Keywords: adrenergic receptors, adult neurogenesis, antidepressants, antipsychotics, depression, neural progenitor cells, norquetiapine, quetiapine
Abstract
Positive modulation of adult hippocampal neurogenesis may contribute to the therapeutic effects of clinically relevant antidepressant drugs, including atypical antipsychotics. Quetiapine, an antipsychotic which represents a therapeutic option in patients who are resistant to classical antidepressants, promotes adult hippocampal neurogenesis in preclinical studies. Norquetiapine, the key active metabolite of quetiapine in humans, has a distinctive receptor profile than the parent compound. The drug is indeed a high affinity norepinephrine transporter inhibitor and such activity has been proposed to contribute to its antidepressant effect. At present, no information is available on the effects of norquetiapine on adult neurogenesis. We extensively investigated the activity of quetiapine and norquetiapine on adult murine neural stem/progenitor cells and their progeny. Additionally, selective antagonists for β2/α2 adrenergic receptors allowed us to evaluate if these receptors could mediate quetiapine and norquetiapine effects. We demonstrated that both drugs elicit in vitro proneurogenic effects but also that norquetiapine had distinctive properties which may depend on its ability to inhibit norepinephrine transporter and involve β2/α2 adrenergic receptors. Animal care and experimental procedures were approved by the Institutional Animal Care and Use Committees (IACUC) at University of Piemonte Orientale, Italy (approval No. 1033/2015PR) on September 29, 2015.
Chinese Library Classification No. R453; R364; R741
Introduction
Major depressive disorder is a debilitating psychiatric disorder with high prevalence (Moussavi et al., 2007). By 2030, according to the World Health Organization, major depressive disorder will rank first amongst the global disability burden of non-fatal diseases (World Health Organization, 2008). Although extensive efforts have advanced our understanding of major depressive disorder pathophysiology, current treatments are still far from ideal, with relapses, residual symptoms, a consistent burden of side effects and about one third of patients which are refractory to pharmacological strategies (Ignácio et al., 2016; Papadimitropoulou et al., 2017). In parallel to discovering new therapies, it is certainly important to further understand the biological mechanisms that are triggered by drugs which provide some additional benefit, such as in adjunct therapy strategies, but also as a monotherapy in patients resistant to classical antidepressant treatments.
The drug quetiapine is an atypical antipsychotic which has shown benefits as a therapeutic option in the treatment of depressed patients who do not present a satisfactory response to classical antidepressants (Wang and Si, 2013; Papadimitropoulou et al., 2017). The drug has a complex and unique multireceptor profile, that makes it unlikely that a single mechanism could explain its antidepressant effects (Saller and Salama, 1993). It is currently proposed that positive modulation of adult neurogenesis may contribute to the therapeutic effects of clinically relevant antidepressant drugs, including atypical antipsychotics (Newton and Duman, 2007; De Oliveira et al., 2020). In line with this hypothesis, quetiapine proved to be able to revert chronic stress-induced reduction of hippocampal neurogenesis in preclinical studies (Luo et al., 2005; Wang et al., 2013).
In humans, norquetiapine is the key active metabolite and the most pharmacologically characterized one (Kim et al., 2016). A pharmacokinetic study revealed that both quetiapine and norquetiapine rapidly cross the blood-brain barrier, with brain distribution of norquetiapine appearing better than the parent compound (Kim et al., 2016). A recent study demonstrated a significant relationship between norquetiapine and the antidepressant effects seen in patients with bipolar disorder (Rovera et al., 2017). Interestingly, norquetiapine largely differs from quetiapine for its receptor binding profile. Norquetiapine profile as a partial agonist for the 5-hydroxytryptamine receptor 5-HT1A as well as a norepinephrine transporter (NET) inhibitor has been proposed to contribute to the antidepressant effects of the drug (Jensen et al., 2008; Winter et al., 2008). In rodents quetiapine is not converted to norquetiapine (López-Muñoz and Álamo, 2013), so the metabolite has to be directly administered to animal models in order to evaluate its effects in comparison to quetiapine. Although in preclinical studies norquetiapine showed antidepressant activity in vivo (Jensen et al., 2008; Cross et al., 2016), no information is currently available on the effects of the drug on adult neurogenesis.
In vitro models allow to directly test the effect of drugs and their metabolites on putative cellular targets and potentially add relevant information on their clinical effects. Herein we extensively investigated the activity of norquetiapine and quetiapine on adult murine neural stem/progenitor cells and their progeny and the potential receptors mediating such in vitro effects.
Materials and Methods
Animals
Male wild type (WT; C57BL/6) and nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) p50–/–(p50KO; C57BL/6j Nfkb1tm1Bal/J) mice were purchased from the Jackson Laboratories (Bar Harbor, ME, USA). Mice, housed in high-efficiency particulate air (HEPA)-filtered Thoren Units (Thoren Caging System Inc., Hazleton, PA, USA), were maintained in number of 3–4 animals/cage, with ad libitum access to food and water, at the University of Piemonte Orientale animal facility. Animal care and experimental procedures were performed in accordance with European Community Directive and approved by the Institutional Animal Care and Use Committees (IACUC) at University of Piemonte Orientale, Italy (approval No. 1033/2015PR) on September 29, 2015.
Culture of adult murine hippocampal and subventricular zone neural progenitor cells
For neural progenitor cell (NPC) isolation, hippocampi or subventricular zone (SVZ) were dissected from brains of three 3–4 month-old male mice and processed as previously described (Meneghini et al., 2010; Valente et al., 2015). NPCs were grown as floating neurospheres which were dissociated for the first time (passage 1, P1) after 7–10 days in vitro (DIV). Thereafter cells were dissociated every five DIV. After each dissociation cells were seeded at a density of 12,000 cells/cm2 in complete culture medium composed by Neurobasal-A medium supplemented with B27 supplement, 2 mM L-glutamine (Gibco, Life Technologies, Monza, IT, USA), recombinant human epidermal growth factor (rhEGF, 20 ng/mL) and recombinant human fibroblast growth factor 2 (rhFGF-2, 10 ng/mL; Peprotech, Rock Hill, NJ, USA), heparin sodium salt (4 µg/mL; Sigma-Aldrich, St. Louis, MO, USA), 100 U/mL penicillin and 100 µg/mL streptomycin (Gibco). For experimental procedures, NPCs were utilized at passages P2–P30.
