Abstract
Background
Concerning viral pneumonia, few large-scale comparative studies have been published describing non-HIV immunocompromised and immunocompetent patients, but the epidemiological characteristics of different viruses or underlying diseases in immunocompromised hosts are lacking.
Methods
We retrospectively recruited patients hospitalised with viral pneumonia from six academic hospitals in China between August 2016 and December 2019. We measured the prevalence of comorbidities, coinfections, nosocomial infections, and in-hospital mortalities.
Results
Of the 806 patients, 370 were immunocompromised and 436 were immunocompetent. The disease severity and in-hospital mortality of immunocompromised patients were higher than those of immunocompetent patients. During the influenza season, an increased number of cases of influenza virus (IFV) infection were found in the immunocompromised group, followed by cases of cytomegalovirus (CMV) and respiratory syncytial virus (RSV) infection. During the non-influenza season, CMV was the main virus detected in the immunocompromised group, while RSV, adenovirus (AdV), parainfluenza virus (PIV), and rhinovirus (HRV) were the main viruses detected in the immunocompetent group. Pneumonia caused by Pneumocystis jirovecii (22.4%), Aspergillus spp. (14.1%), and bacteria (13.8%) were the most frequently observed coinfections in immunocompromised patients but not in immunocompetent patients (Aspergillus spp. [10.8%], bacteria [7.1%], and Mycoplasma spp. [5.3%]). CMV infection and infection with two-or-more viruses were associated with a higher in-hospital mortality rate than non-IFV infection. However, patients with IFV and non-IFV infection in immunocompromised patients had similar disease severity and prognosis.
Conclusions
Immunocompromised patients have a high frequency of coinfections, and a higher mortality rate was observed among those infected with CMV and two-or-more viruses. In addition, patients with IFV and non-IFV infection in immunocompromised patients had similar same disease severity and prognosis. The type of viral infection varied with seasons.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-021-06437-5.
Keywords: Viral pneumonia, Immunocompromised, Immunocompetent, Prognosis
Background
Among transplant recipients and patients with haematological malignancy, viral pneumonia often leads to severe respiratory disease and death [1]. Viral lower respiratory tract infections in immunocompromised patients have generally been ascribed to herpes virus (HSV) and cytomegalovirus (CMV) [2]. In recent years, influenza virus (IFV), parainfluenza virus (PIV), respiratory syncytial virus (RSV), and rhinovirus (HRV) have also been recognised as causes of serious infections, especially in patients undergoing treatment for haematologic malignancies and haematopoietic stem cell transplantation. These patients have a higher tendency to develop severe pneumonia, and a mortality rate as high as 25–70% has been reported [3–7]. These patients might experience prolonged viral shedding that potentially result in a longer duration of infection, a higher nosocomial transmission rate, and a higher mortality rate than those of immunocompetent hosts [8, 9]. Concerning non-HIV immunocompromised patients with viral pneumonia, few large-scale epidemiological studies and comparative studies have investigated different viruses or underlying diseases; however, investigations on the epidemiological and etiologic characteristics are lacking.
The objective of this study was to examine the epidemiological and etiologic characteristics and to identify the most common types of viruses that cause viral pneumonia in non-HIV immunocompromised and immunocompetent patients.
Methods
Study design and participants
We retrospectively recruited patients with community-acquired pneumonia (CAP) who were hospitalised between August 2016 and December 2019 at one of the six secondary and tertiary academic hospitals in China. The diagnosis of CAP was based on the American Thoracic Society and Infectious Disease Society of America (ATS/IDSA) guidelines [10]. Immunocompromised patients were selected if they met any of the following inclusion criteria: (1) solid-organ, stem cell, or bone marrow transplant recipients; (2) undergoing chemotherapy for any haematological disease (including acute lymphocytic leukaemia, acute myeloid leukaemia, chronic lymphocytic leukaemia, myeloma, or lymphoma) or the presence of a solid tumour within 6 months of admission or neutropenia (neutrophil count < 500 cells/mm3); (3) chest radiation therapy within 3 months of admission; (4) an autoimmune disease (including but not limited to systemic lupus erythematosus, rheumatoid arthritis, polymyalgia rheumatica, and interstitial lung disease) and receiving immunosuppressive therapy (including chronic glucocorticoid treatment: oral prednisone > 10 mg/d or the equivalent for ≥3 weeks) or methotrexate > 12.5 mg/week, cyclosporine, azathioprine, or biological modifiers such as etanercept or infliximab within 3 months of admission; and (5) history of splenectomy or cirrhosis [1, 11, 12]. Patients were excluded if they (1) were aged < 14 years, (2) experienced pneumonia onset ≥48 h after admission, or (3) tested positive for human immunodeficiency virus.
Study quality control
Key investigators, including clinicians, statisticians, microbiologists, and radiologists, worked together to draft the protocol and created a single formatted case report form (CRF) that was used by all centres. Before the initiation of the study, all investigators from the six centres received training related to the study protocol, including the screening process, definitions of underlying diseases, and the format of the CRF. After data were collected, CRFs were reviewed by a trained researcher to ensure completeness and data quality.
Data collection
The data were collected and included information on patient and disease characteristics, initial oxygenation strategy, laboratory and microbiological data (blood, nasopharyngeal swabs, sputum, and/or bronchoalveolar lavage samples; bacterial or fungal cultures; viral nucleic acid detection; and antibiotic susceptibility patterns), associated organ dysfunction, and patient outcomes at hospital discharge.
Microbiological methods
Microbiological samplings were performed, bronchoalveolar lavage (BAL) or sputum samples were obtained by the treating physicians, and microorganisms were identified and tested for drug susceptibilities. Bronchoscopic examinations were performed according to general guidelines. Lidocaine spray was applied to the upper airway and carina as a local anaesthetic, and airways were thoroughly examined. BAL was performed by administering 60–120 mL of sterile saline solution 2–4 times into the distal bronchial tree, either at the affected lobe or in the middle lung lobe with more radiographic abnormalities. BAL specimens were aliquoted and immediately transported to laboratories. Sputum, BAL samples, or nasopharyngeal swabs were used for atypical pathogen and viral polymerase chain reaction (PCR) amplification tests. Reverse-transcription real time PCR (RT-PCR) (Shanghai Zhijiang Biological Technology, China) was used to detect respiratory viruses including CMV, RSV, IFV types A and B, PIV, HRV, human metapneumovirus (HMPV), and adenovirus (AdV) and Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella pneumophila, and Pneumocystis jirovecii (PCP) in nasopharyngeal swab, sputum, endotracheal aspirate (ETA), or BAL fluid sample. In addition, sputum, ETA, and BAL samples were cultured to identify the presence of bacterial and fungal organisms; the Platelia Aspergillus test was used for galactomannan detection (Bio-Rad Laboratories, Marnes-la-Coquette, France).
