Abstract
Setting up treatment strategies is the highest concern today to reduce the fatality of COVID-19. Due to a very new kind of virus attack, no specific treatment has been discovered to date. The most crucial way to dominate the disease severity is now the repurposing of drugs. In this review, we focused on the current treatment approaches targeting the crucial causative factors for the disease burden through cytokine storm or cytokine release syndrome. Several vaccines have been developed and have been applied already for prevention purposes, and several are on the way to be developed, although the effects and side effects are under observation. Presently, regulation of the immune response through intervention treatment methods has been adjusted on the basis of the COVID-19 severity stage and generally includes vaccines, immunotherapies including convalescent plasma and immunoglobulin treatment, monoclonal antibodies, cytokine therapy, complement inhibition, regenerative medicine, and repurposed anti-inflammatory and immune-regulatory drugs. Combination therapy is not acceptable in all respects because there is no concrete evidence in clinical trials or in vivo data. Target-specific drug therapies, such as inhibition of cytokine-producing signaling pathways, could be an excellent solution and thus reduce the severity of inflammation and disease severity. Therefore, gathering information about the mechanism of disease progression, possible goals, and drug efficacy of immune-based approaches to combat COVID-19 in the context of orderly review analysis is consequential.
Keywords: Coronavirus, COVID-19, Cytokine storm, NF-κB, Repurposing of drugs, SARS-CoV-2
Abbreviations: ACE, Angiotensin Converting Enzyme; ADAM-17, Disintegrin and Metallopeptidase Domain; Ag, Angiotensin; APCs, Antigen Presenting Cells; ARDS, Acute Respiratory Distress Syndrome; Cathepsin L, Cathepsin of Lysosome; CLR, C-type lectin receptors; COVID, Coronavirus Disease; CSF, Colony Stimulating Factor; HCoV, Human Corona Virus; HGF, Hepatocyte Growth Factor; IFN, Interferons; IKK, IKb kinase Complex; IL, Interleukins; IP, Inducible Protein; JAK, Janus Kinase; MAPK, Mitogen-activated protein kinase; MAPKK, Mitogen activated Protein Kinase Kinase; MAPKKK/MAP3K, Mitogen activated protein Kinase Kinase Kinase; MAPKs, p38 Mitogen Activated Protein Kinases; MCP, Monocyte Chemoattractant Protein; MERS, Middle East Respiratory Syndrome; MIP, Macrophage Inflammatory Protein; Mpro, main protease; MyD88, Myeloid Differentiation Primary Response dependent; NF-κB, Nuclear Factor kappa B; NK cells, Natural Killer cells; NLR, Nucleotide-binding oligomerization domain-like receptors; nsps, nonstructural proteins; PAMPs, Pathogen Associated Molecular Patterns; PLpro, Papain like protease; PORCN, Porcupine O Acetyl transferase.; pp, polyproteins; PRRs, Pattern Recognition Receptors; RBD, Receptor Binding Domain; RdRp, RNA dependent- RNA polymerase; RIG-1, Retinoic acid inducible gene-1; SARS-CoV-2, Severe Acute Respiratory Syndrome Corona Virus-2 (novel corona virus); SARS, Severe Acute Respiratory Syndrome; SFRP, Secreted Frizzled related proteins; SP, Spike Protein; STAT, Signal Transducer and Activator of Transcription Proteins; TLR, Toll like Receptor; TMPRSS2, Type II Transmembrane Serine Protease 2; TNF, Tumor Necrosis Factor; TRIF, TLR domain containing adaptor inducing IFN-β; VEGF, Vascular Endothelial Growth Factor; VUI, Variant Under Investigation; WHO, World Health Organization; Wnt, Wingless related Integration site
1. Introduction
In late December 2019, the Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2) outbreak initially started in the Hubei province of Wuhan, China [1]. It created great havoc in March 2020, and therefore, the World Health Organization (WHO) declared that, following Spanish flu (H1N1) in 1918, Asian flu (H2N2) in 1957, Hong Kong flu (H3N2) in 1968, and Pandemic flu (H1N1) in 2009, SARS-coV-2 can be characterized as a pandemic of 2020 [2]. As per the Johns Hopkins corona virus research center report, there are 191 countries with more than 114,499,553 confirmed cases and at least 2,541,219 deaths have been reported and the count is dramatically increasing [3].
Coronavirus belongs to the order Nidovirales and subfamily Coronavirinae. Coronavirinae is further categorized into alpha, beta, gamma, and delta Coronavirus based on serology where SARS-CoV-2 belongs to the beta coronavirus group [4], [5]. Coronaviruses are zoonotic. Based on past evidence and available literature, it has been hypothesized that coronaviruses are transmitted to humans because of eating bats, and they abruptly spread within humans through respiratory droplets and secretions where the virus remains viable for at least 3 h and also through direct contact [6]. Asymptomatic carriers can also transmit viruses depending on factors, including viral load in their upper respiratory tract. Its incubation period ranges from 1 to 14 days, then symptoms appear [4], [7].
SARS-CoV-2 is associated with high rates of mortality and fatality. It causes fatality in infected individuals by causing respiratory failure, complicated by shock or multiorgan failure. Coronavirus-induced respiratory complications are mostly attributed to its unique host cell entry mechanisms and pathogenesis associated with flush of cytokine release into the body, leading to cytokine storm [8].
In this review, we explored the detailed mechanism of viral cell entry and particularly cytokine storm intending to focus on the importance of repurposing drugs that target viral cell entry mechanisms and drugs that inhibit signaling pathways responsible for the release of cytokines as well as drugs that block potent cytokines involved in cytokine storm with a brief view on clinical trials undergoing on the same.
2. History
The actual history of the human coronavirus began around 1960, when two unusual types of viruses, B814 and 229E, were discovered by Tyrrell and Bynoe, Hamre and Procknow respectively at different time frames in samples obtained from the respiratory tracts of people with colds. Later, Tyrrell, along with a group of virologists, identified that these were similar to those of the bronchitis virus of chickens, mouse hepatitis virus, and gastroenteritis virus of swine, and named this new group of viruses as the Corona virus (corona denoting the crown-like appearance of the surface projections) and later this group of viruses was officially accepted as a new genus of viruses [9]. These coronaviruses cause a wide range of diseases in both animals and humans. Several coronaviruses that cause infections in humans have been discovered, including Human Coronavirus (HCoV) types, i.e., HCoV-229E, HCoV-NL63, HCoV-OC43, SARS-HCoV, HCoV-HKU1, SARS-CoV-1, MERS-CoV and the recently discovered SARS-CoV-2 [5]. Among all these, SARS-CoV-1 and MERS-CoV have been the causative agents of large-scale pandemics that have occurred in the past two decades, i.e., severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) respectively. SARS occurred in 2002 and 2003 in Guangdong Province, China and, after an unprecedented global public health effort, the epidemic was controlled within seven months of its occurrence, whereas MERS happened in 2012 in Middle Eastern countries [1], [10]. Now following them, SARS-CoV-2 caused the ongoing pandemic which has been causing great havoc for almost a year and is acquiring mutations and developing new variants. Recently, a new variant of COVID-19 was detected in the UK, which is named as VUI 202012/01 (VUI-variant under investigation) or B.1.1.7. It is reported that the rate of transmission and viral load of this variant is higher than the existing variants [11].
3. SARS-CoV-2: Structure and cell entry
SARS-CoV-2 is an enveloped spherical virus. Its envelope is formed by the interaction of three glycoproteins-Envelope (E) protein, Membrane (M) protein, and Spike protein (SP) [12]. The SP is club-shaped and protrudes out of the viral envelope, giving it a crown-like appearance under an electron microscope. SP carries the main antigenic epitopes recognized by antibodies and is responsible for host infection and membrane fusion [13]. The internal core of the virus contains a positive-sense single-stranded RNA (+ssRNA) [4].
The virus enters the cell by using SP. It uses angiotensin-converting enzyme 2 (ACE-2) receptors on the surface of targeted cell membranes as cellular receptors enter the cells and then the life cycle of the virus begins [14]. Zhao et al. reported that SP contains two subunits, S1 and S2. S1 contains a receptor-binding domain (RBD), which is responsible for initial contact of the virus with the host cell's surface, and S2 for membrane fusion and intracellular trafficking inside the host cell [15]. The SP will be in metastable prefusion conformation initially. When the S1 subunit of SP fuses with the host cell receptor, it undergoes hinge-like confirmation and enters the host cell [16].
Although, the principal receptor and cofactor for SARS-CoV-2 cellular entrance have been identified as ACE-2 and transmembrane serine protease 2 (TMPRSS2) [17], [18], recent evidence suggests that basigin (CD147) functions as a receptor and furin functions as a cofactor in SARS-CoV-2 pathogenicity [19], [20]. In addition, the VEGF-A receptor neuropilin 1 (NRP1) has also been shown to be the host factor receptor for furin-cleaving SARS-CoV-2 spike peptides recently [21], [22]. Infectivity and entrance are reduced when NRP1 is blocked, and NRP1 reliance is lost when the furin location is changed. In a hamster pathogenesis disease model, deletion of the furin peptide in spike causes decreased replication in Calu3, increased replication and better fitness in Vero E6, and mitigated illness [21], [22].
