Keywords: brain injury, circadian rhythm, hypoxic-ischemic brain damage, miRNA, neonate, pineal gland, sleep, transcription factor
Abstract
Circadian rhythm disorder is a common, but often neglected, consequence of neonatal hypoxic-ischemic brain damage (HIBD). However, the underlying molecular mechanisms remain largely unknown. We previously showed that, in a rat model of HIBD, up-regulation of microRNA-325 (miR-325) in the pineal gland is responsible for the suppression of Aanat, a key enzyme involved in melatonin synthesis and circadian rhythm regulation. To better understand the mechanism by which miR-325 affects circadian rhythms in neonates with HIBD, we compared clinical samples from neonates with HIBD and samples from healthy neonates recruited from the First Affiliated Hospital of Soochow University (Dushuhu Branch) in 2019. We found that circulating miR-325 levels correlated positively with the severity of sleep and circadian rhythm disorders in neonates with HIBD. Furthermore, a luciferase reporter gene assay revealed that LIM homeobox 3 (LHX3) is a novel downstream target of miR-325. In addition, in miR-325 knock-down mice, the transcription factor LHX3 exhibited an miR-325-dependent circadian pattern of expression in the pineal gland. We established a neonatal mouse model of HIBD by performing double-layer ligation of the left common carotid artery and exposing the pups to a low-oxygen environment for 2 hours. Lhx3 mRNA expression was significantly down-regulated in these mice and partially rescued in miR-325 knockout mice subjected to the same conditions. Finally, we showed that improvement in circadian rhythm-related behaviors in animals with HIBD was dependent on both miR-325 and LHX3. Taken together, our findings suggest that the miR-325-LHX3 axis is responsible for regulating circadian rhythms and provide novel insights into the identification of potential therapeutic targets for circadian rhythm disorders in patients with neonatal HIBD. The clinical trial was approved by Institutional Review Board of Children’s Hospital of Soochow University (approval No. 2015028) on July 20, 2015. Animal experiments were approved by Animal Care and Use Committee, School of Medicine, Soochow University, China (approval No. XD-2016-1) on January 15, 2016.
Chinese Library Classification No. R446; R741; Q457
Introduction
Neonatal hypoxic-ischemic brain damage (HIBD) can lead to various post-injury deficits, depending on its severity (de Vries and Jongmans, 2010; Perlman and Shah, 2011; Abbasi and Unsworth, 2020; Jiang et al., 2020; Zhang et al., 2020). While children with severe HIBD often develop overt cognitive and motor impairments, those with moderate or mild HIBD tend to be relatively “asymptotic.” However, patients who appear to lack symptoms often have problems that are overlooked. For example, a substantial proportion of neonatal HIBD patients suffer from circadian dysfunction and chronic sleep problems (Takenouchi et al., 2011; Ding et al., 2016), and little attention has been paid to this topic. Our recent clinical study found that the occurrence of pineal cysts in children with mild to moderate HIBD correlates well with sleep problems and circadian rhythm dysfunctions (Ding et al., 2016). Thus, deciphering the molecular mechanisms of pineal pathophysiology post-HIBD could improve diagnosis of and intervention in circadian disorders in children with HIBD.
Accumulating evidence shows that post-transcriptional mechanisms play important roles in regulating circadian rhythms (Kim et al., 2005; Kim et al., 2007; Kojima et al., 2011). Among these mechanisms, microRNAs (miRNAs), an important class of non-coding RNAs (Gebert and MacRae, 2019), are reported to modulate the expression of key circadian genes in the pineal gland. For instance, miR-182 and miR-483, both of which are abundantly expressed in the pineal gland, target the mRNAs of two master circadian regulators, Clock and Aanat, respectively (Clokie et al., 2012; Ding et al., 2015).
We previously found that up-regulation of miR-325 results in suppression of Aanat and melatonin in the pineal gland in a rat model of HIBD, and disrupts circadian rhythms in children with HIBD (Ding et al., 2015, 2016). Individual miRNAs often co-regulate multiple genes by either degrading or inhibiting their target mRNAs (Gebert and MacRae, 2019). This gives miRNAs the ability to orchestrate the expression of functionally related genes to fine-tune the function of specific pathways in real time. In the current study, we sought to identify novel targets of miR-325 and investigate the relationship between miR-325 expression and the function of its downstream effectors.
Materials and Methods
Clinical sample collection and assessment
This study was approved by the Institutional Review Board of Children’s Hospital of Soochow University (approval No. 2015028) on July 20, 2015 (Additional file 1 (158.3KB, pdf) ). With the parents’ consent, we collected peripheral blood samples at 9:00 a.m. from patients with neonatal HIBD and from age/weight/sex-matched healthy controls born at The First Affiliated Hospital of Soochow University from January to August 2019. The basic characteristics of the HIBD patients and healthy controls are listed in Figure 1A. All of the HIBD patients and healthy controls were evaluated for skin appearance, pulse, grimace, activity, and respiration (APGAR) at 1 and 5 minutes after birth, with a total score from 0 to 10. The inclusion criteria adhered strictly to the standards listed in “Practice of Neonatology,” and the criteria for different degrees of HIBD (moderate/mild versus severe) were based on the Sarnat Grading Scale (Sarnat and Sarnat, 1976). Using density gradient centrifugation, we isolated mononuclear cells and extracted total RNA using a mirVanaTM miRNA isolation kit (Ambion, Austin, TX, USA). The relative levels of miR-325, miR-183, and miR-483 were determined by TaqMan MicroRNA Assay (Applied Biosystems, Waltham, MA, USA), with U6 as a reference. The assay IDs used for miR-325, miR-483-5p, and for miR-183-3p were 478025_mir, 478432_mir, and 477936_mir, respectively. Biological triplicates of all samples were analyzed. We previously (Ding et al., 2016) designed a questionnaire to evaluate the sleep quality of neonates with various degrees of hypoxic ischemic encephalopathy. Two of the questions on the questionnaire evaluated sleep rhythm based on two variables: 1) change in daily sleep schedule (changes in nap-onset time, duration, and nocturnal sleep duration) and 2) unfixed nocturnal sleep-onset time (the time at which the child falls asleep at night) (Ding et al., 2016). For each variable, a 3-point scale was applied: 0, has never happened in the past 6 months; 1, has occurred < 3 times/week in the past 6 months; 2, has occurred > 3 times/week in the past 6 months. For the purpose of this study, the sleep performance score was defined as the sum of the scores for these two variables.
