Abstract
Background
Individuals with Acute Rheumatic Fever (ARF) often report a family history of ARF or Rheumatic Heart Disease (RHD) however the degree of familial susceptibility to RHD is poorly defined. This study aimed to determine RHD prevalence among first degree relatives of ARF patients using echocardiography.
Methods
Children with ARF were recruited from Auckland, New Zealand. Parents and siblings ≥ 4years were offered echocardiography. Echocardiograms were reported according to World Heart Federation 2012 criteria. RHD prevalence in first degree relatives was compared to previously established population rates in the region.
Findings
In total, 70 index cases with ARF were recruited. Echocardiography was performed in 94 parents and 132 siblings. There were 3 siblings with definite RHD and 9 with borderline RHD. There were 4 parents with definite RHD. Overall prevalence of RHD (definite and borderline) in siblings was 90/1,000 (95% CI 45–143/1,000) compared to 36/1,000 (95% CI 30–42/1,000) in New Zealand children from high ARF incidence populations (p 0.001). Prevalence of definite RHD in parents was 42/1,000 (95% CI 7–87/1,000) compared to 22/1,000 (95% CI 9–36/1,000) in adults from a high ARF incidence New Zealand population (p 0.249).
Interpretation
RHD prevalence in siblings and parents of ARF cases is significantly greater than in comparable background populations. The contribution of hereditary versus environmental risk factors remains uncertain. We recommend targeted echocardiographic case-finding among siblings and parents of ARF/RHD cases in order to detect previously unrecognized latent RHD.
Keywords: Acute rheumatic fever, Rheumatic heart disease, Echocardiograms, Definite and borderline
Research in context:
Evidence before this study
Heritability to Acute Rheumatic Fever and Rheumatic Heart Disease has long been suspected, based on observational and twin studies. The prevalence of latent RHD in family members of ARF cases is not well described. New Zealand has high rates of ARF and RHD, with high risk population prevalence of RHD previously established in adults and children.
We performed a PubMed search using the search terms (rheumatic) AND (family* OR genetic) AND echocardiogram*) to identify studies published up until 1 September 2020 published in any language. We identified only one study assessing familial risk of latent Rheumatic Heart Disease. This study from Uganda reported increased prevalence of Definite RHD in siblings of children with RHD compared to controls.
Added value of this study
RHD prevalence in siblings and parents of ARF cases is substantially greater (more than 2x) higher in family members than comparable background New Zealand populations. The results of this study are concordant with the one previously published study, which demonstrated increased prevalence of latent RHD in first degree relatives of children with ARF.
Implications of all the available evidence
This study supports enhanced RHD case finding efforts including the use of echocardiography among siblings and parents of ARF/RHD cases.
Alt-text: Unlabelled box
1. Introduction
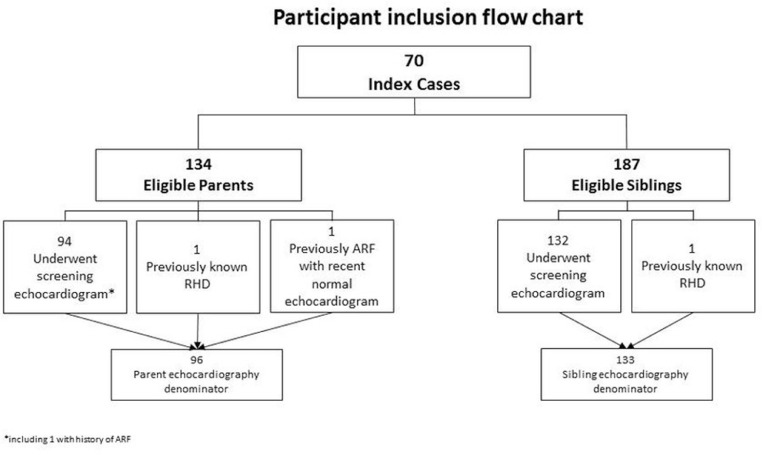
Acute rheumatic fever (ARF) and its sequela chronic rheumatic heart disease (RHD) are important global health problems, accounting for approximately 300–350,000 deaths annually and affecting around 33 million people [1]. Globally, the majority of adults presenting with RHD do not have a documented history of ARF and present instead with features of established valvular heart disease [2]. Echocardiographic screening enables RHD to be detected before the onset of clinical signs and symptoms, and has been undertaken in many high prevalence ARF/RHD populations around the globe [3], including New Zealand [4], [5], [6], to describe RHD burden. However the utility of screening echocardiography in RHD control programmes remains the subject of ongoing debate in high-burden populations [7,8] Fig. 1.