NPC differentiation
For differentiation assessment, a previously described protocol was utilized (Stagni et al., 2017). Briefly, after neurosphere dissociation, NPCs were plated at a 43,750 cells/cm2 density onto laminin-coated (2.5 µg/cm2) Lab-Tek 8 well Permanox chamber slides (NUNC, Thermo Fisher Scientific, Waltham, MA, USA) in differentiation medium [Neurobasal-A medium, B27 supplement, 2 mM L-glutamine, and 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco)]. NPCs were differentiated for 24 hours in presence of vehicle (water), quetiapine fumarate or N-dealkylated norquetiapine used in a wide range of concentrations (0.1, 0.3, 1, 3, 10, 30, 100, 1000, and 10,000 nM). For α2 and β2 adrenergic receptor blockade, the antagonists idazoxan hydrochloride (10 nM) and ICI 118551 (100 nM) were added to NPCs 30 minutes before drug or vehicle addition.
Drugs
N-dealkylated norquetiapine and quetiapine fumarate (ICI 204636) were kindly provided by AstraZeneca (Cambridge, UK); the β2-adrenergic receptor selective antagonist ICI 118551 and the selective α2-adrenergic receptor antagonist idazoxan hydrochloride were purchased from Tocris Bioscience (Bristol, UK). Drug concentrations were chosen based on Ki values at their target receptors (Cross et al., 2016).
Immunocytochemistry
After 24 hours, under differentiation conditions, NPCs were fixed with ice-cold 4% paraformaldehyde/4% sucrose solution for 20 minutes at room temperature and processed by immunocytochemical analysis as previously described (Cvijetic et al., 2017). Cells were incubated overnight at 4°C with the following primary antibodies: anti-nestin (chicken monoclonal, 1:1500, Neuromics, Edina, MN, USA; a marker for undifferentiated neural progenitors), anti-microtuble-associated protein-2 (MAP-2, rabbit polyclonal, 1:600, Millipore, Milan, Italy; a marker for mature neurons), anti-glial fibrillary acidic protein (GFAP, mouse polyclonal, 1:600, Millipore; a marker for astrocytes) and anti-chondroitin sulfate proteoglycan (NG-2, rabbit polyclonal, 1:500, Millipore; a marker for oligodendrocyte precursors). Thereafter cells were incubated for 2 hours at room temperature with the following secondary antibodies: Alexa Fluor 488-conjugated goat anti-chicken IgG (1:1600) Alexa Fluor 555-conjugated goat anti-rabbit IgG (1:1400), Alexa Fluor 555-conjugated goat anti-mouse IgG (1:1600), and Alexa Fluor 488 conjugated goat anti-rabbit IgG (1:1400) (all from Molecular Probes, Life Technologies). In each experiment, five fields/well corresponding to about 150-200 cells were counted using a fluorescence microscope DMIRB (Leica, Wetzal, Germany) with a 60× objective. For each marker, immunopositive cells were quantified and their percentage was calculated over total viable cells. Nuclei were counterstained with 0.8 ng/mL Hoechst 33342 (Thermo Fisher Scientific). Apoptotic nuclei identified by Hoechst staining were counted and the percentage of apoptotic cell was calculated over the total cell number.
Statistical analysis
Data were expressed as the mean ± SD and analyzed by GraphPad Prism 7.0 software (GraphPad Software Inc., La Jolla, CA, USA) using two-tailed Student’s t-test when only two independent groups were compared, or one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test or two-way analysis of variance followed by Bonferroni’s post hoc test when three or more groups were compared. Statistical significance level was set for P values < 0.05.
Results
Quetiapine and its key metabolite norquetiapine significantly promote neuronal differentiation of adult hippocampal NPCs
In the absence of growth factors, multipotent adult hippocampal NPCs (ahNPCs) spontaneously differentiate toward the neuronal lineage, as previously described (Meneghini et al., 2013). Phenotypical characterization of NPC-derived cells can be easily performed by immunocytochemical approach. Double immunostaining with antibodies against the neuronal MAP-2 and nestin, a protein expressed by undifferentiated progenitor cells, allows to identify four different cell subpopulations after 24 hours of differentiation in vitro. More precisely, ahNPCs give rise to i) MAP-2+/nestin– cells (4.6 ± 0.6% of the total number of viable cells), which can be considered as newly generated neurons; ii) MAP-2+/nestin+ cells (23.4 ± 2.5% of the total number of viable cells), a subpopulation of neuroblasts in transition toward neuronal commitment; iii) MAP-2–/nestin+ cells (7.8 ± 0.5% of the total number of viable cells) which correspond to undifferentiated progenitors; iv) MAP-2–/nestin– cells (64.2 ± 2.9% of the total number of viable cells) an heterogenous population including undifferentiated Sox2+ progenitors (Brazel et al., 2005) and cells which differentiated towards glial lineages (Figure 1).
Figure 1.

Adult hippocampal NPC neuronal differentiation rate.
Representative graph of the four different cell subpopulations that originate from adult hippocampal NPCs, differentiated for 24 hours in absence of growth factors and identified by presence and/or absence of microtuble-associated protein-2 (MAP-2) and nestin phenotypic markers. Viable cells were identified by Hoechst nuclear staining. Data are expressed as mean ± SD of five experiments performed in triplicates. NPC: neural progenitor cell.
In these experimental conditions we investigated the effect of quetiapine on ahNPC neuronal differentiation testing a wide range of concentrations (0.1–30 nM). Quetiapine significantly increased, in a concentration dependent manner, the formation of new neurons and neuroblasts as assessed by an increased percentage of MAP-2+/nestin– and MAP-2+/nestin+ cells, when compared to vehicle-treated condition (Figure 2A and B). Then ahNPCs were differentiated in the presence of a wide range of norquetiapine concentrations (0.1–10 nM). In our experimental conditions norquetiapine was a more potent proneurogenic molecules than quetiapine: the drug indeed elicited effects starting at 0.1 nM, with a maximal effect obtained at 1 nM (Figure 2C and D). Like quetiapine, norquetiapine significantly increased both the percentage of MAP-2+/nestin– (Figure 2C) and MAP-2+/nestin+ (Figure 2D) cells. Representative confocal microscopy images of ahNPC immunolabeled with MAP-2 and nestin antibodies show the proneurogenic effect of 1 nM quetiapine (Figure 2F) and norquetiapine (Figure 2G) on both neuronal (MAP-2+/nestin–) and neuroblast (MAP-2+/nestin+) subpopulations, in comparison with vehicle-treated cells (Figure 2E). To exclude a possible neuroprotective component in the in vitro effects elicited by the drug, we quantified the percentage of apoptotic cells in ahNPCs after exposure to norquetiapine and quetiapine. At all tested concentrations, including those endowed with proneurogenic effects, quetiapine (Figure 2H) did not affect the survival rate of hippocampal NPCs and/or their progeny. Similarly, norquetiapine did not significantly change, compared to vehicle, the apoptotic rate in NPC cultures (Figure 2I).
