Abstract
Neurological diseases such as stroke, Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease are among the intractable diseases for which appropriate drugs and treatments are lacking. Proteolysis targeting chimera (PROTAC) technology is a novel strategy to solve this problem. PROTAC technology uses the ubiquitin-protease system to eliminate mutated, denatured, and harmful proteins in cells. It can be reused, and utilizes the protein destruction mechanism of the cells, thus making up for the deficiencies of traditional protein degradation methods. It can effectively target and degrade proteins, including proteins that are difficult to identify and bind. Therefore, it has extremely important implications for drug development and the treatment of neurological diseases. At present, the targeted degradation of mutant BTK, mHTT, Tau, EGFR, and other proteins using PROTAC technology is gaining attention. It is expected that corresponding treatment of nervous system diseases can be achieved. This review first focuses on the recent developments in PROTAC technology in terms of protein degradation, drug production, and treatment of central nervous system diseases, and then discusses its limitations. This review will provide a brief overview of the recent application of PROTAC technology in the treatment of central nervous system diseases.
Keywords: Alzheimer's disease, disease treatment, drug development, Huntington's disease, proteolysis targeting chimera, stroke, targeted degradation
Introduction
Traditional methods of degrading proteins in organisms in the fields of biochemistry and medicine are limited in terms of efficiency and specificity. However, proteolysis targeting chimera (PROTAC) technology can be used to design new drugs with small molecules that can make protein degradation more efficient and specific, thus creating new opportunities in the field of drug development (Lai and Crews, 2017). PROTACs employ heterobifunctional molecules that utilize the UPS by recruiting an E3 ligase to the protein of interest, leading to adjacency-induced ubiquitination and subsequent protein degradation (Buckley and Crews, 2014). Additionally, PROTACs are event-driven, which means that they can cycle through multiple rounds of activity while removing sub-stoichiometric quantities of proteins (Lai and Crews, 2017), and they have been found to have a persistent effect in a xenotransplantation model. In recent years, a number of different PROTAC molecules have been designed, synthesized, and successfully employed in the degradation of different types of targeted proteins, with potential for treating various diseases, including cancer, infections associated with viruses, immune disorders, and neurodegenerative diseases (Zou et al., 2019). Because Tau aggregation is a typical characteristic of neurodegenerative diseases, Tau degradation has been proposed as a promising treatment strategy (Vossel et al., 2010). Qidong and colleagues designed and synthesized a peptide PROTAC by recruiting Kelch-like ECH-associated protein 1 (Keap1) ubiquitin E3 ligase, and applied it in the degradation of intracellular Tau (Lu et al., 2018). Their results indicate that this approach may be beneficial in the treatment of neurodegenerative disease. Further, Kargbo (2020) proposed that the representative PROTAC compounds found in patients could be used treat or prevent neurogenic diseases (e.g., Alzheimer’s disease (AD), Parkinson’s disease, and dementia) related to the accumulation and aggregation of α-synuclein.
PROTAC has a wide range of potential applications in drug development, and its clinical application is a subject of much current investigation. Although a large number of preliminary experiments have demonstrated that PROTAC has high potential for treating nervous system diseases, especially neurodegenerative diseases, the specific clinical applications require further verification. Pertinent topics of investigation include off-target effects, cell permeability, stability, and macromolecular mass. Another concern is the level of difficulty in synthesizing hybrid molecules, including optimizing the length and composition of the linker. According to initial data from tests of the world’s first (ARV-110) and second clinical applications of PROTACs (ARV-471), oral bioavailable PROTAC protein degradation agents are safe and well tolerated in the field of cancer treatment. Using relevant development and research findings, this review paper seeks to elaborate on how PROTAC technology uses the ubiquitin-proteasome pathway to degrade targeted proteins, the determination and optimization of PROTAC molecular structures, the advantages and disadvantages of this technology in application, and directions for improvement.
Data Retrieval
For this review, we collected articles on the use of PROTACs in treating nervous system diseases by analyzing the reference list from Lai and Crews (2017). We also performed an electronic search of the PubMed database for articles on PROTAC technology and the application of PROTACSs for treating central nervous system diseases published from 2009 to 2020. We used the following search conditions: ((PROTAC[Title/Abstract]) OR (Target[Title/Abstract])) AND (disease[Title/Abstract]). The results were further screened by publication date and specific contents. In addition, we conducted an electronic search of the PubMed database for articles published prior to December, 2020, using the following search terms: PROTACs, targeted degradation, drug development, and disease treatment. We completed subsequent searches that were specifically relevant to each study that discussed PROTACs using the following terms: mutant BTK and ibrutinib, PARP1 and iRucaparib-AP6, STAT3 and SD-36, ADC and Her2, Huntington’s disease (HD), Alzheimer’s disease (AD), BET and dBET1.
The Development of PROTACs
The occurrence and progression of many diseases are related to the abnormal expression of target protein. Regulating the expression of target proteins is important in a number of research fields including biology and medicine, and is of great significance to the research and development of new drugs. Although many therapeutic targets have been found in the human proteome, many of these are difficult to target because of limitations related to their structure. The design and application of PROTACs, and particularly small-molecule PROTACs, is likely to offer some important solutions. A number of teams have published papers describing the effects of small-molecule PROTACs in drug development (Bondeson et al., 2015; Buckley et al., 2015; Lu et al., 2015; Winter et al., 2015; Zengerle et al., 2015). The authors described several ways to use PROTACs to induce proteins, such as employing phthalimide conjugation, HaloTag fusion proteins, and Bromodomain and extra-teminal (BET) bromodomain protein BRD4 to induce degradation.
