Keywords: bladder, external urethral sphincter, fluoxetine, micturition, selective serotonin reuptake inhibitor, spinal cord injury, urodynamics, voided stain on paper measurement
Abstract
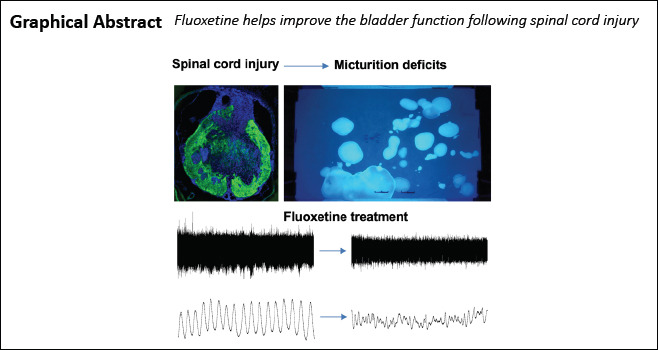
After spinal cord injury, the upward conduction of the spinal cord is lost, resulting in the loss of micturition control, which manifests as detrusor sphincter dyssynergia and insufficient micturition. Studies have shown that serotonergic axons play important roles in the control of the descending urination tract. In this study, mouse models of moderate spinal cord contusions were established. The serotonin agonists quipazine (0.2 mg/kg), 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DAPT, 0.1 mg/kg), buspirone (1 mg/kg), sumatriptan (1 mg/kg), and rizatriptan (50 mg/kg), the serotonin reuptake inhibitors fluoxetine (20 mg/kg) and duloxetine (1 mg/kg), and the dopamine receptor agonist SKF-82197 (0.1 mg/kg) were intraperitoneally administered to the model mice 35 days post-injury in an acute manner. The voided stain on paper method and urodynamics revealed that fluoxetine reduced the amount of residual urine in the bladder and decreased bladder and external urethral sphincter pressure in a mouse model of moderate spinal cord injury. However, fluoxetine did not improve the micturition function in a mouse model of severe spinal cord injury. In contrast, the other serotonergic drugs had no effects on the micturition functions of spinal cord injury model mice. This study was ethically approved by the Institutional Animal Care and Use Committee of Jiangsu Province Hospital of Chinese Medicine (approval No. 2020DW-20-02) on September 11, 2020.
Chinese Library Classification No. R453; R744.2; R971+.2
Introduction
The neural circuits involved in micturition are controlled by the peripheral sensory system, the spinal cord, and the brain. Spinal cord injuries (SCIs) and injuries at the brain-stem level often disrupt the supraspinal tract, which controls micturition. When supraspinal control is lost, the coordinated contraction of the bladder and the external urethral sphincter (EUS) during voiding is greatly affected, often resulting in detrusor sphincter dyssynergia and low void efficiency (Taweel and Seyam, 2015). As a result, SCI patients gradually develop pathologically high intra-bladder pressure, causing bladder hypertrophy, abnormal pain, and urinary tract damage. Therefore, the recovery of the bladder function is often described as highly desirable among SCI patients (Estores, 2003; Anderson, 2004; Han and Xu, 2020).
To improve functional bladder recovery, much attention has focused on pharmacological interventions (Kroll, 2017; White and Holmes, 2019; Tang et al., 2020). For example, several studies demonstrated that β3-adrenergic receptor agonists could alleviate hyperactive detrusor function post-SCI (Frazier et al., 2006; Andersson, 2017). Other studies demonstrated that the administration of serotonergic agonists could enhance micturition function in rats with chronic SCI (Dolber et al., 2007; Norouzi-Javidan et al., 2016). Supporting the impact of serotonergic inputs, treatment with serotonergic receptor antagonists was also shown to block the functional recovery mediated by the robust regeneration/sprouting of descending serotonergic axons (Lee et al., 2013; Lang et al., 2015). However, the development of effective drugs that are able to improve voluntary bladder control post-SCI remains preliminary. In this study, we established spinal contusion injuries of varying severity, and we screened a group of clinically used serotonergic drugs for urinary functional recovery by monitoring voiding patterns and urodynamics.
Materials and Methods
Experimental animals
Owing to the technical difficulty of bladder expression following spinal cord injury when using male mice, this study only included female mice. Adult female C57 mice (n = 99), aged 8 weeks, weighing 22–26 g, were obtained from the Laboratory Animal Center of Nantong University in China [license No. SCXK (Su) 2014-0001]. This study was ethically approved by the Institutional Animal Care and Use Committee of Jiangsu Province Hospital of Chinese Medicine (approval No. 2020DW-20-02) on September 11, 2020. All experiments were designed and reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Age- and weight-matched animals were housed at room temperature (22°C) under a standard 12-hour light/dark cycle and were randomly assigned to sham or experimental groups. A flowchart of the experiments, including detailed information regarding the grouping of animals, the numbers of animals in each group, and the varying injury severities that were established, is presented in Figure 1.
