Abstract
Vision altering diseases, such as glaucoma, diabetic retinopathy, age-related macular degeneration, myopia, retinal vascular disease, traumatic brain injuries and others cripple many lives and are projected to continue to cause anguish in the foreseeable future. Gap junctions serve as an emerging target for neuromodulation and possible regeneration as they directly connect healthy and/or diseased cells, thereby playing a crucial role in pathophysiology. Since they are permeable for macromolecules, able to cross the cellular barriers, they show duality in illness as a cause and as a therapeutic target. In this review, we take recent advancements in gap junction neuromodulation (pharmacological blockade, gene therapy, electrical and light stimulation) into account, to show the gap junction’s role in neuronal cell death and the possible routes of rescuing neuronal and glial cells in the retina succeeding illness or injury.
Keywords: age-related macular degeneration, bystander effect, connexin, diabetic retinopathy, gap junction, glaucoma, neuromodulation, retina, retinal disease, vision
Introduction
Vision is generally the most important sensory modality in vertebrates and as such, it is the most feared sense to lose for humans (Scott et al., 2016). The site for visual perception is the retina and we desperately need new tools to aid retinal regeneration or at least slow down the existing diseases that cripple vision.
Both neuronal (ganglion cells, amacrine cells, bipolar cells, horizontal cells, and photoreceptors) and non-neuronal (astrocytes, microglia, and Müller cells) cell types tend to form gap junctions (GJs) that provide them with conduits for electrical and metabolic communication, thereby serving signaling and metabolism in health as well as in disease (Figure 1). This latter phenomenon has been central to a myriad of studies that identify GJs as possible key participants in developing disease phenotypes. In contrast, only a few studies refer to them as potential targets for regeneration or at least neuromodulation in the retina (Table 1). Their true contribution to cellular regeneration is yet to be unveiled.
Figure 1.
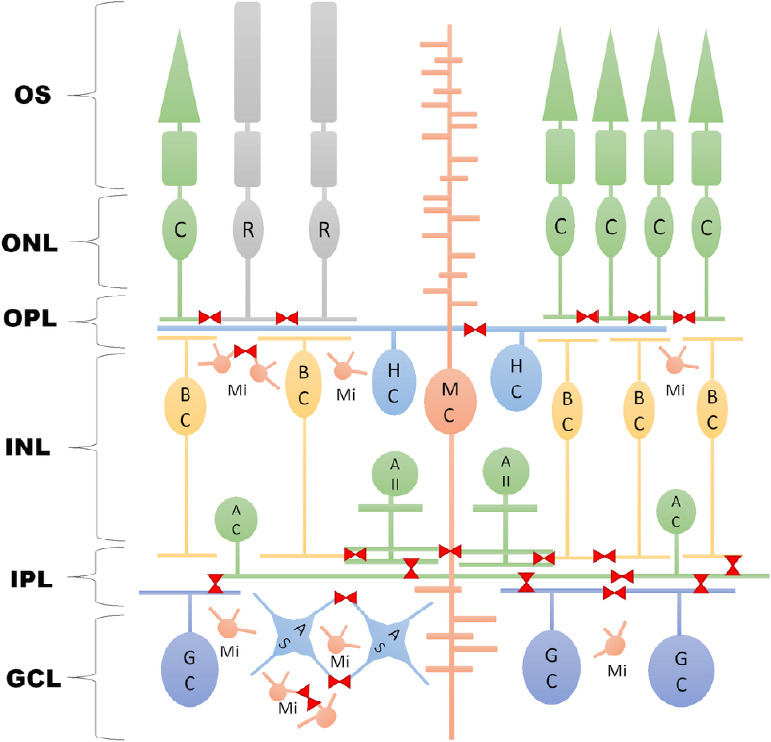
Gap junction connections in the retina.
Neuronal cells and glial cells form diverse GJ connections (red marks) that could serve as sites for neuromodulation in the retina. AC: Amacrine cells; AII: aII amacrine cells; AS: astrocytes; BC: bipolar cells; C: cones; GC: ganglion cells; GCL: ganglion cell layer; HC: horizontal cells; INL: inner nuclear layer; IPL: inner plexiform layer; ONL: outer nuclear layer; OPL: outer plexiform layer; OS: outer segment; R: rods; MC: Müller cell; Mi: microglia.
Table 1.
GJs as possible targets for regeneration and neuromodulation in the retina
| Glaucoma | Diabetic retinopathy | Others (with disease) |
|---|---|---|
| Cx43 (20934339) | Cx43 (15123628) | Cx36, 45 (ischemia, 25100592) |
| Cx36 (28604388) | Cx43 (28674171) | Cx36 (myopia, 32547367) |
| AMD | Cx43 (32566247) | Cx43 (ischemia, 22345088) |
| Cx43 (27490318) | Cx43 (19029021) | Various (injury, inflammation, 22886208) |
| TBI | Cx30.2 (23385797) | Cx43 (inflammation, 27490318) |
| Cx40, 43, 45 (28948071) | Cx43 (24938518) | Cx36, 43, 45 (Huntington, 18816186) |
| Cx43 (31194577) | Cx43 (11431461) | Cx43 (oxidative stress, 22138732) |
| Cx36 (28124625) | Cx26, 43 (28583293) | Cx43 (ischemia, 29891713) |
| Cx36 (16101742) | Cx43 (11978657) | Cx36 (myopia, 31283648) |
| Cx43 (26738943) |
Relevant connexins with PMIDs stated. AMD: Age-related macular degeneration; GJs: gap junctions; TBI: traumatic brain injury.
It is an undisputed fact that GJs permit direct intercellular communication between neighboring cells, allowing them to play a role in neuronal development, communication, metabolic support and normal neuronal function (Völgyi et al., 2013a, b; Roy et al., 2017; Caval-Holme et al., 2019; Tengölics et al., 2019; Trenholm and Awatramani, 2019; Kovács-Öller et al., 2020; Puller et al., 2020; Sigulinsky et al., 2020).
GJs are found between adjacent cells at the sites of intimate plasma-membrane appositions, anchored by the cytoskeletal matrix (Rash et al., 2004; Ciolofan et al., 2007; Li et al., 2008; Völgyi et al., 2013a; Kántor et al., 2017). They are composed of protein monomers of varying molecular weight, called connexins (Cx), in the neuronal tissue and throughout the body. Cx proteins typically have an intracellular loop, two extracellular loops, with both C- and N-terminals facing towards the intracellular space and the cytoplasm. Six Cx subunits are required to form a connexon hemichannel and their Cx composition is rather specific for neuronal or glial cell contact in the nervous tissue. Both interconnected cells contribute with one connexon hemichannel to a functional GJ channel that oppose each other along the two bilayer surfaces. GJs, therefore, form continuous tunnels between connected cells that are permeable to ions and small molecules mostly smaller than 1 kDa, but they could exhibit highly different specificities for different molecules (Goldberg et al., 2002). Interestingly, this latter 1 kDa weight limit for GJ permeability has lately been challenged as a number of reports revealed relatively large molecules (e.g. siRNAs) that can successfully pass-through certain GJs. Homotypic Cx43 GJs have been shown to allow the passage of synthetic oligonucleotides with molecular weights of 2–4 kDa, with a length of 7.6 nm and a diameter of 1–1.1 nm. Since these molecules provide epigenetic sources of information, they might serve as major contributors to neuronal regeneration and modulation (Valiunas et al., 2005, 2015; Zong et al., 2016).
In addition, a transcellular spreading of epigenetic factors among GJ coupled neurons and non-neuronal cells can greatly enhance their therapeutic effects, since GJs are widely expressed between nearly all cell-types of the retina, forming a complex network of direct cellular links (Figure 1). Furthermore, many studies showed that the GJ hemichannels can be activated through stress (ischemic or mechanical), resulting in small-molecule flux (Ca2+, glutamate, ATP, or NAD+), thereby generating a change in cellular signaling and eliciting physiological responses (Orellana et al., 2009; Belousov et al., 2017). Therefore, the above-listed evidence that GJs could potentially be revealed as the most important participants in neuronal degeneration and regeneration processes. GJ coupling is an often-neglected factor in neuronal survival, and thus the major goal of this review is to bring neuronal and non-neuronal GJs of the retina into focus in this regard. To this end, we will first review evidence for the involvement of GJs in retinal degenerative diseases and then the possible therapeutic potential of pharmacological interventions and gene therapy that focus GJs and/or GJ mediated transcellular communication.
Search Strategy and Selection Criteria
The search and the study selection were carried out independently by the authors responsible for each section and matched afterward by the authors. Relevant studies were located in the following databases: PubMed, Google Scholar by using key terms in the title, abstract and by using keywords. In PubMed the Related Citations function and citation tracking was used to retrieve further articles. The reference lists of the retrieved articles were then searched to identify further relevant studies for the review. All articles were manually checked for the relevant parts by at least two authors. The last search was conducted in June 2020.
Diseases and GJs in the Retina
GJs, due to their previously described pivotal role in neuronal network construction and cellular functions, indisputably play a part in a number of degenerative retinal diseases. Even though they may not be causative agents through their regular functioning, by distributing intracellular signals among coupled cells they promote the development of the diseased phenotype. The most prevalent diseases affecting the retina are diabetic retinopathy (DR), age-related macular degeneration (AMD), glaucoma, myopia, retinal vascular disease, and traumatic brain injuries (TBI), next to many other vision-impairing retinal diseases.
Diabetes mellitus (DM), even though 16 years ago it was not even listed among the top 10 malicious diseases, became the 7th most frequent cause of death in 2016 and it is projected to become even more prevalent in the near future (The top 10 causes of death, WHO 2018). Amongst the several metabolic disorders associated with DM, in 34.6% of cases, patients develop at least some level of DR, leading to visual deterioration and blindness (Yau et al., 2012). While appropriate treatment strategies are available for DM, at present, there is no known treatment that allows DR patients to recover even partially (Duh et al., 2017) and therefore it is crucial for all pre-existing conditions to be dealt with adequately. In DR, high glucose mediates the breakdown of GJs in Müller cells, as well as in astrocytes and other metabolically important cell types like pericytes (Ivanova et al., 2017, 2019; Kovács-Öller et al., 2020). These changes contribute to the loss of blood-brain barrier -or in this case blood-retina barrier (BRB)- integrity and vascular failure and eventually the appearance of lesions in the retina (Sato et al., 2002; Li et al., 2003; Fernandes et al., 2004; Li and Roy, 2009; Roy et al., 2017a).
Several studies showed the role of GJs in the pathophysiology of DR (Oku et al., 2001; Manasson et al., 2013; Ivanova et al., 2017; Roy et al., 2017a, b). The Cx43 connexin subunit plays multiple roles in the development of the pathological phenotype, therefore its expression and regulation could become a key factor in combating the disease. In the case of oxygen restricted states, the expression of the channel seems to be upregulated within hours. Similarly, it becomes highly expressed in the case of widespread cell death, inflammation, and vascular leaks (Danesh-Meyer et al., 2012). Oxygen-restricted conditions with significantly reduced choroidal blood flow can develop during DR (Muir et al., 2012). Under prolonged hypoxia, the balance of pro- and anti-angiogenic factors get disrupted, resulting in increased blood vessel growth. The expression of Cx hemichannels also changes during this condition (Tien et al., 2016), for reasons which are yet to be revealed. Elevated Cx levels have been observed in other pathological conditions of the retina as well.
