Abstract
Context:
Bladder cancer (BC) is the sixth most common malignant neoplasm in men. Recently, great effort has been devoted to the study of BC variant histology (VH). Yet, the results from these studies have shown conflicting data and remain unclear whether their presence alters recurrence and survival rates after radical cystectomy (RC).
Aims:
We undertook this study aiming to test the effect on VH on recurrence-free survival (RFS) and overall survival (OS) in single-center RC patients.
Settings and Design:
We have retrospectively analyzed medical records and pathology reports from 331 patients who underwent RC with or without pelvic lymphadenectomy at University Urology Clinic-Skopje, North Macedonia, in the period between 2010 and 2018.
Subjects and Methods:
Microscopic analysis of the specimens involved the evaluation of histological tumor type, tumor grade, pathological tumor node metastasis stage, presence of lymphovascular invasion, and resection margin status.
Statistical Analysis Used:
Univariable and multivariable Cox regression models were applied to test the effect of VH on RFS and OS.
Results:
We found 185 patients who matched our inclusion criteria. At multivariable analyses, lymphovascular invasion and positive resection margins were associated with shorter RFS. Similarly, patients diagnosed with lymphovascular invasion, positive resection margins, and a pelvic lymph node metastasis had poorer OS. VH was not found to be an independent predictor of both RFS and OS (P > 0.05).
Conclusions:
The present study did not reveal prognostic effect of VH on RFS and OS. In our series, histomorphologic parameters including lymphovascular invasion, resection margins, and pelvic lymph node metastasis were the most relevant predictors on survival outcome after RC.
Keywords: Bladder cancer, radical cystectomy, survival, variant histology
INTRODUCTION
Bladder cancer (BC) is the sixth most common malignant neoplasm in men and the 17th most commonly occurring cancer in women, with approximately 550,000 newly diagnosed cases in 2018 worldwide.[1] In North Macedonia, BC ranks as the sixth most prevalent cancer in men, with an estimated incidence of 23.58 in 2018.[2] Due to its high morbidity and mortality rate, especially in muscle-invasive diseases, radical cystectomy (RC) coupled with adjuvant chemotherapy is the treatment of choice.[3]
From pathohistological perspective, pure urothelial carcinoma (PUC) is considered the most frequent histologic type of BC, accounting for 90% of the cases.[4] Remaining cases constitute variant histologies (VHs) which could be present into two forms. The first form is urothelial carcinoma with divergent differentiation (UCDD), in which nonurothelial divergent histology is mixed with at least some degrees of conventional urothelial carcinoma.[4] The second form is pure nonurothelial carcinoma (PNUC), where a single nonurothelial histologic phenotype is present, (e.x., squamous cell carcinoma and adenocarcinoma).[4]
Recently, great effort has been devoted to the study of VH in BC. Yet, the results from these studies have shown conflicting data and remain unclear whether their presence alters recurrence and survival rates after RC.[5] In particular, the majority of the studies have suffered either from a lack of clarity in defining terms or a lack of pathologist's review interpretation.[6]
With this in mind, we undertook this study aiming to test the effect on VH on recurrence-free survival (RFS) and overall survival (OS) in single-center RC patients.
SUBJECTS AND METHODS
Patient's population
After the Institutional Board Approval, we have retrospectively analyzed medical records and pathology reports from 331 patients who underwent RC with or without pelvic lymphadenectomy at University Urology Clinic-Skopje, North Macedonia, in the period between 2010 and 2018.
Inclusion criteria encompassed high-risk nonmuscle or muscle-invasive urinary BC. Exclusion criteria were cystectomies for nononcological indications, cystectomies for nonurologic or mesenchymal malignancies, death within the 1st month of surgery, and previous neoadjuvant chemotherapy.
Diagnostic evaluation of patients was made in compliance with the European Association of Urology (EAU) guidelines for muscle-invasive BC.[3] This involved cystoscopy coupled with biopsy, pelvic/abdominal computed tomography (CT) scan, CT urography, native radiography, and bone scan if clinically indicated.