Fig. 1.
Participant Inclusion flow chart.
A component of genetic susceptibility or heritability to ARF and RHD has been suspected for almost a century, based on reports of families with multiple affected members [9], [10], [11] and studies demonstrating increased risk of ARF in children born to parents with RHD compared to children of unaffected parents [12]. A recent systematic review and meta-analysis reported a concordance risk for ARF of 44% in monozygotic twins and 12% in dizygotic twins, with estimated overall heritability of 60% [13]. It has also been estimated that up to 6% of any population may have a degree of underlying susceptibility to ARF [14,15]. However the sharp reduction in ARF incidence in most high-income countries in the 20th century, and the strong association between environmental risk factors, particularly household crowding, and ARF [16,17] indicate that environmental factors also contribute to ARF susceptibility. Family members of those with ARF or RHD may be at increased risk of RHD compared to the general population, due to the combination of genetic susceptibility and shared environmental predisposing factors.
To date, the prevalence of RHD in relatives of ARF patients is poorly described. Only one previous published study has used echocardiography to evaluate familial RHD risk, reporting increased prevalence of Definite RHD in siblings of children with latent RHD compared to siblings of controls. [18]
In New Zealand, there are high rates of ARF and RHD among indigenous Māori and Pacific peoples [5,19]. The prevalence of definite RHD in schoolchildren from high incidence ARF populations in New Zealand is estimated to be around 1% using widely accepted 2012 WHF criteria for echocardiographic diagnosis of RHD [6,20]. Among young adults of Pacific ethnicity living in Auckland, the prevalence of definite RHD is around 2% [21]. Despite these high local rates of ARF and RHD, and previously described genetic and environmental risks, there are major gaps in knowledge regarding RHD disease burden in New Zealand family members. Furthermore, the clinical approach to family members of individuals with ARF/RHD is not specifically addressed in current New Zealand ARF/RHD management guidelines or in other international clinical practice guidelines [22,23].
This study aimed to determine the prevalence of latent RHD in first degree relatives of children with ARF. We hypothesised that RHD prevalence would be higher in siblings and parents of ARF cases than RHD prevalence in the background population.
2. Materials and methods
2.1. Study setting
This study was conducted in Auckland, New Zealand, where there is a high incidence of ARF/RHD almost exclusively affecting Māori and Pacific peoples, associated with high levels of socio-economic deprivation and household crowding [5,6,21].
2.2. Participant selection and enrolment
Between January 2014 and December 2016, all families of children under 15 years of age with ARF (diagnosed as per New Zealand guidelines) [22] at the three public hospitals in the Auckland region were approached for participation in the study. First degree relatives, including biological parents and siblings, including half-siblings, were deemed eligible. Non-biologic, non-first degree relatives (e.g. cousins, adopted family members, step-parents) were excluded as were children under four years of age. Individuals who were not New Zealand residents were also deemed ineligible, due to inability to ensure appropriate clinical follow-up of echocardiogram findings. Informed consent was obtained from participants. Parental consent was obtained for participants under 16 years, in addition to written assent for children between 10 and 16 years.
A standardised questionnaire regarding family demographics and composition was administered. Past history and family history of ARF/RHD were documented, along with history of non-rheumatic cardiac conditions. Electronic medical records were reviewed to clarify the diagnoses for participants disclosing a past history of ARF/RHD or other cardiac conditions.
2.3. Echocardiography procedures
Echocardiograms were offered to biological parents and siblings aged 4 years or older.
Echocardiograms were performed on either a Vivid™ Q® (GE, General Electric Corporation, Chicago IL) portable platform or on the Philips iE33® (Philips Professional Healthcare, Amsterdam) hospital platform with a 3S 2.2 M Hz transducer by highly experienced cardiac sonographers. Images were acquired and reported using standardised protocols previously utilised in New Zealand by this group of investigators [5,24].
Two dimensional and color Doppler images were obtained in parasternal and apical views, with multiple color sweeps of any mitral or aortic regurgitation identified. Continuous-wave Doppler interrogation of regurgitation was performed to assess peak velocity, duration through the cardiac cycle and spectral envelope. Valve leaflet morphology and thickness was assessed in parasternal long axis (mitral and aortic) and parasternal short axis views (aortic valve) using previously described methods [25]. Images were electronically stored in DICOM format.