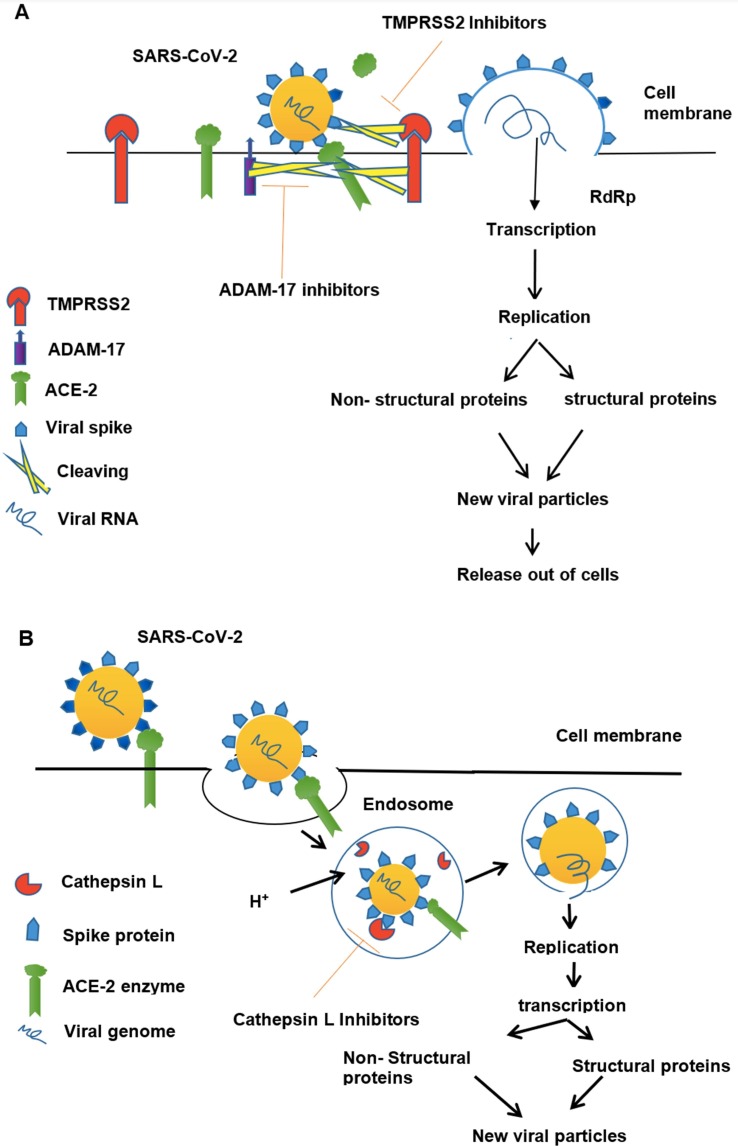
Viral entry occurs by two mechanisms- the non-endosomal pathway and receptor-mediated endocytosis [23]. In the non-endosomal pathway (Fig. 1 A), SP of virus interacts polarly with ACE-2 unlocking receptor-binding domain (RBD) of S1, which is essential for the fusion of the virus to the host cell membrane. After fusing the virus releases its genome into the target host cell [24], [25].
Fig. 1.
Mechanism of SARS-CoV-2 cell entry. (A) Non-endosomal Pathway. SARS- CoV-2 upon contact with host cell surface, spike protein (SP) projecting from the viral cell surface, first undergoes proteolytic cleavage by TMPRSS2 and exposes its receptor binding domain (RBD) and attaches to the ACE-2 receptor on host cell surface via SP. TMPRSS2 also cleaves ACE-2 along with ADAM17 because of which shedding of ACE-2 ectodomain occurs which can still bind to virus. After its attachment, virus fuses with host cell and releases its genome. Viral RNA undergoes transcription, translation and form new viral particles, new viral particles then release from host cell. TMPRSS2 inhibitors may inhibit TMPRSS and thereby exposure of RBD to ACE-2. (B) Endosomal Pathway. SARS-CoV-2 upon contact with host cell surface, signals the host cell and forms a vesicle enclosing virus–ACE-2 complex. Then endocytosis of virus-ACE-2 complex occurs, into the host cell cytoplasm followed by H+ influx into endosome. Decreased pH in endosome activates Cathepsin L, which cleaves SP of virus. Then virus fuses with endosomal membrane and releases its genome. Viral genome transcribes and translates into viral particles, viral particles fuse and release out of host cell. Cathepsin L inhibitors may inhibit Cathepsin L cleavage of viral SP.
In receptor-mediated endocytosis (Fig. 1B), the interaction of SP with ACE-2 leads to the formation of virus – ACE-2 endosome and endocytosis of virus along with ACE-2 occur, when H+ influx occurs in the endosome, then host cell proteases mainly Cysteine protease cathepsin of lysosomes (Cathepsin L) gets activate and, cleaves SP and facilitates viral fusion into host cell leading to +ssRNA release [25], [26].
Along with viral SP, ACE-2 of the host should also undergo cleavage in order to bind with SP. Many studies reported that the internalization of ACE-2 into the host cell gives positive feedback to a host cellular protease called disintegrin and metallopeptidase domain (ADAM17). TMPRSS2 along with ADAM17 cleaves ACE-2 enzyme ectodomain into the extracellular space thus further facilitating viral entry into the host cell [23]. Following their entry, the viral genome translates into two polyproteins (pp) 1a and ab that further undergo proteolysis by the main protease Mpro and Papain-like protease PLpro to yield 16 nonstructural proteins (16 NSPs) [27]. These elements constitute the RNA replicate-transcriptase protein complex and control viral +ssRNA replication and transcription. Out of 16 NSPs, NSP-12 acts as RNA-dependent- RNA polymerase (RdRp) [28] through which +ssRNA replicates and translates into structural and nonstructural proteins. Subsequently, these protein elements, RNA genome, and nucleocapsids assemble in the host cytoplasm and thereby mature viral particles released from the host cell via its internal membrane through exocytosis [5].
Milanetti et al. reported that SARS-CoV-2 has dual entry points i.e., along with ACE-2 receptor, S protein also uses sialic acid as an entry point [29]. As very less literature is published till now on viral utilization of sialic acid as an entry point, advanced research is being suggested in this aspect.
4. Cytokine storm and COVID-19
Cytokines are the protein molecules released by lymphocytes, leukocytes, dendritic cells, T-helper cells (Th) cells, endothelium, epithelium, and leukocytes, play important roles in the inflammatory cascades (Table 1 ). Prolonged and major fatality in COVID-19 has been manifested due to cytokine storm [30], [31], which can be described as the release of several pro-inflammatory cytokines from hyperactive/dysregulated host immune system at levels that are injurious to host cells [32]. Cytokine storm can be broadly defined by three criteria- increased levels of circulating cytokines, acute systemic inflammatory symptoms, and cytokine-driven organ dysfunction [33].
Table 1.
A list of proinflammatory mediators causing cytokine storm in infectious diseases [34], [38], [39], [40], [41], [42], [43], [44].
| Cytokines/Chemokines | Source | Signaling Pathway | Action After Activation |
|---|---|---|---|
| Interleukin (IL)-1 | Macrophages, Dendritic cells, B- cells | Nuclear Factor Kappa -B (NF-κB) pathway, Toll Like Receptor (TLR) signaling pathway | Pro-inflammatory, proliferation and activation of Natural Killer cells (NK cells), T-cells and B-cells |
| IL-2 | T-cells | NF-κB pathway, TLR signaling pathway | proliferation and activation of NK cells, B- and T- cells |
| IL-4 | Th cells | NF-κB pathway, TLR signaling pathway | Stimulates synthesis of IgG and IgE antibodies, proliferation of B- cells and T cells |
| IL-6 (plays vital role in cytokine storm) | Macrophages, fibroblasts, Th cells. | NF-κB pathway, TLR signaling pathway | Stimulates synthesis of IgG antibodies |
| IL-7 | Epithelial cells, Stromal cells. | NF-κB pathway, TLR signaling pathway | T- and B- cell growth factor |
| IL-8 | Macrophages | NF-κB pathway, TLR signaling pathway | Chemotaxis |
| IL-9 | T-cell | NF-κB pathway, TLR signaling pathway | Growth and proliferation of T- cells |
| IL–11 | Bone marrow Stromal cells | NF-κB pathway, TLR signaling pathway | Differentiation of B- cells |
| IL −13 | T-cells | NF-κB pathway, TLR signaling pathway | Activation of NK cells |
| (Tumor necrosis factor) TNF – α | Macrophages | p38 MAPK | Activation of phagocytes |
| (Interferons) IFN – α |
Leukocytes | RIG-I | Shows anti-viral action |
| IFN – γ | RIG-I | Shows anti-viral action and activates monocytes, Neutrophils | |
| (Colony stimulating factors) G-CSF |
Endothelium and fibroblasts. | JAK/STAT pathway, MAPK pathway. | Synthesis of neutrophils, eosinophils and basophils (Granulocytes) |
| GM- CSF | T-cells, Fibroblasts and Macrophages | JAK/STAT pathway, MAPK pathway | Synthesis of granulocytes and monocytes |
| M- CSF | Endothelium and fibroblasts | JAK/STAT pathway, MAPK pathway | Monocyte production and activation |
| Chemokines CCL2/MCP-1 |
Osteoblasts, Macrophages, endothelial cells, adipocytes, fibroblast | NF-κB pathway, p38 MAPK pathway | Chemotaxis of macrophages, monocytes, dendritic cells, basophils, NK-cells, Myeloid cells into tissues |
| CCL3 (MIP-1α) | Monocytes, Dendritic cells, Lymphocytes, mononuclear phagocytes | p38 MAPK pathway | Chemotaxis of Monocytes, eosinophils, basophils, lymphocytes |
| CCL5 (RANTES) | Platelets, T cells, Eosinophils and Basophils | MAPK pathway | Chemotaxis of monocytes and T-lymphocytes |
Cytokines that worsen inflammation are called pro-inflammatory cytokines (IL-1, IL-6, TNF-α, IL-17, IFN-α, INF-β, IFN-γ), and those which serve to reduce inflammation and promotes healing are called anti-inflammatory cytokines (IL-12, IL-10) [34]. Under normal conditions, cytokines are essential to fight against infection, but in the case of a cytokine storm, excessive release of cytokines and chemokines causes infiltration of immune cells and thereby aggravation of inflammation leading to multi-organ complications [35].