In-depth investigations of PROTACs have led to several important developments. For instance, the use of small molecule compounding led to a breakthrough in cancer immunotherapy. Specifically, Powell and colleagues discovered a way to alter the tumor microenvironment by modulating the consequences of the “Warburg effect” (Vander Heiden et al., 2009) and blocking glutamine metabolism to inhibit tumor growth. They developed a small molecule drug that directly enhanced the anti-cancer effect of immune cells. This could be considered a form of immunotherapy, with the ability to modify the microenvironment of the tumor (Altman et al., 2016; Pavlova and Thompson, 2016; Leone et al., 2019).
Mechanism of PROTAC Technology
Structural mechanisms
PROTAC technology centers on targeted protein degradation. The intracellular UPS is used to clean up mutated, denatured, and harmful proteins by recruiting E3 ligases to target proteins, leading to proximity-induced ubiquitination and subsequent protein degradation. PROTAC molecules are composed of three different parts: (1) a ligand for binding to the target protein; (2) a ligand for binding to the E3 ligase (currently, cereblon (CRBN) is the most widely used E3 ubiquitin ligase); and (3) a linker, which is the ligand that connects the above-mentioned two ligands. Ubiquitination and proteasomal degradation of the target protein are catalyzed through the connection of the three parts (Burslem and Crews, 2017) (Figure 1).
Figure 1.
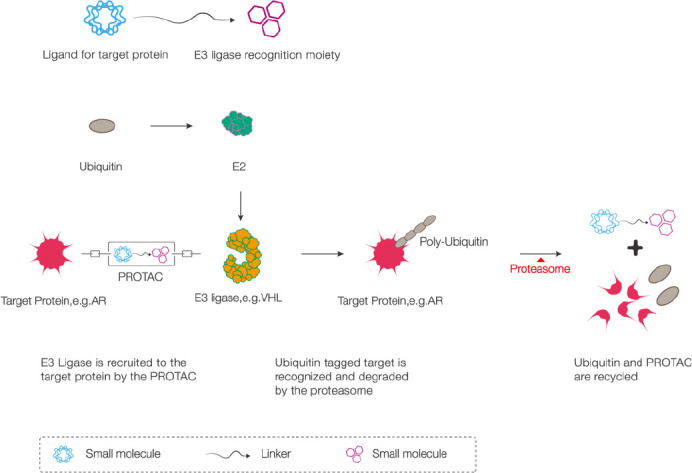
Schematic diagram showing the mechanism of action of PROTAC technology (for this example, we used VHL E3 ubiquitin ligase to decompose the AR).
The PROTAC molecule is like a dumbbell, with a linker in the middle, a targeted protein ligand at one end, and an E3 ligase recognition moiety at the other end. The E3 ligase is recruited to the target protein (AR) by the PROTAC molecule, and then the ubiquitin-tagged target protein (AR) is recognized and degraded by the proteasome. The ubiquitin and PROTAC are subsequently recycled. AR: Androgen receptor; PROTAC: proteolysis-targeting chimeric molecule; VHL: von Hippel–Lindau.
In recent years, the discovery of the crystal structure of the ternary complex has enabled a more comprehensive understanding of the structural mechanisms of PROTACs. Structural studies by von Hippel-Lindau regarding the CRBN have demonstrated that the cullin-RINGE3 ligase forms large, modular compounds that are shaped like a ‘U’. E2 and substrate proteins are located at the near end of this U-shape, which enables targeted ubiquitin transfer. The structure of these large compounds is expected to provide a polyuitination extension radius, which can accommodate substrate proteins of different sizes and shapes (Schapira et al., 2019).
Process
PROTAC technology utilizes a cell’s protein-destruction mechanism to remove specific oncogenic proteins from the cell. Through chemical bonding, the ligands that assist the UPS in finding the substrate protein can be linked to the target protein ligand, degrading the target protein that would otherwise be useless to the body. In contrast to the principles of action of traditional protein inhibitors, PROTAC technology utilizes a bifunctional hybrid compound. This compound, which contains an E3 ligase connected to a target protein, can realize the ubiquitination of a target protein, and then be recognized by the 26S subunit of proteasome and degraded by proteome (Figure 1) (Gadd et al., 2017; Hughes and Ciulli, 2017; Nowak et al., 2018; Smith et al., 2019).
In addition to the classical model of a PROTAC molecule mentioned above, there are other forms of protein degradation strategies, such as molecular glue (Yang et al., 2019), LYTAC (lysosome-targeting chimaeras), and AUTAC (autophagy-targeting chimera) (Ding et al., 2020). In theory, PROTACs only provide binding activity, which is event-driven rather than the traditional possession-driven, and do not directly inhibit the functional activity of the target protein. As they can be reused, high levels of PROTACs activity may not be required (Pettersson and Crews, 2019).