Figure 2.
Quetiapine and norquetiapine effects on neuronal differentiation and survival of ahNPCs.
Twenty-four-hour exposure to quetiapine (0.1–30 nM) significantly increased the percentage of MAP-2+/nestin–(A) and MAP-2+/nestin+ cells (B). Norquetiapine (NORQ)-mediated (0.1–10 nM) increase of MAP-2+/nestin–(C) and MAP-2+/nestin+(D) cell populations. Representative confocal images of ahNPC differentiated in presence of vehicle (E), quetiapine 1 nM (F) and NORQ 1 nM (G) and immunolabelled with the neuronal marker MAP-2 (red) and the marker for undifferentiated progenitor cells nestin (green). MAP-2+/nestin–(arrows) and MAP-2+/nestin+(arrowheads) cells are indicated. Nuclei were stained with Hoechst (blue). Apoptotic nuclei are marked by asterisks. Original magnification: 40×. Scale bars: 20 μm. Quantification of apoptotic rate in adult hippocampal NPCs treated for 24 hours with quetiapine (0.1–10 μM) (H) and norquetiapine (NORQ; 0.1–10 μM) (I). At all tested concentrations both drugs had no effect on the survival rate of ahNPCs and their progeny. Data of experiments performed in triplicates are expressed as percentage of cells normalized to vehicle (mean ± SD); **P < 0.01, ***P < 0.001, vs. vehicle-treated cells (one-way analysis of variance followed by Tukey’s post hoc test). ahNPC: Adult hippocampal NPC; MAP-2: microtuble-associated protein-2; NPC: neural progenitor cell.
Norquetiapine, unlike quetiapine, also promotes neuronal differentiation of NPCs derived from the SVZ
We then investigated if quetiapine and norquetiapine proneurogenic properties were limited to hippocampal NPCs or also to NPCs derived from another adult neurogenic niche, namely the SVZ. NPCs were prepared from adult murine SVZ and then exposed to quetiapine and norquetiapine (0.001–10 μM) under experimental conditions promoting differentiation. Interestingly, quetiapine had no significant effect on SVZ NPC differentiation up to 10 μM (Figure 3A). On the contrary, norquetiapine significantly promoted the formation of MAP-2+/nestin– cells starting at 100 nM with a maximal effect elicited at 1 μM (Figure 3B). Representative confocal microscopy images of MAP-2 (red) and nestin (green) double labelling in SVZ-derived NPC document the proneurogenic effect of 1 nM norquetiapine (Figure 3E) compared to 1 nM quetiapine (Figure 3D) and vehicle (Figure 3C). As in ahNPCs, both drugs had no effect on the survival rate of SVZ-derived NPCs and their progeny (data not shown). These in vitro data suggest distinct proneurogenic properties of quetiapine and its metabolite: quetiapine specifically promotes neuronal differentiation only of hippocampal NPCs while norquetiapine positively affects both hippocampal and SVZ derived NPCs.
Figure 3.
Effect of quetiapine and norquetiapine on neuronal differentiation of SVZ-derived NPCs.
Quantification of the percentage of MAP-2+/nestin– originating from SVZ-derived NPCs differentiated in presence of quetiapine (QTP; 0.001–10 μM) (A) and norquetiapine (NORQ; 0.001–10 μM) (B). Data of experiments performed in triplicates are expressed as percentage of cells normalized to vehicle (mean ± SD); *P < 0.05, **P < 0.01, ***P < 0.001, vs. vehicle-treated cells (one-way analysis of variance followed by Tukey’s post hoc test). Representative confocal images of SVZ-derived NPCs differentiated in presence of vehicle (C), QTP 1 nM (D) and NORQ 1 nM (E) and immunolabelled with MAP-2 (red) and nestin (green). MAP-2+/nestin– neuron (arrows) and MAP-2+/nestin+ neuroblasts (arrowheads) are indicated. Nuclei were stained with Hoechst 33342 (blue). Original magnification: 40×. Scale bars: 20 μm. MAP-2: microtuble-associated protein-2; NPC: neural progenitor cell; SVZ: subventricular zone.
The proneurogenic effect of norquetiapine occurs at the expenses of ahNPC differentiation toward glial lineages
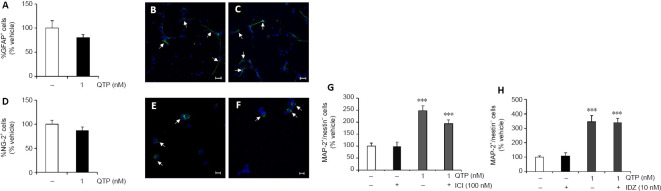
We focused our attention on norquetiapine. In our in vitro systems, ahNPCs can also differentiate toward glial lineages, giving rise to astrocytes (GFAP+ cells) and oligodendrocyte precursors (NG-2+ cells). The percentage of GFAP+ cells was significantly reduced in 1 nM norquetiapine-treated cells (–51.5% vs. vehicle-treated cells, P < 0.01; Figure 4A–C). Similar results were obtained when we evaluated the NG-2+ cell population. 1 nM norquetiapine significantly reduced the percentage of oligodendrocyte precursors compared to vehicle (–43.1% vs. vehicle treated cells, P < 0.001; (Figure 4D–F). Representative confocal microscopy images of norquetiapine-treated GFAP+ (Figure 4C) and NG-2+ cells (Figure 4F) are included for comparison with vehicle-treated conditions (Figure 4B for GFAP and Figure 4E for NG-2 immunostaining). Altogether these data demonstrate that norquetiapine significantly promoted neuronal differentiation of ahNPCs at the expenses of nonneuronal lineages.
Figure 4.
Norquetiapine exerts proneurogenic effects at the expense of glial differentiation and involves β2- and α2-adrenergic receptors.
