Keywords: AKT, cortical neurons, exosome, ischemia, neural progenitor cells, neuronal protection, oxygen and glucose deprivation, pluripotent stem cells, PTEN, signaling pathway
Abstract
Compared with other stem cells, human induced pluripotent stem cells-derived neural progenitor cells (iPSC-NPCs) are more similar to cortical neurons in morphology and immunohistochemistry. Thus, they have greater potential for promoting the survival and growth of neurons and alleviating the proliferation of astrocytes. Transplantation of stem cell exosomes and stem cells themselves have both been shown to effectively repair nerve injury. However, there is no study on the protective effects of exosomes derived from iPSC-NPCs on oxygen and glucose deprived neurons. In this study, we established an oxygen-glucose deprivation model in embryonic cortical neurons of the rat by culturing the neurons in an atmosphere of 95% N2 and 5% CO2 for 1 hour and then treated them with iPSC-NPC-derived exosomes for 30 minutes. Our results showed that iPSC-NPC-derived exosomes increased the survival of oxygen- and glucose-deprived neurons and the level of brain-derived neurotrophic factor in the culture medium. Additionally, it attenuated oxygen and glucose deprivation-induced changes in the expression of the PTEN/AKT signaling pathway as well as synaptic plasticity-related proteins in the neurons. Further, it increased the length of the longest neurite in the oxygen- and glucose-deprived neurons. These findings validate the hypothesis that exosomes from iPSC-NPCs exhibit a neuroprotective effect on oxygen- and glucose-deprived neurons by regulating the PTEN/AKT signaling pathway and neurite outgrowth. This study was approved by the Animal Ethics Committee of Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, China (approval No. SRRSH20191010) on October 10, 2019.
Chinese Library Classification No. R456; R741; Q257
Introduction
Application of stem cells for treatment of ischemic stroke has been shown to be a safe and effective method to improve neurological function (Kalladka and Muir, 2014; Gong an Liu, 2019). Animal experiments have shown that stem cell transplantation can promote cell differentiation, immune regulation, and reduce inflammatory response, and stimulate endogenous repair and lead to both angiogenesis and endogenous neurogenesis (Li et al., 2008; Suda et al., 2020). Many types of stem cells, including bone marrow mesenchymal stem cells (MSCs), embryonic stem cells, neural stem cells, and induced pluripotent stem cells (iPSCs), have been reported to protect neurons from ischemic stroke-induced death (Savitz et al., 2011; Janowski et al., 2015). However, their applications have several limitations. Transplantation of embryonic stem cells can lead to tumorigenicity and/or immune rejection (Ben-David and Benvenisty, 2011; Lv et al., 2015). Few studies, in particular in vivo studies, have addressed the mature and function of neuronal cells that differentiate from MSCs (Raedt et al., 2007; Nojiri et al., 2008), and there is limited direct evidence of neuron regeneration (Matsuse et al., 2011). Transplantation of iPSCs can cause teratomas (Kawai et al., 2010). In vitro and in vivo, neural stem cells have the potential to proliferate and differentiate into neurons, astrocytes, and oligodendrocytes (Tatarishvili et al., 2014), although naturally they are programmed to form olfactory bulb intermediate neurons (Lledo et al., 2008). Neural progenitor cells (NPCs) seem to be a particularly attractive choice as they can rapidly grow into functional cortical neurons. However, it is difficult to obtain NPCs and foreign NPCs are likely to cause a rejection reaction. Therefore, iPSC-derived NPCs (iPSC-NPCs) are a new and potent option that must be evaluated through neurological studies. Compared with the other options, iPSC-NPCs have a greater potential for accelerating the survival and growth of neurocytes and for mitigating astrogliosis (Lee et al., 2017).
In rat models of cerebral infarction, iPSC-NPCs expressing cortical neuron markers have a lower proliferation rate and a higher rate of differentiation into mature neurons as compared with non-fated cells (Tornero et al., 2013). Compared with non-specific cells, iPSC-NPCs are morphologically and immunohistochemically similar to cortical neurons with high axonal projection density and electrophysiological characteristics, which suggests that iPSC-NPCs can more effectively differentiate into cortical neurons and thus improve neurological recovery following ischemic injury (Tornero et al., 2013). Xiao et al. (2018) reported that transplanted iPSC-NPCs can differentiate into astrocytes and oligodendrocytes and promote cognitive function improvement in a mouse model of Alzheimer’s disease. iPSC-NPCs, implanted into a rat model of middle cerebral artery occlusion were found to improve the behavioral disorder following a stroke (Gomi et al., 2012). In addition, iPSC-NPCs transplanted in a pig model of ischemic stroke were found to increase the total score on the post-stroke assessment scale (Lau et al., 2018). The iPSC-NPCs that were transplanted on the opposite side of the injury migrated to the area around the infarct, which demonstrates the characteristics of tissue recovery. Transplantation of iPSC-NPCs initially produced neurotrophic effects on the host brain structure, and then helped to improve the reconstruction of damaged pathways (Polentes et al., 2012). In addition, transplantation of iPSC-NPCs significantly reduced inflammatory responses, microglia activation, glial proliferation, and apoptosis induced by ischemic stroke, which are keys for endogenous neurogenesis (Chang et al., 2013; Hermanto et al., 2018). In the microenvironment of ischemic stroke, iPSC-NPCs also can survive, proliferate, and differentiate into mature neurons or astrocytes (de la Rosa-Prieto et al., 2017; Hermanto et al., 2018). A previous study showed that iPSC-NPCs could be safely transplanted into the brain, without any tumor formation or any obvious rejection during the observation period after cell transplantation (Tornero et al., 2013).
