-1 based therapies including dipeptidyl peptidase (DPP)−4 inhibitors and glucagon-like-peptide (GLP)−1 receptor agonists are glucose-lowering drugs approved for treatment of type 2 diabetes [8]. DPP-4 is a peptide known to cleave a broad range of substrates, including GLP-1 and GLP-2, and is involved in the immune response [9]. GLP-1 is secreted from the intestinal L cells in response to nutrient intake and stimulates insulin release and suppresses glucagon release [10]. Additionally, GLP-1 may reduce systemic inflammation indirectly through improved glucose regulation and body weight loss and directly through binding to GLP-1 receptors on peripheral immune cells. In the gut, GLP-1 may control inflammation through binding to GLP-1 receptors on intestinal intraepithelial lymphocytes [11]. In favour of a clinically relevant role of GLP-1 in reducing systemic inflammation, a meta-analysis of randomised clinical trials concluded that GLP-1 receptor agonists reduce circulating levels of inflammatory biomarkers, including C-reactive protein and tumour necrosis factor (TNF)-α [12]. The clinical impact of GLP-1 in gut inflammation, however, remains uncertain. Although animal studies have shown that treatment with DPP-4 inhibitors and GLP-1 receptor agonists alleviate gut inflammation in mice [13,14], their role in patients with IBD is unclear. Observational studies have examined the association between treatment with DPP-4 inhibitors and risk of incident IBD [15,16], but to our knowledge, no studies have assessed the disease course in IBD patients treated with GLP-1-receptor agonists and/or DPP-4 inhibitors.
We conducted a nationwide population-based cohort study to examine the disease course in patients with IBD and type 2 diabetes treated with GLP-1 based therapies compared with patients treated with other antidiabetic drugs. We hypothesised that treatment with GLP-1 based therapies is associated with an improved disease course in patients with IBD.
2. METHODS
2.1. Study population
We used the Danish National Patient Registry and the Danish National Prescription Registry to identify all patients with a diagnosis of both IBD and type 2 diabetes between 1 January 2007 and 31 March 2019, including both prevalent and incident cases during this period [17,18]. The Danish National Patient Registry contains information on all discharges from hospitals in Denmark since 1977, and from outpatient clinics since 1995. Diagnoses are classified according to the International Classification of Diseases, 8th (ICD-8) and 10th (ICD-10) revision [17]. The Danish National Prescription Registry contains information on all prescribed drugs redeemed at Danish community pharmacies since 1995 [18]. Drugs are coded according to the Anatomical Therapeutic Chemical (ATC) system [19].
IBD patients were defined with recordings back to 1977. However, we required an active disease status defined as being alive and living in Denmark at entry and at least two IBD-related recordings within five years of entry (Supplementary Table 1). Moreover, as previous studies have shown considerable disease heterogeneity according to age of onset of IBD [20], we included only patients with adult onset of IBD disease (>=17 years at first recording). Patients with recordings for both UC and CD were classified with the second recording.
Type 2 diabetes was defined using an algorithm based on data from the Danish National Patient Registry and data from the Danish National Prescription Registry (see Supplementary material for a detailed description of the algorithm). To be eligible for inclusion in the study population, the patients with IBD and type 2 diabetes had to have redeemed a prescription of an antidiabetic drug following their latest diagnosis of IBD or type 2 diabetes dependant of which of the two diseases that were diagnosed the latest between 1 January 2007 and 31 March 2019.
2.2. Exposure and covariates
Patients were considered exposed if they initiated treatment with a GLP-1 receptor agonist and/or DPP-4 inhibitor defined as at least two redemptions of one of these drugs. To emulate a randomised clinical trial and thereby minimise selection bias [21], we excluded prevalent users of GLP-1 based therapies at study entry (n = 296). We used the daily defined dose to estimate exposure duration. Six months after estimated end of the last redemption, we censored follow-up for exposed individuals. The patients were categorised as non-users of GLP-1 based therapies if they redeemed a prescription of an antidiabetic drug other than a GLP-1 receptor agonist and DPP-4 inhibitor. Antidiabetic therapies were identified and classified based on ATC codes from the Danish National Prescription Registry (Supplementary Table 2).
We used the Danish National Patient Registry to obtain information on IBD subtype (CD or UC), age at IBD diagnosis (<30, 30–65, and >65 years), IBD duration (<1, 1–5, 5–10, ≥10 years), IBD-related inpatient hospitalisations one and five years before date of study entry, and IBD-related surgeries (0, 1–2, >2) five years before date of study entry. The Danish National Prescription Registry and the Danish National Patient Registry were used to obtain information on IBD drug treatment one year before the date of study entry. Immunomodulators include mercaptopurine, azathioprine, and methotrexate. Immune suppressants include mycophenolic acid, tofacitinib, vedolizumab, ustekinumab, thalidomide, and ciclosporin. The codes are provided in Supplementary Table 1. As a measure of IBD severity one year before date of study entry, we assessed the number of oral corticosteroid redemptions (0, 1–2, >2), IBD-related inpatient hospitalisations (0, 1–2, >2), IBD-related major surgery defined as colectomies, resections, and other unspecified major intestinal operations (yes, no), and IBD-related minor surgery defined as intra-abdominal abscess drainage, fistula surgery, and surgery for perianal complications (0, 1–2, >2). Those were combined into a baseline severity score divided in mild (highest score=0 and no major surgery), moderate (highest score=1–2 and no major surgery), or severe disease (highest score >2 or major surgery) [22]. This disease severity score has previously been used in a Danish nationwide cohort study of IBD patients [22].