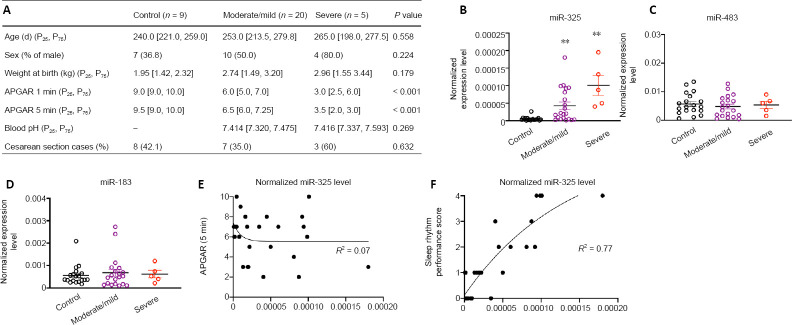
Figure 1.
The level of miR-325 in peripheral mononuclear blood cells correlates with the severity of circadian rhythm disorders in patients with neonatal HIBD.
(A) General characteristics of patients with HIBD and age/sex/weight-matched healthy controls. (B–D) Relative RNA levels (normalized to U6) of miR-325 (B), miR-483 (C), and miR-183 (D) in control subjects (n = 19) and patients with moderate/mild HIBD (n = 20) or severe HIBD (n = 5). Data are expressed as mean ± SEM. **P < 0.01, vs. controls (one-way analysis of variance followed by Bonferroni’s post hoc correction). Correlation (non-linear fit model) between relative miR-325 expression level and neonatal HIBD severity indicated by Appearance, Pulse, Grimace, Activity, Respiration (APGAR) score at 5 minutes (Lv et al., 2020) (E) or severity of sleep rhythm problems (sleep rhythm score) (F) (Ding et al., 2016) in patients with neonatal HIBD (n = 25). In both cases, the R2 value was obtained by one-phase decay, non-linear fit modeling. The data used to generate Figure 1A–F are shown in Additional files 3 (35.7KB, pdf) and 4 (29KB, pdf) . HIBD: Hypoxic-ischemic brain damage.
Luciferase reporter gene assay
To perform the luciferase reporter gene assay, we first constructed plasmids containing a 761-bp fragment of the 3′-untranslated region (UTR) of Lhx3 (luc:Lhx3 3′-UTR) (forward: 5′-AAG GAC CTC TGA GGG AAG GA-3′, reverse: 5′-AGC ACT GAA ACT GAC AAA TT-3′) or the same region containing an 8-bp deletion (TAKARA MutanBEST Kit, TAKARA, luc:Lhx3 3′-UTR del) in the potential miR-325-3p target area (vector: pmirGlo vector; Promega, Madison, WI, USA). We then transfected HEK 293 cells using Lipofectamine Plus (Invitrogen, Carlsbad, CA, USA) with 100 ng pmirGlo-Lhx3 3′-UTR or pmirGlo-Lhx3 3′-UTR del and 20 nmol miR-325-3p mimic (mirVana® miRNA; MC12779; ThermoFisher Scientific, Waltham, MA, USA), miR-325-3p inhibitor (mirVana® miRNA; MH12779; ThermoFisher Scientific), or non-targeted control RNA (5′-UUC UCC GAA CGU GUC ACG U-3′; Genepharma, Shanghai, China). We analyzed the luciferase activity (Dual-Glo® Luciferase Assay System; Promega) at 48 hours post-transfection, and the normalized activity levels were compared with those of a pmirGlo empty vector control.
Generation of miR-325 knock out line
Two single-guide RNAs, TAG CAC AGT GCT TGA TTG ATA GG and CAC AGT GCT TGA TTG ATA GGA GG, were designed to target the miR-325 coding region. These two single-guide RNAs were prepared and co-injected with Cas9 mRNA into zygotes generated from healthy C57Bl6/J female mice (8 weeks old) (Cambridge-SU Genomic Resource Center, Soochow University). Injected zygotes were then transferred into pseudopregnant Institute of Cancer Research–strain female mice. When the pups were born, DNA from tail snips (collected on postnatal day 3) was used to confirm deletion of miR-325.
Establishment of a murine neonatal HIBD model
A mouse model of HIBD was designed based on previous work performed in neonatal rats (Ding et al., 2015; Yang et al., 2017). All surgical procedures performed in the current study were approved by the School of Medicine, Soochow University Institutional Animal Care and Use Committee (approval No. XD-2016-1) on January 15, 2016. Briefly, we identified the left common carotid artery in neonatal mice (postnatal day 5–7) under hypothermal anesthesia and performed a double-layer ligation (in the control mice a sham surgery was performed without ligation). The mice were allowed to recover from the anesthesia on a warming pad in 1–2 minutes, at which point they were immediately transferred to a low-oxygen chamber (a gas mixture of moist 8% nitrogen-oxygen, 1.5 L/min, 37°C) for 2 hours before they were returned to their mother. The mice were then maintained at a 12-hour light/dark (light: 7 a.m.–7 p.m.) photoperiod. In total, 49 and 65 mice were included in the sham and HIBD groups, respectively. Mice of similar body weight and sex were randomized to different treatment groups.
