Research on neurodegenerative diseases such as Alzheimer’s disease, Parkinson´s disease (PD), Huntington’s disease or amyotrophic lateral sclerosis is becoming increasingly important in our society. Due to the ageing of the population, the prevalence of these diseases continue to rise worldwide, and causal cures are not yet available (Erkkinen et al., 2018). This Perspective focusses on PD, a movement disorder of the central nervous system with an estimated prevalence between 65 and 1250/100,000 in Europe, affecting about 1 percent of the population older than 60 years. The clinical symptoms include motor symptoms like bradykinesia, tremor or rigidity which are associated with loss of dopaminergic neurons in the substantia nigra and their innervating axonal fibers to the striatum. Additional non-motor symptoms may consist in depression, hyposmia, cognitive decline or constipation due to impaired motility of the gastrointestinal tract. The aggregation and dysfunction of the protein α-Synuclein (αSyn) in dopaminergic and surrounding cells is the major pathological hallmark of the disease. Its course is significantly influenced by inflammatory processes that also involve non-neuronal cell types such as astroglia, microglia and T cells. Currently, several environmental factors are discussed to influence the development and progression of PD such as the microbiome composition of the gastrointestinal tract. The human gut contains about 160 bacterial species which are essential for the digestion of dietary fibers and the synthesis of several proteins or vitamins. Therefore, the microbiome contributes to the human enteral metabolism and several direct effects on human health have been demonstrated (Rowland et al., 2018).
Is there a link between Parkinson’s disease and the intestinal microbiome? For several years, the contribution of the intestine for the development of sporadic PD has been discussed. Rietdijk et al. (2017) analyzed postmortem tissue of 110 PD patients with focus on pathologic αSyn distribution. According to their hypothesis, a certain pathogen enters the gut and induces αSyn aggregation in the enteric nervous system which finally spreads via the vagus nerve into the central nervous system regions in a certain pattern. This hypothesis has been further modified to the “dual-hit hypothesis”, which includes the olfactory bulb as an additional entry for pathogens. Although there are many supporting data from in vitro and in vivo experiments in favor of this hypothesis, this concept is not accepted unanimously because it could apply only to a subset of human PD patients (Rietdijk et al., 2017). Importantly, PD patients often exhibit gastrointestinal dysfunction like constipation or other alterations of gastric motility which may even occur years before onset of classical motor symptoms.
In order to identify possible causes for this early intestinal involvement, its bacterial microbiome has been studied. In various studies, important and significant differences in the microbiological composition of the intestine in PD in comparison to healthy controls but also to other neurodegenerative diseases have been identified. Prevotellaceae represent one of the main enterotypes of the healthy human gut and are involved in fiber degradation but also in inflammatory processes. After obtaining fecal probes from 72 PD patients and 72 controls the group of Scheperjans observed a reduction of Prevotellaceae by 77.6% in PD subjects as well as a strong abundance of Enterobacteriaceae, both positively correlating with the severity of motor symptoms. In subsequent studies, there were several hints that these alterations of the intestinal microbiome could also be associated with changes in the composition of the intestinal metabolome or, more specifically, short-chain fatty acids (Scheperjans et al., 2015).