Pathogen-specific diagnostic criteria
To diagnose pneumonia caused by Aspergillus, one or more of the following criteria were required: (1) histopathologic or direct microscopic evidence of dichotomous septate hyphae with a positive culture for Aspergillus from tissue sample, (2) a positive Aspergillus culture from BAL fluid sample, (3) a galactomannan optical index in BAL fluid ≥1, (4) a galactomannan optical index in serum ≥0.5; (5) Aspergillus species identified on culture and microscopically [13, 14].
The diagnosis of Pneumocystis jirovecii pneumonia (PCP) required one of the following: (1) high-resolution computed tomography imaging showing diffuse ground glass opacity with patchy distribution; (2) microscopic examination of the respiratory sample revealing the presence of Pneumocystis cystic or trophic forms; or (3) a positive PCR test result for Pneumocystis [15].
Coinfection was considered if bacteria or fungi were isolated from lower respiratory tract specimens (qualified sputum, endotracheal aspirate, and BAL) within 48 h of hospitalisation. A nosocomial infection was diagnosed when patients showed clinical signs or symptoms of pneumonia or bacteraemia and had a positive culture of a new pathogen obtained from lower respiratory tract specimens and/or blood samples taken ≥48 h after admission.
Statistical analysis
The demographics, clinical characteristics, and pathogen testing results are expressed as the mean (± standard deviation), median (interquartile range), or number (percentage). Group comparisons were conducted using Student’s t-test or the Wilcoxon rank-sum test for continuous variables with and without normal distributions, respectively. Categorical variables of the two groups were compared using the χ2 test.
Statistical analyses were performed using SPSS version 19.0 (SPSS, Inc., Chicago, Illinois). All tests were two-sided, and P-values < 0.05 were considered statistically significant.
Patient and public involvement
No patient or the public were involved in the development of the research question, study design, recruitment, and the conduct of the study.
Results
A total of 860 adult patients with positive respiratory viral nucleic acid test results were selected. After excluding patients with upper respiratory tract infections (n = 24) and those who failed to meet the diagnostic criteria for pneumonia (n = 30), 806 patients with viral pneumonia were included in the final analysis. These included 370 immunocompromised and 436 immunocompetent patients. Approximately 34.3% (127/370) of the immunocompromised patients were women with a median age of 60 years. The main presenting symptoms were fever (74.6%), cough (92.4%), and dyspnoea (66.2%). The most common underlying immune-related diseases were connective tissue disease (36.2%), interstitial lung disease (44.6%), solid-organ transplantation (16.2%), and nephrotic syndrome or chronic glomerulonephritis (12.4%). D-dimer levels, pneumonia severity index (PSI) scores, rates of non-invasive mechanical ventilation, septic shock, and in-hospital mortality were higher in the immunocompromised group than in the immunocompetent group (P < 0.05) (Table 1).
Table 1.
Clinical characteristics of viral pneumonia between immunocompetent and immunocompromised group
| Variables | Total, N = 806 | Immunocompromised group, n = 370 |
Immunocompetent group, n = 436 | P-Value |
|---|---|---|---|---|
| Sex, female, n (%) | 290 (36.0) | 127 (34.3) | 163 (37.4) | 0.367 |
| Age, median (IQR) | 62.0 (49.0–71.0) | 60.0 (49.0–68.0) | 63.0 (49.3–75.0) | 0.003 |
| Symptoms and signs, n (%) | ||||
| Fever | 608 (75.4) | 276 (74.6) | 332 (76.1) | 0.610 |
| Cough | 764 (94.8) | 342 (92.4) | 422 (96.8) | 0.006 |
| Expectoration | 732 (90.8) | 322 (87.0) | 410 (94.0) | 0.001 |
| Dyspnea | 542 (67.2) | 245 (66.2) | 297 (68.1) | 0.566 |
| Laboratory examination | ||||
| White blood cell, × 109/L (IQR) | 7.85 (5.62–11.34) | 8.20 (5.73–11.71) | 7.55 (5.43–10.91) | 0.086 |
| Neutrophils, ×109/L (IQR) | 6.17 (3.82–9.22) | 6.73 (4.31–9.80) | 5.52 (3.51–8.95) | 0.014 |
| Lymphocyte, ×109/L (IQR) | 0.95 (0.56–1.52) | 0.84 (0.45–1.40) | 1.03 (0.61–1.58) | 0.001 |
| Persistent lymphocytopenia | 319 (39.6) | 177 (47.8) | 142 (32.6) | < 0.001 |
| Mean hemoglobin±SD, g/L | 117.8 ± 24.5 | 110.6 ± 23.6 | 123.9 ± 23.6 | < 0.001 |
| Mean albumin±SD, g/L | 34.4 ± 6.6 | 33.5 ± 6.6 | 35.2 ± 6.5 | < 0.001 |
| Lactate dehydrogenase, U/L | 302 (217–501) | 357 (245–555) | 263 (199–454) | < 0.001 |
| Blood urea nitrogen, mmol/L | 5.95 (4.18–9.61) | 6.69 (4.61–11.62) | 5.39 (3.90–7.89) | < 0.001 |
| D-Dimer, mmol/L | 1.61 (0.69–4.32) | 2.06 (0.84–9.42) | 1.37 (0.58–3.10) | < 0.001 |
| Procalcitonin, ng/ml | 0.31 (0.17–0.82) | 0.32 (0.16–0.72) | 0.31 (0.18–0.94) | 0.372 |