Much like DR, AMD is also a well-studied but not completely understood retinal disease. The prevalence of AMD rises dramatically after the age of 50. In most developed countries AMD has become a major health issue in the last two decades and is projected to grow even further in the future (Wong et al., 2014). Unlike DR, the role of GJs is not well studied in AMD, but they are likely involved in pathophysiology, since inflammation is involved in the pathogenesis of the disease (Khandhadia et al., 2012), and Guo et al. (2016) showed, using a light damaged retina model, the inflammation affects the retina’s and the choroid’s GJ constitution. During the progressive cell death and degeneration of photoreceptors and epithelial cells in the case of AMD, the expression of the Cx hemichannels increases, with channels appearing in the extracellular matrix and outside blood vessels in stark contrast to healthy tissue (Danesh-Meyer et al., 2016). The same effect has been described in light damaged albino rat models also, with a significant increase in hemichannel expression, where the intravitreal application of mimetic peptides partially restored retinal function through the reduction of the negative effects (Guo et al., 2016).
Glaucoma has been documented as a retinal disease-causing blindness with the second-highest incidence number in the human population (Tham et al., 2014) and is usually not detected until it deteriorates vision. Recent studies highlighted the importance of GJs (Cx36, Cx43) not in the primary insult, but in the secondary cell death wave, precisely through the ablation of the GJ proteins (Kerr et al., 2011; Akopian et al., 2014, 2017).
While Myopia is not considered a retinal disease by default, in more severe cases the retina also becomes affected and recent studies showed the role of GJs in the pathophysiology of this process, much like the other diseases discussed thus far. It has been shown that Cx36 expression and phosphorylation increases in a mouse model of myopia, directly affecting neuronal communication in the retina (Yang et al., 2019; Banerjee et al., 2020).
Retinal vascular disease is an umbrella term for disorders involving vascular effects in the retina. Most of them are caused by hypertensive retinopathy, retinal vein occlusion, central retinal artery occlusion and branch retinal artery occlusion. The role of GJs had been suspected to take part in pathophysiology through the disruption of neurovascular connections (Slavi et al., 2018; Ivanova et al., 2019).
Finally, TBI, a neurological disorder caused by physical accidents, is a disease rarely discussed in connection with the retina, but needs to be addressed regardless, because it has been shown that its effects on the retina may contribute to vision loss (Mohan et al., 2013; Armstrong, 2018; Evans et al., 2018; Harper et al., 2019). Even though our knowledge on the subject is limited, results suggest that GJs are prominent factors in the deterioration of the symptoms (Prochnow, 2014; Chen B et al., 2017). TBI has been shown to affect the expression of Cx43↓, Cx40↑, and Cx45↑ in the brain (Chen B et al., 2017) and Cx36↓ in the retina, which could contribute to cell loss due to TBI (Striedinger et al., 2005).
Based on these results showing Cx expression pattern changes during pathological conditions in the retina, the presence of GJs also offers a potential target for future treatment. In case of elevated expression, reducing the Cx overexpression could – in theory - slow down the progression of the disease. The reason seems to be that the Cx channels play an important role in the initialization of various signal cascades related to all involved pathophysiologies (Gajardo-Gómez et al., 2016). In fact, Cx-like peptides used systematically in order to inhibit hemichannels, in addition to similar treatment modalities, have been proven to be effective in reducing cell death and vascular hemorrhage in cases of ischemia (O’Carroll et al., 2008).
The direct role: what have we learned from knockout animal models?
The body of evidence suggests that normal neuronal function is not possible without the presence of GJs contributing to transcellular metabolic exchange and/or signaling. The direct role of GJs to the maintenance of healthy neuronal functions have been studied in various knock-out (KO) animal models, in which GJ constituent Cx subunits are genetically deleted. In many cases, the embryos or pups of Cx KO mice are not viable due to defects in essential basic life-functions facilitated by the Cx molecules during development. Out of over 20 mammalian Cx subunits, only a handful are abundant in the neuronal retina, including Cx 30, Cx30.2, Cx36, Cx43, Cx45, Cx50, and Cx57 (Güldenagel et al., 2001; Massey et al., 2003; Hombach et al., 2004; Maxeiner et al., 2005; Müller et al., 2010; Kántor et al., 2018). Based on research with KO models, we can determine the exact roles the different Cx subunit types play in retinal mechanisms.
The Cx36 subunit is expressed by most neuronal elements of the retina. Positioned in key signaling pathways, they are responsible for decreasing signal-to-noise ratio in the outer retina in cone-to-cone connections, connecting bipolar cell subtypes to each other, creating populations of retinal ganglion cell (RGC) subtypes (Akopian et al. 2017). Regarding pathophysiology, due to their presence between many cellular subtypes, they play an important role in Glaucoma, Myopia, and TBI.
The constitutive Cx45 KO mice die before birth due to the importance of this Cx type during ontogeny, especially throughout the heart muscle. Thus, the role of Cx45 in retinal functioning has been only studied in conditional Cx45 KOs, where it was only ablated in neurons (Maxeiner et al., 2005; Schubert et al., 2005; Dedek et al., 2006; Li et al., 2008). The lack of Cx45 and Cx36 in the respective KO mice proved their importance in rod-mediated signaling by providing a minimum delay communication system between AII amacrine cells and ON bipolars, with their absence resulting in a form of night blindness (Deans et al., 2002; Bloomfield and Völgyi, 2004; Li et al. 2008).
The ablation of Cx57 induces a reduction in horizontal cell receptive fields, although no obvious change was found regarding the spatial tuning of output ganglion cells (Hombach et al., 2004; Shelley et al., 2006; Dedek et al., 2008). While the neuronal GJs play an important role in the normal retinal sensory mechanisms and have been assumed to have neuroprotective properties (Akopian et al. 2017), their selective KO mutants neither show any obvious phenotypes, nor have been proven to lead to any known pathological alterations or diseases.
The non-neuronal retinal GJs operate using a different set of Cx subunits, including Cx 30, Cx43, Cx45 (Zahs et al., 2003). Much like the Cx45 KO model, the genetic elimination of Cx43 is lethal since it is essential for intercellular communication in the cardiac tissue and in astrocytes (Dermietzel et al., 2000). Using selective KO animals, it has been shown that Cx43 is highly expressed by retinal astrocytes, Müller cells, and pericytes as well (Muto et. al, 2014; Ivanova et al., 2017, 2019; Slavi et al., 2018), but there have been no studies focusing on the morphological aberrations of the retina in the KO mice. All we know is that Cx43 expression is reduced in DR, but whether this reduction is in a causative relationship with the disease or a side-effect is not clear (Ivanova et al., 2017, 2019; Slavi et al., 2018).
Thus, our conclusion here regarding the direct effect of GJs on retinal diseases is two-fold; first, there has been a lack of knowledge on this issue regarding many retinal (neuronal and non-neuronal) Cx subunits, and second, so far, any detected phenotype described in Cx KO mouse strains has been very subtle or otherwise, their underlying role has yet to be proven.
Indirect role: the bystander effect - Myth or reality?
When cells get compromised due to injury or disease, they begin apoptotic or necrotic processes resulting in the release of malicious effector or signal molecules (death signals) that are transferred to neighboring cells, where they induce intracellular mechanisms leading to a secondary cell loss. This largely unknown mechanism is called the ‘bystander effect’, in which cells not affected by the primary insult die because they are located in the vicinity of dying cells. The transcellular transfer of death signal molecules is thought to be mediated by GJs connecting cellular neighbors (Ripps, 2002).
Even though the ‘bystander effect’ is a long-studied phenomenon, it still causes debate amongst scientists. Recent research has supplied strong evidence in support of the involvement of neuronal GJs in the bystander effect in the retina (Ripps, 2002; Akopian et al., 2014). Akopian and colleagues showed that the deletion of Cx36 increased cell survival under excitotoxic conditions, whereas Cx45 offered no protection in similar circumstances. In contrast, after ischemic insult, Cx36 provided no protection, but Cx45 KO mice displayed a greatly reduced neuronal loss in the retina. In addition, the pharmacological blockade of GJs rescued nearly all neighboring amacrine cells and reduced RGC loss by ~70% after the induction of excitotoxic or ischemic conditions (Akopian et al., 2014). These results not only proved the importance of GJs in spreading death-signals via the bystander effect but also showed that the role of various Cx subunits and GJs in these pathological mechanisms is insult-specific.
Apart from ischemic conditions, secondary cell loss appears to be a key component of retinal neurodegeneration in diseases like glaucoma, retinopathies, retinitis pigmentosa (Osborne et al., 2004; Elmore, 2007; Decrock et al., 2009; reviewed by O’Brien and Bloomfield, 2018). Besides progressive genetic and metabolic illnesses, single or repetitive environmental impacts may also be harmful. Excessive light, for example, can induce apoptotic processes in photoreceptors and pigment epithelial cells that are aggravated by their GJ connections via the bystander effect (Ishii and Rohrer, 2017; Ma et al., 2018). This issue is becoming ever more relevant due to the increased time spent exposed to monitor-related light sources in our everyday life. Another example of possible environmental impact, traumatic injury of the surrounding brain tissue (traumatic brain injury - TBI), may have an impact on the optic nerve and the eye, leading to a degeneration of the retina (Chen et al., 2017; Burke et al., 2019). Such an insult can be even more severe as death-signal molecules do not have to travel from the crushed ganglion cell axon to its soma/dendritic region but they can be directly transferred via direct axonal GJs in the optic nerve instead (Smedowski et al., 2020). The GJ permeability for a molecule depends on multiple factors (molecular weight, charge, 3D shape). Nucleotides (e.g. siRNAs) and peptides with molecular weight up to 4 kDa and diameter of 1 nm have been reported to cross GJ channels (Neijssen et al., 2005; Valiunas et al., 2005), however, there is no direct evidence for the passing of cell-survival factors through GJs so far, and neither the effects of excessive light exposure or TBI on retinal degeneration have been studied extensively, and even less is known about the role GJs play in related injurious changes.
In conclusion, the above examples provide evidence that the GJ-mediated bystander effect has a negative impact on the outcome of various retinal diseases and injuries. However, it has generally been ignored that besides death-signals, GJs are intercellular avenues for factors involved in cell survival as well. Therefore, it is possible that healthy cells might also pass certain substances through GJs that, in turn, promote survival.
Glial and vascular support through GJs
GJs could potentially act as bridges between neurons and glial cells (Fróes et al., 1999); however, there is a lack of understanding of how GJs interconnect neuronal and glial cell types in the mammalian retina and how GJ hemichannels can contribute to neuronal-glial interactions. These connections are rare and mostly unexplored, contrary to their great capacity in neuronal rescue and potential treatment strategies to fight progressive retinal diseases.
There are three main types of glial cells in the mammalian retina: astrocytes, Müller cells, and microglia (Figure 1). They are true factotums of the retina, having an enormous impact on retinal health. These cells provide structural support, maintain metabolism by phagocytosis cleaning the neuronal debris, and they also release certain transmitters and trophic factors, not to mention their role in K+ uptake (Vecino et al., 2016).
Müller cells vertically span through the whole retina with their endfeet facing the pigment epithelium and by forming the inner limiting membrane, facing the vitreous. On the other hand, they also ensheath large retinal blood vessels with their endfeet. The side facing the vitreous is forming a basement membrane and is covered with mucopolysaccharides.