Figure 1.
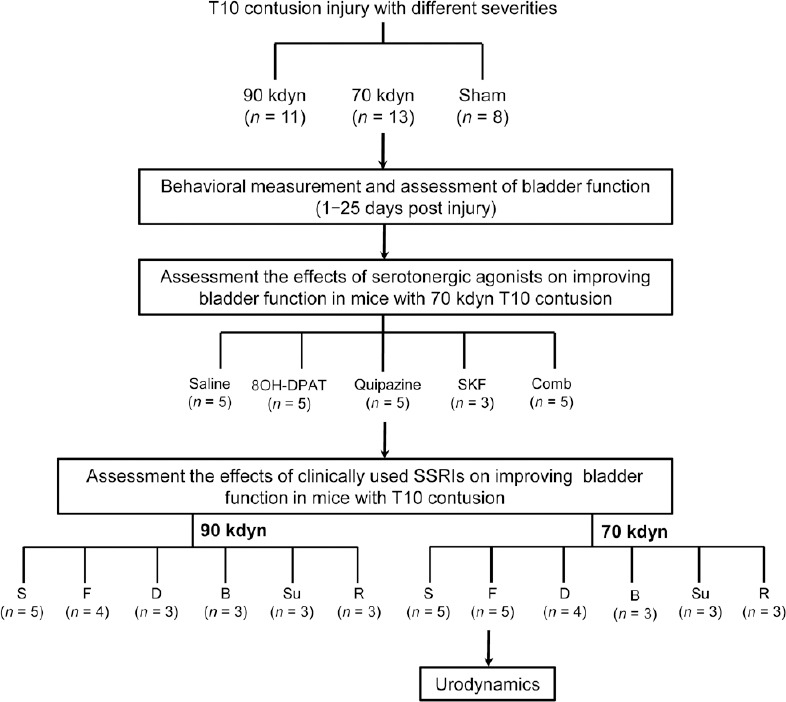
Experimental design flowchart.
Group: B, Buspirone; D, Duloxetine; F, Fluoxetine; R, Rizatriptan; S, Saline; Su, Sumatriptan. 8-OH-DPAT: 8-Hydroxy-2-(di-n-propylamino) tetralin, a 5-hydroxytryptamine 1A agonist; Comb: the combination of 8-OH-DPAT and quipazine; kdyn: kilodyne; quipazine: a 5-hydroxytryptamine 2A agonist; SSRI: selective serotonin reuptake inhibitor; SKF: SKF-82197, a dopamine receptor agonist. 1 dyn = 10–5 N.
Spinal contusion injury model
To generate the spinal contusion injury models, we first anesthetized mice using the nasal application of 2% isoflurane (2 L/min). We then performed a laminectomy at the thoracic level (T10) to remove the vertebra and expose the spinal cord underneath. Lateral clamps were used to stabilize the spinal cord. The contusion spinal injury was induced using an Infinite Horizon spinal cord impactor (Precision Systems and Instrumentation, Fairfax Station, VA, USA), applying either 70 (moderate) or 90 (severe) kilodyne (kdyn; 1 dyn = 10–5 N) of force (Matyas et al., 2017). In the sham group, the animals received a laminectomy but were not subjected to any contusion injury.
Drug treatment
We tested the acute effects of various drugs on voiding. The following drugs were administered intraperitoneally 35 days post-injury: normal saline; quipazine (0.2 mg/kg; Cat# Q1004; Sigma, St. Louis, MO, USA; n = 5) (Liu et al., 2017); 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT; 0.1 mg/kg; Cat# H8520, Sigma; n = 5) (Liu et al., 2017), SKF-82197 (0.1 mg/kg; Cat# S179, Sigma; n = 3) (Liu et al., 2017), fluoxetine (20 mg/kg; Cat# F132, Sigma; n = 9) (Lee et al., 2003), duloxetine (1 mg/kg; Cat# D044, Sigma; n = 7) (Wróbel et al., 2018), buspirone (1 mg/kg; Cat# B7148, Sigma; n = 6) (Myers et al., 2004), sumatriptan (1 mg/kg; Cat# 1642154, Sigma; n = 6) (Lychkova and Pavone, 2013); and rizatriptan (50 mg/kg; Cat# SML0247, Sigma; n = 6) (Langford et al., 2010) in total (see detailed information in Figure 1). All drug treatments were administered 30–120 minutes before the voided stain on paper (VSOP) assay was performed.
Behavioral assessment
We assessed all mice in each group (sham, 70, and 90 kdyn contusion injury) with the Basso Mouse Scale (BMS) (Basso et al., 2006) at days 1, 3, 5, 8, 12, and 20 post-injury. The scores on the BMS range from 0 to 9 (0 indicates complete paralysis/loss of locomotion, and 9 indicates the full recovery of coordinated locomotion). This well-established scale was used to describe spontaneous locomotor recovery post-SCI.