Histopathological evaluation
Operative materials were processed and interpreted in agreement with the International Collaboration on Cancer recommendations for BC reporting.[7] Briefly, after 24 h formalin fixation, cystectomy specimens were inked, and then representative samples of the urinary bladder, including the macroscopically deepest tumor penetration, were taken. In addition, sections of the nonaffected bladder mucosa, resection margins, regional lymph nodes, and eventual adherent organs were submitted.
Microscopic analysis of the specimens involved the evaluation of histological tumor type, tumor grade, pathological tumour node metastasis (pTNM) stage, presence of lymphovascular invasion, and resection margin status. All urinary bladder carcinomas were reassigned with the latest edition of the Union for International Cancer Control classification of malignant tumors.[8] Histologic tumor type and grade were determined according to the 2016 World Health Organization Classification of tumors of the Urinary Tract and Male genital System.[4] Extirpated lymph nodes were histologically validated for the presence of metastases. The presence of intraluminal tumor emboli within endothelium-lined spaces was characterized as a lymphovascular invasion. Histologically confirmed tumor cells at the inked surface of the RC specimen were defined as positive soft-tissue resection margin.
BC patients were subsequently stratified according to their histology into three groups: PUC, UCDD, and PNUC.
Clinical surveillance and treatment
Disease monitoring and treatment were based on the EAU guidelines for muscle-invasive and metastatic BC.[3] Follow-up visits were generally scheduled three times a year, biannually and annually for the first, second, and after the 3rd year of the intervention, respectively. Clinical validation encompassed physical examination, laboratory serum analyses including liver function tests, chest roentgenography, and CT in all patients. When clinically supported, upper urinary tract diagnostic imaging and bone scan were additionally carried out.
Adjuvant chemotherapy was administered depending on patohistologic tumor characteristics, Charlson comorbidity index, as well as patient's preference.
Local recurrence was defined as the presence of tumor in soft tissues at the original surgical site or locoregional lymph nodes.
Distant recurrence was defined as the presence of tumor in distant lymph nodes and/or organs.
RFS was calculated from the date of surgery to the date of detection of the first recurrence.
OS was defined as the time from the date of surgery to the date of the last visit or death from any cause.
In December 2019, we approached both State Statistics Mortality Registry and the Ministry of Health “My Term” software database to assess the exact date of death of all patients. This date was used as a cut-off point for defining the OS.
Statistical analysis
All data were interpreted using the Statistica for Windows software package 13.3 (StatSoft, Inc., Tulsa, OK, USA) and SPSS 22. 0 (IBM, SPSS, Chicago, IL, USA). Descriptive statistics included frequencies and percentages as well as mean and median values with standard deviations. Clinicopathologic characteristics between groups were compared by conducting Fischer's exact test and Chi-square test for categorical variables and Wilcoxon rank-sum test and Mann–Whitney U-test for continuous variables. Simultaneously, we performed Spearman's correlation test to assess the association between pathology stages and VH. Kaplan–Meyer curves with the log-rank test were generated to assess the survival functions. Finally, univariable and multivariable Cox regression models were applied to test the effect of VH on RFS and OS. A value of P < 0.05 was considered statistically significant.
RESULTS
Patient's demographic data and histology groups
A total of 331 patients undergoing cystectomy for BC patients were identified in the period between 2010 and 2018. After excluding 18 individuals who died within the 1st month of surgery and 128 cases with missing follow-up data, we found 185 patients who matched our inclusion criteria. Of them, 159 were male patients (85.95%) and 26 (14.05%) were female patients, with male-to-female ratio 6.1:1. The mean age at surgery of the cohort group was 63.72 years (range 43–83, standard deviation 7.41 years).
Figure 1 illustrates the age-group distribution of the study population at time of cystectomy. Majority of the patients were clustered among the following age groups: 61–65 years (n = 48; 25.95%), 56–60 years (n = 41; 22.16%), and 66–70 years (n = 40; 21.62%). Less frequent were age groups 51–55 years (n = 16; 8.64%), 71–75 years (n = 21; 11.35%). 76–80 years (n = 10; 5.41%). and 46–50 (n = 5; 2.7%). Patients aged 40–45 and 80–85 had equal distribution (n = 2; 1.08%).
Figure 1.