The mechanism through which iPSC-NPCs improve neurological function following an ischemic stroke is currently unknown. Other than differentiating into cortical neurons and glial cells, mechanisms that mediate neuroprotection remain unclear. Co-culture of iPSC-derived endothelial progenitor cells and NPCs protects cerebral endothelial cells in a synergistic manner from hypoxia/reoxygenation injury through activation of the phosphatidylinositol 3 kinase (PI3K)/AKT pathway. Paracrine involving vascular endothelial growth factor and brain-derived neurotrophic factor (BDNF) leads to activation of the PI3K/AKT pathway (Wang et al., 2016). Using comparative whole-genome microarrays and cytokine neutralization, a previous analyzed iPSC-NPCs co-cultured with oxygen-glucose deprived (OGD) primary neurons and reported the identification of a neurorestorative secretome. Further, neutralizing the enriched cytokines abolished the neuroprotective effects in the co-culture (Lee et al., 2017). All these studies suggest that iPSC-NPCs protect co-cultured OGD neurons, possibly through a paracrine mechanism.
Exosomes are an important paracrine factor (Heijnen et al., 1999). They contain a large number of functional proteins, circular RNAs, long non-coding RNAs, microRNAs, DNA fragments, and other bioactive substances, which can shuttle between cells, mediate cell-cell communication, and affect the physiological functions of cells (Mittelbrunn and Sánchez-Madrid, 2012). Exosomes can be directly transferred into ischemic cells, and those derived from stem cells can repair tissue damage similar to how what transplanted stem cells can do. At present, treating ischemic stroke with exosomes derived from stem cells is a topic of intense research (Chen and Chopp, 2018; Cui et al., 2020). However, most studies have focused on exosomes derived from MSCs (Lai et al., 2010; Katsuda et al., 2013) and there is no study on the neuroprotective effect of iPSC-NPC-derived exosomes in ischemic stroke. In this study, we thus focused on this topic, using iPSC-NPCs and their protective potential in primary rat hippocampal neurons under OGD conditions in vitro.
Materials and Methods
Maintenance and differentiation of iPSCs
Human iPSCs DYR0100 were kindly provided by Stem Cell Bank, Chinese Academy of Sciences (Shanghai, China). NPCs were differentiated from iPSCs under DYR0100 by Hopstem Bioengineering (Hangzhou, China) following the differentiation method described previously (Xu et al., 2016). NPCs were plated as monolayers on laminin/poly-D-lysine-coated plates and maintained in HopCell™ Human NPC Medium (Cat# HopCell-NPM-500 Kit; Hopstem Bioengineering) containing Neurobasal/B27 (Gibco/Invitrogen, Carlsbad, CA, USA), brain derived neurotrophic factor (BDNF, 20 ng/mL; Tebu-Bio/PeproTech, Offenbach, Germany), glial cell line-derived neurotrophic factor (20 ng/mL; PeproTech), ascorbic acid (0.2 mM; Sigma-Aldrich, St. Louis, MO, USA), and dibutyryl adenosine 3′,5′-monophosphate (0.5 mM; Sigma-Aldrich). iPSC studies were conducted in accordance with the policy of the Medical School of Zhejiang University. The study was approved by Animal Ethics Committee of Sir Run Run Shaw Hospital (approval No. SRRSH20191010) on Octorber 10, 2019.
Purification of exosomes from culture media
After iPSC-NPCs reached confluence between 70–80%, they were washed three times with phosphate-buffered saline and then incubated in fresh culture media. Following 48 hours of culture, the cells were centrifuged at 800 × g for 10 minutes at 4°C. The supernatant was collected and centrifuged at 2000 × g for 10 minutes at 4°C to remove the cells and cell debris. The supernatant was collected and added into a new centrifuge tube and centrifuged at 3000 × g for 30 minutes, again at 4°C. The supernatant was discarded and the pellet was suspended in phosphate-buffered saline and stored at –80°C. The number of exosomes was measured as the total exosome-associated protein obtained using the Bradford protein assay (Beyotime Biotechnology, Shanghai, China).
Characterization of exosomes
The exosomes were observed under electron microscopy (FEI T12 Spirit TEM, Thermo Fisher Scientific, Waltham, MA, USA). Their particle size was determined using a particle-size analyzer (Brookhaven Instruments Corporation, Holtsville, NY, USA) with Malvern particle-size analysis. After extraction of the exosomes from the supernatant, exosomal protein markers including CD63, tumor susceptibility gene 101 (TSG101), heat shock protein 70 (HSP70), and apoptosis-linked gene-2 interacting protein X (Alix) were detected using western blot assays.
Neuronal cell culture and OGD induction
Cortical neuron cultures of embryonic Sprague-Dawley rats (E18, specific-pathogen-free level) were obtained from Zhejiang Academy of Medical Sciences (China; animal license No. SCXK [Zhe] 2019-0002) in the same way same as previously reported (Sandoval et al., 2011). All procedures were approved by the Animal Ethics Committee of Sir Run Run Shaw Hospital (approval No. SRRSH20191010) on Octorber, 10, 2019. The neurons were divided into control, OGD, and OGD + iPSC-NPC-derived exosomes groups. Primary neurons were injured using the in vitro OGD model, which was established by culturing in glucose-free medium in an atmosphere of 95% N2 and 5% CO2, which was humidified at 37°C for 1 hour. The neurons were then returned to normal culture condition. In the control group, neurons were cultured under normal conditions. The OGD neurons in the OGD + iPSC-NPC-derived exosomes group were cultured with exosomes (100 µg/mL) for 30 minutes (Deng et al., 2018; Pei et al., 2019).
Forty-eight hours after OGD treatment, cell samples were collected for immunofluorescence analysis, enzyme-linked immunosorbent assay (ELISA), and western blot assay. Cell Counting Kit-8 (CCK-8; Beyotime Biotechnology) was used according to the manufacturer’s instructions to evaluate proliferative activity 24, 48, and 72 hours after OGD.