| Oxygenation index | 203 (118–289) | 186 (113–289) | 209 (126–292) | 0.401 |
| Severe pneumonia index score | 78 (59–103) | 83 (62–107) | 75 (56–99) | 0.001 |
| CURB65 score > 1 | 261 (32.4) | 117 (31.6) | 144 (33.0) | 0.671 |
| Underlying Diseases, n (%) | ||||
| Without underlying disease | 106 (13.2) | 0 (0) | 106 (24.3) | < 0.001 |
| Diabetes mellitus | 194 (24.1) | 103 (27.8) | 91 (20.9) | 0.021 |
| Tumor | 62 (7.7) | 41 (11.1) | 21 (4.8) | 0.001 |
| Connective tissue diseasea | 140 (17.4) | 134 (36.2) | 6 (1.4) | < 0.001 |
| Interstitial lung disease | 210 (26.1) | 165 (44.6) | 45 (10.3) | < 0.001 |
| Bronchiectasis | 28 (3.5) | 6 (1.6) | 22 (5.0) | 0.008 |
| Bronchial asthma | 17 (2.1) | 6 (1.6) | 11 (2.5) | 0.375 |
| Chronic obstructive pulmonary disease | 85 (10.5) | 24 (6.5) | 61 (14.0) | 0.001 |
| Cirrhosis | 5 (0.6) | 5 (1.4) | 0 (0) | 0.015 |
| Leukemia | 7 (0.9) | 7 (1.9) | 0 (0) | 0.004 |
| Lymphoma | 17 (2.1) | 16 (4.3) | 1 (0.2) | < 0.001 |
| Nephrotic syndrome or chronic glomerulonephritis | 50 (6.2) | 46 (12.4) | 4 (0.9) | < 0.001 |
| Chronic renal failure | 45 (5.6) | 29 (7.8) | 16 (3.7) | 0.003 |
| After bone marrow or hematopoietic stem cell transplantation | 5 (0.6) | 5 (1.4) | 0 (0) | 0.015 |
| Solid organ transplant | 60 (7.4) | 60 (16.2) | 0 (0) | < 0.001 |
| Current smoker or ex-smoker | 287 (35.6) | 128 (34.6) | 159 (36.5) | 0.599 |
| Bronchoalveolar lavage, n (%) | 609 (75.6) | 271 (73.2) | 338 (77.5) | 0.159 |
| Treatment, before admission, n (%) | ||||
| Antibiotics | 665 (82.5) | 280 (75.7) | 385 (88.3) | < 0.001 |
| Antiviral drugs | 164 (20.3) | 83 (22.4) | 81 (18.6) | 0.176 |
| Treatment, during hospitalization, n (%) | ||||
| Anti - Pseudomonas aeruginosa drugs | 627 (77.8) | 295 (79.7) | 332 (76.1) | 0.223 |
| Voriconazole or caspofungin | 288 (35.7) | 181 (48.9) | 107 (24.5) | < 0.001 |
| Ganciclovir | 254 (31.5) | 221 (59.7) | 33 (7.6) | < 0.001 |
| Trimethoprim | 207 (25.7) | 193 (52.2) | 14 (3.2) | < 0.001 |
| Complications, n (%) | ||||
| Noninvasive ventilation | 146 (18.1) | 90 (24.3) | 56 (12.8) | < 0.001 |
| Invasive mechanical ventilation | 234 (29.0) | 98 (26.5) | 136 (31.2) | 0.183 |
| Mechanical ventilation | 310 (38.5) | 141 (38.1) | 169 (38.8) | 0.982 |
| Respiratory failure during admission | 397 (49.3) | 186 (50.3) | 211 (48.4) | 0.379 |
| ICU admission | 349 (43.3) | 156 (42.2) | 193 (44.3) | 0.532 |
| Septic shock during hospitalization | 170 (21.1) | 91 (24.6) | 79 (18.1) | 0.025 |
| Extracorporeal membrane oxygenation | 58 (7.2) | 24 (6.5) | 34 (7.8) | 0.922 |
| Hospital mortality | 180 (22.3) | 98 (26.5) | 82 (18.8) | 0.008 |
aConnective tissue disorders: rheumatoid arthritis, systemic lupus erythematosus, dermatomyositis, polymyositis, systemic sclerosis, Sjogren’s syndrome, etc.
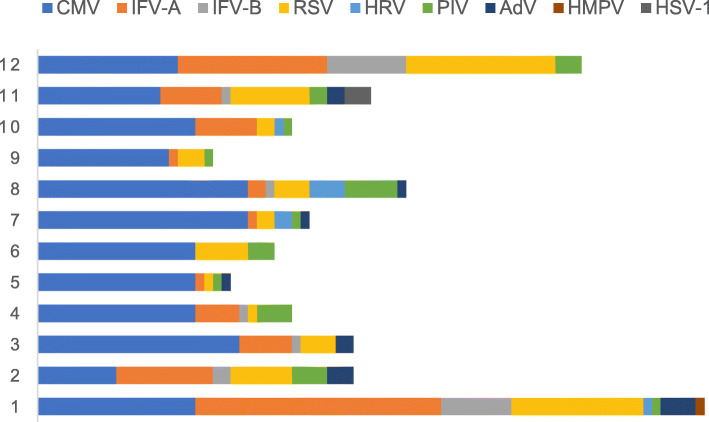
During the influenza season (November, December, January, and February), an increase in the number of IFV infection cases (22.4%) was found in the immunocompromised group, followed by CMV (15.4%) and RSV (13.0%) infection cases. In the immunocompetent group, IFV (43.5%) was most frequently detected, followed by RSV (14.9%). During the non-influenza season, CMV (42.7%) was the main virus detected in the immunocompromised group. However, in the immunocompetent group, there was no dominant virus; the order of detection was as follows: IFV (9.4%), PIV (7.6%), AdV (7.3%) HRV (7.1%), and RSV (5.7%) (Table 2 and Figs. 1 and 2). Regarding coinfections in immunocompromised patients, PCP (22.4%), Aspergillus (14.1%) and bacteria (13.8%) were most frequent, with Klebsiella pneumoniae (4.1%), Pseudomonas aeruginosa (3.0%), and Staphylococcus aureus (3.0%) being the most common bacteria. In the immunocompetent group, Aspergillus (10.8%), bacteria (7.1%), and Mycoplasma (5.3%) were the dominant pathogens, with S. aureus (2.5%), K. pneumoniae (2.1%), and Streptococcus pneumoniae (1.1%) being the dominant bacteria. Among the secondary nosocomial bacterial infections, Acinetobacter baumannii, P. aeruginosa, and K. pneumoniae were most commonly detected as causative agents (Table 2). The CMV infection group had more patients with nephrotic syndrome and high rates of PCP infection and ground glass shadows on computed tomography (CT) (P < 0.05). In the non-IFV group, there were fewer patients who required non-invasive ventilator use and intensive care unit treatment than other groups. Further, the non-IFV group was associated with a lower in-hospital mortality rate than CMV and two-or-more viruses’ groups. However, patients with IFV and non-IFV infection in immunocompromised patients had similar disease severity and prognosis (Table 3).
Table 2.