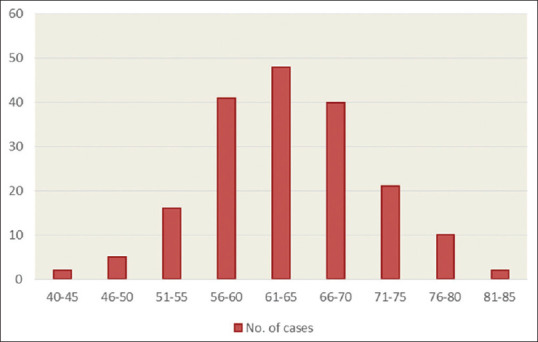
Age group distribution at Cystectomy of the study population
Among these cases, 118 (63.78%) patients lived in urban areas, and the rest of them lived in rural areas.
Figure 2 depicts the classification of the pTNM stage disease of the study population. Arrangement by this system was as follows: stage in situ, 1 (0.54%) patient; Stage I, 3 (1.62%) patients; Stage II, 48 (25.95%) patients; Stage IIIA, 97 (52.43%) patients; Stage IIIB 31 (16.76%), Stage IVA, 2 (1.08%) patients, and Stage IVB 3 (1.62%) patients.
Figure 2.
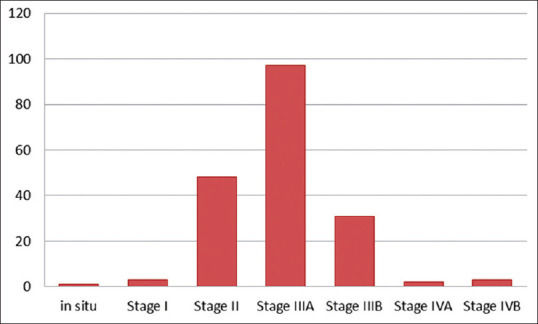
Pathological tumor node metastasis stage classification of the study population undergoing cystectomy
According to the VH, 127 (69%) patients displayed PUC, whereas 37 (20%) and 21 (11%) showed UCDD and PNUC, respectively.
Among patients diagnosed with UCDD, 28 patients had squamous cell differentiation (75.66%), two patients (5,41%) had micropapillary variant, and one patient each (2.70%) had anaplastic, plasmacytoid, lymphoepithelial-like, neuroendocrine, giant cell, glandular, and nested cell variant [Table 1].
Table 1.
Frequency of divergent differentiation histologies in urothelial carcinoma patients undergoing surgery for bladder cancer (2016 World Health Organization classification system)
| Variable | n (%) |
|---|---|
| Squamous cell differentiation | 28 (75.66) |
| Micropapillary variant | 2 (5.41) |
| Anaplastic variant | 1 (2.70) |
| Plasmacytoid variant | 1 (2.70) |
| Lymphoepithelial-like variant | 1 (2.70) |
| Neuroendocrine variant | 1 (2.70) |
| Giant cell variant | 1 (2.70) |
| Glandular variant | 1 (2.70) |
| Nested cell variant | 1 (2.70) |
| Total | 37 (100) |
Conversely, among patients who harbored PNUC, squamous cell carcinoma was the most preponderant type (n = 13; 61.9%), followed by neuroendocrine carcinoma (n = 6; 28.57%) and adenocarcinoma (n = 2; 9.52%) [Table 2].
Table 2.
Frequency of pure nonurothelial histologies in patients undergoing surgery for bladder cancer (2016 World Health Organization classification system)
| Variable | n (%) |
|---|---|
| Squamous cell carcinoma | 13 (61.9) |
| Neuroendocrine carcinoma | 6 (28.57) |
| Adenocarcinoma | 2 (9.52) |
| Total | 21 (100) |
Table 3 compares the clinicopathological features and survival characteristics of patients treated with RC, stratified according to variant histology.
Table 3.