Other immune-mediated inflammatory diseases with similar treatment strategies as to IBD were identified in the Danish National Patient Registry; these include ankylosing spondylitis, psoriasis, psoriasis arthritis, rheumatoid arthritis, and sarcoidosis.
We used the Danish National Patient Registry to obtain information on age at type 2 diabetes diagnosis at study entry which was categorised into three categories (<30, 30–65, >65 years), type 2 diabetes duration at study entry in three categories (<1, 1–5, >5 years), and comorbidities related to type 2 diabetes five years before the date of study entry. We used the Danish National Prescription Registry to obtain information on type 2 diabetes drug treatment one year before the date of study entry. The codes are provided in Supplementary Tables 2 and 3.
2.3. Outcomes
Our primary outcome was a composite of the need for oral corticosteroid treatment, need for TNF-α-inhibitor treatment, IBD-related hospitalisation, or IBD-related major surgery. From the Danish National Prescription Registry, we obtained information on use of corticosteroid treatment defined as a redemption of any oral corticosteroid during follow-up. Information on use of TNF-α-inhibitors was obtained through the Danish National Patient Registry with treatment classification codes for infliximab, adalimumab, and golimumab, and redemptions in the Danish National Prescription Registry for adalimumab (Supplementary Table 1). We used the Danish National Patient Registry to identify all IBD-related hospital admissions and surgical procedures during follow-up (Supplementary Table 1). We used the Danish Civil Registration System to obtain information on date of death. Since 1968, this registry has recorded information on vital status, date of birth, residence, date of emigration, and date of death for all Danish citizens and is updated daily [23].
2.4. Statistical analysis
When patients had a diagnosis of both IBD and type 2 diabetes and had redeemed a prescription for an antidiabetic drug, they fulfilled the inclusion criterion. Follow-up began on 1 January 2007 or the date of the criterion if this was fulfilled later than 1 January 2007. Since we were interested in examining the effect of GLP-1 based therapies on initiation of corticosteroid or TNF-α-inhibitor therapy, patients in current treatment at entry were not considered at risk of subsequent corticosteroid or TNF-α-inhibitor therapy. Patients were considered exposed to oral corticosteroids or TNF-α-inhibitors at entry if the estimated end of the previous treatment period with these drugs exceeded the date of entry. Furthermore, any new or subsequent treatment of these drugs initiated within the first three months of follow-up were not counted as an outcome in the outcome analysis. We followed all patients until the date of the composite outcome, emigration, discontinuation of the antidiabetic drug (+ six months), death, or 31 March 2019, whichever event occurred first.
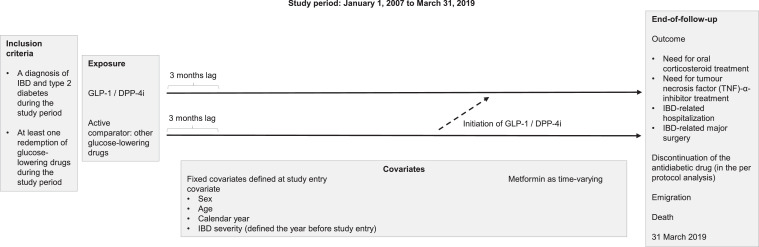
In our main per protocol analysis, exposure to GLP-1 receptor agonists and/or DPP-4 inhibitors was considered a time-varying variable with IBD patients contributing person-time to the relevant exposure group. Patients could switch from the non-user group to the user group, but not in the opposite way. We used a lag period of three months following the date of entry to increase the probability that the outcome was associated with the exposure (Fig. 1).
Fig. 1.
Study design. Follow-up began on 1 January 2007 or when an individual was diagnosed with both IBD and type 2 diabetes and had at least one redemption of antidiabetics if this was fulfilled later than 1 January 2007. Covariates were defined at start to follow up. All IBD patients were contributing with person-time as non-user until the second redemption of treatment with GLP-1 receptor agonists/DPP-4 inhibitors. However, there was no contribution to person-time as exposed until three months after the second redemption due to a lag period implemented to increase the probability of temporality, i.e. that the exposure is prior to the outcome. We followed all patients until the date of the composite outcome, discontinuation of the antidiabetic drug, emigration, death, or 31 March 2019, whichever event occurred first.
We calculated incidence rates (IR) per 1000 person-years (PY). Using Poisson regression models, we estimated incidence rate ratios (IRR) with 95% confidence intervals (CI). The analyses were adjusted for sex, age, calendar year, and IBD severity at entry as fixed covariates, and metformin use as a time-varying covariate. Metformin has previously been shown to be associated with the risk of IBD [24], and due to differences in the prevalence of metformin users between patients exposed to GLP-1 receptor agonists and/or DPP-4 inhibitors and patients exposed to other antidiabetics, metformin was included in the analysis. Because the risk of the composite outcome may differ according to IBD subtype and sex, stratified sub-analyses were also performed.
