Abstract
Background
Recombinant pertussis vaccines inducing long-lasting immune responses could help to control the rise in pertussis. We here report on persisting antibody responses 2 and 3 years after booster vaccination with a new generation recombinant acellular pertussis vaccine.
Methods
Participants of a phase 2/3 randomised-controlled clinical trial with a monovalent pertussis vaccine containing genetically inactivated pertussis toxin (aPgen) or its tetanus and diphtheria toxoids combination (TdaPgen), or a chemically detoxified comparator vaccine (Tdapchem), (originally conducted between July and August 2015) were invited to participate in observational studies of persisting antibody responses 2 and 3 years after vaccination. Serum IgG against pertussis toxin (PT-IgG) and filamentous hemagglutinin (FHA-IgG) were assessed by ELISA, and PT-neutralising antibodies (PT-Nab) by Chinese Hamster Ovary cell assay.
Findings
Waning of antibodies stabilised in aPgen and TdaPgen vaccinees 2 and 3 years after vaccination. Three years post-vaccination PT-neutralising antibodies remained 4·6-fold (95% Confidence Interval (CI) 2·6–8·1) and 3·7-fold (95% CI 2·2–6·1) higher, PT-IgG antibodies 3·0-fold (95% CI 2·2–4·1) and 2·5-fold (95% CI 1·9–3·3) higher, and FHA-IgG antibodies 1·8-fold (95% CI 1·3–2·5) and 1·6-fold (95% CI 1·2–2·1) higher than baseline in aPgen and TdaPgen recipients, respectively. In the Tdapchem group, PT-neutralising and PT-IgG and FHA-IgG antibodies were back at baseline levels 2 years post-vaccination. Three years post-vaccination seroconversion rates for PT-neutralising antibodies were 65·0% (95% CI 44·1–85·9) and 55·0% (95% CI 33·2–76·8) in aPgen and TdaPgen recipients, respectively.
Interpretation
Considering the persistence of elevated antibody responses 3 years post-booster vaccination, genetically detoxified monovalent aPgen and TdaPgen vaccines can be expected to induce longer-lasting protection than chemically inactivated Tdap vaccines.
Funding
BioNet-Asia
Keywords: Pertussis, Acellular, Vaccine, Recombinant, Genetically, Monovalent, Adolescents, Booster, Igg, Persistence, IgG, Waning
Research in context.
Evidence before this study
PubMed was searched for publications before December 1, 2020 involving human trials with pertussis booster vaccines that reported on the persistence of immune responses in adolescents or adults more than 1 year after vaccination. One study was identified that reported on the persistence of pertussis antibody responses in adults aged 18 to 40 years old, approximately 3 years after booster vaccination with investigational acellular pertussis vaccines containing genetically detoxified pertussis toxin. We previously demonstrated that a new generation recombinant acellular pertussis vaccine containing genetically inactivated pertussis toxin, both in a monovalent formulation and combined with tetanus and reduced-dose diphtheria toxoids, was highly immunogenic and that elevated antibody responses persisted at 1-year post-vaccination. However, no studies were found that reported on pertussis antibody persistence in adolescents longer than 1 year after booster vaccination with genetically detoxified pertussis toxin-containing vaccine.
Added value of this study
This is the first study demonstrating that pertussis antibody responses persist at significantly elevated levels in adolescents 3 years after vaccination with recombinant acellular pertussis-based vaccines. In contrast, in adolescents vaccinated with chemically inactivated pertussis booster vaccine antibodies were shown to have returned to baseline by 2 years post-vaccination.
Implications of all the available evidence
Earlier and current findings provide evidence that the new generation recombinant acellular pertussis vaccines, licensed and used in Thailand for active immunisation in adolescents from 11 years of age and adults, induce longer-lasting protection than chemically inactivated booster vaccines in adolescents.
Alt-text: Unlabelled box
1. Introduction
It is increasingly understood that acellular pertussis vaccines are associated with rapid waning of vaccine-induced immune responses and a consequent rise in pertussis disease several years after vaccination [1,2]. All acellular pertussis vaccines contain inactivated pertussis toxin (PT) and most include one up to four additional pertussis antigens (filamentous hemagglutinin, FHA; pertactin, PRN; and fimbriae type 2 and 3, FIM2/3). PT-specific antibodies with toxin-neutralising properties are essential and sufficient to prevent severe pertussis disease [3,4]. To ensure vaccine safety, pertussis toxin must be inactivated for use in humans. In most acellular pertussis vaccines this is accomplished using chemical processes that not only inactivate the toxin but also affect conformational epitopes inducing neutralising antibodies [5,6]. In adolescents vaccinated in childhood with acellular pertussis vaccines containing chemically inactivated PT (PTchem), booster vaccination with a PTchem containing vaccine has been demonstrated to provide moderate protection against pertussis during the first year but then to wane rapidly so that little protection remained 2–3 years after booster vaccination [7]. In contrast to chemical inactivation, genetic inactivation preserves the native epitope structures and immunogenic properties of PT that are important to induce a neutralising antibody response [4,5], and hence may induce longer lasting protection.
A new generation acellular pertussis vaccine containing genetically detoxified PT (PTgen) was developed and licensed as a monovalent recombinant acellular pertussis vaccine (aPgen) or combined with tetanus and reduced-dose diphtheria toxoids (TdaPgen), for immunisation of individuals aged 11 years and older in Thailand [8,9]. Both formulations are used in Thailand to vaccinate adolescents and adults, including pregnant women and the elderly, against pertussis [10]. Pivotal booster vaccination studies in adolescents in Thailand and Switzerland have demonstrated that compared to chemically detoxified pertussis booster vaccine (Tdapchem), aPgen and TdaPgen are more immunogenic and induce PT-specific IgG and PT neutralising antibody responses that persist to be significantly higher 1 year after vaccination [11,12]. To demonstrate that the superior immune responses induced by these new generation recombinant acellular pertussis vaccines last longer, we compared the persistence of vaccine antibody responses in adolescents 2 and 3 years after booster vaccination with one dose of the new generation recombinant PTgen containing pertussis vaccines (aPgen or TdaPgen) or a booster dose of a Tdapchem vaccine.