The Strengths and Weaknesses of PROTACs Technology
Advantages
PROTAC technology combines the advantages of small molecule compounds with those of small molecule nucleic acids to effectively target a protein and degrade it. Traditionally, small molecules are used to block proteins, while protein-targeting antidotes break them down completely by sending them into the proteasome. In theory, PROTACs have certain advantages: they can selectively degrade different proteins expressed by the same gene after protein expression and modification, without requiring large quantities. They do not need to directly inhibit the functional activity of the target protein, or bind to the target protein for a long time or with high intensity. Thus, they can achieve the targets of traditional drugs that are not easy to make. Because they enable selective degradation of target proteins, they can regulate targets that cannot be regulated with small molecules or many antibodies. The PROTAC process is similar to a catalytic reaction and the drug can be reused. Because the inhibition of the target protein does not require a molar amount of the drug, it is possible to generate a highly active drug (Churcher, 2018; Crews, 2018).
Disadvantages
As PROTAC technology uses drugs with dual targets, the administered compounds may have a large molecular weight, molecular rigidity, and high water solubility. As a result, they may not be easily absorbed orally, and may not have high transmurality. These issues represent a major obstacle in pharmacokinetics. Further, chemical synthesis of PROTACS is not easy. In pharmacodynamics, off-target toxicity is an important concern. In general, small molecules, large molecules, and even small nucleotides that target proteins do not completely inhibit protein activity and have little effects on the expression of skeletal proteins. Although this increases the probability of drug resistance, residual activity also guarantees the basic physiological activity of normal cells, tissues, and organs. As PROTAC technology involves a more thorough degradation of target proteins, it may adversely affect other off-target proteins. Therefore, in future clinical trials, it is important to closely monitor the potential toxicity of PROTACs, including that for previously verified targets. The effects of off-target degradation can be difficult to detect and track in preclinical toxicity screenings, and the discovery of toxicity can limit future drug development. Thus, researchers should continue to comprehensively consider pharmacokinetics/pharmacodynamics, and specifically, analyze the kinetic and pharmacodynamic quantitative indicators of PROTAC drugs, so as to ensure the development of drugs that are maximally efficient and minimally toxic.
Applications of PROTACs
Mutations in the Bruton’s tyrosine kinase protein and ibrutinib
Based on PROTACs technology, various studies have verified the advantages of proteolytic agents in terms of drug resistance. External experiments (Sun et al., 2018) revealed that Bruton’s tyrosine kinase (BTK) protein lysator could target and degrade the BTK protein after it had been mutated by C481S (Figure 2A), thus overcoming the resistance of B-cell malignant tumors to the first-line clinical drug ibrutinib. Primary central nervous system lymphoma can enhance the mutation of B cell receptor signals, and ibrutinib, as a BTK inhibitor, can target B cell receptor signals. Experiments have demonstrated that ibrutinib is lethal in most tumors when inhibiting the B cell receptor-signaling pathway. For instance, ibrutinib reduced the tumor mass in patients with primary brain lymphoma by 94% (Lionakis et al., 2017). Sun et al. (2018) reported that they were able to use PROTAC technology to enable the degradation of a variety of mutant BTK proteins, thus offering a way to overcome ibrutinib resistance in patients with non-Hodgkin’s lymphoma. Protein-lowering agents generated based on PROTAC technology are likely to become key drugs in future cancer research (Sun et al., 2019).
Figure 2.
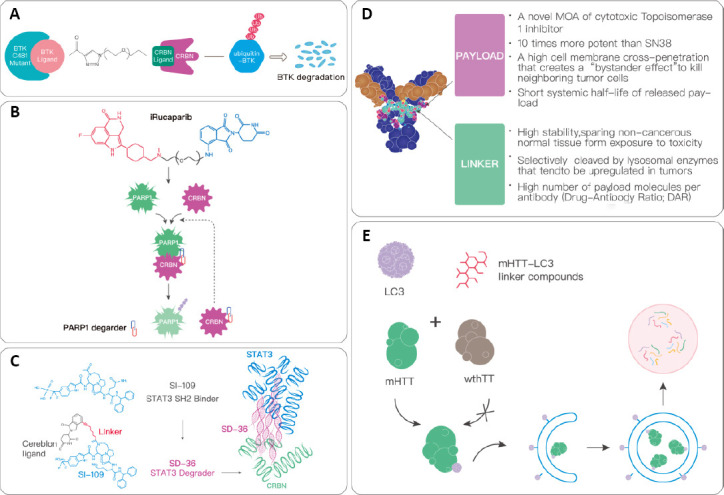
The applications of PROTACs.