The pathogen results of pneumonia between immunocompetent and immunocompromised group
| Variables, n (%) | Immunocompromised group, n = 370 |
Immunocompetent group, n = 436 |
P-Value |
|---|---|---|---|
| One virus | 305 (82.4) | 396 (90.8) | < 0.001 |
| Two or more viruses | 65 (17.6) | 40 (9.2) | < 0.001 |
| Influenza season | |||
| Cytomegalovirus | 57 (15.4) | 12 (2.8) | < 0.001 |
| Influenza A virus | 63 (17.0) | 165 (37.8) | < 0.001 |
| Influenza B virus | 20 (5.4) | 25 (5.7) | 0.840 |
| Rhinovirus | 1 (0.3) | 6 (1.4) | 0.092 |
| Respiratory syncytial virus | 48 (13.0) | 65 (14.9) | 0.430 |
| Adenovirus | 9 (2.4) | 14 (3.2) | 0.508 |
| Parainfluenza virus | 10 (2.7) | 11 (2.5) | 0.873 |
| Human metapneumovirus | 1 (0.3) | 0 (0) | 0.277 |
| HSV-1 | 3 (0.8) | 0 (0) | 0.060 |
| Non-influenza season | |||
| Cytomegalovirus | 158 (42.7) | 10 (2.3) | < 0.001 |
| Influenza A virus | 23 (6.2) | 36 (8.3) | 0.268 |
| Influenza B virus | 3 (0.8) | 5 (1.1) | 0.632 |
| Rhinovirus | 7 (1.9) | 31 (7.1) | < 0.001 |
| Respiratory syncytial virus | 21 (5.7) | 25 (5.7) | 0.972 |
| Adenovirus | 5 (1.4) | 32 (7.3) | < 0.001 |
| Parainfluenza virus | 17 (4.6) | 33 (7.6) | 0.081 |
| Human metapneumovirus | 0 (0) | 3 (0.7) | 0.110 |
| Pathogenic types of coinfections | 204 (55.1) | 101 (23.2) | < 0.001 |
| Bacteria | 51 (13.8) | 31 (7.1) | 0.002 |
| Streptococcus pneumoniae | 1 (0.3) | 5 (1.1) | 0.149 |
| Streptococcus constellatus | 1 (0.3) | 0 (0) | 0.277 |
| Haemophilus influenzae | 1 (0.3) | 0 (0) | 0.277 |
| Staphylococcus aureus | 11 (3.0) | 11 (2.5) | 0.696 |
| Escherichia coli | 3 (0.8) | 1 (0.2) | 0.242 |
| Enterobacter aerogenes | 0 (0) | 1 (0.2) | 0.357 |
| Enterobacter cloacae | 2 (0.5) | 0 (0) | 0.470 |
| Klebsiella pneumoniae | 15 (4.1) | 9 (2.1) | 0.098 |
| Pseudomonas | 11 (3.0) | 4 (0.9) | 0.031 |
| Proteus mirabilis | 2 (0.5) | 0 (0) | 0.470 |
| Acinetobacter | 2 (0.5) | 0 (0) | 0.470 |
| Nocardia | 2 (0.5) | 0 (0) | 0.470 |
| Atypical | 11 (3.0) | 23 (5.3) | 0.105 |
| Mycoplasma pneumoniae | 6 (1.6) | 23 (5.3) | 0.006 |
| Legionella | 5 (1.4) | 0 (0) | 0.015 |
| Pneumocystis | 83 (22.4) | 0 (0) | < 0.001 |
| Aspergillus | 52 (14.1) | 47 (10.8) | 0.158 |
| Mycobacterium tuberculosis | 6 (1.6) | 0 (0) | 0.008 |
| Non-tuberculosis mycobacteria | 1 (0.3) | 0 (0) | 0.277 |
| Pathogens in nosocomial infection | 134 (36.2) | 168 (38.5) | 0.498 |
| Acinetobacter | 31 (8.4) | 52 (11.9) | 0.099 |
| Pseudomonas | 32 (8.6) | 41 (9.4) | 0.710 |
| Klebsiella pneumoniae | 14 (3.8) | 17 (3.9) | 0.932 |
| Burkholderia | 11 (3.0) | 17 (3.9) | 0.474 |
| Enterococcus | 6 (1.6) | 2 (0.5) | 0.097 |
| Enterobacter cloacae | 3 (0.8) | 0 (0) | 0.060 |
| Escherichia coli | 4 (1.1) | 1 (0.2) | 0.125 |
| Proteus mirabilis | 0 (0) | 2 (0.5) | 0.192 |
| Stenotrophomonas maltophilia | 4 (1.1) | 11 (2.5) | 0.131 |
| Corynebacterium striatum | 6 (1.6) | 11 (2.5) | 0.375 |
| Staphylococcus aureus | 4 (1.1) | 0 (0) | 0.030 |
| Rolstonia mannitolytica | 1 (0.3) | 5 (1.1) | 0.149 |
| Other bacteria | 5 (1.4) | 3 (0.7) | 0.344 |
| Aspergillus | 12 (3.2) | 6 (1.4) | 0.074 |
| Trichosporon asahii | 1 (0.3) | 0 (0) | 0.277 |
| Only one virus | 123 (33.2) | 259 (59.4) | < 0.001 |
| >one organism | 247 (66.8) | 177 (40.6) | < 0.001 |
HSV-1 herpes simplex virus type 1
Fig. 1.
Virus detection of immunocompromised hosts in different months
Fig. 2.
Virus detection of immunocompetent hosts in different months
Table 3.
Comparative analysis of different viral pneumonia in immunocompromised patients
| Variables | CMV N = 162 |
IFV N = 65 |
Non-IFV N = 79 |
≥two viruses N = 64 |
P-Value |
|---|---|---|---|---|---|
| Female, n (%) | 59 (36.4) | 18 (27.7) | 30 (38.0) | 20 (31.3) | 0.509 |
| Age, median (IQR), years | 60.0 (47.0, 68.3) | 63.0 (54.0, 69.0) | 59.0 (47.0, 68.0) | 60.0 (50.3, 67.0) | 0.616 |
| Symptoms and signs, n (%) | |||||
| Fever | 138 (85.2) | 48 (73.9) | 48 (60.8) | 42 (65.6) | < 0.001 |
| Cough | 138 (85.2) | 63 (96.9) | 79 (100.0) | 62 (96.9) | < 0.001 |
| Expectoration | 122 (75.3) | 63 (96.9) | 77 (97.5) | 60 (93.8) | < 0.001 |
| Dyspnea | 108 (66.7) | 43 (66.2) | 51 (64.6) | 43 (67.2) | 0.987 |
| Underlying Diseases, n (%) | |||||
| Connective tissue disease | 69 (42.6) | 19 (29.2) | 21 (26.6) | 25 (39.1) | 0.054 |
| Interstitial lung disease | 61 (37.7) | 31 (47.7) | 43 (54.4) | 30 (46.9) | 0.084 |
| Diabetes mellitus | 43 (26.5) | 18 (27.7) | 22 (27.9) | 20 (31.3) | 0.917 |