Significant reduction in the percentages of GFAP+(A) and NG-2+(D) cells after exposure to 1 nM NORQ. Data are expressed as percentage of cells normalized to vehicle (mean ± SD); **P < 0.01, ***P < 0.001, vs. vehicle-treated cells (two-tailed Student’s t-test). Representative confocal microscopy images of ahNPCs differentiated in presence of vehicle (B, E), NORQ 1 nM (C, F) and immunolabelled with GFAP (B, C; original magnification: 40×. Scale bars: 20 μm) and NG-2 (E, F; original magnification: 63×. Scale bars: 10 μm). GFAP+ and NG-2+ cells are indicated (arrows). Nuclei were stained with Hoechst (blue). Effects of NORQ (0.1–10 nM) on ahNPC neuronal differentiation in presence of the β2-adrenergic receptor selective antagonist ICI 118551 (ICI; 100 nM) (G) and in presence of the α2-adrenergic receptor antagonist idazoxan (IDZ; 10 nM) (H). Data are expressed as percentage of cells normalized to vehicle (mean ± SD). ***P < 0.001, vs. vehicle-treated cells (one-way analysis of variance followed by Tukey’s post hoc test). ahNPC: adult hippocampal neural progenitor cell; GFAP: glial fibrillary acidic protein; MAP-2: microtuble-associated protein-2; NG-2: chondroitin sulfate proteoglycan; NORQ: norquetiapine.
Activation of β2 and α2 adrenergic receptors mediate norquetiapine proneurogenic effects
Both quetiapine and norquetiapine are characterized by their ability to interact with multiple receptor systems. Additionally, norquetiapine by blockade of NET increases noradrenergic tone which in turn promotes the formation of new mature neurons in the adult mammalian brain (Nyberg et al., 2013). Recent work from our as well as from other laboratories demonstrated the presence of NET in adult murine NPCs and the contribution of distinct AR receptors in the positive modulation of adult neurogenesis both in vivo and in vitro (Masuda et al., 2012; Meneghini et al., 2014; Bortolotto et al., 2017, 2019). For this purpose, ahNPCs were exposed to norquetiapine in presence of the selective β2-AR antagonist ICI 118551 (100 nM) or its vehicle. In line with such previous studies, ICI 118551 per se had no effect on ahNPC neuronal differentiation (Figure 4G). Conversely, the drug completely prevented norquetiapine-mediated increase of MAP-2+/nestin– cells at both tested concentrations (0.1–1 nM; Figure 4G). Similar effects were observed on the MAP-2+/nestin+ neuroblast population (data not shown). Adult NPCs also express α2-AR (Meneghini et al., 2014). We evaluated the contribution of α2-AR engagement in response to norquetiapine exposure by using the selective antagonist idazoxan at 10 nM concentration. The drug had no effect on ahNPC differentiation per se (Figure 4H). When tested in presence of norquetiapine (0.1–1–10 nM), idazoxan fully counteracted its proneurogenic effects both on MAP-2+/nestin– cells (Figure 4H) and MAP-2+/nestin+ population (data not shown). Altogether these data identify β2- and α2-AR subtypes as mediators of norquetiapine proneurogenic activity in vitro.
Quetiapine and norquetiapine have distinct and unique activity profiles in ahNPC cultures
We further explored potential differences in quetiapine versus norquetiapine activity in our in vitro model. Unlike norquetiapine, proneurogenic concentrations of quetiapine had no significant effect on glial differentiation of ahNPCs as assessed by measuring the percentage of newly formed GFAP+ (Figure 5A–C) and NG-2+ (Figure 5D–F) cells, compared to vehicle conditions. We also tested the potential involvement of α2- e β2-adrenergic receptors in the proneurogenic activity of quetiapine. As shown in Figure 5G, the β2-adrenergic receptor selective antagonist ICI 188551 had no significant effect on quetiapine-mediated proneurogenic activity. Similarly, in presence of the selective α2-adrenergic receptor antagonist idazoxan, the proneurogenic effect of quetiapine was not affected (Figure 5H). These data further support distinct mechanisms of action of quetiapine and its metabolite norquetiapine in primary cultures of ahNPCs.
Figure 5.
Characterization of quetiapine effects in murine hippocampal NPC cultures.
Twenty-four-hour exposure to quetiapine (QTP) 1 nM had no effect on the percentages of GFAP+(A) and NG-2+(D) cells. Data are expressed as percentage of positive cells over total viable cell number, normalized to vehicle (mean ± SD) (two-tailed Student’s t-test). Representative confocal microscopy images of ahNPCs differentiated in presence of vehicle (B, E), QTP 1 nM (C, F) and immunolabelled with GFAP (B, C; original magnification: 40×. Scale bars: 20 μm) and NG-2 (E, F; original magnification: 63×. Scale bars: 10 μm). GFAP+ and NG-2+ cells are indicated (arrows). Nuclei were stained with Hoechst (blue). Effects of QTP (1 nM) on ahNPC neuronal differentiation in presence of the selective β2-adrenergic receptor antagonist ICI 118551 (ICI; 100 nM) (G) or in presence of the α2-AR antagonist idazoxan (IDZ; 10 nM) (H). Data of experiments performed in triplicate are expressed as percentage of cells normalized to vehicle (mean ± SD); ***P < 0.001, vs. vehicle-treated cells (one-way analysis of variance followed by Tukey’s post hoc test). ahNPC: Adult hippocampal NPC; GFAP: glial fibrillary acidic protein; MAP-2: microtuble-associated protein-2; NG-2: chondroitin sulfate proteoglycan; NORQ: norquetiapine; NPC: neural progenitor cell.
Nuclear translocation of NF-κB p50 is not required for the proneurogenic effects induced by norquetiapine and quetiapine
In recent years, our group extensively demonstrated the central role of NF-κB p50 transcription factor in the proneurogenic effect, both in vivo and in vitro, elicited by several clinically relevant drugs (Valente et al., 2012; Cuccurazzu et al., 2013; Bortolotto and Grilli, 2017). Based on those observations, we investigated the requirement of NF-κB p50 expression for quetiapine- and norquetiapine-mediated neuronal differentiation of ahNPCs. To this purpose, we took advantage of NPC derived from the hippocampi of adult p50 knockout mice and their wild type counterpart (Cvjietic et al., 2017). As expected, norquetiapine and quetiapine elicited significant proneurogenic effects also in ahNPC cultures derived from WT C57BL/6j mice (Figure 6A and B). In p50KO ahNPCs, both norquetiapine (Figure 6A) and quetiapine (Figure 6B) significantly increased the percentage of MAP-2+/nestin– cells (and MAP-2+/nestin+ cells, data not shown), compared to vehicle-treated condition. Interestingly, while quetiapine proneurogenic effects were comparable regardless of p50 presence or absence (Figure 6B), in absence of p50 norquetiapine effects were remarkably higher compared to WT condition (P < 0.001; Figure 6A).