(A) CRBN protein is recruited to the BTK protein with a C481S mutation by the BTK protein lysator. This causes the proteins to be ubiquitinated, after which they are recognized and degraded by proteasomes. (B) iRucaparib-AP6 is a PARP1 degrading agent, and is directly connected to the binding molecule of CRBN at the end of rucaparib through a PEG6 linker. PARP1 is finally recognized and degraded by the ubiquitin-protease system. (C) SD-36 is a STAT3 degrading agent. It is composed of a linker that connects the STAT3 SH2 binder (S1–109) and CRBN. It can degrade STAT3 via a proteasome with a ubiquitin tag. (D) Compared with ordinary chemotherapeutics, ADC has seven main advantages. (E) Small molecule drugs degrade mHTT through the autophagy pathway. During autophagy, the key protein LC3 is polymerized and diffused after lipolysis to form the membrane structure, and the degradation targets such as proteins, lipids, and organelles are encapsulated in it to form a complete autophagosome. This is fused with lysosomes and the encapsulated substance is degraded. ADC: Antibody-drug conjugate; BTK: Bruton’s tyrosine kinase; CRBN: cereblon ligand; LC3: microtubule-associated protein 1A/1B-light chain 3; mHTT: mutant huntingtin; MOA: mechanisms of action; PEG6: polyethylene glycol 6; PROTACs: proteolysis-targeting chimeric molecules; STAT3: signal transducer and activator of transcription 3; wthTT: wild type huntingtin protein.
PolyADP-ribose polymerase 1 and iRucaparib-AP6
Generally, it is difficult to effectively degrade polyADP-ribose polymerase 1 (PARP1) because it has high abundance ratios and high stability (Wang et al., 2019). After composing and evaluating a series of approaches based on the PROTAC PARP1 degrader, Wang and colleagues found that a small molecular compound named iRucaparib-AP6, which is highly activity in cells, could specifically initiate degradation of PARP1 (Figure 2B). Therefore, the catalytic inhibition induced by the PARP1 inhibitor can be distinguished from the capturing activity of PARP1. This represents a feasible technical means, along with drug targets, for the study of specific downstream signals and functions mediated by the catalytic inhibition of PARP1 and capture of PARP1, as well as the treatment of diseases such as ischemic tissue injury and neurodegenerative diseases.
Signal transducer and activator of transcription 3 and SD-36
A team of researchers at the University of Michigan discovered a promising new way to target signal transducer and activator of transcription 3 (STAT3). Specifically, they identified a small molecular compound based on PROTAC technology that could completely block the effects of STAT3 using natural “clean-up systems” inside cells. A newly published study showed that a team led by Wang developed a small molecule, known as SD-36, which could degrade STAT3 (Bai et al., 2019). According to experimental results from mouse models of leukemia and lymphoma, SD-36 can almost completely eliminate tumors and has a long-lasting effect. The development of protein-lowering agents using PROTAC technology can avoid some of the problems that might arise from drugs designed to inhibit the activity of STAT3, such as accidentally targeting other proteins in the STAT family (Figure 2C).
Trastuzumab deruxtecan and human epidermal growth factor receptor 2
A recent study by Sankyo and colleagues showed that the administration of trastuzumab deruxtecan led to a remission rate of 60.9% in patients with refractory human epidermal growth factor receptor 2 (Her2)-positive breast cancer in a critical phase-2 clinical trial (de Melo Gagliato et al., 2016). The DXd antibody-drug conjugate technology platform, which includes PROTACs, is the latest innovation in antibody-drug conjugate payloads and linkers, which act as the link between antibodies and cytotoxic drugs. Trastuzumab deruxtecan has very high junction stability, which can ensure that non-tumor tissues are not damaged by toxic drugs (Figure 2D). However, it can be specifically cleaved by lysosomal proteases, which are highly expressed in tumors, and can couple multiple cytotoxic drugs to one antibody molecule, thereby increasing the drug-to-antibody ratio (Modi et al., 2020) (Figure 2D).
Autophagosome-binding compounds and HD
HD is one of the four main neurodegenerative diseases, and is caused by the mutant huntingtin (mHTT) protein and an expanded polyglutamine tract. The use of genetic or RNA methods of targeting large biomolecules or viruses to reduce mHTT levels can improve the pathogenic effects, and has attracted interest as a promising treatment strategy for HD. Nevertheless, new treatment strategies have yet to be discovered. Recently, Lu et al. (2018) proposed a new form of drug research based on autophagosome binding compound (ATTEC). They screened out mHTT-lowering compounds that interact with mHTT and microtubule-associated protein 1A/1B-light chain 3 (LC3), as these compounds target mHTT and phage vectors. Thus, autophagic degradation was possible without affecting the wild type huntingtin level. This strategy represents a starting point for the development of new treatments for HD and similar diseases (Lu et al., 2018; Li et al., 2019).
ATTEC or ‘’small molecule glue’’ is a new concept in drug development, and is expected to be applied to other pathogenic proteins that cannot be targeted, including non-protein pathogenic substances. Compared with PROTAC, which mainly depends on the UPS, the advantages of ATTEC are clear. If the target molecule is not a protein, PROTAC will be ineffective, while ATTEC can degrade non-protein pathogenic molecules through autophagosomes. While it can be difficult to work with large proteins or protein aggregates with PROTAC, ATTEC is universal and effective in many cells. It can specifically bind LC3 and pathogenic proteins (or other pathogenic substances) tightly together, thereby encapsulating the pathogenic proteins in autophagosomes and degrading them instead of binding to wild-type huntingtin proteins, as shown in Figure 2E.