Measurement of Lhx3 RNA expression
Intact adult mice (P60) were euthanized with CO2 for immediate tissue harvest. Total RNA was harvested from different tissues from mice euthanized at 6:00 p.m., from the striatum/pineal gland of control versus miR-325 knockout mice euthanized every 4 hours from zodiac time (ZT) 0 to ZT20 (n = 3 for each time point), or the pineal gland of sham or HIBD animals euthanized at different time points (n = 4 for individual time points), using TRIZOL reagent (Invitrogen). Next, 3 µg of total RNA was used to synthesize complementary DNA. Quantitative real-time polymerase chain reaction was performed in an ABI step-one plus instrument using the SYBR green detection system (Invitrogen). The polymerase chain reaction conditions were: 40 cycles of 95°C, 10 seconds; 60°C, 30 seconds. All results were first normalized to the housekeeping gene gapdh and then compared with expression in the pineal gland, expression at ZT0, or expression at 0 hours post-HIBD. We used the JTK-cycle algorithm (Hughes et al., 2010) to perform circadian analyses of Lhx3 mRNA expression. The primers used were as follows: gapdh: 5′-ACA GCA ACA GGG TGG TGG AC-3′ (forward), 5′-TTT GAG GGT GCA GCG AAC TT-3′ (reverse), 110 bp; Lhx3: 5′-AGT CCG ACA AGG ACA GCA TC-3′ (forward), 5′-GCG TAG CTC TCG GTA CTG CT-3′ (reverse), 215 bp. We verified that each primer pair produced a single, sharp peak that was higher than the suggested threshold.
Measurement of LHX3 protein expression
Pineal gland transverse sections (thickness: 30 µm) were incubated with a rabbit polyclonal anti-LHX3 primary antibody (Cat# ab14555; 1:200; Abcam, Cambridge, MA, USA) at 4°C overnight, and then with a horseradish peroxidase anti-rabbit secondary antibody (Cat# G-21234; anti-rabbit; 1:200; Invitrogen) for 2 hours at room temperature. The specificity of this antibody has been verified in previous publications (Yan et al., 2015; Mukaigasa et al., 2017). As a control, we incubated one sample with phosphate-buffered saline instead of primary antibody, followed by the anti-rabbit secondary antibody, and did not detect any LHX3 signal (data not shown). The relative LHX3 intensity (Figure 2) was calculated as the mean intensity minus the mean background intensity, in arbitrary units, using ImageJ (National Institutes of Health, Bethesda, MD, USA). Five transverse pineal sections were quantified for each individual animal.
Figure 2.

Lhx3 mRNA is a down-stream target of miR-325.
(A) Potential miR-325 binding site in the 3′-UTR of the Lhx3 mRNA. This sequence is highly conserved across species. (B) Normalized luciferase activity under multiple conditions in the presence or absence of miR-325-3p mimic/inhibitor in cells transfected with Lhx3-3′-UTR or its mutated form. Data are expressed as mean ± SEM (n = 4 for each data point). **P < 0.01 (one-way analysis of variance followed by Bonferroni’s post hoc correction). (C, D) Representative transverse pineal gland sections stained with an anti-LHX3 antibody (C) from adult mice (P60) expressing (control) or not expressing (miR-325–/–) miR-325. LHX3 intensity was normalized to the control (D). The data used to generate Figure 2B and D are shown in Additional file 5 (103KB, pdf) . Scale bar: 100 μm. Data are expressed as mean ± SEM (n = 5 for each data point). **P < 0.01 (Student’s t-test). 3′-UTR: 3′-Untranslated region; KO: knockout; Lhx3: LIM homeobox 3; NC: normal control; NS: no statistical significance.
Monitoring the circadian rhythmicity of locomotor activity in juvenile mice post-HIBD
After weaning (P21), mice (control or miR-325 knockout) that had undergone sham or HIBD surgery were individually housed with free access to food and water and supplied with running wheels. Locomotor activity was recorded as revolutions per 5-minute interval. Next, mice were entrained to an initial Light-Dark cycle (light 7:00 a.m.–7:00 p.m.) for two weeks and then placed in constant darkness for 4 weeks. We applied ClockLab software (Actimetrics, Evanston, IL, USA) to analyze the wheel running period. We define the onset of activity for each cycle as the time when the first concentrated bout of activity occurred after a long period of rest. To predict the onset, we applied a linear regression to 15 cycles (days 10–25 under constant darkness) (Colwell et al., 2003; Li et al., 2009). We used two parameters to evaluate the normality of circadian rhythmicity of locomotor activity: first, the wheel running period, which represents the successful establishment of circadian rhythm, and second, the duration of each motor cycle (α), which reflects the degree of the coherence and precision of the circadian rhythmicity of locomotor activities (Colwell et al., 2003). For adeno-associated virus (AAV) preparation, Lhx3 shRNA (5′-GCA CAT CTT GGA CCG TTT CAT-3′) or a scrambled sham sequence (5′-TTC TCC GAA CGT GTC ACG T-3′) was inserted into a pAAV-U6-shRNA-Ubi-eGFP vector and then packaged as AAV2/8 (GenePharma, Shanghai, China). We verified the knockdown efficiency of AAV-shRNA-Lhx3 in miR-325 knock out mice (Additional Figure 1 (909.5KB, tif) ).
Statistical analysis
Error bars in all figures represent mean ± standard error of mean (SEM). Data were analyzed by Student’s t-test or one-way analysis of variance with Bonferroni post hoc correction (Stata version 12, Statistical Software, College Station, TX, USA). The correlation analyses were performed using a non-linear fit model.
