Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes a potentially life-threatening disease, defined as Coronavirus Disease 19 (COVID-19). The most common signs and symptoms of this pathological condition include cough, fever, shortness of breath, and sudden onset of anosmia, ageusia, or dysgeusia. The course of COVID-19 is mild or moderate in more than 80% of cases, but it is severe or critical in about 14% and 5% of infected subjects respectively, with a significant risk of mortality. SARS-CoV-2 related infection is characterized by some pathogenetic events, resembling those detectable in other pathological conditions, such as sepsis and severe acute pancreatitis. All these syndromes are characterized by some similar features, including the coexistence of an exuberant inflammatory- as well as an anti-inflammatory-response with immune depression. Based on current knowledge concerning the onset and the development of acute pancreatitis and sepsis, we have considered these syndromes as a very interesting paradigm for improving our understanding of pathogenetic events detectable in patients with COVID-19.
The aim of our review is:
1)to examine the pathogenetic mechanisms acting during the emergence of inflammatory and anti-inflammatory processes in human pathology;
2)to examine inflammatory and anti-inflammatory events in sepsis, acute pancreatitis, and SARS-CoV-2 infection and clinical manifestations detectable in patients suffering from these syndromes also according to the age and gender of these individuals; as well as to analyze the possible common and different features among these pathological conditions;
3)to obtain insights into our knowledge concerning COVID-19 pathogenesis. This approach may improve the management of patients suffering from this disease and it may suggest more effective diagnostic approaches and schedules of therapy, depending on the different phases and/or on the severity of SARS-CoV-2 infection.
Abbreviations: ALI, Acute Lung Injury; BALF, Bronchoalveolar Lavage Fluid; ARDS, Acute Respiratory Distress Syndrome; CARS, compensatory anti-inflammatory response syndrome; CCL5, Chemokine (C-C motif) ligand 5; cMonocytes, classical Monocytes; CRP, C-reactive Protein; ESR, erythrocyte sedimentation rate; IL-1β, Interleukin 1β; IL-6, Interleukin 6; IL-8, Interleukin 8; IL-10, Interleukin 10; IL-17, Interleukin 17; IL-1R, IL-1 Receptor; IL-1RA, Interleukin-1 Receptor Antagonist; intMonocytes, Intermediate Monocytes; LDH, Lactate Dehydrogenase; MCP-1, Monocyte Chemoattractant Protein-1; MOF, Multiple organ failure; ncMonocytes, non-classical Monocytes; Rantes, regulated on activation, normal T cell expressed and secreted; SIRS, Systemic Inflammatory Response Syndrome; TGF-β, transforming growth factor β; TLRs, Toll-Like Receptors; TNFα, Tumor Necrosis Factor α
Keywords: CoV-2, Covid-19, Sepsis, Acute pancreatitis, Aging
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes a potentially life-threatening disease, defined as Coronavirus Disease 19 (COVID-19). This pathological condition is generally characterized by the presence of several signs and symptoms. The most common ones include cough, sore throat, fever, shortness of breath, sudden onset of anosmia, ageusia or dysgeusia, nausea or vomiting, and diarrhea. The course of COVID-19 is generally mild or moderate in more than 80% of cases, but it is severe or critical in about 14% and 5% of infected subjects respectively, with a significant risk of mortality [1]. The available studies are suggesting that COVID-19 infection induces a host’s defensive reaction, which is characterized by the coexistence of an excessive inflammatory response as well as by the development of immunodepression, with the release of a large spectrum of cytokines, interleukins, and mediators with pro-inflammatory and anti-inflammatory activity. According to these investigations, in their early reports, most of the authors have suggested that the pathogenetic mechanisms, as well as the symptoms and the signs detectable in patients with severe forms of SARS-CoV-2 infection, resemble those occurring in patients with sepsis and serious acute pancreatitis [2]. All these pathological conditions are characterized by a large spectrum of clinical expressions ranging from mild to more severe manifestations such as lung injury, multi-organ failure, and unfavorable prognosis. Based on the current evidence and knowledge concerning the onset and development of acute pancreatitis and sepsis, we have considered these syndromes as a very interesting paradigm for improving our understanding of pathogenetic events detectable in patients with COVID-19. The aim of our paper is:
1)to consider briefly the pathogenetic mechanisms acting during the emergence of inflammatory and anti-inflammatory processes in human pathology;
2)to examine inflammatory and anti-inflammatory events in sepsis, acute pancreatitis, and SARS-CoV-2 infections and clinical manifestations detectable in patients suffering from these syndromes also according to the age and gender of these individuals; as well as to analyze the possible common and different features among these pathological conditions,
3)to obtain insights into our knowledge of COVID-19 pathogenesis; this approach may improve the management of patients with this syndrome and it may suggest more effective diagnostic approaches and schedules of therapy, according to the different phases and/or severity grade of this infectious disease.
2. Pathogenesis and chronobiology of inflammatory and anti-inflammatory events in host’s defensive response against invading pathogens
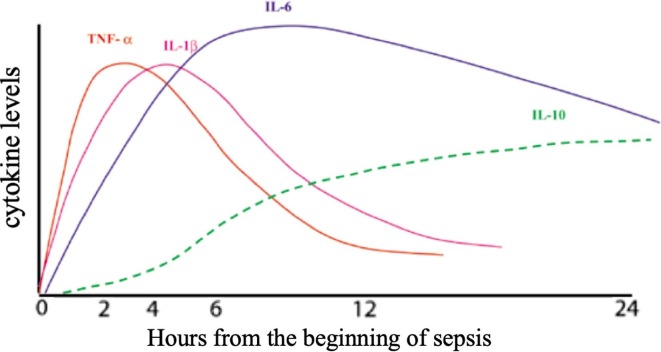
An effective immune response against invading pathogens in humans depends on a tightly regulated and proper balance among inflammatory and anti-inflammatory events involving both innate and adaptive arm of the immune system [3] and it is mediated by the coordinated action of a wide spectrum of immune cells and mediators, like chemokines, cytokines, pro-oxidant and anti-oxidant species and several costimulatory molecules [4]. The goal of the inflammatory response is to fight the pathogen and restore homeostasis. An appropriate activity of all these components, in turn, leads to the resolution of the infectious disease and the recovery of the ill subject. After the host’s infection, invading pathogens induce the expression of warning signals in human tissues. In particular, injured or dying cells release endogenous danger molecules, known as Damage-Associated Molecular Patterns (DAMPs) such as heat-shock proteins, hyaluronan fragments, glycosaminoglycan (GAG)-bearing matrix proteoglycans [5]. Furthermore, invading pathogens themselves generate products, such as glycoconjugates (e.g., bacterial lipo-oligosaccharides) or glycan-based polymers (e.g., bacterial peptidoglycans), including bacterial DNA or viral RNA (deoxy ribose-based polymers). These molecules are defined as “Pathogen Associated Molecular Patterns” (PAMPs) [6]. Some cells of the innate immune system, such as monocytes/macrophages come into contact with DAMPs and PAMPs, recognizing them through the pattern recognition receptors (PRRS) [7]. These events trigger some downstream inflammatory pathways to counteract invading pathogens and restore tissue homeostasis. A complex of intracellular multiprotein signaling platforms is activated. These structures are defined as “Inflammosomes” and represent key components of the innate immune response [8]. NLRP3 is the most studied among these complexes and exerts important functions in the generation of inflammation and the stimulation of effective and protective response of the host against pathogens [9]. After its induction, NLRP3 inflammasome activates caspase-1. This enzyme cleaves the proinflammatory cytokines IL-1/IL-18 and generates the mature forms of these mediators. Interleukin 1, produced by the stimulated macrophages, consists of two forms: IL-1α and IL-1β. Both molecules share the same receptor and, consequently, have the same biological effects. They possess a lot of common biological actions; therefore, they promote the development of inflammation with a synergistic action by binding to specific receptors and activate the gene expression of other inflammatory cytokines (IL-6, IL-8) [10]. IL-1 in association with TNF-α represent the starters in the chronobiology of inflammation ( Fig. 1 ). In particular, IL-1/IL-18 activate numerous intracellular pro-inflammatory signaling pathways. Nuclear Factor kB (NF-kB) translocates into the nucleus and regulates the expression of target genes, including those encoding pro-inflammatory cytokines [11], [12]. Following these events, monocytes and macrophages release a large spectrum of mediators and orchestrate the early steps of the host’s response against invading pathogens. A further host’s defensive process occurs, during the activation of the innate immune response. It is represented by pyroptosis, a highly inflammatory form of programmed cell death [13]. Overall, these cells, molecules, and chemical species constitute the innate arm of the immune response. The local inflammatory response triggered by the infection can extend throughout the body. It is mediated by the cells of the immune system and soluble mediators, released into the circulation [14]. In the early phases of this process, IL-1, in particular, IL-1β, and TNF-α are mainly produced by macrophages, within 30–90 min of interaction with the pathogens [15] and induce the release of other cytokines, eicosanoid, reactive oxygen species (ROS) and adhesion molecules. IL-6 and IL-8 serum levels peak after IL-1β and TNF-α (Fig. 2 ) [16]. Furthermore, IL-6 acts as a pyrogen [10] and induces the synthesis of the acute phase proteins. Pro-inflammatory cytokines, within 6 h, induce the production of C-reactive protein (CRP) by the liver. CRP, in turn, promotes bacterial opsonization and activates complement. However, CRP is a non-specific biomarker of acute conditions and it does not allow the distinction between infectious and non-infectious diseases. On the other hand, procalcitonin begins to increase already 2–4 h after the start of the immune cascade and peaks after 6–24 h [17]. Procalcitonin increases and reaches very high values in the course of bacterial infections. It allows distinguishing between infectious and non-infectious inflammatory diseases and between bacterial and viral pathologies [17], [18]. Only a few cytokines are constitutively expressed under non-pathological conditions. On the other hand, most of these mediators are produced and secreted after the activation of several intra-cell cascades paths. The expression of genes codifying the cytokines is strongly modulated at transcription and translation levels by several regulatory molecules. The transcription factor NF-kB, for example, promotes the expression of IL-1 and IL-6 [19]. IL-8 works by attracting neutrophils to the inflammation site [20]. It has been found in the bronchoalveolar lavage fluid (BALF) of patients with respiratory acute distress syndrome (ARDS) and it is involved in multiple organ dysfunction syndromes (MOF) [21]. In the chronobiology of the inflammation, IL-10 serum levels progressively and significantly increase during this defensive process. This mediator has the role of blocking the inflammation by modulating the synthesis and release of the other interleukins and cytokines (IL-6) [10]. IL-1Ra prevents the effects of IL-1 by competitively binding to the IL-1R receptor [22]. All these cytokines in cooperation with the reactive oxygen species and the antigen presenting cells also regulate the funcion of immune response, by stimulating the components of its adaptive arm, such as different subsets of B and T lymphocytes. The tightly regulated and well-coordinated activities of immune cells and mediators lead to the effective control of the infection and restore the host’s tissue homeostasis. However, an excessive and dysregulated activation of the immune system may generate severe organ damage and cause a life-threatening condition, such as sepsis.
Fig. 1.
Sepsis is a complex and heterogeneous process and it is characterized by the concurrent presence of two types of associated events. An excessive inflammatory response (Hyperinflammation) and an anti-inflammatory response (Immunosuppression). HYPERINFLAMMATION. After the infection of the host’s tissues by invading pathogens, a prompt immune response occurs (1). Some cells (monocytes/macrophages) of the innate immune system recognize a large spectrum of warning molecules, through the pattern recognition receptors (PRRS), such as PAMPS and DAMPs(2). After this event, a large series of intracellular pro-inflammatory signaling pathways are activated (3). NF-kB translocates into the nucleus (4) and regulates the expression of target genes, including those encoding pro-inflammatory cytokines such as TNFα-, IL-6, IL-12, IL-8 (5), and IFNs (6). The release of these soluble pro-inflammatory mediators causes an excessive inflammation known as a “cytokine storm” (7,8, 9). The activation of NLRP3 inflammasome promotes the generation of IL-1 and IL-18 (10) which contribute to amplify the inflammation, leading to the cytokine storm and stimulating the pyroptosis (12), a highly inflammatory form of programmed cell death. The activation of the complement system (13) contributes to the inflammatory reaction and triggers the coagulation cascade (14). The induction of the coagulation pathway can cause (15) disseminated intravascular coagulation (DIC), immunothrombosis, and hemorrhage (16). Neutrophils promote the coagulation cascade (17) and induce immunothrombosis (18) through the release of NETs. Cytokine storm, (19) NETosis, activation of the complement system, and the coagulation cascade can contribute to the development of the microcirculatory disorder (20), with vasodilatation, capillary leakage, thrombosis / microthrombosis, DIC and can culminate in ARDS and multi-organ dysfunction syndrome (MODS), two life-threatening pathological conditions. IMMUNOSUPPRESSION. During the development of sepsis, monocytes/macrophages undergo several epigenetic and functional changes and acquire immunosuppressive properties (a). These modifications are associated with an endotoxin refractory status, defined as Endotoxin Tolerance (b). Circulating monocytes (c) produce lower levels of TNF-⍺, IL-6 and express decreased amounts of HLA-DR (d). An expansion in the number of Immunosuppressive Myeloid-derived suppressor cells (MDSC) occurs(c). This type of Macrophages produce less TNF-⍺ and IL-6 (e). Furthermore, an increase in the release of anti-inflammatory cytokines (IL-10, TGF-β, IL-1RA) occurs (f). T lymphocytes exhibit immunosuppressive characteristics, with impaired production of pro-inflammatory cytokines such as IFN-γ and TNF-α (g) and undergo exhaustion. Their phenotype is now characterized by the increased expression of specific markers, including PD1, TIM-3 (h). Furthermore, a change in NF-kB composition occurs. In particular, its pro-inflammatory heterodimer (including the components p65 and p50) is replaced by an anti-inflammatory homodimer (including two molecules of p50) (i). This condition of immunosuppression is associated with an increased risk of nosocomial infections (l) and subsequent multi-organ failure (MOF) (m). These events are triggered in cooperation by both innate and adaptative arms of the immune system to eliminate pathogens and restore the homeostasis of the host’s damaged tissues. The dysregulation of all these immune mechanisms causes uncontrolled inflammation and represents the main feature for the development of sepsis. Genes encoding pro-inflammatory cytokines such as (TNF-⍺, IL-6, IL-8) and interferons (IFNs) are activated. Furthermore, the recognition of a strict relationship between coagulation and innate immunity, the term “immunothrombosis” has been introduced in clinical practice. Its activation in sepsis can result in microvascular thrombosis, DIC, and hemorrhage. Micro- and macrocirculatory disorders are characterized by vasodilatation, capillary leakage, and coagulopathy. Furthermore, vascular inflammation and pro-coagulation processes are increased by the release of extracellular traps by neutrophils (NETs), consisting of DNA, histones, and serine proteases. All these pathobiological events can result in multi-organ dysfunction syndrome (MODS), a very severe life-threatening condition.
Fig. 2.
Chronobiology of the inflammatory and anti-inflammatory process in sepsis (Boontham et al., 2003). IL-1 and TNF-α represent the chronobiology of inflammation. IL-6 and IL-8 serum levels peak after IL-1β and TNF-α. IL-10 serum levels increase significantly and progressively during sepsis. This interleukin has the role of blocking inflammation by modulating inflammatory cytokines.
3. Changing definitions of sepsis over time
The failure in the control of the pathogens by the immune system may lead to their uncontrolled systemic dissemination through the bloodstream and may cause a severe pleiomorphic syndrome, which is known as sepsis [23]. The definition of sepsis has undergone several changes over the years as a result of the difficulties in characterizing this pathological condition, depending on the evolving concepts and on the knowledge of its pathogenetic mechanisms. In 1991, a consensus conference concluded that sepsis results from the host mounting a systemic inflammatory response to infection [24]. Sepsis complicated by organ dysfunction was termed as “severe sepsis”, with progression to “septic shock”, when hypotension occurred despite adequate fluid resuscitation. In 2001 a task force has expanded the list of diagnostic criteria for this syndrome. However, no alternative definitions to characterize this syndrome have been introduced [25]. In 2016 an International Task Force has considered the more recent progress in understanding the pathobiology of sepsis as well as in the availability of large electronic health record databases and patient cohorts [26]. Therefore, the existing definitions of this syndrome have been reconsidered and revised. In particular, the previously described syndromes, indicated as “systemic inflammatory response syndrome (SIRS)” and “compensatory anti-inflammatory response syndrome (CARS)”, have been replaced by different terms, This process of editing has resulted in the most recent document, entitled: “Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). According to this paper, sepsis is now defined as life-threatening organ dysfunction, which is caused by a dysregulated host response to infection [26], [27]. The presence of the organ dysfunction is a key element for the recognition of this syndrome and this pathological condition may be assessed by the use of the “sepsis-related organ failure assessment (SOFA) score” [28]. This score is based on six different components, one each for the respiratory, cardiovascular, hepatic, coagulation, renal and neurological systems [29]. In particular, sepsis is currently defined by an increase of SOFA score ≥ 2 points due to an infection. Septic shock is determined by persisting hypotension requiring vasopressors to maintain a mean arterial pressure of 65 mm Hg or greater and a serum lactate level greater than 2 mmol/L in the absence of hypovolemia [26]. An early diagnosis and a rapid initiation of treatment represent crucial factors to reduce mortality from sepsis. It is expected that the value of epidemiologic studies and clinical trials will be improved by using the Sepsis-3 definitions [26]. Sepsis is now defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection [26], [27]. The most common cause of sepsis is represented by bacterial infections, whereas a lower number of cases is due to viruses, parasites, and fungi [30].
4. Pathogenesis and chronobiology of the inflammatory and anti-inflammatory processes in sepsis
Sepsis is a complex and heterogeneous process, it is characterized by the concurrent presence of two types of associated events [11]:
a)an excessive inflammatory response (Hyperinflammation) against invading pathogens, mainly in the early phase of this process, it is not protective, as it may induce severe tissue or organ damage in the host and it may lead to a life-threatening condition (Fig. 1);
b)an anti-inflammatory response (Immunosuppression), that may promote suppression of immune response and cause subsequent multiple infections, mainly in the more advanced phases of this process [31], [32] (Fig. 1).
Pro and anti-inflammatory cytokines are produced and released simultaneously since the onset of sepsis [33], as evidenced by transcriptome studies [34], [35]. The extent of the events, during the pro-inflammatory and/or the anti-inflammatory processes, is responsible for the fatal outcomes in this syndrome. An excessive inflammatory response may cause lethal effects, as well as an immunosuppressive response, which can lead to multiple infections, multi-organ failure, and death, mainly in the later stages of sepsis (Fig. 1).