2. Methods
2.1. Study design and study participants
This was an observational follow-up study of the persistence of antibody responses in individuals who had participated through the Vaccine Trial Centre, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand (one of two study sites) in a phase 2/3 multi-centre randomised controlled safety and immunogenicity trial of new generation recombinant pertussis vaccines (originally conducted between July and August 2015). Participants were between 12 and 17 years old when they were enrolled into the phase 2/3 trial and were vaccinated with one dose of aPgen, TdaPgen, or Tdapchem (randomisation 1:1:1). A more detailed description on study participants and inclusion criteria in the phase 2/3 trial has been published previously [9,11]. Of the 225 participants vaccinated through the Vaccine Trial Centre, 223 completed the phase 2/3 trial at 1-year post-vaccination and were invited to participate in the observational follow-up studies: 180 subjects agreed to participate in the 2-year follow-up (aPgen, n = 58; TdaPgen, n = 59; Tdapchem, n = 63), and 181 in the 3-year follow-up study (aPgen, n = 62; TdaPgen, n = 59; Tdapchem, n = 60). To assess the persistence of pertussis vaccine antibody responses, a venous blood sample (5 mL) was collected at each of the 2- and 3-years post-vaccination time points. Laboratory staff was blinded to the vaccine group allocation of specimen they received. Safety and immunogenicity outcomes have been reported previously for the phase 2/3 trial, including PT- and FHA-specific IgG responses at 28 days post-vaccination in all study participants, and PT-neutralising antibodies at 28 days post-vaccination and PT- and FHA-specific IgG and PT neutralising antibody responses at 1-year post-vaccination in a pre-selected subpopulation comprising half of the study cohort [9,11].
2.2. Study vaccines
New generation acellular pertussis vaccines were developed and produced by BioNet-Asia Co., Ltd. (Ayutthaya, Thailand) using a recombinant Bordetella pertussis strain containing a non-toxic S1 gene (R9K and E129G) in the ptx operon [8,13]. The vaccine is available as a monovalent acellular pertussis vaccine (aPgen, Pertagen; Batch No. PE25002–2, and in combination with tetanus and reduced-dose diphtheria toxoids (TdaPgen, Boostagen; Batch No. TD25002–1). A single dose (0·5 mL) of aPgen or TdaPgen contains 5 µg PTgen, 5 µg FHA, and 0·3 mg Al3+as Aluminium hydroxide. TdaPgen in addition contains 7·5 Lf tetanus toxoid (TT) and 2 Lf diphtheria toxoid (DT). One dose (0·5 mL) of the comparator Tdapchem vaccine (Adacel, Sanofi-Pasteur, Canada) (Lot No. U4971AA) contains 2·5 µg PTchem, 5 µg FHA, 3 µg pertactin (PRN), 5 µg Fimbriae types 2 and 3 (FIM), 5 Lf TT, 2 Lf DT, and 0·33 mg Al3+ as Aluminium phosphate. All study vaccines were presented as mono-dose prefilled syringes.
2.3. Assessment of antibody persistence 2 and 3 years after vaccination
Serum PT- and FHA-specific IgG titres were measured using the same validated indirect ELISAs and procedures as used to assess earlier antibody responses [9]. Samples with titres below 5 IU/mL were arbitrarily attributed a titre of 5 IU/mL. PT neutralising antibody titres were assessed using a validated CHO-cell PT neutralisation assay as used to assess responses for earlier timepoints [11]. An assay cut-off at 5 IU/mL was considered a seropositive response. A pertussis booster response was defined as a ≥ 4-fold increase of seropositivity level set at 5 IU/mL, corresponding with antibody titres of ≥ 20 IU/mL. Serum TT- and DT-specific IgG titres were measured for participants vaccinated with Td-containing vaccines using commercially available ELISA kits (Serion ELISA classic Diphtheria IgG kit, ESR130G; and Serion ELISA Classic Tetanus IgG kit, ESR108G, Virion/ Serion, Germany). Samples with titres below the assay cut-off of 0·1 IU/mL were attributed the value of the cut-off. DT and TT IgG titres of > 0·1 IU/mL were considered seroprotective [14,15].
2.4. Ethical considerations
The phase 2/3 trial and follow-up studies were conducted according to ICH-GCP, Declaration of Helsinki and local ethical guidelines. Ethical approval for the extended studies was obtained from the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University. Study participants were re-consented for their participation in the 2 and 3-year follow-up studies. In line with Thai regulation, written informed assent and consent were obtained from study participants and their parents/legal guardians if participants were younger than 18 years old, and written informed consent was obtained from participants if 18 years and older. The initial phase 2/3 clinical study is registered in the Thai Clinical Trial Registry (www.clinicaltrials.in.th), number TCTR20150703002, and the extended 2 and 3-year follow-up studies on ClinicalTrials.gov, numbers NCT04113655 and NCT04102137, respectively.
2.5. Statistical analysis
Data management and statistical analyses were performed by the independent Center of Excellence for Biomedical and Public Health Informatics (BIOPHICS), Bangkok, Thailand, using Statistical Analysis System (SAS) version 9·4. Results were analysed per protocol for all evaluable subjects at any given time point. GMCs/GMTs and proportions were calculated with exact 95% confidence interval (95% CI). An independent t-test or ANOVA were used to test for differences in normally distributed continuous variables between 2 or 3 groups, respectively. Wilcoxon rank sum test or Kruskal Wallis test were used to test for differences in continuous variables that did not follow a normal distribution, comparing 2 or 3 groups, respectively. Paired t-test was used to compare GMCs/GMTs between baseline and post-vaccination data. Bonferroni post-hoc analysis was used to determine which mean or groups of means is/are significantly different. p-values ≤ 0·05 were considered statistically significant.
2.6. Role of funding source
The study funder was involved in all stages of the study, including manuscript development. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors agreed to submit the manuscript for publication. No honorarium, grant, or other form of payment was provided to authors other than those who are employees of BioNet-Asia, except for funding needed to conduct the study.
3. STROBE
This report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement guidelines for reporting on observational studies in cohorts.
