Abstract
Background
Prone positioning is recommended for patients with moderate-to-severe acute respiratory distress syndrome (ARDS) receiving mechanical ventilation. While the debate continues as to whether COVID-19 ARDS is clinically different from non-COVID ARDS, there is little data on whether the physiological effects of prone positioning differ between the two conditions. We aimed to compare the physiological effect of prone positioning between patients with COVID-19 ARDS and those with non-COVID ARDS.
Methods
We retrospectively compared 23 patients with COVID-19 ARDS and 145 patients with non-COVID ARDS treated using prone positioning while on mechanical ventilation. Changes in PaO2/FiO2 ratio and static respiratory system compliance (Crs) after the first session of prone positioning were compared between the two groups: first, using all patients with non-COVID ARDS, and second, using subgroups of patients with non-COVID ARDS matched 1:1 with patients with COVID-19 ARDS for baseline PaO2/FiO2 ratio and static Crs. We also evaluated whether the response to the first prone positioning session was associated with the clinical outcome.
Results
When compared with the entire group of patients with non-COVID ARDS, patients with COVID-19 ARDS showed more pronounced improvement in PaO2/FiO2 ratio [adjusted difference 39.3 (95% CI 5.2–73.5) mmHg] and static Crs [adjusted difference 3.4 (95% CI 1.1–5.6) mL/cmH2O]. However, these between-group differences were not significant when the matched samples (either PaO2/FiO2-matched or compliance-matched) were analyzed. Patients who successfully discontinued mechanical ventilation showed more remarkable improvement in PaO2/FiO2 ratio [median 112 (IQR 85–144) vs. 35 (IQR 6–52) mmHg, P = 0.003] and static compliance [median 5.7 (IQR 3.3–7.7) vs. − 1.0 (IQR − 3.7–3.0) mL/cmH2O, P = 0.006] after prone positioning compared with patients who did not. The association between oxygenation and Crs responses to prone positioning and clinical outcome was also evident in the adjusted competing risk regression.
Conclusions
In patients with COVID-19 ARDS, prone positioning was as effective in improving respiratory physiology as in patients with non-COVID ARDS. Thus, it should be actively considered as a therapeutic option. The physiological response to the first session of prone positioning was predictive of the clinical outcome of patients with COVID-19 ARDS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-021-01819-4.
Keywords: COVID-19, Acute respiratory distress syndrome, Prone position, Oxygenation, Respiratory system compliance
Background
After its first outbreak in Wuhan, China in December 2019, coronavirus disease 2019 (COVID-19) spread rapidly around the world and continues to be a global threat [1]. Although most patients with COVID-19 have mild manifestations, the condition deteriorates in approximately 10–20% of patients, requiring admission to an intensive care unit and invasive mechanical ventilation for acute respiratory distress syndrome (ARDS) [2–4]. Whether ARDS due to COVID-19 (COVID-19 ARDS) is clinically distinct from ARDS due to other causes (non-COVID ARDS) has been a controversial issue [5, 6].
Prone positioning is currently implemented for patients with moderate-to-severe ARDS with the potential to reduce mortality [7]. The beneficial effect of prone positioning on oxygenation has been known for decades, but whether the improvement in oxygenation is directly associated with patients’ survival gain has been questionable [8]. We have recently shown that the extent of improvement in the ratio of partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2) after prone positioning could be a predictor of survival of patients with ARDS [9].
In this study, we aimed to investigate whether physiological responses to prone positioning differ between patients with COVID-19 ARDS and those with non-COVID ARDS, focusing not only on oxygenation, but also on static respiratory system compliance (Crs), considering recent studies that reported a prognostic value of static Crs for COVID-19 ARDS [10, 11]. We also evaluated whether the response to the first session of prone positioning was associated with patients’ clinical outcome.
Methods
Patients with COVID-19 ARDS
This study was a retrospective cohort study using the patients’ medical records conducted at the Seoul National University Hospital, a tertiary referral hospital in South Korea, which has served as a nationally designated hospital for patients with severe and critical COVID-19. This study was approved by the institutional review board of the Seoul National University Hospital (IRB No. 2012-036-1179). We reviewed the records of all patients older than 18 years who were admitted to our center between January and December 2020 after being diagnosed as having COVID-19 using reverse transcription-polymerase chain reaction assay. Among such patients, those for whom mechanical ventilation was initiated and prone positioning was implemented were included in this study.
Treatment and prone positioning
Patients with COVID-19 ARDS were treated based on the most updated evidence at the time of their hospitalization [12, 13]. In patients with worsening respiratory failure, we usually used a high flow nasal cannula at first, but mechanical ventilation with endotracheal intubation was initiated in refractory cases [14]. If PaO2/FiO2 ratio after initiation of mechanical ventilation was less than 200 mmHg, we actively considered prone positioning with neuromuscular blockade [7, 15]. Prone position was maintained for at least 16 h per day [7]. Discontinuation of prone positioning was considered if reduction in ventilator assistance was possible allowing for spontaneous or assisted ventilation.
Comparison with non-COVID ARDS
We reviewed every patient with non-COVID ARDS treated using prone positioning while on mechanical ventilation since January 2014 until December 2020, and the cohort of these patients was used for a comparison between COVID-19 ARDS and non-COVID ARDS. Some of these patients were included in our previous study [9]. First, we used the entire group of patients with non-COVID ARDS while adjusting for the between-group differences. Second, for a more accurate comparison, patients with COVID-19 ARDS were matched with subgroup populations among the non-COVID ARDS group: one matched 1:1 for PaO2/FiO2 ratio and one matched 1:1 for static Crs.
Study outcome and data collection
The primary outcome of this study was the extent of changes in PaO2/FiO2 ratio and static Crs after the first prone positioning session. In each patient, the changes in PaO2/FiO2 ratio and static Crs were tracked during the first prone positioning session. Using the results of arterial blood gas analysis and the ventilator setting at the time of blood sampling, PaO2/FiO2 ratio and static Crs were evaluated at four timepoints for each patient: baseline (before initiation of prone positioning), P1 (approximately 10 h after initiation of prone positioning), P2 (approximately 16 h after initiation of prone positioning, which is the last timepoint before cessation of prone positioning), and S1 (approximately 2 h after changing to supine position). For the main outcome of this study, each patient’s response to the first session of prone positioning was calculated as the difference in PaO2/FiO2 ratio and static Crs between the baseline and P2 timepoints. In addition, we aimed to evaluate whether the physiological responses to prone positioning correlate with the clinical outcomes of patients, given the controversial results of previous studies [9, 16].
Statistical analysis
We assessed the differences between patients with COVID-19 ARDS and those with non-COVID ARDS and P values of < 0.05 for two-tailed tests were considered statistically significant. First, all patients with non-COVID ARDS were compared with patients with COVID-19 ARDS. Then, two subgroup populations of patients with non-COVID ARDS were used for 1:1 matched comparison with patients with COVID-19 ARDS (PaO2/FiO2-matched subgroup and compliance-matched subgroup). The matching was performed using an optimal algorithm without replacement [17].