Immunohistochemistry of tissue sections
To collect specimens for histological analyses, we first performed transcardial perfusion using 4% paraformaldehyde at 20 days post-injury. The whole spinal cord tissues were then post-fixed in 4% paraformaldehyde overnight, cryoprotected with 30% sucrose, and transversely sectioned into 40-µm-thick sections using a cryostat (Leica CM1950, Nussloch. Germany). Sections were blocked with 10% normal donkey serum, incubated with rabbit anti-glial fibrillary acidic protein (1:600; Cat# Z0334; DAKO, Glostrup, Denmark) at 4°C overnight. The sections were then incubated with the secondary antibody Alexa Fluor 488-conjugated donkey anti-rabbit IgG (1:200; Invitrogen, Waltham, MA, USA) at room temperature for 2 hours, and mounted onto slides using Fluoromount-G (Cat# 0100-01, Southern Biotech, Birmingham, AL, USA) mounting solution. Fluorescent images of transverse spinal sections were taken using a 20× lens on a Zeiss 710 Confocal microscope (Carl Zeiss Microcopy, White Plains, NY, USA).
Residual urine and VSOP measurement
We collected residual urine by manually expressing the bladder every 12 hours for 1–25 days post the contusion injury, starting 1 day post-injury. Mice were anesthetized with nasally applied 2% isoflurane (2 L/min) gas during bladder expression (David and Steward, 2010). Expressed urine was collected in a 1.5 mL tube, and the volume was measured. We also performed the VSOP assay (Sugino et al., 2008) to assess the effects of different drug treatments on bladder function. Briefly, we first generated a standard curve by placing different volumes of normal saline (50–800 µL) on filter paper (Whatman Cat# 05-714-5; Sigma-Aldrich, Cambridge, MA, USA). The stain areas were then marked, scanned, and analyzed using ImageJ software (version 1.8.0_172; National Institutes of Health, Bethesda, MD, USA) to obtain relative areas. A standard curve was generated by correlating the saline volume with the stained area. To normalize the hydration status among multiple animals, we supplied mice with distilled water (50 mL/g, subcutaneous injection) before the VSOP measurement. During VSOP, the animals were placed in a wire-netted cage on top of the filter paper (approximately 20 cm above). After 2 hours, the residual urine was manually expressed. The filter paper was placed under an ultraviolet (UV) light to clearly illuminate the urine stain (Hou et al., 2016). Urine stains were then marked, scanned, and measured with ImageJ to determine the relative area. The areas of all urine stains were added together and converted to a micturition volume based on the formula generated using the standard curve. The voiding efficiency was calculated as the ratio of the micturition volume to the sum of the micturition and residual volumes.
Urodynamic recordings and analysis
Terminal urodynamic recordings were performed as described in the literature, with modifications (Lee et al., 2013; Lang et al., 2015). Briefly, the mice were first anesthetized using urethane (0.8 g/kg, intraperitoneal injection), and then we carefully inserted a polyethylene catheter into the bladder through the urethra. To elicit repetitive voids, saline was constantly infused into the bladder at 40 µL/min. To monitor bladder pressure, we used the inserted catheter to infuse saline using a pressure transducer (Grass Technologies, Astronova Inc., West Warwick, RI, USA). To perform electromyography (EMG) recordings, two fine-hook electrodes were positioned surround the EUS. EMG signals were captured with a pre-amplifier (model 1700, A-M Systems) and digitized by Digidata Acquisition System (1440A; Molecular Devices, Silicon Valley, CA, USA). A band-pass filter of 10–1000 Hz and a sampling rate of 2000 Hz were applied to the recording.
Statistical analysis
For all figures, data are presented as the means, and error bars indicate the standard error of the mean (SEM). The number (n) of samples employed is indicated in the figure legends. For two-group comparisons, we chose the Student’s t-test (two-way grouped). For comparisons between multiple groups, we performed a one-way analysis of variance or a repeated-measures of analysis of variance, with Bonferroni correction. Normality and homogeneity of variance were assessed by STATA (version 12; StataCorp LLC., College Station, TX, USA). Statistical analysis was performed using Prism 8.0 (GraphPad Software, San Diego, CA, USA). Significance was set to P < 0.05.