AD
AD is an increasingly urgent global health concern. It is characterized by progressive defects in cognition, memory, and other mental functions, and is one of the main causes of mental disability and death in the elderly. Despite substantial investments in a variety of traditional treatment strategies and success in preclinical models that address the pathological features of the disease, the exact pathogenesis of AD is not yet clear. Various hypotheses have been proposed to explain the complex pathophysiology of AD, including pathogenic tau proteins, β-amyloid (Aβ) cascades, chronic inflammation, oxidative stress, and acetylcholine abnormality. In addition, deficient mitochondrial bioenergy, low brain metabolism, and increased mitochondrial oxidative stress may play important roles in the pathogenesis of AD. The two main protein deposits that are characteristic of AD are senile plagues and neurofibrillary tangles. Senile plaques affect the extracellular accumulation of Aβ in the brain and are the main driving factor in disease progression. Neurofibrillary tangles form as the result of Aβ accumulation, and contain hyperphosphorylated tau protein. Tau protein is abundant in neurons, although its expression level in the central nervous system is very low. It is known to accumulate prior to extensive neuronal damage in individuals with AD. Therefore, tau deposition has become a target for determining the progression and treatment of AD. In addition, the tau hypothesis states that excessive or abnormal phosphorylation of tau breaks down microtubules and separates normal tau, microtubule-associated protein 1, microtubule-associated protein 2, and ubiquitin into pairs of spiral filament tangles. Aβ peptide is produced by amyloid precursor protein and consists of 39–43 amino acids (de la Monte, 2017). PROTAC-induced protein degradation has emerged as a promising strategy for targeting non-enzymatic proteins in cells. Subsequently, the degradation of tau protein based on PROTAC technology has become a focal point in the treatment of AD. For instance, Lu et al. (2018) found a Keap1-dependent peptide PROTAC that enabled knockdown of tau via the ubiquitination-proteasome degradation pathway. Their results suggest that using PROTACs to recruit Keap1 to induce the degradation of Tau may show promise in the treatment of neurodegenerative disease. Keap1 may also be a promising E3 ligase adaptor for use in the design of novel PROTACs (Lu et al., 2018). Kargbo (2019) published two patents concerning the treatment of AD using PROTAC technology. Once involved the use of PROTAC to degrade epidermal growth factor receptors for the treatment of cancer and AD, and the other concerned PROTAC-tau protein degradation for the treatment of AD. Among other new strategies for the treatment of AD, Ordaz et al. (2017) summarized an optogenetic scheme, while Onyango (2018) presented an overview of emerging treatments with the potential to prevent, delay, or reverse the neurodegenerative process by targeting mitochondria. Therefore, PROTAC technology may have broad applications in the treatment of AD, although accessing this potential will require the identification of small PROTAC molecules that can be applied to highly targeted tau proteins.
Degradation of BET Protein by PROTAC dBET1
Neuroinflammation
Activated immune cells initiate the transcription of inflammatory genes through the action of the BET protein. The BET family of proteins includes BRD2, BRD3, BRD4 and testis-specific BRDT. Gene transcription of the inflammatory transcription factor nuclear factor-κB depends on BRD2 and BRD4. Nuclear factor-κB activity can increase the transcription of a series of genes, which is related to neuroinflammation after various types of brain injury (Fujisawa and Filippakopoulos, 2017; Hsu et al., 2017; Zhao et al., 2019). Created using PROTAC technology, dBET1 is a bifunctional compound composed of a phthalimide moiety, which is the ligand of E3 ubiquitin ligase CRBN. It is connected to JQ1, the selective inhibitor of BET protein, which occupies the acetyl lysine-binding pocket. By this design, the BET protein is recognized by JQ1, and can be ubiquitinated and then degraded by proteasome. Studies have shown that the transcription of pro-inflammatory genes induced by lipopolysaccharides clearly weakens when BET protein is inhibited by JQ1. COX-2 and iNOS proteins, which are typical mediators of the pro-inflammatory microglial reaction, are effectively reduced (Winter et al., 2017). DeMars et al. (2018) found that targeting BET protein could effectively block the pro-inflammatory microglial reaction. The degradation of BET protein with dBET1 or inhibition of BET protein with JQ1 can halt the lipopolysaccharide-induced transcription of pro-inflammatory genes in the microglia of SIM-A9 mice. Further, the harmful effects of microglia that have been activated by neuroinflammatory disease are reduced through administration of dBET1. In conclusion, through further experimental research and verification, we expect that it will be possible to use dBET1 for the targeted degradation of BET protein, thus minimizing nervous system damage caused by inflammatory response.
Ischemic stroke
Stroke is a major cause of death in humans, and is also the primary cause of disability for adults (particularly ischemic stroke). During a stroke, cerebral vascular obstruction, hypoxia, and nutrition deficiency in downstream tissues leads to cell death, the release of pro-inflammatory signals by resident microglia, and the infiltration of immune cells from the periphery to damaged tissues. This is the main factor leading to damage to the blood-brain barrier. As BET protein has histone acetyltransferase activity, it can regulate gene transcription after ischemic stroke and promote rehabilitation (Duris et al., 2018). Experiments have shown that the stroke volume and transcription of pro-inflammatory mediators can be reduced when mice with permanent focal cerebral ischemia are treated with dBET1 (DeMars et al., 2019). In addition, the experimenters observed a reduction in the degree of damage in the blood-brain barrier, as well as decreased neutrophil infiltration after a stroke. This indicates that dBET1 may help to reduce neurological deficits when administered within 48 hours after a stroke (DeMars et al., 2019). However, the cellular expression of BET protein after an ischemic stroke has not been specifically described. Thus, more studies are needed to make better use of BET blockers for drug therapy. PROTAC dBET1 is a promising treatment for stroke and other diseases caused by neuroinflammation.