There is evidence for the presence of Müller cell GJs in both lower order vertebrates (Ball and Reynolds, 1998) and a few mammals (rabbit - Zahs and Ceelen, 2006; mouse - Akopian et al., 2014). The involvement of Müller cells in pathophysiological processes is well known (Robinson et al., 1993; Reichenbach and Robinson 1995). As for Müller cell GJs and their Cx subunits, it has been proven that hyperglycemia alters Cx43 expression and promotes Müller cell (and pericyte) apoptosis (Muto et al., 2014). Although it is generally believed that Müller cells lack direct GJ connections towards neurons, one recent research from the Rieke lab (Grimes et al., 2020) showed the coupling of Müller cells to a special kind of amacrine cell, called Müller Associated Cell. This phenomenon makes Müller cells even more important since they might act as a direct bridge towards neurons.
It has been long known that astrocytes communicate with each other through GJs, and similar to Müller cells they take part in K+-signaling (Newman et al., 1984). Astrocytes and Müller cells form heterologous and asymmetric GJ connections (Ceelen et al., 2001). This might be the reason why, in ultrastructural examinations, tracer coupling NB injection experiments, probing retinal astrocytes and pericytes, failed to show any connections between them (Ivanova 2017, 2019; Kovács-Öller et al., 2020).
In the case of neuronal injury, ATP release occurs from astrocytes that stimulate microglial cells, and by activating other astrocytes, influences blood flow and related neuronal function (Gajardo-Gómez et al., 2016). Astrocytes can also trigger reactive gliosis as a result of mechanical perturbation, which likely disrupts their GJ coupling and through secondary signaling, the vascular function (Cahoy et al., 2008; Foo et al., 2011). It has also been shown that phosphorylation of astrocyte Cx43 GJs occurs in proliferative retinopathy and by increasing GJ conductance, it amplifies the effects of the injury (Slavi et al., 2018). Therefore, astrocyte GJ communication clearly has a role in progressive retinal diseases.
Microglia serve as resident immune cells in the retina, having a major role in immune reactions and inflammation. They also establish GJ independent secretory signatures to communicate with neurons and other glial cells, both in healthy and pathological states. Nerve injury and ischemia activate resident microglia in the retina clearly observed in changing their morphology and dispersion (Bosco et al., 2015; Ahmed et al., 2017; Rashid et al., 2019). Through being linked to each other with GJs, they form an ordered system of immune cells looking after retinal health (Gajardo-Gómez et al., 2016).
To summarize, glial and vascular GJ connectivity plays a crucial role in both psychological functioning and in retinal diseases. Probably, the most important GJ-protein in glial and neurovascular interactions is Cx43, which shows a high level of expression within the retina (Kerr et al., 2010; Ivanova et al., 2017, 2019). This pattern of distribution seems to be a feature conserved through evolution, with no significant inter-species differences observable (Janssen-Bienhold et al., 1998). They serve an important role in the proliferation of epithelial and other cells and enable the formation of modulatory Ca2+ waves within cell populations (Pearson et al., 2004; Pocrnich et al., 2012).
Possible Routes for Neuromodulation through GJs
There are only a few possible options to directly affect GJs. Among these are some that are readily available and some that still have not been used in medicine, or omitted due to permeability difficulties. In this next section, we will focus on the promising recent developments in GJ neuromodulation and, if there are any, highlight their limitations.
Pharmacological blockade of GJs
Shortly after their discovery, non-specific pharmacological agents became handy tools for neuroscientists in assessing the role and function of GJs within the retinal tissue. Inhibitory agents, such as different alcohols, quinine, or arachidonic acid have been used to study the function of Cx and pannexin composed intracellular channels and hemichannels, respectively (Hervé and Sarrouilhe, 2005). As an example, the pharmacological blockade of GJs rescued nearly all neighboring amacrine cells and reduced RGC loss by ~70% after induction with excitotoxic or ischemic conditions (Akopian et al., 2014). However, the potential clinical use of these agents is limited due to their non-specific nature, as they cannot differentiate in their effects between Cx hemichannels, GJs, or even other membrane-bound channels in some cases. On the other hand, these same GJ blocking agents have still been widely utilized to reveal the function of neuronal and non-neuronal GJs in experiments in different model animals (Table 2 for the list of agents with targets and off-target effects).
Table 2.
GJ blocker pharmaceutical molecules, their GJ and non-GJ targets
| GJ blocker | Selectivity for Cx Isoforms | Non-GJ target receptors | Non-GJ target channels |
|---|---|---|---|
| Anandamide | 32, 43 | CB1, GABA, glycine, 5-HT | Na+ and Ca2+ |
| Carbenoxolone | Non-selective | p2x7 | Voltage-gated Ca2+ |
| Flufenamic acid | 26, 32, 40, 43, 46, 50 | p2x7, GABA, NMDA | voltage-gated K+Cl–, K+ |
| Gap26, Gap27 | 32, 37, 40, 43 | ||
| Glycyrrhetinic acid | Non-selective | Ca2+ | |
| Heptanol | 32, 43, 45 | p2x7, kainate | Ca2+, K+ |
| Meclofenamic acid | 36, 43, 50 | GABA | Voltage-gated K+ |
| Mefloquine | 36, 43, 50 | Adenosine and p2x7 | ATP-sensitive K+ |
| Niflumic acid | 43, 46, 50 | GABA, NMDA | voltage-gated K+Cl–, Ca2+, Na+ |
| Octanol | 43, 46, 50 | GABA, glycine, AMPA, NMDA, kainate, p2x7 | T-type Ca2+ |
| Oleamide | 32, 43 | CB1, GABA, glycine, 5-HT | |
| Quinidine | 50 | Cholinergic | K+ and Na+ |
| Quinine | 36, 45, 50 | Cholinergic | Voltage-dependent K+ |
| Retinoic acid | 38 | Retinoids, dopamine, 5-HT | L and N-type Ca2+ |
5-HT: 5-Hydroxytryptamine; AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; CB1: cannabinoid receptor 1; GABA: gamma-aminobutyric acid; GJs: gap junctions; NMDA: N-methyl-D-aspartate; p2x7: purinoceptor 7 (Data from Manjarrez-Marmolejo and Franco-Pérez, 2016).
When it comes to downregulating the expression of the channel itself, one of the widely used tools is antisense-oligodeoxynucleotides (AS-ODNs). These are capable of binding to Cx-coding mRNA sequences to stop protein translation. This methodology is effective; however, one of its drawbacks is that the exerted effect is quite transient, lasting only up to 20 minutes (Qiu et al., 2003).
There is a quite wide range of conditions that can benefit from the effects of antisense-oligodeoxynucleotides therapy: the suppressive effect on inflammation has proven to be useful in the treatments of skin wounds, burns, and even spinal cord injuries (Cronin et al., 2008; Ormonde et al., 2012).
The pressure to find more specific and targeted therapeutics led to the discovery and development of the so-called mimetic peptides with channel-selective inhibitory properties. These peptides have amino acid sequences which are mimicking parts of Cx and pannexin proteins (Evans et al., 2012). In most cases, the mimetic peptides match the extracellular docking sequence of Cx molecules and upon binding, they block affected hemichannels from finding partners and forming functional channels with connexons of the neighbor cell membrane (Berthoud et al., 2000). Intracellular mimetic peptides also offer some specific advantages, although they require the presence of additional peptides to pass through the cell membrane. If their transport is successful, they are able to selectively inhibit hemichannels without disrupting the function of GJs (Wang et al., 2013). Danesh-Meyer and colleagues showed the efficacy of mimetic peptides following retinal ischaemia by reducing vascular leakage and RGC death (Danesh-Meyer et al., 2012).
Gene therapy
In the last three decades, a direct gene-level approach to cure diseases has been especially useful for pathologic processes caused by mutations or deficiencies relating to a single gene, of which there are over 200 relating to the retina (Conley and Naash, 2010). GJs have been in the center of a number of research studies and clinical trials, either as a central target or part of a treatment option (Yu et al., 2014; Zhu et al., 2015; Thuringer et al., 2016; Yuan et al., 2016).
Functional copies of the genes causing aberrant behavior can be supplemented using viral vectors, and for this, the retina has proved to be an opportune target (Mast et al., 2010; Han et al., 2015; DiCarlo et al., 2018) due to its location behind the blood-brain barrier providing an immune-privileged state. Immunosuppressive cytokines and cell surface signal molecules produced by the ocular parenchymal cells that interact with regulatory T cells, lowering the eye’s immune responsiveness and creating the aforementioned immune-privileged state (Caspi, 2010). With respect to gene transfection, the blood-brain barrier isolation is an obstacle that can be overcome by intra-arterial infusion with mannitol, which leads to the transient opening of the blood-brain barrier without causing any permanent damage (Fu et al., 2003).
One of the first successful studies on gene supplementation was completed in 1996 by Bennet et al., where they managed to delay photoreceptor degeneration by six weeks using an adenoviral (Ad) vector (Bennett et al., 1996). Ads are non-enveloped, episomal, icosahedral, 90–100 nm viruses with several serotypes (Mast et al., 2010) from which Human Adenovirus 5 (HAd5) is the most studied and popular in clinical use (Ginn et al., 2018). Even if their large genome size (30–40 kb pairs) make them an appealing candidate for gene delivery, some studies have shown the possibility of Ads interacting with blood factors resulting in severe inflammatory responses (Hoffman et al., 1997; Raper et al., 2003). In addition, depending on the population, 30–100% of the people show preexisting immunity to Ads (Mast et al., 2010; Barouch et al., 2011), leading to transient expression. Since the first-time use of Ad vectors for gene supplementation by Bennet et al. in 1996, there have been over 500 clinical trials using the same approach (Mast et al., 2010). However, due to their limitations, Ad vectors have not been extensively used in clinical ocular gene therapy (DiCarlo et al., 2018).
Adeno-associated viruses (AAVs), on the other hand, have provided the most popular gene delivery system throughout the years because of their capability to elicit long-term expression in a safe, non-pathogenic manner (Taymans et al., 2007). AAVs are non-enveloped, helper-dependent parvoviruses with an icosahedral capsid architecture approximately 25 nm in diameter (Murlidharan et al., 2014). The utilization of AAVs provides the default option for integration into host genomes or removing the rep open reading frame, allowing for episomal rather than integrative identity, have high transduction efficiency, and in certain cases cell-type specificity (Nakai et al., 2001; Jüttner et al., 2019). AAVs have been proven to provide long-term gene delivery, studies have shown their persisting effect to last up to 6 years in patients treated with an AAV2-delivered construct for the treatment of Leber congenital amaurosis (Bainbridge et al., 2015). This construct became the basis for the first FDA approved gene therapy in 2017, running under the name luxturna to cure Leber congenital amaurosis (Darrow, 2019). The major limitation in using AAVs results from the genetic payload limit of 4–5 kb pairs. Attempts have been made to overcome this either by splitting transgenes between AAV vectors or using truncated genes (minigenes) (Lai et al., 2009). Alternatively, hybrid AV-AAV vectors have been designed to overcome the size limitation (Gonçalves et al., 2004). In the case of RGC transduction, the transgene will undergo anterograde axonal transport through the optic nerve (Harvey et al., 2002). Considering gene therapy, this is a major drawback of using AAVs.