We conducted sensitivity analyses to examine the robustness of our findings. Firstly, given uncertainties related to the length of the lag period, we used no lag period and a lag period of one year. Secondly, we performed an intention to treat analysis in which we changed the exposure period to last throughout the follow-up (ever-never exposure) to avoid differential censoring between users and non-users. Thirdly, we included prevalent users of GLP-1 receptor agonists and DPP-4 inhibitors to evaluate the implication of including prevalent vs. new users only. Fourthly, we excluded patients who had redeemed a prescription of an insulin or analogue during the study period, since the probability that these patients were prescribed a GLP-1 receptor agonist or DPP-4 inhibitor might be different from those patients who were not treated with an injectable drug. Fifth, to account for a possible incretin effect of GLP-1 receptor agonists or immune effects of DPP-4 inhibitors, we redefined exposure into GLP-1 receptor agonists only or DPP-4 inhibitors only. Sixth, because IBD treatment and contacts may differ with age, we stratified the cohort by predefined age groups at study entry (<50, 50–80, >80 years). Seventh, we stratified the cohort according to mild, moderate, and severe IBD at start of follow-up. Eighth, because patients with other immune-mediated inflammatory diseases may receive similar treatment strategies, patients with one or more of these comorbidities were excluded. Ninth, since corticosteroids may be contraindicated in patients with cardiovascular diseases, we excluded patients with cardiovascular comorbidity. Tenth, because the probability of exposure and treatment may have changed over time, we stratified the follow-up in three predefined periods (2007–2009, 2010–2015, 2015–2019).
We used SAS V.9.4 (SAS Institute, Cary, NC) for statistical analyses.
2.5. Ethical considerations
The study was approved by the Danish Data Protection Agency, and data were analysed on a secure research server at the Danish Health Data Authority. In Denmark, ethical approval is not required for research using pre-existing, routinely collected data. The study adheres to The Strengthening the Reporting of Observational Studies in Epidemiology statement [25].
2.6. Role of the funding source
The funding source had no role in the study design, collection, analysis, interpretation of the data, the writing of this manuscript, or the decision to submit the manuscript for publication. The corresponding author had full access to the data and all authors accepted the responsibility to submit the manuscript for publication.
3. RESULTS
Between 1 January 2007 and 31 March 2019, we identified 3751 IBD patients (CD, n = 960; UC, n = 2791) also diagnosed with type 2 diabetes and receiving antidiabetic drug treatment. Among these, we identified 982 new users of GLP-1 based therapies and 2769 non-users during the study period.
Sex, IBD hospitalisation, IBD surgery, and type 2 diabetes duration were equally distributed amongst patients exposed to GLP-1 receptor agonists and/or DPP-4 inhibitors and patients exposed to other antidiabetic drugs during follow-up. Before start to follow-up, there were no difference in use of oral corticosteroids, TNF-α-inhibitors, immunomodulators, and other immune suppressants, whereas more exposed patients also used 5-aminosalicylic acid and topical corticosteroids before entry. Except for insulin, exposed patients received more type 2 diabetes medication of all types before entry (Table 1).
Table 1.
General, IBD-related, and type-2 diabetes related characteristics of the study population at study entry by treatment with GLP-1 receptor agonists/DPP-4 inhibitors during follow-up.
| Variable | New users of GLP-1-receptor agonists and/or DPP-4 inhibitors during follow-up |
Non-users of GLP-1-receptor agonists and/or DPP-4 inhibitors |
||
|---|---|---|---|---|
| N | % | N | % | |
| 982 | 2769 | |||
| General characteristics | ||||
| Sex | ||||
| Female | 445 | 45·3 | 1298 | 46·9 |
| Male | 537 | 54·7 | 1471 | 53·1 |
| Age at study entry | ||||
| <30 years | 10 | 1·0 | 34 | 1·2 |
| 30–65 years | 701 | 71·4 | 1285 | 46·6 |
| >65 years | 271 | 27·6 | 1450 | 52·4 |
| Cause criterion† | ||||
| IBD before T2D | 611 | 62·2 | 1568 | 56·6 |
| IBD same date as T2D | 10 | 1·0 | 53 | 1·9 |
| IBD after T2D | 361 | 36·8 | 1148 | 41·5 |
| Year criterion† | ||||
| <2007 | 402 | 40·9 | 863 | 31·2 |
| 2007–2010 | 235 | 23·9 | 581 | 21·0 |
| 2011–2014 | 248 | 25·3 | 779 | 28·1 |
| 2015–2019 | 97 | 9·9 | 546 | 19·7 |
| IBD related characteristics | ||||
| IBD subtype | ||||
| CD | 221 | 22·5 | 739 | 26·7 |
| UC | 761 | 77·5 | 2030 | 73·3 |
| Age at IBD diagnosis | ||||
| <30 years | 102 | 10·4 | 159 | 5·7 |
| 30–65 years | 732 | 74·5 | 1590 | 57·4 |
| >65 years | 149 | 17·1 | 1020 | 36·8 |
| IBD duration | ||||