Directions for Improvement of PTOTACs Technology
The molecular weight of PROTAC molecules generally ranges from 800 to 1000 kDa, and the membrane permeability is usually poor, such that it must be modified and screened continuously. According to a review published by Pettersson and Crews (2019), improvements to PROTAC technology could progress in the following four directions:
-
(1)
To optimize the design, synthesis, and evaluation of PROTACs, it is necessary to reveal the discovery principles and establish a stable evaluation platform. This would accelerate and simplify the discovery of PROTACs.
-
(2)
Expand the range of available E3 ligases: it is estimated that there are more than 600 E3 ligases in the human genome. However, only a few have been used or developed for PROTACs. Therefore, finding new ligands for the E3 ligase will likely be a major focus.
-
(3)
Selection and validation of targets: This is one of the most important considerations in drug development. Even if it is possible to target drug-free proteins, it is not possible to ensure that all proteins will be degraded by PROTAC technology if the ligands are absent.
-
(4)
Targeting drug-free proteins: To date, most of the proteins targeted by PROTAC technology have been exploitable drug targets. However, PROTAC technology is likely to be useful in targeting non-exploitable proteins for the treatment of certain diseases.
Summary and Perspectives
New ideas resulting from the use of PROTAC technology for drug research and development have brought certain gains, particularly, a feasible way to treat certain intractable neurological diseases. Considering the shortcomings and challenges of this technology, more suitable E3 ligase ligands are needed, along with the selection and verification of targets, identification of drug-free proteins for use as targets, and generation of solutions for off-target toxicity by changing the structure of PROTACs. Considering the large molecular weight, a new strategy is to shorten PROTACs by clicking to form PROTAC (TCLIPTAC), i.e., dividing large hybrid molecules into two parts. One molecule is the targeted tetrazine-labeled ligand, and the other is a trans-cyclo-octene-tagged ligand for E3. These can “click” together to form a PROTAC (Lebraud et al., 2016). This click response may also provide a more convenient method for the synthesis of PROTACs in vitro (Wurz et al., 2018).
At present, several types of protein degradation products have been examined in neurodegenerative disease and cancer research. These also have high application value in additional fields, and are expected to be useful in solving problems related to the treatment of nervous system diseases for which suitable targets are difficult to identify. In recent years, PROTAC molecules have increasingly entered clinical or pre-clinical stages of research. Further, data regarding ARV-110 and ARV-471 have been promising. It is necessary to constantly modify and screen potential compounds to improve their pharmacokinetic properties, and to continuously verify the efficacy of PROTACs in clinical practice. The realization and application of comprehensive PROTACs technologies will likely be valuable in treating a variety of intractable cancers and nervous system diseases.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was supported by the National Natural Science Foundation of China, No. 81870975 (to SLZ); and the Nantong Science and Technology Innovation Program, China, No. JC2019028 (to XMY). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81870975 (to SLZ); and the Nantong Science and Technology Innovation Program, China, No. JC2019028 (to XMY).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- 1.Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:749. doi: 10.1038/nrc.2016.114. [DOI] [PubMed] [Google Scholar]
- 2.Bai L, Zhou H, Xu R, Zhao Y, Chinnaswamy K, McEachern D, Chen J, Yang CY, Liu Z, Wang M, Liu L, Jiang H, Wen B, Kumar P, Meagher JL, Sun D, Stuckey JA, Wang S. A potent and selective small-molecule degrader of STAT3 achieves complete tumor regression in vivo. Cancer Cell. 2019;36:498–511.e17. doi: 10.1016/j.ccell.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bondeson DP, Mares A, Smith IE, Ko E, Campos S, Miah AH, Mulholland KE, Routly N, Buckley DL, Gustafson JL, Zinn N, Grandi P, Shimamura S, Bergamini G, Faelth-Savitski M, Bantscheff M, Cox C, Gordon DA, Willard RR, Flanagan JJ, et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat Chem Biol. 2015;11:611–617. doi: 10.1038/nchembio.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley DL, Crews CM. Small-molecule control of intracellular protein levels through modulation of the ubiquitin proteasome system. Angew Chem Int Ed Engl. 2014;53:2312–2330. doi: 10.1002/anie.201307761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley DL, Raina K, Darricarrere N, Hines J, Gustafson JL, Smith IE, Miah AH, Harling JD, Crews CM. HaloPROTACS: Use of small molecule PROTACs to induce degradation of halotag fusion proteins. ACS Chem Biol. 2015;10:1831–1837. doi: 10.1021/acschembio.5b00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burslem GM, Crews CM. Small-molecule modulation of protein homeostasis. Chem Rev. 2017;117:11269–11301. doi: 10.1021/acs.chemrev.7b00077. [DOI] [PubMed] [Google Scholar]