What is known about the role of short-chain fatty acids in neurodegenerative diseases? Short-chain fatty acids (SCFA), such as acetic acid, propionic acid, and butyric acid, derive from the digestion of dietary fibers by anaerobic microbiota and are important for the intracellular energy metabolism. In several neurological disease models, important functions of SCFA have been described that go beyond. In a mouse model of stroke, functional recovery was improved by SCFA treatment being associated with increased spine and synapse densities. Additional anti-inflammatory findings were observed that resulted in decreased numbers of microglia and T cells (Sadler et al., 2020). G93A mice, a transgenic mouse model used in amyotrophic lateral sclerosis research, displayed an altered microbiome and intestinal dysfunctions before motor symptoms occurred. Treating these mice with butyrate resulted in a delayed onset of the disease and an extended life-span (Zhang et al., 2017). Also, in a model of Huntington’s disease, butyrate enhanced survival and motor performance of R6/2 mice (Ferrante et al., 2003). During experimental autoimmune encephalomyelitis, a model of multiple sclerosis, SCFA had anti-inflammatory effects on T cell proliferation and differentiation. Treatment of mice with propionic acid ameliorated the disease course and reduced lymphocyte infiltration as well as the degree of demyelination associated with a peripheral induction of Tregs in the small intestine (Haghikia et al., 2015). In a subsequent clinical study in a large cohort of MS patients and healthy controls, serum and stool samples were analyzed concerning bacterial composition, SCFA concentrations and T cell population. MS patients displayed a significant reduction of propionic acid and an altered microbiome which showed a depletion of SCFA producing bacteria like Butyricimonas but had as well an increased amount of pathogenic Flavonifractor or Escherichia. Furthermore, a shift from regulatory T cells to proinflammatory TH17 cells was detected. Treatment with propionic acid over a short time period of only 14 days could restore these alterations. Supplementation with propionic acid for at least 1 year was able to reduce the annual MS relapse rate significantly, from 0.24 to 0.08 (Duscha et al., 2020).
Unger et al. (2016) examined the concentration of several SCFA in fecal samples of PD patients and age matched controls. They found a reduction of acetic acid, propionic acid, and butyric acid in PD patients. Furthermore, they confirmed the changed composition of the bacterial microbiome as observed by others before.
In order to analyze the role of SCFA in the development and progression of PD, several studies have been performed in different models in vivo or in vitro and are listed below. In one study, male C57BL/B6 mice received the toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) for 7 days followed by a 3-week treatment with the SCFA sodium butyrate (200 mg/kg body weight). Liu et al. (2017) observed a protective effect of butyric acid resulting in improved motor performance and less dopaminergic degeneration in substantia nigra and striatum. The authors explained these findings by an increased expression of colonic glucagon-like peptide-1 (GLP-1) which could act as a neurotrophic factor. In a study on LHUMES cells overexpressing αSyn, treatment with butyric acid (150 µM) rescued aSyn-induced DNA damage by inhibition of several histone deacetylases and subsequent upregulation of DNA repair genes (Paiva et al., 2017).
A direct effect or mechanisms of propionic acid on dopaminergic neurons have only been studied in a few projects. In our own study, we could show a protective effect on primary mesencephalic neurons after stress induced damage. Intoxication with 20 nM rotenone for 48 hours lead to a cell death rate of 35 % and reduced neurite outgrowth of 40%. Treatment with propionic acid (300 µM) over 5 days showed clear neuroprotective properties and resulted in a significantly increased survival of tyrosine hydroxylase positive neurons (140%). In addition, an increased neurite outgrowth was detected by trend. Under these treatment conditions, mRNA expression levels of cellular signaling pathways including an analysis of aSyn were partially modified by propionic acid (Figure 1) (Ostendorf et al., 2020).
Figure 1.
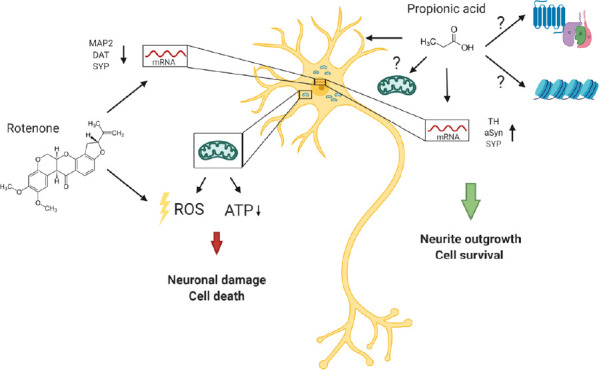
Neuroprotective effects of propionic acid after neuronal damage by rotenone.
Treatment of dopaminergic midbrain neurons with rotenone leads to an inhibition of the mitochondrial respiratory chain and the induction of oxidative stress, resulting in axonal loss and cell death. Propionic acid increases cell survival, neurite outgrowth as well as the gene expression of TH and aSyn. Potential mechanisms of action are via G protein-coupled receptors or modification of histone deacetylase activity. The figure is created with BioRender.com. ATP: Adenosine triphosphate; DAT: dopamine transporter; MAP2: microtubule-associated protein 2; ROS: reactive oxygen species; SYP: synaptophysin; TH: tyrosine hydroxylase.