| < 1 year | 393 | 40·0 | 1426 | 51·5 |
| 1–5 years | 167 | 17·0 | 503 | 18·2 |
| 5–10 years | 126 | 12·8 | 250 | 9·0 |
| > 10 years | 296 | 30·1 | 590 | 21·3 |
| IBD-related inpatient hospitalization | ||||
| 1 year before entry | 40 | 4·1 | 110 | 4·0 |
| 5 years before entry | 139 | 14·2 | 439 | 15·9 |
| No. of IBD related surgeries | ||||
| None | 845 | 86·0 | 2315 | 83·6 |
| 1–2 | 95 | 9·7 | 328 | 11·8 |
| > 2 | 42 | 4·3 | 126 | 4·6 |
| IBD severity | ||||
| Mild | 764 | 77·8 | 2041 | 73·7 |
| Moderate | 110 | 11·2 | 344 | 12·4 |
| Severe | 108 | 11·0 | 384 | 13·9 |
| IBD-related comorbidity | ||||
| Immune-mediated inflammatory diseases^ | 23 | 2·3 | 79 | 2·9 |
| IBD drug treatment | ||||
| 5-ASA | 558 | 56·8 | 1304 | 47·1 |
| Oral corticosteroids | 498 | 50·7 | 1446 | 52·2 |
| Topical corticosteroids | 346 | 35·2 | 862 | 31·1 |
| Immunomodulators | 163 | 16·6 | 395 | 14·3 |
| Other immune suppressants | 7 | 0·7 | 21 | 0·8 |
| TNF-α-inhibitors | 14 | 1·4 | 22 | 0·8 |
| No IBD medication | 235 | 23·9 | 729 | 26·3 |
| Type 2 diabetes related characteristics | ||||
| Age at type 2 diabetes diagnosis | ||||
| < 30 years | 25 | 2·5 | 70 | 2·5 |
| 30–65 years | 777 | 79·1 | 1601 | 57·8 |
| > 65 years | 180 | 18·3 | 1098 | 39·7 |
| Type 2 diabetes duration | ||||
| < 1 year | 477 | 48·6 | 1354 | 48·9 |
| 1–5 years | 256 | 26·1 | 675 | 24·4 |
| > 5 years | 249 | 25·4 | 740 | 26·7 |
| Type 2 diabetes related comorbidities | ||||
| Nephropathy | 19 | 1·9 | 130 | 4·7 |
| Retinopathy | 43 | 4·4 | 91 | 3·3 |
| Neuropathy | 21 | 2·1 | 58 | 2·1 |
| Myocardial infarction | 21 | 2·1 | 80 | 2·9 |
| Heart failure | 23 | 2·3 | 144 | 5·2 |
| Cerebrovascular disease | 35 | 3·6 | 183 | 6·6 |
| Other diabetic complications | 63 | 6·4 | 243 | 8·8 |
| Antihypertensive drugs | 587 | 59·8 | 1822 | 65·8 |
| Lipid-lowering drugs | 441 | 44·9 | 1167 | 42·1 |
| Type 2 diabetes drug treatment | ||||
| Biguanides | 374 | 38·1 | 718 | 25,9 |
| Insulin | 111 | 11·3 | 402 | 14·5 |
| Sulfonylureas | 232 | 23·6 | 438 | 15·8 |
| Thiazolidinediones | 18 | 1·8 | 9 | 0·3 |
| SGLT2 inhibitors | <5 | – | <5 | – |
| Combination therapy | 12 | 1·2 | 14 | 0·5 |
CD Crohn's disease, DPP-4 dipeptidyl peptidase 4, GLP-1 glucagon-like-peptide 1, IBD Inflammatory bowel disease, SGLT2 Sodium-glucose Cotransporter-2, T2D Type 2 diabetes, TNF-α tumour necrosis factor alpha, UC Ulcerative Colitis.
Criterion is when an individual is diagnosed with both IBD and type 2 diabetes, and the latest date of diagnosis with one of the two diseases is the date of criterion.
Immune-mediated inflammatory diseases: Ankylosing spondylitis, psoriasis, psoriasis arthritis, rheumatoid arthritis, sarcoidosis.
3.1. Incidence of hospitalisation, surgery, oral corticosteroids, or TNF-α inhibitors
For the composite outcome (the need for oral corticosteroids, need for TNF-α inhibitors, IBD-related hospitalisation, or IBD-related surgery), we observed 199 events in patients exposed to a GLP-1 receptor agonist and/or DPP-4 inhibitor (IR=106·9 per 1000 PY) vs 2314 events in the patients exposed to other antidiabetics (IR=242·9 per 1000 PY), resulting in a crude IRR of 0·44 (95% CI 0·38–0·51). The adjusted IRR was 0·52 (95% CI 0·42–0·65) after adjustment for sex, age, calendar year, IBD severity, and metformin use (Table 2).
Table 2.
IRRs of composite and specific outcomes comparing treatment with GLP-1 receptor agonists/DPP-4 inhibitors with other antidiabetic therapies.
| New users of GLP-1-receptor agonists and/or DPP-4 inhibitors |
Non-users of GLP-1-receptor agonists and/or DPP-4 inhibitors |
Crude estimate | Adjusted estimate | |||||
|---|---|---|---|---|---|---|---|---|
| Composite outcome | Events | PY | IR per 1000 PY | Events | PY | IR per 1000 PY | IRR (95% CI) | IRR (95% CI) |
| Total | 199 | 1861 | 106·9 | 2333 | 9652 | 241·7 | 0·44 (0·38–0·51) | 0·52 (0·42–0·65) |
| Sex | ||||||||
| Female | 50 | 344 | 145·1 | 1079 | 4325 | 249·5 | 0·44 (0·35–0·54) | 0·49 (0·35–0·69) |
| Male | 149 | 1517 | 98·2 | 1235 | 5202 | 237·4 | 0·45 (0·37–0·55) | 0·55 (0·41–0·73) |
| IBD subtype | ||||||||
| CD | 90 | 836 | 107·6 | 640 | 2144 | 298·5 | 0·49 (0·36–0·65) | 0·62 (0·41–0·92) |
| UC | 109 | 1025 | 106·3 | 1674 | 7382 | 226·8 | 0·43 (0·37–0·51) | 0·50 (0·39–0·65) |
| Separate outcomes | ||||||||
| Hospitalisation | 178 | 2889 | 61·6 | 1445 | 14,024 | 103·0 | 0·60 (0·51–0·70) | 0·73 (0·58–0·91) |
| Surgery | 97 | 3675 | 26·4 | 593 | 17,456 | 34·0 | 0·78 (0·63–0·96) | 0·79 (0·57–1·09) |
| Steroid initiation | 133 | 2813 | 47·3 | 1238 | 13,104 | 94·5 | 0·50 (0·42–0·60) | 0·54 (0·41–0·70) |
| TNF-α-inhibitor initiation | 29 | 4183 | 6·9 | 213 | 18,737 | 11·4 | 0·61 (0·41–0·90) | 0·56 (0·32–1·00) |
CD Crohn's disease, CI confidence interval, IR Incidence rate, IRR Incidence Rate Ratio, PY Person Years, TNF-α tumour necrosis factor alpha, UC Ulcerative Colitis.