Results
Circulating miR-325 levels are tightly correlated with the severity of circadian disorders in patients with neonatal HIBD
We previously showed that miR-325 is significantly up-regulated after the induction of neonatal HIBD in rats (Ding et al., 2015; Yang et al., 2017). To investigate the biological function of miR-325 in a clinical setting, we collected peripheral blood samples from patients with neonatal HIBD with age/weight/sex-matched healthy newborns (Figure 1A). We measured the relative expression of miR-325, as well as that of miRNAs that are known to be highly expressed in the pineal gland (Clokie et al., 2012), in peripheral mononuclear blood cells (Figure 1B–D). Consistent with the results from rats, we detected a significant up-regulation of miR-325 expression in newborns with either moderate/mild or severe HIBD (Figure 1B). In contrast, miR-183 nor miR-483, two miRNAs whose expression is enriched in the pineal gland, showed only non-significant changes in patients with moderate/mild or severe neonatal HIBD compared with healthy controls (Figure 1C and D). We next sought to investigate the correlation between circulating miR-325 levels and different aspects of neonatal HIBD symptoms. Our results indicated that miR-325 levels did not correlate with the severity of neonatal hypoxic ischemic encephalopathy (indicator: APGAR score at 5 minutes) (Figure 1E, R2 = 0.07, non-linear, one-phase decay). Interestingly, we found that the relative expression level of miR-325 correlated well with the degree of sleep rhythm disorders in neonatal HIBD patients (indicator: sleep rhythm performance score (Ding et al., 2016)) (Figure 1F, R2 = 0.77, non-linear, one-phase decay). These results suggest that pineal miR-325 plays a key role in regulating circadian rhythms in patients with neonatal HIBD.
Lhx3 is a novel downstream target of miR-325
In our previous study (Yang et al., 2017) we showed that miR-325-3p interacts with AANAT, a key molecule involved in melatonin synthesis and circadian rhythm regulation. Given that individual miRNAs often regulate functionally related gene clusters, we investigated other downstream targets of miR-325. Bioinformatics analysis showed that the 3′-UTR of the transcription factor Lhx3 contains the consensus miR-325 binding site (Figure 2A). Next, we used a luciferase reporter gene assay to examine the relationship between miR-325 and Lhx3 mRNA. As shown in Figure 2B, in the presence of miR-325 the luciferase activity was approximately 2/3 lower than that seen with a control RNA. In contrast, when either an miR-325-3p inhibitor or a construct with a mutation in the target site of the Lhx3 3′-UTR was used, there was no effect on the expression of the luciferase reporter (Figure 2B). In addition, LHX-3 expression was significantly increased in the pineal gland in miR-325 knock out mice (Figure 2C and D). Taken together, these data suggest that Lhx3 is a direct downstream target of miR-325.
LHX3 exhibits a miR-325-dependent circadian pattern of expression
To further investigate the potential role of LHX3 in regulating circadian rhythms, we first explored its expression in different tissues. In adult animals, Lhx3 was highly expressed in the central nervous system, and was particularly enriched in the pineal gland and striatum (Figure 3A). We then measured the expression of Lhx3 in the striatum of mice raised with 12-hour light/dark cycle and found that it is expressed in a circadian pattern (24 hours/cycle) (Figure 3B). Intriguingly, while this circadian expression pattern was apparent in the pineal gland in control mice, it was disrupted in mice lacking miR-325 (Figure 3C and D). Thus, miR-325-mediated regulation of Lhx3 plays a crucial role in the circadian pattern of expression of this mRNA.
Figure 3.
Circadian pattern of Lhx3 mRNA expression and Lhx3 mRNA down-regulation in the pineal gland in response to HIBD.
(A) Expression of Lhx3 mRNA (normalized to β-actin) in multiple tissues relative to Lhx3 mRNA expression in the pineal gland in adult mice (P60). (B) Circadian pattern of Lhx3 mRNA expression (normalized to β-actin) in the striatum relative to ZT0. (C, D) The circadian pattern of Lhx3 mRNA expression (normalized to β-actin) in the pineal gland relative to ZT0 (left, miR-325+/–(control, C)) was disrupted in miR-325–/– mice (D). The data used to generate this figure are shown in Additional file 6 (12.8KB, pdf) . Data are expressed as mean ± SEM (n = 3 for each data point). Lhx3: LIM homeobox 3; ZT: zodiac time.
miR-325 knock-down rescues LHX3 down-regulation in the pineal gland after HIBD
To assess whether pineal LHX3 plays a role in HIBD pathophysiology, we measured its expression in the pineal gland and found that pineal Lhx3 mRNA expression decreased dramatically beginning 12 hours post-injury (Figure 4A). This down-regulation was negatively correlated with the up-regulation in miR-325 expression observed here (Figure 4B) and in a study by Yang et al. (2017), but not with an up-regulation of miR-483, which is abundantly expressed in the pineal gland (Figure 4C). In miR-325 knock out mice, the down-regulation of Lhx3 post-HIBD was significantly reversed (Figure 4D). These results suggested that pineal LHX3, a downstream target of miR-325, is likely to be involved in the pathophysiology of HIBD.
Figure 4.
Lhx3 mRNA expression post-HIBD in wild-type and miR-325 knockout mice.
(A–C) Changes in Lhx3 mRNA (A), miR-325 (B), and miR-483 (C) expression in the pineal gland at different time points in sham-operated or HIBD in control (with no miR-325 knock out) mice. (D) Changes in Lhx3 mRNA expression in the pineal gland at different time points in sham-operated or HIBD miR-325 knock out mice. The data used to generate this figure are shown in Additional file 7 (16.3KB, pdf) . Data are expressed as mean ± SEM (n = 4 for each data point). **P < 0.01 (repeated measures of analysis of variance followed by Bonferroni’s post hoc correction). HIBD: Hypoxic-ischemic brain damage; LHX3: LIM homeobox 3; n.s.: no statistical significance.