The third popular vector choice, lentiviral vectors, appears to solve the size limitations posed by AAV vectors, allowing for about 10–11 kb gene carrying capacity, which makes the delivery of large transgenes or even multiple genes possible (Coffin et al., 1997; Gonçalves et al., 2004; Counsell et al., 2017). Lentiviruses are pleomorphic, approximately 100 nm in diameter, spherical-shaped and contain two single-stranded positive-sense RNA molecules (Vogt and Simon, 1999). They integrate into the host genome using their own reverse transcriptase and integrase enzymes, making them capable of integrating into both dividing and non-dividing cells, depending on the serogroup (Coffin et al., 1997). Therefore, lentiviral vectors can permanently modify the target cell by integrating into the host genomic DNA, providing stable expression (Saenz et al., 2004). In order to limit the possibility of pathogenicity, non-primate lentiviruses, like equine infectious anemia virus (EIAV), are utilized because they have highly restricted species tropisms and cannot replicate in human cells, or are blocked intracellularly (Ikeda et al., 1996; Shimojima, 2004). The first lentivirus in human clinical trials was applied for human photoreceptor gene delivery using an EIAV (Balaggan et al., 2006; Hashimoto et al., 2007).
In the last decade, a paradigm shift can be observed from gene supplementation strategies to gene surgery, where the host genome or the transcriptome is altered directly, either transiently or permanently (DiCarlo et al., 2018). Gene silencing using endogenously produced micro RNAs (miRNA) and introducing small interfering RNAs (siRNA) or short hairpin RNAs (shRNA) has become an essential technology in studying gene function and a possible approach in gene therapy. Using AAV or Lentiviral vectors siRNAs and shRNAs can be delivered into the cells (Singer and Verma, 2008). GJ mediated transport of shRNA has been proven to be possible in vitro (Valiunas et al., 2005; Wolvetang et al., 2007). Permeability of miRNA molecules has been tested across the GJ channel families comprised of distinct connexin subtypes, showing the following order Cx43 > Cx26/30 > Cx26 > Cx31 > Cx30 (Zong et al., 2016). Since miRNA expression patterns have been associated with most, if not all human malignancies (Calin et al., 2005; Visone and Croce, 2009), the transfer of miRNA transcripts through GJs is an important factor to be considered in cancer research (Katakowski et al., 2010; Lim et al., 2011; Thuringer et al., 2016). Considering retinal diseases, several miRNA targets have been recently identified in cases of AMD and DR, which are either up- or downregulated in patients and pre-clinical models (Liu et al., 2020). Due to this discovery and the validity of the bystander effect mentioned in chapter 2.2, the downregulation of Cx molecule synthesis and its subsequent decrease in GJ numbers using lentiviral mediated RNAi knockdown has been a plausible way of treatment for several illnesses (Lee et al., 1992; Han et al., 2015). While utilizing GJs to transfer and distribute siRNA and miRNAs as a possible treatment modality is an interesting approach, thus far it has not been used not used to its full potential (Thuringer et al., 2016).
Besides the proteome regulating possibilities, direct genome editing techniques have also been made available. One such system, the CRISPR-Cas system borrowed from bacteria, has been gaining popularity in recent years (Ding et al., 2019). In the case of progressive retinal diseases like retinitis pigmentosa or retinal dystrophy, researchers have been testing the potentials of the CRISPR-Cas system-based therapies (Bakondi et al., 2016; Bassuk et al., 2016). However, no matter how powerful this technique might appear, it also poses considerable challenges, like designing guide RNAs (gRNA) that are specific enough to target the mutated gene but avoid the wild type locus (Christie et al., 2017). Delivery of the gRNA and the CAS9 enzyme gene can be achieved using AAV vectors, however, the size limitations described earlier can be troublesome (Platt et al., 2014; Swiech et al., 2015). For this purpose, smaller Cas9 orthologs, originating, for example, from the Staphylococcus aureus, are available (Ran et al., 2015). Injecting CRISPR directly into the human body for the first time has been done during a clinical trial recently, to cure Leber’s congenital amaurosis 10 (Ledford, 2020). The utilization of the CRISPR system has been proven to alter GJ expression levels (Yuan et al., 2016) but much remains to be discovered in this field in the future.
The selective targeting of retinal cell types is essential for precise genetic engineering, especially in in vivo conditions. To combat this issue, members of the Roska lab created a library of 230 AAVs with specific promoters (Jüttner et al., 2019). Recently, an alternative approach to viral vectors has been developed, using an antibody functionalized gold nanoparticles (antibody-AuNP) and ultrafast femtosecond (fs, 10–15 s) 800 nm laser irradiation, called optoporation (Conley and Naash, 2010; Ding et al., 2015). The antibody-AuNP complex selectively binds to the specific extracellular receptor and the irradiation of the AuNP causes nanoscale pore openings in the cell membrane, allowing for extracellular materials, such as siRNA to enter the cell (Conley and Naash, 2010). Due to the retina’s translucent nature, Wilson and colleagues were able to develop an in vivo optoporation system, allowing for selective treatment of RGCs and photoreceptors by optoporating siRNA molecules (Wilson et al., 2018). This method might prove useful in the future to selectively optoporate RGCs and using their GJ connections, affect the whole network of GJ connected cells.
Stimulation
Electrical stimulation has a long-standing history in both neurology and neuroscience (Borchers et al., 2012). While direct or indirect stimulation of cells through GJs is an interesting approach for using GJs as sites of neuromodulation, there are only a few studies addressing this question at any level (Pereda et al., 2013; Roy et al., 2017; Loizos et al., 2018).
The method for stimulation could be electrical, by injecting current in presynaptic cells that will evoke a voltage drop in GJ coupled cells too. This, depending on the transmembrane conductance of GJs, can be accompanied by a change of membrane potential in an extended array of cells (including 2nd, 3rd, and higher-order coupled cells), eventually leading to a multi-level activation of cells within an array. Spontaneous activity waves of cells are known to play a role during development, particularly in the developing retina and the nervous system. Similar spontaneous waves are present in the diseased retina, although their function is not well-characterized (Toychiev et al., 2013; Tsai et al., 2017). They might be pivotal in cell-survival, as GJs are crucial for correlated cellular activity (Völgyi et al., 2013a, b). Inducing such waves through GJs could be beneficial in retrieving cells, although, in retinal degeneration (rd10), GJ blockage eradicates aberrant activity (Toychiev et al., 2013; Ivanova et al., 2016). In the living retina, retinal prostheses could be used to restore vision (Mathieson et al., 2012; Ayton et al., 2014; Lorach et al., 2015), however, we do not know the long term effects of surgically inserted prosthetics, considering that they could alter the GJ connectivity. Since electric and magnetic fields excite neurons, they might affect the retinal GJ’s natural regulatory system (Weiland and Humayun, 2014; Jacoby et al., 2018).
The natural stimulus of the retina, visible light, also takes part in shaping the retinal signal processing by altering GJ connections (Hu et al., 2010; Roy et al., 2017; Zhang et al., 2020). Light induces the coupling and decoupling of GCs (Hu et al., 2010) in amacrine cells, and nitric-oxide release (Jacoby et al., 2018). The circadian response also involves GJ reorganization (Katti et al., 2013; Koo et al., 2015; Ali et al., 2019).
In summary, electrical and light stimulation both actively affect GJ coupling and spontaneous activity, therefore presenting optional routes for neuromodulation in the retina.
Conclusions
Even though there are a number of studies showing multiple links between GJ function and cell survival, the present state of our knowledge regarding the role of GJs in cellular survival in the retina is still insufficient. There are many hereditary diseases associated with Cx mutations that affect GJs and neuronal networks (see review: Srinivas et al., 2018), where it is clearly apparent that GJs are the main origin of the disease. A similar involvement of GJs in non-hereditary diseases is still unexplored and needs to be clarified in order to cure retinal and possibly non-retinal diseases, or at least slow down their progression. The possibility of new treatment modalities focusing on GJs, perhaps through regulating the Cx expression or GJ connectivity, must be designed in the future to rescue otherwise healthy cells from entering the fatal apoptosis process, caused by the spread of death-signals through GJs. At the same time, it appears reasonable to utilize GJ-connected pathways to spread neuroprotective factors, even though this option has so far been neglected, mostly due to the recently overturned size limit.
As an easily accessible part of the CNS that retains a large amount of structural and functional similarity, the retina provides a great model system to reveal yet undiscovered mechanisms of neuronal signaling, as well as of potential treatments for degenerative diseases affecting the nervous tissue. One substantial drawback of using the retina as a model is its limited regenerative capability. On the other hand, GJ-specific pharmacological agents, as well as GJ-related gene therapy and stimulation, could be successfully utilized to limit malicious processes in retinal diseases. These results can later be generalized to restrict the size of affected tissue in other regions of the brain as well. As discussed throughout this article, GJs play a crucial role in a number of pathological conditions, and they are as abundant and diverse in the retina as in any other part of the brain. Although GJ connections differ in their Cx makeup and the cellular elements they connect, they share the potential to utilize this variability to enhance cellular survival, to limit degenerative mechanisms, and perhaps to promote regeneration in the nervous tissue under stress. Certainly, new treatment modalities, described previously, that involve GJs of the retina can easily be adapted to treat diseases affecting other parts of the CNS.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was supported by the Hungarian Brain Research Program 2 (2017-1.2.1.-NKP-2017) (to BV), by the NKFI (OTKA NN128293) from the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TAMOP-4.2.4.A/2-11/1-2012-0001 National Excellence Program (to BV) and by the ÚNKP-20-3-I-PTE-472 New National Excellence Program of the Ministry for Innovation and Technology (to GS).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Marietta Zille, University of Lubeck, Germany.
Funding: This work was supported by the Hungarian Brain Research Program 2 (2017-1.2.1.-NKP-2017) (to BV), by the NKFI (OTKA NN128293) from the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TAMOP-4.2.4.A/2-11/1-2012-0001 National Excellence Program (to BV) and by the ÚNKP-20-3-I-PTE-472 New National Excellence Program of the Ministry for Innovation and Technology (to GS).