Figure 6.

Effects of NORQ and QTP on neuronal differentiation of ahNPCs derived from p50KO and WT mice.
Quantification of the percentage of MAP-2+/nestin– cells derived from WT and p50KO ahNPCs differentiated in presence of NORQ (1 nM) (A), QTP (1 nM) (B), or corresponding vehicle (–) (A, B). Data of experiments performed in triplicates are expressed as percentage of cells normalized vs vehicle (mean ± SD). **P < 0.01, ***P < 0.001, vs. WT vehicle-treated cells; #P < 0.05, ###P < 0.001, vs. p50KO vehicle-treated cells; §§§P < 0.001 (two-way analysis of variance followed by Bonferroni’s post hoc test). ahNPC: Adult hippocampal NPC; KO: knockout; MAP-2: microtuble-associated protein-2; NORQ: norquetiapine; NPC: neural progenitor cell; QTP: quetiapine; WT: wild type.
Discussion
Quetiapine is a second-generation atypical antipsychotic that has demonstrated efficacy as augmentation therapy in treatment-resistant depression (Devarajan et al., 2006; Baune et al., 2007; Doree et al., 2007; Bauer et al., 2009). Quetiapine antidepressant therapeutic efficacy has been ascribed to ability to increase monoamine levels. Indeed the drug enhances central serotonergic neurotransmission via its high affinity 5-HT2A receptor antagonism and partial agonistic activity at the 5-HT1A receptor (McIntyre et al., 2007). Moreover, in the prefrontal cortex its activation of 5-HT1A receptor results in an increase dopaminergic neurotransmission, while affinity for the α2-adrenoceptor mediates a relative increase in extracellular noradrenergic release (Pira et al., 2004; Yatham et al., 2005). Preclinical studies have demonstrated that quetiapine can positively modulate adult hippocampal neurogenesis, a process which is currently proposed to take part into depression pathophysiology (Bortolotto et al., 2014). In vivo experiments in rats demonstrated that quetiapine reverts hippocampal neurogenesis reduction induced by chronic restraint stress (Luo et al., 2005). Even more interestingly, because closely reproducing the clinical settings, quetiapine addition to fluoxetine improved depressive-like behaviour in mice subjected to unpredictable chronic mild stress and, in parallel, increased the number of hippocampal newborn neurons (Wang et al, 2013).
In humans quetiapine has a major active metabolite, norquetiapine, which is endowed with a very distinct pharmacological profile since, in addition to high affinity for 5-HT1A receptors (a property shared with quetiapine), it is a potent inhibitor of the norepinephrine transporter NET (Cross et al., 2016). Blockade of NET represents an important commonality with conventional antidepressant agents and also distinguishes quetiapine from other atypical antipsychotics in humans. This finding also points to the possibility that the clinical antidepressant activity of quetiapine may rely, at least in part, on inhibition of NET elicited by norquetiapine (Prieto et al., 2010).
No information is currently available on the possible effects of norquetiapine on adult neurogenesis in preclinical models or in primary cultures of adult neural progenitor cells, an established in vitro model for assessing neurogenic activity of clinically relevant drugs (Bortolotto et al., 2014). Moreover, no previous study tested quetiapine direct effects in NPC cultures.
In our experimental setting we demonstrated that both drugs have the ability to promote neuronal differentiation of ahNPCs in vitro, in a concentration-dependent manner. Particularly, they significantly increased the percentage of newly generated neurons (MAP-2+/nestin– cells) and neuroblasts (MAP-2+/nestin+ cells). Since neuroprotective effects of atypical antipsychotics have been documented (Chen and Nasrallah, 2019), we also evaluated whether the in vitro proneurogenic activity of norquetiapine and quetiapine could be mediated by increased survival/reduced cell death. Our findings exclude that possibility since the increase in the percentage of newly generated neurons/neuroblasts induced by drugs was not associated with changes in the survival rate of ahNPCs and their progeny. Interestingly, norquetiapine was more potent in vitro than quetiapine, since its proneurogenic effects were apparent at a 10× lower concentration (0.1 nM) than the parent compound. Based on the observation that ahNPC differentiate in vitro both in neuronal and non-neuronal cells, we also evaluated drug effects on the percentage of newly generated astrocytes (GFAP+ cells) and oligodendrocyte precursors (NG-2+ cells). We demonstrated that a proneurogenic concentration of norquetiapine affected ahNPC differentiation toward glial lineages, significantly reducing percentages of GFAP+ and NG-2+ cells. Conversely, under the same experimental conditions, quetiapine had no significant effect on glial differentiation of ahNPCs. These results suggested that, unlike quetiapine, norquetiapine promotes neuronal differentiation of ahNPCs at the expenses of their glial differentiation. These data also suggest that norquetiapine distinctive features, not shared by quetiapine, potentially its activity as NET blocker, may play a role in drug effects elicited in ahNPCs.
NET is expressed by adult NPCs (Meneghini et al., 2014) and norquetiapine binds and blocks NET with high affinity (Jensen et al., 2008). Moreover previous studies from our and other groups demonstrated that several clinically relevant drugs may exert their proneurogenic effects via activation of adrenergic receptors which are also expressed by adult NPCs (Meneghini et al., 2014; Bortolotto et al., 2019; Bortolotto and Grilli, 2020). For these reasons we tested the possibility that adrenergic receptors may also mediate the proneurogenic effect of norquetiapine. It has been demonstrated that norquetiapine has no direct interaction with β2-adrenergic receptors up to 10 μM concentration (Jensen et al., 2008). Herein we showed that the proneurogenic activity of norquetiapine could be abolished in presence of ICI 118551, a selective antagonist for β2-adrenergic receptors. More specifically, β2-adrenergic receptors blockade blunted both norquetiapine-mediated increase in neuronal and neuroblast populations. This finding is in agreement with recent data demonstrating that β2-adrenergic receptors activation by noradrenaline or selective agonists like salmeterol promote positive modulation of adult hippocampal neurogenesis in vitro and in vivo (Masuda et al., 2012; Bortolotto et al., 2019). Moreover, analgesic drugs inhibiting NET like tapentadol also promote neurogenesis in vitro via β2-adrenergic receptor activation in primary hippocampal NPC cultures (Meneghini et al., 2014). Interestingly, ICI 118551 had no effect on the proneurogenic effects of quetiapine which does not interact with NET. Since there is no direct interaction of norquetiapine with β2-adrenergic receptor (Jensen et al., 2008), at the present stage of knowledge we propose that the effects of norquetiapine on neuronal differentiation of hippocampal NPCs may represent an indirect consequence of drug-mediated blockade of noradrenaline reuptake.