As per the literature, plasma levels of COVID-19 patients showed elevated levels of proinflammatory interleukins particularly IL-1, IL-2, IL-4, IL-6, IL-7, IL-13, IL-17, colony-stimulating factors (G-CSF, M-CSF, GM-CSF), Interferon γ inducible protein 10 (IP-10), interferons (IFN-γ, IFN-α), chemokines (CCL2, CCL3, CCl5), tumor necrosis factor (TNF-α), vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) [36], [37], [38].
Drugs targeting the cytokines may play a potential therapeutic role in the treatment of cytokine storm and complications that may be upregulated due to COVID-19.
4.1. Signaling pathways responsible for cytokine/chemokine production
After cellular entry of SARS-CoV-2, pattern recognition receptors (PRRs) of innate immune cells (macrophages, dendritic cells, neutrophils) recognize pathogen-associated molecular patterns (PAMP) present in the S1 subunit of COVID-19. PRRs are further classified into four families i.e., toll-like receptors (TLR), Nucleotide-binding oligomerization domain-like receptors (NLR), C-type lectin receptors (CLR), and retinoic acid-inducible gene-1 (RIG-1), which lead to activation of downstream inflammatory regulation pathways such as nuclear factor kappa B (NF-κB), and mitogen-activated protein kinase (MAPK) pathways, thereby producing pro-inflammatory cytokines [45]. Hyperactivation of these signaling pathways is responsible for cytokine storm in COVID-19.
Along with the pro-inflammatory cytokines released by innate immunity, Antigen-presenting cells (APCs) presents antigen to T-cells, leading to differentiation of T-cells to T-helper (Th) cells and cytotoxic cells. These APCs and Th cells also produce cytokines contributing to cytokine storm [46], [47].
As inflammation and cytokine storm are the major contributors to COVID-19 pathophysiology and post-COVID complications, in our review we focused on signaling pathways responsible for cytokine storm. As hyper-responsiveness of these pathways is responsible for inducing cytokine storm, drug therapies targeting these pathways along with antiviral agents may play a key role in the therapy of COVID-19.
4.1.1. p38 Mitogen Activated Protein Kinase (p38 MAPK)
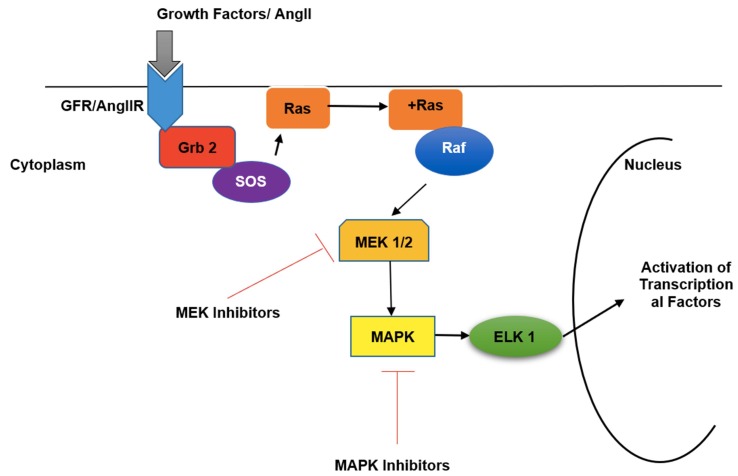
p38 MAPK is an intracellular signaling molecule involved in pro-inflammatory cytokine production. This pathway activates when growth factor receptors (GFR) or Angiotensin-II receptors (AngIIR), activate the Grb2-SOS complex which interacts and activates membrane-bound Ras molecule [46]. The activated Ras molecule stimulates Raf (MAPKKK), which then activates MEK1/2 (MAPKK). MEK1/2 thereby activates ERK1/2 (MAPK) which finally activates terminal molecules responsible for the initiation of transcription of cytokines and other inflammatory mediators [47] (Fig. 2 ). AngII along with vasoconstriction is also responsible for pro-inflammatory cytokine production through activation of p38 MAPK. AngII is converted to Ang 1–7, in the presence of ACE-2. This Ang 1–7 binds to Mas receptors, which inhibits p38 MAPK and thereby inhibits cytokine release from AngII [48]. In the case of COVID-19, because of downregulation of ACE-2 receptors, production of Ang 1–7 decreases, and thereby activation of p38 MAPK occurs by upregulated levels of AngII and thereby increased levels of proinflammatory mediators. Apart from AngII, and growth factors some upstream PRRs and the inflammatory cytokines such as IL-1β, and TNF-α, also stimulates phosphorylation of p38 MAPK leading to activation of transcriptional factors which further mediates inflammatory responses [42].
Fig. 2.
Mechanism of p38 MAPK pathway activation by AngII. Cellular entry of the growth factor or AngII activates the MEK1/2 followed by p38 MAP kinase (MAPK) pathway through the Grb 2-SOS and Ras-Raf signaling pathway. MEK- and MAPK-inhibitors may inhibit the pathway of activation of ELK1 and thereby the activation transcription al factors for the production of pro-inflammatory cytokines can be inhibited in the cell nucleus.
4.1.2. NF-κB signaling pathway
NF-κB belongs to a class of inducible transcription factors that regulate genes involved in the immune and inflammatory response. These proteins are normally segregated and inhibited in the cytosol by the endogenous inhibitor protein family of IκB. Activation of NF-κB completely depends on the degradation of IκB from the NF-κB complex. IκB undergoes inducible degradation by a multi-subunit IκB kinase complex (IKK) when it receives appropriate stimulus (ORF3a, M, ORF7a, and N proteins of SARS-CoV-2) [49]. Many factors such as cytokines, viral RNA, and other particles stimulate IKK which thereby leads to degradation of IκB by the proteasome and induces the NF-κB transcriptional pathway [49]. NF-κB, after activation, translocates into the nucleus and induces transcription of pro-inflammatory cytokines, chemokines, adhesion molecules, and co-stimulatory molecules that activates innate and adaptive immunity [35], [50].
Several cell types have been reported to be affected/activated by the SARS-CoV-2 such as the epithelium of the respiratory tract, endothelium linings, macrophages, mast cells, peripheral mononuclear cells such as monocytes, dendritic cells, and T-cells [20], [51], [52]. The NF-κB signaling has been identified as the major pathway for the pro-inflammatory cytokine/chemokine response caused by SARS-CoV-2 infection in several recent studies [20], [51], [52]. In human bronchial epithelial cells, SARS-CoV-2 spike protein subunit 1 (S1) was found to cause significant levels of NF-κB activation, production of pro-inflammatory cytokines/chemokines such as IL-1, TNF-α, IL-6, and CCL2/MCP-1, and moderate epithelial damage. S1 interaction with the human ACE-2 receptor was needed for NF-κB activation. S1 had greater activity in NF-κB activation than SARS-CoV-S1, which is likely due to the increased binding affinity of S1 to the ACE-2 receptor [53]. In the COVID-19 patients, cytokines/chemokines such as IL-1β, CCL2/MCP-1, CCL3, CCL8, CCL13, CXCL2, CXCL10, and downstream signaling molecules such as IL1R1, TRIF, MYD88, TRAF6, RelA (p65 NF-κB), RelB, NF-κB1 levels have been found significantly higher in the peripheral mononuclear cells while compared with the non-COVID-19 patients. In addition, the simultaneous overexpression of TLR-4, RelA (p65 NF-κB), RelB, NF-κB1, and NF-κB2 genes in COVID-19 patients suggests that TLR-4 mediated NF-κB signaling activation is involved in the development of pro-inflammatory responses in the patients [52], [54]. Drugs targeting these pathways may play a potential role in treating SARS-CoV-2-induced cytokine storm.