Left ventricular size and function was assessed by M mode. Additional images were obtained at the discretion of the sonographer including assessment of valvular abnormality severity.
2.4. Echocardiogram reporting
Echocardiograms were reviewed by one investigator (NCS) and studies showing any potential abnormalities were reported by a panel of cardiologists (NW, JS, TG, RD), blinded to the participant's demographic details and clinical history. Two cardiologists reviewed all potentially abnormal echocardiograms, with a third cardiologist adjudicating in the event of a disagreement as per previously described research protocols. [5,6,20,22] Non-rheumatic abnormalities were interpreted in the context of available clinical information.
2.5. Rheumatic heart disease classification
For participants under 20 years of age, echocardiograms were classified as Definite RHD or Borderline RHD according to WHF Diagnostic Criteria for RHD [20], Normal or Other Abnormal.
For participants over 20 years of age, echocardiograms were classified as Definite RHD, Normal or Other Abnormal, acknowledging that the Borderline RHD category in the WHF criteria applies only to persons under 20 years of age [20].
2.6. Participant management and follow-up
Those under 20 years with definite RHD were offered benzathine penicillin prophylaxis and those with borderline RHD were recommended enhanced surveillance for sore throats and follow-up echocardiography, in keeping with global best-practice recommendations at the time the study was conducted [23]. Those diagnosed with non-RHD cardiac abnormalities were counselled and referred for cardiology review as appropriate.
2.7. Determination of background population RHD prevalence
Comparative data were previously established by population-based echocardiographic studies using the same techniques for children[6] and young adults [21] in similar high prevalence ARF/RHD regions in New Zealand. The previously established prevalence of definite and borderline RHD in children is 36 per 1000 (95% CI 30–42 per 1000) [6] and in young adults of Pacific ethnicity aged less than 40 years is 22 per 1000 (95% CI 9–36 per 1000) [21].
2.8. Statistical methods
Prevalence of RHD in siblings was calculated by the sum of the sibling RHD cases detected by echocardiography, plus those with clinically diagnosed RHD, expressed as a proportion of those siblings scanned, i.e. siblings not participating were not included in the denominator. The sibling prevalence of RHD was compared to the background population prevalence.
Prevalence of RHD in parents was calculated by the sum of the echocardiographic RHD cases plus those with clinically diagnosed RHD, expressed as a proportion of those parents scanned, i.e. parents not participating were not included in the denominator. The parent prevalence of RHD was compared to the background population prevalence.
Categorical data are expressed as proportions. Relative risks were calculated to determine prevalence of RHD among different groups. To compare the prevalence of RHD between siblings (sample study) and New Zealand children and between those with definite RHD and borderline RHD, a chi-square (χ2) test of independence (equality of proportions) was used. Significance was set at p < 0.05 for all analyses. Statistical Analysis System (SAS) Version 9.4 from SAS Institute Inc., Cary, NC, USA was used for data analysis.
2.9. Ethics
Ethics approval was obtained from the New Zealand Health and Disability Ethics Committee (13/STH/189/AM04) and locality approval obtained from the research offices of each participating hospital.
2.10. STROBE statement
This study was conducted and reported according to STROBE guidelines for observational studies.
2.11. Role of the funding source
This study was funded by the Health Research Council of New Zealand ([Ref. 13]/965). The funding source had no role in study design, conduct analysis or interpretation of results. All authors had full access to the data and accept responsibility to submit for publication
3. Results
3.1. Demographics of index cases and family members
There were 70 children (index cases of ARF) and families who participated in the study. The median age of index cases was 11 years (range 4–15 years). Forty patients (57%) were male. The majority of children with ARF were of Pacific ethnicity (55/70, 79%) and 15/70 (21%) were Māori.
There were 134 eligible parents and 187 eligible siblings aged 4 years or older. The median number of eligible siblings per index case was 2 (range 0–9).
There were 132/187 (71%) of eligible siblings and 94/134 (70%) of eligible parents who underwent echocardiography (Table 1). The median age of participating parents was 37 years (range 22–61 years, IQR 33–43). The median age of participating siblings was 10 years (range 4–23 years, IQR 7–13). 57/133 (43%) of enrolled siblings were male and 38/96 (40%) of parents were male.
Table 1.