The potential benefit of GLP-1 receptor agonists and/or DPP-4 inhibitors was similar amongst females (adjusted IRR 0·49, 95% CI 0·35–0·69) and males (adjusted IRR 0·55, 95% CI 0·41–0·73); test for homogeneity, pinteraction=0·91.
When analysing the risk of UC and CD separately, the adjusted IRR of the composite outcome was 0·50 (95% CI 0·39–0·65) amongst individuals diagnosed with UC and 0·62 (95% CI 0·41–0·92) amongst individuals diagnosed with CD; test for homogeneity, pinteraction=0·81.
In analyses of the individual outcomes included in the composite outcome, need for oral corticosteroids and hospitalisation were lower in individuals exposed to GLP-1 receptor agonists and/or DPP-4 inhibitors: adjusted IRR=0·54 (95% CI 0·41–0·70) and adjusted IRR=0·73 (95% 0·58–0·91), respectively. The IRRs of new treatment with TNF-α inhibitors and surgery were likewise lower with adjusted IRRs of 0·56 (95% CI 0·32–1·00) and 0·79 (0·57–1·09), respectively, although not statistically significant.
Sensitivity analyses yielded results that were consistent with those of the main analysis with adjusted IRRs ranging between 0·38 and 0·62 (Table 3). Accordingly, we found similar results when using no lag period or a lag period of one year; when applying an intention to treat analysis in which the exposure period lasted throughout the follow-up period (ever-never exposure); when including prevalent users of GLP-1 receptor agonists and DPP-4 inhibitors; when analysing exposure to GLP-1 receptor agonists and DPP-4 inhibitors separately; when excluding individuals who had redeemed a prescription of insulin during the study period; and when stratifying by age, disease severity, and calendar period at study entry. Exclusion of 102 patients with an immune-mediated inflammatory comorbidity yielded an adjusted IRR=0·51 (95% CI 0·40–0·63). When excluding 433 patients with a cardiovascular comorbidity, the adjusted IRR=0·55 (95% CI 0·39–0·63).
Table 3.
IRRs of the composite outcome comparing treatment with GLP-1 receptor agonists/DPP-4 inhibitors with other antidiabetic therapies.
| New users of GLP-1-receptor agonists and/or DPP-4 inhibitors |
Non-users of GLP-1-receptor agonists and/or DPP-4 inhibitors |
Crude estimate | Adjusted estimate | |||||
|---|---|---|---|---|---|---|---|---|
| Events | PY | IR per 1000 PY | Events | PY | IR per 1000 PY | IRR (95% CI) | IRR (95% CI) | |
| No lag | 218 | 1987 | 109·7 | 2314 | 9526 | 242·9 | 0·45 (0·39–0·52) | 0·55 (0·45–0·68) |
| One year lag | 147 | 1525 | 96·4 | 2385 | 9989 | 238·8 | 0·40 (0·34–0·47) | 0·46 (0·35–0·60) |
| Ever/never use | 200 | 1861 | 107·5 | 2320 | 9526 | 243·5 | 0·44 (0·38–0·51) | 0·52 (0·42–0·64) |
| Prevalent users | 368 | 2309 | 159·4 | 2314 | 9526 | 242·9 | 0·66 (0·59–0·73) | 0·79 (0·67–0·93) |
| Only GLP-1 receptor agonists | 46 | 465 | 99·0 | 2652 | 11,439 | 231·8 | 0·43 (0·32–0·57) | 0·56 (0·39–0·83) |
| Only DPP-4 inhibitors | 153 | 1396 | 109·6 | 2511 | 10,426 | 240·8 | 0·45 (0·39–0·54) | 0·51 (0·39–0·66) |
| No insulin users | 69 | 752 | 91·8 | 1318 | 5812 | 226·8 | 0·40 (0·32–0·52) | 0·55 (0·39–0·79) |
| <30 years of age | <5 | – | – | <5 | – | – | – | – |
| 30–65 years of age | 85 | 930 | 91·4 | 971 | 4229 | 229·6 | 0·40 (0·32–0·50) | 0·51 (0·41–0·64) |
| >65 years of age | 107 | 902 | 118·7 | 1281 | 5078 | 252·3 | 0·47 (0·39–0·57) | 0·53 (0·39–0·72) |
| Mild IBD severity | 169 | 1709 | 98·9 | 1581 | 8213 | 192·5 | 0·41 (0·25–0·69) | 0·55 (0·43–0·70) |
| Moderate IBD severity | 15 | 89 | 168·8 | 331 | 810 | 408·7 | 0·51 (0·44–0·60) | 0·38 (0·19–0·77) |
| Severe IBD severity | 15 | 63 | 236·7 | 402 | 504 | 798·0 | 0·44 (0·38–0·51) | 0·38 (0·18–0·82) |
| No patients with IMID | 193 | 1842 | 104·8 | 2237 | 9386 | 238·3 | 0·44 (0·38–0·51) | 0·51 (0·40–0·63) |
| No patients with CVD | 182 | 1721 | 105·7 | 2052 | 8774 | 233·9 | 0·45 (0·39–0·53) | 0·55 (0·39–0·63) |
| Follow-up 2007–2009 | 10 | 78 | 127·7 | 785 | 2513 | 312·4 | 0·41 (0·22–0·76) | 0·62 (0·31–1·25) |
| Follow-up 2010–2014 | 88 | 755 | 116·5 | 924 | 4265 | 216·7 | 0·54 (0·43–0·67) | 0·55 (0·40–0·77) |
| Follow-up 2015–2019 | 101 | 1028 | 98·3 | 605 | 2749 | 220·1 | 0·45 (0·36–0·55) | 0·45 (0·33–0·62) |
CI confidence interval, CVD Cardiovascular disease, DPP-4 dipeptidyl peptidase 4, GLP-1 glucagon-like-peptide 1, IMID Immune-mediated inflammatory diseases, IR Incidence rate, IRR Incidence Rate Ratio, PY Person Years.