4.1.3. Toll Like Receptor (TLR) signaling
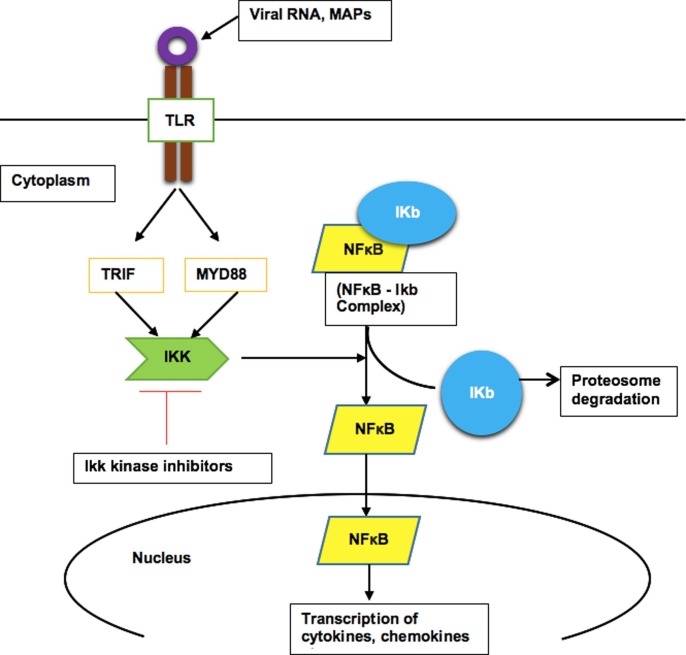
TLRs are expressed in immune cells such as macrophages and dendritic cells as well as non-immune cells such as epithelial cells and fibroblasts. TLRs are of two types, cell surface TLRs and intracellular TLRs. Cell surface TLRs recognize viruses through PAMPs on the S1 subunit of SP, whereas intracellular TLRs recognize viral RNA and get activated [55]. TLR-4, a cell surface pattern recognition receptor (PRR), plays an important role in the pathogenesis of COVID-19-induced cytokine storm. Upon stimulator binding to the TLR-4, activation of two pathways- myeloid differentiation primary response (MYD88) dependent- and TLR domain-containing adaptor inducing IFN-β (TRIF)-dependent- pathways lead to activation of intracellular signaling cascade and release cytokines/chemokines, and interleukins. These substances further activate and recruit inflammatory cells such as macrophages, neutrophils, mast cells, and NK-cells leading to activation of downstream NF-κB, and/or MAPK pathway and thereby release of pro-inflammatory cytokines and reactive oxygen species, which is responsible for host cell damage [56], [57]. Drugs targeting this pathway may inhibit the activation of NF-κB signaling pathways, which is well-recognized as the major transcriptional factor to produce pro-inflammatory cytokines/chemokines (Fig. 3 ).
Fig. 3.
Mechanism of activation/translocation of NF-κB signaling through toll like receptor (TLR) pathway. Upon stimulator binding (either viral components, or other stimuli) to the TLR-4, activation of two pathways- MYD88 dependent- and TRIF-dependent- pathways lead to activation of NF-κB signaling cascade and produce cytokines/chemokines. Inhibition of the IKK kinase can be a possible pathway to inhibit the NF-κB activation, and thereby inhibit the pro-inflammatory mediator-production.
Literature is evidencing that hyperstimulation of TLR-4 plays an important role in COVID-19-induced pulmonary injury as stimulation of TLR-4 increases ACE-2 expression. As ACE-2 expression is more in type-2 alveolar cells of the lung, it is the primary organ susceptible to infection and complications [58]. A recent report showed that TLRs are responsible for the expression of pro-inflammatory cytokines such as IL‐1β, and IL-6 in COVID-19 infection [59]. In addition, the interaction of TLRs with virus particles causes immunopathological effects that result in mortality in COVID-19 patients. TLR-3/TLR-4 adapter, TRIF deficient mice are very sensitive to SARS-CoV-2 and have a high risk of death., and TLR3-/-, and TLR4-/- mice are highly vulnerable to SARS-CoV-2 as well. Moreover, individuals with poor outcomes in SARS-CoV-2 infection have been found with activation of proinflammatory signaling pathways and proinflammatory cytokine production after infection with TRIF-/- mice [60]. However, TLR-4 has been found as a key factor in the COVID-19 pathophysiology causing increment of inflammation through induction of NETosis and inflammasome activation [54], [61].
TLR agonists might possibly be utilized as a COVID-19 prevention medication. According to Proud et al., TLR2/6 agonist prophylactic treatment decreases SARS-CoV-2 transmission and protects against the infection [62].
4.1.4. JAK/STAT pathway
JAK/STAT (Janus Kinase- signal transducers and activation of transcription pathway) plays an important role in inflammation by inducing the production of growth factors, cytokines, and is essential for many cellular processes such as hematopoiesis, and lactation. In general, STAT proteins remain inactive in the cytoplasm. JAK/STAT pathway initiates when the cytokines produced by SARS-CoV-2 bind to their corresponding receptor [41].
JAKs transmit extracellular signals from pro-inflammatory cytokines to phosphorylating STATs. These phosphorylated STATs dimerize and then the complex translocates into the nucleus and acts as transcription factors responsible for inflammation, synthesis, and maturity of inflammatory cells (B- and T- cells), and interferon-induced gene expression. Activation of the JAK/STAT pathway in COVID-19 is one of the factors responsible for cytokine storm [63].
4.1.5. Wingless related integration site (Wnt)/β-catenin signaling
Wnt/β-catenin signaling plays an important role in cellular development, differentiation, and cell cycle control. This pathway gets activated by binding of Wnt ligands (Wnt-3a, Wnt-5a, β-catenin) to frizzled family of receptors. In normal cells, Wnt is inactive, in the cytoplasm and β-catenin exists in a destruction complex along with APC, CK1α, GSK3-β. β-catenin undergoes ubiquitination and then gets degraded by proteasome thereby maintaining low levels of β-catenin. Wnt after binding to frizzled receptors stimulates dissociation of this destruction complex leading to increased availability of β-catenin in the cytoplasm. β-catenin then undergoes phosphorylation and translocates to the nucleus and interacts with transcription factors (T - cell factor) and then regulates the cell cycle. More et al., by their study, reported that activation of Wnt/β-catenin signaling increases influenza virus mRNA production in mouse lung epithelial cells, whereas inhibition of Wnt/β-catenin signaling reduces virus expression and production [64]. Although the role of this pathway is less studied, literature is indicating that it plays an important role in viral infections.
It is also evident in the literature that Wnt ligands are secreted from immune cells. Choi and the group reported that Wnt-5a is a promising diagnostic marker in SARS-CoV-2 induced acute respiratory distress syndrome (ARDS), indicating its important role in the disease. It is hypothesized that Wnt-5a gets activate in ARDS leading to further inflammation and fibrosis. So, drug therapy targeting this pathway may help in the disease prognosis of COVID-19 [65].
4.1.6. TGF – β/Smad signaling
Even though there are very few studies on the role of TGF-β/Smad signaling in COVID-19, literature is evidencing that TGF-β plays a very important role in lung fibrosis by stimulating fibroblast proliferation and development [66]. TGF-β gets generated in response to tissue injury and then TGF-β/Smad signaling gets initiate when TGF-β1 binds to a receptor and after activation, it propagates signal through Smad protein cascade phosphorylation. The activated Smad complexes translocate into the nucleus and regulate the transcription of genes responsible for the progression of ARDS and pulmonary fibrosis. Therapeutically targeting the TGF-β/Smad pathway in COVID-19 may prevent and regulate the progression of ARDS to fibrosis and damage [67], [68].
4.2. Complement system
As the complement system acts as the interplay between innate immunity and adaptive immunity, it also activates several immune cells and pro-inflammatory cytokines. The anaphylatoxins, C3a and C5a act by binding to C3aR, and C5aR respectively, and activates neutrophils, macrophages, mast cells, Lymphocytes, and basophils; thereby leading to the release of pro-inflammatory cytokines/chemokines [69]. C3a and C5a are formed by cleaving C3 and C5 by convertases or serine proteases. They seem to be responsible for COVID-19 related lung injury and blood levels of patients with ARDS are detected with C3a and C5a [70].
5. Drugs targeting cytokine storm
Currently, COVID-19 therapy mostly relied on US Food and Drug Administration (FDA) approved drugs. Repurposing of drugs that inhibit cytokine signaling pathways and viral entry increases therapeutic options for COVID-19.
5.1. Immunotherapy checkpoint inhibitors
5.1.1. NF-κB inhibitors
To date, naturally available products Nobiletin, Curcumin, Resveratrol, are found to inhibit the NF-κB pathway [71], [72], [73], [74], and clinical trials are ongoing, although the role of these drugs at molecular levels of the NF-κB pathway is still unknown [75], [76], [77], [78], [79]. The previous report indicates that emetine, fluorosalan, sunitinib, bithionol, tribromsalan, and lestaurtinib inhibit IκBα phosphorylation either reversibly or irreversibly and prevent activation of the NF-κB pathway [80].
However, concrete studies with double-blind clinical trials are suggested to be carried out before administration to the COVID-19 patients. Although many clinical trials are undergoing on the role of NF-κB inhibitors and IKK inhibitors in cancer therapy, very few studies are conducting on the potential role of these drugs in COVID-19 [81].
5.1.2. p38 MAPK inhibitors
MAPK inhibitors- Losmapimod, Pamapimod, and Semapimod blocks p38 MAPK and downregulates ACE-2 inhibitors thereby decrease the release of pro-inflammatory cytokines, platelet aggregation, and vasodilation [82]. Other drugs that inhibit the MAPK pathway are MEK inhibitors, and they include, Refametinib, Selumetinib, and Trametinib, which can act as potential therapeutic add-ons for treating COVID-19 [63]. Many clinical trials are undergoing on these drugs for the treatment of cancer and other inflammatory drugs, which can be repurposed on COVID-19 (Table 2 ).