| Tumor | 19 (11.7) | 7 (10.8) | 11 (13.9) | 4 (6.3) | 0.524 |
| Bronchial asthma | 6 (3.7) | 0 (0) | 0 (0) | 0 (0) | 0.050 |
| COPD | 13 (8.0) | 6 (9.2) | 3 (3.8) | 2 (3.1) | 0.311 |
| Leukemia | 2 (1.2) | 0 (0) | 4 (5.1) | 1 (1.6) | 0.114 |
| Lymphoma | 5 (3.1) | 2 (3.1) | 5 (6.3) | 4 (6.3) | 0.535 |
| After bone marrow or HSCT | 2 (1.2) | 0 (0) | 1 (1.3) | 2 (3.1) | 0.490 |
| Nephrotic syndrome or chronic glomerulonephritis | 36 (22.2) | 2 (3.1) | 4 (5.1) | 4 (6.3) | < 0.001 |
| Solid organ transplant | 7 (4.3) | 17 (26.2) | 23 (29.1) | 13 (20.3) | < 0.001 |
| Cirrhosis | 0 (0) | 3 (4.6) | 1 (1.3) | 1 (1.6) | 0.059 |
| Laboratory examination | |||||
| White blood cell, ×109/L (IQR) | 8.50 (5.70, 12.52) | 7.95 (5.08, 11.07) | 7.57 (5.69, 11.47) | 8.50 (6.35, 11.45) | 0.587 |
| Neutrophils, ×109/L (IQR) | 7.08 (4.52, 10.94) | 6.80 (3.80, 9.24) | 5.69 (3.51, 8.81) | 6.90 (4.86, 9.77) | 0.081 |
| Lymphocyte, × 109/L (IQR) | 0.73 (0.41, 1.40) | 0.81 (0.41, 1.31) | 1.11 (0.60, 1.83) | 0.80 (0.45, 1.32) | 0.048 |
| Persistent lymphocytopenia | 84 (51.9) | 32 (49.2) | 28 (35.4) | 33 (51.6) | 0.097 |
| D-Dimer, mg/L | 1.78 (0.78, 3.08) | 1.52 (0.58, 3.09) | 1.12 (0.55, 2.68) | 1.34 (0.60, 2.57) | 0.288 |
| Lactate dehydrogenase, U/L | 395.5 (255.8, 590.0) | 325.0 (228.0, 482.0) | 300.0 (206.0, 430.0) | 386.0 (276.0, 553.9) | 0.007 |
| Oxygenation index | 184.2 (113.5, 286.0) | 285.7 (154.1375.9) | 244.1 (96.3, 277.1) | 122.4 (92.5, 272.8) | 0.067 |
| Severe pneumonia index score | 75.0 (58.0, 107.0) | 79.0 (60.0, 99.0) | 79.0 (61.0, 104.0) | 80.5 (57.8, 105.3) | 0.508 |
| CURB65 score > 1 | 55 (34.0) | 25 (38.5) | 19 (24.1) | 18 (28.1) | 0.234 |
| Imaging features, n (%),24 missing | |||||
| Consolidation or mass | 71 (43.8) | 24 (36.9) | 39 (49.4) | 34 (53.1) | 0.176 |
| Ground-glass opacity | 99 (61.1) | 30 (46.2) | 42 (53.2) | 35 (54.7) | 0.004 |
| Viral-PCP co-infection | 64 (39.5) | 4 (6.2) | 3 (3.8) | 7 (10.9) | < 0.001 |
| Viral-aspergillus co-infection | 16 (9.9) | 9 (13.8) | 12 (15.2) | 15 (23.4) | 0.069 |
| Viral-bacteria co-infection | 22 (13.6) | 7 (10.8) | 10 (12.7) | 9 (14.1) | 0.939 |
| Viral-atypical co-infection | 6 (3.7) | 1 (1.5) | 3 (3.8) | 1 (1.6) | 0.708 |
| Nosocomial bacterial infection | 36 (22.2) | 17 (26.2) | 17 (21.5) | 22 (34.4) | 0.237 |
| Complications, n (%) | |||||
| Noninvasive ventilation | 54 (33.3) | 10 (15.4) | 9 (11.4) | 17 (26.6) | 0.001 |
| Invasive mechanical ventilation | 45 (27.8) | 20 (30.8) | 16 (20.3) | 19 (29.7) | 0.462 |
| Respiratory failure | 91 (56.2) | 33 (50.8) | 26 (32.9) | 36 (56.3) | 0.001 |
| ICU care | 89 (54.9) | 22 (33.8) | 19 (24.1) | 26 (40.6) | < 0.001 |
| Septic shock | 40 (24.7) | 17 (26.2) | 14 (17.7) | 20 (31.3) | 0.305 |
| Extracorporeal membrane oxygenation | 4 (2.5) | 7 (10.8) | 7 (8.9) | 6 (9.4) | 0.268 |
| In-hospital mortality | 50 (30.9) | 14 (21.5) | 12 (15.2) | 22 (34.4) | 0.022a |
IFV influenza A virus, influenza B virus; Non-IFV virus respiratory syncytial virus (RSV), HPIV human parainfluenza virus, HRV human rhinovirus, ADV adenovirus and HSV-1 herpes simplex virus type 1, HSCT hematopoietic stem cell transplantation, COPD Chronic obstructive pulmonary disease.
aThe in-hospital mortality between non-IFV and IFV patients was not statistically different (P = 0.324), but the non-IFV group was associated with a lower in -hospital rate than that of CMV group and two or more viruses' group (P<0.05)
Patients with nephrotic syndrome and chronic glomerulonephritis had the highest rate of CMV infection (89.1%), organ transplant patients had the highest rate of RSV infection (35.0%), patients with haematopoiesis diseases had the highest rates of AdV (22.7%) and HRV (18.2%) infections, and malignant solid patients with radiotherapy and chemotherapy had the highest rate of PIV infection (23.5%). Patients with nephrotic syndrome and chronic glomerulonephritis had a low oxygenation index and lymphocyte count, high rate of CMV and PCP infection, were more likely to require additional non-invasive ventilator use and intensive care unit treatment, and had a high in-hospital mortality rate. The in-hospital mortality rate of patients with connective tissue disease was the second highest (30%), while that of solid-organ transplantation patients was the lowest (10%) (Table 4). Viral shedding was significantly longer in immunocompromised hosts than in immunocompetent hosts (Table 5).
Table 4.