NLRP3 displays an important role in the development of this syndrome [36]. Several studies have shown that NLRP3 null animals are protected against organ damage during the emergence of septic shock [37], [38] . After activation of the inflammasome complexes, the process of inflammation starts. However, excessive stimulation of the immune system is associated with the production of high cytokine levels and mediators. This event may drive an excessive inflammation, known as “cytokine storm”. A self-amplifying cytokine cascade develops and it represents a crucial pathogenetic mechanism by which infections can cause sepsis [39]. Several immune system cells and their related molecules, mediators of inflammation, and factors of coagulation are involved in the pathogenesis of sepsis [40]. In particular, the release of cytokines, such as TNF-α, IL-6, IL-12, IL-8, interferons (IFNs), mediators of inflammation such as prostaglandins and reactive oxygen species as well as the activation of additional important systems, including coagulation and complement pathways characterize the development of sepsis [41]. The complement system is involved in the host's innate defense responses. It stimulates the inflammatory reaction mainly through the activation of cells such as leukocytes and endothelial cells [42] and contributes to the development of the cytokine storm [43], [44]. The induction of the coagulation cascade is also part of the host’s defense systems against pathogens [45]. This occurrence arises during the development of sepsis and may be associated with the appearance of disseminated intravascular coagulation (DIC), microvascular thrombosis, and hemorrhage [46]. Neutrophils trigger the coagulation cascade and induce immunothrombosis through the release of NETs (complexes of DNA, histones, and serine proteases) and contribute to amplification of the inflammatory process [47], [48], [49]. Therefore, during sepsis, cytokine storm, NETosis, the activation of the complement system and coagulation cascade can contribute to the development of a microcirculatory disorder, with vasodilatation, capillary leakage, thrombosis/microthrombosis, DIC and can lead to the highly lethal multi-organ dysfunction syndrome (MODS) [50], [51] . Overall, inflammation, as well as coagulation and complement pathways, are tightly inter-connected processes and their interactions should be considered within a broader perspective [52], [53]. During the development of sepsis, a hyperinflammatory status coexists with an immune suppressive response. Monocytes/macrophages undergo genetic and epigenetic changes and exhibit functional modifications [54] and acquire immunosuppressive properties and characteristics. These modifications are associated with an endotoxin refractory state, defined Endotoxin Tolerance (ET) [55]. Various studies have shown the possibility of inducing ET following pre-exposure to sub-lethal doses of lipopolysaccharide (LPS). Overall, ET is associated with the presence of reprogrammed monocytes/macrophages with modifications in their activities and in their abilities to secrete pro-inflammatory and anti-inflammatory mediators. These cells modulate both the innate and adaptative arms of immune systems and change their functions. In particular, during the immunosuppressive response in sepsis the monocyte/macrophages exhibit the following activities:
1)up-regulate the release of inhibitory molecules, including glucocorticoids, prostaglandins, IL-10, and transforming growth factor-beta [56], [57];
2)down-regulate the Toll-like receptor signaling cascades [58], [59] and the expression of the Class II histocompatibility antigens (HLA-DR) [60];
3)turn the composition of the pro-inflammatory molecule NFkB (the heterodimer p65: p50 is changed into the anti-inflammatory homodimer p50:p50) [61], [62]. It has been shown that p50: p50 homodimers decrease the transcriptional activity of pro-inflammatory genes and stimulate the transcription of IL-10, whereas the expression of the heterodimer p65: p50 in response to LPS in peripheral blood mononuclear cells (PBMCs) of subjects with sepsis is decreased [63], [64]. However, the macrophage maintains unchanged phagocytic activity [65], [66]. A hallmark of endotoxin tolerance in vitro is the inability of monocytes/macrophages obtained from individuals with sepsis to produce pro-inflammatory cytokines TNF-α after their exposure to endotoxin in vivo [67]. Higher plasma levels of inflammatory cytokines (TNF-alpha, IL-6, IL-8), IL-10, and the IL-10/TNF-alpha ratio and decreased expression of HLA-DR on membrane surfaces of monocytes correlate with more elevated mortality in sepsis [68], [69], [70], [71]. As a consequence of all these events, heterogeneous populations of inducible immature myeloid-derived suppressor cells (MDSC) expand. These cells display a reduced antigen presentation capacity, and they are characterized by an anti-inflammatory phenotype, exerting a prominent role in the development of acquired immunodeficiency associated with sepsis [72], [73]. MDSCs modulate, attenuate or inhibit the excessive inflammatory responses and contribute to preventing the harmful effects of uncontrolled activation of immune responses [74]. An increase in MDSC was also found in sepsis contributing to immunosuppressive status and this event is associated with clinical worsening [75], [76] as well as with an increased risk of nosocomial infections and subsequent multi-organ failure (MOF), a common cause of mortality in most patients with sepsis [77], [78], [79], [80]. Furthermore, T lymphocytes detectable in patients with sepsis exhibit an exhausted phenotype, exerting immunosuppressive functions. In particular, these immune cells display an impaired production of pro-inflammatory cytokines, such as IFN-γ and TNF-α as well as with an increased expression of PD-1 and TIM-3 [81], [82], [83], [84]. A proper understanding and knowledge of chronobiology and patterns of components involved in inflammatory and anti-inflammatory events in the host’s defensive response, such as cytokine profile, NETosis, complement, and coagulation pathways involved in the sepsis are needed with the purpose to improve our knowledge of pathogenetic mechanisms causing the development of sepsis and it can help to optimize therapeutic strategy. In synthesis, when a bacterial infection occurs the innate immune response develops, and it is followed by the activation of adaptive immunity. During these events, both pro- and anti-inflammatory cytokines are produced, inducing a coexistent hyperinflammatory status to counteract the invading pathogens and an immunosuppressive response to reduce hyperinflammation and its potentially harmful effects [85]. The main pro-inflammatory biomarkers are represented by TNF-α, IL-1β, IL-6, IL-8. These interleukins are not specifically produced during sepsis, but they are well-known prognostic factors in patients with sepsis. The main mediators with immunosuppressive activities include IL-10, IL1ra, TGF-β [15]. IL-10 acts by decreasing hyper inflammation [86], [87], [88], [89]. The cytokine profile in the septic individuals could provide useful information about the most prevalent response (hyperinflammatory or immunosuppressive responses) and the patients’ prognosis, contributing to improving their management (Fig. 2).
5. Severe acute pancreatitis
Severe acute pancreatitis is a very serious pathological condition associated with a systemic immune response, including both inflammatory and anti-inflammatory components. These events are simultaneous and are dominated by the production and release of pro-inflammatory and anti-inflammatory cytokines and chemokines [90], [91], [92]. Although in recent years there has been an improvement in the knowledge and the therapeutic strategy of this disease, its specific pathogenesis is still unclear and the mortality rate remains high. Severe acute pancreatitis is burdened by severe complications, as its development may be associated with the onset of life-threatening conditions [93], [94]. This disease begins with the local damage, involving the pancreatic acini and ducts, due to the abnormal and early activation of pancreatic proteases [95]. This local damage causes the development of a defensive event in the injured organ. In particular, pancreatic tissue is infiltrated by different types of cells, belonging to the innate arm of the immune system. Macrophages and neutrophils are the most important components involved in the triggering of inflammation. Severe acute pancreatitis is characterized by the coexistence of an inflammatory reaction (sterile inflammation), predominant in the earliest phase of the syndrome, and an anti-inflammatory response, prevalent in a more advanced period of the disease. Once these responses are triggered, self-amplification and self-amplifying mechanisms arise and are associated with clinical manifestations detectable in patients with this pathological condition. Both these phases may evolve into highly lethal clinical manifestation. The inflammatory state is associated with the development of ALI/ARDS (Acute Lung Injury/Acute Respiratory Distress Syndrome), MOF (Multiple Organ Failure) and causes the patient’s death. ALI/ARDS are the leading causes of early mortality in patients with severe acute pancreatitis. On the other hand, the state of immunosuppression may promote the secondary infection of necrotic pancreatic tissue and induce the onset of septic shock. The cells, interleukins, cytokines, and other mediators as well as pathogenetic mechanisms involved in the onset of severe acute pancreatitis and the development of pro-inflammatory and anti-inflammatory responses have been already described in the previous section and will be not discussed further [96].
6. SARS-CoV-2 and COVID 19
At the end of 2019, a novel coronavirus with the capability to infect humans has emerged in Wuhan, Hubei Province in China, causing a pneumonia epidemic outbreak. This pathogen has been defined as SARS-CoV-2 (Severe Acute Respiratory Syndrome Corona Virus 2) [97]. It has rapidly spread across the world and the clinical syndrome associated with this infection has been named ‘coronavirus disease 2019′ (or ‘COVID-19′) and declared as a “public health emergency of international concern” by The International Health Regulations Emergency Committee of the World Health Organization [98]. The SARS-CoV-2 infection causes a multiorgan involvement with pulmonary symptoms, ranging from dyspnoea, chest discomfort, and dry cough to acute respiratory failure and severe respiratory distress syndrome as well as extrapulmonary ones, such as neurological-, cardiac-, renal-, gastrointestinal-, ocular-, vascular- and olfactory- clinical manifestations. Furthermore, patients with COVID-19 often display a systemic sign of disease, represented by fever. The multiorgan involvement has been associated with the abundance of the ACE2 receptors on the cell membranes in the distinct human tissues [99], [100]. Most patients with COVID-19 exhibit a proper innate and adaptive anti-viral response and successfully control the pathogen. Unfortunately, in some patients, immune system is unable to exert a protective function and a process of persistent inflammation develops, potentially leading to severe SARS-CoV-2 infection with an increased risk of death. Proper knowledge of COVID-19 immunopathogenesis is needed to improve the management and outcome of these patients.
7. COVID-19 immunopathogenesis
In the last months, a large series of studies are investigating the immunopathogenesis of COVID-19, concerning both the innate and adaptive arms of the immune system [101]. In patients with SARS-CoV-2 infection, the activation of the inflammation process develops, involving dendritic cells, monocytes, and macrophages [102]. Some differences are detectable in the immune system function, depending on sex and age, affecting the outcomes of COVID-19. The prevalence of COVID-19 is superimposable in both sexes. However, male sex, is a risk factor for severe disease and death, regardless of age [103], [104]. Age is also an independent risk factor for severe COVID-19. Human monocytes represent a heterogeneous population of cells belonging to the innate immune system arm. To date, at least three monocyte subpopulations have been identified, based on the expression of the CD14 and CD16 receptors on the cell membrane [105]:
-
i)
CD14++ CD16- - cMonocytes (classic monocytes) (in normal condition they represent the prevailing subpopulation: 80%);
-
ii)
CD14+ CD16+ - IntMonocytes (intermediate monocytes)
-
iii)
CD14+ CD16++ - ncMonocytes (non-classic monocytes).
A higher percentage of ncMonocytes is generally detectable in healthy male than in female individuals. This difference is associated with more elevated levels of cytokines and inflammatory chemokines such as IL-8 and CCL5 (Rantes) in the male sex in comparison with the female gender [106]. The CCL5 chemokine (Rantes) has several effects on innate and adaptive immunity. It promotes phagocytosis by macrophages in the inflamed lung, contributes to the migration of dendritic cells from the lung to the draining lymph nodes, recruits the effector T lymphocytes, promoting the adaptive immune response in the lung [107]. IL-8 is a chemokine capable to recruit neutrophils at the infection site and the presence of elevated levels of neutrophils is associated with a more severe prognosis for COVID-19. The role of monocytes, monocyte-derived cells, and DCs in COVID-19 pathogenesis is emerging from a large series of studies [108], [109].
In patients with non-infectious inflammatory pathological conditions or with infectious diseases associated or not with SARS-CoV-2, Nc-Monocytes contribute to endothelial inflammation and can promote micro thrombosis and thromboembolism [110], [111]. Table 1 shows the main differences between male and female gender in the pattern of the immune response in COVID-19 patients. The following elements are considered: IntMonocytes, ncMonocytes, inflammatory cytokines, and cell-mediated immunity. Male subjects have a strong inflammatory response, with increased production of inflammatory cytokines of the innate immune system. In female subjects, there is a robust cell-mediated response, with a prevalence of CD8+ T-lymphocytes (Table 1). Consistent with the effects of these peculiar immune responses, male subjects with poorer cell-mediated responses have a worse prognosis. On the other hand, the prognosis is worse in female subjects with a higher level of cytokines, produced and released by the cells belonging to the innate arm of the immune system. Furthermore, the impairment of the cell-mediated response correlates with age in male subjects but not in female subjects.
Table 1.
Differences in the pattern of the immune response in COVID-19 subjects, according to gender. IntMonocytes: intermediate monocytes; Red: Non-classical monocytes and inflammatory cytokines responses are associated with a pathological effect. Green: cell-mediated immunity is protective.
| Men | Women | |
|---|---|---|
| IntMonocytes | +/- | ++/- |
| ncMonocytes | +++ | +/- |
| Inflammatory Cytokines | +++ | +/- |
| Cell-mediated immunity | +/- | +++ |
The transition from the classic to the intermediate and non-classic phenotype is associated with the progressive loss of the CD14 receptor and the gain of the CD16 receptor. In vitro studies have shown that non-classical monocytes can induce a stronger inflammatory response following the binding to Toll-like Receptor (TLR) by the pathogen in comparison with the other subclasses of monocytes [112]. Senescence, NF-kB, and IL-1β promote the phenotypic switch towards non-classic monocytes. The rise in the proportion of ncMonocytes is associated with the increase in the production of TNF-α, IL-1β, and IL-8 [112], [113], [114], [115], [116], [117], [118], [119]. In subjects with severe COVID-19 the number of circulating intermediate monocytes CD14+ CD16+ and the synthesis of the tissue factor CD142 increase. On the other hand, non-classical CD14- CD16++ and HLA-DR expression on the cell surface monocytes decrease. DCs levels are reduced in both blood and lung. In the alveoli, the recruitment of monocytes occurs, and this event is associated with an increase in Monocyte-Derived Macrophages with pro-inflammatory phenotype [108], [120], [121]. A recent study has shown that an excessive switch towards the ncMonocyte phenotype is observed in patients with type 2 diabetes, suffering from COVID-19. Diabetes is one of the major risk factors for the development of severe COVID-19 disease [122], [123]. A new investigation has examined the different characteristics of the immune response to SARS CoV-2 in both sexes, during the early stages of the disease [124]. High levels of GM-CSF are produced by CD14+ CD16+ HLA-DHRlow inflammatory monocytes that express high levels of IL-6 and can infiltrate the lungs by amplifying inflammation and potentiating alveolar damage. Dendritic cells (DCs) are crucial in orchestrating both innate and adaptive immune responses during viral infection [106]. DCs are classified into conventional DCs (cDCs) and type I interferon- (IFN-) producing plasmacytoid DCs (pDCs) [125]. SARS-CoV-2 Infection causes a decrease in the numbers of circulating cDCs, of their progenitors and pDCs as well as induces a reduced expression of HLA-DR [126]. Furthermore, severe forms of COVID-19 are characterized by the expansion of the MDSCs suppressing T cell proliferation and IFN-γ production [127], [128]. This event is associated with a decrease in the number of T lymphocytes (CD4+ and CD8+) and NK cells as well as with their dysfunction [129]. All these alterations correlate with the mortality of patients suffering from COVID-19 [130]. In these subjects, circulating CD4+ and CD8+ T lymphocytes and NK cells exhibit the upregulation of markers of exhaustion (PD-1, TIM-3, LAG-3). These immune cells (monocytes, T cells, and NK cells) do not express significant amounts of pro-inflammatory cytokines [130], [131], [132]. On the other hand, activated CD4+ CTLs accumulate in the lungs [133] and correlate with disease severity and progression. CD4+ CTLs exhibit a high expression of transcripts encoding chemokines that are involved in the recruitment of myeloid cells and dendritic cells to the sites of viral infection. It determines a greater expression of HLA-DR on lung epithelial and endothelial cells and increases apoptosis of epithelial cells [134]. Furthermore, several ILs support inflammation and cause changes in the pattern of lymphocyte populations. In particular, IL-6 has an inhibitory effect on early steps, during the process of lymphopoiesis and promotes the depletion of some lymphocyte subclasses, decreasing their effector and cytotoxic activities, via a self-maintaining process [108], [135]. In particular, IL-6 induces the differentiation of some subpopulations of naive lymphocytes T, stimulating the generation of Th17 cell subsets with the capability to secrete further mediators, such as IL-17, IL-21, IL-22 [136]. IL-6, in association with IL-8, promotes the recruitment of neutrophils and monocytes into the lung. These mediators support inflammation and also cause lymphocyte depletion and reduce the expression of HLA-DR [137]. An increase in the Neutrophil/Lymphocyte ratio (NLR) has been also reported in these patients [138]. It has been reported that this parameter correlates with the severity of their pneumonia and systemic disease [139], [140]. On the other hand, the number, as well as the normal phenotype of T lymphocytes, is restored in the peripheral blood of patients recovering from COVID-19 [141]. Complement and coagulation systems, as well as platelets, form a closely interconnected network and the activation of the components belonging to one of these cascades is associated with the stimulation of other elements involved in this complex axis [142]. In particular, some elements of the complement pathway, such as C3a and C5a, may stimulate the coagulation process [143]. Thrombin can activate complement C3 and C5, independently of each other [144], as well as von Willebrand Factor (vWF) exerts a complex role in the process of coagulation and regulates the function of the complement system [145]. Whereas small vWF multimers increase the activity of the complement inhibitor factor I and the inactivation of C3b, large vWF ones stimulate the C3b to activate some components of the complement system [146], [147] . Some activated factors of the coagulation cascade (FIXa, FXa, FXIa as well as plasmin) can cleave C3 and C5, originating C3a and C5a, respectively [148]. C5a exerts a prothrombotic effect by upregulating TF [149] and plasminogen activator inhibitor-1 (PAI-1) expression by endothelial cells, neutrophils, and monocytes [150], [151] as well as plasminogen activator inhibitor-1 (PAI-1) [148]. Platelets also are involved in the complement activation [152]. The disruption of endothelial cells and platelets by the membrane attack complex (MAC) provides a nidus for prothrombinase assembly [153]. C3 and C5 are potent chemoattractant for neutrophils and monocytes that are recruited at the pulmonary level. Activated neutrophils generate NETs that activate the alternative complement pathway triggering a feedback loop and inducing a state of hypercoagulability. The MAC also induces endotheliitis and tissue damage. Endothelial damage leads to the generation of vWF and the release of PAI-1, exacerbating thrombosis. The interactions between complement activation, hypercoagulability, endotheliopathy, and thrombotic microangiopathy cause tissue damage like ARDS [154]. In vitro and in vivo investigations have also shown that SARS CoV-2 causes a robust activation of the complement pathway and activation and this event plays a critical role in the pathogenesis and the severity of COVID-19 [142]. The SARS-CoV-2 infection produces a strong impact on the functions of the complement system and platelet/neutrophil extracellular traps (NETs)/coagulation axis, causing dysregulation in their biological activities with severe pathological effects [142], [155]. In particular, the stimulation of complement cascade contributes to the genesis of coagulopathy and endotheliopathy, as detectable in patients with severe forms of COVID-19 [154], [156]. The role of the complement system activation in the pathogenesis of respiratory dysfunction has been demonstrated in mouse models of SARS CoV-1 [157] and MERS infections [158]. Furthermore, the activation and the deposition [156] of the complement components in several organs, such as lungs, heart, kidneys, brain, and skin have also been reported in SARS CoV-2 infection [159]. The interaction of the complement system and neutrophils play an important role in the pathogenesis of immunothrombosis in patients with severe COVID-19, as the neutrophils of these subjects express high amounts of Tissue Factor (TF) and release elevated levels of this component and neutrophil extracellular traps (NETs) [160]. Collectively, these data may suggest the use of strategies aimed at blocking or attenuating the activation of complement and/or coagulation pathways in COVID-19 patients, by acting on the components of these systems [156], [161], [162], [163], [164].
8. Weaker and delayed IFN response occurs in severe COVID-19 patients
A large series of studies have suggested that type I-III IFNs may have an effective and crucial role in counteracting SARS-CoV-2-associated infection. In particular: 1) defects in the function of Type I interferon pathway and autoantibodies against type I IFNs promote the development of severe forms of COVID-19 [165], [166]; 2) the results of some trials suggest that the use of recombinant Type I or Type III interferons in the treatment of patients with COVID-19 at an early stage of infection has beneficial effects on the clinical outcome of subjects suffering from this syndrome [167], [168].
3) Furthermore, some SARS CoV-2 proteins can antagonize the host’s antiviral response to interferons, delaying its onset and reducing its extent and duration [169], [170], [171].
According to the current knowledge, a crucial event in the host’s protective response against SARS CoV-2 infection depends on a tightly regulated and well-coordinated early activation of the innate arm of the immune system. In particular, a key step in this defensive process is represented by the production and secretion of type I-III IFNs. Some studies have investigated the chronobiology, concerning the release of these molecules as well as the magnitude and the time course of this event. It has been reported that a decreased and delayed IFN-I response causes the infiltration of the lungs by pathogenic inflammatory monocyte-macrophages (IMMs). These cells secrete elevated lung cytokine/chemokine amounts, causing damage in the endothelial wall of the vessels and the impairment of specific T-cell responses against the virus [172]. Recent research has confirmed the beneficial effects of timely production or administration of IFN in comparison with deleterious ones observed with its late synthesis or use. The study has been performed in 446 patients and it has shown a favorable clinical outcome and a decreased hospital mortality in individuals with COVID-19, who have been treated early with IFN-⍺ (IFN-2ab). On the other hand, an increase in mortality and a slower recovery has been observed in the individuals undergoing a delayed administration of this drug [173].
SARS CoV-2 infection causes a disease with a clinical course distinct from that elicited by other pathogens, such as influenza virus: (1) a longer incubation period, (2) a slower onset of symptoms, (3) a longer persistence of the virus in the respiratory tract, (4) a longer duration of disease, (5) a longer window of positivity, (6) a more prolonged hyper-inflammatory response, (7) a higher incidence of cases with more severe clinical forms and more elevated rates of mortality [174], [175], [176], [177], [178], [179].
Several reasons may contribute to explain these discrepancies. The defensive response induced by the IFN system in patients with COVID-19 differs from one detectable in individuals with flu. In particular, SARS-CoV-2 infection induces a weaker and delayed IFN type I-III response in comparison with that elicited by the influenza virus (about 1–3 days in influenza; 7–10 days in COVID-19). Furthermore, SARS CoV-2 stimulates the release of a cytokine pattern by immune cells, including TNF-⍺, IL-6, IL-8, IL-10. The spectrum of these mediators is similar to that elicited by the influenza virus, but the induced inflammatory response in individuals with COVID-19 is more prolonged and is associated with longer hospitalization, higher incidence of critical disease, and mortality in comparison with that detectable in subjects with flu (Fig. 3 ) [175].
Fig. 3.
The defensive response induced by the IFN system in patients with COVID-19 differs from one detectable in individuals with flu. SARS-CoV-2 infection induces a weaker and delayed IFN type I-III response in comparison with that elicited by the influenza virus (about 1–3 days in influenza; 7–10 days in COVID-19). Furthermore, SARS CoV-2 stimulates the release of a cytokine pattern by immune cells, including TNF-⍺, IL-6, IL-8, IL-10. The spectrum of these mediators is similar to that elicited by the influenza virus, but the induced inflammatory response in individuals with COVID-19 is more prolonged and is associated with longer hospitalization, higher incidence of critical disease, and mortality in comparison with that detectable in subjects with flu.