Demographic data of siblings and parents who underwent echocardiography.
| Siblings (n = 133) |
Parents (n = 96) |
||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years) | Median | 10 | 37 | ||
| Range | 4–23 | 22–61 | |||
| Sex | Male | 57 | (43%) | 38 | (40%) |
| Ethnicity | NZ Māori | 26 | (20%) | 14 | (15%) |
| Pacific | 107 | (80%) | 81 | (84%) | |
| NZ European | 1 | (1%) | |||
| Previously known ARF/RHD | 1 | 2 | |||
A family history of ARF/RHD in one or more biologic relatives was reported in 18/70 (26%) of index cases.
3.2. Clinical features of index cases and family members
Index cases: All 70 ARF patients were first episodes of ARF and there were no recurrent episodes. There were 34 patients (48%) with mild carditis and 24 (34%) had moderate or severe carditis as defined by New Zealand guidelines [22]. In this cohort, 12 of 70 patients (17%) underwent cardiac surgery for severe carditis or persisting severe RHD within 12 months following their ARF diagnosis. Only 12 patients (17%) had ARF without carditis. Chorea occurred in six patients (9%).
Family members: One sibling had a clinical history of ARF with known chronic RHD and did not undergo a screening echocardiogram but is included in prevalence data. Among parents, three had a medically confirmed history of ARF or RHD. One parent had known clinically diagnosed RHD confirmed by recent clinical echocardiogram, and is included in prevalence data. One parent had a documented history of ARF with a normal echocardiogram conducted as part of prior routine clinical care, and the third parent had a confirmed prior history of ARF with a normal echocardiogram conducted as part of this study.
3.3. Rheumatic heart disease prevalence in siblings and parents
Of the 132 siblings who underwent echocardiography, two siblings were found to have definite RHD (both cases of moderate severity) and nine had borderline RHD detected by screening. One additional sibling had an established diagnosis of moderately severe RHD prior to participation in the study. In two families, two siblings were found to have borderline RHD. Siblings with RHD detected by echocardiography had a median age of 11 years (range 5–17 years). The prevalence of total RHD (definite and borderline) in siblings was 90 per 1000 (95% CI 45–143 per 1000) compared to 36 per 1000 (95% CI 30–42 per 1000) background population children (p 0.001) (Table 2).
Table 2.
Prevalence of rheumatic heart disease in siblings vs. background population children.
| Borderline RHD | Definite RHD | Total RHD (Borderline + Definite) | ||||
|---|---|---|---|---|---|---|
| Siblings | 9/133 | 68 per 1000 95% CI 36–124 per 1000 |
3/133 | 23 per 1000 95% CI 8–64 per 1000 |
12/133 | 90 per 1000 95% CI 45–143 per 1000 |
| NZ children (6) | 90/3634 | 25 per 1000 95% CI 20–30 per 1000 |
40/3634 | 11 per 1000 95% CI 8–15 per 1000 |
130/3634 | 36 per 1000 95% CI 30–42 per 1000 |
| p-value | 0.002 | 0.218 | 0.001 | |||
Of the 94 parents who underwent echocardiography, three had definite RHD (2 mild, 1 moderate severity). One additional parent with clinically diagnosed RHD was included in prevalence data. The overall prevalence of definite RHD in parents was 43 per 1000 (95% CI 7–87 per 1000) compared to 22 per 1000 (95% CI 9–36 per 1000) background population adults (p 0.249) (Table 3).
Table 3.
Prevalence of rheumatic heart disease in parents vs. background adult population.
| Definite RHD | ||
|---|---|---|
| Parents | 4/96 | 42 per 1000 95% CI 7–87 per 1000 |
| NZ adults (21) | 10/465 | 22 per 1000 95% CI 9–36 per 1000 |
| p-value | 0.249 | |
Following echocardiography, there were 16 families with two first-degree family members with RHD and two families with three affected first-degree family members.
The relative risk of any latent RHD for siblings compared to the background population was 2.52 (p 0.01) (Table 4). The relative risk of definite RHD for parents compared to the background population was 1.94 (p 0.255) (Table 4). The relative risk of RHD for siblings compared to the background population if the index case required cardiac surgery was 4.78 (p 0.003) (Table 5).
Table 4.
Relative risk of rheumatic heart disease for siblings and parents compared to New Zealand children and adults.
| Relative risk (95% confidence interval) | p value | ||
|---|---|---|---|
| All RHD | 2.52 (1.43–4.44) | 0.001 | |
| Siblings | Definite RHD | 2.05 (0.64–6.54) | 0.226 |
| Borderline RHD | 2.73 (1.41–5.30) | 0.003 | |
| Parents | Definite RHD | 1.94 (0.62–6.05) | 0.255 |
Table 5.