Results
Generation of spinal contusion injuries of different severities at T10
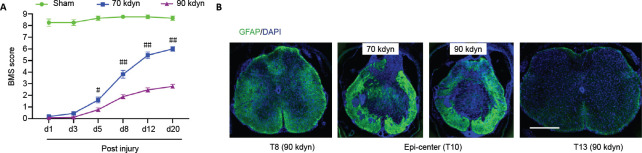
First, we assessed the behavioral outcomes of inducing contusion injuries with different forces. Although in both groups, the BMS scores steadily increased post-injury, the hind limb locomotor recovery was significantly better in the group subjected to moderate force (70 kdyn) compared with the group subjected to a stronger (90 kdyn) force (Figure 2A). At 3 weeks post-injury, the mice in the moderate contusion injury group showed coordinated plantar stepping, achieving a mean BMS score of 6. In contrast, those in the stronger force contusion group only showed occasional plantar stepping, with a mean BMS score of 3 (Figure 2A). Histologically, immunoreactivity against glial fibrillary acidic protein, which serves as a marker for lesion-induced inflammation, showed that the area of the lesion epicenter was smaller in mice from the moderate contusion group (70 kdyn) compared with the group with more severe (90 kdyn) contusion injury (Figure 2B). These results suggested that the moderate contusion injury spared more descending tracts than the severe contusion injury.
Figure 2.
Characterization of T10 spinal contusion injury severity.
(A) Time course of BMS scores in animals in the sham, 70 kdyn, or 90 kdyn spinal contusion injury groups. Data are expressed as the mean ± SEM (n = 8, 11, and 13 for sham, 90 kdyn, and 70 kdyn groups, respectively). #P < 0.05, ##P < 0.01, vs. 90 kdyn group (repeated-measures analysis of variance followed by Bonferroni correction). Lower BMS scores indicated worse behavioral outcomes. (B) Representative images of transverse spinal cord sections, rostral to, ant the epicenter, and caudal to the lesion site, stained with anti-GFAP (green, Alexa Fluor 488), a glial inflammation marker. Scale bar: 500 μm. BMS: Basso Mouse Scale; DAPI: 4′,6-diamidino-2-phenylindole; GFAP: glial fibrillary acidic protein; kdyn: kilodyne. 1 dyn = 10–5 N.
Bladder function following spinal contusion injuries of different severities
We next assessed the bladder functions of animals following the generation of varying severities of spinal contusion injuries. Consistent with previous studies (David and Steward, 2010), both moderate and severe contusion injuries resulted in compromised bladder function, as indicated by high residual urine volumes. In the moderate contusion group, the volume of expressible urine peaked 4 days post-injury and then declined until reaching a plateau at approximately 12 days post-injury, which remained significantly higher than that for the sham group (Figure 3). In contrast, the residual urine volume of mice in the severe contusion injury group remained high from days 4–20 post-injury (Figure 3), suggesting no spontaneous recovery of bladder function.
Figure 3.
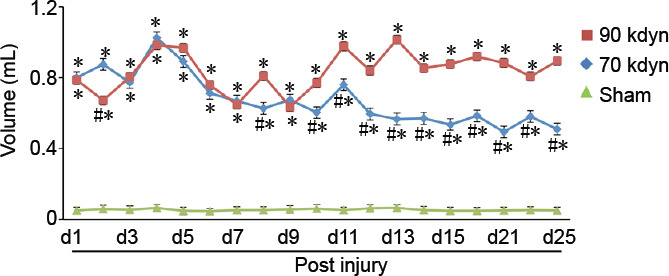
Time course of spontaneous voiding recovery following spinal contusion injuries of different severities.
Starting 1 day post-injury and continuing for 25 days, residual urine was expressed each morning, and the volume was measured. Data are expressed as the mean ± SEM (n = 8, 11, and 13 for sham, 90 kdyn, and 70 kdyn groups, respectively). *P < 0.05, vs. sham group; #P < 0.05, vs. 90 kdyn group (repeated-measures analysis of variance followed by Bonferroni post hoc test). kdyn: Kilodyne. 1 dyn = 10–5 N.
Serotonergic agonists improve micturition function in mice with moderate spinal contusion injuries
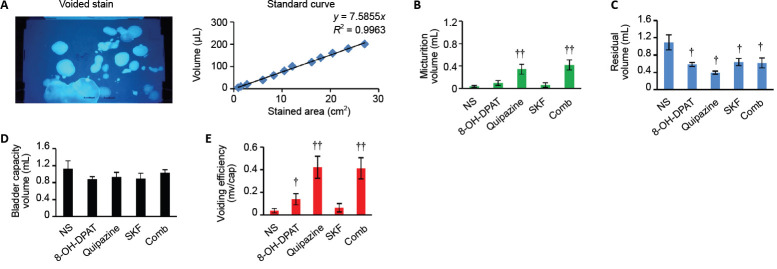
Previous studies suggested that the activation of serotonergic receptors could improve micturition function post-SCI (Dolber et al., 2007; Norouzi-Javidan et al., 2016). To further test this hypothesis, we treated mice with moderate spinal contusion injury with various serotonergic agonists, including quipazine and 8-OH-DPAT, which are both 5-hydroxytryptamine receptor 2A agonist. SKF-8219, a dopamine receptor (D1/D5) agonist, was used as a control. To assess the micturition function, we measured VSOP (Figure 4A). Both quipazine and 8-OH-DPAT significantly improved the voiding efficiency of mice with moderate spinal contusion injury (Figure 4B–E). In contrast, neither normal saline nor SKF-8219 showed any effects (Figure 4B–E), indicating the specific improvement of bladder functional recovery post-SCI in response to serotonergic agents.