Pneumonia associated with Influenza A virus has a shorter incubation period, a more rapid onset, a shorter course, a lower risk of developing severe forms of the disease, and lower mortality [175], [176]. Further components of the immune system are involved in the development of an effective antiviral response. Recent studies have suggested that “bystander activated T cells” may display a crucial defensive role in this context. In particular, these subpopulations are induced in a cytokine-dependent- and in a TCR-independent way and produce IFN-α [180]. Type I interferons, interleukin-18, and interleukin-15 are mediators capable of rapidly inducing a «bystander activity». Even if these T cells are activated independently of their cognate antigen and exhibit no specific immune response, they can strongly affect the course of the SARS-CoV-2 infection. While antigen-specific T populations, belonging to the adaptive arm of the immune response, take several days to develop and exert their function, bystander activation of memory T cells can occur rapidly in response to cytokines of innate immune response (e.g., IFNs type I, IL-18, and IL-15), establishing the first line of defense. Therefore, it has been suggested that “bystander activated T lymphocytes” may promote an early protective role against several viruses with beneficial effects for the host [181], [182], [183], [184], [185]. However, long-lasting induction of ’’bystander T cells” may mediate the tissue injury in patients suffering from distinct infections, by exerting cytotoxic functions [180]. The activity of these T lymphocytes has been detected and studied e in subjects with acute and chronic HBV or HCV infections [186]. It has been reported that HBV-unrelated CD8+ T cells infiltrating the liver may contribute to hepatic injury [187]. As IFNs I is the main inducer of “activation of bystander T lymphocytes”, a proper and coordinated kinetic of production and release of these molecules, according to the stage of infection and to the microenvironmental context, display a crucial role in the pathogenesis of COVID-19. A recent study has investigated the function of this type of T cells in 207 patients with SARS-CoV2 infection of different severity. These individuals have been followed for 12 weeks by the onset of symptoms. The research has suggested that individuals with asymptomatic or mild forms of COVID-19, develop an early and robust immune response mediated by bystander-activated CD8 T cells, whereas their delayed activation and persistent alteration of these lymphocytes is associated with severe forms of COVID-19. Furthermore, Grant has reported that SARS-CoV-2 induces an alveolitis with a slow and spatially limited development. In particular, alveolar macrophages, which have engulfed the virions of SARS-CoV-2, and T-lymphocytes generate a self-maintaining loop, causing the persistence of alveoplar inflammation [188].
Fig. 4 depicts the profiles of viral load, cytokine pattern, IFN type I-III response, and disease severity in patients with Influenza A virus infection in comparison with individuals with COVID-19. Patients with SARS-CoV-2 infection, who remain asymptomatic or mildly symptomatic and who experiment no progression towards a life-threatening disease generally mount an early and robust activation of bystander CD8+ T cells. On the other hand, a dysregulated function of this type of T lymphocytes is associated with a higher risk of developing severe forms of COVID-19. Therefore, protective immune response in subjects with a self-limiting SARS-CoV-2 infection is probably characterized by early and well-coordinated activation of the IFN-I-III system and bystander CD8+ T cells.
Fig. 4.
Time profiles describing viral load, cytokine pattern, IFN type I-III response, and disease severity in patients with serious pneumonia associated with Influenza A virus infection (IAV) in comparison with individuals with COVID-19. Yellow line: magnitude of IFN I-III response; Purple line: viral load; Red line: magnitude of cytokines response; red box: severity of the disease. In IAV pneumonia, the IFN I-III mediated response is more prompt and more robust, precedes the pro-inflammatory one, the course of the disease is shorter, and the disease is less severe. In COVID-19 individuals with pneumonia, the IFN type I-III mediated response is weaker and delayed as well as it follows the pro-inflammatory one, the course is longer, and the disease is more severe.
9. Similarities and differences between severe COVID-19 and sepsis
There are both similarities and differences between severe COVID-19 and sepsis. Many patients with COVID-19 present the involvement of multiple organs and systems: lungs, liver, immune system, kidneys, brain, digestive system, heart, vessels. Furthermore, they may suffer from thromboembolism [189], [190] and develop some clinical manifestations, resembling septic shock: severe metabolic acidosis, cold extremities, and weak peripheral pulses as well as evident hypotension. Therefore, according to Li's observations and hypotheses [191] and based on the pathogenesis of a known and already widely studied pathological condition, such as sepsis, it is possible to draw useful lessons for a better understanding of disease mechanisms and clinical manifestations in patients with COVID-19. IL-6, IL-8, IFN-α, and TNF-α are important mediators involved in the immunopathogenesis of a wide spectrum of inflammatory diseases, including sepsis and SARS-CoV-2 associated infection. In particular, IL-6 significantly contributes to increasing vascular permeability and impairing the functions of multiple organs [192]. This mediator constitutes a useful biomarker as a prognostic factor in sepsis and serious COVID-19 and its levels are enhanced in both syndromes but by a different order of magnitude. After the outbreak of the SARS-CoV-2 pandemic, some studies have suggested the concept that subjects with severe forms of this infection present an inflammatory process with clinical and laboratory features similar to those detectable in CRS, sepsis, or acute pancreatitis and known as “cytokine storm”. The acceptance of this assumption has led to the use of powerful treatment, to counteract the process of cytokine release. However, this therapeutic approach may not reflect the actual profile in the serum levels of these mediators, even in patients with life-threatening conditions of COVID-19 and may have the theoretical risk of impairing the viral control by host’s immune response and of promoting the development of secondary infections. Available studies have shown increased levels of pro-inflammatory cytokines (IL-1, TNF-α, IL-6, IL-8) in patients with SARS-CoV-2, but the amounts of these mediators are usually lower than those found in sepsis, CRS, ARDS non-COVID-19, severe acute pancreatitis. In a meta-analysis, Leisman and colleagues have compared the levels of some pro-inflammatory cytokines and other biomarkers in patients with serious SARS-CoV-2-related infections with those reported in studies, enrolling individuals with sepsis, ARDS unrelated to COVID-19 and CRS [193]. In another meta-analysis, Hegyi and colleagues have compared inflammatory cytokine levels and disease severity in COVID-19 and acute pancreatitis [194]. Serum median levels of IL-6 in patients with these syndromes are about 10 until 100 times higher than ones in patients with severe COVID-19 (Table 2 ).
Table 2.
Immune-inflammatory profile in patients with COVID-19, Sepsis, ARDS unrelated-COVID, Cytokine Release Syndrome and Severe Acute Pancreatitis. Immune-inflammatory profiles and the mean serum concentration of some inflammatory cytokines in patients with COVID-19, Sepsis, ARDS unrelated sepsis, Cytokine release Syndrome, and Severe Acute Pancreatitis, according to Daniel Leisman’s meta-analysis. Mean serum IL-6 concentration is about 27, 43, 86, and 10 times higher in patients with sepsis, ARDS unrelated to COVID-19 respectively in patients with Cytokine Release Syndrome and with Severe Acute Pancreatitis in comparison with individuals with COVID-19.
| COVID-19 | SEPSIS | ARDS unrelated COVID-19 | Cytokine Release Syndrome | Severe Acute Pancreatitis | |
|---|---|---|---|---|---|
| Interleukin-6 (pg/ml) | 36.7 | 983.6 | 1,558.2 | 3,110.5 | 391.21 |
| Interleukin-8 (pg/ml) | 22 | 228 | 196 | 575 | 16.45 |
| TNF-α | 5 | 34.6 | 32 | 52.2 | _ |
| IFN-α | 10.8 | _ | _ | 3,722.1 | _ |
| s-IL2R | 506 | _ | _ | 12,396 | _ |
| CRP | ↑ | ↑ | ↑↑↑ | ||
| D-Dimer | ↑↑↑ | ↑ | |||
| Ferritin | ↑↑ | ↑↑↑ | |||
| Lactate dehydrogenase | ↑↑ | _ | _ | ↑↑↑ | _ |
| Pro-calcitonin | ↑ | ↑↑↑ | _ | _ | _ |
Based on available data, some researchers have questioned the existence of a “cytokine storm” in individuals with severe cases of SARS-CoV-2 infection and they have suggested that these subjects experience a “quasi cytokine storm” [193]. This assumption seems to be confirmed even in patients with ARDS associated with COVID-19, who require mechanical ventilation. Although serum levels of IL-6 and other mediators are significantly elevated in these individuals, they are relatively low compared to ones detectable in subjects with septic shock, acute pancreatitis, or CAR-T syndrome [195], [196]. However, in individuals with SARS-CoV-2 infection, IL-6 values tend to increase over time, depending on disease severity and deterioration of lung function [197], but they never reach ones detectable in the above-mentioned pathological conditions. In these subjects, moderately elevated IL-6 levels are a prognostic factor associated with respiratory failure and the need for mechanical ventilation (cutoff: ≥80 pg/ml). The 92% of patients with IL-6 values ≥ 80 pg/ml require mechanical ventilation within a median time of 1.5 days (range 0–4 days) [198]. IL-6 concentration ≥ 100 pg/mL has exclusively observed in critically ill patients and extremely high IL-6 level was closely correlated with viral load and mortality [199]. However, the serum levels and the kinetics of IL-6 clearly distinguish the response of subjects with SARS CoV 2 infection from septic patients [195]. A crucial element in the pathobiology of patients with COVID-19 is represented by the virus-mediated endotheliitis. SARS-CoV-2 can directly infect Endothelial Cells (ECs), by binding to the ACE2 receptor (an enzyme counteracting angiotensin vasopressors) [200] and may cause widespread endotheliitis and EC dysfunction. The presence of these signs has been demonstrated in several organs of patients who died from the virus [201].
However, further Authors have suggested an important role of an excessive cytokine response as a possible cause of organ damage not only in patients with sepsis but also in individuals with SARS-CoV-2 infection. Therefore, it has been speculated that the suppression of the inflammatory response by targeting IL-6, TNF-alpha, IL-1, could have a beneficial effect on the treatment of individuals with COVID-19.
Fig. 5 summarizes the events and pathogenetic mechanisms during the development of sepsis and COVID-19.
Fig. 5.
The picture summarizes peculiar (A and C) as well as common (B) events and pathogenetic mechanisms involved in the development of sepsis and COVID-19.
10. Possible treatments for COVID-19.
According to available data, several immunomodulatory treatments have been tested or about to be tested for the treatment of COVID-19 and they are listed in this paragraph.
-
1)
Complement system
The complement-mediated inflammatory response can cause damage of different severity in the host’s organs and tissues. These harmful effects are characterized by the development of endothelial damage [202], thrombosis, and microthrombosis [203], [204], [205], potentially leading to the onset of sepsis [42], [206], [207], [208], ARDS [157], [209], and multiorgan failure [210]. These pathogenetic mechanisms and clinical manifestations are detectable also in subjects with COVID-19 [159], [211], [212]. These pathological conditions are the main cause of death in patients with COVID-19 [213] . Several studies have shown the widespread activation of the complement system in severe COVID-19 patients, with increased serum levels of its components and extensive deposition in autopsy samples [155], [156], [214], [215]. Taking advantage of these results, several trials evaluating the efficacy of complement inhibitors as a therapeutic strategy in adults with severe COVID-19 have been performed, although only a small number of patients have been enrolled in these studies. These drugs act at different levels of the complement cascade and include: a) AMY-101, a synthetic peptide that inhibits C3; b) eculizumab and ravulizumab, monoclonal antibodies against C5; c) Vilobelimab (IFX-1), a monoclonal antibody specifically targeting C5a; d) Narsoplimab, a human monoclonal antibody against MASP-2, acting at the level of the Lectin pathway. Clinical trials are currently in progress examining complement inhibitors [154]. The major concern in the use of these drugs is the risk of secondary infections. Eculizumab is an inhibitor of the complement system c5 fraction. This drug has been shown to be effective in the treatment of 4 patients suffering from ARDS associated with COVID-19 [216]. Vilobelimab (IFX-1) is a chimeric monoclonal IgG4 antibody that specifically binds with high affinity to the soluble form of human C5a. This drug blocks the biological activity of C5a, whereas the formation of the membrane attack complex (C5b-9) remains intact. Therefore, this drug prevents the inhibition of this important defense mechanism. Further drugs are under consideration. They include i) C3 inhibitors: AMY-101 (NCT04395456), APL-9 (NCT04402060); ii) C5 inhibitors: Ravulizumab (NCT04369469 and NCT04390464), and Zilucoplan (NCT04382755); iii) C5a receptor inhibitor: avdoralimab (NCT04371367); iv) C1 esterase inhibitors: Conestat Alfa (NCT04414631), ruconest (NCT04530136) [217].
-
2)
Monoclonal antibodies against cytokines
An excessive immune response with the overproduction of pro-inflammatory cytokines and chemokines, such as IL-1, IL-6, and TNF-α, has been described in severely ill patients with SARS-CoV-2 infection. Several studies have suggested the concept that in these subjects an inflammatory process, with features similar to those detectable in CRS, sepsis, or acute pancreatitis and known as cytokine storm, may occur [218]. This event has been proposed as a crucial pathogenetic mechanism, during the development of severe forms of COVID-19. The acceptance of this assumption has lead to the use of powerful therapies, based on counteracting the process of cytokine release. However, although the amounts of IL-1, IL-6, and TNF-α are usually lower than those detectable in sepsis, CRS, ARDS non-COVID-19, and severe acute pancreatitis [193]. Based on the concept that the inhibition in the activity of these ILs may represent a promising strategy for the treatment of patients with SARS-CoV-2, some Authors have promoted the search of drugs, to target these pro-inflammatory cytokines and of modulating their activities [219], [220], [221]. Some observational or randomized studies have been already performed or are in progress. After SARS CoV-2 infection, IL-1 is activated and induces the secretion of other pro-inflammatory cytokines (TNF-α, IL-16), with possible deleterious effects on the host’s tissues and organs [222]. The IL-1α release represents an early event in the activation of the inflammatory pathway, whether mechanisms for proper control of this cascade are overcome, a self-maintaining and a self-amplification loop may arise. Therefore, this process becomes dysregulated and may persist in an uncontrolled way, increasing the severity of the disease. Therefore, inhibition of IL-1 could become a promising strategy. Treatment with anakinra did not make better clinical outcomes in patients with mild to moderate COVID-19 (CORIMUNO), but it improved overall survival and invasive ventilation-free survival and was well tolerated in patients with ARDS associated with COVID-19 infection [223], [224]. The main challenge is to identify patients who might have benefits from this treatment, either through correct timing of administration or through the correct identification of molecules involved in the inflammatory responses of each individual [225]. Besides anakinra, available strategies to inhibit IL-1 include the monoclonal antibody canakinumab and the soluble IL-1 trap rilonacept. The DAMPs released by the damaged epithelium in the lung activate the NLRP3 inflammasome, This event turns the inactive IL-1 into active IL-1, leading to the production of an array of chemokines and cytokines [226], [227], [228], [229]. The upregulation of pro-inflammatory cytokines/chemokines in severe COVID-19 forms promotes lung infiltration by macrophages/neutrophils. This occurrence is associated with the development of a hypercoagulation state, with organ damage, and with the possible development of fibrosis [230]. The use of Anakinra (IL-1R blocker) has been shown to decrease mortality and the need for invasive mechanical ventilation as well as to improve the oxygenation status in patients with severe forms of COVID-19 in small clinical studies [231], [232]. Furthermore, it has been suggested that IL-6 also displays a critical role in the immune-pathogenesis of subjects with severe forms of COVID-19 [233]. The correlation between increased pro-inflammatory cytokines, disease severity, and fatal outcome in hospitalized COVID-19 patients has been suggested in some studies [234]. The actual existence of cytokine storm in patients with COVID-19 has been reconsidered and debunked by some studies [193] and the indiscriminate suppression of inflammation in COVID-19 patients admitted to ICU has been questioned, due to the risk of increasing secondary infections [235], [236] Tocilizumab is a monoclonal antibody against the receptor for the IL-6 and its use has been approved for the treatment of patients with cytokine release syndrome, following the chimeric antigen receptor-T (CAR-T) therapy. After the outbreak of the SARS-CoV-2 pandemic, the administration of this drug has been begun soon in patients with severe forms of COVID-19 in a compassionate manner [237]. Preliminary observational studies had suggested possible benefits of tocilizumab in improving the outcome and mortality rate in patients with COVID-19, but some further randomized clinical trials [236], [238], [239], have not confirmed these promising results [240], [241], [242]. Further randomized clinical trials will have to clarify several crucial points for the proper use of these drugs: their efficacy, their profile of safety, the appropriate time for their administration as well as the identification of predictive biomarkers. TNF-α levels are increased in COVID-19, and levels in the early phase of infection predict the severity and mortality rate of the disease [243]. Anti-TNF therapy reduces the formation of NETs in vitro as well as in murine models and patients with immune-mediated diseases [244], [245]. Furthermore, some observational clinical studies support the use of antibodies anti-TNF-α for the treatment of COVID-19 [246]. A number of clinical trials on the use of anti-TNF alpha (infliximab and adalimumab) in covid-19 are ongoing: CATALYST (infliximab): ISRCTN40580903), ACTIV-1 (renedesivir + infliximab): NCT04593940; Tufts (infliximab): NCT04425538; Xu (adalimumab): ChiCTR2000030089; AVID-CC (adalimumab): ISRCTN33260034.
-
3)
ICI
Checkpoints inhibitors (ICI) are drugs capable to block proteins defined as checkpoints, such as the PD-1 protein. These molecules are produced by some cells of the immune system and are involved in the regulation and control of the immune response. Lymphopenia, overexpression of several pro-inflammatory cytokines, and dysregulation of a large spectrum of regulatory proteins detectable in patients with COVID-19 make the administration of ICI a plausible therapeutic strategy [247]. In severe COVID-19, immunosuppression overlaps with hyper inflammation, and it is characterized by two important events: lymphopenia and overexpression of checkpoint molecules such as PD-1, TIM-3, LAG (signs of exhausted T populations) on membrane cell of these T lymphocytes. ICI can prevent the induction of apoptosis and depletion of T lymphocytes and subsequently stimulate the immunity of T lymphocytes against the SARS CoV-2 virus. Normal function and activities of T cell populations are induced and maintained by the presence of IL-7, IL-15, and Thymosin [248]. Thymosin and anti-PD-1 antibodies have been administered in the NCT04268537 clinical study. Treatment by means of ICI of patients with SARS-CoV-2 infection leads to the reactivation of exhausted T lymphocytes and the improvement of immune system activities. However, the most important concern of this therapeutic approach is represented by the possible increased release of mediators, exacerbating the inflammatory process and promoting the host’s tissue and organ damage. Nivolumab, an inhibitor of ICI, has been well-tolerated and it has not caused hypercytokinemia in patients with sepsis [82].
PD-1 and CTLA-4 promote the progression of the disease, by blocking the antiviral activity of T lymphocytes. CTLA-4, expressed by CD8+ T lymphocytes, can bind to its ligand (CD-86), which is expressed on the membrane of DCs or monocytes. This interaction suppresses the activity of the CD8+ T-cells and down-regulates the antiviral activity of these lymphocytes [249], [250], [251], [252]. Therefore, PD-1 inhibition is capable to remove the state of energy in peripheral effector CD8+ T lymphocytes and it promotes their reactivation, while CTLA-4 inhibition leads to an increased in the number of CD4+ and CD8+ T cells [253].
-
4)
JAK inhibitors
The activation of JAK-STAT (Janus kinase–signal transducer and activator of transcription) signaling pathways transmit information from the extracellular environment into intracellular compartments, including the nucleus. This event is mediated by chemical signals and is associated with a modification in the expression of several genes, encoding a large number of proteins. In particular, these molecules regulate a lot of crucial cell functions, such as their proliferation, differentiation, apoptosis, and production of energy (Fig. 6 ).
Fig. 6.
JAK-STAT signaling pathways. Receptors (binding the external chemical molecules), Janus kinases (JAKs), Signal Transducer, and Activator of Transcription proteins (STATs) are indicated. JAKs specifically binds to the intracellular domains of the cytokine receptor and catalyzes ligand-induced phosphorylation, recruiting STAT molecules (STATs). Phosphorylated STATs undergo either homo- or heterodimerization, enter the nucleus, regulating the expression of target genes.
The functions of these cytokines have been identified in the last years, through the study of some inherited immunodeficiency syndromes. These pathological conditions are caused by mutations that block either receptor-JAK interactions or the kinase activity of the JAKs (Fig. 7 ) [254].
Fig. 7.
Immunomodulation: targeting cytokines, Jak, and complement cascade.
JAKs specifically binds to the intracellular domains of the cytokine receptor and catalyzes ligand-induced phosphorylation, recruiting STAT molecules (STATs). Phosphorylated STATs undergo either homo- or heterodimerization and translocate into the nucleus, modulating the expression of target genes [255], [256], [257]. The use of Jak inhibitors for the treatment of COVID-19 patients presents three possible side effects: 1) cytopenia [258], [259]; 2) increased risk of infectious complications, associated with immunosuppression [260], [261]; 3) enhanced thromboembolic risk [262], [263], [264].
Although the inhibitors of the JAKs pathway cause lymphopenia, anemia, and neutropenia in patients with rheumatoid arthritis, some studies have shown that the use of this class of drugs may induce some potentially beneficial effects in patients with SARS-CoV-2 infection. In particular, Baricitinib and Ruxolitinib, two of these Jaks inhibitors, increase the levels of lymphocytes in individuals with severe forms of COVID-19 [265], [266], [267].
The use of baricitinib for a prolonged period causes a slight increase in the rate of herpes simplex virus and of varicella-zoster virus infections [261]. On the other hand, a shorter exposure to this drug, as indicated for the treatment of COVID-19 patients, should present a good safety profile and low numbers of side effects [261].
Data on the risk of thromboembolic events in patients in therapy with Jak inhibitors are inconclusive [263], [268], [269]. Jaks inhibitors could lower the thromboembolic risk in treated individuals, by decreasing the severity of inflammation as well as the rate of intubation and bed rest syndrome. It has been also shown that baricitinib accelerates the reduction in D-dimer levels [270].
-
5)
Corticosteroids
The anti-inflammatory and immunosuppressive properties of corticosteroids have been widely exploited in rheumatic diseases [271]. Some studies have assessed the effectiveness and safety of corticosteroids use in patients with SARS-CoV-2 infection. However, to date, the available data are still considered inconclusive, although an increasing number of observational, as well as randomized controlled studies, have been performed or are in progress. A recent trial concerning the use of dexamethasone in subjects with COVID-19 has reported that this drug has reduced the mortality rates in intubated patients in comparison with standard treatments [272]. On the other hand, dexamethasone had no effect versus standard therapies in patients who required no respiratory support. Furthermore, a recent systematic review and meta-analysis have assessed the effectiveness and safety of corticosteroids in COVID-19. Forty-four studies have been considered, including 20.197 subjects. Twenty-two studies have also quantified the effect of corticosteroid administration on mortality rates. This meta-analysis has shown beneficial effects with the administration of corticosteroids on short-term mortality and a decreased need for mechanical ventilation in subjects with SAR-CoV-2 infection, although it has been described a slightly higher risk of infections and more elevated use of antibiotics.