Relative risk of rheumatic heart disease for siblings with other factors.
| RHD in sibling | Relative risk (95% confidence interval) | p value |
|---|---|---|
| If reported family history of ARF/RHD | 1.10 (0.32–3.80) | 0.884 |
| If moderate/severe carditis in index case | 1.83 (0.62–5.36) | 0.270 |
| If RHD surgery in index case | 4.78 (1.69–13.51) | 0.003 |
3.4. Non-rheumatic echocardiographic abnormalities
Three siblings had non-rheumatic abnormalities detected by echocardiography: two had minor congenital anomalies of the mitral valve and one had a dilated aortic root.
There were 10 parents with non-rheumatic abnormalities detected by echocardiography: two congenital valvular abnormalities (one mitral, one aortic), three with dilated ascending aorta, two left ventricular hypertrophy (one mild, one moderate), two with aortic valve sclerosis (one with aortic stenosis) and one regional wall motion abnormality.
4. Discussion
This study found that RHD prevalence (definite and borderline) among siblings of children with ARF was 2.5 times the background population prevalence (9% compared to 3.5%). Restated, siblings and parents of ARF patients are themselves at increased risk of latent RHD. Of note, the relative risk of RHD in siblings was markedly elevated (4.8, see Table 5) if the index ARF case in the family had also undergone RHD surgery, suggesting the potential for a gradient in familial RHD risk. Whilst Definite RHD prevalence in biologic parents of children with ARF was nearly twice the background population rate (4% compared to 2.3%), this did not reach statistical significance.
The one previously published study from Uganda using echocardiography to evaluate familial RHD risk had important differences in methodology [18]. Index cases with RHD were identified via echocardiographic studies, in contrast to the children in our study who were recruited following a clinical diagnosis of ARF. The relative risk of definite RHD for siblings of cases with any latent RHD (definite or borderline) was 4.6, when compared to siblings of controls with normal echocardiograms. The concordant finding of these two studies confirms a familial risk of RHD. The current study found that sibling risk of RHD increases with increasing severity of cardiac involvement in the index case, similar to the Ugandan study where siblings of children with definite RHD had a relative risk of definite RHD of 5.3 [18,26].
A genetic component of ARF has long been recognised [9], [10], [11]. The concordance risk for ARF is estimated to be 44% for monozygotic twins and 12% for in dizygotic twins, with a calculated heritability of 60% [13]. Population-based studies of susceptibility to ARF and RHD suggest that environmental exposure to Streptococci and overcrowding may be more important than genetic susceptibility [15,16]. Our findings are concordant with previous descriptions of familial susceptibility [18].
The current study cannot discount a heritable component for susceptibility for ARF but was not designed to elucidate the relative contributions of heritability, environmental and epigenetic factors to ARF and RHD susceptibility.
Our findings have substantial impact regarding the clinical management of relatives of persons newly diagnosed with ARF. Upon diagnosis of ARF, a detailed family history may not be known, in settings where resources permit, active case finding among first degree relatives using echocardiography should also be considered and may be incorporated into local ARF and RHD clinical practice guidelines. It is logical that echocardiography is also offered to siblings of children diagnosed with chronic RHD, in addition to siblings of those with ARF, as factors contributing to elevated susceptibility, whether genetic or environmental, will be the same.
Echocardiography to detect latent RHD has previously been shown to have a high degree of acceptability to families [27]. The current study demonstrates high uptake by family members when offered echocardiography. Participation was similar for eligible siblings and eligible parents, at around 70%. It should be noted that the majority of families were offered participation whilst the index case was an inpatient with ARF. It is uncertain whether uptake would be as high if families were offered echocardiography as part of routine clinical care, outside a research setting.
Reported family history may not be always be reliable and interestingly we found that a family history of ARF/RHD on the study questionnaire was not associated with increased relative risk of RHD. Recall bias and prior unrecognized episodes of ARF in family members may have contributed to this observation. Several families in our study had multiple affected first degree relatives, in keeping with findings from Uganda [18]. This scenario presents a strong mandate for active case finding among family members and may also inform prioritization of echocardiography in resource-limited settings. Family history may also assist clinicians and individuals to make decisions regarding initiation of benzathine penicillin secondary prophylaxis when individuals with suspected ARF do not meet full diagnostic criteria.