Figure 4.
Serotonergic receptor agonists increase the voiding efficiency in mice with moderate spinal contusion injuries.
(A) Representative image of a voiding stain (left) and the standard curve (right) generated for VSOP measurements. (B–E) Comparisons of micturition volumes (B), residual volumes (C), bladder capacities (D), and voiding efficiencies (E) in animals with moderate T10 spinal contusion injuries treated with normal saline (NS), 8-OH-DPAT, quipazine, SKF, or comb (n = 5, 5, 5, 3, and 5, respectively). Data are expressed as the mean ± SEM. †P < 0.05, ††P < 0.01, vs. NS (one-way analysis of variance followed by Bonferroni’s post hoc test). 8-OH-DPAT: 8-Hydroxy-2-(di-n-propylamino) tetralin, a 5-hydroxytryptamine 1A agonist; cap: bladder capacity; kdyn: kilodyne; mv: micturition volume; NS: normal saline; quipazine: a 5-hydroxytryptamine 2A agonist; SKF: SKF-82197, a dopamine receptor agonist; VSOP: voided stain on paper. 1 dyn = 10–5 N.
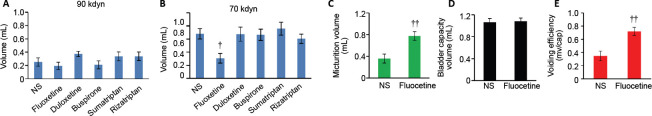
Fluoxetine, but not other clinically approved serotonergic drugs, improves micturition function in mice with moderate spinal contusion injuries
To promote the translatability of this study, we tested the effects of a group of serotonergic drugs that are currently clinically approved. Among these drugs, the widely used selective serotonin reuptake inhibitor (SSRI) fluoxetine increased the voiding volume during the VSOP test, indicating improved voiding efficiency (Figure 5A–E). In contrast, the tested clinically approved serotonergic agonists did not improve micturition function (Figure 5B), likely because of a lack of specificity for the activation of serotonergic supraspinal axons, which is mediated by 5-hydroxytryptamine receptors. Fluoxetine treatment did not have any effects on micturition function in mice with severe spinal contusion injury (Figure 5A), which may indicate that all descending serotonergic axons might be severed.
Figure 5.
Fluoxetine specifically increased the voiding efficiency in animals with moderate T10 spinal contusion injury.
(A, B) Expressed residual urine after VSOP from mice with severe (A) or moderate (B) T10 spinal contusion injuries following treatment with normal saline (NS), fluoxetine, duloxetine, buspirone, sumatriptan, or rizatriptan. (C–E) Comparisons of micturition volumes (C), bladder capacities (D), and voiding efficiencies (E) among animals with moderate T10 spinal contusion injuries treated with NS and fluoxetine. Data are expressed as the mean ± SEM (5A: n = 5, 4, 3, 3, 3, and 3; 5B: n = 5, 5, 4, 3, 3, and 3, in NS, saline, fluoxetine, duloxetine, buspirone, sumatriptan, and rizatriptan treatment groups, respectively). †P < 0.05, vs. NS group (one-way analysis of variance followed by Bonferroni’s post hoc test), ††P < 0.01, vs. normal saline group (Student’s t-test). cap: Bladder capacity; kdyn: kilodyne; mv: micturition volume; NS: normal saline; VSOP: voided stain on paper. 1 dyn = 10–5 N.
Fluoxetine improves the quality of bladder function following moderate spinal contusion injury
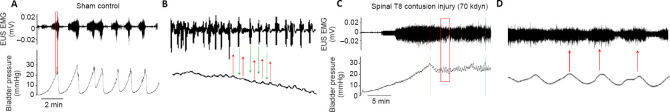
To further investigate the quality of bladder function, we performed urodynamics assessments to measure bladder contractions and EUS activity during voiding in the sham and moderate spinal contusion injury groups. As shown in Figure 6, in the sham group, the EUS showed a basal level of tonic activity. Starting from the onset of voiding, when the bladder pressure rapidly increases, the EUS bursts phasically to coordinate with bladder contractions (Figure 6A and B). In contrast, following moderate spinal contusion injury, animals developed hyperactive, atypical EUS EMG bursting activity during both the non-voiding and voiding periods (Figure 6C and D). The administration of fluoxetine significantly diminished the amplitudes of bladder contractions and EUS EMG bursting activity during voiding (Figure 7A–D). However, we did not observe any overt improvements in coordinated EUS-bladder contractions during micturition. These results suggested that fluoxetine treatment might reduce bladder hyperreflexia and EUS hyperactivation post-SCI.
Figure 6.
Urodynamics of mice in sham or moderate T10 spinal contusion injury groups.