For each patient, the Wilcoxon singed-rank test was used to compare the PaO2/FiO2 ratio and static Crs between different timepoints Then, the extent of changes in these parameters from baseline to P2 timepoints was compared between the COVID-19 ARDS and non-COVID ARDS groups using multivariable linear regression analysis. Comparisons between the matched samples were performed similarly [18]. Because there are no definite well-known predictors for response to prone positioning, we adjusted for age, sex, body mass index, duration of mechanical ventilation before the initiation of prone positioning, sequential organ failure assessment (SOFA) score, Charlson comorbidity index (CCI), and baseline setting of mechanical ventilator (positive end-expiratory pressure [PEEP] and tidal volume) as well as baseline PaO2/FiO2 ratio, static Crs, and ventilatory ratio. Ventilatory ratio was selected as a parameter to assess the efficacy of ventilation because we did not routinely monitor the expired CO2 level [19].
For patients with COVID-19 ARDS treated using prone positioning, we assessed whether the response of PaO2/FiO2 and static Crs could predict patients’ probability of successful discontinuation of mechanical ventilation within 90 days using a receiver operating characteristic (ROC) analysis. In addition, the Fine and Gray competing risk regression analysis was performed to calculate the subdistribution hazard ratio (SHR) and 95% confidence interval (CI) with adjustment for age, sex, SOFA score, CCI, and baseline PaO2/FiO2 ratio and static Crs [20, 21]. Death occurring during mechanical ventilation was considered as the competing event. Patients who were still dependent on mechanical ventilation were censored at 90 days after the first prone positioning session. All statistical analyses were performed using STATA software (version 14.0; StataCorp LP, College Station, TX, USA).
Results
Clinical characteristics of patients
Until December 2020, 46 patients with COVID-19 ARDS were treated at our center using mechanical ventilation. Among them, 23 patients (50%) did not start prone positioning because their oxygenation status rapidly improved after initiation of mechanical ventilation. The remaining 23 patients (50%) were treated using prone positioning for persistent moderate-to-severe ARDS. The median interval between the diagnosis of COVID-19 and initiation of prone positioning was 9 (interquartile range [IQR] 4–12) days. To compare with patients with COVID-19 ARDS, 145 patients with non-COVID ARDS treated using prone positioning were reviewed and among them, two subgroups of 23 patients (1:1 matched for PaO2/FiO2 ratio and static Crs, respectively) were selected.
Comparison of baseline characteristics and respiratory mechanics between these groups are described in Tables 1, 2. The patients with non-COVID ARDS had more comorbidities and they were more severely ill with more organ dysfunctions and higher SOFA scores than the patients with COVID-19 ARDS. They also showed worse oxygenation (median PaO2/FiO2 ratio 96 vs. 107 mmHg, P = 0.037) and lower static Crs (median 21.9 vs. 27.2 mL/cmH2O, P = 0.005). All patients in both groups received ventilation with low tidal volume, but patients with non-COVID ARDS had higher ventilatory ratio (median 2.2 vs. 1.7, P < 0.001), requiring higher minute ventilation (median 177 vs. 140 mL/kg/min, P < 0.001). Among the patients with non-COVID ARDS, 1:1 matching was well performed, showing no between-group differences in the median values of PaO2/FiO2 ratio and static Crs in PaO2/FiO2-matched and compliance-matched samples, respectively.
Table 1.
Patient characteristics
| Variables | COVID-19 ARDS | Non-COVID ARDS | |||||
|---|---|---|---|---|---|---|---|
| Entire group N = 23 | Entire group N = 145 | P valuea | PaO2/FiO2-matched N = 23 | P valuea | Compliance-matched N = 23 | P valuea | |
| Age, years | 70 (63–74) | 67 (59–74) | 0.222 | 75 (70–79) | 0.092 | 66 (60–74) | 0.159 |
| Male sex | 15 (65.2%) | 97 (66.9%) | 0.874 | 17 (73.9%) | 0.522 | 16 (69.6%) | 0.753 |
| Height, cm | 165 ± 9 | 163 ± 8 | 0.374 | 163 ± 10 | 0.614 | 164 ± 8 | 0.791 |
| Body weight, kg | 70 (58–79) | 61 (53–70) | 0.018 | 57 (51–69) | 0.015 | 64 (55–69) | 0.132 |
| Body mass index, kg/m2 | 25.6 (22.9–27.4) | 22.9 (20.8–26.0) | 0.024 | 22.5 (19.3–24.7) | 0.009 | 22.7 (20.5–27.2) | 0.121 |
| Interval between intubation and the first prone positioning session, days | 1 (0–2) | 2 (1–5) | 0.009 | 1 (0–3) | 0.116 | 2 (1–4) | 0.047 |
| Total number of sessions of prone positioning | 4 (3–9) | 2 (1–4) | < 0.001 | 2 (1–4) | 0.008 | 2 (1–4) | 0.011 |
| Mean duration of prone positioning per session, hours | 18 (17–19) | 18 (16–19) | 0.653 | 17 (16–18) | 0.180 | 17 (16–20) | 0.231 |
| Charlson comorbidity index | 4 (3–4) | 5 (3–8) | 0.012 | 5 (4–6) | 0.011 | 5 (4–7) | 0.032 |
| APACHE II score | 20 (12–25) | 29 (25–33) | < 0.001 | 30 (26–35) | < 0.001 | 29 (25–37) | < 0.001 |
| SAPS II score | 42 (31–61) | 65 (55–71) | < 0.001 | 66 (62–78) | < 0.001 | 65 (58–78) | < 0.001 |
| SOFA score | 8 (5–11) | 12 (9–14) | < 0.001 | 12 (9–14) | 0.002 | 13 (11–15) | < 0.001 |
APACHE acute physiology and chronic health evaluation, ARDS acute respiratory distress syndrome, SAPS Simplified Acute Physiology Score, SOFA sequential organ failure assessment
aP values are for comparison between patients with COVID-19 ARDS and patients with non-COVID ARDS
Table 2.