Table 2.
Clinical trial data on MAPK and MEK inhibitors in COVID-19.
| Drugs Under Investigation | Aim of The Trial | Status of Clinical Trial | Location | Results from The Trial | References |
|---|---|---|---|---|---|
| Losmapimod | To assess whether the early initiation of p38 inhibitor therapy in patients with moderate COVID-19 will prevent further clinical deterioration and reduce the need for both increased respiratory support as well as mortality as it showed increased survival rate in mice infected with COVID-19. | Recruiting | Brazil, Mexico, United States | No results posted | [83] |
5.1.3. TLR antagonists
Natural surfactant proteins present in the lungs act as innate immunity and removes viruses from the lungs. Literature is saying that lack of surfactants may be one of the responsible factors for viral infection and using these surfactants as therapy may help in removing the virus from bronchi and in inhibiting TLR-4 from activation by viral particles [84], [85]. Therefore, investigating the use of pulmonary surfactants in the therapy of COVID-19 is highly warranted, and therapeutic developments based on this concept may develop drugs against TLR-4 receptors (Table 3 ).
Table 3.
Clinical trial data on pulmonary surfactants in COVID-19.
| Drug Under Investigation | Aim of The Study | Status of Clinical Trial | Location | Results from The Trial | References |
|---|---|---|---|---|---|
| Pulmonary surfactant delivered through COVsurf Drug delivery system. | To assess whether the exogenous surfactant administration to lungs is potential treatment option in COVID-19 patients in terms of both severity and improvement of oxygenation. | Recruiting | United Kingdom | No results posted | [86] |
| Surfactant | To prove the efficacy and safety Surfactant-BL, administered by inhalation in adult hospitalized patients with ARDS due to COVID-19. | Recruiting | Russia | No results posted | [87] |
5.1.4. JAK/STAT inhibitors
JAK/STAT inhibitors may be administered in treating cytokine storm that may make the disease condition less severe. They may act as a potential therapeutic agent for COVID-19 treatment. Many clinical trials are currently undergoing on Roxolitinib for its utilization in COVID-19 therapy [88]. Besides, there are some other drugs currently are in the trial as JAK/STAT inhibitors (Table 4 ).
Table 4.
Clinical trial data on JAK/STAT inhibitors in COVID-19.
| Drug Under Investigation | Aim of The Trial. | Status of Clinical Trial | Location | Results from The Trial | References |
|---|---|---|---|---|---|
| Baricitinib | To assess the efficacy of baricitinib in hospitalized COVID-19 patients. | Recruiting | Argentina, Brazil, India, Germany, Japan, Italy, Korea, Mexico, Russian Federation, Puerto Rico, Spain, United States, United Kingdom. | No results posted | [89] |
| Baricitinib and Remdesivir | To evaluate the combination of Baricitinib and Remdesivir compared to remdesivir alone in COVID-19 patients. | Completed | Denmark, Korea, Japan, Republic of, Spain, Mexico, Singapore, United States, United Kingdom. | Baricitinib plus Remdesivir was superior to Remdesivir alone in reducing recovery time and increased improvement in clinical status | [90] |
| Tofacitinib | To assess the safety and efficacy of tofacitinib plus standard pharmacologic and supportive measures in treating hospitalized participants with COVID-19 pneumonia. | Active, not recruiting | Brazil | No results posted | [91] |
| To study the efficacy of tofacitinib in reducing the risk of mechanical ventilation and/or death in patients with moderately severe COVID-19 pneumonia who received standard of care treatment | Recruitment Completed | Russian Federation | No results posted | [92] | |
| To assess the efficacy and safety of tofacitinib in hospitalized adult patients with SARS-CoV-2 and pneumonia who require supplemental oxygen and have serologic markers of inflammation but do not need mechanical ventilation. | Recruiting | United States | No results posted | [93] | |
| Ruxolitinib | To evaluate the efficacy and safety of Ruxolitinib in the treatment of patients with COVID-19 severe pneumonia. | Active, Not recruiting (Phase II) | Germany | No results posted | [94] |
| To study the reversal of hyperinflammation to improve pulmonary function, reduce respiratory dependency and reduce mortality. | Recruiting | Germany | No results posted | [95] |
5.1.5. Wnt/β-catenin signaling inhibitors
Drugs that inhibit Wnt/β-catenin pathway are under investigation to treat cancers and other autoimmune diseases as it plays a vital role in immune cell infiltration and regulates the expression of a number of genes essential for immune cell proliferation and differentiation [96], [97]. It is essential to Investigate drugs that may inhibit this pathway in the view of COVID-19. Porcupine-O-Acetyl transferase (PORCN) inhibitors, Secreted Frizzled related proteins (SFRP), FZD antagonist or monoclonal antibodies, β-catenin transcriptional activity inhibitors are under investigation for their role in cancer [98]. Conducting trials on these drugs is essential to develop potential therapeutic targets for COVID-19.
5.1.6. TGF/Smad inhibitors
TGF/Smad signaling blockade is finding its significance in lung fibrosis and heart diseases. AS Activation of TGF is the main factor responsible for ARDS, drugs blocking this pathway such as Fresolimumab, Galunisertib [99], may occupy their role in preventing and treating COVID-19 induced pulmonary ARDS and fibrosis as well as cardiac complications.
5.1.7. Complement system inhibitors
Drugs inhibiting the complement system have their scope in treating several immune-related disorders such as Rheumatoid Arthritis, Inflammatory bowel disease, and asthma [100]. Anti-C5-monoclonal antibodies such as Eculizumab, C5a receptor blocker Avacopan [101], [102], and drugs that target C3a and C3a receptors should be widely investigated in terms of COVID-19. Currently, Eculizumab is under trial to sees its effect in the treatment of COVID-19 patients in the US and France (Table 5 ).
Table 5.
Clinical trial data on complement system inhibitors in COVID-19.
| Drug Under Investigation | Aim of The Trial. | Status of Clinical Trial | Location | Results from The Trial | References |
|---|---|---|---|---|---|
| Eculizumab | To assess whether by modulating the activity of immune response with Eculizumab, can mortality be halted while the patient has time to recover from the virus with supportive medical care. | Recruiting | United States, France | No results posted | [103] |
5.2. Immunomodulators against cytokine storm
During SARS-CoV-2 induced cytokine storm, specific cytokines are being observed in patient blood samples. So, therapeutically it is beneficial to target those specific cytokines in treating and preventing cytokine storm-induced complications, thus several drugs are now in the clinical trials (Table 6 ).
Table 6.
Clinical trial data on immunomodulators in COVID-19.
| Drug Under Investigation | Aim of The Trial | Status of Clinical Trial | Location | Results from The Trial | References |
|---|---|---|---|---|---|
| Anakinra (IL-1 antagonist) | To determine the therapeutic efficacy and tolerance of Anakinra in patients with moderate, severe pneumonia or critical pneumonia associated with COVID-19. | Recruitment completed | France | No results posted | [104] |
| To assess the efficacy of Anakinra in the Management of COVID-19 patients. | Recruiting | Qatar | No results posted | [105] | |
| Tocilizumab (IL-6 antagonist) | To study the efficacy and tolerability of Tocilizumab in the treatment of patients with COVID-19 pneumonia. | Active, not recruiting | Italy | No results posted. | [106] |
| To evaluate the efficacy, safety, pharmacodynamics, and pharmacokinetics of Tocilizumab compared with a matching placebo in combination with standard of care (SOC) in hospitalized patients with severe COVID-19 pneumonia. | Recruitment Completed | Canada, France, Denmark, Spain, UK, United states | In this randomized trial which involved hospitalized severe Covid-19 pneumonia patients, the use of tocilizumab didn’t result in significantly better clinical status or lower mortality than placebo at 28 days. | [107] | |
| Pegylated interferon-α2b (Interferon antagonist) | To evaluate the efficacy and safety of Pegylated Interferon -α2b in the treatment of adult patients diagnosed with SARS-CoV2. | Recruiting | Mexico | No results posted. | [108] |
| To evaluate the efficacy of a single dose of subcutaneous injections of 180 ug of Peginterferon Lambda-1a, compared with placebo in reducing the duration of viral shedding of SARS-CoV-2 virus in uncomplicated patients. | Active, not recruiting | United States | No results posted. | [109] | |
| To investigate the efficacy of a single 180 µg subcutaneous injection of peginterferon lambda or placebo in outpatients with COVID-19. | Recruiting | Canada | Peginterferon lambda increased viral decline in COVID 19 outpatients. Increased the proportion of patients with viral clearance by day 7, particularly in those with high baseline viral load. It prevents clinical deterioration and shorten duration of viral shedding. | [110] |
6. Cell entry inhibitors
6.1. TMPRSS2 inhibitors (Camostat, Nafamostat, Aprotinin, Bromhexine, Bicalutamide)
As TMPRSS2 protease plays a pivotal role in the novel coronavirus cell entry by lysing SP and ACE-2 along with ADAM-17. Drugs that inhibit TMPRSS2 can act as a potential treatment in COVID-19 therapy. TMPRSS2 inhibitors can partially block SARS-CoV-2-SP driven cell entry (Fig. 1A). Nafamostat is already an established drug in treating COVID-19 unrelated conditions such as chronic pancreatitis, and prostate cancer, in many parts of the world [111]. Hoffmann et al. is the first to provide evidence from their study that blocking of TMPRSS2 by Camostat significantly reduced SARS-CoV-2 infection into lungs [17]. Later Yamamoto et al., by their quantitative fusion assay reported that Nafamostat is more potentially blocking viral entry when compared to Camostat [112]. Many clinical trials are currently ongoing, to evaluate the more suitability of these agents in treating COVID-19 (Table 7 ).