Siblings <20 years of age diagnosed with definite RHD were recommended to commence benzathine penicillin secondary prophylaxis as per guidelines [22], based on the rationale that benzathine penicillin secondary prophylaxis prevents recurrences of ARF and worsening of RHD. New Zealand has a proven record of high adherence to benzathine penicillin secondary prophylaxis for individuals with RHD detected by echocardiography [28]. We shared the uncertainty of the diagnosis of borderline RHD with the families and recommended enhanced surveillance with interval follow up echocardiography and education regarding the importance of primary prevention [23]. Some families still chose to embark on secondary prevention usually influenced by the diagnosis of ARF in the index case or other family members with RHD.
Our study did not address the wider issue of population screening for RHD, nor the detail of which subsets of RHD should be offered secondary prophylaxis.
There is also currently a lack of international consensus regarding the appropriateness (or otherwise) of commencing adults with newly diagnosed and previously unrecognized RHD on benzathine penicillin secondary prophylaxis. However, there are other benefits of making a new diagnosis in adulthood including referral to cardiology services, potentially also improving pregnancy outcomes for women with RHD in their childbearing years.
Non-rheumatic cardiac abnormalities were detected in 2% of siblings and 10% of parents. We have previously emphasised that not all valvular heart disease found by echocardiographic screening in children is rheumatic [5]. The high prevalence of non-rheumatic abnormalities in adults is in keeping with previous New Zealand echocardiographic screening studies in young adults of Māori and Pacific ethnicity [21,29,30]. It must be anticipated that a number of non-rheumatic abnormalities will be found when echocardiographic screening is undertaken, some of which require physician follow-up.
New Zealand is in a unique position globally as a high income country with a high incidence of ARF/RHD. New Zealand has a history of significant efforts in RHD control with primary prevention efforts via intensive management of sore throats in schools in high-ARF incidence areas and previous experience with echocardiographic screening for RHD undertaken in schools in high-ARF incidence areas.
First degree relatives within the same household share common environmental exposures and are likely exposed to the same strains of streptococcus over time. In addition to family Group A streptococcal contact management as already occurs with throat swabbing in New Zealand, echocardiographic screening of family members offers a more comprehensive risk management strategy when a household member is diagnosed with ARF.
There is ongoing uncertainty regarding the natural history and clinical significance of RHD detected by echocardiography. Findings from a randomised trial of benzathine penicillin in latent RHD currently underway in Uganda are expected to further inform future global approaches to the clinical management of latent RHD detected by echocardiography [26].
The study exemplifies the concept of enhanced case detection for high risk persons, as distinct from whole population screening for RHD. The high participation rate demonstrates the acceptability of echocardiography to families affected by ARF/RHD. This study is just the second study to determine the familial susceptibility of ARF/RHD using echocardiographic methodology [18].
This study was not designed to determine whether increased familial risk of RHD was due to genetic or environmental factors and involved a relatively small number of ARF patients and family members. There is uncertainty associated with the limited sample size. Although a high proportion of eligible family members underwent echocardiography, it remains uncertain whether family members who did not undergo echocardiography would have had a higher or lower chance of having RHD than those who had echocardiograms.
Echocardiography of first degree relatives of individuals with ARF detects 2,3 times the RHD prevalence of the background population, although contribution of heritability, environmental and epigenetic factors cannot be differentiated. Where feasible, active case detection for RHD using echocardiography should be offered to family members after a new diagnosis of ARF or RHD.
Funding
Health Research Council of New Zealand ([Ref. 13]/965). The Funders had no input into the study design, analyses or interpretation of findings, nor into the writing of the final report.
Data sharing statement
The study protocol and participant materials may be made available upon email request to the corresponding author. De-identified data may be made available upon email request to the corresponding author within a formal data sharing agreement, subject to all of the following: (1) agreement by Māori and Pacific advisors to the study, particularly regarding matters pertaining to indigenous data sovereignty, (2) ethical approval by the New Zealand Health and Disability Ethics Committee (13/STH/189/AM04), (3) conduct and dissemination of additional analyses to be undertaken with involvement of senior investigators NW and RW, together with Māori and Pacific advisors and 4) commitment to not publish or share any potentially identifiable information.
Declaration of Competing Interest
The authors have no financial or personal conflicts of interest to declare.
Acknowledgments
The study was funded by the Health Research Council of New Zealand, the Ministry of Health, New Zealand, Cure Kids New Zealand, Te Puni Kokiri and the National Heart Foundation of New Zealand (HRC [Ref. 13]/965). RND is the holder of the NZ Heart Foundation Chair of Heart Health.