4. DISCUSSION
In this nationwide cohort study of patients with IBD and type 2 diabetes, we observed a lower risk of adverse clinical events (a composite of the need for oral corticosteroid treatment, need for TNF-α-inhibitor treatment, IBD-related hospitalisation, or IBD-related major surgery) amongst patients treated with GLP-1 receptor agonists and/or DPP-4 inhibitors compared with treatment with other antidiabetic therapies. Specifically, we observed a decreased risk of hospitalisation and the need for oral corticosteroid treatment. The risk of TNF-α-inhibitor treatment and surgery was likewise decreased, although not statistically significant. Stratified analyses indicated that the decreased risk was similar in patients diagnosed with CD and UC and in females and males.
Conflicting results exits on the association between DPP-4 inhibitors and risk of IBD with one study showing increased risk of IBD in patients treated with DPP-4 inhibitors [15] and another study showing no association [16]. However, to the best of our knowledge, no previous studies have examined the disease course in IBD patients treated with GLP-1-receptor agonists and/or DPP-4 inhibitors. Animal studies have shown that treatment with DPP-4 inhibitors or GLP-1 receptor agonists alleviate gut inflammation in mice [13,14]. Whether the same mechanism is responsible for the novel potential beneficial effect observed in humans with IBD and type 2 diabetes in the present study is unknown.
DPP-4 is expressed in the immune cells and is involved in the immune response [9]. It has been reported that DPP-4 inhibitors in vitro inhibit T cell proliferation and cytokine production [26,27], and that patients with IBD had altered DPP-4 expressions compared with healthy controls [28]. Regarding GLP-1, it has been suggested that GLP-1 reduces intestinal and systemic inflammation through interaction with GLP-1 receptors expressed in intraepithelial lymphocytes and in other organs and cells [11]. In addition, GLP-2 has in animals been found to possess intestinal reparative and protective properties [29]. Further investigations are needed to establish whether the lower risk of adverse clinical events that we observed amongst patients treated with GLP-1 receptor agonists and/or DPP-4 inhibitors compared with treatment with other antidiabetic therapies is due to a reduced intestinal and systemic inflammation.
When analysing the individual outcomes, we observed a decreased risk of hospitalisation and the need for oral corticosteroid treatment amongst users of GLP-1 based therapies, and a likewise decreased risk for TNF-α inhibitor treatment and surgery, although the latter two were not statistically significant. We speculate whether these findings reflect that treatment with GLP-1 based therapies protects against clinical worsening of IBD, or whether the patients treated with GLP-1 based therapies in general received a more successful maintenance therapy of their IBD avoiding frequent corticosteroid treatment episodes and hospitalisations. Another possible explanation might also be that patients treated with GLP-1 based therapies possibly have more uncontrolled diabetes compared with patients treated with other antidiabetics, and steroids might therefore be more carefully used in these patients due to concerns about hyperglycaemia. Since any causal associations cannot be drawn from this observational study, the results need to be confirmed in clinical trials, e.g. pragmatic trials or a phase 1 clinical trial including IBD patients only.
In 2015, Saxenda™ (Novo Nordisk, Bagsvaerd, Denmark), which is a GLP-1 receptor agonist containing the active substance liraglutide, was introduced for the treatment of obesity and overweight [30]. Although it is not yet entirely clear how obesity impacts IBD disease course [31], recently published studies concur that obesity has an unfavourable impact on the disease course of IBD. Accordingly, an internet-based cohort study of >7000 patients with IBD showed worse disease course in patients with obesity [32], and a cohort study of >40,000 patients with IBD linked obesity to higher burden and costs of hospitalisations [33]. Yet, we observed a similar lower risk of adverse clinical outcomes when analysing GLP-1 receptor agonists and DPP-4 inhibitors individually.
A major strength of the present study is its unselected nature as it included a study population covering the entire Danish population with IBD and type 2 diabetes treated with antidiabetic drugs with complete information on follow-up. The quality of IBD diagnoses coded in the Danish National Patient Registry has previously been validated and found to be high [34]. Some patients with type 2 diabetes are treated in primary care only and therefore, it is likely that this diagnosis is under-represented in the Danish National Patient Registry. However, we identified patients with type 2 diabetes through diagnoses in the Danish National Patient Registry as well as through prescriptions of glucose-lowering drugs recorded in the Danish National Prescription Registry in order to reduce misclassification.