4. Results
4.1. Population characteristics
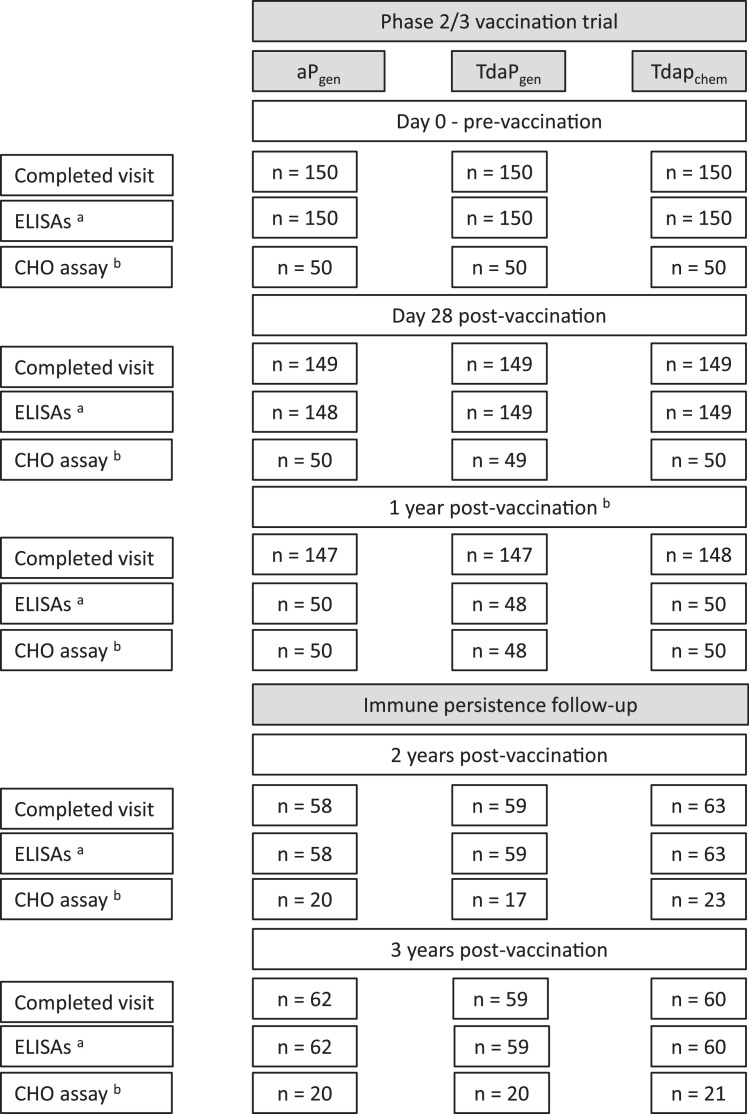
Study participant disposition and the number of participants with evaluable antibody responses at different time points during the 3-year study period are shown in Fig. 1. Study participants were on average 16 years and 11 months old (ranging between 13 years and 11 months, and 19 years and 11 months) at the 2-year follow-up, and 17 years and 10 months (ranging between 15 years and 21 years) at the 3-year follow-up, with no differences between the vaccine groups.
Fig. 1.
Subject disposition for initial trial and extended follow-up studies.
Provided are the number of participants who completed study visits and the number of blood samples analysed for IgG responses against pertussis antigens (PT and FHA) and diphtheria and tetanus toxoids as assessed by ELISA (a), and PT-neutralising antibody titres as assessed by the CHO neutralisation assay (b) as part of the initial trial (pre-vaccination, and Day 28 and 1-year post-vaccination) and the follow-up studies (2-year and 3-year post-vaccination).
4.2. Kinetics of pertussis antibody responses over 3 years after booster vaccination
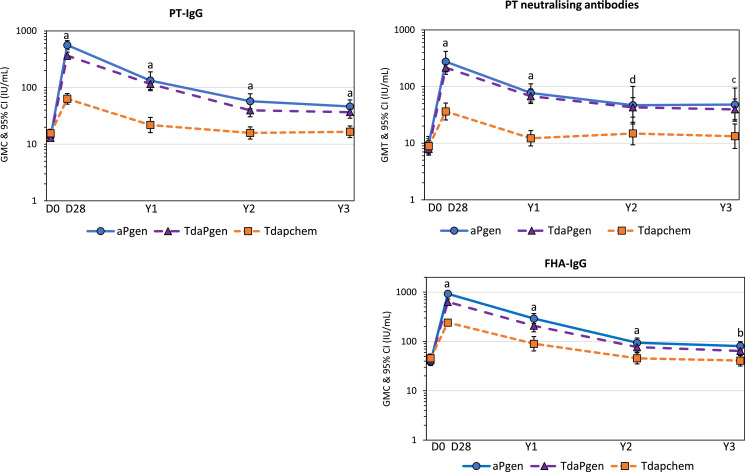
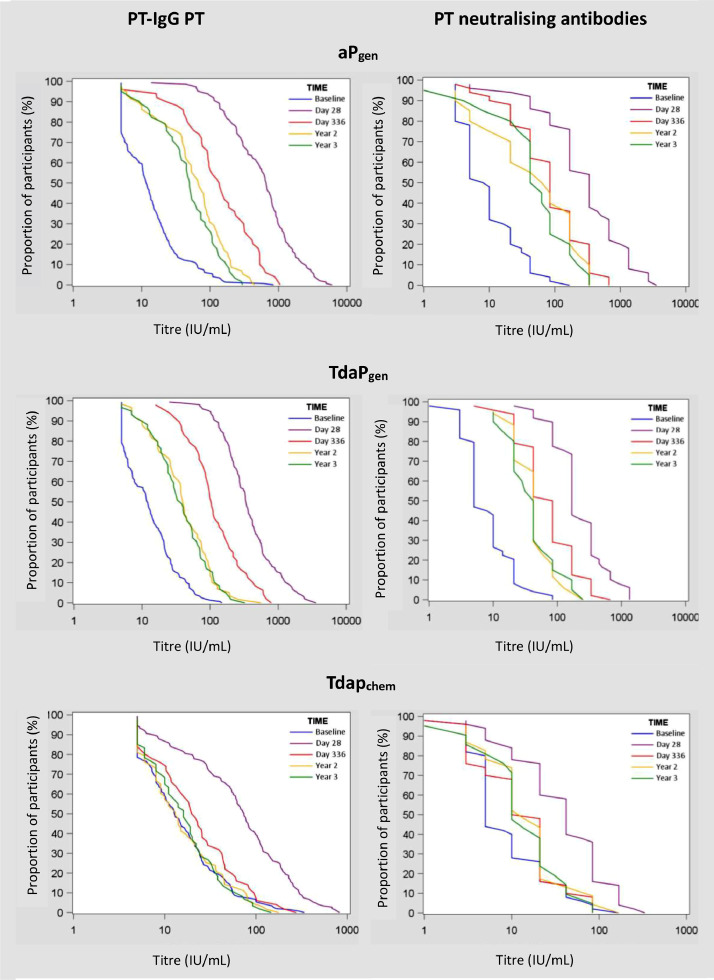
The kinetics of pertussis antibody responses over 3 years after booster vaccination, including previously reported results on antibody responses at 28 days and 1 year after vaccination [9,11], are presented in GMCs in Fig. 2, and in reverse cumulative distribution curves (RCDCs) in Fig. 3. PT- and FHA-specific antibodies declined in the first year after vaccination in all groups. In aPgen and TdaPgen recipients, antibody waning stabilized 2- and 3-years post-vaccination at levels significantly above baseline (Figs. 2 and 3, Table 2, and Supplementary Table 1). At all time-points after vaccination, PT-specific IgG and PT neutralising antibody responses were significantly higher in the aPgen and TdaPgen. groups compared to the Tdapchem group. Studying seroconversion rates (≥ 4-fold change from baseline), none of the Tdapchem recipients remained seroconverted for PT neutralising antibodies 3 years post-vaccination (0·0%, 95% 0·0–0·0), compared to 65·0% (95% CI 44·1–85·9) of the aPgen recipients and 55·0% (95% CI 33·2–76·8) of the TdaPgen recipients (Supplementary Table 2).
Fig. 2.
Kinetics of pertussis antibody responses over a 3-year period after booster vaccination with recombinant acellular pertussis or chemically detoxified Tdap.
Graphs show geometric mean concentrations or titres (and 95% confidence intervals) of IgG antibodies against pertussis toxin (PT-IgG), filamentous hemagglutinin (FHA-IgG), and PT-neutralising antibodies (PT-Nab) for individuals vaccinated at Day 0 (pre-vaccination) with recombinant aPgen vaccine (blue line; circles), recombinant TdaPgen vaccine (purple line; triangles), or comparator Tdapchem vaccine (orange line; squares) with evaluable data at different time points. Differences in GMCs between groups were compared using the Bonferroni post-hoc test, with observed p-values corresponding to a) p < 0·0001; b) p = 0.0001; c) p = 0·0015, and d) p = 0·0047.