ELISA
The culture medium from the various groups, namely control, OGD and OGD + iPSC-NPC-derived exosomes groups, was collected to measure the level of BDNF using an ELISA kit (SEKH-0101; Solarbio, Beijing, China) according to the manufacturer’s instructions.
Quantitative reverse transcription-polymerase chain reaction
Total RNA was extracted with TRIzol reagent (Invitrogen). cDNAs were synthesized with RevertAid™ First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). Quantitative reverse transcription polymerase chain reaction (PCR) was performed using FastStart Universal SYBR green Master (Roche, Basel Switzerland). All procedures were conducted following the manufacturers’ instructions. The primers were designed by Primer Premier 5.0 (Premier, Palo Alto, CA, USA) (Table 1). The mRNA level of the target gene was determined using methods described elsewhere (Wang et al., 2008; Brevet et al., 2010). In general, the process was performed as follows: denaturation at 95°C for 15 seconds and primer annealing at 60°C for 30 seconds. The data were analyzed using the 2–ΔΔCt method.
Table 1.
Primers for quantitative reverse transcription-polymerase chain reaction
| Gene | Primers sequence (5′–3′) | Product size (bp) |
|---|---|---|
| PTEN | Forward: GTG CAG ATA ATG ACA AG | 147 |
| Reverse: GAT TTG ACG GCT CCT CT | ||
| Akt | Forward: AGG AGG AGG AGG AGA TGG A | 327 |
| Reverse: GGT CGT GGG TCT GGA AAG | ||
| Synapsin1 | Forward: GCA GTT TGG TCA TTG GGC TG | 202 |
| Reverse: ACA GGG TAT GTT GTG CTG CT | ||
| PSD95 | Forward: AGA CGG TGA CGC AGA TGG AA | 100 |
| Reverse: TCG GGG AAC TCG GAG AGA AG | ||
| NF200 | Forward: GAG GCA CTG AAA AGC ACC A | 250 |
| Reverse: CAA AGC CAA TCC GAC ACT CT | ||
| GAP43 | Forward: GAT GGT GTC AAA CCG GAG GAT | 77 |
| Reverse: CTT GTT ATG TGT CCA CGG AAG C | ||
| GAPDH | Forward: GGA GCG AGA TCC CTC CAA AAT | 197 |
| Reverse: GGC TGT TGT CAT ACT TCT CAT GG |
GAP43: Growth associated protein 43; GAPDH: glyceraldehyde-3phosphate dehydrogenase; NF200: neurofilament 200; PSD95: postsynaptic density protein 95; PTEN: phosphatase and tensin homolog deleted on chromosome ten.
Western blot assay
To characterize exosomes, exosomal protein markers were detected by western blot assay (Hu et al., 2019). 20 µg exosomes were separated, and the exosome-free lysates were incubated in a 10% sodium dodecyl sulfate and subjected to polyacrylamide gel electrophoresis. After transfer, the samples were incubated with primary antibodies at 4°C overnight: mouse anti-CD63 (monoclonal, 1:1000, Cat# ab213090, Abcam, Cambridge, UK), rabbit anti-Hsp70 (monoclonal, 1:1000, Cat# ab181606, Abcam), rabbit anti-TSG101 (monoclonal, 1:1000, Cat# ab125011, Abcam), and rabbit anti-Alix (monoclonal, 1:1000, Cat# ab275377, Abcam). Then, the samples were incubated with goat anti-mouse IgG-horseradish peroxidase (1:500; Cat# ab205718; Abcam) and goat anti-rabbit IgG-horseradish peroxidase (1:500; Cat# 1662408; BioRad Laboratories, Hercules, CA, USA) at room temperature for 2 hours. The blots were visualized with enhanced chemiluminescence (ECL, Amersham, Uppsala, Sweden). Densitometric analysis was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
To determine whether iPSC-NPC exosomes protect neurons from OGD, we checked for the expression of phosphatase and tensin homolog deleted on chromosome ten (PTEN), Akt, phosphor-Akt (p-Akt), neurofilament 200 (NF200), growth associated protein 43 (GAP43), synapsin, and postsynaptic density protein 95 (PSD95). The samples from the three groups of neurons were incubated with primary antibodies at 4°C overnight: PTEN (rabbit polyclonal, 1:1000, Cat# 9552, CST, Danvers, MA, USA), Akt (rabbit monoclonal, 1:1000, Cat# 4685, CST), p-Akt (rabbit monoclonal, 1:2000, Cat# 4060, CST), NF200 (rabbit polyclonal, 1:1000, Cat# DF6060, Affinity Bioscience, Cincinnati, OH, USA), GAP43 (rabbit polyclonal, 1:1000, Cat# DF7766, Affinity Bioscience), synapsin (rabbit polyclonal, 1:1000, Cat# AF6201, Affinity Bioscience), PSD95 (rabbit polyclonal, 1:1000, Cat# AF5283, Affinity Bioscience), and β-actin (rabbit monoclonal, 1:1000, Cat#4970, CST).
Immunofluorescence analysis
Neurons were fixed and incubated with 4% paraformaldehyde as described previously (Lopez-Verrilli et al., 2013). Then, they were incubated with anti-β-tubulin 3 (rabbit polyclonal, 1:2000, Cat# ab18207, Abcam) at 4°C overnight and with secondary antibodies (Alexa 488-conjugated goat anti-rabbit IgG; 1:500; Cat# 4412, CST) at 37°C for 1 hour. The nucleus was stained with 4′,6-diamidino-2-phenylindole (DAPI) and the sections were observed under a microscope (FV3000; Olympus, Tokyo, Japan). After 48 hours of treatment, the lengths of the ten longest neurites were measured and the maximum branch number was counted using Image-ProPlus 5.1 software (Media Cybernetics, Rockville, MD, USA).