Clinicopathological features and survival characteristic of cystectomy patients, stratified according to variant histology
| Characteristic | PUC | UCDD | PNUC | P PUC versus UCDD | P UCDD versus PNUC | P PUC versus PNUC |
|---|---|---|---|---|---|---|
| Number of patients (%) | 127 (70.09) | 37 (20) | 21 (9.97) | |||
| Age (years), mean±SD | 64.48±7.34 | 61.24±6.83 | 63.42±8.12 | 0.018* | 0.28* | 0.55* |
| Gender | 0.76** | 0.48** | 0.56** | |||
| Male | 109 | 31 | 19 | |||
| Female | 18 | 6 | 2 | |||
| Place of residency | 0.42** | 0.31** | 0.61** | |||
| Urban | 80 | 26 | 12 | |||
| Rural | 47 | 11 | 9 | |||
| Primary pT tumour | 0.45** | 0.96** | 0.52** | |||
| ≤T2 | 39 | 9 | 5 | |||
| ≥T3 | 88 | 28 | 16 | |||
| Pathologic nodal status | 0.18** | 0.27** | 0.79** | |||
| Node-positive | 40 | 16 | 6 | |||
| Node-negative | 87 | 21 | 15 | |||
| Pathologic TNM stage | 0.4** | 0.36** | 0.11** | |||
| Stage ≤2 | 40 | 9 | 3 | |||
| Stage ≥3 | 87 | 28 | 18 | |||
| Surgical margin status | 0.56** | 0.65** | 0.94** | |||
| Margin positive | 19 | 7 | 3 | |||
| Margin negative | 108 | 30 | 18 | |||
| Lymphovascular invasion | 0.07** | 0.31** | 0.76** | |||
| Present | 68 | 26 | 12 | |||
| Absent | 59 | 11 | 9 | |||
| RFS mean (median) | 22.25 (12) | 22.89 (12) | 16.42 (10) | 0.26*** | 0.32*** | 0.34*** |
| Overall survival in months, mean (median) | 30.04 (20) | 28.9 (17) | 22.14 (15) | 0.95*** | 0.26*** | 0.19*** |
*Independent samples t-test, **Pearson Chi-square test, ***Mann–Whitney test. SD: Standard deviation, PUC: Pure urothelial carcinoma, UCDD: Urothelial carcinoma with divergent differentiation, PNUC: Pure nonurothelial carcinoma, RFS: Recurrence-free survival
Comparative statistical analyses revealed a younger age at surgery in patients with UCDD contrasted to PUC patients (P < 0.018). With this exception, we did not identify statistically significant differences concerning patient's gender, place of residency, primary tumor extent, regional lymph node metastasis (pN), pathological (pTNM) stage, RFS, and OS (all P > 0.5).
Patient outcome
After a median follow-up of 28.91 months (range: 1–185 months), 85 patients (85%) experienced tumor recurrence; of them, 59 (31.89%) patients developed distant metastases.
At the end of the follow-up period, 105 (54.05%) patients died of any cause, among them, 10 (5.41%) deceased 2 months after surgery.
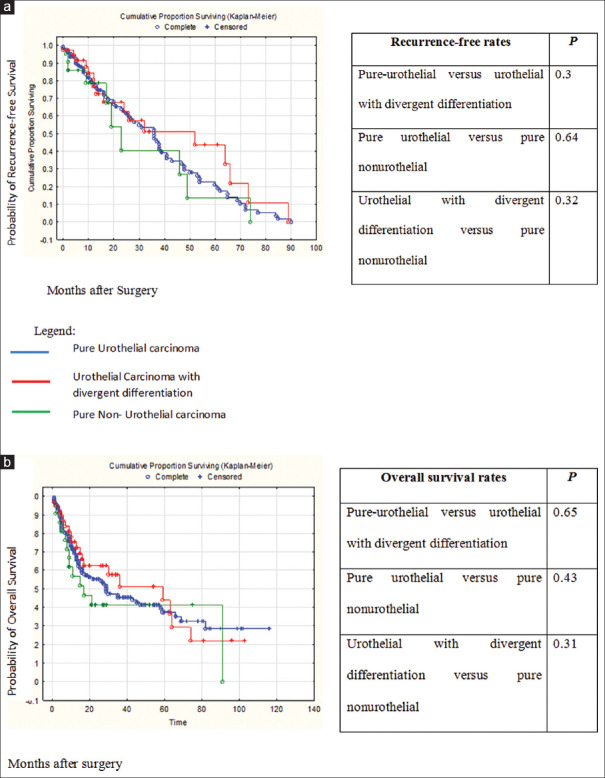
Figure 3 shows Kaplan–Meier plots assessing outcome characteristics between patients, stratified, according to VH (PUC vs. UCDD vs. PNUC). This analysis did not find statistical differences between groups in terms of RFS (P = 0.69) and OS (P = 0.41).