Fig. 3.
Reverse cumulative distribution curves (RCDCs) for PT-IgG and PT-neutralising antibody titres before and after booster vaccination with recombinant acellular pertussis (aPgen or TdaPgen) or chemically detoxified Tdap.
Blue lines present RCDCs before vaccination, purple lines 28 days after vaccination, red lines 1 year after vaccination, yellow lines 2 years after vaccination, and green lines 3 years after vaccination.
Table 2.
Pertussis seropositivity, pertussis booster responses, and seroprotection for tetanus and diphtheria.
| aPgen,% (95% CI) |
TdaPgen,% (95% CI) |
Tdapchem,% (95% CI) |
||||
|---|---|---|---|---|---|---|
| Pertussis seropositive a | 2-years | 3-years | 2-years | 3-years | 2-years | 3-years |
| PT > 5 IU/mL a | 96·6 (91·9–100) |
95·2 (89·8–100) |
95·2 (89·8–100) |
96·6 (92·0–100) |
81·0 (71·3–90·7) |
85·0 (76·0–94·0) |
| FHA > 5 IU/mL a | 98·3 (94·9–100) |
98·4 (95·3–100) |
96·6 (92·0–100) |
96·6 (92·0–100) |
96·8 (92·5–100) |
98·3 (95·1–100) |
| Pertussis booster responsec | ||||||
| PT ≥ 20 IU/mL a |
81·0 (71·0–91·1) |
80·7 (70·8–90·5) |
74·6 (63·5–85·7) |
74·6 (63·5–85·7) |
38·1 (26·1–50·1) |
40·0 (27·6–52·4) |
| FHA ≥ 20 IU/mL a | 96·6 (91·9–100) |
98·4 (95·3–100) |
96·6 (92·0–100) |
96·6 (92·0–100) |
81·0 (71·3–90·7) |
76·7 (66·0–87·4) |
| Nab ≥ 20 IU/mL b | 75·0 (56·0–94·0) |
85·0 (69·4–100) |
94·1 (82·9–100) |
85·0 (69·4–100) |
47·8 (27·4–68·2) |
42·9 (21·7–64·0) |
| Tetanus & Diphtheria seroprotectiona | ||||||
| TT > 0·1 IU/mL | – | – | 100 (100–100) |
100 (100–100) |
100 (100–100) |
100 (100–100) |
| DT > 0·1 IU/mL | – | – | 96·6 (92·0–100) |
86·2 (77·3–95·1) |
92·1 (85·4–98·7) |
88·3 (80·2–96·5) |
Antibody IgG measured by ELISA assay.
PT-neutralising antibodies (Nab) measured by CHO assay.
Antibody titres ≥ 20 IU/mL correspond with a 4-fold increase (post-booster) of seropositivity level set at 5 IU/mL.
4.3. Differences in persistence of pertussis antibody responses after vaccination with chemically and genetically inactivated vaccines
Studying GMC ratios (GMCRs) of antibody responses after vaccination to responses before vaccination, PT-specific IgG at 3-years post-vaccination persisted at levels 3·0-fold (95% CI 2·2–4·1) and 2·5-fold (95% CI 1·9–3·3) higher than baseline in aPgen and TdaPgen recipients, respectively (Table 1). For FHA-specific IgG, GMCRs at 3-years post-vaccination were 1·8 (95% CI 1·3–2·5) and 1·6 (95% CI 1·2–2·1) in aPgen and TdaPgen recipients, respectively (Table 1). Antibody persistence was the greatest for PT neutralising antibodies that remained 4·6-fold (95% CI 2·6–8·1) and 3·7-fold (95% CI 2·2–6·1) elevated at 3-years post-vaccination in the aPgen and TdaPgen recipients, respectively (Table 1). In the Tdapchem group, PT- and FHA-specific antibodies were back at or below pre-vaccination levels 2 years after vaccination (Table 1).