11. Discussion
SARS-CoV-2 infection represents a very serious health problem worldwide as it causes potentially life-threatening diseases. Although our knowledge concerning the pathogenesis, the symptoms, and the signs of COVID-19 has increased in the last months with the introduction in clinical practice of preventive strategies, such as vaccines, and of therapeutic options with the purpose to attenuate the severity of SARS-CoV-2 infection or to block its spreading across the globe, to date no undoubted approaches are available for obtaining the definitive eradication of this pathogen. It is likely that SARS-CoV-2 has now become endemic in the world population, such as HBV, HCV, and HIV and that in the next years, it will cause further outbreaks with possible subsequent waves, mainly in the cold season. This risk depends on the mechanisms involved in the replication of this pathogen. In particular, SARS-CoV-2 is an RNA virus and it is now well-known that RNA viruses generally exhibit high mutation rates and error-prone genomic replication, as their RNA polymerase is lacking proofreading activity or systems for post replicative RNA repair [273]. As a consequence, the replication of RNA viruses is characterized by the incorporation of nucleotide random substitutions into the nascent genome. The poor fidelity of viral RNA polymerase in carrying out this event is associated with a high risk of generating different variants. SARS-CoV-2 as the other Coronaviruses has a peculiarity. The replicative machinery of this pathogen consists of 2 crucial subunits: 1) nsp12, exerting the RNA-dependent RNA polymerase (RdRp) activity with poor efficiency, 2) nsp14 (a 3′-5′ exoribonuclease), displaying a proofreading function with error-correcting capability during viral transcription [274], [275]. To date, despite the introduction in clinical practice of several antivirals with potential activity against this pathogen, none of the existing compounds has been definitively approved for the treatment of patients with COVID-19. These drugs are nucleoside analogs when incorporated into the nascent viral chains, cause the block of viral replication. It has been suggested that one of the critical reasons for the ineffectiveness of these antivirals may depend on the existence and of the activity of nsp14, it catalyzes the excision of nucleoside analogs from the nascent viral chains and restore viral replication [276], [277]. Therefore, based on these observations, to date, one of the strategies for the treatment of patients with COVID-19 may be the modulation of the pathogenetic mechanisms, acting in this syndrome. Excessive inflammation in association with immune depression characterizes sepsis, severe forms of acute pancreatitis, and COVID-19. In particular, these three syndromes share common pathogenetic mechanisms, but they also present peculiar and distinct characteristics. This paper has aimed to examine the pathogenetic events and clinical manifestations detectable in patients who suffer from these syndromes, also according to the age and the gender of these individuals, as well as to analyze the possible common and different features among these pathological conditions. This approach may improve the management of patients with COVID-19, and it may suggest more effective diagnostic strategies and schedules of therapy, depending on the different phases and/or on the severity of SARS-CoV-2 infection. According to current evidence, the following points may contribute to explain the pathogenesis of the disease associated with this virus and some of its peculiar characteristics in comparison with sepsis and acute pancreatitis. This virus enters human cells by binding to the Angiotensin-Converting Enzyme 2 (ACE-2) complex, an integral membrane glycoprotein with the catalytic site at the N end, facing the extracellular microenvironment and with a cytoplasmic C- terminus domain [278]. ACE-2 is a member of the Renin-Angiotensin-System (RAS), in association with angiotensinogen (ANG), angiotensin (Ang) I, Ang II, renin, Ang II type 1 receptor (AT1R), Ang II type 2 receptor (AT2R), and the Angiotensin-Converting Enzyme (ACE). This is a vital very complex network, regulating several pivotal functions in the human body [279]. Briefly, renin catalyzes the conversion of angiotensinogen to Ang I. Subsequently ACE cleaves Ang I and generates Ang II. This molecule exerts its function through two receptors (AT1R and AT2R). Both of them are G protein-coupled receptors, but the activation of AT1R mediates the most important biological actions, including the control of plasma sodium concentration, arterial blood pressure, extracellular volume, cell proliferation, inflammation as well as the processes of coagulation, fibrogenesis, and of cellular oxidative stress [280]. Persistent or excessive activation of Ang II causes deleterious effects. Therefore, tightly regulated mechanisms modulate the synthesized amounts and functionality of this molecule. In particular, ACE 2 converts Ang II into Ang 1–7. These metabolites downregulate NF-kB, decrease the generation of reactive oxygen species and fibroblast activation and collagen production. These events are associated with antiproliferative effects and counteract the profibrotic and proliferative activities of Ang II. Furthermore, ACE-2 antagonizes the release of several proinflammatory cytokines in vitro, including Tumor Necrosis Factor (TNF-α) and Interleukin-6 (IL-6) as well as reduce macrophage tissue recruitment. During the development of COVID-19, SARS-CoV-2 downregulates ACE2, increases macrophage expression and pro-inflammatory responses [281], [282], [283]. Furthermore, it has been shown that ACE-2 exerts antithrombotic activity and its inactivation could promote the development of thrombosis as it is observed in an elevated number of patients with COVID-19 [284]. However, not only SARS-CoV-2 affects the activity and function of this enzyme and RAS. Influenza virus infection also is associated with the down-regulation of ACE-2. In particular, H1N1, H3N2, H5N1, and H7N9 types have been demonstrated to affect its activity [285] and induce acute lung injury in a way similar to the damage observed in ARDS [286]. A recent study has reported that, during a median of 8.7 years of follow-up, individuals treated with ACE inhibitors experienced a reduced risk of influenza virus infection in comparison with controls not receiving this therapy [287]. However, some reports investigating the type of injury detectable in lung samples from patients with SARS-CoV-2 and influenza viruses have shown very interesting features. In particular, fibrin microthrombi can be seen in the alveolar capillaries of all lungs from both COVID-19 and H1N1. Alveolar capillary microthrombi were 9 times more prevalent in patients with COVID-19 than in H1N1. 3D micro-CT of lung samples that confirmed histological results that both COVID-19 and H1N1 showed occlusions of precapillary and postcapillary vessels [288]. Teuwen and colleagues have proposed some mechanisms to explain the processes leading to thrombosis in subjects with SARS-CoV-2 infection [289]. Based on current evidence, several similar microscopic histological alterations with different degrees of severity have been described in the lung tissue of individuals with COVID-19 and the H1N1 influenza virus. The most common ones are represented by 1) microthrombi observed in pre-capillary venules, alveolar capillaries, and post capillaries, 2) patterns of diffuse alveolar damage. However, some differences exist between these individuals. Patients with SARS-CoV-2 present a significantly higher prevalence of microthrombi in alveolar capillaries and more severe endothelial involvement, diffuse thrombosis, and microangiopathy (9 times more prevalent) than individuals with H1N1 [289]. The reasons for these differences are not well-known, although some hypotheses may be considered. At a microscopic level, both these viruses down-regulates ACE-2 function, but with different mechanisms. In particular, it has been reported that neuraminidase protein of H1N1 induces ACE-2 protein degradation by proteasome pathway, without directly affecting viral replication, whereas SARS-CoV2 may promote the down-regulation of this protein and stimulates the shedding of its ectodomain via a TNF-α converting enzyme (TACE)-dependent way, as shown for SARS-CoV. The cytoplasmic tail of ACE2 is need for its shedding. This process is associated with the release of TNF-α and with the activation of several intracellular signaling cascades, leading to the tissue SARS-CoV-2 related damage [290]. To date, no definitive data are available about the biological activities and overall effects caused by ACE-2 shedding in the virus-mediated damage of human organs. The mechanisms described above may contribute to explain not only the pathogenetic events developing in patients with SARS-CoV-2 and Influenza virus infections but also in individuals with sepsis and with acute pancreatitis. Sepsis is a potentially life-threatening syndrome with a high risk of death, but its course may be impaired by the emergence of an additional pathological condition, defined as septic shock. It further increases the mortality rate of the affected subjects. In the last years, some studies have suggested the activation of RAS and ACE-angiotensin receptor II- T1 axis in patients with sepsis. These events represent a physiologic response to the infections, and it is characterized by the production of ROS and by the induction of structural modifications in endothelial cells. However, uncontrolled hyperstimulation of this network may lead to dysfunction in the micro-vascular tissue environment and organ failure in individuals suffering from sepsis [291]. According to the results in experimental animal models, a systemic downregulation of both AT-1 and AT-2 receptors occurs, with secondary hypotension, although plasma Ang II and renin levels are increased in these individuals. Furthermore, adrenal AT-2 receptors are down-regulated with alteration in catecholamine release by the adrenal medulla and consequent hypotension [292]. However, in animal models, the infusion of Ang II has been reported to be effective as vasoactive drugs, such as norepinephrine, to restore arterial blood pressure, and cardiac output, whereas it induces a decrease in renal blood flow and cardiac output in healthy animals [293]. Some studies have shown that the use of Ang II in patients with vasoplegic shock and with severe acute kidney injury was associated with improved survival [294]. On the other hand, other investigations have suggested that individuals with ARDS present an increase of ACE levels in the broncho-alveolar lavage, and a reduction in the serum and elevated Ang I concentrations have been associated with higher mortality [295]. Although only a few studies are available and no definitive conclusion may be drawn, the stimulation of the Ang II/ACE pathways in experimental ARDS animal models is associated with an alteration of the ventilation to perfusion ratio and increases the severity of the inflammatory process. The use of drugs to counteract the ACE-Ang I axis has been reported to decrease lung injury and the extent of the inflammatory process in animal models, but no definitive conclusions and data concerning their effective usefulness are available in clinical practice [296], [297]. The modulation of the ACE-2 and Ang (1–7) axis, with the infusion of metabolites of Ang II, has been reported to prevent RAS-mediated lung injury and to provide a protective effect in animal models. Furthermore, the use in a pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in patients with ARDS has been associated with beneficial effects in these individuals, including the decrease of Ang II and IL-6 concentrations and an increase of surfactant protein D levels [298]. Based on all these shreds of evidence, although no definitive results are available, it is possible to hypothesize that the modulation of RAS function may be a very useful strategy to counteract the harmful effects associated with the hyperactivation of this system, during the development of sepsis. Current data from studies in animal models suggest that RAS may have a crucial role also in the development of acute pancreatitis [299]. In particular, increased activity of RAS and an imbalance in ACE and ACE2 is associated with a large spectrum of events involved in the onset of this pathological, such as the generation of a hyperinflammatory response, the arising of oxidative stress, and the release of pancreatic enzymes, as well as with its severity [300], [301]. The blockade of ACE–Ang II–angiotensin II type 1 receptor (AT1R) has been shown to prevent the development of pancreatitis in experimental studies [302]. A further investigation in an experimental mouse model of severe acute pancreatitis (SAP) has shown that ACE2 and its derivative products, such as angiotensin-(1–7) may mitigate the harmful effects of the hyperactivation of ACE-angiotensin receptor II- T1 axis [303]. It has been suggested that high levels of Ang-(1–7) in pancreatic tissue display anti-inflammatory and antioxidative activities [304]. Taking advantage of all this evidence, a possible role for RAS modulation also in patients with severe SARS-CoV-2 infection has been proposed and some small trials concerning the use of Ang II for the treatment of these individuals have been carried. These studies have shown that this treatment was associated with the improvement in cardiovascular and respiratory status in these individuals [305], [306]. Furthermore, the administration of ACE inhibitors and angiotensin receptor 1 blockers has been suggested to prevent or treat COVID-19 [307]. A further very interesting therapeutic option is represented by the use of soluble engineered variants of ACE2 (sACE2), lacking the transmembrane domain. These forms could interact with SARS-CoV-2 particles, seizing them, preventing their binding to the ACE-2 receptors on the surface of cell membranes, and reducing their entry into the cells. As a consequence, the native ACE-2 may exert its activity, catalyzing the production of Ang 1–7 and down-regulating the excessive inflammatory events associated with COVID-19 [308]. This promising strategy has been reported to be effective in “in vitro” cultured cells [309]. Further experimental evidence has shown that patients with SARS-CoV-2 infection and with vitamin D deficiency present a worst clinical outcome in comparison with subjects with normal levels of this fat-soluble compound [310]. A beneficial role of vitamin D as a treatment or as a preventive strategy to counteract SARS-CoV-2 infection has been first hypothesized [39], [311] and then reported in clinical practice [312], [313]. Interestingly, some studies have shown that an interplay may exist between ACE-2-axis and vitamin D and that this fat-soluble micronutrient may modulate this interaction, the function of RAS and exert a therapeutic effect [314], [315]. In particular, vitamin D may counteract the unbalance induced by SARS-CoV-2 via the stimulation of ACE2/Ang-(1–7)/pathway activity and inhibition of renin and the ACE/Ang II/AT1R axis. The up-regulation of ACE2 expression induces a protective anti-inflammatory and anti-fibrogenic effect and may contribute to preventing acute lung injury [316]. Further trials and experimental studies are requested to confirm these preliminary promising data, that are emerging also in clinical practice. Furthermore, the improvement of the RAS pathophysiology, obtained from these investigations can be used for a better understanding of pathogenesis concerning other pathological processes, such as malignancies, infections, and inflammatory diseases, with potentially useful diagnostic and therapeutic effects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The Authors thank: Dr. Simonetta Righi, Biblioteca Centralizzata, Policlinico S. Orsola-Malpighi, Università di Bologna, Bologna, Italy for her support in the search of scientific bibliography.
References
- 1.Xia L., Chen J., Friedemann T., Yang Z., Ling Y., Liu X., Lu S., Li T., Song Z., Huang W., Lu Y., Schroder S., Lu H. The Course of Mild and Moderate COVID-19 Infections-The Unexpected Long-Lasting Challenge, Open Forum. Infect Dis. 2020;7(9):ofaa286. doi: 10.1093/ofid/ofaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimabukuro-Vornhagen A., Godel P., Subklewe M., Stemmler H.J., Schlosser H.A., Schlaak M., Kochanek M., Boll B., von Bergwelt-Baildon M.S. Cytokine release syndrome. J. ImmunoTher. Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams M.A., Bevan M.J. Effector and memory CTL differentiation. Annu. Rev. Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 4.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venereau E., Ceriotti C., Bianchi M.E. DAMPs from Cell Death to New Life. Front. Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar H., Kawai T., Akira S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011;30(1):16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 7.Arpaia N., Barton G.M. Toll-like receptors: key players in antiviral immunity. Curr Opin Virol. 2011;1(6):447–454. doi: 10.1016/j.coviro.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019;20(13) doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackwell T.S., Christman J.W. Sepsis and cytokines: current status. Br. J. Anaesth. 1996;77(1):110–117. doi: 10.1093/bja/77.1.110. [DOI] [PubMed] [Google Scholar]
- 11.Hotchkiss R.S., Moldawer L.L., Opal S.M., Reinhart K., Turnbull I.R., Vincent J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinhagen F., Rodriguez L.G., Tross D., Tewary P., Bode C., Klinman D.M. IRF5 and IRF8 modulate the CAL-1 human plasmacytoid dendritic cell line response following TLR9 ligation. Eur. J. Immunol. 2016;46(3):647–655. doi: 10.1002/eji.201545911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar V. Inflammasomes: Pandora's box for sepsis. J Inflamm Res. 2018;11:477–502. doi: 10.2147/JIR.S178084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39(5):517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 15.Ramnath R.D., Weing S., He M., Sun J., Zhang H., Bawa M.S., Madhav Bhatia M. Inflammatory mediators in sepsis: Cytokines, chemokines, adhesion molecules and gases. Journal of Organ Dysfunction. 2006;2(2):80–92. [Google Scholar]
- 16.Boontham P., Chandran P., Rowlands B., Eremin O. Surgical sepsis: dysregulation of immune function and therapeutic implications. Surgeon. 2003;1(4):187–206. doi: 10.1016/s1479-666x(03)80018-5. [DOI] [PubMed] [Google Scholar]
- 17.Mehanic S., Baljic R. The importance of serum procalcitonin in diagnosis and treatment of serious bacterial infections and sepsis. Mater Sociomed. 2013;25(4):277–281. doi: 10.5455/msm.2013.25.277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi S.T., Song J.S. Serum Procalcitonin as a Useful Serologic Marker for Differential Diagnosis between Acute Gouty Attack and Bacterial Infection. Yonsei Med. J. 2016;57(5):1139–1144. doi: 10.3349/ymj.2016.57.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burkovskiy I., Sardinha J., Zhou J., Lehmann C. Cytokine release in sepsis. Advances in Bioscience and Biotechnology. 2013;4:860–865. [Google Scholar]
- 20.Gomes A.P., Balbino Miguel P.S., Souza Alves D.L., Inoue V.H., de Paiva Oliveira A., Cerqueira F.R., Correia Lopes T.C., Santana L.A., Geller M., Siqueira-Batista R. Pro-Inflammatory Cytokines in Sepsis: Biological Studies and Prospects From In Silico Research, Biological Systems: Open. Access. 2015;5(1):1–7. [Google Scholar]
- 21.Miller E.J., Cohen A.B., Matthay M.A. Increased interleukin-8 concentrations in the pulmonary edema fluid of patients with acute respiratory distress syndrome from sepsis. Crit. Care Med. 1996;24(9):1448–1454. doi: 10.1097/00003246-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Dinarello C.A. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int. Rev. Immunol. 1998;16(5–6):457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- 23.Cecconi M., Evans L., Levy M., Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. doi: 10.1016/S0140-6736(18)30696-2. [DOI] [PubMed] [Google Scholar]
- 24.Crit. Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 25.Levy M.M., Fink M.P., Marshall J.C., Abraham E., Angus D., Cook D., Cohen J., Opal S.M., Vincent J.L., Ramsay G. Sccm/Esicm/Accp/Ats/Sis, 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 26.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., Hotchkiss R.S., Levy M.M., Marshall J.C., Martin G.S., Opal S.M., Rubenfeld G.D., van der Poll T., Vincent J.L., Angus D.C. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seymour C.W., Liu V.X., Iwashyna T.J., Brunkhorst F.M., Rea T.D., Scherag A., Rubenfeld G., Kahn J.M., Shankar-Hari M., Singer M., Deutschman C.S., Escobar G.J., Angus D.C. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.M. Shankar-Hari, G.S. Phillips, M.L. Levy, C.W. Seymour, V.X. Liu, C.S. Deutschman, D.C. Angus, G.D. Rubenfeld, M. Singer, F. Sepsis Definitions Task, Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), JAMA 315(8) (2016) 775-87. [DOI] [PMC free article] [PubMed]
- 29.Vincent J.L., Moreno R., Takala J., Willatts S., De Mendonca A., Bruining H., Reinhart C.K., Suter P.M., Thijs L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine, Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 30.Beale R., Reinhart K., Brunkhorst F.M., Dobb G., Levy M., Martin G., Martin C., Ramsey G., Silva E., Vallet B., Vincent J.L., Janes J.M., Sarwat S., Williams M.D., Board P.A. Promoting Global Research Excellence in Severe Sepsis (PROGRESS): lessons from an international sepsis registry. Infection. 2009;37(3):222–232. doi: 10.1007/s15010-008-8203-z. [DOI] [PubMed] [Google Scholar]
- 31.Clere-Jehl R., Mariotte A., Meziani F., Bahram S., Georgel P., Helms J. JAK-STAT Targeting Offers Novel Therapeutic Opportunities in Sepsis. Trends Mol. Med. 2020;26(11):987–1002. doi: 10.1016/j.molmed.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Webster N.R., Galley H.F. Immunomodulation in the critically ill. Br. J. Anaesth. 2009;103(1):70–81. doi: 10.1093/bja/aep128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamayo E., Fernandez A., Almansa R., Carrasco E., Heredia M., Lajo C., Goncalves L., Gomez-Herreras J.I., de Lejarazu R.O., Bermejo-Martin J.F. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur. Cytokine Netw. 2011;22(2):82–87. doi: 10.1684/ecn.2011.0281. [DOI] [PubMed] [Google Scholar]