Figure 3.
Kaplan–Meier curves depicting recurrence-free survival (a), and overall survival (b), stratified by variant histologies
Results from univariable and multivariable Cox regression models predicting factors associated with RFS and OS are shown in Tables 4 and 5, respectively. At multivariable analyses, lymphovascular invasion (hazard ratio [HR] = 1.837, 95% confidence interval [CI] 1.002–3.368; P = 0.049) and positive resection margins (HR = 1.98, 95% CI [1.093–3.587], P = 0.024) were associated with shorter RFS. Similarly, patients diagnosed with lymphovascular invasion (HR = 1.879, 95% CI 1.081–3.265, P =.025), positive resection margins (HR = 2.229, 95% CI [1.289–3.855] P = 0.004), and a pelvic lymph node metastasis (HR = 1.936, CI 1.014–3.694, P = 0.045) had poorer OS. Importantly, VH was not found to be independent predictor of both RFS and OS (P > 0.05).
Table 4.
Univariate and multivariate cox regression analyses for predictors of recurrence-free survival
| Covariates | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender (reference: Males) | 1.430 (0.805-2.538) | 0.222 | ||
| Place of residency (reference: Urban) | 0.871 (0.554-1.371) | 0.551 | ||
| Age | 1.019 (0.987-1.051) | 0.246 | ||
| Pure urothelial versus urothelial with divergent differentiation | 1.229 (0.735-2.054)) | 0.431 | ||
| Pure urothelial versus pure nonurothelial | 1.464 (0.764-2.807) | 0.250 | ||
| Pathologic N status (reference: N0) | ||||
| N1 | 2.068 (1.143-3.742) | 0.016 | ||
| N2 | 2.890 (1.662-5.027) | 0.000 | ||
| N3 | 3.218 (1.132-9.142) | 0.028 | ||
| Lymhovascular invasion | 2.514 (1.562-4.045) | 0.000 | 1.837 (1.002-3.368) | 0.0049 |
| Number of isolated lymph nodes | 1.022 (0.979-1.066) | 0.324 | ||
| Number of positive lymph nodes | 1.129 (1.047-1.218) | 0.002 | ||
| Positive resection margins | 2.234 (1.324-3.769) | 0.003 | 1.980 (1.093-3.587) | 0.024 |
HR: Hazard ratio, CI: Confidence interval
Table 5.
Univariate and multivariate cox regression analyses for predictors of overall survival
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender (reference: Males) | 1.417 (0.830-2.419) | 0.201 | ||
| Place of residency (reference: Urban) | 0.901 (0.600-1.352) | 0.614 | ||
| Age | 8.495 (2.384-30.278) | 0.065 | ||
| Pure urothelial versus urothelial with divergent differentiation | 0.886 (0.534-1.468) | 0.637 | ||
| Pure urothelial versus pure nonurothellial | 1.292 (0.715-2.334) | 0.397 | ||
| Primary p? tumour (reference: pTis) | ||||
| 1 | 9.067 (0.563-146.07) | 0.120 | ||
| 2a | 1.841 (0.222-15.301) | 0.572 | ||
| 2b | 3.054 (0.401-23.256) | 0.281 | ||
| 3a | 3.657 (0.439-30.456) | 0.231 | ||
| 3b | 4.327 (0.587-31.904) | 0.151 | ||
| 4a | 8.382 (1.152-61.007) | 0.036 | ||
| 4b | 20.490 (1.812-231.6) | 0.015 | ||
| Pathologic nodal status (reference: pN0) | ||||
| N1 | 2.238 (1.298-3.858) | 0.004 | 1.936 (1.014-3.694) | 0.045 |
| N2 | 2.70 (1.588-4.589) | 0.000 | ||
| N3 | 3.065 (1.082-8.677) | 0.035 | ||
| Lymhovascular invasion | 2.496 (1.633-3.815) | 0.000 | 1.879 (1.081-3.265) | 0.025 |
| Number of isolated lymph nodes | 0.965 (0.924-1.009) | 0.115 | ||
| Number of positive lymph nodes | 1.090 (1.006-1.182) | 0.036 | ||
| Positive resection margins | 2.607 (1.630-4.169) | 0.000 | 2.229 (1.289-3.855) | 0.004 |
HR: Hazard ratio, CI: Confidence interval, is: In situ
DISCUSSION
The 2016 WHO Classification of tumors of the urinary bladder emphasizes the usefulness of accurate reporting of VH. The presence of VH in BC serves as an important clue for intratumoral molecular heterogeneity, which might influence patient survival.[9] Although there has been an increased overall tendency of reporting VH, data regarding their prognostic impact have not been elucidated.