R-Reviewer: Zille M; C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
References
- 1.Ahmed A, Wang LL, Abdelmaksoud S, Aboelgheit A, Saeed S, Zhang CL. Minocycline modulates microglia polarization in ischemia-reperfusion model of retinal degeneration and induces neuroprotection. Sci Rep. 2017;7:14065. doi: 10.1038/s41598-017-14450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akopian A, Atlasz T, Pan F, Wong S, Zhang Y, Völgyi B, Paul DL, Bloomfield SA. Gap junction-mediated death of retinal neurons is connexin and insult specific: a potential target for neuroprotection. J Neurosci. 2014;34:10582–10591. doi: 10.1523/JNEUROSCI.1912-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akopian A, Kumar S, Ramakrishnan H, Roy K, Viswanathan S, Bloomfield SA. Targeting neuronal gap junctions in mouse retina offers neuroprotection in glaucoma. J Clin Invest. 2017;127:2647–2661. doi: 10.1172/JCI91948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali AAH, Stahr A, Ingenwerth M, Theis M, Steinhäuser C, von Gall C. Connexin30 and Connexin43 show a time-of-day dependent expression in the mouse suprachiasmatic nucleus and modulate rhythmic locomotor activity in the context of chronodisruption. Cell Commun Signal. 2019;17:61. doi: 10.1186/s12964-019-0370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong RA. Visual problems associated with traumatic brain injury. Clin Exp Optom. 2018;101:716–726. doi: 10.1111/cxo.12670. [DOI] [PubMed] [Google Scholar]
- 6.Ayton LN, Blamey PJ, Guymer RH, Luu CD, Nayagam DA, Sinclair NC, Shivdasani MN, Yeoh J, McCombe MF, Briggs RJ, Opie NL, Villalobos J, Dimitrov PN, Varsamidis M, Petoe MA, McCarthy CD, Walker JG, Barnes N, Burkitt AN, Williams CE, et al. First-in-human trial of a novel suprachoroidal retinal prosthesis. PLoS One. 2014;9:e115239. doi: 10.1371/journal.pone.0115239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, Georgiadis A, Mowat FM, Beattie SG, Gardner PJ, Feathers KL, Luong VA, Yzer S, Balaggan K, Viswanathan A, de Ravel TJ, Casteels I, Holder GE, Tyler N, Fitzke FW, et al. Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med. 2015;372:1887–1897. doi: 10.1056/NEJMoa1414221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakondi B, Lv W, Lu B, Jones MK, Tsai Y, Kim KJ, Levy R, Akhtar AA, Breunig JJ, Svendsen CN, Wang S. In vivo CRISPR/Cas9 gene editing corrects retinal dystrophy in the S334ter-3 rat model of autosomal dominant retinitis pigmentosa. Mol Ther. 2016;24:556–563. doi: 10.1038/mt.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balaggan KS, Binley K, Esapa M, Iqball S, Askham Z, Kan O, Tschernutter M, Bainbridge JWB, Naylor S, Ali RR. Stable and efficient intraocular gene transfer using pseudotyped EIAV lentiviral vectors. J Gene Med. 2006;8:275–285. doi: 10.1002/jgm.845. [DOI] [PubMed] [Google Scholar]
- 10.Ball AK, McReynolds JS. Localization of gap junctions and tracer coupling in retinal Müller cells. J Comp Neurol. 1998;393:48–57. doi: 10.1002/(sici)1096-9861(19980330)393:1<48::aid-cne5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee S, Wang Q, Zhao F, Tang G, So C, Tse D, To CH, Feng Y, Zhou X, Pan F. Increased Connexin36 phosphorylation in AII amacrine cell coupling of the mouse myopic retina. Front Cell Neurosci. 2020;14:124. doi: 10.3389/fncel.2020.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, Clark S, Ng’ang’a D, Brandariz KL, Abbink P, Sinangil F, de Bruyn G, Gray GE, Roux S, Bekker LG, Dilraj A, Kibuuka H, Robb ML, Michael NL, Anzala O, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–5209. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassuk AG, Zheng A, Li Y, Tsang SH, Mahajan VB. Precision medicine: genetic repair of retinitis pigmentosa in patient-derived stem cells. Sci Rep. 2016;6:19969. doi: 10.1038/srep19969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belousov AB, Fontes JD, Freitas-Andrade M, Naus CC. Gap junctions and hemichannels: communicating cell death in neurodevelopment and disease. BMC Cell Biol. 2017;18:4. doi: 10.1186/s12860-016-0120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett J, Tanabe T, Sun D, Zeng Y, Kjeldbye H, Gouras P, Maguire AM. Photoreceptor cell rescue in retinal degeneration (rd) mice by in vivo gene therapy. Nat Med. 1996;2:649–654. doi: 10.1038/nm0696-649. [DOI] [PubMed] [Google Scholar]
- 16.Berthoud VM, Tadros PN, Beyer EC. Connexin and gap junction degradation. Methods. 2000;20:180–187. doi: 10.1006/meth.1999.0935. [DOI] [PubMed] [Google Scholar]
- 17.Bloomfield SA, Völgyi B. Function and plasticity of homologous coupling between AII amacrine cells. Vision Res. 2004;44:3297–3306. doi: 10.1016/j.visres.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Borchers S, Himmelbach M, Logothetis N, Karnath HO. Direct electrical stimulation of human cortex — the gold standard for mapping brain functions. Nat Rev Neurosci. 2012;13:63–70. doi: 10.1038/nrn3140. [DOI] [PubMed] [Google Scholar]
- 19.Bosco A, Romero CO, Ambati BK, Vetter ML. In vivo dynamics of retinal microglial activation during neurodegeneration: confocal ophthalmoscopic imaging and cell morphometry in mouse glaucoma. J Vis Exp. 2015:e52731. doi: 10.3791/52731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke EG, Cansler SM, Evanson NK. Indirect traumatic optic neuropathy: modeling optic nerve injury in the context of closed head trauma. Neural Regen Res. 2019;14:593–594. doi: 10.4103/1673-5374.247463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, Iuliano R, Palumbo T, Pichiorri F, Roldo C, Garzon R, Sevignani C, Rassenti L, Alder H, Volinia S, Liu CG, et al. A microRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 23.Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120:3073–3083. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caval-Holme F, Zhang Y, Feller MB. Gap junction coupling shapes the encoding of light in the developing retina. Curr Biol. 2019;29:4024–4035. doi: 10.1016/j.cub.2019.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceelen PW, Lockridge A, Newman EA. Electrical coupling between glial cells in the rat retina. Glia. 2001;35:1–13. doi: 10.1002/glia.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen B, Sun L, Wu X, Ma J. Correlation between connexin and traumatic brain injury in patients. Brain Behav. 2017;7:e00770. doi: 10.1002/brb3.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YJ, Liang CM, Tai MC, Chang YH, Lin TY, Chung CH, Lin FH, Tsao CH, Chien WC. Longitudinal relationship between traumatic brain injury and the risk of incident optic neuropathy: a 10-year follow-up nationally representative Taiwan survey. Oncotarget. 2017;8:86924–86933. doi: 10.18632/oncotarget.21008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christie KA, Courtney DG, DeDionisio LA, Shern CC, De Majumdar S, Mairs LC, Nesbit MA, Moore CBT. Towards personalised allele-specific CRISPR gene editing to treat autosomal dominant disorders. Sci Rep. 2017;7:16174. doi: 10.1038/s41598-017-16279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciolofan C, Lynn BD, Wellershaus K, Willecke K, Nagy JI. Spatial relationships of connexin36 connexin57 and zonula occludens-1 in the outer plexiform layer of mouse retina. Neuroscience. 2007;148:473–488. doi: 10.1016/j.neuroscience.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Coffin JM, Hughes SH, Varmus HE. Retroviruses. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- 31.Conley SM, Naash MI. Nanoparticles for retinal gene therapy. Prog Retin Eye Res. 2010;29:376–397. doi: 10.1016/j.preteyeres.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Counsell JR, Asgarian Z, Meng J, Ferrer V, Vink CA, Howe SJ, Waddington SN, Thrasher AJ, Muntoni F, Morgan JE, Danos O. Lentiviral vectors can be used for full-length dystrophin gene therapy. Sci Rep. 2017;7:44775. doi: 10.1038/srep44775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cronin M, Anderson PN, Cook JE, Green CR, Becker DL. Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Mol Cell Neurosci. 2008;39:152–160. doi: 10.1016/j.mcn.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Danesh-Meyer HV, Kerr NM, Zhang J, Eady EK, O’Carroll SJ, Nicholson LF, Johnson CS, Green CR. Connexin43 mimetic peptide reduces vascular leak and retinal ganglion cell death following retinal ischaemia. Brain. 2012;135:506–520. doi: 10.1093/brain/awr338. [DOI] [PubMed] [Google Scholar]
- 35.Danesh-Meyer HV, Zhang J, Acosta ML, Rupenthal ID, Green CR. Connexin43 in retinal injury and disease. Prog Retin Eye Res. 2016;51:41–68. doi: 10.1016/j.preteyeres.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Darrow JJ. Luxturna: FDA documents reveal the value of a costly gene therapy. Drug Discov Today. 2019;24:949–954. doi: 10.1016/j.drudis.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Decrock E, Vinken M, De Vuyst E, Krysko DV, D’Herde K, Vanhaecke T, Vandenabeele P, Rogiers V, Leybaert L. Connexin-related signaling in cell death: to live or let die. Cell Death Differ. 2009;16:524–536. doi: 10.1038/cdd.2008.196. [DOI] [PubMed] [Google Scholar]
- 39.Dedek K, Pandarinath C, Alam NM, Wellershaus K, Schubert T, Willecke K, Prusky GT, Weiler R, Nirenberg S. Ganglion cell adaptability: does the coupling of horizontal cells play a role. PLoS One. 2008;3:e1714. doi: 10.1371/journal.pone.0001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dedek K, Schultz K, Pieper M, Dirks P, Maxeiner S, Willecke K, Weiler R, Janssen-Bienhold U. Localization of heterotypic gap junctions composed of connexin45 and connexin36 in the rod pathway of the mouse retina. Eur J Neurosci. 2006;24:1675–1686. doi: 10.1111/j.1460-9568.2006.05052.x. [DOI] [PubMed] [Google Scholar]
- 41.Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, Bennett MV, Spray DC. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Brain Res Rev. 2000;32:45–56. doi: 10.1016/s0165-0173(99)00067-3. [DOI] [PubMed] [Google Scholar]
- 42.DiCarlo JE, Mahajan VB, Tsang SH. Gene therapy and genome surgery in the retina. J Clin Invest. 2018;128:2177–2188. doi: 10.1172/JCI120429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding W, Bergeron E, Lachaine R, Meunier M. Nanomaterial-assisted light-induced poration and transfection of mammalian cells. In: Hamblin MR, Avci P, editors. Applications of Nanoscience in Photomedicine. Oxford: Chandos Publishing, Elsevier; 2015. pp. 331–376. [Google Scholar]
- 44.Ding X, Seebeck T, Feng Y, Jiang Y, Davis GD, Chen F. Improving CRISPR-Cas9 genome editing efficiency by fusion with chromatin-modulating peptides. CRISPR J. 2019;2:51–63. doi: 10.1089/crispr.2018.0036. [DOI] [PubMed] [Google Scholar]