The improvement post-HIBD circadian dysfunction seen in miR-325-depleted mice is disrupted by LHX3 knockdown
Previous studies have shown that HIBD often causes circadian rhythm dysfunctions in juvenile and adolescent humans and animals (Newman et al., 2006; Angriman et al., 2015; Ding et al., 2016; Yang et al., 2017). Because miR-325, which is crucial for regulation of AANAT expression, is known to be up-regulated post-HIBD, we first sought to investigate whether depletion of miR-325 rescues post-HIBD circadian dysfunction, using the wheel running assay (Verwey et al., 2013; Yang et al., 2017). We found that miR-325 depletion did not alter the mice’s entrained sleep/wake cycle when they were transitioned to constant darkness (Figure 5A, C and G). Consistent with previous studies (Yang et al., 2017), HIBD mice exhibited significantly greater cycle-to-cycle variability in the onset of daily activity, without any apparent changes in the wheel running period (Figure 5A, B and G). However, this high variability was reversed in miR-325 knockout animals (Figure 5B, D and G), suggesting that miR-325 depletion improves post-HIBD circadian dysfunction.
Figure 5.
Circadian rhythmicity of locomotor activity in HIBD mice.
(A, B) Representative wheel running activity of sham-operated or neonatal HIBD juvenile mice (P21) maintained in constant dark (DD) conditions. The onset time of activity in the mice with neonatal HIBD became erratic. (C, D) Representative wheel running activity in sham-operated or neonatal HIBD juvenile miR-325–/– mice maintained in DD conditions. Note that, while the erratic onset time of wheel running improved, the duration of the wheel running period was altered. (E, F) Representative wheel running activity of juvenile miR-325–/– mice with neonatal HIBD maintained in DD conditions and injected with AAV-shRNA-sham or -LHX3. (G) Circadian rhythmicity of locomotor activity in control or miR-325–/– sham-operated or neonatal HIBD mice maintained in DD conditions. The data used to generate this figure are shown in Additional file 8 (13.9KB, pdf) . Data are expressed as mean ± SEM (n = 4 for each data point). **P < 0.01, vs. sham group (Student’s t- test); ##P < 0.01, vs. AAV-shRNA-sham group (one-way analysis of variance followed by Bonferroni’s post hoc correction comparison). AAV: Adeno-associated viruses; HIBD: hypoxic-ischemic brain damage; LHX3: LIM homeobox 3.
Next, we examined whether LHX3 expression is required for functional rescue post-HIBD in miR-325 knock out mice by injecting AAV2/8-shRNA-Lhx3 into the lateral ventricle via ultrasound guidance (Arlotta et al., 2005) at embryonic day 18. After verifying knockdown in the pineal gland (Additional Figure 1 (909.5KB, tif) ), we assessed the circadian rhythm of mice with LHX3 knock down post-HIBD. Compared with AAV-sham-shRNA injection, LHX3 knockdown almost completely disrupted the rescue effect of miR-325 knockout on circadian rhythm (Figure 5E, F, and G). These results suggest that LHX3, as a down-stream target of miR-325, is required for re-establishing a normal circadian rhythm after HIBD.
Discussion
By establishing a positive correlation between circulating miR-325 levels and the severity of sleep rhythm problems in patients with neonatal HIBD, we have confirmed our previous findings that miR-325 plays a critical role in regulating circadian rhythms. Next, we identified that LHX3, an LIM-homeodomain transcription factor, is a downstream target of miR-325 and exhibits a miR-325-dependent circadian pattern of expression in the pineal gland. Functionally, knockdown of LHX3 substantially reversed the rescue effect that miR-325 depletion had on establishing normal circadian rhythms in post-HIBD mice. Taken together, the findings from this study demonstrate a novel role for a circadian transcriptional factor in fine-tuning a key element regulating circadian rhythms.
To maintain homeostasis in general, and circadian rhythms in specific, the expression of multiple functionally related genes is highly coordinated in response to environmental changes. Therefore, the ability to fine-tune the expression of a group of mRNAs that participate in the same functional pathway can help orchestrate their expression in real time. In line with this concept, multiple RNA binding proteins are transcribed from post-transcriptional RNA modification operons to co-regulate the expression of functionally related mRNAs (Keene, 2007; Liu et al., 2008; Liu and Szaro, 2011; Bisogno and Keene, 2018). Similar regulatory mechanisms have been identified for miRNAs, which are a crucial component of post-transcriptional regulation. For example, miR-126 serves as a key regulator of metastasis by regulating the expression of MERTK, IGFBP2, and PITPNC1, thereby affecting tumor angiogenesis (Png et al., 2011). Another example is miR-19b, which suppresses the expression of four genes that are key regulators of the nuclear factor-κB signaling pathway (Gantier et al., 2012). We have shown that miR-325 regulates the expression of Aanat and Lhx3, suggesting that it is a key regulator of circadian rhythms. We therefore suggest that quantifying circulating miR-325 levels could help diagnose sleep disorders in patients with neonatal HIBD.
LHX3, a LIM-homeodomain transcription factor, is known to play a determining role in motor neuron/V2 interneuron fate specification during spinal cord development (Pfaff et al., 1996; Sharma et al., 1998; Thaler et al., 2002). In addition, LHX3, along with LHX4, regulates the development of hormone-secreting cells in the mammalian pituitary gland (Mullen et al., 2007). However, it is unknown whether and how it might play a role in circadian regulation. Interestingly, Welsh et al. (2010) found that LHX1, a closely related member of the LIM-homeodomain transcription factor family, is required to synchronize circadian oscillator neurons in the hypothalamic suprachiasmatic nucleus, the master organ for setting the circadian clock. Therefore, LIM-homeodomain transcriptional factors may be involved in regulating circadian rhythm at multiple levels through distinct mechanisms.