The primary objective of the study was to compare the clinicopathologic characteristics and survival features of patients stratified, according to VH, on RC specimens.
There are several findings worth mentioning in this report. First, VH was present in every third patient. Of these, pure nonurothelial histologies comprised 9.7% of the cases. These findings are in line with the previous report of Moschini et al.[10] who evaluated data from 1067 patients from a single tertiary center and reported an incidence of 31.7% of VH. In contrast, a large retrospective study encompassing 163,683 patients demonstrated a VH incidence of 6.4%.[11] A possible explanation for this disconcordance includes a lack of definitive microscopic criteria for VH, high interobserver variability, and suboptimal tumor sampling.
Second, apart from a younger age at surgery in the UCDD group, compared to the PUC group, we did not reveal demographic differences and dissimilarities in pathologic tumor characteristics between examined groups. Of note, pathologic features including lymphovascular invasion, positive resection margins, and pelvic lymph node metastasis indicated an approximately two-fold greater risk of shorter RFS and OS. These results are consistent with the findings of a recent systematic review[12] covering 19,702 patients who sought to identify the most relevant contributing factors for BC specific survival. Namely, Zhang et al.[12] underlined that gender, VH, and adjuvant chemotherapy do not influence on cancer-specific survival. Instead, advanced age and unfavorable pathological parameters were found to be independent predictors for cancer-specific survival. Likewise, another meta-analysis[13] confirmed that VH histology does not affect the prognosis of BC patients. Several authors have also suggested that, although patients with VH initially presented with worse pathologic features, overall and cancer-specific survival remained similar compared to patients with PUC.[14,15,16] Furthermore, many studies have provided evidence that selected VH including small cell carcinoma,[17,18,19] squamous, signet ring cell and spindle cell carcinoma,[19] and nonmucinous adenocarcinoma[18] were independent predictors of both RFS and cancer-specific survival. On the contrary, several reports have found a negative impact of VH on RFS and cancer-specific survival.[20,21,22] The aforementioned discrepancies could be attributed to nonuniform pathology reporting, data pooled from national data registries, or nondefinitive cutoff criteria of pathology percentage of VH.
Finally, contrary to the previously reported data stating that PNUC portends worse prognostic characteristics,[23,24] no variabilities in survival outcomes between patients of various groups were identified. Plausible theory for this finding could be the limited number of patients within the pure nonurothelial group, which might have influenced the statistical interpretation.