- 45.Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding mechanisms, and treatment strategies. JCI Insight. 2017;2:e93751. doi: 10.1172/jci.insight.93751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans LP, Newell EA, Mahajan M, Tsang SH, Ferguson PJ, Mahoney J, Hue CD, Vogel EW, 3rd, Morrison B, 3rd, Arancio O, Nichols R, Bassuk AG, Mahajan VB. Acute vitreoretinal trauma and inflammation after traumatic brain injury in mice. Ann Clin Transl Neurol. 2018;5:240–251. doi: 10.1002/acn3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans WH, Bultynck G, Leybaert L. Manipulating connexin communication channels: use of peptidomimetics and the translational outputs. J Membr Biol. 2012;245:437–449. doi: 10.1007/s00232-012-9488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandes R, Girão H, Pereira P. High glucose down-regulates intercellular communication in retinal endothelial cells by enhancing degradation of connexin 43 by a proteasome-dependent mechanism. J Biol Chem. 2004;279:27219–27224. doi: 10.1074/jbc.M400446200. [DOI] [PubMed] [Google Scholar]
- 50.Foo LC, Allen NJ, Bushong EA, Ventura PB, Chung WS, Zhou L, Cahoy JD, Daneman R, Zong H, Ellisman MH, Barres BA. Development of a method for the purification and culture of rodent astrocytes. Neuron. 2011;71:799–811. doi: 10.1016/j.neuron.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fróes MM, Correia AH, Garcia-Abreu J, Spray DC, Campos de Carvalho AC, Neto MV. Gap-junctional coupling between neurons and astrocytes in primary central nervous system cultures. Proc Natl Acad Sci U S A. 1999;96:7541–7546. doi: 10.1073/pnas.96.13.7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu H, Muenzer J, Samulski RJ, Breese G, Sifford J, Zeng X, McCarty DM. Self-complementary adeno-associated virus serotype 2 vector: global distribution and broad dispersion of AAV-mediated transgene expression in mouse brain. Mol Ther. 2003;8:911–917. doi: 10.1016/j.ymthe.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 53.Gajardo-Gómez R, Labra VC, Orellana JA. Connexins and pannexins: new insights into microglial functions and dysfunctions. Front Mol Neurosci. 2016;9:86. doi: 10.3389/fnmol.2016.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene therapy clinical trials worldwide to 2017: An update. J Gene Med. 2018;20:e3015. doi: 10.1002/jgm.3015. [DOI] [PubMed] [Google Scholar]
- 55.Goldberg GS, Moreno AP, Lampe PD. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J Biol Chem. 2002;277:36725–36730. doi: 10.1074/jbc.M109797200. [DOI] [PubMed] [Google Scholar]
- 56.Gonçalves MAFV, van der Velde I, Knaän-Shanzer S, Valerio D, de Vries AAF. Stable transduction of large DNA by high-capacity adeno-associated virus/adenovirus hybrid vectors. Virology. 2004;321:287–296. doi: 10.1016/j.virol.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 57.Grimes WN, Aytürk DG, Hoom M, Yoshimatsu T, Gamlin C, Carrera D, Ahlquist RM, Sabnis A, Diamond JS, Wong RO, Cepko C, Rieke F. A high-density narrow-field inhibitory retinal interneuron with direct coupling to Müller glia. bioRxiv [Preprint] January 24, 2020 [accessed 2020 November] Available from: https://doiorg/101101/20200123917096 . [DOI] [PMC free article] [PubMed]
- 58.Guo CX, Mat Nor MN, Danesh-Meyer HV, Vessey KA, Fletcher EL, O’Carroll SJ, Acosta ML, Green , CR Connexin43 mimetic peptide improves retinal function and reduces inflammation in a light-damaged albino rat model. Invest Ophthalmol Vis Sci. 2016;57:3961–3973. doi: 10.1167/iovs.15-16643. [DOI] [PubMed] [Google Scholar]
- 59.Güldenagel M, Ammermüller J, Feigenspan A, Teubner B, Degen J, Söhl G, Willecke K, Weiler R. Visual transmission deficits in mice with targeted disruption of the gap junction gene connexin36. J Neurosci. 2001;21:6036–6044. doi: 10.1523/JNEUROSCI.21-16-06036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han XJ, Chen M, Hong T, Zhu LY, He D, Feng JG, Jiang LP. Lentivirus-mediated RNAi knockdown of the gap junction protein Cx43, attenuates the development of vascular restenosis following balloon injury. Int J Mol Med. 2015;35:885–892. doi: 10.3892/ijmm.2015.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harper MM, Hedberg-Buenz A, Herlein J, Abrahamson EE, Anderson MG, Kuehn MH, Kardon RH, Poolman P, Ikonomovic MD (2019) Blast-mediated traumatic brain injury exacerbates retinal damage and amyloidosis in the APPswePSENd19e mouse model of Alzheimer’s disease. Invest Ophthalmol Vis Sci. 60:2716–2725. doi: 10.1167/iovs.18-26353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harvey AR, Kamphuis W, Eggers R, Symons NA, Blits B, Niclou S, Boer GJ, Verhaagen J. Intravitreal injection of adeno-associated viral vectors results in the transduction of different types of retinal neurons in neonatal and adult rats: a comparison with lentiviral vectors. Mol Cell Neurosci. 2002;21:141–157. doi: 10.1006/mcne.2002.1168. [DOI] [PubMed] [Google Scholar]
- 63.Hashimoto T, Gibbs D, Lillo C, Azarian SM, Legacki E, Zhang XM, Yang XJ, Williams DS. Lentiviral gene replacement therapy of retinas in a mouse model for Usher syndrome type 1B. Gene Ther. 2007;14:584–594. doi: 10.1038/sj.gt.3302897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hervé JC, Sarrouilhe D. Connexin-made channels as pharmacological targets. Curr Pharm Des. 2005;11:1941–1958. doi: 10.2174/1381612054021060. [DOI] [PubMed] [Google Scholar]
- 65.Hoffman LM, Maguire AM, Bennett J. Cell-mediated immune response and stability of intraocular transgene expression after adenovirus-mediated delivery. Invest Ophthalmol Vis Sci. 1997;38:2224–2233. [PubMed] [Google Scholar]
- 66.Hombach S, Janssen-Bienhold U, Söhl G, Schubert T, Büssow H, Ott T, Weiler R, Willecke K. Functional expression of connexin57 in horizontal cells of the mouse retina. Eur J Neurosci. 2004;19:2633–2640. doi: 10.1111/j.0953-816X.2004.03360.x. [DOI] [PubMed] [Google Scholar]
- 67.Hu EH, Pan F, Völgyi B, Bloomfield SA. Light increases the gap junctional coupling of retinal ganglion cells: Light increases coupling between retinal ganglion cells. J Physiol. 2010;588:4145–4163. doi: 10.1113/jphysiol.2010.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ikeda Y, Tomonaga K, Kawaguchi Y, Kohmoto M, Inoshima Y, Tohya Y, Miyazawa T, Kai C, Mikami T. Feline immunodeficiency virus can infect a human cell line (MOLT-4) but establishes a state of latency in the cells. J Gen Virol. 1996;77:1623–1630. doi: 10.1099/0022-1317-77-8-1623. [DOI] [PubMed] [Google Scholar]
- 69.Ishii M, Rohrer B. Bystander effects elicited by single-cell photo-oxidative blue-light stimulation in retinal pigment epithelium cell networks. Cell Death Discov. 2017;3:16071. doi: 10.1038/cddiscovery.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ivanova E, Kovács-Öller T, Sagdullaev BT. vascular pericyte impairment and Connexin43 gap junction deficit contribute to vasomotor decline in diabetic retinopathy. J Neurosci. 2017;37:7580–7594. doi: 10.1523/JNEUROSCI.0187-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ivanova E, Kovács-Öller T, Sagdullaev BT. Domain-specific distribution of gap junctions defines cellular coupling to establish a vascular relay in the retina. J Comp Neurol. 2019;527:2675–2693. doi: 10.1002/cne.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ivanova E, Yee CW, Baldoni R, Sagdullaev BT. Aberrant activity in retinal degeneration impairs central visual processing and relies on Cx36-containing gap junctions. Exp Eye Res. 2016;150:81–89. doi: 10.1016/j.exer.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacoby J, Nath A, Jessen ZF, Schwartz GW. A self-regulating gap junction network of amacrine cells controls nitric oxide release in the retina. Neuron. 2018;100:1149–1162. doi: 10.1016/j.neuron.2018.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Janssen-Bienhold U, Dermietzel R, Weiler R. Distribution of connexin43 immunoreactivity in the retinas of different vertebrates. J Comp Neurol. 1998;396:310–321. [PubMed] [Google Scholar]
- 75.Jüttner J, Szabo A, Gross-Scherf B, Morikawa RK, Rompani SB, Hantz P, Szikra T, Esposti F, Cowan CS, Bharioke A, Patino-Alvarez CP, Keles Ö, Kusnyerik A, Azoulay T, Hartl D, Krebs AR, Schübeler D, Hajdu RI, Lukats A, Nemeth J, et al. Targeting neuronal and glial cell types with synthetic promoter AAVs in mice, non-human primates and humans. Nat Neurosci. 2019;22:1345–1356. doi: 10.1038/s41593-019-0431-2. [DOI] [PubMed] [Google Scholar]
- 76.Kántor O, Szarka G, Benkő Z, Somogyvári Z, Pálfi E, Baksa G, Rácz G, Nitschke R, Debertin G, Völgyi B. Strategic positioning of Connexin36 Gap junctions across human retinal ganglion cell dendritic arbors. Front Cell Neurosci. 2018;12:409. doi: 10.3389/fncel.2018.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kántor O, Varga A, Nitschke R, Naumann A, Énzsöly A, Lukáts Á, Szabó A, Németh J, Völgyi B. Bipolar cell gap junctions serve major signaling pathways in the human retina. Brain Struct Funct. 2017;222:2603–2624. doi: 10.1007/s00429-016-1360-4. [DOI] [PubMed] [Google Scholar]
- 78.Katakowski M, Buller B, Wang X, Rogers T, Chopp M. Functional microRNA is transferred between glioma cells. Cancer Res. 2010;70:8259–8263. doi: 10.1158/0008-5472.CAN-10-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katti C, Butler R, Sekaran S. Diurnal and circadian regulation of Connexin 36 transcript and protein in the mammalian retina. Invest Ophthalmol Vis Sci. 2013;54:821. doi: 10.1167/iovs.12-10375. [DOI] [PubMed] [Google Scholar]
- 80.Kerr NM, Johnson CS, de Souza CF, Chee KS, Good WR, Green CR, Danesh-Meyer HV. Immunolocalization of gap junction protein connexin43 (GJA1) in the human retina and optic nerve. Invest Ophthalmol Vis Sci. 2010;51:4028–4034. doi: 10.1167/iovs.09-4847. [DOI] [PubMed] [Google Scholar]
- 81.Kerr NM, Johnson CS, Green CR, Danesh-Meyer HV. Gap junction protein connexin43 (GJA1) in the human glaucomatous optic nerve head and retina. J Clin Neurosci. 2011;18:102–108. doi: 10.1016/j.jocn.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 82.Khandhadia S, Cipriani V, Yates JR, Lotery AJ. Age-related macular degeneration and the complement system. Immunobiology. 2012;217:127–146. doi: 10.1016/j.imbio.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 83.Koo J, Choe HK, Kim HD, Chun SK, Son GH, Kim K. Effect of mefloquine a Gap junction blocker, on circadian Period2 gene oscillation in the mouse suprachiasmatic nucleus ex vivo. Endocrinol Metab. 2015;30:361. doi: 10.3803/EnM.2015.30.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kovács-Öller T, Ivanova E, Bianchimano P, Sagdullaev BT. The pericyte connectome: spatial precision of neurovascular coupling is driven by selective connectivity maps of pericytes and endothelial cells and is disrupted in diabetes. Cell Discov. 2020;6:39. doi: 10.1038/s41421-020-0180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, Judge L, Bostick B, Chamberlain JS, Terjung RL, Duan D. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest. 2009;119:624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ledford H. CRISPR treatment inserted directly into the body for first time. Nature. 2020;579:185–185. doi: 10.1038/d41586-020-00655-8. [DOI] [PubMed] [Google Scholar]