Baseline respiratory mechanics and clinical outcomes
| Variables | COVID-19 ARDS | Non-COVID ARDS | |||||
|---|---|---|---|---|---|---|---|
| Entire group N = 23 | Entire group N = 145 | P valuea | PaO2/FiO2-matched N = 23 | P valuea | Compliance-matched N = 23 | P valuea | |
| Arterial blood gas analysis | |||||||
| pH | 7.37 (7.34–7.39) | 7.34 (7.28–7.40) | 0.173 | 7.36 (7.26–7.40) | 0.291 | 7.34 (7.28–7.41) | 0.568 |
| PaCO2, mmHg | 44 (40–49) | 49 (40–55) | 0.139 | 48 (45–54) | 0.071 | 46 (37–54) | 0.860 |
| PaO2, mmHg | 75 (66–80) | 71 (62–85) | 0.467 | 79 (65–93) | 0.391 | 69 (55–78) | 0.097 |
| HCO3, mEq/L | 24.9 (24.1–28.4) | 24.2 (21.5–27.3) | 0.161 | 23.3 (21–28.6) | 0.191 | 23.3 (18.6–28.6) | 0.240 |
| Ventilator FiO2 | 0.7 (0.6–0.8) | 0.8 (0.65–1.0) | 0.047 | 0.75 (0.6–0.9) | 0.537 | 0.75 (0.7–1.0) | 0.221 |
| PaO2/FiO2 ratio, mmHg | 107 (92–132) | 96 (74–120) | 0.037 | 107 (92–131) | 1.000 | 90 (72–104) | 0.007 |
| PEEP, cmH2O | 12 (9–13) | 10 (8–11) | 0.016 | 10 (7–10) | 0.063 | 10 (8–11) | 0.135 |
| Driving pressure, cmH2O | 13 (12–16) | 18 (15–21) | < 0.001 | 18 (15–22) | 0.001 | 15 (13–18) | 0.096 |
| Respiratory rate, breaths/min | 21 (19–27) | 27 (24–30) | 0.002 | 26 (25–30) | 0.012 | 24 (21–30) | 0.116 |
| Tidal volume per PBW, mL/kg | 6.3 (5.6–7.0) | 6.6 (6.0–7.3) | 0.126 | 6.4 (5.9–7.2) | 0.734 | 6.5 (5.9–9.0) | 0.177 |
| Minute ventilation per PBW, mL/kg/min | 140 (123–171) | 177 (145–200) | < 0.001 | 167 (133–193) | 0.044 | 173 (141–194) | 0.003 |
| Static respiratory system compliance, mL/cmH2O | 27.2 (21.9–32.7) | 21.9 (18.2–26.5) | 0.005 | 20.0 (15.6–27.2) | 0.008 | 27.2 (21.9–32.7) | 0.983 |
| Ventilatory ratio | 1.7 (1.4–2.0) | 2.2 (1.7–2.7) | < 0.001 | 2.1 (1.7–2.5) | 0.015 | 2.2 (1.7–2.6) | 0.002 |
| Laboratory results | |||||||
| White blood cell, 103/μL | 10.46 (6.53–16.04) | 13.36 (5.73–17.77) | 0.717 | 15.73 (13.14–23.71) | 0.008 | 10.01 (5.73–16.86) | 0.904 |
| Segmented neutrophil, 103/μL | 9.66 (5.88–14.57) | 11.42 (4.93–15.06) | 0.906 | 14.40 (11.53–19.56) | 0.014 | 9.56 (4.53–14.43) | 0.684 |
| Lymphocyte, 103/μL | 0.69 (0.48–0.86) | 0.43 (0.18–0.84) | 0.047 | 0.70 (0.27–0.98) | 0.895 | 0.51 (0.16–0.88) | 0.249 |
| C-reactive protein, mg/dL | 10.9 (6.3–19.8) | 13.5 (6.6–21.5) | 0.234 | 13.8 (7.6–21.5) | 0.449 | 19.2 (9.1–25.7) | 0.037 |
| RT-PCR for SARS-CoV-2 | |||||||
| Ct value for env gene | 23.01 ± 4.99 | ||||||
| Ct value for RdRp gene | 22.60 ± 5.22 | ||||||
| Adjunctive therapies | |||||||
| Inhaled nitric oxide | 8 (34.8%) | 56 (38.6%) | 0.725 | 4 (17.4%) | 0.179 | 8 (34.8%) | 1.000 |
| Renal replacement therapy | 4 (17.4%) | 29 (20.0%) | 1.000 | 2 (8.7%) | 0.665 | 7 (30.4%) | 0.491 |
| ECMO or ECCO2R | 3 (13.0%) | 7 (4.8%) | 0.141 | 0 (0.0%) | 0.233 | 2 (8.7%) | 1.000 |
| Tracheostomy | 12 (52.2%) | 59 (40.7%) | 0.300 | 15 (65.2%) | 0.369 | 6 (26.1%) | 0.070 |
| 90-days clinical outcome | |||||||
| Successful discontinuation of mechanical ventilation | 16 (69.6%) | 27 (18.6%) | < 0.001 | 5 (21.7%) | 0.005 | 4 (17.4%) | 0.001 |
| Dependent on mechanical ventilation | 2 (8.7%) | 11 (7.6%) | 5 (21.7%) | 1 (4.3%) | |||
| Death | 5 (21.7%) | 107 (73.8%) | 13 (56.6%) | 18 (78.3%) | |||
| Ventilator free days | 45 (0–82) | 0 (0–0) | < 0.001 | 0 (0–0) | 0.002 | 0 (0–0) | 0.002 |
ARDS acute respiratory distress syndrome, Ct value cycle threshold value, ECCO2R extracorporeal carbon dioxide removal, ECMO extracorporeal membrane oxygenation, FiO2 fraction of inspired oxygen, PaCO2 partial pressure of carbon dioxide, PaO2 partial pressure of oxygen, PBW predicted body weight, PEEP positive end expiratory pressure, RT-PCR reverse transcription polymerase chain reaction
aP values are for comparison between patients with COVID-19 ARDS and patients with non-COVID ARDS
Oxygenation and static compliance responses
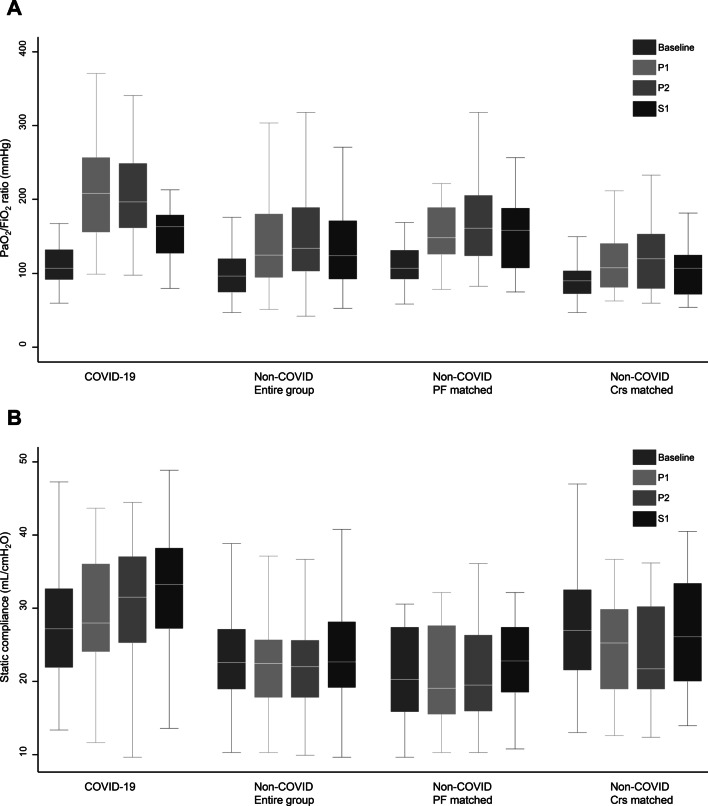
The changes in PaO2/FiO2 ratio and static Crs after the first session of prone positioning are described in Fig. 1 and Additional file 1: Table S1. Baseline measurements were performed at a median of 1.3 (IQR 0.7–2.8) hours before initiation of prone positioning. Measurements for P1 and P2 timepoints were performed at a median of 9.7 (IQR 7.6–11.2) hours and 16.0 (IQR 13.5–17.8) hours after initiation of prone positioning, respectively. Most patients with COVID-19 ARDS showed improvement in both PaO2/FiO2 ratio and static Crs after prone positioning. The increase in PaO2/FiO2 ratio was the most prominent at the P1 timepoint and it slightly decreased when patients were moved to a supine position (Fig. 1A and Additional file 1: Table S1). The static Crs showed a continuous gradual increase during the first prone positioning session (Fig. 1B and Additional file 1: Table S1). A detailed comparison between patients with COVID-19 ARDS and those with non-COVID ARDS is presented in Table 3.