We thank Dr Florina Chan Mow and Dr Rebecca Somerville for contributing to recruitment at Counties Manukau District Health Board and Waitemata District Health Board. We also thank the cardiac sonographers at Starship Children's hospital for performing study echocardiograms.
References
- 1.Watkins D.A., Johnson C.O., Colquhoun S.M., Karthikeyan G., Beaton A., Bukhman G. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017;377:713–722. doi: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 2.Zuhlke L., Engel M.E., Karthikeyan G., Rangarajan S., Mackie P., Cupido B. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the global rheumatic heart disease registry (the REMEDY study) Eur Heart J. 2015;36:1115–1122. doi: 10.1093/eurheartj/ehu449. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothenbuhler M., O'Sullivan C.J., Stortecky S., Stefanini G.G., Spitzer E., Estill J. Active surveillance for rheumatic heart disease in endemic regions: a systematic review and meta-analysis of prevalence among children and adolescents. Lancet Glob Health. 2014;2:e717–e726. doi: 10.1016/S2214-109X(14)70310-9. [DOI] [PubMed] [Google Scholar]
- 4.Wu X.D., Zeng Z.Y., Gong D.P., Wen J.L., Huang F. Potential involvement of S1PR1/STAT3 signaling pathway in cardiac valve damage due to rheumatic heart disease. mSphere. 2019:1–6. doi: 10.1080/10520295.2019.1574028. [DOI] [PubMed] [Google Scholar]
- 5.Webb R.H., Wilson N.J., Lennon D.R., Wilson E.M., Nicholson R.W., Gentles T.L. Optimising echocardiographic screening for rheumatic heart disease in New Zealand: not all valve disease is rheumatic. Cardiol Young. 2011;21:436–443. doi: 10.1017/S1047951111000266. [DOI] [PubMed] [Google Scholar]
- 6.Culliford-Semmens N., Nicholson R., Tilton E., Stirling J., Sidhu K., Webb R. The World Heart Federation criteria raise the threshold of diagnosis for mild rheumatic heart disease: three reviewers are better than one. Int J Cardiol. 2019;291:112–118. doi: 10.1016/j.ijcard.2019.02.058. [DOI] [PubMed] [Google Scholar]
- 7.Marangou J., Beaton A., Aliku T.O., Nunes M.C.P., Kangaharan N., Remenyi B. Echocardiography in indigenous populations and resource poor settings. Heart Lung Circ. 2019;28:1427–1435. doi: 10.1016/j.hlc.2019.05.176. [DOI] [PubMed] [Google Scholar]
- 8.Roberts K., Colquhoun S.M., Steer A., Remenyi B., Carapetis J. Screening for rheumatic heart disease: current approaches and controversies. Nat Rev Cardiol. 2013;10:49–58. doi: 10.1038/nrcardio.2012.157. [DOI] [PubMed] [Google Scholar]
- 9.Paul J.R., Salinger R. The spread of rheumatic fever through families. J Clin Invest. 1931;10:33–51. doi: 10.1172/JCI100340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson M.G., Schweitzer M.D., Lubschez R. The familial epidemiology of rheumatic fever:genetic and epidemiologic studies: I. genetic studies. J Pediatr. 1943;22:468–492. [Google Scholar]
- 11.Markowitz M. Susceptibility to rheumatic fever. Circulation. 1968;38:3–4. doi: 10.1161/01.cir.38.1.3. [DOI] [PubMed] [Google Scholar]
- 12.Davies A.M., Lazarov E. Heredity, infection and chemoprophylaxis in rheumatic carditis: an epidemiological study of a communal settlement. J Hyg (Lond) 1960;58:263–276. doi: 10.1017/s0022172400038377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engel M.E., Stander R., Vogel J., Adeyemo A.A., Mayosi B.M. Genetic susceptibility to acute rheumatic fever: a systematic review and meta-analysis of twin studies. PLoS ONE. 2011;6:e25326. doi: 10.1371/journal.pone.0025326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryant P.A., Robins-Browne R., Carapetis J.R., Curtis N., Bryant P.A., Robins-Browne R. Some of the people, some of the time: susceptibility to acute rheumatic fever. Circulation. 2009;119:742–753. doi: 10.1161/CIRCULATIONAHA.108.792135. [DOI] [PubMed] [Google Scholar]
- 15.Carapetis J.R., Currie B.J., Mathews J.D. Cumulative incidence of rheumatic fever in an endemic region: a guide to the susceptibility of the population? Epidemiol Infect. 2000;124:239–244. doi: 10.1017/s0950268800003514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaine R., Baker M., Venugopal K. Acute rheumatic fever associated with household crowding in a developed country. Pediatr Infect Dis J. 2011;30:315–319. doi: 10.1097/INF.0b013e3181fbd85b. [DOI] [PubMed] [Google Scholar]