We believe that the strength of the study was the multidisciplinary approach in a single tertiary center, exploiting the experience of dedicated surgeons and uropathologists, thus minimizing the risk of bias. Furthermore, taking into consideration ongoing controversies surrounding optimal treatment for patients for VH, our report could contribute to the growing body of evidence-based research that strengthens the link between the VH and patients’ survival outcomes. Namely, in the 2019 European Society of Urology and European Society of Medical Oncology multidisciplinary meeting, only 5 of 14 statements concerning the management of BC with VH had reached consensus.[25]
Our study is not devoid of limitations. First, we acknowledge the inherent biases with retrospective reviews and the small number size included, specifically of patients within the PNUC group. However, such retrospective studies could initiate more extensive prospective researches, encompassing a larger group of cohort patients. Second, we decided to not investigate cancer-specific survival because of the lack of clinical data concerning the eventual associated patient's comorbidities. Consequently, these results should be interpreted in this context. However, our findings were comparable with the previous reports. Finally, VH was reported regardless of the estimated percentage. This approach was used since currently no definitive cutoff criteria exist.[26]
CONCLUSIONS
The present study did not reveal a prognostic effect of VH on RFS and OS. In our series, histomorphologic parameters including lymphovascular invasion, resection margins, and pelvic lymph node metastasis were the most relevant predictors on survival outcome after RC. Larger prospective studies, using combined histologic and molecular methodology, are required to further investigate the effect of VH. This seems to be a reasonable strategy for designing a tailored treatment approach and for improving patient outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Public Health in the Republic of North Macedonia. Cancer in the Republic of North Macedonia, 2008-2018; 2019. [Last accessed on 2020 Jan 15]. Available from: http://iph.mk/wp-content/uploads/2014/09/IZVESTAJ-ZA-RAK-2008-2018-VER-4.pdf .
- 3.Alfred Witjes J, Lebret T, Compérat EM, Cowan NC, De Santis M, Bruins HM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71:462–75. doi: 10.1016/j.eururo.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: Prostate and bladder tumours. Eur Urol. 2016;70:106–19. doi: 10.1016/j.eururo.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Moschini M, Shariat SF, Lucianò R, D’Andrea D, Foerster B, Abufaraj M, et al. Pure but not mixed histologic variants are associated with poor survival at radical cystectomy in bladder cancer patients. Clin Genitourin Cancer. 2017;15:e603–7. doi: 10.1016/j.clgc.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Matulay JT, Narayan VM, Kamat AM. Clinical and genomic considerations for variant histology in bladder cancer. Curr Oncol Rep. 2019;21:23. doi: 10.1007/s11912-019-0772-8. [DOI] [PubMed] [Google Scholar]
- 7.Compérat E, Srigley JR, Brimo F, Delahunt B, Koch M, Lopez-Beltran A, et al. Dataset for the reporting of carcinoma of the bladder-cystectomy, cystoprostatectomy and diverticulectomy specimens: Recommendations from the International Collaboration on Cancer Reporting (ICCR) Virchows Arch. 2020;476:521–34. doi: 10.1007/s00428-019-02727-1. [DOI] [PubMed] [Google Scholar]
- 8.Brierley JD, Gospodarowicz MK, Wittekind C, O'Sullivan B, Mason M, Asamura H, et al., editors. TNM Classification of Malignant Tumours. 8th ed. Oxford, UK: Wiley; 2017. [Google Scholar]
- 9.Tiwari RV, Ngo NT, Lee LS. The optimal management of variant histology in muscle invasive bladder cancer. Transl Androl Urol. Publ Ahead Print. 2020 doi: 10.21037/tau.2020.01.02. http://tau.amegroups.com/article/view/35953 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moschini M, Dell’Oglio P, Luciano’ R, Gandaglia G, Soria F, Mattei A, et al. Incidence and effect of variant histology on oncological outcomes in patients with bladder cancer treated with radical cystectomy. Urol Oncol. 2017;35:335–41. doi: 10.1016/j.urolonc.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Royce TJ, Lin CC, Gray PJ, Shipley WU, Jemal A, Efstathiou JA. Clinical characteristics and outcomes of nonurothelial cell carcinoma of the bladder: Results from the National Cancer Data Base. Urol Oncol. 2018;36:78.e1. doi: 10.1016/j.urolonc.