Fig. 1.
Changes in PaO2/FiO2 ratio and static respiratory system compliance after the first session of prone positioning. A PaO2/FiO2 ratio; B Static respiratory system compliance
Table 3.
PaO2/FiO2 ratio and static respiratory system compliance responses after the first prone positioning session
| Entire group | PaO2/FiO2-matched | Compliance-matched | ||||
|---|---|---|---|---|---|---|
| N = 168 | P value | N = 46 | P value | N = 46 | P value | |
| PaO2/FiO2 ratio | ||||||
| Change at the end of the first prone positioning session, mmHg | ||||||
| COVID-19 ARDS, median (IQR) | 89.8 (35.3–135.2) | 0.003 | 89.8 (35.3–135.2) | 0.091 | 89.8 (35.3–135.2) | < 0.001 |
| Non-COVID ARDS, median (IQR) | 40.1 (7.0–77.5) | 48.7 (5.0–87.0) | 21.1 (0.0–43.8) | |||
| Regression coefficient (COVID vs. non-COVID) | ||||||
| Unadjusted (95% CI) | 46.7 (17.8–75.6) | 0.002 | 32.5 (− 14.7–79.8) | 0.172 | 69.7 (31.2–108.2) | 0.001 |
| Adjusted (95% CI)a | 39.3 (5.2–73.5) | 0.024 | 4.0 (− 62.9–71.0) | 0.903 | 58.4 (− 13.5–130.2) | 0.108 |
| Static respiratory system compliance | ||||||
| Change at the end of the first prone positioning session, mL/cmH2O | ||||||
| COVID-19 ARDS, median (IQR) | 3.7 (− 1.0–7.3) | < 0.001 | 3.7 (− 1.0–7.3) | 0.044 | 3.7 (− 1.0–7.3) | < 0.001 |
| Non-COVID ARDS, median (IQR) | − 0.3 (− 4.0–1.9) | 0.4 (− 1.2–2.3) | − 2.3 (− 7.2–0.0) | |||
| Regression coefficient (COVID vs. non-COVID) | ||||||
| Unadjusted (95% CI) | 4.2 (2.3–6.2) | < 0.001 | 2.7 (0.0–5.5) | 0.047 | 6.6 (3.8–9.3) | < 0.001 |
| Adjusted (95% CI)a | 3.4 (1.1–5.6) | 0.003 | 0.3 (− 3.3–3.8) | 0.883 | 2.2 (− 2.4–6.7) | 0.340 |
CI confidence interval, FiO2 fraction of inspired oxygen, IQR interquartile range, PaO2 partial pressure of oxygen
aFor multivariable linear regression, the following variables were adjusted: age, sex, body mass index, duration of mechanical ventilation before initiation of prone positioning, sequential organ failure assessment (SOFA) score, Charlson comorbidity index (CCI), baseline setting of mechanical ventilator (positive end-expiratory pressure and tidal volume), and baseline respiratory mechanics before initiation of prone positioning (PaO2/FiO2, static compliance, and ventilatory ratio)
When comparing baseline and P2 timepoints, the absolute improvement in PaO2/FiO2 ratio was higher in patients with COVID-19 ARDS [median 89.8 (IQR 35.3–135.2) mmHg] than in patients with non-COVID ARDS [median 40.1 (IQR 7.0–77.5) mmHg]. The difference between the two groups remained significant after adjusting for other variables when the analysis was conducted using the entire group of patients with non-COVID ARDS [adjusted difference 39.3 (95% CI 5.2–73.5) mmHg]. However, when compared with the matched subgroups of patients with non-COVID ARDS, it was not significant [adjusted difference 4.0 (95% CI − 62.9–71.0) mmHg in PaO2/FiO2-matched samples and 58.4 (95% CI − 13.5–130.2) mmHg in compliance-matched samples].
The absolute change in static Crs between baseline and P2 timepoints was also higher in patients with COVID-19 ARDS [median 3.7 (IQR − 1.0–7.3) mL/cmH2O] than in patients with non-COVID ARDS [median − 0.3 (IQR − 4.0–1.9) mL/cmH2O]. However, similar to the case of PaO2/FiO2 ratio, after adjusting for other variables, this difference in static Crs was significant only when the analysis was conducted using the entire group of patients with non-COVID ARDS [adjusted difference 3.4 (95% CI 1.1–5.6) mL/cmH2O]. The significance was lost in the analysis of matched samples [adjusted difference 0.3 (95% CI − 3.3–3.8) mL/cmH2O in PaO2/FiO2-matched samples and 2.2 (95% CI − 2.4–6.7) mL/cmH2O in compliance-matched samples].
As a sensitivity analysis, we compared the relative percentage changes in PaO2/FiO2 ratio and static Crs between the two groups (COVID-19 ARDS and non-COVID ARDS). The relative percentage change was calculated as the absolute change divided by the baseline reference value. The results of this sensitivity analysis were similar to those of the main analysis (Additional file 1: Table S2). The unmatched analysis suggested that the relative percentage improvement in PaO2/FiO2 ratio and static Crs was more prominent in patients with COVID-19 ARDS than in patients with non-COVID ARDS. However, the significance was lost in the analysis of matched samples.