Table 7.
Clinical trial data on TMPRSS2 inhibitors in COVID-19.
| Drug Under Investigation | Aim of The Trial. | Status of Clinical Trial | Location | Results from The Trial | References |
|---|---|---|---|---|---|
| Camostat | To determine the therapeutic effect and tolerance of Camostat mesylate, compared to placebo in adult patients with ambulatory COVID-19 disease | Recruiting | France | No results posted | [113] |
| To assess the impact of Camostat in COVID-19 disease. | Active, not recruiting | Denmark, Sweden | No results posted | [114] | |
| The assess the potential of Oral Camostat in Early COVID-19 Disease in an Ambulatory Setting to Reduce Viral Load and Disease Burden | Recruiting | Belgium | No results posted | [115] | |
| To determine if camostat can reduce the clinical progression of COVID-19 and therefore the need for hospital admission and supplemental oxygen. | Recruiting | United Kingdom | No results posted. | [116] | |
| Convalescent plasma and Camostat mesylate. | To evaluate the safety and efficacy of convalescent serum (CP) or Camostat mesylate with control or placebo in adult patients diagnosed with SARS-CoV-2 and high risk for moderate/severe COVID-19. | Recruiting | Germany | No results posted | [117] |
6.2. ADAM-17 inhibitors (INCB7839, ZLDI 8)
ADAM-17 inhibitors have a well-established role in their usage in oncology, and other immune disorders. Along with ACE-2, ADAM-17 enzyme also cleaves TNF precursors and ectodomains of many membrane-bound cytokines and other inflammatory mediators; thus, inhibiting ADAM-17 may exert protective inhibition against COVID-19, both in terms of cell entry (Fig. 1A) and cytokine storm, however, studies and trials are lacking on them [118], [119].
6.3. Cathepsin L inhibitors (Teicoplanin) and trypsin inhibitors (Ulinastatin, Aprotinin, α-1 antitrypsin
Many studies are evidencing that Cathepsin L (Cat L) and Trypsin is essential to cleave spike protein, in the endosomal pathway, and aids the virus to bind with the ACE-2 receptor, whereas the role of other proteases such as elastase is still under investigation. The use of Cathepsin (Fig. 1B) and trypsin inhibitors may add therapeutic benefits to the COVID-19 therapy. Liu et al. proved that combined use of TMPRSS2 and Cat L inhibitors can block coronavirus host cell entry and intracellular replication, without affecting the host immune system [120]. Bojkova et al. analyzed and proved that Aprotinin significantly inhibits viral entry in culture cells, thus providing evidence that they may play an important role in COVID-19 therapy [71]. Currently, Teicoplanin is under clinical trial to assess its effect against COVID-19 (Table 8 ).
Table 8.
Clinical trial data on Cathepsin inhibitors in COVID-19.
| Drug Under Investigation | Aim of The Trial. | Status of Clinical Trial | Location | Results from The Trial | References |
|---|---|---|---|---|---|
| Teicoplanin | To Evaluate the effect of Ticoplanin in patients with COVID-19 | Recruitment complete | Iran | No results posted | [121] |
7. Discussion and future directions
Cytokine storm is a hazardous systemic inflammatory syndrome that involves overexpression of circulating cytokines resulting in immune cell activation, adhesion and transmigration that sets off by monogenic abnormalities, certain drug therapies, pathogen invasion, cancers, and autoimmune states. COVID-19 infection triggers an inflammatory response that includes the release of a huge number of pro-inflammatory cytokines. A number of research examining cytokine profiles from COVID-19 patients found that the cytokine storm was linked to lung damage, multi-organ failure, and a hostile prognosis in serious COVID-19 patients [38], [122], [123].
Among the proinflammatory mediators IL-1, IL-6, TNF-α, and CCL2/MCP-1 are considered as the important cytokines of the innate immune feedback [30], [124]. Principal sources of these cytokines are the macrophages, mast cells, neutrophils, endothelial cells, and epithelial cells [30], [124], [125]. Overexpression of the complement protein C5a also has been found crucial in the ARDS development in COVID-19 patients [124]. Upregulation of these mediators in the body causes cellular recruitment of the leukocytes especially neutrophils, monocytes/macrophages, and T-cells to the site of infection/injury, and consequently damage the vascular endothelium, alveolar cell linings, multiple organs, and finally take towards death. Lung abnormality, especially ARDS is one of the severe health conditions found in the COVID-19 patients [124]. Certain mechanism of development of ARDS in the COVID-19 patients is still under investigation, even then cytokine storm is considered as the principal factors in this disease severity. To use drugs to reduce the disease severity, several approaches should be considered.
Primarily, it was reported that children specially under teenage or teenagers are less affected by the SARS-CoV-2, whereas a recent study on children with inflammatory syndrome with COVID-19, showed elevated levels of expression of IL-1β, IL-6, IL-8, IL-17, and IFN-γ on myeloid cells [126]. In case of management of the inflammatory syndrome in children, resisting the cytokines by administrating anti-inflammatory cytokines (such as IL-37 and IL-38) have been suggested, rather than using other anti-viral drugs as a crucial treatment aspect [127].
Various immunoregulatory management strategies have been taken in action to resist cytokines mostly found in the COVID-19 patients are IL-6- [112], [113], [114] or IL-1 –receptor antagonists [128]. Since, several other cytokines have been found prominent in the COVID-19 patients, treatment targeting only a single pro-inflammatory factor has arisen question on the management strategy of the uncontrollable cytokine storm.
In a recent report, crosstalk between IL-6 with the STAT and NF-κB has been manifested in the COVID-19 disease burden [129]. Several recent literatures revealed that the cytokines involved in the COVID-19 mostly are IL-6, IL-18, IFN-γ, IL-15, TNF-α, IL-1α, IL-1β, and IL-2, where none of them singly high concentration gradient to exert the pro-inflammatory effects as well as cellular death. At the same time, when TNF-α along with IFN-γ was administered, that suggested synergistic effects between several other cytokines on the targeted cell levels [130].
Although, several signaling pathways can be involved in the production of inflammatory cytokines, i.e. JAK/STAT, p38 MAPK, along with other MAPKs [131], NF-κB signaling pathway has been reported as the central player to produce most of the pro-inflammatory cytokines associated with SARS-CoV-2 infection. Particularly, inflammatory mediators such as IL-1, IL-6, TNF-α, CCL2/MCP-1, CCL3/MIP-1α, and −1β/CCL4 are expressed through this pathway during acute stage of COVID-19 patients [52].
However, JAK signaling pathway inhibition has been considered as an important treatment strategy against cytokine storm in COVID-19 patients [132], whereas NF-κB signaling inhibition has been found inhibiting dominant inflammatory cytokines/chemokines such as IL-1, IL-6, CCL2/MCP-1, and TNF-α, which are mainly related with the disease exacerbation at cellular level [133] associated with the SARS-CoV-2 infection, rather than cytokines which responds initially to the antiviral treatments e.g., IFN-γ [52], [134], [135] which is primarily JAK/STAT signaling pathway dependent.
Despite the fact that the cytokine storm is not the primary focus of any of the medications commonly used to treat COVID-19, there is increasing evidence that it may have a substantial impact on the disease progression especially in patients with severe condition [136], [137]. In the treatment of COVID-19 patients, several approaches are being followed now a day. The treatment options include anti-viral, anti-inflammatory, anti-cytokine, antibiotic/anti-parasitic, and ACE inhibitors/angiotensin receptor blockers. Most medicines recommended for the treatment of COVID-19 have an anti-inflammatory profile, and the bulk of them would reduce the levels of IL-6 and TNF-α, cytokines that are important targets for COVID-19 drugs [137]. Favipiravir acts to reduce the level of TNF-α [138]; immunomodulatory antiviral drug IFN-α2b inhibits the replication of SARS-CoV, and increase the level of IL-10 [139], and reduce the level of TNF-α [140], Remdesivir lowers the levels of IL-1β, IL-6, and TNF-α [137], [141], [142]; antineoplastic drug Ruxolitinib reduces the IL-6 and TNF-α level by inhibiting the JAK signaling pathway [88], [143].
In addition, Azithromycine, Ivermectin, Corticosteroids, Hydroxychloroquines are being used broadly in different countries, based on the previously discovered basic mechanism of action as immunosuppressor or anti-inflammatory effects, and act on reducing levels of IL-1β, IL-6, and TNF-α [137]; however, concrete data on the effects against the COVID-19 such as multiple in vivo data or clinical trial data have not been revealed to date may be due to lack of time or medical facilities.
However, several reports claimed combination therapy as the effective ways of treating critical COVID-19 patients. An in vitro study by Wang et al. showed the use of Remdesivir and Chloroquine as a combination therapy may control the SARS-CoV-2 infection [141]. Whereas Cantini et al. proposed in their systematic review that Remdesivir, Dexamethasone, and Baricitinib are the best combination therapy applicable in multiple steps of the disease progression. On the other hand, Sarilumab, Ruxolitinib, and Baricitinib have been withdrawn based on their primary data as having extra immunosuppressive properties [144], [145].