Adult NPCs express adrenergic receptors other than β2-adrenergic receptor, including α2-adrenergic receptor (Meneghini et al., 2014). We tested the effects of norquetiapine in presence of idazoxan, a selective α2-adrenergic receptor antagonist. Idazoxan per se had no effect, but in presence of norquetiapine it completely abolished its proneurogenic effects. Although quetiapine has a moderate α2-adrenergic receptor antagonistic activity, for comparison with norquetiapine we also tested its effects in presence of idazoxan. The α2-adrenergic receptor antagonist had no effect on quetiapine proneurogenic effects, pointing again to a different profile for the two drugs.
Activity at intracellular targets, including signal transduction pathways, may also mediate the distinct in vitro effects of quetiapine and norquetiapine. Previous data of our group demonstrated that NF-κB mediated transcription, and in particular the p50 subunit, may play a central role in the modulation of adult hippocampal neurogenesis by several clinically relevant drugs (Valente et al., 2012; Cuccurazzu et al., 2013; Bortolotto et al., 2014; Meneghini et al., 2014; Chiechio et al., 2017). Moreover NF-κB signalling has been proposed to lie downstream of quetiapine effects in the modulation of hippocampal neurogenesis (Bi et al., 2009). We therefore evaluated the differentiation of ahNPCs derived from NF-κB p50 knock out mice hippocampi in presence of proneurogenic concentrations of quetiapine and norquetiapine. We demonstrated no difference in quetiapine proneurogenic activity in WT and p50KO ahNPC. On the contrary, norquetiapine promoted neuronal differentiation of p50KO ahNPCs more effectively than of WT NPCs. In this regard norquetiapine and quetiapine are different from several drugs whose in vitro proneurogenic activity is blunted in absence of p50 protein (Bortolotto et al., 2014). The reasons why norquetiapine is more effective in absence of p50 may deserve future investigation, but, again, this result points to distinct intracellular mechanisms for the drug and its parent compound.
Finally, our studies disclosed another distinctive feature of norquetiapine compared to quetiapine when the two drugs were tested on adult SVZ-derived NPC cultures. Surprisingly we observed that only norquetiapine, and not quetiapine, significantly increased neuronal differentiation of SVZ-derived NPCs. Of note, much higher norquetiapine concentrations were required for promoting neuronal differentiation of adult SVZ NPCs. Adult hippocampal neurogenesis has been demonstrated to occur in humans and proposed to play a physiolopgical role in cognition and mood regulation (Anacker and Hen, 2017). At present the physiological relevance of adult SVZ neurogenesis in human is questioned (Akter et al., 2020). Nevertheless, our in vitro data further highlight differences between the two adult neurogenic areas in their response to pharmacological treatments and/or for receptor expression patterns.
Altogether, our data unravel a distinct profile for the proneurogenic activities of norquetiapine and its parent compound quetiapine. Although they both promote neuronal differentiation of adult murine NPCs in vitro, only norquetiapine effects are mediated by β2- and α2- adrenergic receptors. Since it has been demonstrated that norquetiapine does not bind β2-adrenergic receptors (Jensen et al., 2008), it is possible that such effect is secondary to an increased noradrenergic tone caused by NET blockade, a norquetiapine property which is not shared by quetiapine. Also blockade of norquetiapine effects by idazoxan may be explained by increased extracellular noradrenergic concentrations after NET blockade. Furthermore norquetiapine, and not quetiapine, proneurogenic effects occurred at the expense of glial differentiation and they were augmented in absence of NF-κB p50.
The limitation of this study is that, at present, we have no definitive data on the mechanisms underlying quetiapine proneurogenic effects in vitro. Since we recently demonstrated that blockade of 5-HT2A and 5-HT2C receptors may result in proneurogenic effects (Bortolotto et al., 2017), it is possible that these receptors may mediate the proneurogenic effects of quetiapine.
Altogether these data further support the idea that norquetiapine may contribute to the antidepressant effect of quetiapine in the clinical setting. Moreover they highlight the importance of studying drugs already in clinic and their active metabolites to better understand their profile of efficacy and, potentially, to unravel novel targets for future drug interventions.
Footnotes
Conflicts of interest: All authors declare no conflicts of interest.
Financial support: This work was partially supported by the pharmaceutical company Astra Zeneca and by Fondazione Generali. The content is solely the responsibility of the authors and does not represent the official views of the funding agency.
Institutional review board statement: Animal care and experimental procedures were approved by the Institutional Animal Care and Use Committees (IACUC) at University of Piemonte Orientale, Italy (approval No. 1033/2015PR) on September 29, 2015.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was partially supported by the pharmaceutical company Astra Zeneca and by Fondazione Generali.