Table 1.
The persistence of pertussis antibody responses 2 and 3 years after vaccination in adolescents vaccinated with recombinant pertussis (aPgen or TdaPgen) or chemically detoxified pertussis vaccine (Tdapchem).
| GMC ratio post/pre-vaccination (95% CI) |
||||||
|---|---|---|---|---|---|---|
| aPgen | TdaPgen | Tdapchem | p-value | aPgen vs. Tdapchem | TdaPgen vs. Tdapchem | |
| PT-IgG | ||||||
| 2-years post-vaccination | 3·6 (2·6–5·1) | 2·5 (1·9–3·2) | 0·8 (0·6–0·9) | <0·0001 | 4·7 (3.0–7·3) | 3·2 (2·0–5·0) |
| 3-years post-vaccination | 3·0 (2·2–4·1) | 2·5 (1·9–3·3) | 0·8 (0·7–1·0) | <0·0001 | 3·7 (2·4–5·7) | 3·1 (2·0–4·8) |
| PT-Nab | ||||||
| 2-years post-vaccination | 4·5 (2·5–8·1) | 3·7 (2·3–5·9) | 1·1 (0·8–1·7) | 0·0001 | 3·9 (1·8–8·6) | 3·2 (1·4–7·4) |
| 3-years post-vaccination | 4·6 (2·6–8·1) | 3·7 (2·2–6·1) | 0·9 (0·7–1·2) | <0·0001 | 4·9 (2·3–10·5) | 4.0 (1·9–8·4) |
| FHA-IgG | ||||||
| 2-years post-vaccination | 2·2 (1·5–3·1) | 1·8 (1·4–2·5) | 0·7 (0·5–1·0) | <0·0001 | 3·0 (1·7–5·3) | 2·5 (1·4–4·4) |
| 3-years post-vaccination | 1·8 (1·3–2·5) | 1·6 (1·2–2·1) | 0·7 (0·5–1·0) | <0·0001 | 2·6 (1·5–4·5) | 2·3 (1·4–4·1) |
The persistence of pertussis antibody responses was assessed by calculating geometric mean concentration ratios (GMCRs) and 95% CIs of antibody responses after vaccination to before vaccination. To compare differences in antibody persistence between recombinant pertussis (aPgen or TdaPgen) and chemically detoxified pertussis vaccine (Tdapchem), fold-differences in GMCRs and 95% CIs were calculated.
Comparing GMCRs for genetically inactivated vaccines versus chemically inactivated vaccine recipients at 3-years after vaccination (Table 1), there was a 3·7-fold (95% CI 2·4–5·7) difference for PT-specific IgG between aPgen vs. Tdapchem, and a 3.1-fold (95% CI 2·0–4·8) difference for PT-specific IgG between TdaPgen and Tdapchem. For PT neutralising antibodies the difference (GMCR) was 4·9-fold (95% CI 2·3–10·5) between aPgen vs. Tdapchem and 4·0-fold (95% CI 1·9–8·4) between TdaPgen and Tdapchem (Table 1). GMCRs for FHA-specific IgG were 2·6 (95% CI 1·4–4·1) for aPgen vs. Tdapchem recipients, and 2·3 (95% CI 1·4–4·1) for TdaPgen vs. Tdapchem recipients at 3-years post-vaccination.
4.4. Pertussis seropositivity and booster responses
Three years after vaccination 95·2% (95% CI 89·8–100) of aPgen recipients, 96·6% (95% CI 92·0–100) of TdaPgen recipients, and 85·0% (95% CI 76·0–94·0) of Tdapchem recipients remained seropositive for PT-IgG (Table 2). Seropositivity for FHA at 3 years post-vaccination was 98·4% (95% CI 95·3–100), 96·6% (95% CI 92·0–100), and 98·3% (95% CI 95·1–100) in the aPgen, TdaPgen and Tdapchem groups, respectively.
Pertussis booster responses (≥ 20 IU/mL) for PT-specific IgG persisted 3-years post-vaccination in 40·0% (95% CI 27·6–52·4) of Tdapchem recipients compared to 80·7% (95% CI 70·8–90·5) and 74·6% (95% CI 63·5–85·7) of aPgen or TdaPgen recipients, respectively (Table 2). Booster responses for PT neutralising antibodies persisted in 42·9% (95% CI 21·7–64·0) of the Tdapchem group compared to 85·0% (95% CI 69·4–100) in both the aPgen and TdaPgen groups. FHA-specific IgG booster responses were significantly higher in aPgen (98·4%; 95% CI 95·3–100) and TdaPgen recipients (96·6%; 95% CI 92·0–100) than Tdapchem recipients (76.7%; 95% CI 66·0–87·4).
4.5. Seroprotection against tetanus and diphtheria
All participants remained seroprotected against tetanus toxoid 3 years after booster vaccination with TdaPgen or Tdapchem (100% seroprotection) (Table 2). Seroprotection against diphtheria toxoid 3 years after was vaccination was 86·2% (95% CI 77·3–95·1) for TdaPgen, and 88·3% (95% CI 80·2–96·5) for Tdapchem. TT- and DT-specific IgG GMCs at 2 and 3 years after vaccination with TdaPgen or Tdapchem are presented in Supplementary Table 3.
5. Discussion
These extended follow-up studies show that PT-specific IgG and PT neutralising antibody responses persisted at elevated levels at least up to 3 years after an adolescent booster dose of recombinant pertussis vaccine. In line with reports from other studies [16], [17], [18], in recipients of an adolescent booster dose of Tdapchem PT-specific antibodies had returned to baseline levels after 2 years. While the persistence of PT-specific IgG has been demonstrated for adults vaccinated with another genetically detoxified vaccine [19], to our knowledge this is the first study demonstrating the persistence of antibody responses after vaccination with genetically inactivated pertussis vaccine in adolescents.