Given that the respiratory distress in patients with non-COVID ARDS had various underlying causes, we performed a subgroup analysis according to the etiology of ARDS in patients with non-COVID ARDS. Of 145 patients with non-COVID ARDS, pneumonia was the leading cause of ARDS in 124 patients (85.5%). We compared patients by dividing them into three groups: COVID-19 ARDS, non-COVID ARDS due to pneumonia, and non-COVID ARDS not due to pneumonia (Additional file 1: Table S3). Among the patients with non-COVID ARDS, oxygenation and Crs responses to prone positioning did not differ regardless of whether the underlying cause was pneumonia or not.
Association between clinical outcomes
In patients with COVID-19 ARDS, successful discontinuation of mechanical ventilation was achieved in 16 of 23 patients (69.6%) within 90 days after the first session of prone positioning. We evaluated whether the changes in PaO2/FiO2 ratio and static Crs after the first prone positioning session were associated with successful discontinuation of mechanical ventilation. Improvement in PaO2/FiO2 ratio was more pronounced in patients who successfully discontinued mechanical ventilation than in those who did not [median 112 (IQR 85–144) vs. 35 (IQR 6–52) mmHg, P = 0.003]. In patients who successfully discontinued mechanical ventilation, static Crs increased by a median of 5.7 (IQR 3.3–7.7) mL/cmH2O, whereas in patients who did not, it decreased by a median of 1.0 (IQR − 3.0–3.7) mL/cmH2O (P = 0.006).
In ROC analysis, the areas under the curve were 0.893 (0.754–1.000) for the change in PaO2/FiO2 ratio and 0.866 (0.714–1.000) for the change in static Crs in predicting successful discontinuation of mechanical ventilation within 90 days (Fig. 2). In competing risk regression analysis, the extent of improvement in PaO2/FiO2 ratio (SHR 1.19, 95% CI 1.08–1.30 per 10 mmHg increase) and static Crs (SHR 1.57, 95% CI 1.29–1.91 per 1 mL/cmH2O increase) after the first prone positioning session were both associated with successful discontinuation of mechanical ventilation (Table 4). Among other variables, female sex, lower SOFA score, and higher baseline static Crs were associated with higher probability of successful discontinuation of mechanical ventilation.
Fig. 2.
Receiver operating characteristic curve for changes in PaO2/FiO2 ratio and static respiratory system compliance in predicting the successful discontinuation of mechanical ventilation. A PaO2/FiO2 ratio; B Static respiratory system compliance
Table 4.
Predictors of successful discontinuation of mechanical ventilation for patients with COVID-19 ARDS
| Predictors (N = 23) | Subdistribution hazard ratioa | P value |
|---|---|---|
| Age (per 1 year) | 1.18 (0.99–1.40) | 0.063 |
| Female sex (vs. Male sex) | 13.92 (1.17–165.15) | 0.037 |
| SOFA score (per 1 point) | 0.68 (0.49–0.95) | 0.022 |
| Charlson comorbidity index (per 1 point) | 0.48 (0.17–1.39) | 0.176 |
| Baseline PaO2/FiO2 ratio (per 10 mmHg) | 0.73 (0.53–1.00) | 0.054 |
| Baseline static respiratory system compliance (per 1 mL/cmH2O) | 1.40 (1.10–1.79) | 0.006 |
| Increase in PaO2/FiO2 ratio after the first prone positioning session (per 10 mmHg) | 1.19 (1.08–1.30) | < 0.001 |
| Increase in static respiratory system compliance after the first prone positioning session (per 1 mL/cmH2O) | 1.57 (1.29–1.91) | < 0.001 |
FiO2 fraction of inspired oxygen, PaO2 partial pressure of oxygen, SOFA sequential organ failure assessment
aSubdistribution hazard ratios are described with their 95% confidence intervals
Literature review for related studies
Given the limited sample size of our study, we performed additional literature review for other related studies investigating the physiological effects of prone positioning in mechanically ventilated patients with COVID-19 ARDS (Table 5). As of June 2021, we were able to identify 16 studies, and all studies retrieved showed that prone positioning substantially improves oxygenation in patients with COVID-19 ARDS. However, responses of static Crs varied between the studies.
Table 5.
Literature review for studies evaluating efficacy of prone positioning in mechanically ventilated patients with COVID-19 ARDS
| Study author | Number of patients | Study region | Timing of response evaluation | Change in PaO2/FiO2 ratio (mmHg) | Change in compliance (mL/cmH2O) |
|---|---|---|---|---|---|
| Present study by Jimyung Park | 23 | South Korea | End of first proning session |
Median 107 (IQR 92–132) → median 196 (IQR 161–248) |
Median 27.2 (IQR 21.9–32.7) → median 31.5 (IQR 25.2–37.0) |
| Osama Abou-Arab [41] | 25 | France | End of first proning session |
Median 91 (95% CI 78–137) → median 124 (95% CI 97–149) |
Median 32 (95% CI 21–38) → median 32 (95% CI 23–40) |
| Alfredo J Astua [42] | 29 | U.S.A | End of first proning session |
Mean 107.5 ± 5.6 → mean 142.0 ± 10.8 |
N.A |
| Max Berrill [43] | 34 | U.K | End of every proning session |
Mean 99.8 ± 100 → mean 151.9 ± 58.9 |
N.A |
| Jennifer Clarke [44] | 20 | Ireland | During first proning session |
Median 123 (IQR 100–154) → median 286 (IQR 195–348) |
Median 33.7 (IQR 30.1–43.0) → median 32.5 (IQR 26.7–37.5) |
| Ivor S Douglas [45] | 61 | U.S.A | 2 h after starting proning |
Median 99 (IQR 73–128) → median 136 (IQR 105–164) |
N.A |
| Helena Gleissman [46] | 44 | Sweden | End of first proning session |
Median 104 (IQR 86–122) → median 161 (IQR 127–207) |
N.A |
| Rohit Khullar [47] | 23 | U.S.A | End of last proning session |
Mean 84.8 (SD N.A.) → mean 202.0 (SD N.A.) |
N.A |
| Thomas Langer [48] | 78 | Italy | End of first proning session |
Median 98 (IQR 72–121) → median 158 (IQR 112–220) |
Median 43 (IQR 31–50) → median 42 (IQR 35–48) |
| Mirja Mittermaier [49] | 9 | Germany | 12 h after starting proning |
Mean 118.4 ± 41.9 → mean 181.8 ± 63.2 |
N.A |
| François Perier [50] | 9 | France | 3 h after starting proning | N.A |
Median 44 (IQR 38–55) → median 39 (IQR 32–53) |
| Ling Sang [51] | 20 | China | End of first proning session |
Mean 68.0 ± 10.3 → mean 82.4 ± 15.5 |
Mean 17.5 ± 3.5 → mean 20.6 ± 4.4 |
| Gaetano Scaramuzzo [52] | 191 | Italy | 3 h after resupination | Median 49% improvement (IQR 19–100%) | N.A |
| Mehdi C Shelhamer [53] | 62 | U.S.A | During first proning session | Improvement by 36.4 mmHg (49% improvement) | N.A |
| Richard Vollenberg [54] | 13 | Germany | 6 h after starting proning | Median 58% improvement (IQR 31–95%) |
Median 38 (IQR 26–58) → median 39 (IQR 27–59) |
| Tyler T Weiss [29] | 36 | U.S.A | 2 h after starting proning |
Median 131 (IQR 87–144) → median 208 (IQR 146–268) |
Median 29.2 (IQR 23.3–35.5) → median 29.2 (IQR 24.0–36.2) |
| David R Ziehr [55] | 122 | U.S.A | End of first proning session |
Median 149 (IQR 123–170) → median 235 (IQR 186–285) |
Median 31 (IQR 27–39) → median 33 (IQR 28–38) |
CI confidence interval, FiO2 fraction of inspired oxygen, IQR interquartile range, N.A. not available, PaO2 partial pressure of oxygen, SD standard deviation
Discussion
In this study, we compared the physiological response of prone positioning between patients with COVID-19 ARDS and non-COVID ARDS, focusing on changes in oxygenation and static Crs. Most patients with COVID-19 ARDS showed improvement in PaO2/FiO2 ratio and static Crs after the first session of prone positioning. The extent of improvement in these parameters appeared to be higher in patients with COVID-19 ARDS when compared crudely with the entire group of patients with non-COVID ARDS. However, when 1:1 matched samples (PaO2/FiO2-matched and compliance-matched) were analyzed, the physiological response to prone positioning was not different between patients with COVID-19 ARDS and those with non-COVID ARDS.