- 34.Tang B.M., Huang S.J., McLean A.S. Genome-wide transcription profiling of human sepsis: a systematic review. Crit. Care. 2010;14(6):R237. doi: 10.1186/cc9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao W., Mindrinos M.N., Seok J., Cuschieri J., Cuenca A.G., Gao H., Hayden D.L., Hennessy L., Moore E.E., Minei J.P., Bankey P.E., Johnson J.L., Sperry J., Nathens A.B., Billiar T.R., West M.A., Brownstein B.H., Mason P.H., Baker H.V., Finnerty C.C., Jeschke M.G., Lopez M.C., Klein M.B., Gamelli R.L., Gibran N.S., Arnoldo B., Xu W., Zhang Y., Calvano S.E., McDonald-Smith G.P., Schoenfeld D.A., Storey J.D., Cobb J.P., Warren H.S., Moldawer L.L., Herndon D.N., Lowry S.F., Maier R.V., Davis R.W., Tompkins R.G. Inflammation, P. Host Response to Injury Large-Scale Collaborative Research, A genomic storm in critically injured humans, J Exp Med. 2011;208(13):2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long H., Xu B., Luo Y., Luo K. Artemisinin protects mice against burn sepsis through inhibiting NLRP3 inflammasome activation. Am. J. Emerg. Med. 2016;34(5):772–777. doi: 10.1016/j.ajem.2015.12.075. [DOI] [PubMed] [Google Scholar]
- 37.Luo Y.P., Jiang L., Kang K., Fei D.S., Meng X.L., Nan C.C., Pan S.H., Zhao M.R., Zhao M.Y. Hemin inhibits NLRP3 inflammasome activation in sepsis-induced acute lung injury, involving heme oxygenase-1. Int. Immunopharmacol. 2014;20(1):24–32. doi: 10.1016/j.intimp.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Gong Z., Zhou J., Li H., Gao Y., Xu C., Zhao S., Chen Y., Cai W., Wu J. Curcumin suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Mol. Nutr. Food Res. 2015;59(11):2132–2142. doi: 10.1002/mnfr.201500316. [DOI] [PubMed] [Google Scholar]
- 39.Fiorino S., Gallo C., Zippi M., Sabbatani S., Manfredi R., Moretti R., Fogacci E., Maggioli C., Travasoni Loffredo F., Giampieri E., Corazza I., Dickmans C., Denitto C., Cammarosano M., Battilana M., Orlandi P.E., Del Forno F., Miceli F., Visani M., Acquaviva G., De Leo A., Leandri P., Hong W., Brand T., Tallini G., Jovine E., Jovine R., de Biase D. Cytokine storm in aged people with CoV-2: possible role of vitamins as therapy or preventive strategy. Aging Clin Exp Res. 2020;32(10):2115–2131. doi: 10.1007/s40520-020-01669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hotchkiss R.S., Monneret G., Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lupu F., Keshari R.S., Lambris J.D., Coggeshall K.M. Crosstalk between the coagulation and complement systems in sepsis. Thromb. Res. 2014;133(Suppl 1):S28–S31. doi: 10.1016/j.thromres.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mollnes T.E., Huber-Lang M. Complement in sepsis-when science meets clinics. FEBS Lett. 2020;594(16):2621–2632. doi: 10.1002/1873-3468.13881. [DOI] [PubMed] [Google Scholar]
- 43.Charchaflieh J., Rushbrook J., Worah S., Zhang M. Activated Complement Factors as Disease Markers for Sepsis. Dis. Markers. 2015;2015 doi: 10.1155/2015/382463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reis E.S., Mastellos D.C., Hajishengallis G., Lambris J.D. New insights into the immune functions of complement. Nat. Rev. Immunol. 2019;19(8):503–516. doi: 10.1038/s41577-019-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013;13(1):34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 46.Semeraro N., Ammollo C.T., Semeraro F., Colucci M. Sepsis-associated disseminated intravascular coagulation and thromboembolic disease. Mediterr J Hematol Infect Dis. 2010;2(3) doi: 10.4084/MJHID.2010.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimball A.S., Obi A.T., Diaz J.A., Henke P.K. The Emerging Role of NETs in Venous Thrombosis and Immunothrombosis. Front. Immunol. 2016;7:236. doi: 10.3389/fimmu.2016.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kapoor S., Opneja A., Nayak L. The role of neutrophils in thrombosis. Thromb. Res. 2018;170:87–96. doi: 10.1016/j.thromres.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J., Wu Z., Long Q., Huang J., Hong T., Liu W., Lin J. Insights Into Immunothrombosis: The Interplay Among Neutrophil Extracellular Trap, von Willebrand Factor, and ADAMTS13. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.610696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camicia G., Pozner R., de Larranaga G. Neutrophil extracellular traps in sepsis. Shock. 2014;42(4):286–294. doi: 10.1097/SHK.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 51.Zucoloto A.Z., Jenne C.N. Platelet-Neutrophil Interplay: Insights Into Neutrophil Extracellular Trap (NET)-Driven Coagulation in Infection. Front Cardiovasc Med. 2019;6:85. doi: 10.3389/fcvm.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Bont C.M., Boelens W.C., Pruijn G.J.M. NETosis, complement, and coagulation: a triangular relationship. Cell. Mol. Immunol. 2019;16(1):19–27. doi: 10.1038/s41423-018-0024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lo M.W., Kemper C., Woodruff T.M. COVID-19: Complement, Coagulation, and Collateral Damage. J. Immunol. 2020;205(6):1488–1495. doi: 10.4049/jimmunol.2000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li L.L., Dai B., Sun Y.H., Zhang T.T. Monocytes Undergo Functional Reprogramming to Generate Immunosuppression through HIF-1alpha Signaling Pathway in the Late Phase of Sepsis. Mediators Inflamm. 2020;2020:4235909. doi: 10.1155/2020/4235909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biswas S.K., Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30(10):475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 56.Cavaillon J.M., Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit. Care. 2006;10(5):233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marchant A., Deviere J., Byl B., De Groote D., Vincent J.L., Goldman M. Interleukin-10 production during septicaemia. Lancet. 1994;343(8899):707–708. doi: 10.1016/s0140-6736(94)91584-9. [DOI] [PubMed] [Google Scholar]
- 58.Dobrovolskaia M.A., Vogel S.N. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4(9):903–914. doi: 10.1016/s1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- 59.Foster S.L., Hargreaves D.C., Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447(7147):972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 60.del Fresno C., Garcia-Rio F., Gomez-Pina V., Soares-Schanoski A., Fernandez-Ruiz I., Jurado T., Kajiji T., Shu C., Marin E., Gutierrez del Arroyo A., Prados C., Arnalich F., Fuentes-Prior P., Biswas S.K., Lopez-Collazo E. Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J. Immunol. 2009;182(10):6494–6507. doi: 10.4049/jimmunol.0803350. [DOI] [PubMed] [Google Scholar]
- 61.Bohuslav J., Kravchenko V.V., Parry G.C., Erlich J.H., Gerondakis S., Mackman N., Ulevitch R.J. Regulation of an essential innate immune response by the p50 subunit of NF-kappaB. J Clin Invest. 1998;102(9):1645–1652. doi: 10.1172/JCI3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ziegler-Heitbrock H.W. Molecular mechanism in tolerance to lipopolysaccharide. J. Inflamm. 1995;45(1):13–26. [PubMed] [Google Scholar]
- 63.Adib-Conquy M., Adrie C., Moine P., Asehnoune K., Fitting C., Pinsky M.R., Dhainaut J.F., Cavaillon J.M. NF-kappaB expression in mononuclear cells of patients with sepsis resembles that observed in lipopolysaccharide tolerance. Am. J. Respir. Crit. Care Med. 2000;162(5):1877–1883. doi: 10.1164/ajrccm.162.5.2003058. [DOI] [PubMed] [Google Scholar]
- 64.McCall C.E., Grosso-Wilmoth L.M., LaRue K., Guzman R.N., Cousart S.L. Tolerance to endotoxin-induced expression of the interleukin-1 beta gene in blood neutrophils of humans with the sepsis syndrome. J Clin Invest. 1993;91(3):853–861. doi: 10.1172/JCI116306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Danikas D.D., Karakantza M., Theodorou G.L., Sakellaropoulos G.C., Gogos C.A. Prognostic value of phagocytic activity of neutrophils and monocytes in sepsis. Correlation to CD64 and CD14 antigen expression. Clin. Exp. Immunol. 2008;154(1):87–97. doi: 10.1111/j.1365-2249.2008.03737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manjuck J., Saha D.C., Astiz M., Eales L.J., Rackow E.C. Decreased response to recall antigens is associated with depressed costimulatory receptor expression in septic critically ill patients. J. Lab. Clin. Med. 2000;135(2):153–160. doi: 10.1067/mlc.2000.104306. [DOI] [PubMed] [Google Scholar]
- 67.Mathison J.C., Virca G.D., Wolfson E., Tobias P.S., Glaser K., Ulevitch R.J. Adaptation to bacterial lipopolysaccharide controls lipopolysaccharide-induced tumor necrosis factor production in rabbit macrophages. J Clin Invest. 1990;85(4):1108–1118. doi: 10.1172/JCI114542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lehmann A.K., Halstensen A., Sornes S., Rokke O., Waage A. High levels of interleukin 10 in serum are associated with fatality in meningococcal disease. Infect. Immun. 1995;63(6):2109–2112. doi: 10.1128/iai.63.6.2109-2112.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monneret G., Finck M.E., Venet F., Debard A.L., Bohe J., Bienvenu J., Lepape A. The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol. Lett. 2004;95(2):193–198. doi: 10.1016/j.imlet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 70.van Deuren M., van der Ven-Jongekrijg J., Bartelink A.K., van Dalen R., Sauerwein R.W., van der Meer J.W. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J. Infect. Dis. 1995;172(2):433–439. doi: 10.1093/infdis/172.2.433. [DOI] [PubMed] [Google Scholar]
- 71.van Dissel J.T., van Langevelde P., Westendorp R.G., Kwappenberg K., Frolich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351(9107):950–953. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 72.Greten T.F., Manns M.P., Korangy F. Myeloid derived suppressor cells in human diseases. Int. Immunopharmacol. 2011;11(7):802–807. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Montero A.J., Diaz-Montero C.M., Kyriakopoulos C.E., Bronte V., Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J. Immunother. 2012;35(2):107–115. doi: 10.1097/CJI.0b013e318242169f. [DOI] [PubMed] [Google Scholar]
- 74.Schrijver I.T., Theroude C., Roger T. Myeloid-Derived Suppressor Cells in Sepsis. Front. Immunol. 2019;10:327. doi: 10.3389/fimmu.2019.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mira J.C., Gentile L.F., Mathias B.J., Efron P.A., Brakenridge S.C., Mohr A.M., Moore F.A., Moldawer L.L. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit. Care Med. 2017;45(2):253–262. doi: 10.1097/CCM.0000000000002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boomer J.S., To K., Chang K.C., Takasu O., Osborne D.F., Walton A.H., Bricker T.L., Jarman S.D., 2nd, Kreisel D., Krupnick A.S., Srivastava A., Swanson P.E., Green J.M., Hotchkiss R.S. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drewry A.M., Samra N., Skrupky L.P., Fuller B.M., Compton S.M., Hotchkiss R.S. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42(5):383–391. doi: 10.1097/SHK.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gentile L.F., Cuenca A.G., Efron P.A., Ang D., Bihorac A., McKinley B.A., Moldawer L.L., Moore F.A. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krautz C., Maier S.L., Brunner M., Langheinrich M., Giamarellos-Bourboulis E.J., Gogos C., Armaganidis A., Kunath F., Grutzmann R., Weber G.F. Reduced circulating B cells and plasma IgM levels are associated with decreased survival in sepsis - A meta-analysis. J. Crit. Care. 2018;45:71–75. doi: 10.1016/j.jcrc.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 81.Fieren M.W., Van den Bemd G.J., Bonta I.L. Endotoxin-stimulated peritoneal macrophages obtained from continuous ambulatory peritoneal dialysis patients show an increased capacity to release interleukin-1 beta in vitro during infectious peritonitis. Eur. J. Clin. Invest. 1990;20(4):453–457. doi: 10.1111/j.1365-2362.1990.tb01883.x. [DOI] [PubMed] [Google Scholar]
- 82.Hotchkiss R.S., Colston E., Yende S., Angus D.C., Moldawer L.L., Crouser E.D., Martin G.S., Coopersmith C.M., Brakenridge S., Mayr F.B., Park P.K., Ye J., Catlett I.M., Girgis I.G., Grasela D.M. Immune Checkpoint Inhibition in Sepsis: A Phase 1b Randomized, Placebo-Controlled, Single Ascending Dose Study of Antiprogrammed Cell Death-Ligand 1 Antibody (BMS-936559) Crit. Care Med. 2019;47(5):632–642. doi: 10.1097/CCM.0000000000003685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jacobs R.F., Tabor D.R., Burks A.W., Campbell G.D. Elevated interleukin-1 release by human alveolar macrophages during the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1989;140(6):1686–1692. doi: 10.1164/ajrccm/140.6.1686. [DOI] [PubMed] [Google Scholar]
- 84.Suzuki T., Shimizu T., Szalay L., Choudhry M.A., Rue L.W., 3rd, Bland K.I., Chaudry I.H. Androstenediol ameliorates alterations in immune cells cytokine production capacity in a two-hit model of trauma-hemorrhage and sepsis. Cytokine. 2006;34(1–2):76–84. doi: 10.1016/j.cyto.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 85.Biron B.M., Ayala A., Lomas-Neira J.L. Biomarkers for Sepsis: What Is and What Might Be? Biomark Insights. 2015;10(Suppl 4):7–17. doi: 10.4137/BMI.S29519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adib-Conquy M., Cavaillon J.M. Compensatory anti-inflammatory response syndrome. Thromb. Haemost. 2009;101(1):36–47. [PubMed] [Google Scholar]
- 87.Berner R., Niemeyer C.M., Leititis J.U., Funke A., Schwab C., Rau U., Richter K., Tawfeek M.S., Clad A., Brandis M. Plasma levels and gene expression of granulocyte colony-stimulating factor, tumor necrosis factor-alpha, interleukin (IL)-1beta, IL-6, IL-8, and soluble intercellular adhesion molecule-1 in neonatal early onset sepsis. Pediatr. Res. 1998;44(4):469–477. doi: 10.1203/00006450-199810000-00002. [DOI] [PubMed] [Google Scholar]
- 88.Lusyati S., Hulzebos C.V., Zandvoort J., Sukandar H., Sauer P.J. Cytokines patterns in newborn infants with late onset sepsis. J Neonatal Perinatal Med. 2013;6(2):153–163. doi: 10.3233/NPM-1364112. [DOI] [PubMed] [Google Scholar]
- 89.Machado J.R., Soave D.F., da Silva M.V., de Menezes L.B., Etchebehere R.M., Monteiro M.L., dos Reis M.A., Correa R.R., Celes M.R. Neonatal sepsis and inflammatory mediators. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/269681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mofidi R., Duff M.D., Wigmore S.J., Madhavan K.K., Garden O.J., Parks R.W. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br. J. Surg. 2006;93(6):738–744. doi: 10.1002/bjs.5290. [DOI] [PubMed] [Google Scholar]
- 91.Singh V.K., Wu B.U., Bollen T.L., Repas K., Maurer R., Mortele K.J., Banks P.A. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol. 2009;7(11):1247–1251. doi: 10.1016/j.cgh.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 92.Zippi M., Hong W., Traversa G., Maccioni F., De Biase D., Gallo C., Fiorino S. Involvement of the exocrine pancreas during COVID-19 infection and possible pathogenetic hypothesis: a concise review. Infez Med. 2020;28(4):507–515. [PubMed] [Google Scholar]
- 93.Fu Q., Zhai Z., Wang Y., Xu L., Jia P., Xia P., Liu C., Zhang X., Qin T., Zhang H. NLRP3 Deficiency Alleviates Severe Acute Pancreatitis and Pancreatitis-Associated Lung Injury in a Mouse Model. Biomed Res. Int. 2018;2018:1294951. doi: 10.1155/2018/1294951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.S.M. van Dijk, N.D.L. Hallensleben, H.C. van Santvoort, P. Fockens, H. van Goor, M.J. Bruno, M.G. Besselink, G. Dutch Pancreatitis Study, Acute pancreatitis: recent advances through randomised trials, Gut 66(11) (2017) 2024-2032. [DOI] [PubMed]
- 95.Sendler M., Maertin S., John D., Persike M., Weiss F.U., Kruger B., Wartmann T., Wagh P., Halangk W., Schaschke N., Mayerle J., Lerch M.M. Cathepsin B Activity Initiates Apoptosis via Digestive Protease Activation in Pancreatic Acinar Cells and Experimental Pancreatitis. J. Biol. Chem. 2016;291(28):14717–14731. doi: 10.1074/jbc.M116.718999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.M. Sendler, C. van den Brandt, J. Glaubitz, A. Wilden, J. Golchert, F.U. Weiss, G. Homuth, L.L. De Freitas Chama, N. Mishra, U.M. Mahajan, L. Bossaller, U. Volker, B.M. Broker, J. Mayerle, M.M. Lerch, NLRP3 Inflammasome Regulates Development of Systemic Inflammatory Response and Compensatory Anti-Inflammatory Response Syndromes in Mice With Acute Pancreatitis, Gastroenterology 158(1) (2020) 253-269 e14. [DOI] [PubMed]
- 97.N. Zhu, D. Zhang, W. Wang, X. Li, B. Yang, J. Song, X. Zhao, B. Huang, W. Shi, R. Lu, P. Niu, F. Zhan, X. Ma, D. Wang, W. Xu, G. Wu, G.F. Gao, W. Tan, I. China Novel Coronavirus, T. Research, A Novel Coronavirus from Patients with Pneumonia in China, 2019, N Engl J Med 382(8) (2020) 727-733. [DOI] [PMC free article] [PubMed]
- 98.Cheng Z.J., Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48(2):155–163. doi: 10.1007/s15010-020-01401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 100.Thakur V., Ratho R.K., Kumar P., Bhatia S.K., Bora I., Mohi G.K., Saxena S.K., Devi M., Yadav D., Mehariya S. Multi-Organ Involvement in COVID-19: Beyond Pulmonary Manifestations. J Clin Med. 2021;10(3) doi: 10.3390/jcm10030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Z. Zhou, L. Ren, L. Zhang, J. Zhong, Y. Xiao, Z. Jia, L. Guo, J. Yang, C. Wang, S. Jiang, D. Yang, G. Zhang, H. Li, F. Chen, Y. Xu, M. Chen, Z. Gao, J. Yang, J. Dong, B. Liu, X. Zhang, W. Wang, K. He, Q. Jin, M. Li, J. Wang, Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients, Cell Host Microbe 27(6) (2020) 883-890 e2. [DOI] [PMC free article] [PubMed]
- 102.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jin J.M., Bai P., He W., Wu F., Liu X.F., Han D.M., Liu S., Yang J.K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meng Y., Wu P., Lu W., Liu K., Ma K., Huang L., Cai J., Zhang H., Qin Y., Sun H., Ding W., Gui L., Wu P. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: A retrospective study of 168 severe patients. PLoS Pathog. 2020;16(4) doi: 10.1371/journal.ppat.1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wong K.L., Tai J.J., Wong W.C., Han H., Sem X., Yeap W.H., Kourilsky P., Wong S.C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118(5):e16–e31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 106.Guilliams M., Ginhoux F., Jakubzick C., Naik S.H., Onai N., Schraml B.U., Segura E., Tussiwand R., Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014;14(8):571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chu K.L., Batista N.V., Girard M., Watts T.H. Monocyte-Derived Cells in Tissue-Resident Memory T Cell Formation. J. Immunol. 2020;204(3):477–485. doi: 10.4049/jimmunol.1901046. [DOI] [PubMed] [Google Scholar]
- 108.E.J. Giamarellos-Bourboulis, M.G. Netea, N. Rovina, K. Akinosoglou, A. Antoniadou, N. Antonakos, G. Damoraki, T. Gkavogianni, M.E. Adami, P. Katsaounou, M. Ntaganou, M. Kyriakopoulou, G. Dimopoulos, I. Koutsodimitropoulos, D. Velissaris, P. Koufargyris, A. Karageorgos, K. Katrini, V. Lekakis, M. Lupse, A. Kotsaki, G. Renieris, D. Theodoulou, V. Panou, E. Koukaki, N. Koulouris, C. Gogos, A. Koutsoukou, Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure, Cell Host Microbe 27(6) (2020) 992-1000 e3. [DOI] [PMC free article] [PubMed]
- 109.Wilk A.J., Rustagi A., Zhao N.Q., Roque J., Martinez-Colon G.J., McKechnie J.L., Ivison G.T., Ranganath T., Vergara R., Hollis T., Simpson L.J., Grant P., Subramanian A., Rogers A.J., Blish C.A. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020;26(7):1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Buscher K., Marcovecchio P., Hedrick C.C., Ley K. Patrolling Mechanics of Non-Classical Monocytes in Vascular Inflammation. Front Cardiovasc Med. 2017;4:80. doi: 10.3389/fcvm.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chimen M., Yates C.M., McGettrick H.M., Ward L.S., Harrison M.J., Apta B., Dib L.H., Imhof B.A., Harrison P., Nash G.B., Rainger G.E. Monocyte Subsets Coregulate Inflammatory Responses by Integrated Signaling through TNF and IL-6 at the Endothelial Cell Interface. J. Immunol. 2017;198(7):2834–2843. doi: 10.4049/jimmunol.1601281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ong S.M., Hadadi E., Dang T.M., Yeap W.H., Tan C.T., Ng T.P., Larbi A., Wong S.C. The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell Death Dis. 2018;9(3):266. doi: 10.1038/s41419-018-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pence B.D. Severe COVID-19 and aging: are monocytes the key? Geroscience. 2020;42(4):1051–1061. doi: 10.1007/s11357-020-00213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alzaid F., Julla J., Diedisheim M., Potier C., Potier L., Velho G., Gaborit B., Manivet P., Germain S., Vidal-Trecan T., Roussel R., Riveline J., Dalmas E., Venteclef N., Gautier J. Monocyte class switch and hyperinflammation characterise severe COVID-19 in type 2 diabetes. MedRxiv. 2020 doi: 10.15252/emmm.202013038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Belge K.U., Dayyani F., Horelt A., Siedlar M., Frankenberger M., Frankenberger B., Espevik T., Ziegler-Heitbrock L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J. Immunol. 2002;168(7):3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 116.Frankenberger M., Sternsdorf T., Pechumer H., Pforte A., Ziegler-Heitbrock H.W. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood. 1996;87(1):373–377. [PubMed] [Google Scholar]
- 117.Hijdra D., Vorselaars A.D., Grutters J.C., Claessen A.M., Rijkers G.T. Phenotypic characterization of human intermediate monocytes. Front. Immunol. 2013;4:339. doi: 10.3389/fimmu.2013.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mukherjee R., Kanti Barman P., Kumar Thatoi P., Tripathy R., Kumar Das B., Ravindran B. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci. Rep. 2015;5:13886. doi: 10.1038/srep13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stansfield B.K., Ingram D.A. Clinical significance of monocyte heterogeneity. Clin Transl Med. 2015;4:5. doi: 10.1186/s40169-014-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26(6):842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 121.I. Sanchez-Cerrillo, P. Landete, B. Aldave, S. Sanchez-Alonso, A. Sanchez-Azofra, A. Marcos-Jimenez, E. Avalos, A. Alcaraz-Serna, I. de Los Santos, T. Mateu-Albero, L. Esparcia, C. Lopez-Sanz, P. Martinez-Fleta, L. Gabrie, L. Del Campo Guerola, H. de la Fuente, M.J. Calzada, I. Gonzalez-Alvaro, A. Alfranca, F. Sanchez-Madrid, C. Munoz-Calleja, J.B. Soriano, J. Ancochea, E. Martin-Gayo, C. Reinmun, E. groups, COVID-19 severity associates with pulmonary redistribution of CD1c+ DCs and inflammatory transitional and nonclassical monocytes, J Clin Invest 130(12) (2020) 6290-6300. [DOI] [PMC free article] [PubMed]
- 122.Banerjee A., Pasea L., Harris S., Gonzalez-Izquierdo A., Torralbo A., Shallcross L., Noursadeghi M., Pillay D., Sebire N., Holmes C., Pagel C., Wong W.K., Langenberg C., Williams B., Denaxas S., Hemingway H. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet. 2020;395(10238):1715–1725. doi: 10.1016/S0140-6736(20)30854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pinto L.C., Bertoluci M.C. Type 2 diabetes as a major risk factor for COVID-19 severity: a meta-analysis. Arch Endocrinol Metab. 2020;64(3):199–200. doi: 10.20945/2359-3997000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.T. Takahashi, P. Wong, M.K. Ellingson, C. Lucas, J. Klein, B. Israelow, J. Silva, J.E. Oh, T. Mao, M. Tokuyama, P. Lu, A. Venkataraman, A. Park, F. Liu, A. Meir, J. Sun, E.Y. Wang, A.L. Wyllie, C.B.F. Vogels, R. Earnest, S. Lapidus, I.M. Ott, A.J. Moore, A. Casanovas-Massana, C.D. Cruz, J.B. Fournier, C.D. Odio, S. Farhadian, N.D. Grubaugh, W.L. Schulz, A.I. Ko, A.M. Ring, S.B. Omer, A. Iwasaki, I.r.t. Yale, Sex differences in immune responses to SARS-CoV-2 that underlie disease outcomes, medRxiv (2020).