Statistical analysis
The data are presented as the mean ± standard error of mean (SEM). The cell viability of neurons, the levels of BDNF in culture medium, mRNA and protein expression along the PTEN/AKT signaling pathway and that of synaptic plasticity-related proteins, as well as quantitative analysis of cortical neurons morphology (longest neurite and maximum branch number) were analyzed by one-way analysis of variance with post hoc Bonferroni test. Statistical significance was considered as P < 0.05.
Results
Identification of exosomes from iPSC-NPCs
The results of electron microscopy and the Malvern particle-size analysis of the exosomes are shown in Figure 1A and B. The particles were revealed as cup-shaped vesicles with a double-layer membrane structure. The mean diameter of the exosomes was approximately 200 nm (173.82 ± 12.87 nm, n = 8). Results from western blot assay revealed exosome protein markers (CD63, TSG101, and HSP70) in the exosomes. Additionally, Alix was enriched in the exosomes, but not in the exosome-free media (Figure 1C).
Figure 1.
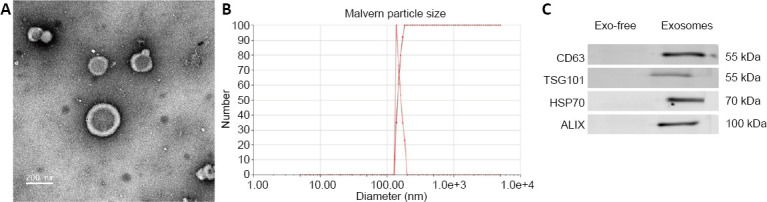
Characterization of exosomes.
(A) Exosomes were visualized using electron microscopy. The diameters of the exosomes were approximately 200 nm. Scale bar: 200 nm. (B) Particle size analysis of exosomes. (C) Western blot results for specific marker (CD63, TSG101, HSP70, and Alix) expression in the exosomes and exosome-free media (Exo-free). All experiments were repeated three times. Alix: Apoptosis-linked gene-2 interacting protein X; HSP70: heat shock protein 70; TSG101: tumor susceptibility gene 101.
Protective effects of iPSC-NPC exosomes on neuronal death induced by OGD
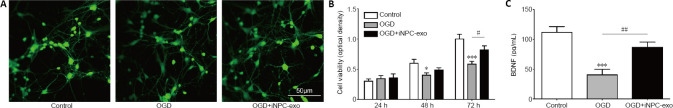
To evaluate the effect of exosomes that were derived from iPSC-NPCs on neuronal death caused by OGD, an OGD model of primary rat cortex neuron culture in vitro was used and cells were treated with iPSC-NPCs-derived exosomes following OGD treatment. The neurons were observed with β-tubulin 48 hours after treatment using immunofluorescence and a confocal microscope (Figure 2A). The results showed that immunopositivity of β-tubulin was lower after OGD treatment but significantly higher when iPSC-NPC-derived exosomes were present.
Figure 2.
Effect of iPSC-NPC-derived exosomes on OGD-induced neuronal viability.
(A) Immunopositive expression of β-tubulin 3 (green, stained by Alexa 488) in neurons 48 hours after oxygen-glucose deprivation (OGD) treatment. The results indicated that OGD treatment led to lower immunopositivity of β-tubulin, while iPSC-NPC-derived exosomes (OGD+iNPC-exo) led to significantly higher β-tubulin immunopositivity. Scale bar: 50 μm. (B) The cell viability of neurons was determined using the CCK-8 assay. (C) Quantitative results for brain-derived neurotrophic factor (BDNF) levels in the culture medium of cultured neurons. Data are presented as mean ± SD. *P < 0.05, ***P < 0.001, vs. control group; #P < 0.05, ##P < 0.01, vs. OGD group (one-way analysis of variance with post hoc Bonferroni test). All experiments were repeated three times.
To investigate the effect of exosomes on neuronal death caused by OGD, cell viability was measured using the CCK-8 assay. As shown in Figure 2B, compared with the control group, cell viability in the OGD group was significantly lower at 48 and 72 hours after OGD (P < 0.05 and P < 0.001, respectively). However, when iPSC-NPC-derived exosomes were present, this decrease was significantly less at 72 hours after OGD (P < 0.05, vs. OGD group). This suggests that exosomes derived from iPSC-NPCs can protect neurons from ischemic injury and restore neuronal survival
Improved levels of BDNF in the iPSC-NPC-derived exosome medium conditioned with primary neurons following OGD
To identify the mechanism underlying the protective effect of iPSC-NPC-derived exosomes, we performed an ELISA to measure the BDNF level in the culture medium. We found that expression of BDNF was lower than controls for both the OGD and OGD + iPSC-NPC-derived exosomes groups (both P < 0.001). Importantly, BDNF levels in the OGD + iPSC-NPC-derived exosomes group were significantly higher than those in the OGD group (P < 0.01; Figure 2C).