In another study, an association between the composition of the intestinal flora and the extent of Parkinson’s pathology in mice overexpressing αSyn (Thy1-αSyn mice) could be shown. Under germ-free conditions, mice demonstrated significantly reduced neuropathology which was accompanied by reduced microglia activation. Interestingly, lower concentrations of SCFA were observed in fecal samples of the germ-free mice. To evaluate the relevance of this finding, a mixture of acetic acid (67.5 mM), propionic acid (25 mM), and butyric acid (40 mM) was administered to the mice. This resulted in impaired motor performance as well as in increased αSyn aggregation and microglia activation. Treatment of Thy1-αSyn mice with stool microbiome samples of PD patients further enhanced these αSyn-mediated motor deficits, implicating a rather unfavorable involvement of SCFA for the development of PD-like pathology in mice (Sampson et al., 2016).
A just recently published study analyzed the influence of butyric acid (165 mg/kg body weight) treatment in the mouse MPTP model. Here, significant dopaminergic pathology in the substantia nigra and striatum was accompanied by increased microglia and astrocyte activation. Additionally, intestinal permeability in mice was exacerbated and expression of IL6 and IL18 were enhanced. An accompanying in vitro treatment of BV2 microglia cells with butyric acid and lipopolysaccharide resulted in an increased expression of the proinflammatory cytokines IL1β and IL18 (Qiao et al., 2020).
What molecular mechanisms are influenced by SCFA? SCFA have been shown to be able to bind to four different receptors belonging to the GPCR family which are mainly expressed on intestinal epithelial cells and on immune cells. Activation of these receptors is important for gut inflammatory homeostasis and maintenance of intestinal barrier integrity (Ratajczak et al., 2019). In a study of autism spectrum disorder, the receptor GPR41 was detected on human stem cells. Treatment with SCFA increased proliferation and differentiation of these cells. The observed effect was abolished by treatment with an GPR41 inhibitor (Abdelli et al., 2019). Furthermore, modification of histone deacetylase (HDAC) activity could be verified for propionic and butyric acid in several studies, but the involvement in Parkinson’s disease pathology requires further investigation (reviewed by Ratajczak et al., 2019)
Conclusion and perspective: So far, PD disease progression cannot be halted by any pharmacological means and the disease itself cannot be modified to shift to a more favorable disease course. Therefore, the continuous investigation of new causal treatment options is of great importance.
A strong advantage of agents such as propionic acid is the potential to quickly translate and repurpose into clinical study programs because it is already approved for human application as a food preservative. The application of SCFA in PD disease models has shown heterogeneous results with both beneficial but also unfavorable effects. A reason for these findings could be that in some cases SCFA were applied in a mixture and treatment with a single SCFA such as propionate have not been tested. In human stem cells for example, propionic acid promotes glial differentiation while butyric acid shifted differentiation towards a neuronal phenotype and ameliorated neurite outgrowth. It could be shown, that both SCFA act via GPR41 but activated different downstream signaling pathways (Abdelli et al., 2019).
Furthermore, dosages of single SCFA varied and different dosages could be optimal in distinct disease models or in human application. Haghikia et al. (2015) analyzed the potential of different propionic acid (PA) concentrations on T cell differentiation in vitro. While 100 µM PA promoted polarization of naïve T cells towards Tregs, a high dosage of 1 mM rather reversed the effect. Interestingly, longer- chain fatty acids all had negative impact on T cell differentiation. In our rotenone-based in vitro study, we observed a neuroprotective outcome with prolonged neurite outgrowth in propionic acid-treated cells, but the responsible mechanisms have still to be delineated. We assume an involvement of mitochondrial function as PA was able to improve mitochondrial respiration rates in lymphocytes in another study (Duscha et al., 2020).
Taken together, there is strong evidence that the gastrointestinal microbiome is altered in PD and this is similarly true for the composition of the intestinal metabolome. SCFAs are increasingly studied intestinal metabolites that have shown great potential to influence disease processes in neuroinflammatory disorders. Their role for neurodegenerative movement disorders such as PD is still unclear but both analyses in translational disease models and in human clinical trials are on the way to improve our understanding.