- 125.Rodrigues P.F., Alberti-Servera L., Eremin A., Grajales-Reyes G.E., Ivanek R., Tussiwand R. Distinct progenitor lineages contribute to the heterogeneity of plasmacytoid dendritic cells. Nat. Immunol. 2018;19(7):711–722. doi: 10.1038/s41590-018-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kvedaraite E., Hertwig L., Sinha I., Ponzetta A., Hed Myrberg I., Lourda M., Dzidic M., Akber M., Klingstrom J., Folkesson E., Muvva J.R., Chen P., Gredmark-Russ S., Brighenti S., Norrby-Teglund A., Eriksson L.I., Rooyackers O., Aleman S., Stralin K., Ljunggren H.G., Ginhoux F., Bjorkstrom N.K., Henter J.I., Svensson M., Karolinska K.I.K.C.-S.G. Major alterations in the mononuclear phagocyte landscape associated with COVID-19 severity. Proc Natl Acad Sci U S A. 2021;118(6) doi: 10.1073/pnas.2018587118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Falck-Jones S., Vangeti S., Yu M., Falck-Jones R., Cagigi A., Badolati I., Osterberg B., Lautenbach M.J., Ahlberg E., Lin A., Lepzien R., Szurgot I., Lenart K., Hellgren F., Maecker H., Salde J., Albert J., Johansson N., Bell M., Lore K., Farnert A., Smed-Sorensen A. Functional monocytic myeloid-derived suppressor cells increase in blood but not airways and predict COVID-19 severity. J Clin Invest. 2021;131(6) doi: 10.1172/JCI144734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sacchi A., Grassi G., Bordoni V., Lorenzini P., Cimini E., Casetti R., Tartaglia E., Marchioni L., Petrosillo N., Palmieri F., D'Offizi G., Notari S., Tempestilli M., Capobianchi M.R., Nicastri E., Maeurer M., Zumla A., Locatelli F., Antinori A., Ippolito G., Agrati C. Early expansion of myeloid-derived suppressor cells inhibits SARS-CoV-2 specific T-cell response and may predict fatal COVID-19 outcome. Cell Death Dis. 2020;11(10):921. doi: 10.1038/s41419-020-03125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ahmed F., Jo D.H., Lee S.H. Can Natural Killer Cells Be a Principal Player in Anti-SARS-CoV-2 Immunity? Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.586765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li M., Guo W., Dong Y., Wang X., Dai D., Liu X., Wu Y., Li M., Zhang W., Zhou H., Zhang Z., Lin L., Kang Z., Yu T., Tian C., Qin R., Gui Y., Jiang F., Fan H., Heissmeyer V., Sarapultsev A., Wang L., Luo S., Hu D. Elevated Exhaustion Levels of NK and CD8(+) T Cells as Indicators for Progression and Prognosis of COVID-19 Disease. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.580237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wolf Y., Anderson A.C., Kuchroo V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2020;20(3):173–185. doi: 10.1038/s41577-019-0224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meckiff B.J., Ramirez-Suastegui C., Fajardo V., Chee S.J., Kusnadi A., Simon H., Grifoni A., Pelosi E., Weiskopf D., Sette A., Ay F., Seumois G., Ottensmeier C.H., Vijayanand P. Single-Cell Transcriptomic Analysis of SARS-CoV-2 Reactive CD4 (+) T Cells. SSRN. 2020:3641939. doi: 10.1016/j.cell.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.N. Kaneko, J. Boucau, H.H. Kuo, C. Perugino, V.S. Mahajan, J.R. Farmer, H. Liu, T.J. Diefenbach, A. Piechocka-Trocha, K. Lefteri, M.T. Waring, K.R. Premo, B.D. Walker, J.Z. Li, G. Gaiha, X.G. Yu, M. Lichterfeld, R.F. Padera, S. Pillai, Expansion of Cytotoxic CD4+ T cells in the lungs in severe COVID-19, medRxiv (2021). [DOI] [PMC free article] [PubMed]
- 135.Maeda K., Baba Y., Nagai Y., Miyazaki K., Malykhin A., Nakamura K., Kincade P.W., Sakaguchi N., Coggeshall K.M. IL-6 blocks a discrete early step in lymphopoiesis. Blood. 2005;106(3):879–885. doi: 10.1182/blood-2005-02-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Harbour S.N., DiToro D.F., Witte S.J., Zindl C.L., Gao M., Schoeb T.R., Jones G.W., Jones S.A., Hatton R.D., Weaver C.T. TH17 cells require ongoing classic IL-6 receptor signaling to retain transcriptional and functional identity. Sci. Immunol. 2020;5(49) doi: 10.1126/sciimmunol.aaw2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Glynn P.C., Henney E.M., Hall I.P. Peripheral blood neutrophils are hyperresponsive to IL-8 and Gro-alpha in cryptogenic fibrosing alveolitis. Eur. Respir. J. 2001;18(3):522–529. doi: 10.1183/09031936.01.00057901. [DOI] [PubMed] [Google Scholar]
- 138.A. Ciccullo, A. Borghetti, L. Zileri Dal Verme, A. Tosoni, F. Lombardi, M. Garcovich, F. Biscetti, M. Montalto, R. Cauda, S. Di Giambenedetto, G.A.C. Group, Neutrophil-to-lymphocyte ratio and clinical outcome in COVID-19: a report from the Italian front line, Int J Antimicrob Agents 56(2) (2020) 106017. [DOI] [PMC free article] [PubMed]
- 139.Imran M.M., Ahmad U., Usman U., Ali M., Shaukat A., Gul N. Neutrophil/lymphocyte ratio-A marker of COVID-19 pneumonia severity. Int. J. Clin. Pract. 2021;75(4) doi: 10.1111/ijcp.13698. [DOI] [PubMed] [Google Scholar]
- 140.Zahorec R., Hulin I., Zahorec P. Rationale Use of Neutrophil-to-lymphocyte ratio for early diagnosis and stratification of COVID-19. Bratisl. Lek. Listy. 2020;121(7):466–470. doi: 10.4149/BLL_2020_077. [DOI] [PubMed] [Google Scholar]
- 141.T. Sekine, A. Perez-Potti, O. Rivera-Ballesteros, K. Stralin, J.B. Gorin, A. Olsson, S. Llewellyn-Lacey, H. Kamal, G. Bogdanovic, S. Muschiol, D.J. Wullimann, T. Kammann, J. Emgard, T. Parrot, E. Folkesson, C.-S.G. Karolinska, O. Rooyackers, L.I. Eriksson, J.I. Henter, A. Sonnerborg, T. Allander, J. Albert, M. Nielsen, J. Klingstrom, S. Gredmark-Russ, N.K. Bjorkstrom, J.K. Sandberg, D.A. Price, H.G. Ljunggren, S. Aleman, M. Buggert, Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19, Cell 183(1) (2020) 158-168 e14. [DOI] [PMC free article] [PubMed]
- 142.Amara U., Rittirsch D., Flierl M., Bruckner U., Klos A., Gebhard F., Lambris J.D., Huber-Lang M. Interaction between the coagulation and complement system. Adv. Exp. Med. Biol. 2008;632:71–79. doi: 10.1007/978-0-387-78952-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ganter M.T., Brohi K., Cohen M.J., Shaffer L.A., Walsh M.C., Stahl G.L., Pittet J.F. Role of the alternative pathway in the early complement activation following major trauma. Shock. 2007;28(1):29–34. doi: 10.1097/shk.0b013e3180342439. [DOI] [PubMed] [Google Scholar]
- 144.Huber-Lang M., Sarma J.V., Zetoune F.S., Rittirsch D., Neff T.A., McGuire S.R., Lambris J.D., Warner R.L., Flierl M.A., Hoesel L.M., Gebhard F., Younger J.G., Drouin S.M., Wetsel R.A., Ward P.A. Generation of C5a in the absence of C3: a new complement activation pathway. Nat. Med. 2006;12(6):682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 145.Hattori R., Hamilton K.K., McEver R.P., Sims P.J. Complement proteins C5b–9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J. Biol. Chem. 1989;264(15):9053–9060. [PubMed] [Google Scholar]
- 146.Bettoni S., Galbusera M., Gastoldi S., Donadelli R., Tentori C., Sparta G., Bresin E., Mele C., Alberti M., Tortajada A., Yebenes H., Remuzzi G., Noris M. Interaction between Multimeric von Willebrand Factor and Complement: A Fresh Look to the Pathophysiology of Microvascular Thrombosis. J. Immunol. 2017;199(3):1021–1040. doi: 10.4049/jimmunol.1601121. [DOI] [PubMed] [Google Scholar]
- 147.Feng S., Liang X., Kroll M.H., Chung D.W., Afshar-Kharghan V. von Willebrand factor is a cofactor in complement regulation. Blood. 2015;125(6):1034–1037. doi: 10.1182/blood-2014-06-585430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wojta J., Kaun C., Zorn G., Ghannadan M., Hauswirth A.W., Sperr W.R., Fritsch G., Printz D., Binder B.R., Schatzl G., Zwirner J., Maurer G., Huber K., Valent P. C5a stimulates production of plasminogen activator inhibitor-1 in human mast cells and basophils. Blood. 2002;100(2):517–523. doi: 10.1182/blood.v100.2.517. [DOI] [PubMed] [Google Scholar]
- 149.Lee K.G., Lee H., Ha J.M., Lee Y.K., Kang H.J., Park C.G., Kim S.J. Increased human tumor necrosis factor-alpha levels induce procoagulant change in porcine endothelial cells in vitro. Xenotransplantation. 2012;19(3):186–195. doi: 10.1111/j.1399-3089.2012.00704.x. [DOI] [PubMed] [Google Scholar]
- 150.Ritis K., Doumas M., Mastellos D., Micheli A., Giaglis S., Magotti P., Rafail S., Kartalis G., Sideras P., Lambris J.D. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 2006;177(7):4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 151.Foley J.H., Conway E.M. Cross Talk Pathways Between Coagulation and Inflammation. Circ. Res. 2016;118(9):1392–1408. doi: 10.1161/CIRCRESAHA.116.306853. [DOI] [PubMed] [Google Scholar]
- 152.Peerschke E.I., Yin W., Ghebrehiwet B. Platelet mediated complement activation. Adv. Exp. Med. Biol. 2008;632:81–91. doi: 10.1007/978-0-387-78952-1_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wiedmer T., Esmon C.T., Sims P.J. Complement proteins C5b–9 stimulate procoagulant activity through platelet prothrombinase. Blood. 1986;68(4):875–880. [PubMed] [Google Scholar]
- 154.Java A., Apicelli A.J., Liszewski M.K., Coler-Reilly A., Atkinson J.P., Kim A.H., Kulkarni H.S. The complement system in COVID-19: friend and foe? JCI Insight. 2020;5(15) doi: 10.1172/jci.insight.140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.J. Carvelli, O. Demaria, F. Vely, L. Batista, N. Chouaki Benmansour, J. Fares, S. Carpentier, M.L. Thibult, A. Morel, R. Remark, P. Andre, A. Represa, C. Piperoglou, C.-I.P.H.g. Explore, C.-M.I.g. Explore, P.Y. Cordier, E. Le Dault, C. Guervilly, P. Simeone, M. Gainnier, Y. Morel, M. Ebbo, N. Schleinitz, E. Vivier, Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis, Nature 588(7836) (2020) 146-150. [DOI] [PMC free article] [PubMed]
- 156.Cugno M., Meroni P.L., Gualtierotti R., Griffini S., Grovetti E., Torri A., Panigada M., Aliberti S., Blasi F., Tedesco F., Peyvandi F. Complement activation in patients with COVID-19: A novel therapeutic target. J. Allergy Clin. Immunol. 2020;146(1):215–217. doi: 10.1016/j.jaci.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery V.D., Jensen K., Leist S.R., Whitmore A., Heise M.T., Baric R.S. Complement Activation Contributes to Severe Acute Respiratory Syndrome Coronavirus Pathogenesis, mBio. 2018;9(5) doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jiang Y., Li J., Teng Y., Sun H., Tian G., He L., Li P., Chen Y., Guo Y., Li J., Zhao G., Zhou Y., Sun S. Complement Receptor C5aR1 Inhibition Reduces Pyroptosis in hDPP4-Transgenic Mice Infected with MERS-CoV. Viruses. 2019;11(1) doi: 10.3390/v11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Noris M., Benigni A., Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98(2):314–322. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Skendros P., Mitsios A., Chrysanthopoulou A., Mastellos D.C., Metallidis S., Rafailidis P., Ntinopoulou M., Sertaridou E., Tsironidou V., Tsigalou C., Tektonidou M., Konstantinidis T., Papagoras C., Mitroulis I., Germanidis G., Lambris J.D., Ritis K. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130(11):6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ricklin D., Mastellos D.C., Lambris J.D. Therapeutic targeting of the complement system. Nat Rev Drug Discov. 2019 doi: 10.1038/s41573-019-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kleczko E.K., Kwak J.W., Schenk E.L., Nemenoff R.A. Targeting the Complement Pathway as a Therapeutic Strategy in Lung Cancer. Front. Immunol. 2019;10:954. doi: 10.3389/fimmu.2019.00954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stakos D., Skendros P., Konstantinides S., Ritis K. Traps N' Clots: NET-Mediated Thrombosis and Related Diseases. Thromb. Haemost. 2020;120(3):373–383. doi: 10.1055/s-0039-3402731. [DOI] [PubMed] [Google Scholar]
- 164.Zelek W.M., Xie L., Morgan B.P., Harris C.L. Compendium of current complement therapeutics. Mol. Immunol. 2019;114:341–352. doi: 10.1016/j.molimm.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 165.P. Bastard, L.B. Rosen, Q. Zhang, E. Michailidis, H.H. Hoffmann, Y. Zhang, K. Dorgham, Q. Philippot, J. Rosain, V. Beziat, J. Manry, E. Shaw, L. Haljasmagi, P. Peterson, L. Lorenzo, L. Bizien, S. Trouillet-Assant, K. Dobbs, A.A. de Jesus, A. Belot, A. Kallaste, E. Catherinot, Y. Tandjaoui-Lambiotte, J. Le Pen, G. Kerner, B. Bigio, Y. Seeleuthner, R. Yang, A. Bolze, A.N. Spaan, O.M. Delmonte, M.S. Abers, A. Aiuti, G. Casari, V. Lampasona, L. Piemonti, F. Ciceri, K. Bilguvar, R.P. Lifton, M. Vasse, D.M. Smadja, M. Migaud, J. Hadjadj, B. Terrier, D. Duffy, L. Quintana-Murci, D. van de Beek, L. Roussel, D.C. Vinh, S.G. Tangye, F. Haerynck, D. Dalmau, J. Martinez-Picado, P. Brodin, M.C. Nussenzweig, S. Boisson-Dupuis, C. Rodriguez-Gallego, G. Vogt, T.H. Mogensen, A.J. Oler, J. Gu, P.D. Burbelo, J.I. Cohen, A. Biondi, L.R. Bettini, M. D'Angio, P. Bonfanti, P. Rossignol, J. Mayaux, F. Rieux-Laucat, E.S. Husebye, F. Fusco, M.V. Ursini, L. Imberti, A. Sottini, S. Paghera, E. Quiros-Roldan, C. Rossi, R. Castagnoli, D. Montagna, A. Licari, G.L. Marseglia, X. Duval, J. Ghosn, H. Lab, N.-U.I.R.t.C. Group, C. Clinicians, C.-S. Clinicians, C.G. Imagine, C.C.S.G. French, C. Milieu Interieur, V.C.C. Co, U.M.C.C.-B. Amsterdam, C.H.G. Effort, J.S. Tsang, R. Goldbach-Mansky, K. Kisand, M.S. Lionakis, A. Puel, S.Y. Zhang, S.M. Holland, G. Gorochov, E. Jouanguy, C.M. Rice, A. Cobat, L.D. Notarangelo, L. Abel, H.C. Su, J.L. Casanova, Autoantibodies against type I IFNs in patients with life-threatening COVID-19, Science 370(6515) (2020). [DOI] [PMC free article] [PubMed]
- 166.Q. Zhang, P. Bastard, Z. Liu, J. Le Pen, M. Moncada-Velez, J. Chen, M. Ogishi, I.K.D. Sabli, S. Hodeib, C. Korol, J. Rosain, K. Bilguvar, J. Ye, A. Bolze, B. Bigio, R. Yang, A.A. Arias, Q. Zhou, Y. Zhang, F. Onodi, S. Korniotis, L. Karpf, Q. Philippot, M. Chbihi, L. Bonnet-Madin, K. Dorgham, N. Smith, W.M. Schneider, B.S. Razooky, H.H. Hoffmann, E. Michailidis, L. Moens, J.E. Han, L. Lorenzo, L. Bizien, P. Meade, A.L. Neehus, A.C. Ugurbil, A. Corneau, G. Kerner, P. Zhang, F. Rapaport, Y. Seeleuthner, J. Manry, C. Masson, Y. Schmitt, A. Schluter, T. Le Voyer, T. Khan, J. Li, J. Fellay, L. Roussel, M. Shahrooei, M.F. Alosaimi, D. Mansouri, H. Al-Saud, F. Al-Mulla, F. Almourfi, S.Z. Al-Muhsen, F. Alsohime, S. Al Turki, R. Hasanato, D. van de Beek, A. Biondi, L.R. Bettini, M. D'Angio, P. Bonfanti, L. Imberti, A. Sottini, S. Paghera, E. Quiros-Roldan, C. Rossi, A.J. Oler, M.F. Tompkins, C. Alba, I. Vandernoot, J.C. Goffard, G. Smits, I. Migeotte, F. Haerynck, P. Soler-Palacin, A. Martin-Nalda, R. Colobran, P.E. Morange, S. Keles, F. Colkesen, T. Ozcelik, K.K. Yasar, S. Senoglu, S.N. Karabela, C. Rodriguez-Gallego, G. Novelli, S. Hraiech, Y. Tandjaoui-Lambiotte, X. Duval, C. Laouenan, C.-S. Clinicians, C. Clinicians, C.G. Imagine, C.C.S.G. French, V.C.C. Co, U.M.C.C.-B. Amsterdam, C.H.G. Effort, N.-U.T.C.I. Group, A.L. Snow, C.L. Dalgard, J.D. Milner, D.C. Vinh, T.H. Mogensen, N. Marr, A.N. Spaan, B. Boisson, S. Boisson-Dupuis, J. Bustamante, A. Puel, M.J. Ciancanelli, I. Meyts, T. Maniatis, V. Soumelis, A. Amara, M. Nussenzweig, A. Garcia-Sastre, F. Krammer, A. Pujol, D. Duffy, R.P. Lifton, S.Y. Zhang, G. Gorochov, V. Beziat, E. Jouanguy, V. Sancho-Shimizu, C.M. Rice, L. Abel, L.D. Notarangelo, A. Cobat, H.C. Su, J.L. Casanova, Inborn errors of type I IFN immunity in patients with life-threatening COVID-19, Science 370(6515) (2020). [DOI] [PMC free article] [PubMed]
- 167.P.D. Monk, R.J. Marsden, V.J. Tear, J. Brookes, T.N. Batten, M. Mankowski, F.J. Gabbay, D.E. Davies, S.T. Holgate, L.P. Ho, T. Clark, R. Djukanovic, T.M.A. Wilkinson, C.-S.G. Inhaled Interferon Beta, Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial, Lancet Respir Med 9(2) (2021) 196-206. [DOI] [PMC free article] [PubMed]
- 168.Rahmani H., Davoudi-Monfared E., Nourian A., Khalili H., Hajizadeh N., Jalalabadi N.Z., Fazeli M.R., Ghazaeian M., Yekaninejad M.S. Interferon beta-1b in treatment of severe COVID-19: A randomized clinical trial. Int. Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Y. Konno, I. Kimura, K. Uriu, M. Fukushi, T. Irie, Y. Koyanagi, D. Sauter, R.J. Gifford, U.-C. Consortium, S. Nakagawa, K. Sato SARS-CoV-2 ORF3b Is a Potent Interferon Antagonist Whose Activity Is Increased by a Naturally Occurring Elongation Variant. Cell Rep. 2020;32(12) doi: 10.1016/j.celrep.2020.108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lei X., Dong X., Ma R., Wang W., Xiao X., Tian Z., Wang C., Wang Y., Li L., Ren L., Guo F., Zhao Z., Zhou Z., Xiang Z., Wang J. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 2020;11(1):3810. doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Xia H., Cao Z., Xie X., Zhang X., Chen J.Y., Wang H., Menachery V.D., Rajsbaum R., Shi P.Y. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 2020;33(1) doi: 10.1016/j.celrep.2020.108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.N. Wang, Y. Zhan, L. Zhu, Z. Hou, F. Liu, P. Song, F. Qiu, X. Wang, X. Zou, D. Wan, X. Qian, S. Wang, Y. Guo, H. Yu, M. Cui, G. Tong, Y. Xu, Z. Zheng, Y. Lu, P. Hong, Retrospective Multicenter Cohort Study Shows Early Interferon Therapy Is Associated with Favorable Clinical Responses in COVID-19 Patients, Cell Host Microbe 28(3) (2020) 455-464 e2. [DOI] [PMC free article] [PubMed]
- 174.T.T. Brehm, M. van der Meirschen, A. Hennigs, K. Roedl, D. Jarczak, D. Wichmann, D. Frings, A. Nierhaus, T. Oqueka, W. Fiedler, M. Christopeit, C. Kraef, A. Schultze, M. Lutgehetmann, M.M. Addo, S. Schmiedel, S. Kluge, J. Schulze Zur Wiesch, Comparison of clinical characteristics and disease outcome of COVID-19 and seasonal influenza, Sci Rep 11(1) (2021) 5803. [DOI] [PMC free article] [PubMed]
- 175.Galani I.E., Rovina N., Lampropoulou V., Triantafyllia V., Manioudaki M., Pavlos E., Koukaki E., Fragkou P.C., Panou V., Rapti V., Koltsida O., Mentis A., Koulouris N., Tsiodras S., Koutsoukou A., Andreakos E. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 2021;22(1):32–40. doi: 10.1038/s41590-020-00840-x. [DOI] [PubMed] [Google Scholar]
- 176.I.E. Galani, V. Triantafyllia, E.E. Eleminiadou, O. Koltsida, A. Stavropoulos, M. Manioudaki, D. Thanos, S.E. Doyle, S.V. Kotenko, K. Thanopoulou, E. Andreakos, Interferon-lambda Mediates Non-redundant Front-Line Antiviral Protection against Influenza Virus Infection without Compromising Host Fitness, Immunity 46(5) (2017) 875-890 e6. [DOI] [PubMed]