Whether patients with COVID-19 ARDS have a clinically different phenotype compared with those with typical non-COVID ARDS continues to be a controversial issue [5, 22]. One of the issues related to this controversy is regarding static Crs. Since the COVID-19 pandemic started, some patients with COVID-19 ARDS have been reported to have preserved static Crs despite impaired oxygenation, which is referred to as “type L (low elastance) phenotype” compared with “type H (high elastance) phenotype” [22, 23]. A multicenter study in Italy reported that patients with COVID-19 ARDS had higher median static Crs than those with non-COVID ARDS (41 vs. 32 mL/cmH2O), although there was a substantial overlap between the two groups [11]. However, in several other studies, patients with COVID-19 ARDS presented with static Crs of approximately 30–35 mL/cmH2O, which is similar to that in previous reports of typical non-COVID ARDS [6, 10, 24–27].
In our study, patients in both groups showed substantially reduced static Crs (median 27.2 and 21.9 mL/cmH2O in COVID-19 and non-COVID group, respectively). Especially, patients with non-COVID ARDS in this study had extremely poor static Crs considering that a recent secondary analysis of the LUNG SAFE study, which included a large multinational cohort of patients, reported the median static Crs of 30 mL/cmH2O [28]. This may be due to the selection bias that occurs in single-center studies. In fact, we could not identify any patient in either group (COVID-19 or non-COVID) who can be classified as having type L phenotype (static Crs ≥ 50 mL/cmH2O). Therefore, our findings may not be applicable to patients with type L phenotype.
Almost every patient with COVID-19 ARDS in this study showed improvement in PaO2/FiO2 ratio after prone positioning. Such improvement was rapid and most noticeable after 10 h of prone positioning. This finding is consistent with that of another single-center study of intubated patients with COVID-19 treated using prone positioning, which reported that PaO2/FiO2 ratio improved within 2 h after initiation of prone positioning [29]. In a prospective study of prone positioning in nonintubated patients, improvement in oxygenation was observed even 10 min after initiation of prone positioning [30]. In contrast, a previous study on non-COVID ARDS showed that the oxygenation status was not always improved immediately after initiation of prone positioning [31]. In other studies, including the PROSEVA trial, PaO2/FiO2 ratio was higher at the end of the prone positioning session than at 1 h after initiation of prone positioning, which is similar to our findings for patients with non-COVID ARDS [7, 32]. Based on these findings, it can be suggested that the speed of the oxygenation response after prone positioning may differ between patients with COVID-19 ARDS and those with non-COVID ARDS. Because PaO2/FiO2 ratio cannot be monitored on real-time basis, monitoring oxygenation based on SpO2/FiO2 ratio might provide more information on this issue.
The change in static Crs after prone positioning has not been studied as much as the change in oxygenation. In one study, static Crs was improved with prone positioning when it was accompanied only with application of high PEEP, but not with low PEEP [33]. Crs is determined by compliance of the chest wall and lung. Because chest wall compliance usually decreases during prone positioning, the overall change in Crs after prone positioning depends on how much the compliance of the lung improves, which may be related to lung recruitability [8]. In our study, the extent of improvement in static Crs after prone positioning appeared to be higher in patients with COVID-19 ARDS than in patients with non-COVID ARDS in a crude analysis. However, the difference was not significant when the analysis was performed using the matched samples. In addition to static Crs, it may be useful to monitor the lung recruitability while implementing prone positioning [34–37].
The major finding of our study was that oxygenation and Crs responses after prone positioning were not different between patients with COVID-19 ARDS and those with non-COVID ARDS after careful matching and adjusting for baseline between-group differences. It is intriguing that the unmatched analysis suggested that prone positioning was more effective in patients with COVID-19 ARDS than in those with non-COVID ARDS. However, this finding may have resulted from the effects of unmeasured confounding factors, suggesting that our 1:1 matched analysis is more appropriate for a proper comparison. Taking the findings of both unmatched and 1:1 matched analyses into account, the physiological effects of prone positioning in COVID-19 ARDS may be comparable with, or at least not inferior to, those in typical non-COVID ARDS.
In fact, because non-COVID ARDS comprises lung injuries from very heterogeneous causes, it is not easy to make a proper comparison between the two groups. Furthermore, although COVID-19 ARDS occurs by infection caused by a common single pathogen, results of several studies indicated that respiratory mechanics of patients with COVID-19 ARDS show a substantial interindividual variability, highlighting the importance of individualization in ventilator management [38]. As in our study, it may be because of this interindividual variability that other studies also failed to identify significant differences between COVID-19 ARDS and non-COVID ARDS [39, 40].
We have recently reported that the extent of improvement in oxygenation after the first session of prone positioning could be predictive of clinical outcome for patients with non-COVID ARDS [9]. In this study, we confirmed this finding in patients with COVID-19 ARDS. In addition, we found that the improvement in static Crs after prone positioning was also associated with clinical outcome. Our findings suggest that if the physiological effect of prone positioning is not substantial at the end of the first session, intensivists may have to consider other therapeutic options. By comparison, a post hoc analysis of the PROSEVA trial found no association between the improvement in oxygenation after 1 h of prone positioning and survival outcomes [16]. This discrepancy may have arisen from the difference in the timing of evaluating the response to prone positioning. Given that it is not clear which timepoint after initiating prone positioning is most appropriate for response evaluation, more studies are needed to clarify this issue.