- 17.Oliver J., Pierse N., Baker M.G. Estimating rheumatic fever incidence in New Zealand using multiple data sources. Epidemiol Infect. 2015;143:167–177. doi: 10.1017/S0950268814000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aliku T., Sable C., Scheel A., Tompsett A., Lwabi P., Okello E. Targeted echocardiographic screening for latent rheumatic heart disease in Northern Uganda: evaluating familial risk following identification of an index case. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milne R.J., Lennon D.R., Stewart J.M., Vander Hoorn S., Scuffham P.A. Incidence of acute rheumatic fever in New Zealand children and youth. J Paediatr Child Health. 2012;48:685–691. doi: 10.1111/j.1440-1754.2012.02447.x. [DOI] [PubMed] [Google Scholar]
- 20.Remenyi B., Wilson N., Steer A., Ferreira B., Kado J., Kumar K. World heart federation criteria for echocardiographic diagnosis of rheumatic heart disease-an evidence-based guideline. Nat Rev Cardiol. 2012;9:297–309. doi: 10.1038/nrcardio.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webb R., Culliford-Semmens N., ChanMow A., Doughty R., Tilton E., Peat B. PS286 Prevalence of rheumatic heart disease and other echocardiographic abnormalities in polynesian young adults in South Auckland, New Zealand. Glob Heart. 2016;11:e63. [Google Scholar]
- 22.Heart Foundation of New Zealand . Heart Foundation of New Zealand; Auckland: 2014. New zealand guidelines for rheumatic fever: diagnosis, management and secondary prevention of acute rheumatic fever and rheumatic heart disease: 2014 update. [Google Scholar]
- 23.Saxena A., Zuhlke L., Wilson N. Echocardiographic screening for rheumatic heart disease: issues for the cardiology community. Glob Heart. 2013;8:197–202. doi: 10.1016/j.gheart.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Webb R.H., Gentles T.L., Stirling J.W., Lee M., O'Donnell C., Wilson N.J. Valvular regurgitation using portable echocardiography in a healthy student population: implications for rheumatic heart disease screening. J Am Soc Echocardiogr. 2015;28:981–988. doi: 10.1016/j.echo.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Webb R.H., Culliford-Semmens N., Sidhu K., Wilson N.J. Normal echocardiographic mitral and aortic valve thickness in children. Heart Asia. 2017;9:70–75. doi: 10.1136/heartasia-2016-010872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaton A., Okello E., Engelman D., Grobler A., Scheel A., DeWyer A. Determining the impact of Benzathine penicillin G prophylaxis in children with latent rheumatic heart disease (GOAL trial): study protocol for a randomized controlled trial. Am Heart J. 2019;215:95–105. doi: 10.1016/j.ahj.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Perelini F., Blair N., Wilson N., Farrell A., Aitken A. Family acceptability of school-based echocardiographic screening for rheumatic heart disease in a high-risk population in New Zealand. J Paediatr Child Health. 2015;51:682–688. doi: 10.1111/jpc.12829. [DOI] [PubMed] [Google Scholar]
- 28.Culliford-Semmens N., Tilton E., Webb R., Lennon D., Paku B., Malcolm J. Adequate adherence to benzathine penicillin secondary prophylaxis following the diagnosis of rheumatic heart disease by echocardiographic screening. N Z Med J. 2017;130:50–57. [PubMed] [Google Scholar]
- 29.Faatoese A.F., Pitama S.G., Gillies T.W., Robertson P.J., Huria T.M., Tikao-Mason K.N. Community screening for cardiovascular risk factors and levels of treatment in a rural Maori cohort. Aust N Z J Public Health. 2011;35:517–523. doi: 10.1111/j.1753-6405.2011.00777.x. [DOI] [PubMed] [Google Scholar]
- 30.Whalley G.A., Pitama S., Troughton R.W., Doughty R.N., Gamble G.D., Gillies T. Higher prevalence of left ventricular hypertrophy in two Maori cohorts: findings from the Hauora Manawa/community heart study. Aust N Z J Public Health. 2015;39:26–31. doi: 10.1111/1753-6405.12300. [DOI] [PubMed] [Google Scholar]