Although brain imaging may help predict the occurrence of circadian rhythm-related sleep disorders in children with HIBD (Ding et al., 2016), it is not a straight-forward or cost-effective approach. Our findings that miR-325 expression levels specifically correlate with the occurrence of rhythmic sleep disorders indicate that miR-325 could serve as a prognostic indicator. However, this study was limited by the relatively small sample size, especially the small number of patients with severe HIBD who were included. Future studies will focus on collecting more data to verify the findings from this study and ideally develop an easy-to-use diagnostic kit that could facilitate early intervention for patients with circadian rhythm–related sleep disorders caused by neonatal HIBD.
| Groups | ID | Sex | Age | Weight | APGAR 1 min | APGAR 5 min | Blood PH | Cesarean Section |
|---|---|---|---|---|---|---|---|---|
| Control | ||||||||
| 1 | 670213 | f | 196 | 1.15 | 7 | 10 | y | |
| 1 | 671755 | m | 204 | 1.3 | 9 | 9 | n | |
| 1 | 667610 | m | 212 | 1.25 | 9 | 9 | n | |
| 1 | 671034 | f | 214 | 1.4 | 9 | 9 | y | |
| 1 | 664887 | m | 261 | 1.69 | 10 | 9 | y | |
| 1 | 670798 | f | 221 | 1.45 | 9 | 10 | y | |
| 1 | 666425 | f | 230 | 1.63 | 10 | 10 | n | |
| 1 | 664966 | m | 238 | 1.74 | 9 | 10 | n | |
| 1 | 673457 | m | 238 | 2.38 | 8 | 8 | n | |
| 1 | 677048 | f | 240 | 1.95 | 10 | 10 | n | |
| 1 | 667057 | f | 240 | 2.2 | 10 | 10 | y | |
| 1 | 675174 | f | 242 | 1.42 | 10 | 9 | y | |
| 1 | 675620 | f | 246 | 2.3 | 10 | 10 | n | |
| 1 | 670832 | f | 252 | 2.76 | 10 | 10 | n | |
| 1 | 666620 | f | 259 | 2.21 | 9 | 10 | n | |
| 1 | 670519 | m | 259 | 2.1 | 10 | 10 | n | |
| 1 | 671536 | f | 266 | 2.32 | 10 | 10 | y | |
| 1 | 665904 | m | 271 | 2.95 | 9 | 10 | y | |
| 1 | 669367 | f | 274 | 3.06 | 6 | 8 | n | |
| moderate/mild | ||||||||
| 2 | 668319 | f | 290 | 3 | 6 | 9 | 7.484 | n |
| 2 | 668847 | m | 171 | 0.85 | 6 | 8 | 7.289 | n |
| 2 | 670790 | f | 283 | 3.94 | 6 | 10 | 7.533 | n |
| 2 | 666640 | m | 213 | 1.45 | 6 | 6 | 7.319 | n |
| 2 | 668335 | f | 200 | 1.26 | 5 | 7 | 7.46 | y |
| 2 | 670740 | f | 283 | 3.87 | 7 | 7 | 7.438 | y |
| 2 | 666641 | m | 213 | 1.16 | 6 | 6 | 7.448 | n |
| 2 | 674157 | m | 290 | 3.5 | 8 | 8 | 7.181 | n |
| 2 | 674762 | f | 215 | 1.61 | 6 | 6 | 7.23 | y |
| 2 | 672901 | m | 196 | 1.08 | 4 | 4 | 7.358 | n |
| 2 | 673920 | f | 269 | 1.47 | 1 | 3 | 7.398 | n |
| 2 | 673271 | m | 280 | 3.9 | 5 | 5 | 7.324 | n |
| 2 | 672622 | m | 256 | 2.9 | 5 | 6 | 7.607 | y |
| 2 | 673836 | m | 236 | 1.6 | 1 | 3 | 7.437 | y |
| 2 | 674123 | f | 273 | 2.78 | 7 | 7 | 7.519 | y |
| 2 | 676376 | f | 269 | 1.7 | 6 | 6 | 7.48 | n |
| 2 | 676231 | m | 276 | 3.8 | 4 | 6 | 7.482 | y |
| 2 | 675013 | m | 287 | 3.78 | 7 | 8 | 7.36 | n |
| 2 | 675676 | f | 271 | 2.6 | 9 | 10 | 7.364 | n |
| 2 | 676292 | f | 289 | 3.2 | 7 | 7 | 7.48 | n |
| severe | ||||||||
| 3 | 667608 | m | 197 | 1.51 | 3 | 3 | 7.593 | y |
| 3 | 669945 | f | 289 | 3.97 | 3 | 3 | 7.229 | y |
| 3 | 669217 | m | 290 | 3.91 | 2 | 2 | 7.33 | n |
Additional files:
Additional Figure 1 (909.5KB, tif) : Knock down of LHX3 in the pineal gland.
Knock down of LHX3 in the pineal gland.
(A, B) Representative transverse section of pineal gland showing GFP signal (A) or stained with anti-LHX3 (B) in miR-325-/- mice with ventricle AAV2/8-shRNA-sham (left column) or AAV-2/8-shRNA-LHX3 (right column) injection at P1. Scale bar: 100 μm. AAV: Adeno-associated viruses; GFP: green fluorescent protein; LHX3: LIM homeobox 3.
Additional file 1 (158.3KB, pdf) : Hospital ethics approval (Chinese).
Additional file 2: Open peer review reports 1 (80.7KB, pdf) and 2 (81.3KB, pdf) .
Additional file 3 (35.7KB, pdf) : Original data of Figure 1A.
Additional file 4 (29KB, pdf) : Original data of Figure 1B-F.
Additional file 5 (103KB, pdf) : Original data of Figure 2B and D.