iPSC-NPCs-derived exosomes attenuate OGD-induced changes in the PTEN/AKT signaling pathway and in synaptic plasticity-related proteins
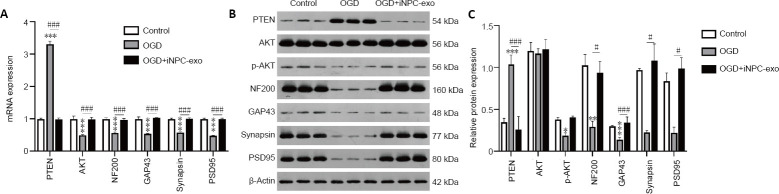
Expression of PTEN mRNA and protein was increased following OGD treatment (P < 0.001). However, after adding iPSC-NPC-derived exosomes, this increase was attenuated relative to OGD treatment alone (P < 0.001). OGD treatment decreased AKT mRNA expression as well as p-AKT protein expression (P < 0.001 and P < 0.05, respectively), and this decrease of AKT mRNA was also attenuated by iPSC-NPC-derived exosomes (P < 0.001), while the effect on p-AKT protein level was significant (P = 0.064). The expression of AKT protein was similar among control, OGD, and OGD + iPSC-NPC-derived exosomes groups (P > 0.05; Figure 3). mRNA expression of NF200, GAP43, synapsin, and PSD95 was lower after OGD treatment (P < 0.001) and this decrease was also attenuated by iPSC-NPC-derived exosome treatment (P < 0.001, Figure 3A). Additionally, the expression of NF200 and GAP43 protein was significantly lower after OGD treatment (P < 0.01 and P < 0.001, vs. control group). The expression of NF200, GAP43, synapsin, and PSD95 protein was higher in OGD neurons after treatment with iPSC-NPC-derived exosomes (P < 0.05, vs. OGD group; Figure 3B and C).
Figure 3.
Effect of iPSC-NPC-derived exosomes (OGD+iNPC-exo) on expression of the PTEN/AKT signaling pathway and of synaptic plasticity-related proteins in OGD induced neurons.
(A–C) mRNA expression (A) and protein expression (B, C) of the PTEN/AKT signaling pathway and of synaptic plasticity-related proteins (NF200, GAP-43, Synapsin, and PSD95) analyzed by polymerase chain reaction and western blot assay. The mRNA expression is described by the optical density ratio relative to the control group. Protein expression was described by the optical density ratio relative to β-actin. Data are presented as mean ± SD. ***P < 0.001, vs. control group; ###P < 0.001 (one-way analysis of variance with post hoc Bonferroni test). All experiments were repeated three times. GAP43: growth associated protein 43; iPSC-NPCs: induced pluripotent stem cells-derived neural progenitor cells; NF200: neurofilament 200; OGD: oxygen-glucose deprivation; p-Akt: phosphor-Akt; PSD95: postsynaptic density protein 95; PTEN: phosphatase and tensin homolog deleted on chromosome ten.
Effect of iPSC-NPC exosomes on neurite outgrowth in OGD primary neurons
To demonstrate the effect of iPSC-NPCs on neurite outgrowth, we measured the longest neurite and the maximum branch number using immunofluorescence staining of neurons with β-tubulin antibodies (Figure 4A). The results showed that β-tubulin immunopositivity was down-regulated in the OGD group. However, the addition of iPSC-NPC-derived exosomes significantly up-regulated β-tubulin immunopositivity. The longest neurite in the control group was significantly longer than that in the OGD group (P < 0.05), while this difference was significantly reduced with the addition of iPSC-NPC-derived exosome (P < 0.05, vs. OGD group; Figure 4B). However, neither OGD nor the addition of iPSC-NPC-derived exosomes affected the maximum branch number (P > 0.05; Figure 4C).
Figure 4.
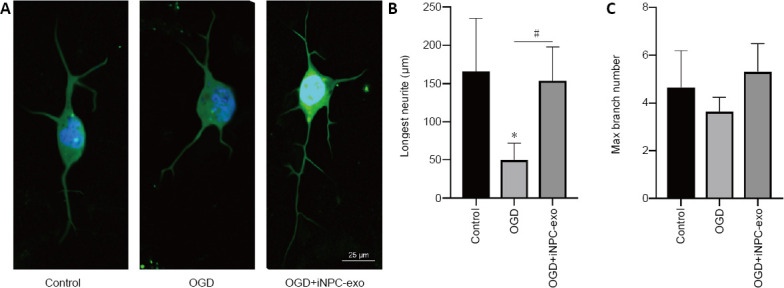
Effect of iPSC-NPC-derived exosomes on neuronal growth in OGD-induced neurons.
(A) Cortical neurons immunostained with anti-β-tubulin 3 to visualize the neurites. The immunopositivity of β-tubulin was down-regulated in the OGD group. However, iPSC-NPC-derived exosomes significantly up-regulated β-tubulin immunopositivity. Scar bar: 25 μm. (B, C) Quantitative analysis of cortical neuron morphology (longest neurite and maximum branch number). Data are presented as mean ± SD. *P < 0.05, vs. control group; #P < 0.05 (one-way analysis of variance with post hoc Bonferroni test). All experiments were repeated three times. iPSC-NPCs: Induced pluripotent stem cells-derived neural progenitor cells; OGD: oxygen-glucose deprivation; OGD+iNPC-exo: oxygen-glucose deprivation and induced pluripotent stem cells-derived exosome.
Discussion
Ischemic stroke can cause hypoxic-ischemic injury to the central nervous system, which leads to dysfunction of neurocytes and even death (Iglesias-Rey and Castillo, 2020; Mo et al., 2020). Treatment with stem cell is a new and promising therapeutic prospect for reducing ischemic neuronal damage. Stem cells and stem cell-secreted exosomes can participate in several signaling pathways and have roles in reducing ischemia-induced damage. iPSC-NPCs are a particularly attractive therapeutic option not only because they are easily obtained and do not induce a rejection reaction, but also because of their immunomodulatory properties and neurotrophic effects. However, the paracrine mechanism of action mediated by iPSC-NPCs to protect ischemic neurons remains unknown.