We obtained information on oral corticosteroids through the Danish National Prescription Registry. Glucocorticoids dispensed at hospitals and at outpatient clinical visits are not recorded in this registry, which may result in an underestimation of the actual use. However, outpatient clinic and in-hospital treatment with corticosteroids is normally followed by prescription of gradual reduction corticosteroid treatment, which is registered in the Danish National Prescription Registry. To our knowledge, the validity of the use of TNF-α inhibitors registered in the Danish National Patient Registry has not been evaluated. This class of medications is expensive, and we, therefore, assume that the validity of drug registration is high since correct coding is necessary in order to assure reimbursement for the hospitals. Also, in our cohort, few patients were treated anti integrins, JAK inhibitors, or anti IL12/23 inhibitors precluding assessment of these types of therapies. The quality of IBD related surgeries has to our knowledge not been validated, but we expect that the validity is high, as it has been shown to be high for general surgery [17]. The mortality data are complete [23]. The available data for the present study lack information regarding clinical parameters and patient-reported symptoms that would allow the calculation of validated disease severity scores. To account for this, we evaluated IBD disease severity assessing previous use of oral corticosteroid, surgery, and hospitalisation.
It is unclear which impact treatment with GLP-1 based therapies has on the development of IBD [15,16] and subsequent disease course. We therefore also applied a design, including both prevalent and new users, and here, we observed a similar lower IRR as compared with the main analysis including new users only. For our main analysis, we used a per-protocol approach where patients were censored when they stopped using GLP-1 based therapies. Yet, when using an intention to treat approach (that is, never-ever use), we observed similar results. Also, per design we chose to analyse time to first event. However, it could also be relevant to study dose escalation and repeated treatments with corticosteroids and TNF-α-inhibitors.
Although we adjusted for several potential confounders, such as age, sex, calendar year, IBD severity, and metformin use, we cannot rule out unknown, unmeasured, or residual confounding. We used an active comparator design to reduce the risk of confounding by indication [35] and therefore, only individuals with a disease severity requiring glucose-lowering drug treatment were included in order to make the treatment groups similar. However, the active comparator group included heterogenous glucose-lowering drugs which could influence our results. Future well-powered studies should therefore preferably conduct head-to-head comparison of GLP-1 receptor agonists / DPP-4 inhibitors vs. specific groups of other glucose-lowering drugs. Moreover, it would be relevant to examine whether GLP-1 based therapy was associated with better glycaemic control than other glucose-lowering drugs and whether that was associated with improved disease course of IBD.
In conclusion, in this nationwide cohort study of patients with IBD and type 2 diabetes, we observed a lower risk of adverse clinical events amongst patients treated with GLP-1 receptor agonists and/or DPP-4 inhibitors compared with treatment with other antidiabetic therapies. Specifically, the risk of new corticosteroid treatment and hospitalisations was decreased. These findings suggest that treatment with GLP-1 receptor agonists and/or DPP-4 inhibitors may improve the disease course of IBD.
Declaration of Competing Interest
EJS is co-author of studies sponsored by pharmaceutical companies; Eli Lilly, Amgen, UCB, Novartis and Janssen. EJS has not personally received any economic compensation from any pharmaceutical company in the last 36 months. MV, ABS, KHA, TJ have no conflicts of interest to disclose.
Acknowledgments
Funding
The study was funded by the Novo Nordisk Foundation [grant number NNF16OC0022586 and NNF17OC0029768] and The Danish National Research Foundation [grant number DNRF148].
Data Sharing
Because of data protection regulation, data cannot be shared directly by the authors. Data is accessible to researchers from an authorised institution after application to the Danish Health Data Authority. To apply for data and help with the application process, please apply through the Danish Health Data Authority.
Contributors
TJ and KHA conceived the study idea. MV, ABS, and KHA designed the study. MV conducted data management and statistical analyses. KHA verified the underlying data. ABS and MV drafted the first manuscript. All authors participated in the interpretation of the results as well as critical revision of the manuscript. All authors approved the final version of the article. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Footnotes
Funding: The study was funded by the Novo Nordisk Foundation [grant number NNF16OC0022586 and NNF17OC0029768] and The Danish National Research Foundation [grant number DNRF148].
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100979.
Appendix. Supplementary materials
REFERENCES
- 1.Kaplan G.G. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart D.C., Sandborn W.J. Crohn's disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 3.Danese S., Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 4.Podolsky D.K. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 5.Wallace E., Salisbury C., Guthrie B., Lewis C., Fahey T., Smith S.M. Managing patients with multimorbidity in primary care. BMJ. 2015;350:h176. doi: 10.1136/bmj.h176. [DOI] [PubMed] [Google Scholar]
- 6.Anon. Ibuprofen “Actavis”. danish summary of product characteristics. The Danish Medicines Agency.
- 7.Anon. Metoprolol “GEA”. Medicin.dk.