- 7.Churcher I. Protac-induced protein degradation in drug discovery: breaking the rules or just making new ones. J Med Chem. 2018;61:444–452. doi: 10.1021/acs.jmedchem.7b01272. [DOI] [PubMed] [Google Scholar]
- 8.Crews CM. Inducing protein degradation as a therapeutic strategy. J Med Chem. 2018;61:403–404. doi: 10.1021/acs.jmedchem.7b01333. [DOI] [PubMed] [Google Scholar]
- 9.de la Monte SM. Insulin resistance and neurodegeneration: progress towards the development of new therapeutics for Alzheimer’s disease. Drugs. 2017;77:47–65. doi: 10.1007/s40265-016-0674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Melo Gagliato D, Jardim DL, Marchesi MS, Hortobagyi GN. Mechanisms of resistance and sensitivity to anti-HER2 therapies in HER2+ breast cancer. Oncotarget. 2016;7:64431–64446. doi: 10.18632/oncotarget.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeMars KM, Yang C, Candelario-Jalil E. Neuroprotective effects of targeting BET proteins for degradation with dBET1 in aged mice subjected to ischemic stroke. Neurochem Int. 2019;127:94–102. doi: 10.1016/j.neuint.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 12.DeMars KM, Yang C, Castro-Rivera CI, Candelario-Jalil E. Selective degradation of BET proteins with dBET1 a proteolysis-targeting chimera, potently reduces pro-inflammatory responses in lipopolysaccharide-activated microglia. Biochem Biophys Res Commun. 2018;497:410–415. doi: 10.1016/j.bbrc.2018.02.096. [DOI] [PubMed] [Google Scholar]
- 13.Ding Y, Fei Y, Lu B. Emerging new concepts of degrader technologies. Trends Pharmacol Sci. 2020;41:464–474. doi: 10.1016/j.tips.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duris K, Splichal Z, Jurajda M. The role of inflammatory response in stroke associated programmed cell death. Curr Neuropharmacol. 2018;16:1365–1374. doi: 10.2174/1570159X16666180222155833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujisawa T, Filippakopoulos P. Functions of bromodomain-containing proteins and their roles in homeostasis and cancer. Nat Rev Mol Cell Biol. 2017;18:246–262. doi: 10.1038/nrm.2016.143. [DOI] [PubMed] [Google Scholar]
- 16.Gadd MS, Testa A, Lucas X, Chan KH, Chen W, Lamont DJ, Zengerle M, Ciulli A. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat Chem Biol. 2017;13:514–521. doi: 10.1038/nchembio.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu SC, Gilgenast TG, Bartman CR, Edwards CR, Stonestrom AJ, Huang P, Emerson DJ, Evans P, Werner MT, Keller CA, Giardine B, Hardison RC, Raj A, Phillips-Cremins JE, Blobel GA. The BET protein BRD2 cooperates with CTCF to enforce transcriptional and architectural boundaries. Mol Cell. 2017;66:102–116.e7. doi: 10.1016/j.molcel.2017.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes SJ, Ciulli A. Molecular recognition of ternary complexes: a new dimension in the structure-guided design of chemical degraders. Essays Biochem. 2017;61:505–516. doi: 10.1042/EBC20170041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kargbo RB. Treatment of cancer and Alzheimer’s disease by PROTAC degradation of EGFR. ACS Med Chem Lett. 2019;10:1098–1099. doi: 10.1021/acsmedchemlett.9b00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kargbo RB. PROTAC compounds targeting α-synuclein protein for treating neurogenerative disorders: Alzheimer’s and Parkinson’s diseases. ACS Med Chem Lett. 2020;11:1086–1087. doi: 10.1021/acsmedchemlett.0c00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai AC, Crews CM. Induced protein degradation: an emerging drug discovery paradigm. Nat Rev Drug Discov. 2017;16:101–114. doi: 10.1038/nrd.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebraud H, Wright DJ, Johnson CN, Heightman TD. Protein degradation by in-cell self-assembly of proteolysis targeting chimeras. ACS Cent Sci. 2016;2:927–934. doi: 10.1021/acscentsci.6b00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, Arwood ML, Bettencourt IA, Patel CH, Wen J, Tam A, Blosser RL, Prchalova E, Alt J, Rais R, Slusher BS, Powell JD. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366:1013–1021. doi: 10.1126/science.aav2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Wang C, Wang Z, Zhu C, Li J, Sha T, Ma L, Gao C, Yang Y, Sun Y, Wang J, Sun X, Lu C, Difiglia M, Mei Y, Ding C, Luo S, Dang Y, Ding Y, Fei Y, et al. Allele-selective lowering of mutant HTT protein by HTT-LC3 linker compounds. Nature. 2019;575:203–209. doi: 10.1038/s41586-019-1722-1. [DOI] [PubMed] [Google Scholar]