Clinical characteristics of pneumonia with immunocompromised patients in different underlying disease
| Variables | Connective tissue disease, N = 134 | Solid organ transplant, N = 60 | Nephrotic syndrome or chronic glomerulonephritis, N = 46 |
Hematopoiesis diseasesa N = 22 |
Idiopathic interstitial pneumonia, N = 51 |
Radiotherapy and chemotherapy of malignant solid tumor, N = 17 |
P value |
|---|---|---|---|---|---|---|---|
| Sex, female, n (%) | 64 (47.8) | 11 (18.3) | 11 (23.9) | 7 (31.8) | 16 (31.4) | 3 (17.6) | < 0.001 |
| Age, median (IGR) | 62.0 (45.0, 70.3) | 58.0 (47.0, 63.0) | 58.0 (47.8, 65.3) | 55.0 (32.8, 69.5) | 59.0 (53.0, 69.0) | 64.0 (57.0, 67.0) | 0.043 |
| Laboratory examination | |||||||
| White blood cell, × 109/L (IQR) | 8.59 (6.30, 11.72) | 6.79 (4.47, 9.81) | 8.83 (6.44, 11.97) | 5.58 (3.21, 9.85) | 7.85 (5.73, 11.48) | 8.01 (4.21, 10.77) | 0.005 |
| Neutrophils, × 109/L (IQR) | 6.99 (5.05, 9.80) | 4.63 (3.11, 7.70) | 8.20 (5.2, 10.9) | 4.19 (1.89, 7.51) | 6.45 (4.60, 9.58) | 6.73 (2.91, 8.30) | 0.001 |
| Lymphocyte, × 109/L (IQR) | 0.81 (0.44, 1.45) | 0.95 (0.36, 1.62) | 0.62 (0.33, 0.96) | 0.70 (0.22, 1.34) | 1.09 (0.70, 1.83) | 0.80 (0.46, 1.21) | 0.039 |
| Oxygenation index | 212.4 (116.8, 291.8) | 244.1 (142.4, 338.1) | 122.0 (78.6, 206.2) | 225.8 (116.1, 368.2) | 209.2 (111.3, 328.5) | 327.4 (296.2, 413.6) | 0.026 |
| Severe pneumonia index score | 76.0 (50.8, 103.0) | 83.0 (64.3, 100.0) | 89.5 (66.8, 119.0) | 87.5 (59.3, 119.0) | 79.0 (63.0, 91.0) | 107.0 (80.0, 125.0) | 0.018 |
| CURB65 score > 1 | 37 (27.6) | 17 (28.3) | 19 (41.3) | 4 (18.2) | 15 (29.4) | 6 (35.3) | 0.421 |
| Imaging features, n (%) | 126 (94.0) | 59 (98.3) | 37 (80.4) | 18 (81.8) | 51 (100.0) | 15 (88.2) | – |
| Consolidation or mass | 86 (64.2) | 26 (43.3) | 27 (58.7) | 5 (22.7) | 38 (74.5) | 8 (47.1) | < 0.001 |
| Ground-glass opacity | 65 (48.5) | 22 (36.7) | 21 (45.7) | 11 (50.0) | 18 (35.3) | 10 (58.8) | 0.049 |
| CMV | 89 (66.4) | 18 (30.0) | 41 (89.1) | 11 (50.0) | 25 (49.0) | 7 (41.2) | < 0.001 |
| IFV-A | 24 (17.9) | 18 (30.0) | 5 (10.9) | 3 (13.6) | 12 (23.5) | 6 (35.3) | 0.086 |
| IFV-B | 9 (6.7) | 5 (8.3) | 0 (0) | 0 (0) | 5 (9.8) | 2 (11.8) | 0.229 |
| RSV | 26 (19.4) | 21 (35.0) | 3 (6.5) | 2 (9.1) | 14 (27.5) | 0 (0) | 0.001 |
| AdV | 3 (2.2) | 3 (5.0) | 0 (0) | 5 (22.7) | 0 (0) | 1 (5.9) | < 0.001 |
| HRV | 2 (1.5) | 1 (1.7) | 0 (0) | 4 (18.2) | 0 (0) | 0 (0) | < 0.001 |
| PIV | 9 (6.7) | 6 (10.0) | 0 (0) | 3 (13.6) | 3 (5.9) | 4 (23.5) | 0.035 |
| Viral-PCP co-infection | 30 (22.4) | 3 (5.0) | 24 (52.2) | 3 (13.6) | 9 (17.6) | 4 (23.5) | < 0.001 |
| Viral-aspergillus co-infection | 13 (9.7) | 17 (28.3) | 5 (10.9) | 1 (4.5) | 6 (11.8) | 2 (11.8) | 0.010 |
| Viral-bacteria co-infection | 16 (11.9) | 12 (20.0) | 6 (13.0) | 1 (4.5) | 2 (3.9) | 3 (17.6) | 0.134 |
| Viral-atypical co-infection | 4 (3.0) | 2 (3.3) | 3 (6.5) | 1 (4.5) | 0 (0) | 0 (0) | 0.518 |
| Nosocomial bacterial infection | 26 (19.4) | 25 (41.7) | 12 (26.1) | 5 (22.7) | 11 (21.6) | 2 (11.8) | 0.021 |
| Complications, n (%) | |||||||
| NIV | 41 (30.6) | 8 (13.3) | 17 (37.0) | 4 (18.2) | 15 (29.4) | 2 (11.8) | 0.035 |
| IMV | 41 (30.6) | 9 (15.0) | 12 (26.1) | 3 (13.6) | 17 (33.3) | 1 (5.9) | 0.033 |
| Respiratory failure | 78 (58.2) | 21 (35.0) | 24 (52.2) | 6 (27.3) | 28 (54.9) | 6 (35.3) | 0.004 |
| ICU care | 66 (49.3) | 10 (16.7) | 28 (60.9) | 8 (36.4) | 25 (49.0) | 2 (11.8) | < 0.001 |
| Septic shock | 31 (23.1) | 12 (20.0) | 17 (37.0) | 3 (13.6) | 11 (21.6) | 5 (29.4) | 0.256 |
| ECMO | 8 (6.0) | 4 (6.7) | 3 (6.5) | 1 (4.5) | 7 (13.7) | 0 (0) | 0.297 |
| In-hospital mortality | 40 (30.0) | 6 (10.0) | 18 (39.1) | 3 (13.6) | 13 (25.5) | 4 (23.5) | 0.011 |
NIV Noninvasive ventilation, IMV Invasive mechanical ventilation, ECMO Extracorporeal membrane oxygenation
aHematopoiesis diseases: Leukemia, lymphoma, bone marrow or hematopoietic stem cell transplantation
Table 5.
viral shedding in of different groups
| Variables | Viral shedding in immunocompromised group(d) |
Viral shedding in immunocompetent group(d) |
P value |
|---|---|---|---|
| IFV | 12.0 (6.5, 26.5) | 8.5 (5.0, 13.0) | 0.022 |
| RSV | 14.0 (6.0, 30.0) | 6.5 (3.0, 14.0) | 0.024 |
Discussion
This study was a large-scale, multicentre, retrospective study of the aetiology of and clinical risk factors for CAP in immunocompromised patients. The main findings were as follows: (1) The disease severity and in-hospital mortality rate of immunocompromised patients were higher than those of immunocompetent patients; (2) during the influenza and non-influenza seasons, the distribution of viruses in the immunocompromised group differed; (3) among the coinfections of immunocompromised patients, PCP was the main pathogen, followed by Aspergillus and bacteria, and in the immunocompetent group, Aspergillus was the most common pathogen, followed by bacteria and Mycoplasma; (4) the in-hospital mortality rate of the non-IFV infection group was lower than those of the CMV group and the two-or-more viruses group, but had similar prognosis with IFV group; (5) the type of virus infection varied according to the underlying diseases detected; (6) viral shedding was significantly longer in immunocompromised hosts than in immunocompetent hosts.