The figure is created with BioRender.com.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Song LP; T-Editor: Jia Y
References
- 1.Abdelli LS, Samsam A, Naser SA. Propionic acid induces gliosis and neuro-inflammation through modulation of pten/akt pathway in autism spectrum disorder. Sci Rep. 2019;9:8824. doi: 10.1038/s41598-019-45348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duscha A, Gisevius B, Hirschberg S, Yissachar N, Stangl GI, Eilers E, Bader V, Haase S, Kaisler J, David C, Schneider R, Troisi R, Zent D, Hegelmaier T, Dokalis N, Gerstein S, Del Mare-Roumani S, Amidror S, Staszewski O, Poschmann G, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. 2020;180:1067–1080.e16. doi: 10.1016/j.cell.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 3.Ferrante RJ, Kubilus JK, Lee J, Ryu H, Beesen A, Zucker B, Smith K, Kowall NW, Ratan RR, Luthi-Carter R, Hersch SM. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J Neurosci. 2003;23:9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haghikia A, Jörg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee DH, May C, Wilck N, Balogh A, Ostermann AI, Schebb NH, Akkad DA, Grohme DA, Kleinewietfeld M, Kempa S, Thöne J, Demir S, Müller DN. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2015;43:817–829. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Wang F, Liu S, Du J, Hu X, Xiong J, Fang R, Chen W, Sun J. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J Neurol Sci. 2017;381:176–181. doi: 10.1016/j.jns.2017.08.3235. [DOI] [PubMed] [Google Scholar]
- 6.Ostendorf F, Metzdorf J, Gold R, Haghikia A, Tönges L. Propionic acid and fasudil as treatment against rotenone toxicity in an in vitro model of parkinson’s disease. Molecules. 2020:25. doi: 10.3390/molecules25112502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paiva I, Pinho R, Pavlou MA, Hennion M, Wales P, Schütz AL, Rajput A, Szego É, Kerimoglu C, Gerhardt E, Rego AC, Fischer A, Bonn S, Outeiro TF. Sodium butyrate rescues dopaminergic cells from alpha-synuclein-induced transcriptional deregulation and DNA damage. Hum Mol Genet. 2017;26:2231–2246. doi: 10.1093/hmg/ddx114. [DOI] [PubMed] [Google Scholar]
- 8.Qiao CM, Sun MF, Jia XB, Li Y, Zhang BP, Zhao LP, Shi Y, Zhou ZL, Zhu YL, Cui C, Shen YQ. Sodium butyrate exacerbates parkinson’s disease by aggravating neuroinflammation and colonic inflammation in mptp-induced mice model. Neurochem Res. 2020;45:2128–2142. doi: 10.1007/s11064-020-03074-3. [DOI] [PubMed] [Google Scholar]
- 9.Ratajczak W, Rył A, Mizerski A, Walczakiewicz K, Sipak O, Laszczyńska M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs) Acta Biochim Pol. 2019;66:1–12. doi: 10.18388/abp.2018_2648. [DOI] [PubMed] [Google Scholar]
- 10.Rietdijk CD, Perez-Pardo P, Garssen J, van Wezel RJ, Kraneveld AD. Exploring Braak’s hypothesis of Parkinson’s disease. Front Neurol. 2017;8:37. doi: 10.3389/fneur.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadler R, Cramer JV, Heindl S, Kostidis S, Betz D, Zuurbier KR, Northoff BH, Heijink M, Goldberg MP, Plautz EJ, Roth S, Malik R, Dichgans M, Holdt LM, Benakis C, Giera M, Stowe AM, Liesz A. Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J Neurosci. 2020;40:1162–1173. doi: 10.1523/JNEUROSCI.1359-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–1480.e1412. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 14.Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, Schäfer KH. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Zhang YG, Wu S, Yi J, Xia Y, Jin D, Zhou J, Sun J. Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin Ther. 2017;39:322–336. doi: 10.1016/j.clinthera.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