- 87.Lee SW, Tomasetto C, Paul D, Keyomarsi K, Sager R. Transcriptional downregulation of gap-junction proteins blocks junctional communication in human mammary tumor cell lines. J Cell Biol. 1992;118:1213–1221. doi: 10.1083/jcb.118.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li AF, Roy S. High glucose-induced downregulation of connexin 43 expression promotes apoptosis in microvascular endothelial cells. Invest Ophthalmol Vis Sci. 2009;50:1400–1407. doi: 10.1167/iovs.07-1519. [DOI] [PubMed] [Google Scholar]
- 89.Li AF, Sato T, Haimovici R, Okamoto T, Roy S. High glucose alters connexin 43 expression and gap junction intercellular communication activity in retinal pericytes. Invest Ophthalmol Vis Sci. 2003;44:5376–5382. doi: 10.1167/iovs.03-0360. [DOI] [PubMed] [Google Scholar]
- 90.Li X, Kamasawa N, Ciolofan C, Olson CO, Lu S, Davidson KGV, Yasumura T, Shigemoto R, Rash JE, Nagy JI. Connexin45-containing neuronal gap junctions in rodent retina also contain connexin36 in both apposing hemiplaques forming bihomotypic gap junctions, with scaffolding contributed by zonula occludens-1. J Neurosci. 2008;28:9769–9789. doi: 10.1523/JNEUROSCI.2137-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, Greco SJ, Bryan M, Patel PS, Rameshwar P. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71:1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 92.Liu CH, Huang S, Britton WR, Chen J. MicroRNAs in vascular eye diseases. Int J Mol Sci. 2020;21:649. doi: 10.3390/ijms21020649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Loizos K, Marc R, Humayun M, Anderson JR, Jones BW, Lazzi G. Increasing electrical stimulation efficacy in degenerated retina: stimulus waveform design in a multiscale computational model. IEEE Trans Neural Syst Rehabil Eng. 2018;26:1111–1120. doi: 10.1109/TNSRE.2018.2832055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lorach H, Goetz G, Smith R, Lei X, Mandel Y, Kamins T, Mathieson K, Huie P, Harris J, Sher A, Palanker D. Photovoltaic restoration of sight with high visual acuity. Nat Med. 2015;21:476–482. doi: 10.1038/nm.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma Y, Han X, de Castro RB, Zhang P, Zhang K, Hu Z, Qin L. Analysis of the bystander effect in cone photoreceptors via a guided neural network platform. Sci Adv. 2018;4:eaas9274. doi: 10.1126/sciadv.aas9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Manasson J, Tien T, Moore C, Kumar NM, Roy S. High glucose-induced downregulation of connexin 30. 2 promotes retinal vascular lesions: implications for diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54:2361–2366. doi: 10.1167/iovs.12-10815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manjarrez-Marmolejo J, Franco-Pérez J. Gap junction blockers: an overview of their effects on induced seizures in animal models. Curr Neuropharmacol. 2016;14:759–771. doi: 10.2174/1570159X14666160603115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Massey SC, O’Brien JJ, Trexler EB, Li W, Keung JW, Mills SL, O’Brien J. Multiple neuronal connexins in the mammalian retina. Cell Commun Adhes. 2003;10:425–430. doi: 10.1080/cac.10.4-6.425.430. [DOI] [PubMed] [Google Scholar]
- 99.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, Mbewe B, Pitisuttithum P, Schechter M, Vardas E, Wolfe ND, Aste-Amezaga M, Casimiro DR, Coplan P, Straus WL, Shiver JW. International epidemiology of human pre-existing adenovirus (Ad) type-5 type-6, type-26 and type-36 neutralizing antibodies: Correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28:950–957. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 100.Mathieson K, Loudin J, Goetz G, Huie P, Wang L, Kamins TI, Galambos L, Smith R, Harris JS, Sher A, Palanker D. Photovoltaic retinal prosthesis with high pixel density. Nature Photon. 2012;6:391–397. doi: 10.1038/nphoton.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maxeiner S, Dedek K, Janssen-Bienhold U, Ammermüller J, Brune H, Kirsch T, Pieper M, Degen J, Krüger O, Willecke K, Weiler R. Deletion of connexin45 in mouse retinal neurons disrupts the rod/cone signaling pathway between AII amacrine and ON cone bipolar cells and leads to impaired visual transmission. J Neurosci. 2005;25:566–576. doi: 10.1523/JNEUROSCI.3232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mohan K, Kecova H, Hernandez-Merino E, Kardon RH, Harper MM. Retinal ganglion cell damage in an experimental rodent model of blast-mediated traumatic brain injury. Invest Ophthalmol Vis Sci. 2013;54:3440–3450. doi: 10.1167/iovs.12-11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Muir ER, Rentería RC, Duong TQ. Reduced ocular blood flow as an early indicator of diabetic retinopathy in a mouse model of diabetes. Invest Ophthalmol Vis Sci. 2012;53:6488–6494. doi: 10.1167/iovs.12-9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murlidharan G, Samulski RJ, Asokan A. Biology of adeno-associated viral vectors in the central nervous system. Front Mol Neurosci. 2014;7:76. doi: 10.3389/fnmol.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muto T, Tien T, Kim D, Sarthy VP, Roy S. High glucose alters Cx43 expression and gap junction intercellular communication in retinal Müller cells: promotes Müller cell and pericyte apoptosis. Invest Ophthalmol Vis Sci. 2014;55:4327–4337. doi: 10.1167/iovs.14-14606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Müller LP, Dedek K, Janssen-Bienhold U, Meyer A, Kreuzberg MM, Lorenz S, Willecke K, Weiler R. Expression and modulation of connexin 30. 2, a novel gap junction protein in the mouse retina. Vis Neurosci. 2010;27:91–101. doi: 10.1017/S0952523810000131. [DOI] [PubMed] [Google Scholar]
- 107.Nakai H, Yant SR, Storm TA, Fuess S, Meuse L, Kay MA. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol. 2001;75:6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, Neefjes J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 109.Newman EA, Frambach DA, Odette LL. Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science 1984. 1984;225:1174–1175. doi: 10.1126/science.6474173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.O’Brien J, Bloomfield SA. Plasticity of retinal Gap junctions: roles in synaptic physiology and disease. Annu Rev Vis Sci. 2018;4:79–100. doi: 10.1146/annurev-vision-091517-034133. [DOI] [PubMed] [Google Scholar]
- 111.O’Carroll SJ, Alkadhi M, Nicholson LF, Green CR. Connexin 43 mimetic peptides reduce swelling astrogliosis, and neuronal cell death after spinal cord injury. Cell Commun Adhes. 2008;15:27–42. doi: 10.1080/15419060802014164. [DOI] [PubMed] [Google Scholar]
- 112.Oku H, Kodama T, Sakagami K, Puro DG. Diabetes-induced disruption of gap junction pathways within the retinal microvasculature. Invest Ophthalmol Vis Sci. 2001;42:1915–1920. [PubMed] [Google Scholar]
- 113.Orellana JA, Sáez PJ, Shoji KF, Schalper KA, Palacios-Prado N, Velarde V, Giaume C, Bennett MV, Sáez JC. Modulation of brain hemichannels and gap junction channels by pro-inflammatory agents and their possible role in neurodegeneration. Antioxid Redox Signal. 2009;11:369–399. doi: 10.1089/ars.2008.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ormonde S, Chou CY, Goold L, Petsoglou C, Al-Taie R, Sherwin T, McGhee CN, Green CR. Regulation of connexin43 gap junction protein triggers vascular recovery and healing in human ocular persistent epithelial defect wounds. J Membr Biol. 2012;245:381–388. doi: 10.1007/s00232-012-9460-4. [DOI] [PubMed] [Google Scholar]
- 115.Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 116.Pearson RA, Catsicas M, Becker DL, Bayley P, Lüneborg NL, Mobbs P. Ca(2+) signalling and gap junction coupling within and between pigment epithelium and neural retina in the developing chick. Eur J Neurosci. 2004;19:2435–2445. doi: 10.1111/j.0953-816X.2004.03338.x. [DOI] [PubMed] [Google Scholar]
- 117.Pereda AE, Curti S, Hoge G, Cachope R, Flores CE, Rash JE. Gap junction-mediated electrical transmission: Regulatory mechanisms and plasticity. Biochim Biophys Acta. 2013;1828:134–146. doi: 10.1016/j.bbamem.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, Jovanovic M, Graham DB, Jhunjhunwala S, Heidenreich M, Xavier RJ, Langer R, Anderson DG, Hacohen N, Regev A, Feng G, Sharp PA, et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell. 2014;159:440–455. doi: 10.1016/j.cell.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pocrnich CE, Shao Q, Liu H, Feng MM, Harasym S, Savage M, Khimdas S, Laird DW, Hutnik CM. The effect of connexin43 on the level of vascular endothelial growth factor in human retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2012;250:515–522. doi: 10.1007/s00417-011-1871-x. [DOI] [PubMed] [Google Scholar]
- 120.Prochnow N. Relevance of gap junctions and large pore channels in traumatic brain injury. Front Physiol. 2014;5:31. doi: 10.3389/fphys.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Puller C, Duda S, Lotfi E, Arzhangnia Y, Block CT, Ahlers MT, Greschner M. Electrical coupling of heterotypic ganglion cells in the mammalian retina. J Neurosci. 2020;40:1302–1310. doi: 10.1523/JNEUROSCI.1374-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qiu C, Coutinho P, Frank S, Franke S, Law LY, Martin P, Green CR, Becker DL. Targeting connexin43 expression accelerates the rate of wound repair. Curr Biol. 2003;13:1697–1703. doi: 10.1016/j.cub.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 123.Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS, Koonin EV, Sharp PA, Zhang F. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–191. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao G, Wilson JM, Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 125.Rash JE, Pereda A, Kamasawa N, Furman CS, Yasumura T, Davidson KGV, Dudek FE, Olson C, Li X, Nagy JI. High-resolution proteomic mapping in the vertebrate central nervous system: close proximity of connexin35 to NMDA glutamate receptor clusters and co-localization of connexin36 with immunoreactivity for zonula occludens protein-1 (ZO-1) J Neurocytol. 2004;33:131–151. doi: 10.1023/B:NEUR.0000029653.34094.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rashid K, Akhtar-Schaefer I, Langmann T. Microglia in retinal degeneration. Front Immunol. 2019;10:1975. doi: 10.3389/fimmu.2019.01975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reichenbach A, Robinson SR. Phylogenetic constraints on retinal organisation and development. Prog Retin Eye Res. 1995;15:139–171. [Google Scholar]
- 128.Ripps H. Cell death in retinitis pigmentosa: gap junctions and the ‘bystander’ effect. Exp Eye Res. 2002;74:327–336. doi: 10.1006/exer.2002.1155. [DOI] [PubMed] [Google Scholar]
- 129.Robinson SR, Hampson EC, Munro MN, Vaney DI. Unidirectional coupling of gap junctions between neuroglia. Science. 1993;262:1072–1074. doi: 10.1126/science.8093125. [DOI] [PubMed] [Google Scholar]