Additional file 6 (12.8KB, pdf) : Original data of Figure 3.
Additional file 7 (16.3KB, pdf) : Original data of Figure 4.
Additional file 8 (13.9KB, pdf) : Original data of Figure 5G.
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
Financial support: This study is supported by the National Natural Science Foundation of China, Nos. 81871193 (to XD), 81671532 (to BS), 81771625 & 81701490 (to XF), 81801505 (to MG); Jiangsu Provincial Medical Youth Talent of China, Nos. QNRC2016763 (to XD), QNRC2016758 (to LXX), QNRC2016762 (to ML); the Science and Technology Project of Suzhou City of China, No. SS201709 (to XD); the Natural Science Foundation of Jiangsu Province of China, No. BK20180205 (to XD); the Training Program Foundation for Health Talents of Gusu of China, No. GSWS2019049 (to XD); the Jiangsu Provincial Key Medical Discipline of China, No. ZDXKA2016013 (to XF); the Jiangsu Province Women and Children Health Research Project of China, No. F201750 (to LXX); the Pediatric Clinical Center of Suzhou City of China, No. Szzx201504 (to XF); Suzhou Industrial Technology Innovation Project of China, No. SYS201765 (to LZ), and the Project of Suzhou Science, Education and Health and Technology, China, No. KJXW2018018 (to LL). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The clinical research was approved by Institutional Review Board of Children’s Hospital of Soochow University (approval No. 2015028) on July 20, 2015, and animal experiment was approved by School of Medicine, Soochow University Institutional Animal Care and Use Committee (approval No. XD-2016-1) on January 15, 2016.
Declaration of participant consent: The authors certify that they have obtained the consent forms from their parents. In the form, parents have given their consent for the participants’ images and other clinical information to be reported in the journal. The parents understand that the participants’ names and initials will not be published.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Makoto Nabetani, Yodogawa Christian Hospital, Japan; Luminita Paduraru, Universitatea de Medicină şi Farmacie “Grigore T. Popa”, Romania.
Funding: This study was supported by the National Natural Science Foundation of China, Nos. 81871193 (to XD), 81671532 (to BS), 81771625 & 81701490 (to XF), 81801505 (to MG); Jiangsu Provincial Medical Youth Talent of China, Nos. QNRC2016763 (to XD), QNRC2016758 (to LXX), QNRC2016762 (to ML); the Science and Technology Project of Suzhou City of China, No. SS201709 (to XD); the Natural Science Foundation of Jiangsu Province of China, No. BK20180205 (to XD); the Training Program Foundation for Health Talents of Gusu of China, No. GSWS2019049 (to XD); the Jiangsu Provincial Key Medical Discipline of China, No. ZDXKA2016013 (to XF); the Jiangsu Province Women and Children Health Research Project of China, No. F201750 (to LXX); the Pediatric Clinical Center of Suzhou City of China, No. Szzx201504 (to XF); Suzhou Industrial Technology Innovation Project of China, No. SYS201765 (to LZ), and the Project of Suzhou Science, Education and Health and Technology, China, No. KJXW2018018 (to ML).
P-Reviewer: Nabetani M, Paduraru L; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Crow E, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Abbasi H, Unsworth CP. Electroencephalogram studies of hypoxic ischemia in fetal and neonatal animal models. Neural Regen Res. 2020;15:828–837. doi: 10.4103/1673-5374.268892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angriman M, Caravale B, Novelli L, Ferri R, Bruni O. Sleep in children with neurodevelopmental disabilities. Neuropediatrics. 2015;46:199–210. doi: 10.1055/s-0035-1550151. [DOI] [PubMed] [Google Scholar]
- 3.Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 4.Bisogno LS, Keene JD. RNA regulons in cancer and inflammation. Curr Opin Genet Dev. 2018;48:97–103. doi: 10.1016/j.gde.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clokie SJ, Lau P, Kim HH, Coon SL, Klein DC. MicroRNAs in the pineal gland: miR-483 regulates melatonin synthesis by targeting arylalkylamine N-acetyltransferase. J Biol Chem. 2012;287:25312–25324. doi: 10.1074/jbc.M112.356733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R939–949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- 7.de Vries LS, Jongmans MJ. Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010;95:F220–224. doi: 10.1136/adc.2008.148205. [DOI] [PubMed] [Google Scholar]
- 8.Ding X, Sun B, Huang J, Xu L, Pan J, Fang C, Tao Y, Hu S, Li R, Han X, Miao P, Wang Y, Yu J, Feng X. The role of miR-182 in regulating pineal CLOCK expression after hypoxia-ischemia brain injury in neonatal rats. Neurosci Lett. 2015;591:75–80. doi: 10.1016/j.neulet.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Ding X, Cheng Z, Sun B, Huang J, Wang L, Han X, Yang Y, Xu W, Cao X, Miao P, Wang Y, Guo W, Gu Q, Feng X. Distinctive sleep problems in children with perinatal moderate or mild hypoxic-ischemia. Neurosci Lett. 2016;614:60–64. doi: 10.1016/j.neulet.2015.12.061. [DOI] [PubMed] [Google Scholar]