- 25.Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R, Yang Y, Cole DE, Melani C, Higham CS, Desai JV, Ceribelli M, Chen L, Thomas CJ, Little RF, Gea-Banacloche J, Bhaumik S, Stetler-Stevenson M, Pittaluga S, Jaffe ES, et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31:833–843.e5. doi: 10.1016/j.ccell.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu J, Qian Y, Altieri M, Dong H, Wang J, Raina K, Hines J, Winkler JD, Crew AP, Coleman K, Crews CM. Hijacking the E3 ubiquitin ligase cereblon to efficiently target BRD4. Chem Biol. 2015;22:755–763. doi: 10.1016/j.chembiol.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu M, Liu T, Jiao Q, Ji J, Tao M, Liu Y, You Q, Jiang Z. Discovery of a Keap1-dependent peptide PROTAC to knockdown Tau by ubiquitination-proteasome degradation pathway. Eur J Med Chem. 2018;146:251–259. doi: 10.1016/j.ejmech.2018.01.063. [DOI] [PubMed] [Google Scholar]
- 28.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, Sohn J, Denduluri N, Perrin C, Aogi K, Tokunaga E, Im SA, Lee KS, Hurvitz SA, Cortes J, Lee C, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nowak RP, DeAngelo SL, Buckley D, He Z, Donovan KA, An J, Safaee N, Jedrychowski MP, Ponthier CM, Ishoey M, Zhang T, Mancias JD, Gray NS, Bradner JE, Fischer ES. Plasticity in binding confers selectivity in ligand-induced protein degradation. Nat Chem Biol. 2018;14:706–714. doi: 10.1038/s41589-018-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onyango IG. Modulation of mitochondrial bioenergetics as a therapeutic strategy in Alzheimer’s disease. Neural Regen Res. 2018;13:19–25. doi: 10.4103/1673-5374.224362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ordaz JD, Wu W, Xu XM. Optogenetics and its application in neural degeneration and regeneration. Neural Regen Res. 2017;12:1197–1209. doi: 10.4103/1673-5374.213532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pettersson M, Crews CM. PROteolysis TArgeting Chimeras (PROTACs) - Past present and future. Drug Discov Today Technol. 2019;31:15–27. doi: 10.1016/j.ddtec.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schapira M, Calabrese MF, Bullock AN, Crews CM. Targeted protein degradation: expanding the toolbox. Nat Rev Drug Discov. 2019;18:949–963. doi: 10.1038/s41573-019-0047-y. [DOI] [PubMed] [Google Scholar]
- 35.Smith BE, Wang SL, Jaime-Figueroa S, Harbin A, Wang J, Hamman BD, Crews CM. Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase. Nat Commun. 2019;10:131. doi: 10.1038/s41467-018-08027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Y, Ding N, Song Y, Yang Z, Liu W, Zhu J, Rao Y. Degradation of Bruton’s tyrosine kinase mutants by PROTACs for potential treatment of ibrutinib-resistant non-Hodgkin lymphomas. Leukemia. 2019;33:2105–2110. doi: 10.1038/s41375-019-0440-x. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Zhao X, Ding N, Gao H, Wu Y, Yang Y, Zhao M, Hwang J, Song Y, Liu W, Rao Y. PROTAC-induced BTK degradation as a novel therapy for mutated BTK C481S induced ibrutinib-resistant B-cell malignancies. Cell Res. 2018;28:779–781. doi: 10.1038/s41422-018-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, Cui B, Mucke L. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330:198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S, Han L, Han J, Li P, Ding Q, Zhang QJ, Liu ZP, Chen C, Yu Y. Uncoupling of PARP1 trapping and inhibition using selective PARP1 degradation. Nat Chem Biol. 2019;15:1223–1231. doi: 10.1038/s41589-019-0379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winter GE, Buckley DL, Paulk J, Roberts JM, Souza A, Dhe-Paganon S, Bradner JE. Drug development. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science. 2015;348:1376–1381. doi: 10.1126/science.aab1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winter GE, Mayer A, Buckley DL, Erb MA, Roderick JE, Vittori S, Reyes JM, di Iulio J, Souza A, Ott CJ, Roberts JM, Zeid R, Scott TG, Paulk J, Lachance K, Olson CM, Dastjerdi S, Bauer S, Lin CY, Gray NS, et al. BET bromodomain proteins function as master transcription elongation factors independent of CDK9 recruitment. Mol Cell. 2017;67:5–18.e19. doi: 10.1016/j.molcel.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wurz RP, Dellamaggiore K, Dou H, Javier N, Lo MC, McCarter JD, Mohl D, Sastri C, Lipford JR, Cee VJ. A “Click Chemistry Platform” for the rapid synthesis of bispecific molecules for inducing protein degradation. J Med Chem. 2018;61:453–461. doi: 10.1021/acs.jmedchem.6b01781. [DOI] [PubMed] [Google Scholar]
- 44.Yang J, Li Y, Aguilar A, Liu Z, Yang CY, Wang S. Simple structural modifications converting a bona fide MDM2 PROTAC degrader into a molecular glue molecule: a cautionary tale in the design of PROTAC degraders. J Med Chem. 2019;62:9471–9487. doi: 10.1021/acs.jmedchem.9b00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zengerle M, Chan KH, Ciulli A. Selective small molecule induced degradation of the BET bromodomain protein BRD4. ACS Chem Biol. 2015;10:1770–1777. doi: 10.1021/acschembio.5b00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao CP, Zhu ML, Wang TT, Liu XL, Liu CL. A chemical radiculitis model in the rat: establishment and evaluation. Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23:1030–1034. [Google Scholar]
- 47.Zou Y, Ma D, Wang Y. The PROTAC technology in drug development. Cell Biochem Funct. 2019;37:21–30. doi: 10.1002/cbf.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]