In recent years, several studies have focused on respiratory virus infection in patients after haematopoietic cell transplantation (HCT) [16–21]. Sachiko studied HRV in the lower respiratory tract of patients with HCT and found that 55% of patients had coinfections and that the 90-day mortality rate was 41% [16], which was similar to that of lower respiratory tract infections caused by RSV, PIV, or IFV [17–19]. Among the immunocompromised patients with IFV pneumonia, approximately 60% had an associated infection with at least one other organism, and the mortality rate among these patients was 15–30% [20]. The mortality rate among haematologic malignancy patients with RSV is approximately 18%, and in HCT recipients who developed RSV lower respiratory tract infections, it can be as high as 83% [21]. Similarly, our study showed that the disease severity and in-hospital mortality (26.5% vs 18.8%) of immunocompromised patients were higher than those of immunocompetent patients.
CMV, especially with PCP coinfection, has a high mortality rate in immunocompromised patients [22, 23]. However, at present, there are few comparative studies examining CMV and other respiratory viruses. Our findings indicated that during the influenza season, IFV, CMV, and RSV were the main viruses detected in immunocompromised hosts, while during the non-influenza season, we need to pay attention to CMV, IFV, PIV, AdV, HRV and RSV as these were more readily detected. Non-CMV viral infections may also exist with a PCP coinfection, albeit less frequent. Comparably we found no difference in the rate of virus-Aspergillus coinfections irrespective of the type of viral infection [13, 24].
The disease severity in, complications in, and outcomes of immunocompetent patients with CAP were similar between IFV- and non-IFV-related respiratory diseases [25–27]. We found that the in-hospital mortality rate was significantly higher in immunocompromised patients with CMV or two-or-more viral infections than the non-IFV infections. This suggests that when a viral infection is suspected in an immunocompromised patient, healthcare providers should also determine the presence of CMV and other viral aetiologies, as early diagnosis and treatment are essential in improving the outcomes. In addition, the highest mortality rate was observed among patients with nephrotic syndrome or chronic glomerulonephritis, for which there was a higher rate of CMV and PCP infection. This indicates that routinely screening for PCP and CMV infections should be considered for this group of patients. Moreover, the higher incidence of CMV and PCP and mortality rates associated with nephrotic syndrome patients may be related to the lack of routine prevention of infection when using immunosuppressants or glucocorticoids.
It has been suggested that viral respiratory infections in immunocompromised patients involve persistent viral shedding, rendering these patients contagious for prolonged periods [28–30]. Memoli et al. reported that the viral shedding period of immunocompromised patients was longer than that of immunocompetent patients with IFV pneumonia (19.04 vs. 6.38 days, respectively; P < 0.05) [29]. Virus detection for ≥30 days was reported in 29% of infected patients with haematological disorders [28]. In this study, we demonstrated that both influenza A virus subtype H1N1 and RSV infections had a longer viral shedding period in immunocompromised hosts, which made it necessary to extend the duration of antiviral therapy.
There were some limitations to this study. First, it had a retrospective design and might not have included all patients. Second, as it was a multicentre research, not every patient with pneumonia underwent a full array of pathogen testing. Therefore, pathogen identification and diagnosis could have been incomplete. Third, many patients had been previously administered antibiotics. Despite these limitations, our results were consistent with the literature and provide a detail insight into the clinical and pathogenic characteristics and outcomes of different viral infections in immunocompromised hosts.
Conclusions
Immunocompromised patients have high frequencies of coinfections, nosocomial infections, and mortality rates. A longer viral shedding duration may lead to a prolonged period of infectivity.
Supplementary Information
Additional file 1: Supplementary Table 1: virus detection in immunocompetent and immunocompromised group.
Acknowledgements
Not applicable.
Abbreviations
- CAP
Community-acquired pneumonia
- CMV
Cytomegalovirus
- Flu A
Influenza A virus
- Flu B
Influenza B virus
- PIV
Parainfluenza virus
- RSV
Respiratory syncytial virus
- AdV
Adenovirus
- HRV
Rhinovirus
- HMPV
Human metapneumovirus
- HSV
Herpes virus
- PCP
Pneumocystis jirovecii pneumonia
- ETA
Endotracheal aspirate
- BAL
Bronchoalveolar lavage
- PSI
Pneumonia severity index
- HCT
Haematopoietic cell transplantation
- RT-PCR
Reverse-transcription real time polymerase chain reaction
Authors’ contributions
Study design: LL. Data collection: LL, WC, LB, SL, JS, YR, JW, XZ, JL. Statistical analysis: LL. Writing: LL, SH. All authors take full responsibility for the study design, data analysis and interpretation, and preparation of the manuscript. All authors approved the final draft manuscript.
Funding
This work was supported by the Ministry of Science and Technology Support Program [grant number 2015BAI12B11] and the Beijing Science and Technology Commission Key Project [grant number D151100002115004].
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Ethics Committee of China-Japan Friendship Hospital (no. 2015–86) granted approval for this retrospective study and orchestrated the centralised collaboration and approval of all participating institutions. The data used in this study were anonymised before use. The use of raw data in this study was approved by China-Japan Friendship Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Camps Serra M, Cervera C, Pumarola T, Moreno A, Perello R, Torres A, Jimenez de Anta MT, Marcos MA. Virological diagnosis in community-acquired pneumonia in immunocompromised patients. Eur Respir J. 2008;31(3):618–624. doi: 10.1183/09031936.00073807. [DOI] [PubMed] [Google Scholar]
- 2.Barton TD, Blumberg EA. Viral pneumonias other than cytomegalovirus in transplant recipients. Clin Chest Med. 2005;26(4):707–720. doi: 10.1016/j.ccm.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcolini JA, Malik S, Suki D, Whimbey E, Bodey GP. Respiratory disease due to parainfluenza virus in adult leukemia patients. Eur J Clin Microbiol Infect Dis. 2003;22(2):79–84. doi: 10.1007/s10096-002-0864-4. [DOI] [PubMed] [Google Scholar]
- 4.Ebbert JO, Limper AH. Respiratory syncytial virus pneumonitis in immunocompromised adults: clinical features and outcome. Respiration. 2005;72(3):263–269. doi: 10.1159/000085367. [DOI] [PubMed] [Google Scholar]
- 5.Ljungman P. Prevention and treatment of viral infections in stem cell transplant recipients. Br J Haematol. 2002;118(1):44–57. doi: 10.1046/j.1365-2141.2002.03515.x. [DOI] [PubMed] [Google Scholar]