- 10.Gantier MP, Stunden HJ, McCoy CE, Behlke MA, Wang D, Kaparakis-Liaskos M, Sarvestani ST, Yang YH, Xu D, Corr SC, Morand EF, Williams BR. A miR-19 regulon that controls NF-κB signaling. Nucleic Acids Res. 2012;40:8048–8058. doi: 10.1093/nar/gks521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20:21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang LJ, Xu ZX, Wu MF, Dong GQ, Zhang LL, Gao JY, Feng CX, Feng X. Resatorvid protects against hypoxic-ischemic brain damage in neonatal rats. Neural Regen Res. 2020;15:1316–1325. doi: 10.4103/1673-5374.272615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keene JD. RNA regulons: coordination of post-transcriptional events. Nat Rev Genet. 2007;8:533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- 14.Kim TD, Woo KC, Cho S, Ha DC, Jang SK, Kim KT. Rhythmic control of AANAT translation by hnRNP Q in circadian melatonin production. Genes Dev. 2007;21:797–810. doi: 10.1101/gad.1519507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim TD, Kim JS, Kim JH, Myung J, Chae HD, Woo KC, Jang SK, Koh DS, Kim KT. Rhythmic serotonin N-acetyltransferase mRNA degradation is essential for the maintenance of its circadian oscillation. Mol Cell Biol. 2005;25:3232–3246. doi: 10.1128/MCB.25.8.3232-3246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kojima S, Shingle DL, Green CB. Post-transcriptional control of circadian rhythms. J Cell Sci. 2011;124:311–320. doi: 10.1242/jcs.065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li JD, Burton KJ, Zhang C, Hu SB, Zhou QY. Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei. Am J Physiol Regul Integr Comp Physiol. 2009;296:R824–830. doi: 10.1152/ajpregu.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Szaro BG. hnRNP K post-transcriptionally co-regulates multiple cytoskeletal genes needed for axonogenesis. Development. 2011;138:3079–3090. doi: 10.1242/dev.066993. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Gervasi C, Szaro BG. A crucial role for hnRNP K in axon development in Xenopus laevis. Development. 2008;135:3125–3135. doi: 10.1242/dev.022236. [DOI] [PubMed] [Google Scholar]
- 20.Lv Y, Sun B, Lu XX, Liu YL, Li M, Xu LX, Feng CX, Ding X, Feng X. The role of microglia mediated pyroptosis in neonatal hypoxic-ischemic brain damage. Biochem Biophys Res Commun. 2020;521:933–938. doi: 10.1016/j.bbrc.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Mukaigasa K, Sakuma C, Okada T, Homma S, Shimada T, Nishiyama K, Sato N, Yaginuma H. Motor neurons with limb-innervating character in the cervical spinal cord are sculpted by apoptosis based on the Hox code in chick embryo. Development. 2017;144:4645–4657. doi: 10.1242/dev.158873. [DOI] [PubMed] [Google Scholar]
- 22.Mullen RD, Colvin SC, Hunter CS, Savage JJ, Walvoord EC, Bhangoo AP, Ten S, Weigel J, Pfäffle RW, Rhodes SJ. Roles of the LHX3 and LHX4 LIM-homeodomain factors in pituitary development. Mol Cell Endocrinol. 2007;265-266:190–195. doi: 10.1016/j.mce.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman CJ, O’Regan M, Hensey O. Sleep disorders in children with cerebral palsy. Dev Med Child Neurol. 2006;48:564–568. doi: 10.1017/S0012162206001198. [DOI] [PubMed] [Google Scholar]
- 24.Perlman M, Shah PS. Hypoxic-ischemic encephalopathy: challenges in outcome and prediction. J Pediatr. 2011;158:e51–54. doi: 10.1016/j.jpeds.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84:309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- 26.Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2011;481:190–194. doi: 10.1038/nature10661. [DOI] [PubMed] [Google Scholar]
- 27.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 28.Sharma K, Sheng HZ, Lettieri K, Li H, Karavanov A, Potter S, Westphal H, Pfaff SL. LIM homeodomain factors Lhx3 and Lhx4 assign subtype identities for motor neurons. Cell. 1998;95:817–828. doi: 10.1016/s0092-8674(00)81704-3. [DOI] [PubMed] [Google Scholar]
- 29.Takenouchi T, Rubens EO, Yap VL, Ross G, Engel M, Perlman JM. Delayed onset of sleep-wake cycling with favorable outcome in hypothermic-treated neonates with encephalopathy. J Pediatr. 2011;159:232–237. doi: 10.1016/j.jpeds.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Thaler JP, Lee SK, Jurata LW, Gill GN, Pfaff SL. LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein-protein interactions. Cell. 2002;110:237–249. doi: 10.1016/s0092-8674(02)00823-1. [DOI] [PubMed] [Google Scholar]
- 31.Verwey M, Robinson B, Amir S. Recording and analysis of circadian rhythms in running-wheel activity in rodents. J Vis Exp. 2013:50186. doi: 10.3791/50186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan Y, Wladyka C, Fujii J, Sockanathan S. Prdx4 is a compartment-specific H2O2 sensor that regulates neurogenesis by controlling surface expression of GDE2. Nat Commun. 2015;6:7006. doi: 10.1038/ncomms8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Sun B, Huang J, Xu L, Pan J, Fang C, Li M, Li G, Tao Y, Yang X, Wu Y, Miao P, Wang Y, Li H, Ren J, Zhan M, Fang Y, Feng X, Ding X. Up-regulation of miR-325-3p suppresses pineal aralkylamine N-acetyltransferase (Aanat) after neonatal hypoxia-ischemia brain injury in rats. Brain Res. 2017;1668:28–35. doi: 10.1016/j.brainres.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Zhang FH, Fan WJ, Hua XY, Chen XD. Compound brain peptide ganglioside can improve intrauterine hypoxia-induced neonatal brain injury and promote synapse regeneration in a mouse model. Zhongguo Zuzhi Gongcheng Yanjiu. 2020;24:1689–1694. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Knock down of LHX3 in the pineal gland.
(A, B) Representative transverse section of pineal gland showing GFP signal (A) or stained with anti-LHX3 (B) in miR-325-/- mice with ventricle AAV2/8-shRNA-sham (left column) or AAV-2/8-shRNA-LHX3 (right column) injection at P1. Scale bar: 100 μm. AAV: Adeno-associated viruses; GFP: green fluorescent protein; LHX3: LIM homeobox 3.