C-Editors: Zhao M, Li CH; T-Editor: Jia Y
References
- 1.Akter M, Kaneko N, Sawamoto K. Neurogenesis and neuronal migration in the postnatal ventricular-subventricular zone: Similarities and dissimilarities between rodents and primates. Neurosci Res. 2020 doi: 10.1016/j.neures.2020.06.001. S0168-0102(20)30379-5. [DOI] [PubMed] [Google Scholar]
- 2.Anacker C, Hen R. Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat Rev Neurosci. 2017;18:335–346. doi: 10.1038/nrn.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer M, Dell’osso L, Kasper S, Pitchot W, Dencker Vansvik E, Köhler J, Jørgensen L, Montgomery SA. Extended-release quetiapine fumarate (quetiapine XR) monotherapy and quetiapine XR or lithium as add-on to antidepressants in patients with treatment-resistant major depressive disorder. J Affect Disord. 2009;151:209–219. doi: 10.1016/j.jad.2013.05.079. [DOI] [PubMed] [Google Scholar]
- 4.Baune BT, Caliskan S, Todder D. Effects of adjunctive antidepressant therapy with quetiapine on clinical outcome quality of sleep and daytime motor activity in patients with treatment-resistant depression. Hum Psychopharmacol. 2007;22:1–9. doi: 10.1002/hup.817. [DOI] [PubMed] [Google Scholar]
- 5.Bi X, Yan B, Fang S, Yang Y, He J, Li XM, Kong J. Quetiapine regulates neurogenesis in ischemic mice by inhibiting NF-kappaB p65/p50 expression. Neurol Res. 2009;31:159–166. doi: 10.1179/174313209X393573. [DOI] [PubMed] [Google Scholar]
- 6.Bortolotto V, Bondi H, Cuccurazzu B, Rinaldi M, Canonico PL, Grilli M. Salmeterol, a β2 adrenergic agonist, promotes adult hippocampal neurogenesis in a region-specific manner. Front Pharmacol. 2019;10:1000. doi: 10.3389/fphar.2019.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bortolotto V, Cuccurazzu B, Canonico PL, Grilli M. NF-κB mediated regulation of adult hippocampal neurogenesis: relevance to mood disorders and antidepressant activity. Biomed Res Int. 2014;2014:612798. doi: 10.1155/2014/612798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bortolotto V, Grilli M. Every cloud has a silver lining: proneurogenic effects of Aβ oligomers and HMGB-1 via activation of the RAGE-NF-κB axis. CNS Neurol Disord Drug Targets. 2017;16:1066–1079. doi: 10.2174/1871527315666160803153459. [DOI] [PubMed] [Google Scholar]
- 9.Bortolotto V, Grilli M. Activation of β2 adrenergic receptors promotes adult hippocampal neurogenesis. Neural Regen Res. 2020;15:2258–2259. doi: 10.4103/1673-5374.284991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bortolotto V, Mancini F, Mangano G, Salem R, Xia E, Del Grosso E, Bianchi M, Canonico PL, Polenzani L, Grilli M. Proneurogenic effects of trazodone in murine and human neural progenitor cells. ACS Chem Neurosci. 2017;8:2027–2038. doi: 10.1021/acschemneuro.7b00175. [DOI] [PubMed] [Google Scholar]
- 11.Brazel CY, Limke TL, Osborne JK, Miura T, Cai J, Pevny L, Rao MS. Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell. 2005;4:197–207. doi: 10.1111/j.1474-9726.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1–7. doi: 10.1016/j.schres.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Chiechio S, Canonico PL, Grilli M. l-Acetylcarnitine: a mechanistically distinctive and potentially rapid-acting antidepressant drug. Int J Mol Sci. 2017;19:11. doi: 10.3390/ijms19010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cross AJ, Widzowski D, Maciag C, Zacco A, Hudzik T, Liu J, Nyberg S, Wood MW. Quetiapine and its metabolite norquetiapine: translation from in vitro pharmacology to in vivo efficacy in rodent models. Br J Pharmacol. 2016;173:155–166. doi: 10.1111/bph.13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuccurazzu B, Bortolotto V, Valente MM, Ubezio F, Koverech A, Canonico PL, Grilli M. Upregulation of mGlu2 receptors via NF-κB p65 acetylation is involved in the Proneurogenic and antidepressant effects of acetyl-L-carnitine. Neuropsychopharmacology. 2013;38:2220–2230. doi: 10.1038/npp.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cvijetic S, Bortolotto V, Manfredi M, Ranzato E, Marengo E, Salem R, Canonico PL, Grilli M. Cell autonomous and noncell-autonomous role of NF-κB p50 in astrocyte-mediated fate specification of adult neural progenitor cells. Glia. 2017;65:169–181. doi: 10.1002/glia.23085. [DOI] [PubMed] [Google Scholar]
- 17.De Oliveira CL, Bolzan JA, Surget A, Belzung C. Do antidepressants promote neurogenesis in adult hippocampus. A systematic review and meta-analysis on naive rodents? Pharmacol Ther. 2020;210:107515. doi: 10.1016/j.pharmthera.2020.107515. [DOI] [PubMed] [Google Scholar]
- 18.Devarajan S, Ali J, Dursun SM. Quetiapine plus SSRI in treatment-resistant depression: possible mechanisms. Psychopharmacology (Berl) 2006;185:402–403. doi: 10.1007/s00213-006-0314-6. [DOI] [PubMed] [Google Scholar]
- 19.Dorée JP, Des Rosiers J, Lew V, Gendron A, Elie R, Stip E, Tourjman SV. Quetiapine augmentation of treatment-resistant depression: a comparison with lithium. Curr Med Res Opin. 2007;23:333–341. doi: 10.1185/030079906X162809. [DOI] [PubMed] [Google Scholar]
- 20.Ignàcio ZM, Réus GZ, Arent CO, Abelaria HM, Pitcher MR, Quecedo J. New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br J Clin Pharmacol. 2016;82:1280–1290. doi: 10.1111/bcp.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen NH, Rodriguiz RM, Caron MG, Wetsel WC, Rothman RB, Roth BL. N-desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine’s antidepressant activity. Neuropsychopharmacology. 2008;33:2303–2312. doi: 10.1038/sj.npp.1301646. [DOI] [PubMed] [Google Scholar]
- 22.Kim DW, Weon KY, Hong EP, Chung EK, Lee KT. Comparative physicochemical and pharmacokinetic properties of quetiapine and its active metabolite norquetiapine. Chem Pharm Bull (Tokyo) 2016;64:1546–1554. doi: 10.1248/cpb.c16-00223. [DOI] [PubMed] [Google Scholar]