(A and B) Representative EUS EMG activity and bladder pressure recordings (A) with magnified portions (B, rectangle in A) during micturition in the sham groups. Arrows in B (red: bladder contraction; green: EUS contraction) indicate the alternating (coordinated) contractions of the detrusor and the EUS during voiding. (C and D) Representative EUS EMG activity and bladder pressure recordings (C) with magnified portions (D, rectangle in C) during micturition in animals with moderate (70 kdyn) spinal contusion injuries. Arrows in D indicated the co-contractions (dyssynergia) of the detrusor and the EUS during voiding. n = 5 for sham or mice treated with 70 kdyn T10 contusional spinal contusion injuries tested for urodynamics. EMG: Electromyography; EUS: external urethral sphincter; kdyn: kilodyne. 1 dyn = 10–5 N.
Figure 7.
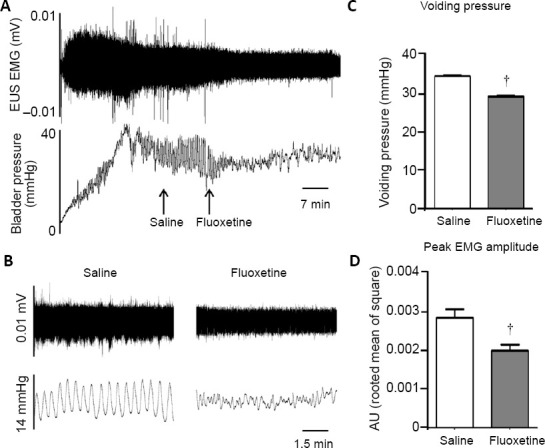
Fluoxetine improves bladder function recovery in mice with moderate T10 spinal contusion injuries.
(A, B) Representative EUS EMG activity and bladder pressure recordings (A) with magnified portions (B) during micturition. Arrows indicated the timing of saline or fluoxetine infusions. Note that fluoxetine treatment reduced the amplitudes of bladder contraction and EUS EMG activity. (C and D) Quantification of voiding pressure (C) and peak EMG amplitude (D) during voiding when saline or fluoxetine was administrated. Data are expressed as the mean ± SEM (n = 5). †P < 0.05, vs. normal saline group (Student’s t-test). EMG: Electromyography; EUS: external urethral sphincter; NS: normal saline.
Discussion
In this study, after establishing T10 spinal contusion injuries of varying severities, we observed different levels of functional micturition deficits. The pharmacological study showed that the activation of serotonergic, but not dopaminergic, receptors was able to significantly improve void volumes and voiding efficiency. We then screened multiple clinically approved serotonergic drugs and identified the SSRI fluoxetine as a positive effector that was able to improve bladder function following moderate spinal contusion injury. These results indicate that fluoxetine may serve as a translatable treatment for improving micturition function post-SCI.
Multiple supraspinal nuclei are involved in micturition control, including both the bladder and the urethral sphincter. The most studied structure is the pontine micturition center (or Barrington’s nucleus), a nucleus located in the pontine area (Fowler et al., 2008). Other descending neurons, including serotonergic neurons that originate from the medullary raphe nuclei and noradrenergic neurons that originate from the locus coeruleus or the brain stem A5 area, are known to be involved in micturition control (Malykhina, 2017; Manohar et al., 2017). However, the specific roles played by distinct supraspinal tracts in micturition control remain largely unknown (Fowler et al., 2008; Hou et al., 2016; Keller et al., 2018; Yao et al., 2018). The SSRI fluoxetine improved functional bladder recovery but not duloxetine, which is a serotonin and norepinephrine reuptake inhibitor, suggesting that serotonergic and noradrenergic descending axons might play distinct and possible contradictory roles in micturition control.
Serotonin receptors are broadly expressed in the central nervous system, including in the lumbosacral spinal cord. Consistent with a previous study (Lang et al., 2015), our results showed that two traditional 5-hydroxytryptamine 1A/2A receptor agonists substantially increased both voiding volume and efficiency following moderate spinal contusion injury. However, the use of clinically approved 5-hydroxytryptamine receptor agonists at safe doses was unable to achieve a similar effect. In contrast, one clinically approved SSRI, fluoxetine, was able to significantly improve micturition function. Fluoxetine had no overt effects in animals with severe contusion spinal cord injury, suggesting that very few descending axons survived in the severe injury model. These results suggested that the specific activation of serotonergic supraspinal circuits is crucial for improved bladder function post-SCI.