This study is the first to report that iPSC-NPC-derived exosomes can protect rat primary cortical neurons from OGD-induced death. Further, they increase BDNF secretion in the conditioned medium, attenuate OGD-induced changes in the PTEN/AKT signaling pathway and expression of synaptic plasticity-related proteins, as well as improve OGD-induced neurite damage. Our results provide evidence for the hypothesis that iPSC-NPC-derived exosomes are associated with neurotrophic effects observed in hypoxic-ischemic neurons in vitro, which may support the future application of iPSC-NPC-derived exosome therapy in patients with stroke. The current study alone cannot determine whether human iPSC-NPC-derived exosomes play the same role on other types of neurons or on neurons from other species. These questions must be answered through further investigation.
Exosomes derived from stem cells have great potential to be used in the treatment of ischemic neuronal injury. Previous studies have shown that stem cells and exosomes play neuroprotective, angiogenic, and anti-inflammatory roles in ischemic stroke therapy (Hao et al., 2014). Our study mainly focused on the neuroprotective role of iPSC-NPC-derived exosomes in ischemic neuronal injury. BDNF is responsible for the beneficial effects of primary neurons (Deyama et al., 2019). As previous studies reported, MSC-derived exosomes increased BDNF in cell models of cerebral injury and disease (Wei et al., 2016; Zhao et al., 2019). Our work is the first study to report an increase in BDNF following the addition of human iPSC-NPC-derived exosomes, which supports the idea that human iPSC-NPC exosomes can protect the neurons in the rat model of ischemia. Previous studies showed that the benefits of BDNF depended on BDNF/TrkB signals. The PI3K/AKT pathway is downstream of TrkB, which mediates several cellular activities, including cell survival and proliferation (Holmes et al., 2007; Massa et al., 2010). In this study, we found that iPSC-NPC-derived exosomes attenuate OGD-induced changes in PTEN/AKT signaling, which demonstrates the neurotrophic effects of iPSC-NPCs through the AKT pathway. These findings are in accordance with the results of previous studies that focused on exosomes from other type of stem cells (Deng et al., 2018).
We also found that iPSC-NPC exosomes attenuate changes caused by OGD in the expression of synaptic plasticity-related proteins and neurite outgrowth, which supports the claim that iPSC-NPC-derived exosomes have a positive effect on synaptic plasticity in hypoxic-ischemic neurons. However, how the iPSC-NPC-derived exosomes promote neurite outgrowth has not been identified previously and needs further investigation. One study found that MSC-derived exosomes have selectively effects on the promotion of neurite outgrowth (Lopez-Verrilli et al., 2016).
The current study has several limitations. First, we did not investigate whether the neuroprotective effect of iNPC-derived exosomes is comparable to that of iNPCs. Second, we did not do experiments with the exosomes derived from other stem cells to investigate whether iPSC-NPC-derived exosomes are superior or not. Third, this was an in vitro study, and in vivo studies are needed to determine the effects of iPSC-NPC-derived exosomes on neurons, as well as other cells like endothelial cells, astrocytes, and microglia in animal models of ischemic stroke. Finally, our study showed that the neuroprotective effects of iPSC-NPC-derived exosomes on OGD rat primary cortex neurons might depend on the BDNF/PI3K/AKT pathway. Better designed experiments that can test the importance of specific molecules in the pathway using inhibitors are needed to confirm the results.
In conclusion, using an in vitro preclinical OGD model, we demonstrated that iPSC-NPC-derived exosomes confer a neuroprotective effect on OGD rat primary cortical neurons. The protective effect might result from increasing BDNF levels and/or molecules in the PI3K/AKT pathway and result in improved survival of neurons and neurite outgrowth. Our study provides evidence that the beneficial neuroprotective effect of iPSC-NPC-derived exosomes on OGD rat primary cortical neurons are vital for indicating the proper selection use of iPSC-NPCs sources and their exosomes for the treatment of stroke. Our findings will be useful for future clinical research into the application of iPSC-NPC-derived exosomes for treating stroke in humans.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest.
Financial support: This work was supported by the Foundation of Zhejiang Provincial Basic Public Welfare Research Program of China, No. LGF19H090024 (to XYX); the Natural Science Foundation of Zhejiang Province of China, No. LY17H090006 (to WYL); and National Natural Science foundation of China, No. 81901073 (to QBZ). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The study was approved by Animal Ethics Committee of Sir Run Run Shaw Hospital (approval No. SRRSH20191010) on Octorber, 10, 2019.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the Foundation of Zhejiang Provincial Basic Public Welfare Research Program of China, No. LGF19H090024 (to XYX); the Natural Science Foundation of Zhejiang Province of China, No. LY17H090006 (to WYL); and National Natural Science foundation of China, No. 81901073 (to QBZ).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Philips A, Song LP; T-Editor: Jia Y
References
- 1.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 2.Brevet M, Kojima H, Asakawa A, Atsuchi K, Ushikai M, Ataka K, Inui A, Kimura H, Sevestre H, Fujimiya M. Chronic foot-shock stress potentiates the influx of bone marrow-derived microglia into hippocampus. J Neurosci Res. 2010;88:1890–1897. doi: 10.1002/jnr.22362. [DOI] [PubMed] [Google Scholar]