Taken together, in this article we tried to shed a light on the recent management strategies of COVID-19 in the context of cytokine storm induced disease burden. Although, it is considered that vaccines are the best way to prevent the pandemic situation, due to insufficient production and distribution facilities compared with the world demand, prevention are not being gained successfully within a short. At the same time, several reports claiming the side effects about the vaccines in various countries, although concrete proof has not been found, people became confused to take the vaccine for their protection against COVID-19. Therefore, repurposing of drugs has become the major concern to lessen disease severity and mortality, although during convalescent plasma treatment, there was evidence of negative viral loading, proper source/donor is difficult sometimes when needed. Similarly, selecting treatment strategies based on concrete research is recommended. In fine, when treating the SARS-CoV-2 infected patients, proper knowledge on the drug safety and use, drug-drug interaction, side effects, and patient tolerance must be considered as the priority to reduce the post-treatment drug-caused health hazard.
CRediT authorship contribution statement
Haripriya Sunkara: Methodology, Writing - Original Draft, Visualization. Syed Masudur Rahman Dewan: Conceptualization, Methodology, Writing - Original Draft, Writing - Review & Editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to pay tribute to all front-liners who are devoted and involved in the treatment of COVID-19 patients. They would also like to thank fellow researchers working to combat the current pandemic situation for humankind.
References
- 1.Liu Y.-C., Kuo R.-L., Shih S.-R. COVID-19: The first documented coronavirus pandemic in history. Biomed. J. 2020;43:328–333. doi: 10.1016/j.bj.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Decaro N., Lorusso A. Novel human coronavirus (SARS-CoV-2): A lesson from animal coronaviruses. Vet. Microbiol. 2020;244 doi: 10.1016/j.vetmic.2020.108693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 Map, Johns Hopkins Coronavirus Resour. Cent., n.d. https://coronavirus.jhu.edu/map.html (accessed February 23, 2021).
- 4.Berekaa M.M. Insights into the COVID-19 pandemic: Origin, pathogenesis, diagnosis, and therapeutic interventions. Front. Biosci. Elite Ed. 2021;13:117–139. doi: 10.2741/874. [DOI] [PubMed] [Google Scholar]
- 5.C.J. Burrell, C.R. Howard, F.A. Murphy, Fenner and White’s medical virology, fifth ed., Elsevier/AP, Academic Press is an imprint of Elsevier, Amsterdam ; Boston, 2017.
- 6.Chen L., Liu B., Yang J., Jin Q. DBatVir: the database of bat-associated viruses. Database J. Biol. Databases Curation. 2014;2014 doi: 10.1093/database/bau021. bau021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karia R., Gupta I., Khandait H., Yadav A., Yadav A. COVID-19 and its modes of transmission. SN Compr. Clin. Med. 2020:1–4. doi: 10.1007/s42399-020-00498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent J.-L., Taccone F.S. Understanding pathways to death in patients with COVID-19. Lancet Respir. Med. 2020;8:430–432. doi: 10.1016/S2213-2600(20)30165-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn J.S., McIntosh K. History and recent advances in coronavirus discovery. Pediatr. Infect. Dis. J. 2005;24:223–227. doi: 10.1097/01.inf.0000188166.17324.60. discussion S226. [DOI] [PubMed] [Google Scholar]
- 10.Lauxmann M.A., Santucci N.E., Autrán-Gómez A.M. The SARS-CoV-2 Coronavirus and the COVID-19 Outbreak. Int. Braz. J. Urol. 2020;46:6–18. doi: 10.1590/s1677-5538.ibju.2020.s101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wise J. Covid-19: New coronavirus variant is identified in UK. BMJ. 2020:m4857. doi: 10.1136/bmj.m4857. [DOI] [PubMed] [Google Scholar]
- 12.Ji T., Liu Z., Wang G., Guo X., Akbar Khan S., Lai C., Chen H., Huang S., Xia S., Chen B., Jia H., Chen Y., Zhou Q. Detection of COVID-19: a review of the current literature and future perspectives. Biosens. Bioelectron. 2020;166 doi: 10.1016/j.bios.2020.112455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittal A., Manjunath K., Ranjan R.K., Kaushik S., Kumar S., Verma V. COVID-19 pandemic: insights into structure, function, and hACE2 receptor recognition by SARS-CoV-2. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou M.-Y., Xie X.-L., Peng Y.-G., Wu M.-J., Deng X.-Z., Wu Y., Xiong L.-J., Shang L.-H. From SARS to COVID-19: what we have learned about children infected with COVID-19. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020;96:710–714. doi: 10.1016/j.ijid.2020.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao P., Praissman J.L., Grant O.C., Cai Y., Xiao T., Rosenbalm K.E., Aoki K., Kellman B.P., Bridger R., Barouch D.H., Brindley M.A., Lewis N.E., Tiemeyer M., Chen B., Woods R.J., Wells L. Virus-receptor interactions of glycosylated SARS-CoV-2 spike and human ACE2 receptor. BioRxiv Prepr. Serv. Biol. 2020 doi: 10.1101/2020.06.25.172403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung J.M., Yang C.X., Tam A., Shaipanich T., Hackett T.-L., Singhera G.K., Dorscheid D.R., Sin D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur. Respir. J. 2020;55:2000688. doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K., Chen W., Zhou Y.-S., Lian J.-Q., Zhang Z., Du P., Gong L., Zhang Y., Cui H.-Y., Geng J.-J., Wang B., Sun X.-X., Wang C.-F., Yang X., Lin P., Deng Y.-Q., Wei D., Yang X.-M., Zhu Y.-M., Zhang K., Zheng Z.-H., Miao J.-L., Guo T., Shi Y., Zhang J., Fu L., Wang Q.-Y., Bian H., Zhu P., Chen Z.-N. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. Microbiology. 2020 doi: 10.1101/2020.03.14.988345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J., Hume A.J., Abo K.M., Werder R.B., Villacorta-Martin C., Alysandratos K.-D., Beermann M.L., Simone-Roach C., Lindstrom-Vautrin J., Olejnik J., Suder E.L., Bullitt E., Hinds A., Sharma A., Bosmann M., Wang R., Hawkins F., Burks E.J., Saeed M., Wilson A.A., Mühlberger E., Kotton D.N. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell. 2020;27 doi: 10.1016/j.stem.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daly J.L., Simonetti B., Klein K., Chen K.-E., Williamson M.K., Antón-Plágaro C., Shoemark D.K., Simón-Gracia L., Bauer M., Hollandi R., Greber U.F., Horvath P., Sessions R.B., Helenius A., Hiscox J.A., Teesalu T., Matthews D.A., Davidson A.D., Collins B.M., Cullen P.J., Yamauchi Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moutal A., Martin L.F., Boinon L., Gomez K., Ran D., Zhou Y., Stratton H.J., Cai S., Luo S., Gonzalez K.B., Perez-Miller S., Patwardhan A., Ibrahim M.M., Khanna R. SARS-CoV-2 spike protein co-opts VEGF-A/neuropilin-1 receptor signaling to induce analgesia. Neuroscience. 2020 doi: 10.1101/2020.07.17.209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J., Xu X., Jiang L., Dua K., Hansbro P.M., Liu G. SARS-CoV-2 induces transcriptional signatures in human lung epithelial cells that promote lung fibrosis. Respir. Res. 2020;21:182. doi: 10.1186/s12931-020-01445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinto B.G.G., Oliveira A.E.R., Singh Y., Jimenez L., Gonçalves A.N.A., Ogava R.L.T., Creighton R., Peron J.P.S., Nakaya H.I. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. MedRxiv Prepr. Serv. Health Sci. 2020 doi: 10.1101/2020.03.21.20040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmoud I.S., Jarrar Y.B., Alshaer W., Ismail S. SARS-CoV-2 entry in host cells-multiple targets for treatment and prevention. Biochimie. 2020;175:93–98. doi: 10.1016/j.biochi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zipeto D., Palmeira J. da F., Argañaraz G.A., Argañaraz E.R. ACE2/ADAM17/TMPRSS2 interplay may be the main risk factor for COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.576745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ullrich S., Nitsche C. The SARS-CoV-2 main protease as drug target. Bioorg. Med. Chem. Lett. 2020;30 doi: 10.1016/j.bmcl.2020.127377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y., Yin W., Xu H.E. RNA-dependent RNA polymerase: structure, mechanism, and drug discovery for COVID-19. Biochem. Biophys. Res. Commun. 2021;538:47–53. doi: 10.1016/j.bbrc.2020.08.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milanetti E., Miotto M., Di Rienzo L., Monti M., Gosti G., Ruocco G. In-Silico evidence for two receptors based strategy of SARS-CoV-2. Phys. Q-Bio. 2020 doi: 10.3389/fmolb.2021.690655. http://arxiv.org/abs/2003.11107 ArXiv200311107 (accessed February 24, 2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; What we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front. Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinha P., Matthay M.A., Calfee C.S. Is a “Cytokine Storm” relevant to COVID-19? JAMA Intern. Med. 2020;180:1152. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 33.Fajgenbaum D.C., June C.H. Cytokine storm. N. Engl. J. Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2 doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satarker S., Tom A.A., Shaji R.A., Alosious A., Luvis M., Nampoothiri M. JAK-STAT pathway inhibition and their implications in COVID-19 therapy. Postgrad. Med. 2020:1–19. doi: 10.1080/00325481.2020.1855921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pahan P., Pahan K. Smooth or risky revisit of an old malaria drug for COVID-19? J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2020;15:174–180. doi: 10.1007/s11481-020-09923-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hariharan A., Hakeem A.R., Radhakrishnan S., Reddy M.S., Rela M. The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 patients. Inflammopharmacology. 2021;29:91–100. doi: 10.1007/s10787-020-00773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 20*19 novel coronavirus in Wuhan. China, The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta. 1843;2014:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 40.M.J. Cameron, D.J. Kelvin, Cytokines, Chemokines and Their Receptors, Landes Bioscience, 2013. https://www.ncbi.nlm.nih.gov/books/NBK6294/ (accessed February 24, 2021).