Our study has several limitations. First, our study was conducted at a single center and the number of patients studied was limited, although we enrolled every consecutive patient treated using prone positioning until December 2020. To compensate for this limitation, we performed additional literature review for other related studies. All studies retrieved consistently showed that prone positioning is effective in improving oxygenation in patients with COVID-19 ARDS. Second, despite our efforts to adjust for between-group differences including 1:1 matched analysis, we cannot exclude the possibility that uncontrolled individual factors affected our study findings. Third, we could not evaluate the effect of prone positioning in patients with preserved static Crs (type L phenotype), because there were no such patients in our cohort.
Conclusions
In conclusion, in patients with COVID-19 ARDS, prone positioning was as effective in improving oxygenation and static Crs as in patients with non-COVID ARDS. Although interindividual variability in respiratory mechanics indicates the need for more individualized approaches in ventilator management, our study findings suggest that prone positioning should be actively considered for patients with moderate-to-severe COVID-19 ARDS. In addition, the physiological response to the first session of prone positioning should be monitored to predict the future clinical outcome.
Supplementary Information
Additional file 1:Table S1. Change in PaO2/FiO2 ratio and static respiratory system compliance after prone positioning; Table S2. Relative percentage change in PaO2/FiO2 ratio and static respiratory system compliance; Table S3. Subgroup analysis according to underlying cause of non-COVID ARDS.
Acknowledgements
Not applicable.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CCI
Charlson comorbidity index
- CI
Confidence interval
- COVID-19
Coronavirus disease 2019
- FiO2
Fraction of inspired oxygen
- IQR
Interquartile range
- PaO2
Partial pressure of arterial oxygen
- PEEP
Positive end-expiratory pressure
- ROC
Receiver operating characteristics
- Crs
Respiratory system compliance
- SOFA
Sequential organ failure assessment
- SHR
Subdistribution hazard ratio
Authors’ contributions
SML is the study lead and guarantor for this paper. JP, HYL, JL, and SML contributed to conception and design of the study. JP and HYL contributed to acquisition, analysis, and interpretation of data. JP and HYL performed the main statistical analysis, and JL and SML critically appraised those results. JP wrote the first draft of this paper, and HYL, JL, and SML revised it critically for important intellectual content. JP, HYL, JL, and SML had access to final version of this paper and approved it to be published. JP, HYL, JL, and SML reached agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Funding
There was no funding for this study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional review board and informed consent was waived because this study was considered as posing minimal risk to the study participants because of its retrospective study design.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, et al. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan E, Song J, Deane AM, Plummer MP. Global impact of coronavirus disease 2019 infection requiring admission to the ICU: a systematic review and meta-analysis. Chest. 2021;159(2):524–536. doi: 10.1016/j.chest.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 5.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “Typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernandez M, Gea A, Arruti E, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020;46(12):2200–2211. doi: 10.1007/s00134-020-06192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 8.Guerin C, Albert RK, Beitler J, Gattinoni L, Jaber S, Marini JJ, et al. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020;46(12):2385–2396. doi: 10.1007/s00134-020-06306-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HY, Cho J, Kwak N, Choi SM, Lee J, Park YS, et al. Improved oxygenation after prone positioning may be a predictor of survival in patients with acute respiratory distress syndrome. Crit Care Med. 2020;48(12):1729–1736. doi: 10.1097/CCM.0000000000004611. [DOI] [PubMed] [Google Scholar]
- 10.Botta M, Tsonas AM, Pillay J, Boers LS, Algera AG, Bos LDJ, et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med. 2021;9(2):139–148. doi: 10.1016/S2213-2600(20)30459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grasselli G, Tonetti T, Protti A, Langer T, Girardis M, Bellani G, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8(12):1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Group RC. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernandez G, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199(11):1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 15.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 16.Albert RK, Keniston A, Baboi L, Ayzac L, Guerin C, Proseva I. Prone position-induced improvement in gas exchange does not predict improved survival in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189(4):494–496. doi: 10.1164/rccm.201311-2056LE. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum PR. Optimal matching for observational studies. J Am Stat Assoc. 1989;84(408):1024–1032. doi: 10.1080/01621459.1989.10478868. [DOI] [Google Scholar]
- 18.Pearce N. Analysis of matched case-control studies. BMJ. 2016;352:969. doi: 10.1136/bmj.i969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinha P, Calfee CS, Beitler JR, Soni N, Ho K, Matthay MA, et al. Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2019;199(3):333–341. doi: 10.1164/rccm.201804-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 21.Yehya N, Harhay MO, Curley MAQ, Schoenfeld DA, Reeder RW. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200(7):828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24(1):154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schenck EJ, Hoffman K, Goyal P, Choi J, Torres L, Rajwani K, et al. Respiratory mechanics and gas exchange in COVID-19-associated respiratory failure. Ann Am Thorac Soc. 2020;17(9):1158–1161. doi: 10.1513/AnnalsATS.202005-427RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandenbunder B, Ehrmann S, Piagnerelli M, Sauneuf B, Serck N, Soumagne T, et al. Static compliance of the respiratory system in COVID-19 related ARDS: an international multicenter study. Crit Care. 2021;25(1):52. doi: 10.1186/s13054-020-03433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Network C-IGobotR, the C-ICUI Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47(1):60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panwar R, Madotto F, Laffey JG, van Haren FMP. Compliance phenotypes in early acute respiratory distress syndrome before the COVID-19 pandemic. Am J Respir Crit Care Med. 2020;202(9):1244–1252. doi: 10.1164/rccm.202005-2046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss TT, Cerda F, Scott JB, Kaur R, Sungurlu S, Mirza SH, et al. Prone positioning for patients intubated for severe acute respiratory distress syndrome (ARDS) secondary to COVID-19: a retrospective observational cohort study. Br J Anaesth. 2021;126(1):48–55. doi: 10.1016/j.bja.2020.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coppo A, Bellani G, Winterton D, Di Pierro M, Soria A, Faverio P, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765–774. doi: 10.1016/S2213-2600(20)30268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albert RK, Keniston A, Baboi L, Ayzac L, Guérin C. Prone position–induced improvement in gas exchange does not predict improved survival in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189(4):494–496. doi: 10.1164/rccm.201311-2056LE. [DOI] [PubMed] [Google Scholar]