- 8.Blind E., Janssen H., Dunder K., de Graeff P.A. The European Medicines Agency's approval of new medicines for type 2 diabetes. Diabetes Obes Metab. 2018;20:2059–2063. doi: 10.1111/dom.13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klemann C., Wagner L., Stephan M., Hörsten S von. Cut to the chase: a review of CD26/dipeptidyl peptidase-4’s (DPP4) entanglement in the immune system. Clinical & Experimental Immunology. 2016;185:1–21. doi: 10.1111/cei.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes, Obesity and Metabolism. 2016;18:203–216. doi: 10.1111/dom.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drucker D.J. The Cardiovascular Biology of Glucagon-like Peptide-1. Cell Metab. 2016;24:15–30. doi: 10.1016/j.cmet.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Bray J.J.H., Foster-Davies H., Salem A. Glucagon-like peptide-1 receptor agonists improve biomarkers of inflammation and oxidative stress: a systematic review and meta-analysis of randomised controlled trials. Diabetes Obes Metab. 2021 doi: 10.1111/dom.14399. [DOI] [PubMed] [Google Scholar]
- 13.Anbazhagan A.N., Thaqi M., Priyamvada S. GLP-1 nanomedicine alleviates gut inflammation. Nanomedicine. 2017;13:659–665. doi: 10.1016/j.nano.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yazbeck R., Howarth G.S., Geier M.S., Demuth H.-.U., Abbott C.A. Inhibiting dipeptidyl peptidase activity partially ameliorates colitis in mice. Front Biosci. 2008;13:6850–6858. doi: 10.2741/3193. [DOI] [PubMed] [Google Scholar]
- 15.Abrahami D., Douros A., Yin H. Dipeptidyl peptidase-4 inhibitors and incidence of inflammatory bowel disease among patients with type 2 diabetes: population based cohort study. BMJ. 2018;360:k872. doi: 10.1136/bmj.k872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T., Yang J.Y., Buse J.B. Dipeptidyl Peptidase 4 Inhibitors and Risk of Inflammatory Bowel Disease: real-world Evidence in U.S. Adults. Dia Care. 2019;42:2065–2074. doi: 10.2337/dc19-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt M., Schmidt S.A.J., Sandegaard J.L., Ehrenstein V., Pedersen L., Sørensen H.T. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pottegård A., Schmidt S.A.J., Wallach-Kildemoes H., Sørensen H.T., Hallas J., Schmidt M. Data Resource Profile: the Danish National Prescription Registry. Int J Epidemiol. 2017:798. doi: 10.1093/ije/dyw213. –798f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anon. WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC classification and DDD assignment 2019. Oslo, Norway, 2018.
- 20.Duricova D., Burisch J., Jess T., Gower-Rousseau C., Lakatos P.L. ECCO-EpiCom. Age-related differences in presentation and course of inflammatory bowel disease: an update on the population-based literature. J Crohns Colitis. 2014;8:1351–1361. doi: 10.1016/j.crohns.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Danaei G., Tavakkoli M., Hernán M.A. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 2012;175:250–262. doi: 10.1093/aje/kwr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomsen S.B., Allin K.H., Burisch J. Outcome of concomitant treatment with thiopurines and allopurinol in patients with inflammatory bowel disease: a nationwide Danish cohort study. United European Gastroenterol J. 2020;8:68–76. doi: 10.1177/2050640619868387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt M., Pedersen L., Sørensen H.T. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 24.Tseng C.-.H. Metformin use is associated with a lower risk of inflammatory bowel disease in patients with type 2 diabetes mellitus. J Crohns Colitis. 2021;15(1):64–73. doi: 10.1093/ecco-jcc/jjaa136. [DOI] [PubMed] [Google Scholar]
- 25.von Elm E., Altman D.G., Egger M. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18:800–804. doi: 10.1097/EDE.0b013e3181577654. [DOI] [PubMed] [Google Scholar]
- 26.Yazbeck R., Howarth G.S., Abbott C.A. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol. Sci. 2009;30:600–607. doi: 10.1016/j.tips.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Reinhold D., Biton A., Goihl A. Dual Inhibition of Dipeptidyl Peptidase IV and Aminopeptidase N Suppresses Inflammatory Immune Responses. Ann. N. Y. Acad. Sci. 2007;1110:402–409. doi: 10.1196/annals.1423.042. [DOI] [PubMed] [Google Scholar]
- 28.Hildebrandt BFK M Rose M., Rüter J., Salama A., Mönnikes H. Dipeptidyl Peptidase IV (DP IV, CD26) in Patients with Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2001;36:1067–1072. doi: 10.1080/003655201750422675. [DOI] [PubMed] [Google Scholar]
- 29.Drucker D.J. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology. 2002;122:531–544. doi: 10.1053/gast.2002.31068. [DOI] [PubMed] [Google Scholar]
- 30.Anon. Saxenda. summary of product characteristics. The European Medicines Agency. https://www.ema.europa.eu/en/documents/product-information/saxenda-epar-product-information_en.pdf.
- 31.Singh S., Dulai P.S., Zarrinpar A., Ramamoorthy S., Sandborn W.J. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nature Reviews Gastroenterology & Hepatology. 2017;14:110–121. doi: 10.1038/nrgastro.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain A., Nguyen N.H., Proudfoot J.A. Impact of Obesity on Disease Activity and Patient-Reported Outcomes Measurement Information System (PROMIS) in Inflammatory Bowel Diseases. Am J Gastroenterol. 2019;114:630–639. doi: 10.14309/ajg.0000000000000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen N.H., Ohno-Machado L., Sandborn W.J., Singh S. Obesity Is Independently Associated With Higher Annual Burden and Costs of Hospitalization in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2019;17:709–718.e7. doi: 10.1016/j.cgh.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Fonager K., Sørensen H.T., Rasmussen S.N., Møller-Petersen J., Vyberg M. Assessment of the diagnoses of Crohn's disease and ulcerative colitis in a Danish hospital information system. Scand J Gastroenterol. 1996;31:154–159. doi: 10.3109/00365529609031980. [DOI] [PubMed] [Google Scholar]
- 35.Lund J.L., Richardson D.B., Stürmer T. The Active Comparator, New User Study Design in Pharmacoepidemiology: historical Foundations and Contemporary Application. Curr Epidemiol Rep. 2015;2:221–228. doi: 10.1007/s40471-015-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.