- 3.Chang DJ, Lee N, Park IH, Choi C, Jeon I, Kwon J, Oh SH, Shin DA, Do JT, Lee DR, Lee H, Moon H, Hong KS, Daley GQ, Song J. Therapeutic potential of human induced pluripotent stem cells in experimental stroke. Cell Transplant. 2013;22:1427–1440. doi: 10.3727/096368912X657314. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Chopp M. Exosome Therapy for Stroke. Stroke. 2018;49:1083–1090. doi: 10.1161/STROKEAHA.117.018292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui GN, Liu XP, Hu JR, Li PQ. Research and progress in the correlation between exosomes from different sources and tumorigenesis. Zuzhi Gongcheng Yanjiu. 2020;24:21095–2101. [Google Scholar]
- 6.de la Rosa-Prieto C, Laterza C, Gonzalez-Ramos A, Wattananit S, Ge R, Lindvall O, Tornero D, Kokaia Z. Stroke alters behavior of human skin-derived neural progenitors after transplantation adjacent to neurogenic area in rat brain. Stem Cell Res Ther. 2017;8:59. doi: 10.1186/s13287-017-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng M, Xiao H, Peng H, Yuan H, Xu Y, Zhang G, Tang J, Hu Z. Preservation of neuronal functions by exosomes derived from different human neural cell types under ischemic conditions. Eur J Neurosci. 2018;47:150–157. doi: 10.1111/ejn.13784. [DOI] [PubMed] [Google Scholar]
- 8.Deyama S, Bang E, Kato T, Li XY, Duman RS. Neurotrophic and antidepressant actions of brain-derived neurotrophic factor require vascular endothelial growth factor. Biol Psychiatry. 2019;86:143–152. doi: 10.1016/j.biopsych.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomi M, Takagi Y, Morizane A, Doi D, Nishimura M, Miyamoto S, Takahashi J. Functional recovery of the murine brain ischemia model using human induced pluripotent stem cell-derived telencephalic progenitors. Brain Res. 2012;1459:52–60. doi: 10.1016/j.brainres.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 10.Gong J, Liu M. Human induced pluripotent stem cell transplantation for hypoxic-ischemic encephalopathy in neonatal mice. Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23:5322–5327. [Google Scholar]
- 11.Hao L, Zou Z, Tian H, Zhang Y, Zhou H, Liu L. Stem cell-based therapies for ischemic stroke. Biomed Res Int. 2014;2014:468748. doi: 10.1155/2014/468748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 13.Hermanto Y, Sunohara T, Faried A, Takagi Y, Takahashi J, Maki T, Miyamoto S. Transplantation of feeder-free human induced pluripotent stem cell-derived cortical neuron progenitors in adult male Wistar rats with focal brain ischemia. J Neurosci Res. 2018;96:863–874. doi: 10.1002/jnr.24197. [DOI] [PubMed] [Google Scholar]
- 14.Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19:2003–2012. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Hu YT, Chen XL, Huang SH, Zhu QB, Yu SY, Shen Y, Sluiter A, Verhaagen J, Zhao J, Swaab D, Bao AM. Early growth response-1 regulates acetylcholinesterase and its relation with the course of Alzheimer’s disease. Brain Pathol. 2019;29:502–512. doi: 10.1111/bpa.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iglesias-Rey R, Castillo J. New strategies for ischemic stroke: internal photobiomodulation therapy. Neural Regen Res. 2020;15:1658–1659. doi: 10.4103/1673-5374.276328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janowski M, Wagner DC, Boltze J. Stem cell-based tissue replacement after stroke: factual necessity or notorious fiction. Stroke. 2015;46:2354–2363. doi: 10.1161/STROKEAHA.114.007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalladka D, Muir KW. Brain repair: cell therapy in stroke. Stem Cells Cloning. 2014;7:31–44. doi: 10.2147/SCCAA.S38003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katsuda T, Kosaka N, Takeshita F, Ochiya T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13:1637–1653. doi: 10.1002/pmic.201200373. [DOI] [PubMed] [Google Scholar]
- 20.Kawai H, Yamashita T, Ohta Y, Deguchi K, Nagotani S, Zhang X, Ikeda Y, Matsuura T, Abe K. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J Cereb Blood Flow Metab. 2010;30:1487–1493. doi: 10.1038/jcbfm.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Lau VW, Platt SR, Grace HE, Baker EW, West FD. Human iNPC therapy leads to improvement in functional neurologic outcomes in a pig ischemic stroke model. Brain Behav. 2018;8:e00972. doi: 10.1002/brb3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee IH, Huang SS, Chuang CY, Liao KH, Chang LH, Chuang CC, Su YS, Lin HJ, Hsieh JY, Su SH, Lee OK, Kuo HC. Delayed epidural transplantation of human induced pluripotent stem cell-derived neural progenitors enhances functional recovery after stroke. Sci Rep. 2017;7:1943. doi: 10.1038/s41598-017-02137-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li WY, Choi YJ, Lee PH, Huh K, Kang YM, Kim HS, Ahn YH, Lee G, Bang OY. Mesenchymal stem cells for ischemic stroke: changes in effects after ex vivo culturing. Cell Transplant. 2008;17:1045–1059. doi: 10.3727/096368908786991551. [DOI] [PubMed] [Google Scholar]