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Wu B, Zha Z, Qu W, Zhao H, Yuan J. Clinicopathological factors in bladder cancer for cancer-specific survival outcomes following radical cystectomy: A systematic review and meta-analysis. BMC Cancer. 2019;19:716. doi: 10.1186/s12885-019-5924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Q, Li L, Wang G, Hu J, Sun T, Fu B. Do histological variants in urothelial carcinoma of the bladder portend poor prognosis? A systematic review and meta-analysis. Oncotarget. 2017;8:48263–71. doi: 10.18632/oncotarget.17593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobo N, Shariat SF, Guo CC, Fernandez MI, Kassouf W, Choudhury A, et al. What Is the significance of variant histology in Urothelial carcinoma? Eur Urol Focus. 2020;6:653–63. doi: 10.1016/j.euf.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Han JH, Choi SY, Yoo S, Baek SH, Ryu J, Kyung YS, et al. Impact of histologic variants of bladder cancer on oncology outcome after radical cystectomy. Korean J Urol Oncol. 2017;15:121–30. [Google Scholar]
- 16.Nechifor-Boilă IA, Chibelean BC, Loghin A, Nechifor-Boilă AC, Voidăzan TS, Rădulescu MF, et al. Histopathological predictive factors for the overall survival rate in patients with urothelial carcinoma of the bladder treated by radical cystectomy: A Romanian cohort study. Rom J Morphol Embryol. 2019;60:1183–90. [PubMed] [Google Scholar]
- 17.Jue JS, Koru-Sengul T, Moore KJ, Miao F, Alameddine M, Nahar B, et al. Sociodemographic and survival disparities for histologic variants of bladder cancer. Can J Urol. 2018;25:9179–85. [PubMed] [Google Scholar]
- 18.Vetterlein MW, Seisen T, Leow JJ, Preston MA, Sun M, Friedlander DF, et al. Effect of nonurothelial histologic variants on the outcomes of radical cystectomy for nonmetastatic muscle-invasive urinary bladder cancer. Clin Genitourin Cancer. 2018;16 doi: 10.1016/j.clgc.2017.08.007. doi:10.1016/j.clgc.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Veskimäe E, Espinos EL, Bruins HM, Yuan Y, Sylvester R, Kamat AM, et al. What Is the prognostic and clinical importance of urothelial and nonurothelial histological variants of bladder cancer in predicting oncological outcomes in patients with muscle-invasive and metastatic bladder cancer? a european association of urology muscle invasive and metastatic bladder cancer guidelines panel systematic review. Eur Urol Oncol. 2019;2:625–42. doi: 10.1016/j.euo.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Koguchi D, Matsumoto K, Ikeda M, Taoka Y, Hirayama T, Murakami Y, et al. Histologic variants associated with biological aggressiveness and poor prognosis in patients treated with radical cystectomy. Jpn J Clin Oncol. 2019;49:373–8. doi: 10.1093/jjco/hyz015. [DOI] [PubMed] [Google Scholar]
- 21.Böyük A, Şanlı í, Erdem S, Tefik T, ízcan F, ízlük Y, et al. The association between variant urothelial histologies, pathological stage and disease specific survival in patients with bladder cancer. Turk J Urol. 2018;44:24–30. doi: 10.5152/tud.2017.48107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ku JH, Yuk HD, Godoy G, Amiel GE, Lerner SP. Prognostication in patients treated with radical cystectomy for urothelial bladder carcinoma: A new simplified model incorporating histological variants. Bladder Cancer. 2018;4:195–203. doi: 10.3233/BLC-170156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroman L, Nair R, Russell B, Malik N, Desai A, Chandra A, et al. The impact of non-urothelial variant histology on oncological outcomes following radical cystectomy. BJU Int. 2019;124:418–23. doi: 10.1111/bju.14704. [DOI] [PubMed] [Google Scholar]
- 24.Martin JW, Jefferson FA, Huang M, Sung JM, Chang J, Piranviseh K, et al. A California cancer registry analysis of urothelial and non-urothelial bladder cancer subtypes: Epidemiology, treatment, and survival. Clin Genitourin Cancer. 2020;18:e330–6. doi: 10.1016/j.clgc.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Horwich A, Babjuk M, Bellmunt J, Bruins HM, De Reijke TM, De Santis M, et al. EAU-ESMO consensus statements on the management of advanced and variant bladder cancer-an international collaborative multi-stakeholder effort: Under the auspices of the EAU and ESMO Guidelines Committees†. Ann Oncol. 2019;30:1697–727. doi: 10.1093/annonc/mdz296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang G, McKenney JK. Urinary bladder pathology: World health organization classification and American joint committee on cancer staging update. Arch Pathol Lab Med. 2019;143:571–7. doi: 10.5858/arpa.2017-0539-RA. [DOI] [PubMed] [Google Scholar]