- 177.Kato H., Shimizu H., Shibue Y., Hosoda T., Iwabuchi K., Nagamine K., Saito H., Sawada R., Oishi T., Tsukiji J., Fujita H., Furuya R., Masuda M., Akasaka O., Ikeda Y., Sakamoto M., Sakai K., Uchiyama M., Watanabe H., Yamaguchi N., Higa R., Sasaki A., Tanaka K., Toyoda Y., Hamanaka S., Miyazawa N., Shimizu A., Fukase F., Iwai S., Komase Y., Kawasaki T., Nagata I., Nakayama Y., Takei T., Kimura K., Kunisaki R., Kudo M., Takeuchi I., Nakajima H. Clinical course of 2019 novel coronavirus disease (COVID-19) in individuals present during the outbreak on the Diamond Princess cruise ship. J Infect Chemother. 2020;26(8):865–869. doi: 10.1016/j.jiac.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li Y., Wang H., Wang F., Du H., Liu X., Chen P., Wang Y., Lu X. Comparison of hospitalized patients with pneumonia caused by COVID-19 and influenza A in children under 5 years. Int J Infect Dis. 2020;98:80–83. doi: 10.1016/j.ijid.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim T.S., Shin E.C. The activation of bystander CD8(+) T cells and their roles in viral infection. Exp. Mol. Med. 2019;51(12):1–9. doi: 10.1038/s12276-019-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Berg R.E., Crossley E., Murray S., Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J. Exp. Med. 2003;198(10):1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lertmemongkolchai G., Cai G., Hunter C.A., Bancroft G.J. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J. Immunol. 2001;166(2):1097–1105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 183.Raue H.P., Brien J.D., Hammarlund E., Slifka M.K. Activation of virus-specific CD8+ T cells by lipopolysaccharide-induced IL-12 and IL-18. J. Immunol. 2004;173(11):6873–6881. doi: 10.4049/jimmunol.173.11.6873. [DOI] [PubMed] [Google Scholar]
- 184.Soudja S.M., Ruiz A.L., Marie J.C., Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37(3):549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tough D.F., Borrow P., Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272(5270):1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 186.Shin E.C., Sung P.S., Park S.H. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat. Rev. Immunol. 2016;16(8):509–523. doi: 10.1038/nri.2016.69. [DOI] [PubMed] [Google Scholar]
- 187.Maini M.K., Boni C., Lee C.K., Larrubia J.R., Reignat S., Ogg G.S., King A.S., Herberg J., Gilson R., Alisa A., Williams R., Vergani D., Naoumov N.V., Ferrari C., Bertoletti A. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 2000;191(8):1269–1280. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Grant R.A., Morales-Nebreda L., Markov N.S., Swaminathan S., Querrey M., Guzman E.R., Abbott D.A., Donnelly H.K., Donayre A., Goldberg I.A., Klug Z.M., Borkowski N., Lu Z., Kihshen H., Politanska Y., Sichizya L., Kang M., Shilatifard A., Qi C., Lomasney J.W., Argento A.C., Kruser J.M., Malsin E.S., Pickens C.O., Smith S.B., Walter J.M., Pawlowski A.E., Schneider D., Nannapaneni P., Abdala-Valencia H., Bharat A., Gottardi C.J., Budinger G.R.S., Misharin A.V., Singer B.D., Wunderink R.G., Investigators N.S.S. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature. 2021;590(7847):635–641. doi: 10.1038/s41586-020-03148-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., Greninger A.L., Pipavath S., Wurfel M.M., Evans L., Kritek P.A., West T.E., Luks A., Gerbino A., Dale C.R., Goldman J.D., O'Mahony S., Mikacenic C. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N. Engl. J. Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Puelles V.G., Lutgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S., Braun F., Lu S., Pfefferle S., Schroder A.S., Edler C., Gross O., Glatzel M., Wichmann D., Wiech T., Kluge S., Pueschel K., Aepfelbacher M., Huber T.B. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., Su X., Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Coomes E.A., Haghbayan H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020;30(6):1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S., Hirayama A.V., Mastroiani F., Turtle C.J., Harhay M.O., Legrand M., Deutschman C.S. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8(12):1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hegyi P., Szakacs Z., Sahin-Toth M. Lipotoxicity and Cytokine Storm in Severe Acute Pancreatitis and COVID-19. Gastroenterology. 2020;159(3):824–827. doi: 10.1053/j.gastro.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Remy K.E., Brakenridge S.C., Francois B., Daix T., Deutschman C.S., Monneret G., Jeannet R., Laterre P.F., Hotchkiss R.S., Moldawer L.L. Immunotherapies for COVID-19: lessons learned from sepsis. Lancet Respir Med. 2020;8(10):946–949. doi: 10.1016/S2213-2600(20)30217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Surbatovic M., Popovic N., Vojvodic D., Milosevic I., Acimovic G., Stojicic M., Veljovic M., Jevdjic J., Djordjevic D., Radakovic S. Cytokine profile in severe Gram-positive and Gram-negative abdominal sepsis. Sci. Rep. 2015;5:11355. doi: 10.1038/srep11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.T. Herold, V. Jurinovic, C. Arnreich, B.J. Lipworth, J.C. Hellmuth, M. von Bergwelt-Baildon, M. Klein, T. Weinberger, Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19, J Allergy Clin Immunol 146(1) (2020) 128-136 e4. [DOI] [PMC free article] [PubMed]
- 199.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., Men D., Huang Q., Liu Y., Yang B., Ding J., Li F. Detectable Serum Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load (RNAemia) Is Closely Correlated With Drastically Elevated Interleukin 6 Level in Critically Ill Patients With Coronavirus Disease 2019. Clin. Infect. Dis. 2020;71(8):1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.M. Hoffmann, H. Kleine-Weber, S. Schroeder, N. Kruger, T. Herrler, S. Erichsen, T.S. Schiergens, G. Herrler, N.H. Wu, A. Nitsche, M.A. Muller, C. Drosten, S. Pohlmann, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell 181(2) (2020) 271-280 e8. [DOI] [PMC free article] [PubMed]
- 201.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hawley H.B., Chang J.C. Complement Induced Endotheliopathy Associated Vascular Microthrombosis in Coronavirus 2019 Disease. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Afshar-Kharghan V. Complement and clot. Blood. 2017;129(16):2214–2215. doi: 10.1182/blood-2017-03-771501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Conway E.M. Reincarnation of ancient links between coagulation and complement. J. Thromb. Haemost. 2015;13(Suppl 1):S121–S132. doi: 10.1111/jth.12950. [DOI] [PubMed] [Google Scholar]
- 205.Amara U., Flierl M.A., Rittirsch D., Klos A., Chen H., Acker B., Bruckner U.B., Nilsson B., Gebhard F., Lambris J.D., Huber-Lang M. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 2010;185(9):5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Agrawal P., Nawadkar R., Ojha H., Kumar J., Sahu A. Complement Evasion Strategies of Viruses: An Overview. Front. Microbiol. 2017;8:1117. doi: 10.3389/fmicb.2017.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Charchaflieh J., Wei J., Labaze G., Hou Y.J., Babarsh B., Stutz H., Lee H., Worah S., Zhang M. The role of complement system in septic shock. Clin Dev Immunol. 2012;2012 doi: 10.1155/2012/407324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Markiewski M.M., DeAngelis R.A., Lambris J.D. Complexity of complement activation in sepsis. J. Cell Mol. Med. 2008;12(6A):2245–2254. doi: 10.1111/j.1582-4934.2008.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Raghunandan S., Josephson C.D., Verkerke H., Linam W.M., Ingram T.C., Zerra P.E., Arthur C.M., Stowell S.R., Briones M., Chonat S. Complement Inhibition in Severe COVID-19 Acute Respiratory Distress Syndrome. Front. Pediatr. 2020;8 doi: 10.3389/fped.2020.616731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rittirsch D., Redl H., Huber-Lang M. Role of complement in multiorgan failure. Clin Dev Immunol. 2012;2012 doi: 10.1155/2012/962927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ghebrehiwet B., Peerschke E.I. Complement and coagulation: key triggers of COVID-19-induced multiorgan pathology. J Clin Invest. 2020;130(11):5674–5676. doi: 10.1172/JCI142780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Holter J.C., Pischke S.E., de Boer E., Lind A., Jenum S., Holten A.R., Tonby K., Barratt-Due A., Sokolova M., Schjalm C., Chaban V., Kolderup A., Tran T., Tollefsrud Gjolberg T., Skeie L.G., Hesstvedt L., Ormasen V., Fevang B., Austad C., Muller K.E., Fladeby C., Holberg-Petersen M., Halvorsen B., Muller F., Aukrust P., Dudman S., Ueland T., Andersen J.T., Lund-Johansen F., Heggelund L., Dyrhol-Riise A.M., Mollnes T.E. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci U S A. 2020;117(40):25018–25025. doi: 10.1073/pnas.2010540117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chen W., Pan J.Y. Anatomical and Pathological Observation and Analysis of SARS and COVID-19: Microthrombosis Is the Main Cause of Death. Biol Proced Online. 2021;23(1):4. doi: 10.1186/s12575-021-00142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.B. Shen, X. Yi, Y. Sun, X. Bi, J. Du, C. Zhang, S. Quan, F. Zhang, R. Sun, L. Qian, W. Ge, W. Liu, S. Liang, H. Chen, Y. Zhang, J. Li, J. Xu, Z. He, B. Chen, J. Wang, H. Yan, Y. Zheng, D. Wang, J. Zhu, Z. Kong, Z. Kang, X. Liang, X. Ding, G. Ruan, N. Xiang, X. Cai, H. Gao, L. Li, S. Li, Q. Xiao, T. Lu, Y. Zhu, H. Liu, H. Chen, T. Guo, Proteomic and Metabolomic Characterization of COVID-19 Patient Sera, Cell 182(1) (2020) 59-72 e15. [DOI] [PMC free article] [PubMed]
- 216.F. Diurno, F.G. Numis, G. Porta, F. Cirillo, S. Maddaluno, A. Ragozzino, P. De Negri, C. Di Gennaro, A. Pagano, E. Allegorico, L. Bressy, G. Bosso, A. Ferrara, C. Serra, A. Montisci, M. D'Amico, S. Schiano Lo Morello, G. Di Costanzo, A.G. Tucci, P. Marchetti, U. Di Vincenzo, I. Sorrentino, A. Casciotta, M. Fusco, C. Buonerba, M. Berretta, M. Ceccarelli, G. Nunnari, Y. Diessa, S. Cicala, G. Facchini, Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience, Eur Rev Med Pharmacol Sci 24(7) (2020) 4040-4047. [DOI] [PubMed]
- 217.S. Ram Kumar Pandian, S. Arunachalam, V. Deepak, S. Kunjiappan, K. Sundar, Targeting complement cascade: an alternative strategy for COVID-19, 3 Biotech 10(11) (2020) 479. [DOI] [PMC free article] [PubMed]
- 218.Rabaan A.A., Al-Ahmed S.H., Muhammad J., Khan A., Sule A.A., Tirupathi R., Mutair A.A., Alhumaid S., Al-Omari A., Dhawan M., Tiwari R., Sharun K., Mohapatra R.K., Mitra S., Bilal M., Alyami S.A., Emran T.B., Moni M.A., Dhama K. Role of Inflammatory Cytokines in COVID-19 Patients: A Review on Molecular Mechanisms, Immune Functions, Immunopathology and Immunomodulatory Drugs to Counter Cytokine Storm. Vaccines (Basel) 2021;9(5) doi: 10.3390/vaccines9050436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bao J., Li C., Zhang K., Kang H., Chen W., Gu B. Comparative analysis of laboratory indexes of severe and non-severe patients infected with COVID-19. Clin. Chim. Acta. 2020;509:180–194. doi: 10.1016/j.cca.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gustine J.N., Jones D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 2021;191(1):4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Manson J.J., Crooks C., Naja M., Ledlie A., Goulden B., Liddle T., Khan E., Mehta P., Martin-Gutierrez L., Waddington K.E., Robinson G.A., Ribeiro Santos L., McLoughlin E., Snell A., Adeney C., Schim van der Loeff I., Baker K.F., Duncan C.J.A., Hanrath A.T., Lendrem B.C., De Soyza A., Peng J., J'Bari H., Greenwood M., Hawkins E., Peckham H., Marks M., Rampling T., Luintel A., Williams B., Brown M., Singer M., West J., Jury E.C., Collin M., Tattersall R.S. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study, Lancet. Rheumatol. 2020;2(10):e594–e602. doi: 10.1016/S2665-9913(20)30275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Conti P., Caraffa A., Gallenga C.E., Ross R., Kritas S.K., Frydas I., Younes A., Ronconi G. Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: a promising inhibitory strategy. J. Biol. Regul. Homeost. Agents. 2020;34(6):1971–1975. doi: 10.23812/20-1-E. [DOI] [PubMed] [Google Scholar]
- 223.C.-C. group, Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial, Lancet Respir Med 9(3) (2021) 295-304. [DOI] [PMC free article] [PubMed]
- 224.Franzetti M., Forastieri A., Borsa N., Pandolfo A., Molteni C., Borghesi L., Pontiggia S., Evasi G., Guiotto L., Erba M., Pozzetti U., Ronchetti A., Valsecchi L., Castaldo G., Longoni E., Colombo D., Soncini M., Crespi S., Maggiolini S., Guzzon D., Piconi S. IL-1 Receptor Antagonist Anakinra in the Treatment of COVID-19 Acute Respiratory Distress Syndrome: A Retrospective. Observational Study, J Immunol. 2021;206(7):1569–1575. doi: 10.4049/jimmunol.2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Cavalli G., Dagna L. The right place for IL-1 inhibition in COVID-19. Lancet Respir Med. 2021;9(3):223–224. doi: 10.1016/S2213-2600(21)00035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yang Y., Wang H., Kouadir M., Song H., Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10(2):128. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jo E.K., Kim J.K., Shin D.M., Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016;13(2):148–159. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Martin S.J. Cell death and inflammation: the case for IL-1 family cytokines as the canonical DAMPs of the immune system. FEBS J. 2016;283(14):2599–2615. doi: 10.1111/febs.13775. [DOI] [PubMed] [Google Scholar]
- 229.Di Paolo N.C., Shayakhmetov D.M. Interleukin 1alpha and the inflammatory process. Nat. Immunol. 2016;17(8):906–913. doi: 10.1038/ni.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lukens J.R., Gross J.M., Kanneganti T.D. IL-1 family cytokines trigger sterile inflammatory disease. Front. Immunol. 2012;3:315. doi: 10.3389/fimmu.2012.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cauchois R., Koubi M., Delarbre D., Manet C., Carvelli J., Blasco V.B., Jean R., Fouche L., Bornet C., Pauly V., Mazodier K., Pestre V., Jarrot P.A., Dinarello C.A., Kaplanski G. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci U S A. 2020;117(32):18951–18953. doi: 10.1073/pnas.2009017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.van de Veerdonk F.L., Netea M.G. Blocking IL-1 to prevent respiratory failure in COVID-19. Crit. Care. 2020;24(1):445. doi: 10.1186/s13054-020-03166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kimmig L.M., Wu D., Gold M., Pettit N.N., Pitrak D., Mueller J., Husain A.N., Mutlu E.A., Mutlu G.M. IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.583897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Somers E.C., Eschenauer G.A., Troost J.P., Golob J.L., Gandhi T.N., Wang L., Zhou N., Petty L.A., Baang J.H., Dillman N.O., Frame D., Gregg K.S., Kaul D.R., Nagel J., Patel T.S., Zhou S., Lauring A.S., Hanauer D.A., Martin E., Sharma P., Fung C.M., Pogue J.M. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.P. Mehta, D.F. McAuley, M. Brown, E. Sanchez, R.S. Tattersall, J.J. Manson, U.K. Hlh Across Speciality Collaboration, COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet 395(10229) (2020) 1033-1034. [DOI] [PMC free article] [PubMed]
- 238.Biran N., Ip A., Ahn J., Go R.C., Wang S., Mathura S., Sinclaire B.A., Bednarz U., Marafelias M., Hansen E., Siegel D.S., Goy A.H., Pecora A.L., Sawczuk I.S., Koniaris L.S., Simwenyi M., Varga D.W., Tank L.K., Stein A.A., Allusson V., Lin G.S., Oser W.F., Tuma R.A., Reichman J., Brusco L., Jr., Carpenter K.L., Costanzo E.J., Vivona V., Goldberg S.L. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2(10):e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., Franceschini E., Cuomo G., Orlando G., Borghi V., Santoro A., Di Gaetano M., Puzzolante C., Carli F., Bedini A., Corradi L., Fantini R., Castaniere I., Tabbi L., Girardis M., Tedeschi S., Giannella M., Bartoletti M., Pascale R., Dolci G., Brugioni L., Pietrangelo A., Cossarizza A., Pea F., Clini E., Salvarani C., Massari M., Viale P.L., Mussini C. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.C. Salvarani, G. Dolci, M. Massari, D.F. Merlo, S. Cavuto, L. Savoldi, P. Bruzzi, F. Boni, L. Braglia, C. Turra, P.F. Ballerini, R. Sciascia, L. Zammarchi, O. Para, P.G. Scotton, W.O. Inojosa, V. Ravagnani, N.D. Salerno, P.P. Sainaghi, A. Brignone, M. Codeluppi, E. Teopompi, M. Milesi, P. Bertomoro, N. Claudio, M. Salio, M. Falcone, G. Cenderello, L. Donghi, V. Del Bono, P.L. Colombelli, A. Angheben, A. Passaro, G. Secondo, R. Pascale, I. Piazza, N. Facciolongo, M. Costantini, R.-T.-C.-S. Group, Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial, JAMA Intern Med 181(1) (2021) 24-31. [DOI] [PMC free article] [PubMed]
- 241.O. Hermine, X. Mariette, P.L. Tharaux, M. Resche-Rigon, R. Porcher, P. Ravaud, C.-C. Group, Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial, JAMA Intern Med 181(1) (2021) 32-40. [DOI] [PMC free article] [PubMed]
- 242.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., Horick N.K., Healy B.C., Shah R., Bensaci A.M., Woolley A.E., Nikiforow S., Lin N., Sagar M., Schrager H., Huckins D.S., Axelrod M., Pincus M.D., Fleisher J., Sacks C.A., Dougan M., North C.M., Halvorsen Y.D., Thurber T.K., Dagher Z., Scherer A., Wallwork R.S., Kim A.Y., Schoenfeld S., Sen P., Neilan T.G., Perugino C.A., Unizony S.H., Collier D.S., Matza M.A., Yinh J.M., Bowman K.A., Meyerowitz E., Zafar A., Drobni Z.D., Bolster M.B., Kohler M., D'Silva K.M., Dau J., Lockwood M.M., Cubbison C., Weber B.N., Mansour M.K., Investigators B.B.T.T. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N. Engl. J. Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Del Valle D.M., Kim-Schulze S., Huang H.H., Beckmann N.D., Nirenberg S., Wang B., Lavin Y., Swartz T.H., Madduri D., Stock A., Marron T.U., Xie H., Patel M., Tuballes K., Van Oekelen O., Rahman A., Kovatch P., Aberg J.A., Schadt E., Jagannath S., Mazumdar M., Charney A.W., Firpo-Betancourt A., Mendu D.R., Jhang J., Reich D., Sigel K., Cordon-Cardo C., Feldmann M., Parekh S., Merad M., Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Keshari R.S., Jyoti A., Dubey M., Kothari N., Kohli M., Bogra J., Barthwal M.K., Dikshit M. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS ONE. 2012;7(10) doi: 10.1371/journal.pone.0048111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ruiz-Limon P., Ladehesa-Pineda M.L., Castro-Villegas M.D.C., Abalos-Aguilera M.D.C., Lopez-Medina C., Lopez-Pedrera C., Barbarroja N., Espejo-Peralbo D., Gonzalez-Reyes J.A., Villalba J.M., Perez-Sanchez C., Escudero-Contreras A., Collantes-Estevez E., Font-Ugalde P., Jimenez-Gomez Y. Enhanced NETosis generation in radiographic axial spondyloarthritis: utility as biomarker for disease activity and anti-TNF-alpha therapy effectiveness. J. Biomed. Sci. 2020;27(1):54. doi: 10.1186/s12929-020-00634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.E.J. Brenner, R.C. Ungaro, R.B. Gearry, G.G. Kaplan, M. Kissous-Hunt, J.D. Lewis, S.C. Ng, J.F. Rahier, W. Reinisch, F.M. Ruemmele, F. Steinwurz, F.E. Underwood, X. Zhang, J.F. Colombel, M.D. Kappelman, Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry, Gastroenterology 159(2) (2020) 481-491 e3. [DOI] [PMC free article] [PubMed]