As demonstrated by others, most adolescents and adults still have detectable antibodies against at least one pertussis vaccine antigen 10 years after a booster dose of Tdapchem, and as such are considered seropositive [17,18]. This mostly concerns antibodies against pertussis antigens other than PT, against which antibodies have often waned and are no longer detectable [16], [17], [18]. In parallel with the waning of PT-specific antibodies [16,20,21], the effectiveness of a Tdapchem booster dose wanes rapidly with little protection remaining after 2 to 3 years after vaccination [7]. This can imply that the persistence of PT-specific antibody responses is paramount for the duration of pertussis booster effectiveness.
The rate at which antibodies wane is related to the immunogenicity of the antigen [20,22]. Compared to PT that has been inactivated using formaldehyde/glutaraldehyde, PTgen in the new generation recombinant pertussis vaccine is 6-to-9 fold more immunogenic [9,12,13,19]. As has come to light by pertussis outbreaks in middle and high schools in the US [23,24], waning of antibody responses in the years following completion of childhood pertussis vaccination leaves children at increased risk for pertussis as they become older [25]. In 2006 the US Advisory Committee on Immunization Practices (ACIP) recommended the introduction of a Tdap booster for adolescents between 11 and 12 years of age [26]. However, the effectiveness of currently used Tdapchem is estimated to be less than 10% after 3 years [7]. This raises the question whether a subsequent pertussis booster is needed at older adolescence (age 15–17 years). An alternative may be replacing the current Tdapchem booster vaccines with recombinant pertussis booster vaccines that may offer longer lasting protection.
Recombinant acellular pertussis booster vaccines are of interest in the context of maternal immunisation. In line with WHO recommendations, maternal pertussis vaccination is introduced in an increasing number of countries to prevent severe pertussis and reduce pertussis mortality in young infants [27], [28], [29]. The duration of protection induced by maternal pertussis vaccination depends largely on the level of PT-specific antibodies transferred from mother to child [30,31]. Maternal vaccination with the new recombinant pertussis vaccine may provide infants with better and longer-lasting protection than chemically inactivated pertussis vaccines, and possibly may induce immune protection over multiple pregnancies. The new generation recombinant pertussis vaccines could therefore be highly beneficial in low- and middle-income countries where the breadth of vaccination programs is limited for economic reasons.
Pertussis booster vaccines when first developed for use in adolescent and adults have only been available in combination with tetanus and diphtheria toxoids. However, booster vaccination with diphtheria and tetanus is not needed in adults who have received 6 doses of tetanus and diphtheria toxoids-containing vaccines (3-dose primary series plus 3-dose booster series) prior to adolescence [14], [15], [32]. This includes vaccination of pregnant women in high-income countries, where maternal tetanus vaccination to prevent neonatal tetanus is not required. There is an increasing call for a pertussis-only vaccine for use in adults, especially pregnant women [33,34]. The recombinant aPgen vaccine used in this study is currently the only stand-alone pertussis vaccine available worldwide and is used safely in adolescents and adults including pregnant women in Thailand [10].
As supported by findings from another study that the new generation recombinant pertussis vaccines also induced higher PT-specific antibody responses than a different comparator Tdapchem vaccine with a higher PT content [12], the difference in PT contents cannot explain the higher and longer persisting PT-specific antibody responses induced by the recombinant vaccines reported here. Instead, this is mostly attributed to the use of recombinant DNA technology to inactivate the toxic property of PT and that preserves the native conformational epitopes of PT [8,35]. The high concentrations of formaldehyde used in Tdapchem vaccines to inactivate residual PT co-eluted with FHA also affects the protein formation and immune recognition of FHA [36] and explains why the recombinant vaccines induce higher and longer lasting FHA-specific antibodies despite containing the same amount of FHA as the comparator vaccine.
Our study has a few limitations. Firstly, we do not know if participants have been exposed to B. pertussis over the 3 years of follow-up, which may have boosted antibody responses. However, exposure, if any, can be expected to have happened at the same rate within the different vaccine groups. Secondly, while inducing longer lasting immune responses in adolescents, it remains to be shown that these new generation recombinant pertussis vaccines also induce longer persisting antibodies than currently used acellular pertussis vaccines when given as a booster dose to younger children. Current field studies show that immunity is fast waning after booster vaccination with chemically inactivated pertussis vaccines in 4-to-6 year old schoolchildren [25].
As a final remark, while PT-specific IgG seropositivity was comparable for recombinant and chemically inactivated pertussis vaccines 3 years after vaccination, only 40% of Tdapchem recipients versus 81% of aPgen and 75% of TdaPgen recipients, maintained PT-IgG booster responses 3 years post-vaccination. This implies that seropositivity as defined by the presence of antibodies at or above the lower limit of quantitation (LLOQ) may not be the best measure to study the long-term persistence of vaccine-induced immunity induced by different vaccines. Instead, using more stringent criteria such as a pertussis booster response may be more adequate and allow to differentiate between the capacity of different vaccines.
In summary, new generation recombinant acellular pertussis booster vaccines, available as a pertussis-only and in combination with tetanus and diphtheria, are associated with stabilizing PT antibody responses at levels 2 and 3 years after vaccination that are well above pre-vaccination. It may therefore be expected that the duration of immune protection and hence vaccine effectiveness induced by this new generation recombinant pertussis vaccine is longer than that of currently used chemically detoxified Tdap booster vaccines. Follow-up studies to assess persistence of antibodies at 5 years after booster vaccination are planned.
Funding
This study was funded by BioNet-Asia Co., Ltd., Thailand.
Data sharing statement
Datasets used in the presented study may be made available, depending on researchers submitting a research proposal (to Clinical@BioNet-Asia.com) outlining the scientific objectives and planned analyses and subsequent approval by a relevant human research ethics committee.