- 25.Lledo PM, Merkle FT, Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 2008;31:392–400. doi: 10.1016/j.tins.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61:1795–1806. doi: 10.1002/glia.22558. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Verrilli MA, Caviedes A, Cabrera A, Sandoval S, Wyneken U, Khoury M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 2016;320:129–139. doi: 10.1016/j.neuroscience.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 28.Lv W, Li WY, Xu XY, Jiang H, Bang OY. Bone marrow mesenchymal stem cells transplantation promotes the release of endogenous erythropoietin after ischemic stroke. Neural Regen Res. 2015;10:1265–1270. doi: 10.4103/1673-5374.162759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massa SM, Yang T, Xie Y, Shi J, Bilgen M, Joyce JN, Nehama D, Rajadas J, Longo FM. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J Clin Invest. 2010;120:1774–1785. doi: 10.1172/JCI41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuse D, Kitada M, Ogura F, Wakao S, Kohama M, Kira J, Tabata Y, Dezawa M. Combined transplantation of bone marrow stromal cell-derived neural progenitor cells with a collagen sponge and basic fibroblast growth factor releasing microspheres enhances recovery after cerebral ischemia in rats. Tissue Eng Part A. 2011;17:1993–2004. doi: 10.1089/ten.TEA.2010.0585. [DOI] [PubMed] [Google Scholar]
- 31.Mittelbrunn M, Sánchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13:328–335. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mo Y, Sun YY, Liu KY. Autophagy and inflammation in ischemic stroke. Neural Regen Res. 2020;15:1388–1396. doi: 10.4103/1673-5374.274331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nojiri Y, Takeda S, Enomoto M, Nishitsuji H, Masuda T, Sotome S, Shinomiya K. Co-overexpression of GDNF and GFRalpha1 induces neural differentiation in neural progenitor cells in comparison to bone marrow stromal cells. J Med Dent Sci. 2008;55:121–128. [PubMed] [Google Scholar]
- 34.Pei X, Li Y, Zhu L, Zhou Z. Astrocyte-derived exosomes suppress autophagy and ameliorate neuronal damage in experimental ischemic stroke. Exp Cell Res. 2019;382:111474. doi: 10.1016/j.yexcr.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Polentes J, Jendelova P, Cailleret M, Braun H, Romanyuk N, Tropel P, Brenot M, Itier V, Seminatore C, Baldauf K, Turnovcova K, Jirak D, Teletin M, Côme J, Tournois J, Reymann K, Sykova E, Viville S, Onteniente B. Human induced pluripotent stem cells improve stroke outcome and reduce secondary degeneration in the recipient brain. Cell Transplant. 2012;21:2587–2602. doi: 10.3727/096368912X653228. [DOI] [PubMed] [Google Scholar]
- 36.Raedt R, Pinxteren J, Van Dycke A, Waeytens A, Craeye D, Timmermans F, Vonck K, Vandekerckhove B, Plum J, Boon P. Differentiation assays of bone marrow-derived multipotent adult progenitor cell (MAPC)-like cells towards neural cells cannot depend on morphology and a limited set of neural markers. Exp Neurol. 2007;203:542–554. doi: 10.1016/j.expneurol.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Sandoval R, González A, Caviedes A, Pancetti F, Smalla KH, Kaehne T, Michea L, Gundelfinger ED, Wyneken U. Homeostatic NMDA receptor down-regulation via brain derived neurotrophic factor and nitric oxide-dependent signalling in cortical but not in hippocampal neurons. J Neurochem. 2011;118:760–772. doi: 10.1111/j.1471-4159.2011.07365.x. [DOI] [PubMed] [Google Scholar]
- 38.Savitz SI, Chopp M, Deans R, Carmichael T, Phinney D, Wechsler L. Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke. 2011;42:825–829. doi: 10.1161/STROKEAHA.110.601914. [DOI] [PubMed] [Google Scholar]
- 39.Suda S, Nito C, Yokobori S, Sakamoto Y, Nakajima M, Sowa K, Obinata H, Sasaki K, Savitz SI, Kimura K. Recent advances in cell-based therapies for ischemic stroke. Int J Mol Sci. 2020;21:6718. doi: 10.3390/ijms21186718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatarishvili J, Oki K, Monni E, Koch P, Memanishvili T, Buga AM, Verma V, Popa-Wagner A, Brüstle O, Lindvall O, Kokaia Z. Human induced pluripotent stem cells improve recovery in stroke-injured aged rats. Restor Neurol Neurosci. 2014;32:547–558. doi: 10.3233/RNN-140404. [DOI] [PubMed] [Google Scholar]
- 41.Tornero D, Wattananit S, Grønning Madsen M, Koch P, Wood J, Tatarishvili J, Mine Y, Ge R, Monni E, Devaraju K, Hevner RF, Brüstle O, Lindvall O, Kokaia Z. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain. 2013;136:3561–3577. doi: 10.1093/brain/awt278. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Chen Y, Yang Y, Xiao X, Chen S, Zhang C, Jacobs B, Zhao B, Bihl J, Chen Y. Endothelial progenitor cells and neural progenitor cells synergistically protect cerebral endothelial cells from hypoxia/reoxygenation-induced injury via activating the PI3K/Akt pathway. Mol Brain. 2016;9:12. doi: 10.1186/s13041-016-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol Psychiatry. 2008;13:786–799. doi: 10.1038/mp.2008.38. 741. [DOI] [PubMed] [Google Scholar]
- 44.Wei JJ, Chen YF, Xue CL, Ma BT, Shen YM, Guan J, Bao XJ, Wu H, Han Q, Wang RZ, Zhao CH. Protection of nerve injury with exosome extracted from mesenchymal stem cell. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38:33–36. doi: 10.3881/j.issn.1000-503X.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Xiao D, Liu X, Zhang M, Zou M, Deng Q, Sun D, Bian X, Cai Y, Guo Y, Liu S, Li S, Shiang E, Zhong H, Cheng L, Xu H, Jin K, Xiang M. Direct reprogramming of fibroblasts into neural stem cells by single non-neural progenitor transcription factor Ptf1a. Nat Commun. 2018;9:2865. doi: 10.1038/s41467-018-05209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu JC, Fan J, Wang X, Eacker SM, Kam TI, Chen L, Yin X, Zhu J, Chi Z, Jiang H, Chen R, Dawson TM, Dawson VL. Cultured networks of excitatory projection neurons and inhibitory interneurons for studying human cortical neurotoxicity. Sci Transl Med. 2016;8:333ra348. doi: 10.1126/scitranslmed.aad0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao H, Li Y, Chen L, Shen C, Xiao Z, Xu R, Wang J, Luo Y. HucMSCs-derived miR-206-knockdown exosomes contribute to neuroprotection in subarachnoid hemorrhage induced early brain injury by targeting BDNF. Neuroscience. 2019;417:11–23. doi: 10.1016/j.neuroscience.2019.07.051. [DOI] [PubMed] [Google Scholar]