- 23.López-Muñoz F, Alamo C. Active metabolites as antidepressant drugs: the role of norquetiapine in the mechanism of action of quetiapine in the treatment of mood disorders. Front Psychiatry. 2013;4:102. doi: 10.3389/fpsyt.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lou C, Xu H, Li XM. Quetiapine reverses the suppression of hippocampal neurogenesis caused by repeated restraint stress. Brain Res. 2005;1063:32–39. doi: 10.1016/j.brainres.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 25.Masuda T, Nakagawa S, Boku S, Nishikawa H, Takamura N, Kato A, Inoue T, Koyama T. Noradrenaline increases neural precursor cells derived from adult rat dentate gyrus through β2 receptor. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:44–51. doi: 10.1016/j.pnpbp.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 26.McIntyre RS, Konarski JZ, Jones M, Paulsson B. Quetiapine in the treatment of acute bipolar mania: efficacy across a broad range of symptoms. J Affect Disord 100 Suppl. 2007;1:S5–14. doi: 10.1016/j.jad.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Meneghini V, Bortolotto V, Francese MT, Dellarole A, Carraro L, Terzieva S, Grilli M. High-mobility group box-1 protein and β-amyloid oligomers promote neuronal differentiation of adult hippocampal neural progenitors via receptor for advanced glycation end products/nuclear factor-κB axis: relevance for Alzheimer’s disease. J Neurosci. 2013;33:6047–6059. doi: 10.1523/JNEUROSCI.2052-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meneghini V, Cuccurazzu B, Bortolotto V, Ramazzotti V, Ubezio F, Tzschentke TM, Canonico PL, Grilli M. The noradrenergic component in tapentadol action counteracts μ-opioid receptor-mediated adverse effects on adult neurogenesis. Mol Pharmacol. 2014;85:658–670. doi: 10.1124/mol.113.091520. [DOI] [PubMed] [Google Scholar]
- 29.Meneghini V, Francese MT, Carraro L, Grilli M. A novel role for the Receptor for Advanced Glycation End-products in neural progenitor cells derived from adult SubVentricular Zone. Mol Cell Neurosci. 2010;45:139–150. doi: 10.1016/j.mcn.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 31.Newton SS, Duman RS. Neurogenic actions of atypical antipsychotic drugs and therapeutic implications. CNS Drugs. 2007;21:715–725. doi: 10.2165/00023210-200721090-00002. [DOI] [PubMed] [Google Scholar]
- 32.Nyberg S, Jucaite A, Takano A, Kågedal M, Cselényi Z, Halldin C, Farde L. Norepinephrine transporter occupancy in the human brain after oral administration of quetiapine XR. Int J Neuropsychopharmacol. 2013;16:2235–2244. doi: 10.1017/S1461145713000680. [DOI] [PubMed] [Google Scholar]
- 33.Papadimitropoulou K, Vossen C, Karabis A, Donatti C, Kubitz N. Comparative efficacy and tolerability of pharmacological and somatic interventions in adult patients with treatment-resistant depression: a systematic review and network meta-analysis. Curr Med Res Opin. 2017;33:701–711. doi: 10.1080/03007995.2016.1277201. [DOI] [PubMed] [Google Scholar]
- 34.Pira L, Mongeau R, Pani L. The atypical antipsychotic quetiapine increases both noradrenaline and dopamine release in the rat prefrontal cortex. Eur J Pharmacol. 2004;504:61–64. doi: 10.1016/j.ejphar.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 35.Prieto E, Micó JA, Meana JJ, Majadas S. Neurobiological bases of quetiapine antidepresant effect in the bipolar disorder. Actas Esp Psiquiatr. 2010;38:22–32. [PubMed] [Google Scholar]
- 36.Rizk P, Salazar J, Raisman-Vozari R, Marien M, Ruberg M, Colpaert F, Debeir T. The alpha2-adrenoceptor antagonist dexefaroxan enhances hippocampal neurogenesis by increasing the survival and differentiation of new granule cells. Neuropsychopharmacology. 2006;31:1146–1157. doi: 10.1038/sj.npp.1300954. [DOI] [PubMed] [Google Scholar]
- 37.Rovera C, Esposito CM, Ciappolino V, Cattaneo D, Baldelli S, Clementi E, Altamura AC, Buoli M. Quetiapine-induced hypomania and its association with quetiapine/norquetiapine plasma concentrations: a case series of bipolar type 2 patients. Drug Saf Cas Rep. 2017;4:13. doi: 10.1007/s40800-017-0057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saller CS, Salama AI. Seroquel: biochemical profile of a potential atypical antipsychotic. Psycophamracology (Berl) 1993;112:285–292. doi: 10.1007/BF02244923. [DOI] [PubMed] [Google Scholar]
- 39.Stagni F, Giacomini A, Guidi S, Emili M, Uguagliati B, Salvalai ME, Bortolotto V, Grilli M, Rimondini R, Bartesaghi R. A flavonoid agonist of the TrkB receptor for BDNF improves hippocampal neurogenesis and hippocampus-dependent memory in the Ts65Dn mouse model of DS. Exp Neurol. 2017;298(Pt A):79–96. doi: 10.1016/j.expneurol.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Valente MM, Bortolotto V, Cuccurazzu B, Ubezio F, Meneghini V, Francese MT, Canonico PL, Grilli M. α2δ ligands act as positive modulators of adult hippocampal neurogenesis and prevent depression-like behavior induced by chronic restraint stress. Mol Pharmacol. 2012;82:271–280. doi: 10.1124/mol.112.077636. [DOI] [PubMed] [Google Scholar]
- 41.Valente MM, Allen M, Bortolotto V, Lim ST, Conant K, Grilli M. The MMP-1/PAR-1 axis enhances proliferation and neuronal differentiation of adult hippocampal neural progenitor cells. Neural Plast. 2015;2015:646595. doi: 10.1155/2015/646595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang P, Si T. Use of antipsychotics in the treatment of depressive disorders. Shangai Arch Psychiatry. 2013;25:134–140. doi: 10.3969/j.issn.1002-0829.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Chang T, Chen YC, Zhang RG, Wang HN, WuWJ , Peng ZW, Tan QR. Quetiapine add-on therapy improves the depressive behaviors and hippocampal neurogenesis in fluoxetine treatment resistant depressive rats. Behav Brain Res. 2013;253:206–211. doi: 10.1016/j.bbr.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 44.Winter HR, Earley WR, Hamer-Maansson JE, Davis PC, Smith MA. Steady-state pharmacokinetic safety, and tolerability profiles of quetiapine, norquetiapine, and other quetiapine metabolites in pediatric and adult patients with psychotic disorders. J Child Adolesc Psychopharmacol. 2008;18:81–98. doi: 10.1089/cap.2007.0084. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization. The Global Burden of Disease: 2004 Update. Geneva: WHO; 2008. [Google Scholar]
- 46.Yanpallewar SU, Fernandes K, Marathe SV, Vadodaria KC, Jhaveri D, Rommelfanger K, Ladiwala U, Jha S, Muthig V, Hein L, Bartlett P, Weinshenker D, Vaidya VA. Alpha2-adrenoceptor blockade accelerates the neurogenic neurotrophic, and behavioral effects of chronic antidepressant treatment. J Neurosci. 2010;30:1096–1109. doi: 10.1523/JNEUROSCI.2309-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yatham LN, Goldstein JM, Vieta E, Bowden CL, Grunze H, Post RM, Suppes T, Calabrese JR. Atypical antipsychotics in bipolar depression: potential mechanisms of action. J Clin Psychiatry. 2005;66(Suppl 5):40–48. [PubMed] [Google Scholar]