Fluoxetine did not improve dyssynergia post-SCI, which suggested a low efficiency for the reformation of spontaneous supraspinal inputs originating from the medullary raphe nuclei or other micturition related nuclei. The extent of axonal regeneration or sprouting beyond the lesion site has been shown to be extremely restricted in the adult mammalian central nervous system (He and Jin, 2016; Tran et al., 2018; Courtine and Sofroniew, 2019). Various strategies have been applied to the promotion of axonal regeneration in the central nervous system, such as enhancing intrinsic neuronal growth properties or alleviating the extrinsic inhibitory environment (Lang et al., 2015; He and Jin, 2016; Carmichael et al., 2017; Liu et al., 2017; Rosenzweig et al., 2019). Combinatory strategies that include both clinically approved pharmacological treatments and methods that promote the regeneration of descending tract axons might achieve improved functional recovery for both moderate and severe SCI conditions. This study was limited by the focus on acute SSRI effects, and future studies will investigate whether the chronic administration of fluoxetine can facilitate the recovery of bladder function post-SCI. Our results showed that fluoxetine, a commonly used anti-depression drug, might help improve bladder function post-SCI, thus shedding light on potential therapeutic targets for the development of novel drugs to promote the functional recovery of micturition following SCI or other traumatic central nervous system injuries.
Additional file: Open peer review report 1 (85.7KB, pdf) .
Acknowledgments:
We thank the General Clinical Research Center at Jiangsu Province Hospital of Chinese Medicine, China for technical assistance.
Footnotes
Conflicts of interest: The authors declare no competing interests.
Financial support: This work was supported by Jiangsu Province Hospital of Chinese Medicine, No. Y19061 (to LM). The funder had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The study was approved by the Institutional Animal Care and Use Committee of Jiangsu Province Hospital of Chinese Medicine (approval No. 2020DW-20-02) on September 11, 2020.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Luc Bauchet, Montpellier University Medical Center, France; Hızır Ufuk Akdemir, Ondokuz Mayis University, Turkey.
Funding: This work was supported by Jiangsu Province Hospital of Chinese Medicine, No. Y19061 (to LM).
P-Reviewers: Bauchet L, Akdemir HU; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Giles L, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE. On the site and mechanism of action of β(3)-adrenoceptor agonists in the bladder. Int Neurourol J. 2017;21:6–11. doi: 10.5213/inj.1734850.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 4.Carmichael ST, Kathirvelu B, Schweppe CA, Nie EH. Molecular, cellular and functional events in axonal sprouting after stroke. Exp Neurol. 2017;287:384–394. doi: 10.1016/j.expneurol.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courtine G, Sofroniew MV. Spinal cord repair: advances in biology and technology. Nat Med. 2019;25:898–908. doi: 10.1038/s41591-019-0475-6. [DOI] [PubMed] [Google Scholar]
- 6.David BT, Steward O. Deficits in bladder function following spinal cord injury vary depending on the level of the injury. Exp Neurol. 2010;226:128–135. doi: 10.1016/j.expneurol.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolber PC, Gu B, Zhang X, Fraser MO, Thor KB, Reiter JP. Activation of the external urethral sphincter central pattern generator by a 5-HT(1A) receptor agonist in rats with chronic spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1699–1706. doi: 10.1152/ajpregu.00142.2006. [DOI] [PubMed] [Google Scholar]
- 8.Estores IM. The consumer’s perspective and the professional literature: what do persons with spinal cord injury want. J Rehabil Res Dev. 2003;40:93–98. doi: 10.1682/jrrd.2003.08.0093. [DOI] [PubMed] [Google Scholar]
- 9.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frazier EP, Schneider T, Michel MC. Effects of gender age and hypertension on beta-adrenergic receptor function in rat urinary bladder. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:300–309. doi: 10.1007/s00210-006-0077-y. [DOI] [PubMed] [Google Scholar]
- 11.Han Q, Xu XM. Neurotrophin-3-mediated locomotor recovery: a novel therapeutic strategy targeting lumbar neural circuitry after spinal cord injury. Neural Regen Res. 2020;15:2241–2242. doi: 10.4103/1673-5374.284985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He Z, Jin Y. Intrinsic control of axon regeneration. Neuron. 2016;90:437–451. doi: 10.1016/j.neuron.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Hou XH, Hyun M, Taranda J, Huang KW, Todd E, Feng D, Atwater E, Croney D, Zeidel ML, Osten P, Sabatini BL. Central control circuit for context-dependent micturition. Cell. 2016;167:73–86.e12. doi: 10.1016/j.cell.2016.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller JA, Chen J, Simpson S, Wang EH, Lilascharoen V, George O, Lim BK, Stowers L. Voluntary urination control by brain-stem neurons that relax the urethral sphincter. Nat Neurosci. 