- 247.Vivarelli S., Falzone L., Torino F., Scandurra G., Russo G., Bordonaro R., Pappalardo F., Spandidos D.A., Raciti G., Libra M. Immune-checkpoint inhibitors from cancer to COVID19: A promising avenue for the treatment of patients with COVID19 (Review) Int. J. Oncol. 2021;58(2):145–157. doi: 10.3892/ijo.2020.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Aghbash P.S., Eslami N., Shamekh A., Entezari-Maleki T., Baghi H.B. SARS-CoV-2 infection: The role of PD-1/PD-L1 and CTLA-4 axis. Life Sci. 2021;270 doi: 10.1016/j.lfs.2021.119124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Parry R.V., Chemnitz J.M., Frauwirth K.A., Lanfranco A.R., Braunstein I., Kobayashi S.V., Linsley P.S., Thompson C.B., Riley J.L. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 2005;25(21):9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ahn E., Araki K., Hashimoto M., Li W., Riley J.L., Cheung J., Sharpe A.H., Freeman G.J., Irving B.A., Ahmed R. Role of PD-1 during effector CD8 T cell differentiation. Proc Natl Acad Sci U S A. 2018;115(18):4749–4754. doi: 10.1073/pnas.1718217115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Carter L., Fouser L.A., Jussif J., Fitz L., Deng B., Wood C.R., Collins M., Honjo T., Freeman G.J., Carreno B.M. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur. J. Immunol. 2002;32(3):634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 252.Vandenborre K., Van Gool S.W., Kasran A., Ceuppens J.L., Boogaerts M.A., Vandenberghe P. Interaction of CTLA-4 (CD152) with CD80 or CD86 inhibits human T-cell activation. Immunology. 1999;98(3):413–421. doi: 10.1046/j.1365-2567.1999.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.McLane L.M., Abdel-Hakeem M.S., Wherry E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 254.Hammaren H.M., Virtanen A.T., Raivola J., Silvennoinen O. The regulation of JAKs in cytokine signaling and its breakdown in disease. Cytokine. 2019;118:48–63. doi: 10.1016/j.cyto.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 255.Aaronson D.S., Horvath C.M. A road map for those who don't know JAK-STAT. Science. 2002;296(5573):1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 256.Jatiani S.S., Baker S.J., Silverman L.R., Reddy E.P. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1(10):979–993. doi: 10.1177/1947601910397187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Leonard W.J. Role of Jak kinases and STATs in cytokine signal transduction. Int. J. Hematol. 2001;73(3):271–277. doi: 10.1007/BF02981951. [DOI] [PubMed] [Google Scholar]
- 258.O'Shea J.J., Kontzias A., Yamaoka K., Tanaka Y., Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann. Rheum. Dis. 2013;72 Suppl 2:ii111-5. doi: 10.1136/annrheumdis-2012-202576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.van Vollenhoven R., Lee E.B., Strengholt S., Mojcik C., Valdez H., Krishnaswami S., Biswas P., Lazariciu I., Hazra A., Clark J.D., Hodge J., Wang L., Choy E. Evaluation of the Short-, Mid-, and Long-Term Effects of Tofacitinib on Lymphocytes in Patients With Rheumatoid Arthritis, Arthritis. Rheumatol. 2019;71(5):685–695. doi: 10.1002/art.40780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.F. Sunzini, I. McInnes, S. Siebert, JAK inhibitors and infections risk: focus on herpes zoster, Ther Adv Musculoskelet Dis 12 (2020) 1759720X20936059. [DOI] [PMC free article] [PubMed]
- 261.Winthrop K.L., Harigai M., Genovese M.C., Lindsey S., Takeuchi T., Fleischmann R., Bradley J.D., Byers N.L., Hyslop D.L., Issa M., Nishikawa A., Rooney T.P., Witt S., Dickson C.L., Smolen J.S., Dougados M. Infections in baricitinib clinical trials for patients with active rheumatoid arthritis. Ann. Rheum. Dis. 2020;79(10):1290–1297. doi: 10.1136/annrheumdis-2019-216852. [DOI] [PubMed] [Google Scholar]
- 262.Jorgensen S.C.J., Tse C.L.Y., Burry L., Dresser L.D. Baricitinib: A Review of Pharmacology, Safety, and Emerging Clinical Experience in COVID-19. Pharmacotherapy. 2020;40(8):843–856. doi: 10.1002/phar.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Scott I.C., Hider S.L., Scott D.L. Thromboembolism with Janus Kinase (JAK) Inhibitors for Rheumatoid Arthritis: How Real is the Risk? Drug Saf. 2018;41(7):645–653. doi: 10.1007/s40264-018-0651-5. [DOI] [PubMed] [Google Scholar]
- 264.Verden A., Dimbil M., Kyle R., Overstreet B., Hoffman K.B. Analysis of Spontaneous Postmarket Case Reports Submitted to the FDA Regarding Thromboembolic Adverse Events and JAK Inhibitors. Drug Saf. 2018;41(4):357–361. doi: 10.1007/s40264-017-0622-2. [DOI] [PubMed] [Google Scholar]
- 265.Bronte V., Ugel S., Tinazzi E., Vella A., De Sanctis F., Cane S., Batani V., Trovato R., Fiore A., Petrova V., Hofer F., Barouni R.M., Musiu C., Caligola S., Pinton L., Torroni L., Polati E., Donadello K., Friso S., Pizzolo F., Iezzi M., Facciotti F., Pelicci P.G., Righetti D., Bazzoni P., Rampudda M., Comel A., Mosaner W., Lunardi C., Olivieri O. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest. 2020;130(12):6409–6416. doi: 10.1172/JCI141772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Cantini F., Niccoli L., Matarrese D., Nicastri E., Stobbione P., Goletti D. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J. Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Y. Cao, J. Wei, L. Zou, T. Jiang, G. Wang, L. Chen, L. Huang, F. Meng, L. Huang, N. Wang, X. Zhou, H. Luo, Z. Mao, X. Chen, J. Xie, J. Liu, H. Cheng, J. Zhao, G. Huang, W. Wang, J. Zhou, Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial, J Allergy Clin Immunol 146(1) (2020) 137-146 e3. [DOI] [PMC free article] [PubMed]
- 268.Kotyla P.J., Engelmann M., Giemza-Stoklosa J., Wnuk B., Islam M.A. Thromboembolic Adverse Drug Reactions in Janus Kinase (JAK) Inhibitors: Does the Inhibitor Specificity Play a Role? Int. J. Mol. Sci. 2021;22(5) doi: 10.3390/ijms22052449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Rajasimhan S., Pamuk O., Katz J.D. Safety of Janus Kinase Inhibitors in Older Patients: A Focus on the Thromboembolic Risk. Drugs Aging. 2020;37(8):551–558. doi: 10.1007/s40266-020-00775-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rodriguez-Garcia J.L., Sanchez-Nievas G., Arevalo-Serrano J., Garcia-Gomez C., Jimenez-Vizuete J.M., Martinez-Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology (Oxford) 2021;60(1):399–407. doi: 10.1093/rheumatology/keaa587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hardy R.S., Raza K., Cooper M.S. Therapeutic glucocorticoids: mechanisms of actions in rheumatic diseases. Nat. Rev. Rheumatol. 2020;16(3):133–144. doi: 10.1038/s41584-020-0371-y. [DOI] [PubMed] [Google Scholar]
- 272.R.C. Group, P. Horby, W.S. Lim, J.R. Emberson, M. Mafham, J.L. Bell, L. Linsell, N. Staplin, C. Brightling, A. Ustianowski, E. Elmahi, B. Prudon, C. Green, T. Felton, D. Chadwick, K. Rege, C. Fegan, L.C. Chappell, S.N. Faust, T. Jaki, K. Jeffery, A. Montgomery, K. Rowan, E. Juszczak, J.K. Baillie, R. Haynes, M.J. Landray, Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med 384(8) (2021) 693-704. [DOI] [PMC free article] [PubMed]
- 273.Steinhauer D.A., Domingo E., Holland J.J. Lack of evidence for proofreading mechanisms associated with an RNA virus polymerase. Gene. 1992;122(2):281–288. doi: 10.1016/0378-1119(92)90216-c. [DOI] [PubMed] [Google Scholar]
- 274.Ogando N.S., Zevenhoven-Dobbe J.C., van der Meer Y., Bredenbeek P.J., Posthuma C.C., Snijder E.J. The Enzymatic Activity of the nsp14 Exoribonuclease Is Critical for Replication of MERS-CoV and SARS-CoV-2. J. Virol. 2020;94(23) doi: 10.1128/JVI.01246-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Eskier D., Suner A., Oktay Y., Karakulah G. Mutations of SARS-CoV-2 nsp14 exhibit strong association with increased genome-wide mutation load. PeerJ. 2020;8 doi: 10.7717/peerj.10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Frediansyah A., Tiwari R., Sharun K., Dhama K., Harapan H. Antivirals for COVID-19: A critical review. Clin Epidemiol Glob Health. 2021;9:90–98. doi: 10.1016/j.cegh.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hussain N., Yoganathan A., Hewage S., Alom S., Harky A. The effect of antivirals on COVID-19: a systematic review. Expert Rev Anti Infect Ther. 2021;19(4):473–486. doi: 10.1080/14787210.2021.1823832. [DOI] [PubMed] [Google Scholar]
- 278.Hamming I., Cooper M.E., Haagmans B.L., Hooper N.M., Korstanje R., Osterhaus A.D., Timens W., Turner A.J., Navis G., van Goor H. The emerging role of ACE2 in physiology and disease. J. Pathol. 2007;212(1):1–11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Miller A.J., Arnold A.C. The renin-angiotensin system in cardiovascular autonomic control: recent developments and clinical implications. Clin. Auton. Res. 2019;29(2):231–243. doi: 10.1007/s10286-018-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Patel S., Rauf A., Khan H., Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017;94:317–325. doi: 10.1016/j.biopha.2017.07.091. [DOI] [PubMed] [Google Scholar]
- 281.Kittana N. Angiotensin-converting enzyme 2-Angiotensin 1–7/1-9 system: novel promising targets for heart failure treatment. Fundam. Clin. Pharmacol. 2018;32(1):14–25. doi: 10.1111/fcp.12318. [DOI] [PubMed] [Google Scholar]
- 282.Banu N., Panikar S.S., Leal L.R., Leal A.R. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to Macrophage Activation Syndrome: Therapeutic implications. Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Cheng H., Wang Y., Wang G.Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020;92(7):726–730. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Fraga-Silva R.A., Sorg B.S., Wankhede M., Dedeugd C., Jun J.Y., Baker M.B., Li Y., Castellano R.K., Katovich M.J., Raizada M.K., Ferreira A.J. ACE2 activation promotes antithrombotic activity. Mol. Med. 2010;16(5–6):210–215. doi: 10.2119/molmed.2009.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Liu X., Yang N., Tang J., Liu S., Luo D., Duan Q., Wang X. Downregulation of angiotensin-converting enzyme 2 by the neuraminidase protein of influenza A (H1N1) virus. Virus Res. 2014;185:64–71. doi: 10.1016/j.virusres.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yang P., Gu H., Zhao Z., Wang W., Cao B., Lai C., Yang X., Zhang L., Duan Y., Zhang S., Chen W., Zhen W., Cai M., Penninger J.M., Jiang C., Wang X. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci. Rep. 2014;4:7027. doi: 10.1038/srep07027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Chung S.C., Providencia R., Sofat R. Association between Angiotensin Blockade and Incidence of Influenza in the United Kingdom. N. Engl. J. Med. 2020;383(4):397–400. doi: 10.1056/NEJMc2005396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20(7):389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci U S A. 2008;105(22):7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zhang W., Chen X., Huang L., Lu N., Zhou L., Wu G., Chen Y. Severe sepsis: Low expression of the renin-angiotensin system is associated with poor prognosis. Exp Ther Med. 2014;7(5):1342–1348. doi: 10.3892/etm.2014.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lee H.W., Suh J.K., Jang E., Lee S.M. Effect of angiotensin converting enzyme inhibitor and angiotensin II receptor blocker on the patients with sepsis. Korean J. Intern. Med. 2021;36(2):371–381. doi: 10.3904/kjim.2019.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Correa T.D., Takala J., Jakob S.M. Angiotensin II in septic shock. Crit. Care. 2015;19:98. doi: 10.1186/s13054-015-0802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Khanna A., English S.W., Wang X.S., Ham K., Tumlin J., Szerlip H., Busse L.W., Altaweel L., Albertson T.E., Mackey C., McCurdy M.T., Boldt D.W., Chock S., Young P.J., Krell K., Wunderink R.G., Ostermann M., Murugan R., Gong M.N., Panwar R., Hastbacka J., Favory R., Venkatesh B., Thompson B.T., Bellomo R., Jensen J., Kroll S., Chawla L.S., Tidmarsh G.F., Deane A.M., Investigators A-. Angiotensin II for the Treatment of Vasodilatory Shock. N. Engl. J. Med. 2017;377(5):419–430. doi: 10.1056/NEJMoa1704154. [DOI] [PubMed] [Google Scholar]
- 295.Bitker L., Burrell L.M. Classic and Nonclassic Renin-Angiotensin Systems in the Critically Ill. Crit. Care Clin. 2019;35(2):213–227. doi: 10.1016/j.ccc.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Raiden S., Nahmod K., Nahmod V., Semeniuk G., Pereira Y., Alvarez C., Giordano M., Geffner J.R. Nonpeptide antagonists of AT1 receptor for angiotensin II delay the onset of acute respiratory distress syndrome. J. Pharmacol. Exp. Ther. 2002;303(1):45–51. doi: 10.1124/jpet.102.037382. [DOI] [PubMed] [Google Scholar]
- 297.Shen L., Mo H., Cai L., Kong T., Zheng W., Ye J., Qi J., Xiao Z. Losartan prevents sepsis-induced acute lung injury and decreases activation of nuclear factor kappaB and mitogen-activated protein kinases. Shock. 2009;31(5):500–506. doi: 10.1097/SHK.0b013e318189017a. [DOI] [PubMed] [Google Scholar]
- 298.Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., Hall R., Poirier G., Ronco J.J., Tidswell M., Hardes K., Powley W.M., Wright T.J., Siederer S.K., Fairman D.A., Lipson D.A., Bayliffe A.I., Lazaar A.L. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care. 2017;21(1):234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Liu R., Qi H., Wang J., Wang Y., Cui L., Wen Y., Yin C. Angiotensin-converting enzyme (ACE and ACE2) imbalance correlates with the severity of cerulein-induced acute pancreatitis in mice. Exp. Physiol. 2014;99(4):651–663. doi: 10.1113/expphysiol.2013.074815. [DOI] [PubMed] [Google Scholar]
- 300.Chan Y.C., Leung P.S. Co-operative effects of angiotensin II and caerulein in NFkappaB activation in pancreatic acinar cells in vitro. Regul. Pept. 2011;166(1–3):128–134. doi: 10.1016/j.regpep.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 301.Chan Y.C., Leung P.S. Involvement of redox-sensitive extracellular-regulated kinases in angiotensin II-induced interleukin-6 expression in pancreatic acinar cells. J. Pharmacol. Exp. Ther. 2009;329(2):450–458. doi: 10.1124/jpet.108.148353. [DOI] [PubMed] [Google Scholar]
- 302.Sjoberg Bexelius T., Garcia Rodriguez L.A., Lindblad M. Use of angiotensin II receptor blockers and the risk of acute pancreatitis: a nested case-control study. Pancreatology. 2009;9(6):786–792. doi: 10.1159/000225906. [DOI] [PubMed] [Google Scholar]
- 303.Wang Y., Wang J., Liu R., Qi H., Wen Y., Sun F., Yin C. Severe acute pancreatitis is associated with upregulation of the ACE2-angiotensin-(1–7)-Mas axis and promotes increased circulating angiotensin-(1–7) Pancreatology. 2012;12(5):451–457. doi: 10.1016/j.pan.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 304.A.C. Simoes e Silva, K.D. Silveira, A.J. Ferreira, M.M. Teixeira, ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis, Br J Pharmacol 169(3) (2013) 477-92. [DOI] [PMC free article] [PubMed]
- 305.A. Zangrillo, G. Landoni, L. Beretta, F. Morselli, A. Serpa Neto, R. Bellomo, C.O.-B.S. Group, Angiotensin II infusion in COVID-19-associated vasodilatory shock: a case series, Crit Care 24(1) (2020) 227. [DOI] [PMC free article] [PubMed]
- 306.Leisman D.E., Mastroianni F., Fisler G., Shah S., Hasan Z., Narasimhan M., Taylor M.D., Deutschman C.S. Physiologic Response to Angiotensin II Treatment for Coronavirus Disease 2019-Induced Vasodilatory Shock: A Retrospective Matched Cohort Study. Crit Care Explor. 2020;2(10) doi: 10.1097/CCE.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Bloch M.J. Renin-Angiotensin System Blockade in COVID-19: Good, Bad, or Indifferent? J. Am. Coll. Cardiol. 2020;76(3):277–279. doi: 10.1016/j.jacc.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Krishnamurthy S., Lockey R.F., Kolliputi N. Soluble ACE2 as a potential therapy for COVID-19. Am. J. Physiol. Cell Physiol. 2021;320(3):C279–C281. doi: 10.1152/ajpcell.00478.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Miller A., Leach A., Thomas J., McAndrew C., Bentley E., Mattiuzzo G., John L., Mirazimi A., Harris G., Gamage N., Carr S., Ali H., Van Montfort R., Rabbitts T. A super-potent tetramerized ACE2 protein displays enhanced neutralization of SARS-CoV-2 virus infection. Sci. Rep. 2021;11(1):10617. doi: 10.1038/s41598-021-89957-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.N. Liu, J. Sun, X. Wang, T. Zhang, M. Zhao, H. Li, Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis, Int J Infect Dis 104 (2021) 58-64. [DOI] [PMC free article] [PubMed]
- 311.Fiorino S., Zippi M., Gallo C., Sifo D., Sabbatani S., Manfredi R., Rasciti E., Rasciti L., Giampieri E., Corazza I., Leandri P., de Biase D. The rationale for a multi-step therapeutic approach based on antivirals, drugs and nutrients with immunomodulatory activity in patients with coronavirus-SARS2-induced disease of different severities. Br. J. Nutr. 2021;125(3):275–293. doi: 10.1017/S0007114520002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.C. Annweiler, B. Hanotte, C. Grandin de l'Eprevier, J.M. Sabatier, L. Lafaie, T. Celarier, Vitamin D and survival in COVID-19 patients: A quasi-experimental study, J Steroid Biochem Mol Biol 204 (2020) 105771. [DOI] [PMC free article] [PubMed]
- 313.Annweiler G., Corvaisier M., Gautier J., Dubee V., Legrand E., Sacco G., Annweiler C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients. 2020;12(11) doi: 10.3390/nu12113377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Getachew B., Tizabi Y. Vitamin D and COVID-19: Role of ACE2, age, gender, and ethnicity. J. Med. Virol. 2021 doi: 10.1002/jmv.27075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Aygun H. Vitamin D can prevent COVID-19 infection-induced multiple organ damage. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(7):1157–1160. doi: 10.1007/s00210-020-01911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Malek Mahdavi A. A brief review of interplay between vitamin D and angiotensin-converting enzyme 2: Implications for a potential treatment for COVID-19. Rev. Med. Virol. 2020;30(5) doi: 10.1002/rmv.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]