- 6.Vakil E, Sheshadri A, Faiz SA, Shah DP, Zhu Y, Li L, Kmeid J, Azzi J, Balagani A, Bashoura L, Ariza-Heredia E, Chemaly RF. Risk factors for mortality after respiratory syncytial virus lower respiratory tract infection in adults with hematologic malignancies. Transpl Infect Dis. 2018;20(6):e12994. doi: 10.1111/tid.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCann S, Byrne JL, Rovira M, et al. Infectious diseases working party of the EBMT. Outbreaks of infectious diseases in stem cell transplant units: a silent cause of death for patients and transplant programmes. Bone Marrow Transplant. 2004;33(5):519–529. doi: 10.1038/sj.bmt.1704380. [DOI] [PubMed] [Google Scholar]
- 8.Couch RB, Englund JA, Whimbey E. Respiratory viral infections in immunocompetent and immunocompromised persons. Am J Med. 1997;102(3A):2–9. doi: 10.1016/S0002-9343(97)00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vigil KJ, Adachi JA, Chemaly RF. Viral pneumonias in immunocompromised adult hosts. J Intensive Care Med. 2010;25(6):307–326. doi: 10.1177/0885066610377969. [DOI] [PubMed] [Google Scholar]
- 10.Society AT, et al. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2015;171(40):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 11.Sousa D, Justo I, Domínguez A, Manzur A, Izquierdo C, Ruiz L, Nebot M, Bayas JM, Celorrio JM, Varona W, Llinares P, Miguez E, Sánchez E, Carratalá J. Community-acquired pneumonia in immunocompromised older patients: incidence, causative organisms and outcome. Clin Microbiol Infect. 2013;19(2):187–192. doi: 10.1111/j.1469-0691.2012.03765.x. [DOI] [PubMed] [Google Scholar]
- 12.Silva DR, Menegotto DM, Schulz LF, Gazzana MB, Dalcin PTR. Clinical characteristics and evolution of non-HIV-infected immunocompromised patients with an in-hospital diagnosis of tuberculosis. J Bras Pneumol. 2010;36(4):475–484. doi: 10.1590/S1806-37132010000400013. [DOI] [PubMed] [Google Scholar]
- 13.Schauwvlieghe AFAD, Rijnders BJA, Nele P, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 14.Patterson TF, Thompson GR, 3rd, Denning DW. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo F, Chen Y, Yang SL, Xia H, Li XW, Tong ZH. Pneumocystis pneumonia in HIV-infected and immunocompromised non-HIV infected patients: a retrospective study of two centers in China. PLoS One. 2014;9(7):e101943. doi: 10.1371/journal.pone.0101943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo S, Waghmare A, Scott EM, Xie H, Kuypers JM, Hackman RC, Campbell AP, Choi SM, Leisenring WM, Jerome KR, Englund JA, Boeckh M. Human rhinovirus detection in the lower respiratory tract of hematopoietic cell transplant recipients: association with mortality. Haematologica. 2017;102(6):1120–1130. doi: 10.3324/haematol.2016.153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martino R, Ramila E, Rabella N, et al. Respiratory virus infections in adults with hematologic malignancies: a prospective study. Clin Infect Dis. 2003;36(1):1–8. doi: 10.1086/344899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39(9):1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 19.Chemaly RF, Ghosh S, Bodey GP, Rohatgi N, Safdar A, Keating MJ, Champlin RE, Aguilera EA, Tarrand JJ, Raad II. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006;85(5):278–287. doi: 10.1097/01.md.0000232560.22098.4e. [DOI] [PubMed] [Google Scholar]
- 20.Schnell D, Mayaux J, de Bazelaire C, Legoff J, Feuillet S, Scieux C, Andreu-Gallien J, Darmon M, Baruchel A, Schlemmer B, Azoulay É. Risk factors for pneumonia in immunocompromised patients with influenza. Respir Med. 2010;104(7):1050–1056. doi: 10.1016/j.rmed.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Khawaja F, Chemaly RF. Respiratory syncytial virus in hematopoietic cell transplant recipients and patients with hematologic malignancies. Haematologica. 2019;104(7):1322–1331. doi: 10.3324/haematol.2018.215152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu Q, Jia P, Su L, Zhao H, Que C. Outcomes and prognostic factors of non-HIV patients with pneumocystis jirovecii pneumonia and pulmonary CMV co-infection: a retrospective cohort study. BMC Infect Dis. 2017;17(1):392. doi: 10.1186/s12879-017-2492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korkmaz Ekren P, Töreyin ZN, Nahid P, Doskaya M, Caner A, Turgay N, Zeytinoglu A, Toz S, Bacakoglu F, Guruz Y, Erensoy S. The association between cytomegalovirus co-infection with pneumocystis pneumonia and mortality in immunocompromised non-HIV patients. Clin Respir J. 2018;12(11):2590–2597. doi: 10.1111/crj.12961. [DOI] [PubMed] [Google Scholar]
- 24.Ustun C, Slabý J, Shanley RM, Vydra J, Smith AR, Wagner JE, Weisdorf DJ, Young JAH. Human parainfluenza virus infection after hematopoietic stem cell transplantation: risk factors, management, mortality, and changes over time. Biol Blood Marrow Transplant. 2012;18(10):1580–1588. doi: 10.1016/j.bbmt.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou F, Wang Y, Liu Y, Liu X, Gu L, Zhang X, Pu Z, Yang G, Liu B, Nie Q, Xue B, Feng J, Guo Q, Liu J, Fan H, Chen J, Zhang Y, Xu Z, Pang M, Chen Y, Nie X, Cai Z, Xu J, Peng K, Li X, Xiang P, Zhang Z, Jiang S, Su X, Zhang J, Li Y, Jin X, Jiang R, Dong J, Song Y, Zhou H, Wang C, Cao B. Disease severity and clinical outcomes of community acquired pneumonia caused by non-influenza respiratory viruses in adults: a multicenter prospective registry study from CAP-China network. Eur Respir J. 2019;54(2):1802406. doi: 10.1183/13993003.02406-2018. [DOI] [PubMed] [Google Scholar]
- 26.Skowronski DM, De Serres G. Other respiratory viruses are important contributors to adult respiratory hospitalizations and mortality even during peak weeks of the influenza season. Open Forum Infect Dis. 2014;1:ofu086. doi: 10.1093/ofid/ofu086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjarnason A, Westin J, Lindh M, et al. Incidence, Etiology, and Outcomes of Community-Acquired Pneumonia: A Population-Based Study. Open Forum Infect Dis. 2018;5(2):ofy010. doi: 10.1093/ofid/ofy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehners N, Tabatabai J, Prifert C, Wedde M, Puthenparambil J, Weissbrich B, Biere B, Schweiger B, Egerer G, Schnitzler P. Long-term shedding of influenza virus, parainfluenza virus, respiratory syncytial virus and nosocomial epidemiology in patients with hematological disorders. PLoS One. 2016;11(2):e0148258. doi: 10.1371/journal.pone.0148258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Memoli MJ, Athota R, Reed S, Czajkowski L, Bristol T, Proudfoot K, Hagey R, Voell J, Fiorentino C, Ademposi A, Shoham S, Taubenberger JK. The natural history of influenza infection in the severely immunocompromised vs nonimmunocompromised hosts. Clin Infect Dis. 2014;58(2):214–224. doi: 10.1093/cid/cit725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Lima CR, Mirandolli TB, Carneiro LC, et al. Prolonged respiratory viral shedding in transplant patients. Transpl Infect Dis. 2014;16(1):165–169. doi: 10.1111/tid.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1: virus detection in immunocompetent and immunocompromised group.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.