2018;21:1229–1238. doi: 10.1038/s41593-018-0204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroll P. Pharmacotherapy for pediatric neurogenic bladder. Paediatr Drugs. 2017;19:463–478. doi: 10.1007/s40272-017-0249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang BT, Cregg JM, DePaul MA, Tran AP, Xu K, Dyck SM, Madalena KM, Brown BP, Weng YL, Li S, Karimi-Abdolrezaee S, Busch SA, Shen Y, Silver J. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature. 2015;518:404–408. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 18.Lee KS, Na YG, Dean-McKinney T, Klausner AP, Tuttle JB, Steers WD. Alterations in voiding frequency and cystometry in the clomipramine induced model of endogenous depression and reversal with fluoxetine. J Urol. 2003;170:2067–2071. doi: 10.1097/01.ju.0000080648.01911.9a. [DOI] [PubMed] [Google Scholar]
- 19.Lee YS, Lin CY, Jiang HH, Depaul M, Lin VW, Silver J. Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J Neurosci. 2013;33:10591–10606. doi: 10.1523/JNEUROSCI.1116-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Wang X, Li W, Zhang Q, Li Y, Zhang Z, Zhu J, Chen B, Williams PR, Zhang Y, Yu B, Gu X, He Z. A sensitized IGF1 treatment restores corticospinal axon-dependent functions. Neuron. 2017;95:817–833.e4. doi: 10.1016/j.neuron.2017.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lychkova AE, Pavone LM. Role of serotonin receptors in regulation of contractile activity of urinary bladder in rabbits. Urology. 2013;81:696.e13–18. doi: 10.1016/j.urology.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 22.Malykhina AP. How the brain controls urination. Elife. 2017;6:e33219. doi: 10.7554/eLife.33219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manohar A, Curtis AL, Zderic SA, Valentino RJ. Brain-stem network dynamics underlying the encoding of bladder information. Elife. 2017;6:e29917. doi: 10.7554/eLife.29917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matyas JJ, O’Driscoll CM, Yu L, Coll-Miro M, Daugherty S, Renn CL, Faden AI, Dorsey SG, Wu J. Truncated TrkB. T1-mediated astrocyte dysfunction contributes to impaired motor function and neuropathic pain after spinal cord injury. J Neurosci. 2017;37:3956–3971. doi: 10.1523/JNEUROSCI.3353-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers RA, Plym MJ, Signor LJ, Lodge NJ. 1-(2-pyrimidinyl)-piperazine, a buspirone metabolite, modulates bladder function in the anesthetized rat. Neurourol Urodyn. 2004;23:709–715. doi: 10.1002/nau.20037. [DOI] [PubMed] [Google Scholar]
- 26.Norouzi-Javidan A, Javanbakht J, Barati F, Fakhraei N, Mohammadi F, Dehpour AR. Effect of 5-HT7 receptor agonist LP-211, on micturition following spinal cord injury in male rats. Am J Transl Res. 2016;8:2525–2533. [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenzweig ES, Salegio EA, Liang JJ, Weber JL, Weinholtz CA, Brock JH, Moseanko R, Hawbecker S, Pender R, Cruzen CL, Iaci JF, Caggiano AO, Blight AR, Haenzi B, Huie JR, Havton LA, Nout-Lomas YS, Fawcett JW, Ferguson AR, Beattie MS, et al. Chondroitinase improves anatomical and functional outcomes after primate spinal cord injury. Nat Neurosci. 2019;22:1269–1275. doi: 10.1038/s41593-019-0424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugino Y, Kanematsu A, Hayashi Y, Haga H, Yoshimura N, Yoshimura K, Ogawa O. Voided stain on paper method for analysis of mouse urination. Neurourol Urodyn. 2008;27:548–552. doi: 10.1002/nau.20552. [DOI] [PubMed] [Google Scholar]
- 29.Tang MM, Chen HC, Xie HC, Zhang Y, Tan XS, Sun YX, Huang YN. Histocompatibility of poly(L-lactide-co-ε-caprolactone)/cross-linked polyvinylpyrrolidone ureteral stent grafted into the rat bladder. Zhongguo Zuzhi Gongcheng Yanjiu. 2020;24:583–588. [Google Scholar]
- 30.Taweel WA, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Rep Urol. 2015;7:85–99. doi: 10.2147/RRU.S29644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran AP, Warren PM, Silver J. The biology of regeneration failure and success after spinal cord injury. Physiol Rev. 2018;98:881–917. doi: 10.1152/physrev.00017.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White AR, Holmes GM. Investigating neurogenic bowel in experimental spinal cord injury: where to begin? Neural Regen Res. 2019;14:222–226. doi: 10.4103/1673-5374.244779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wróbel A, Rechberger E, Rechberger T. The influence of duloxetine on detrusor overactivity in rats with depression induced by 13-cis-retinoic acid. Int Urogynecol J. 2018;29:987–995. doi: 10.1007/s00192-017-3424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao J, Zhang Q, Liao X, Li Q, Liang S, Li X, Zhang Y, Li X, Wang H, Qin H, Wang M, Li J, Zhang J, He W, Zhang W, Li T, Xu F, Gong H, Jia H, Xu X, et al. A corticopontine circuit for initiation of urination. Nat Neurosci. 2018;21:1541–1550. doi: 10.1038/s41593-018-0256-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.