Contributors
PC, JS, SM, SV, WW and HTP were responsible for the conceptualization and design of the study; PP, PC, LF, and SM were responsible for study coordination and supervision; PP was the principal investigator for the study site; PP, CS, JD, AP, YS, CK, and PC were responsible for conducting the study; LF was responsible for pharmacovigilance management during the study; IKP was responsible for designing and overseeing the immunological assays; MC was responsible for manufacturing of the investigational products; PC and CK were responsible for data verification; all authors contributed to data interpretation; AvdB prepared the first draft and final manuscript; all authors reviewed, edited, and approved the final manuscript.
Declaration of Competing Interest
AvdB, CK, HTP, IKP, JS, LF, MC, PC, SM and WW were employed by BioNet-Asia Co., Ltd at the time the study was conducted. PP, JD, CS, AP, SV and YS declare to have received funding from BioNet-Asia Co., Ltd. for the conduct of this study.
Acknowledgments
The authors thank all study participants, study investigators and clinical study site staff at the Faculty of Tropical Medicine, Mahidol University for their contribution to the study, and BIOPHICS for data management and statistical analysis. The authors would also like to thank BioNet-Asia's study monitoring team, human serological laboratory team, manufacturing team, regulatory affairs team, and pharmacovigilance team for their contributions.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.100976.
Appendix. Supplementary materials
References
- 1.World Health Organization (WHO). WHO SAGE pertussis working group. Background paper SAGE April 2014. Available at: http://www.who.int/immunization/sage/meetings/2014/april/1_Pertussis_background_FINAL4_web.pdf. Accessed 2 Oct 2019.
- 2.Esposito S., Stefanelli P., Fry N.K. Pertussis prevention: reasons for resurgence, and differences in the current acellular pertussis vaccines. Front Immunol. 2019;10:1344. doi: 10.3389/fimmu.2019.01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taranger J., Trollfors B., Lagergard T., Sundh V., Bryla D.A., Schneerson R. Correlation between pertussis toxin IgG antibodies in postvaccination sera and subsequent protection against pertussis. J Infect Dis. 2000;181(3):1010–1013. doi: 10.1086/315318. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen A.W., DiVenere A.M., Papin J.F., Connelly S., Kaleko M., Maynard J.A. Neutralization of pertussis toxin by a single antibody prevents clinical pertussis in neonatal baboons. Sci Adv. 2020;6(6):eaay9258. doi: 10.1126/sciadv.aay9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibsen P.H. The effect of formaldehyde, hydrogen peroxide and genetic detoxification of pertussis toxin on epitope recognition by murine monoclonal antibodies. Vaccine. 1996;14(5):359–368. doi: 10.1016/0264-410x(95)00230-x. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland J.N., Chang C., Yoder S.M., Rock M.T., Maynard J.A. Antibodies recognizing protective pertussis toxin epitopes are preferentially elicited by natural infection versus acellular immunization. Clin Vaccine Immunol. 2011;18(6):954–962. doi: 10.1128/CVI.00561-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein N.P., Bartlett J., Fireman B., Baxter R. Waning Tdap effectiveness in adolescents. Pediatrics. 2016;137(3) doi: 10.1542/peds.2015-3326. [DOI] [PubMed] [Google Scholar]
- 8.Buasri W., Impoolsup A., Boonchird C., Luengchaichawange A., Prompiboon P., Petre J. Construction of Bordetella pertussis strains with enhanced production of genetically-inactivated Pertussis Toxin and Pertactin by unmarked allelic exchange. BMC Microbiol. 2012;12:61. doi: 10.1186/1471-2180-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sricharoenchai S., Sirivichayakul C., Chokephaibulkit K., Pitisuttithum P., Dhitavat J., Pitisuthitham A. A genetically inactivated two-component acellular pertussis vaccine, alone or combined with tetanus and reduced-dose diphtheria vaccines, in adolescents: a phase 2/3, randomised controlled non-inferiority trial. Lancet Infect Dis. 2018;18(1):58–67. doi: 10.1016/S1473-3099(17)30612-6. [DOI] [PubMed] [Google Scholar]
- 10.Fortuna L., Chaithongwongwatthana S., Soonthornworasiri N., Spiegel J., Wijagkanalan W., Mansouri S. Enhanced post-licensure safety surveillance of a new recombinant acellular pertussis vaccine licensed as a monovalent (aP, Pertagen) and tetanus, reduced-dose diphtheria combination (TdaP, Boostagen) vaccine for immunization of adolescents and adults in Thailand. Vaccine. 2020;38(51):8194–8199. doi: 10.1016/j.vaccine.2020.10.070. [DOI] [PubMed] [Google Scholar]
- 11.Pitisuttithum P., Chokephaibulkit K., Sirivichayakul C., Sricharoenchai S., Dhitavat J., Pitisuthitham A. Antibody persistence after vaccination of adolescents with monovalent and combined acellular pertussis vaccines containing genetically inactivated pertussis toxin: a phase 2/3 randomised, controlled, non-inferiority trial. Lancet Infect Dis. 2018;18(11):1260–1268. doi: 10.1016/S1473-3099(18)30375-X. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard Rohner G., Chatzis O., Chinwangso P., Rohr M., Grillet S., Salomon C. Boosting teenagers with acellular pertussis vaccines containing recombinant or chemically inactivated pertussis toxin: a randomized clinical trial. Clin Infect Dis. 2018;68(7):1213–1222. doi: 10.1093/cid/ciy594. [DOI] [PubMed] [Google Scholar]
- 13.Sirivichayakul C., Chanthavanich P., Limkittikul K., Siegrist CA., Wijagkanalan W., Chinwangso P. Safety and immunogenicity of a combined Tetanus, Diphtheria, recombinant acellular Pertussis vaccine (TdaP) in healthy Thai adults. Hum Vaccin Immunother. 2017;13(1):136–143. doi: 10.1080/21645515.2016.1234555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO) Diphtheria vaccine: WHO position paper – August 2017. Wkly Epidemiol Rec. 2017;92(31):417–435. [Google Scholar]
- 15.World Health Organization (WHO) Tetanus vaccines: WHO position paper – February 2017. Wkly Epidemiol Rec. 2017;92(6):53–76. [PubMed] [Google Scholar]