- 130.Roy K, Kumar S, Bloomfield SA. Gap junctional coupling between retinal amacrine and ganglion cells underlies coherent activity integral to global object perception. Proc Natl Acad Sci U S A. 2017;114:E10484–10493. doi: 10.1073/pnas.1708261114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roy S, Jiang JX, Li AF, Kim D. Connexin channel and its role in diabetic retinopathy. Prog Retin Eye Res. 2017b;61:35–59. doi: 10.1016/j.preteyeres.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roy S, Kim D, Lim R. Cell-cell communication in diabetic retinopathy. Vision Res. 2017a;139:115–122. doi: 10.1016/j.visres.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saenz DT, Loewen N, Peretz M, Whitwam T, Barraza R, Howell KG, Holmes JM, Good M, Poeschla EM. Unintegrated lentivirus DNA persistence and accessibility to expression in nondividing cells: analysis with Class I integrase mutants. J Virol. 2004;78:2906–2920. doi: 10.1128/JVI.78.6.2906-2920.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sato T, Haimovici R, Kao R, Li AF, Roy S. Downregulation of connexin 43 expression by high glucose reduces gap junction activity in microvascular endothelial cells. Diabetes. 2002;51:1565–1571. doi: 10.2337/diabetes.51.5.1565. [DOI] [PubMed] [Google Scholar]
- 135.Schubert T, Maxeiner S, Krüger O, Willecke K, Weiler R. Connexin45 mediates gap junctional coupling of bistratified ganglion cells in the mouse retina. J Comp Neurol. 2005;490:29–39. doi: 10.1002/cne.20621. [DOI] [PubMed] [Google Scholar]
- 136.Scott AW, Bressler NM, Ffolkes S, Wittenborn J S, Jorkasky J. Public attitudes about eye and vision health. JAMA Ophthalmol. 2016;134:1111. doi: 10.1001/jamaophthalmol.2016.2627. [DOI] [PubMed] [Google Scholar]
- 137.Shelley J, Dedek K, Schubert T, Feigenspan A, Schultz K, Hombach S, Willecke K, Weiler R. Horizontal cell receptive fields are reduced in connexin57-deficient mice. Eur J Neurosci. 2006;23:3176–3186. doi: 10.1111/j.1460-9568.2006.04848.x. [DOI] [PubMed] [Google Scholar]
- 138.Shimojima M. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science. 2004;303:1192–1195. doi: 10.1126/science.1092124. [DOI] [PubMed] [Google Scholar]
- 139.Sigulinsky CL, Anderson JR, Kerzner E, Rapp CN, Pfeiffer RL, Rodman TM, Emrich DP, Rapp KD, Nelson NT, Lauritzen JS, Meyer M, Marc RE, Jones BW. Network architecture of gap junctional coupling among parallel processing channels in the mammalian retina. J Neurosci. 2020;40:4483–4511. doi: 10.1523/JNEUROSCI.1810-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Singer O, Verma IM. Applications of lentiviral vectors for shRNA delivery and transgenesis. Curr Gene Ther. 2008;8:483–488. doi: 10.2174/156652308786848067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Slavi N, Toychiev AH, Kosmidis S, Ackert J, Bloomfield SA, Wulff H, Viswanathan S, Lampe PD, Srinivas M. Suppression of connexin 43 phosphorylation promotes astrocyte survival and vascular regeneration in proliferative retinopathy. Proc Natl Acad Sci U S A. 2018;115:E5934–5943. doi: 10.1073/pnas.1803907115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Smedowski A, Akhtar S, Liu X, Pietrucha-Dutczak M, Podracka L, Toropainen E, Alkanaan A, Ruponen M, Urtti A, Varjosalo M, Kaarniranta K, Lewin-Kowalik J. Electrical synapses interconnecting axons revealed in the optic nerve head - a novel model of gap junctions’ involvement in optic nerve function. Acta Ophthalmol. 2020;98:408–417. doi: 10.1111/aos.14272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Srinivas M, Verselis VK, White TW. Human diseases associated with connexin mutations. Biochim Biophys Acta Biomembr. 2018;1860:192–201. doi: 10.1016/j.bbamem.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Striedinger K, Petrasch-Parwez E, Zoidl G, Napirei M, Meier C, Eysel UT, Dermietzel R. Loss of connexin36 increases retinal cell vulnerability to secondary cell loss. Eur J Neurosci. 2005;22:605–616. doi: 10.1111/j.1460-9568.2005.04228.x. [DOI] [PubMed] [Google Scholar]
- 145.Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, Sur M, Zhang F. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol. 2015;33:102–106. doi: 10.1038/nbt.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Taymans J-M, Vandenberghe LH, Haute CVD, Thiry I, Deroose CM, Mortelmans L, Wilson JM, Debyser Z, Baekelandt V. Comparative analysis of adeno-associated viral vector serotypes 1 2, 5, 7 and 8 in mouse brain. Hum Gene Ther. 2007;18:195–206. doi: 10.1089/hum.2006.178. [DOI] [PubMed] [Google Scholar]
- 147.Tengölics ÁJ, Szarka G, Ganczer A, Szabó-Meleg E, Nyitrai M, Kovács-Öller T, Völgyi B. Response latency tuning by retinal circuits modulates signal efficiency. Sci Rep. 2019;9:15110. doi: 10.1038/s41598-019-51756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 149.Thuringer D, Jego G, Berthenet K, Hammann A, Solary E, Garrido C. 28160-28168. Gap junction-mediated transfer of miR-145-5p from microvascular endothelial cells to colon cancer cells inhibits angiogenesis. Oncotarget. 2016;7:28160–28168. doi: 10.18632/oncotarget.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tien T, Muto T, Zhang J, Sohn EH, Mullins RF, Roy S. Association of reduced Connexin 43 expression with retinal vascular lesions in human diabetic retinopathy. Exp Eye Res. 2016;146:103–106. doi: 10.1016/j.exer.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 151.Toychiev AH, Ivanova E, Yee CW, Sagdullaev BT. 13972-13977. Block of Gap junctions eliminates aberrant activity and restores light responses during retinal degeneration. J Neurosci. 2013;33:13972–13977. doi: 10.1523/JNEUROSCI.2399-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Trenholm S, Awatramani GB. Myriad roles for gap junctions in retinal circuits (2019) Myriad roles for gap junctions in retinal circuits. In: Kolb H, Fernandez E, Nelson R, editors. Webvision: The Organization of the Retina and Visual System. Salt Lake City (UT): University of Utah Health Sciences Center; 2019. [PubMed] [Google Scholar]
- 153.Tsai D, Morley JW, Suaning GJ, Lovell NH. Survey of electrically evoked responses in the retina - stimulus preferences and oscillation among neurons. Sci Rep. 2017;7:13802. doi: 10.1038/s41598-017-14357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, Mathias RT, Robinson RB, Rosen MR, Cohen IS, Brink PR. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005;568:459–468. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Valiunas V, Wang HZ, Li L, Gordon C, Valiuniene L, Cohen IS, Brink PR. A comparison of two cellular delivery mechanisms for small interfering RNA. Physiol Rep. 2015;3:e12286. doi: 10.14814/phy2.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res. 2016;51:1–40. doi: 10.1016/j.preteyeres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 157.Visone R, Croce CM. MiRNAs and cancer. Am J Pathol. 2009;174:1131–1138. doi: 10.2353/ajpath.2009.080794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vogt VM, Simon MN. Mass determination of rous sarcoma virus virions by scanning transmission electron microscopy. J Virol. 1999;73:7050–7055. doi: 10.1128/jvi.73.8.7050-7055.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Völgyi B, Kovács-Öller T, Atlasz T, Wilhelm M, Gábriel R. Gap junctional coupling in the vertebrate retina: variations on one theme. Prog Retin Eye Res. 2013a;34:1–18. doi: 10.1016/j.preteyeres.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 160.Völgyi B, Pan F, Paul DL, Wang JT, Huberman AD, Bloomfield SA. Gap junctions are essential for generating the correlated spike activity of neighboring retinal ganglion cells. PLoS One. 2013b;8:e69426. doi: 10.1371/journal.pone.0069426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang N, De Bock M, Decrock E, Bol M, Gadicherla A, Bultynck G, Leybaert L. Connexin targeting peptides as inhibitors of voltage- and intracellular Ca2+-triggered Cx43 hemichannel opening. Neuropharmacology. 2013;75:506–516. doi: 10.1016/j.neuropharm.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 162.Weiland JD, Humayun MS. Retinal prosthesis. IEEE Trans Biomed Eng. 2014;61:1412–1424. doi: 10.1109/TBME.2014.2314733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wilson AM, Mazzaferri J, Bergeron É, Patskovsky S, Marcoux-Valiquette P, Costantino S, Sapieha P, Meunier M. In vivo laser-mediated retinal ganglion cell optoporation using K V 1. 1 conjugated gold nanoparticles. Nano Lett. 2018;18:6981–6988. doi: 10.1021/acs.nanolett.8b02896. [DOI] [PubMed] [Google Scholar]
- 164.Wolvetang EJ, Pera MF, Zuckerman KS. Gap junction mediated transport of shRNA between human embryonic stem cells. Biochem Biophys Res Commun. 2007;363:610–615. doi: 10.1016/j.bbrc.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 165.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 166.World Health Organization (2018) Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000-2016. Geneva. 2018 [Google Scholar]
- 167.Yang GY, Liu FY, Li X, Zhu QR, Chen BJ, Liu LQ. Decreased expression of gap junction delta-2 (GJD2) messenger RNA and connexin 36 protein in form-deprivation myopia of guinea pigs. Chin Med J (Engl) 2019;132:1700–1705. doi: 10.1097/CM9.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O’Hare JP, Orchard TJ, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yu Q, Wang Y, Chang Q, Wang J, Gong S, Li H, Lin X. Virally expressed connexin26 restores gap junction function in the cochlea of conditional Gjb2 knockout mice. Gene Ther. 2014;21:71–80. doi: 10.1038/gt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yuan L, Sui T, Chen M, Deng J, Huang Y, Zeng J, Lv Q, Song Y, Li Z, Lai L. CRISPR/Cas9-mediated GJA8 knockout in rabbits recapitulates human congenital cataracts. Sci Rep. 2016;6:22024. doi: 10.1038/srep22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yungher BJ, Ribeiro M, Park KK. Regenerative responses and axon pathfinding of retinal ganglion cells in chronically injured mice. Invest Ophthalmol Vis Sci. 2017;58:1743–1750. doi: 10.1167/iovs.16-19873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zahs KR, Ceelen PW. Gap junctional coupling and connexin immunoreactivity in rabbit retinal glia. Vis Neurosci. 2006;23:1–10. doi: 10.1017/S0952523806231018. [DOI] [PubMed] [Google Scholar]
- 173.Zahs KR, Kofuji P, Meier C, Dermietzel R. Connexin immunoreactivity in glial cells of the rat retina. J Comp Neurol. 2003;455:531–546. doi: 10.1002/cne.10524. [DOI] [PubMed] [Google Scholar]
- 174.Zhang L, Wu Q, Zhang Y. Early visual motion experience shapes the gap junction connections among direction selective ganglion cells. PLoS Biol. 2020;18:e3000692. doi: 10.1371/journal.pbio.3000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhu Y, Zong L, Mei L, Zhao HB. Connexin26 gap junction mediates miRNA intercellular genetic communication in the cochlea and is required for inner ear development. Sci Rep. 2015;5:15647. doi: 10.1038/srep15647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zong L, Zhu Y, Liang R, Zhao HB. Gap junction mediated miRNA intercellular transfer and gene regulation: A novel mechanism for intercellular genetic communication. Sci Rep. 2016;6:19884. doi: 10.1038/srep19884. [DOI] [PMC free article] [PubMed] [Google Scholar]