- 32.Haddam M, Zieleskiewicz L, Perbet S, Baldovini A, Guervilly C, Arbelot C, et al. Lung ultrasonography for assessment of oxygenation response to prone position ventilation in ARDS. Intensive Care Med. 2016;42(10):1546–1556. doi: 10.1007/s00134-016-4411-7. [DOI] [PubMed] [Google Scholar]
- 33.Cornejo RA, Diaz JC, Tobar EA, Bruhn AR, Ramos CA, Gonzalez RA, et al. Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2013;188(4):440–448. doi: 10.1164/rccm.201207-1279OC. [DOI] [PubMed] [Google Scholar]
- 34.Haudebourg AF, Perier F, Tuffet S, de Prost N, Razazi K, Mekontso Dessap A, et al. Respiratory mechanics of COVID-19- versus non-COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202(2):287–290. doi: 10.1164/rccm.202004-1226LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen L, Del Sorbo L, Grieco DL, Junhasavasdikul D, Rittayamai N, Soliman I, et al. Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome. A clinical trial. Am J Respir Crit Care Med. 2020;201(2):178–187. doi: 10.1164/rccm.201902-0334OC. [DOI] [PubMed] [Google Scholar]
- 36.Pan C, Chen L, Lu C, Zhang W, Xia JA, Sklar MC, et al. Lung recruitability in COVID-19-associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Crit Care Med. 2020;201(10):1294–1297. doi: 10.1164/rccm.202003-0527LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauri T, Spinelli E, Scotti E, Colussi G, Basile MC, Crotti S, et al. Potential for lung recruitment and ventilation-perfusion mismatch in patients with the acute respiratory distress syndrome from coronavirus disease 2019. Crit Care Med. 2020;48(8):1129–1134. doi: 10.1097/CCM.0000000000004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan E, Beitler JR, Brochard L, Calfee CS, Ferguson ND, Slutsky AS, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8(8):816–821. doi: 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bain W, Yang H, Shah FA, Suber T, Drohan C, Al-Yousif N, et al. COVID-19 versus non-COVID ARDS: comparison of demographics, physiologic parameters, inflammatory biomarkers and clinical outcomes. Ann Am Thorac Soc. 2021;18(7):1202–10. [DOI] [PMC free article] [PubMed]
- 40.Grieco DL, Bongiovanni F, Chen L, Menga LS, Cutuli SL, Pintaudi G, et al. Respiratory physiology of COVID-19-induced respiratory failure compared to ARDS of other etiologies. Crit Care. 2020;24(1):529. doi: 10.1186/s13054-020-03253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abou-Arab O, Haye G, Beyls C, Huette P, Roger PA, Guilbart M, et al. Hypoxemia and prone position in mechanically ventilated COVID-19 patients: a prospective cohort study. Can J Anaesth. 2021;68(2):262–263. doi: 10.1007/s12630-020-01844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Astua AJ, Michaels EK, Michaels AJ. Prone during pandemic: development and implementation of a quality-based protocol for proning severe COVID-19 hypoxic lung failure patients in situationally or historically low resource hospitals. BMC Pulm Med. 2021;21(1):25. doi: 10.1186/s12890-021-01401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berrill M. Evaluation of oxygenation in 129 proning sessions in 34 mechanically ventilated COVID-19 patients. J Intensive Care Med. 2021;36(2):229–232. doi: 10.1177/0885066620955137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clarke J, Geoghegan P, McEvoy N, Boylan M, Orna NC, Mulligan M, et al. Prone positioning improves oxygenation and lung recruitment in patients with SARS-CoV-2 acute respiratory distress syndrome; a single centre cohort study of 20 consecutive patients. BMC Res Notes. 2021;14(1):20. doi: 10.1186/s13104-020-05426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douglas IS, Rosenthal CA, Swanson DD, Hiller T, Oakes J, Bach J, et al. Safety and outcomes of prolonged usual care prone position mechanical ventilation to treat acute coronavirus disease 2019 hypoxemic respiratory failure. Crit Care Med. 2021;49(3):490–502. doi: 10.1097/CCM.0000000000004818. [DOI] [PubMed] [Google Scholar]
- 46.Gleissman H, Forsgren A, Andersson E, Lindqvist E, Lipka Falck A, Cronhjort M, et al. Prone positioning in mechanically ventilated patients with severe acute respiratory distress syndrome and coronavirus disease 2019. Acta Anaesthesiol Scand. 2021;65(3):360–363. doi: 10.1111/aas.13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khullar R, Shah S, Singh G, Bae J, Gattu R, Jain S, et al. Effects of prone ventilation on oxygenation, inflammation, and lung infiltrates in COVID-19 related acute respiratory distress syndrome: a retrospective cohort study. J Clin Med. 2020;9(12):4129. [DOI] [PMC free article] [PubMed]
- 48.Langer T, Brioni M, Guzzardella A, Carlesso E, Cabrini L, Castelli G, et al. Prone position in intubated, mechanically ventilated patients with COVID-19: a multi-centric study of more than 1000 patients. Crit Care. 2021;25(1):128. doi: 10.1186/s13054-021-03552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mittermaier M, Pickerodt P, Kurth F, de Jarcy LB, Uhrig A, Garcia C, et al. Evaluation of PEEP and prone positioning in early COVID-19 ARDS. EClinicalMedicine. 2020;28:100579. doi: 10.1016/j.eclinm.2020.100579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perier F, Tuffet S, Maraffi T, Alcala G, Victor M, Haudebourg AF, et al. Effect of positive end-expiratory pressure and proning on ventilation and perfusion in COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202(12):1713–1717. doi: 10.1164/rccm.202008-3058LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sang L, Zheng X, Zhao Z, Zhong M, Jiang L, Huang Y, et al. Lung recruitment, individualized PEEP, and prone position ventilation for COVID-19-associated severe ARDS: a single center observational study. Front Med (Lausanne). 2020;7:603943. doi: 10.3389/fmed.2020.603943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scaramuzzo G, Gamberini L, Tonetti T, Zani G, Ottaviani I, Mazzoli CA, et al. Sustained oxygenation improvement after first prone positioning is associated with liberation from mechanical ventilation and mortality in critically ill COVID-19 patients: a cohort study. Ann Intensive Care. 2021;11(1):63. doi: 10.1186/s13613-021-00853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shelhamer MC, Wesson PD, Solari IL, Jensen DL, Steele WA, Dimitrov VG, et al. Prone positioning in moderate to severe acute respiratory distress syndrome due to COVID-19: a cohort study and analysis of physiology. J Intensive Care Med. 2021;36(2):241–252. doi: 10.1177/0885066620980399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vollenberg R, Matern P, Nowacki T, Fuhrmann V, Padberg JS, Ochs K, et al. Prone position in mechanically ventilated COVID-19 patients: a multicenter study. J Clin Med. 2021;10(5):1046. [DOI] [PMC free article] [PubMed]
- 55.Ziehr DR, Alladina J, Wolf ME, Brait KL, Malhotra A, La Vita C, et al. Respiratory physiology of prone positioning with and without inhaled nitric oxide across the coronavirus disease 2019 acute respiratory distress syndrome severity spectrum. Crit Care Explor. 2021;3(6):0471. doi: 10.1097/CCE.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1:Table S1. Change in PaO2/FiO2 ratio and static respiratory system compliance after prone positioning; Table S2. Relative percentage change in PaO2/FiO2 ratio and static respiratory system compliance; Table S3. Subgroup analysis according to underlying cause of non-COVID ARDS.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.