- 16.Embree J., Law B., Voloshen T., Tomovici A. Immunogenicity, safety, and antibody persistence at 3, 5, and 10 years postvaccination in adolescents randomized to booster immunization with a combined tetanus, diphtheria, 5-component acellular pertussis, and inactivated poliomyelitis vaccine administered with a hepatitis B virus vaccine concurrently or 1 month apart. Clin Vaccine Immunol. 2015;22(3):282–290. doi: 10.1128/CVI.00682-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pool V., Tomovici A., Johnson D.R., Greenberg D.P., Decker M.D. Humoral immunity 10 years after booster immunization with an adolescent and adult formulation combined tetanus, diphtheria, and 5-component acellular pertussis vaccine in the USA. Vaccine. 2018;36(17):2282–2287. doi: 10.1016/j.vaccine.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 18.Tomovici A., Barreto L., Zickler P. Humoral immunity 10 years after booster immunization with an adolescent and adult formulation combined tetanus, diphtheria, and 5-component acellular pertussis vaccine. Vaccine. 2012;30(16):2647–2653. doi: 10.1016/j.vaccine.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Leroux-Roels G., Lattanzi M., Solis C.D., Contorni M., Costantini M., Moraschini L. A phase I, randomized, controlled, dose-ranging study of investigational acellular pertussis (aP) and reduced tetanus-diphtheria-acellular pertussis (TdaP) booster vaccines in adults. Hum Vaccin Immunother. 2017;14(1):45–58. doi: 10.1080/21645515.2017.1385686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelman K., He Q., Makinen J., Sahlberg A., Haanpera M., Schuerman L. Immunity to pertussis 5 years after booster immunization during adolescence. Clin Infect Dis. 2007;44(10):1271–1277. doi: 10.1086/514338. [DOI] [PubMed] [Google Scholar]
- 21.Mertsola J., Van Der Meeren O., He Q., Linko-Parvinen A., Ramakrishnan G., Mannermaa L. Decennial administration of a reduced antigen content diphtheria and tetanus toxoids and acellular pertussis vaccine in young adults. Clin Infect Dis. 2010;51(6):656–662. doi: 10.1086/655825. [DOI] [PubMed] [Google Scholar]
- 22.Brandon D., Kimmel M., Kuriyakose S.O., Kostanyan L., Mesaros N. Antibody persistence and safety and immunogenicity of a second booster dose nine years after a first booster vaccination with a reduced antigen diphtheria-tetanus-acellular pertussis vaccine (Tdap) in adults. Vaccine. 2018;36(42):6325–6333. doi: 10.1016/j.vaccine.2018.08.051. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) School-associated pertussis outbreak–Yavapai County, Arizona, September 2002-February 2003. MMWR Morb Mortal Wkly Rep. 2004;53(10):216–219. [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC). Use of mass Tdap vaccination to control an outbreak of pertussis in a high school–Cook County, Illinois, September 2006-January 2007. MMWR Morb Mortal Wkly Rep2008; 57(29): 796–9. [PubMed]
- 25.Zerbo O., Bartlett J., Goddard K., Fireman B., Lewis E., Klein N.P. Acellular pertussis vaccine effectiveness over time. Pediatrics. 2019;144(1) doi: 10.1542/peds.2018-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broder K.R., Cortese M.M., Iskander J.K., Kretsinger K., Slade B.A., Brown K.H. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(Rr-3):1–34. [PubMed] [Google Scholar]
- 27.Amirthalingam G., Campbell H., Ribeiro S., Fry N.F., Ramsay M., Miller E. Sustained effectiveness of the maternal pertussis immunization program in England 3 years following introduction. Clin Infect Dis. 2016;63(suppl 4):S236–S243. doi: 10.1093/cid/ciw559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skoff T.H., Blain A.E., Watt J., Scherzinger K., McMahon M., Zansky S.M. Impact of the US maternal tetanus, diphtheria, and acellular pertussis vaccination program on preventing pertussis in iInfants <2 months of age: a case-control evaluation. Clin Infect Dis. 2017;65(12):1977–1983. doi: 10.1093/cid/cix724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saul N., Wang K., Bag S., Baldwin H., Alexander K., Chandra M. Effectiveness of maternal pertussis vaccination in preventing infection and disease in infants: the NSW Public Health Network case-control study. Vaccine. 2018;36(14):1887–1892. doi: 10.1016/j.vaccine.2018.02.047. [DOI] [PubMed] [Google Scholar]
- 30.Eberhardt C.S., Blanchard-Rohner G., Lemaitre B., Boukrid M., Combescure C., Othenin-Girard V. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin Infect Dis. 2016;62(7):829–836. doi: 10.1093/cid/ciw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcellini V., Piano Mortari E., Fedele G., Gesualdo F., Pandolfi E., Midulla F. Protection against pertussis in humans correlates to elevated serum antibodies and memory B cells. Front Immunol. 2017;8:1158. doi: 10.3389/fimmu.2017.01158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slifka A.M., Park B., Gao L., Slifka M.K. Incidence of tetanus and diphtheria in relation to adult vaccination schedules. Clin Infect Dis. 2021;72(2):285–292. doi: 10.1093/cid/ciaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McIntyre P.B., Edwards K.M. Genetically modified pertussis toxin: a quantum leap? Lancet Infect Dis. 2018;18(11):1169–1171. doi: 10.1016/S1473-3099(18)30426-2. [DOI] [PubMed] [Google Scholar]
- 34.Heininger U. Diphtheria, tetanus, and pertussis: unequal vaccine siblings with distinct characteristics. Clin Infect Dis. 2021;72(3):534. doi: 10.1093/cid/ciaa642. [DOI] [PubMed] [Google Scholar]
- 35.Nencioni L., Pizza M., Bugnoli M., De Magistris T., Di Tommaso A., Giovannoni F. Characterization of genetically inactivated pertussis toxin mutants: candidates for a new vaccine against whooping cough. Infect Immun. 1990;58(5):1308–1315. doi: 10.1128/iai.58.5.1308-1315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolgiano B., Crane D.T., Xing D., Williams L., Jones C., Corbel M.J. Physico-chemical analysis of Bordetella pertussis antigens. Biologicals. 1999;27(2):155–162. doi: 10.1006/biol.1999.0201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.