- 41.Wenjun W., Xiaoqing L., Sipei W., Puyi L., Liyan H., Yimin L., Linling C., Sibei C., Lingbo N., Yongping L., Jianxing H. The definition and risks of Cytokine Release Syndrome-Like in 11 COVID-19-Infected Pneumonia critically ill patients: disease characteristics and retrospective analysis. Intens. Care Critical Care Med. 2020 doi: 10.1101/2020.02.26.20026989. [DOI] [Google Scholar]
- 42.Liu Y., Holdbrooks A.T., De Sarno P., Rowse A.L., Yanagisawa L.L., McFarland B.C., Harrington L.E., Raman C., Sabbaj S., Benveniste E.N., Qin H. Therapeutic efficacy of suppressing the Jak/STAT pathway in multiple models of experimental autoimmune encephalomyelitis. J. Immunol. Baltim. Md. 2014;1950(192):59–72. doi: 10.4049/jimmunol.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lorenzo J. Osteoimmunology. Elsevier; 2016. The effects of immune cell products (Cytokines and Hematopoietic Cell Growth Factors) on bone cells; pp. 143–167. [DOI] [Google Scholar]
- 44.F. van Roy, V. Nimmrich, A. Bespalov, A. Möller, H. Hara, J.P. Turowec, N.A. St. Denis, D.W. Litchfield, D. Boucher, J.-B. Denault, K. Matsuda, M. Yuzaki, C. Repeke, T. Garlet, A.P. Trombone, G. Garlet, C. Repeke, T. Garlet, E.M. Silveira, G. Garlet, T. Garlet, C. Repeke, A. Vieira, F. Cunha, G. Garlet, S. Kubota, M. Takigawa, H. Soares, S. Nolasco, J. Gonçalves, A. Bensussan, A. Marie-Cardine, S. Deswal, W.W.A. Schamel, L. Santos-Argumedo, S. Deswal, W.W.A. Schamel, G.A. Bishop, D.A. Decker, B.S. Hostager, M.E. Bravo-Adame, M.A. Sandoval-Hernandez, O.A. Migueles-Lozano, Y. Rosenstein, P. Johnson, A. Samarakoon, A.E. Saunders, K.W. Harder, D.D. Roberts, D.R. Soto-Pantoja, J.S. Isenberg, P.A. Lazo, R. Barcia, H.-J. Wu, N. Muthusamy, S. Bondada, S. Levy, S. Pawaria, R.J. Binder, H. Masai, D. Hu, J.M. Lahti, B.B. Singer, R. Horuk, L.J. Miller, J. Morisset, D.W. Litchfield, A.R. Mistry, C.A. O’Callaghan, A.E. Fenton-May, C.A. O’Callaghan, A.R. Mistry, C.A. O’Callaghan, M. Reschen, C.A. O’Callaghan, J.A. Willment, G.D. Brown, L. Rabinow, S.A. Ness, C.E. Creutz, N. Yagishita-Kyo, M. Inoue, M. Nonaka, H. Okuno, H. Bito, M. Okada, H.-C. Cheng, M.I. Hossain, M.A. Kamaruddin, Y.-P. Chong, B. Sen, F.M. Johnson, P.A. Pino, A.E. Cardona, F. Paroni, K. Maedler, R.Y.C. Poon, CCL5, in: S. Choi (Ed.), Encycl. Signal. Mol., Springer New York, New York, NY, 2012, pp. 269–273. 10.1007/978-1-4419-0461-4_580. [DOI]
- 45.Esmaeilzadeh A., Elahi R. Immunobiology and immunotherapy of COVID-19: A clinically updated overview. J. Cell. Physiol. 2021;236:2519–2543. doi: 10.1002/jcp.30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodríguez-Carballo E., Gámez B., Ventura F. p38 MAPK signaling in osteoblast differentiation. Front. Cell Dev. Biol. 2016;4 doi: 10.3389/fcell.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuadrado A., Nebreda A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 48.Lei X., Dong X., Ma R., Wang W., Xiao X., Tian Z., Wang C., Wang Y., Li L., Ren L., Guo F., Zhao Z., Zhou Z., Xiang Z., Wang J. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 2020;11:3810. doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nunes A.T., Annunziata C.M. Proteasome inhibitors: structure and function. Semin. Oncol. 2017;44:377–380. doi: 10.1053/j.seminoncol.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerard C., Rollins B.J. Chemokines and disease. Nat. Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 51.Ingraham N.E., Lotfi-Emran S., Thielen B.K., Techar K., Morris R.S., Holtan S.G., Dudley R.A., Tignanelli C.J. Immunomodulation in COVID-19. Lancet Respir. Med. 2020;8:544–546. doi: 10.1016/S2213-2600(20)30226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kircheis R., Haasbach E., Lueftenegger D., Heyken W.T., Ocker M., Planz O. NF-κB pathway as a potential target for treatment of critical stage COVID-19 patients. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.598444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu A.C.-Y., Wang G., Reid A.T., Veerati P.C., Pathinayake P.S., Daly K., Mayall J.R., Hansbro P.M., Horvat J.C., Wang F., Wark P.A. SARS-CoV-2 Spike protein promotes hyper-inflammatory response that can be ameliorated by Spike-antagonistic peptide and FDA-approved ER stress and MAP kinase inhibitors in vitro. Immunology. 2020 doi: 10.1101/2020.09.30.317818. [DOI] [Google Scholar]
- 54.Sohn K.M., Lee S.-G., Kim H.J., Cheon S., Jeong H., Lee J., Kim I.S., Silwal P., Kim Y.J., Paik S., Chung C., Park C., Kim Y.-S., Jo E.-K. COVID-19 patients upregulate toll-like receptor 4-mediated inflammatory signaling that mimics bacterial sepsis. J. Korean Med. Sci. 2020;35 doi: 10.3346/jkms.2020.35.e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pålsson-McDermott E.M., O’Neill L.A.J. Signal transduction by the lipopolysaccharide receptor, toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Azam S., Jakaria M., Kim I.-S., Kim J., Haque M.E., Choi D.-K. Regulation of Toll-Like Receptor (TLR) signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: focus on TLR4 signaling. Front. Immunol. 2019;10:1000. doi: 10.3389/fimmu.2019.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patra R., Chandra Das N., Mukherjee S. Targeting human TLRs to combat COVID-19: a solution? J. Med. Virol. 2021;93:615–617. doi: 10.1002/jmv.26387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aboudounya M.M., Heads R.J. COVID-19 and toll-like receptor 4 (TLR4): SARS-CoV-2 may bind and activate TLR4 to increase ACE2 expression, facilitating entry and causing hyperinflammation. Mediators Inflamm. 2021;2021:8874339. doi: 10.1155/2021/8874339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I., Kritas S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34:327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 60.Totura A.L., Whitmore A., Agnihothram S., Schäfer A., Katze M.G., Heise M.T., Baric R.S. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. MBio. 2015;6:e00638–00615. doi: 10.1128/mBio.00638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khadke S., Ahmed N., Ahmed N., Ratts R., Raju S., Gallogly M., de Lima M., Sohail M.R. Harnessing the immune system to overcome cytokine storm and reduce viral load in COVID-19: a review of the phases of illness and therapeutic agents. Virol. J. 2020;17:154. doi: 10.1186/s12985-020-01415-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Proud P.C., Tsitoura D., Watson R.J., Chua B.Y., Aram M.J., Bewley K.R., Cavell B.E., Cobb R., Dowall S., Fotheringham S.A., Ho C.M.K., Lucas V., Ngabo D., Rayner E., Ryan K.A., Slack G.S., Thomas S., Wand N.I., Yeates P., Demaison C., Zeng W., Holmes I., Jackson D.C., Bartlett N.W., Mercuri F., Carroll M.W. Prophylactic intranasal administration of a TLR2/6 agonist reduces upper respiratory tract viral shedding in a SARS-CoV-2 challenge ferret model. EBioMedicine. 2021;63 doi: 10.1016/j.ebiom.2020.103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y., Kim S.C., Yu T., Yi Y.-S., Rhee M.H., Sung G.-H., Yoo B.C., Cho J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/352371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.More S., Yang X., Zhu Z., Bamunuarachchi G., Guo Y., Huang C., Bailey K., Metcalf J.P., Liu L. Regulation of influenza virus replication by Wnt/β-catenin signaling. PloS One. 2018;13 doi: 10.1371/journal.pone.0191010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi E.Y., Park H.H., Kim H., Kim H.N., Kim I., Jeon S., Kim W., Bae J.-S., Lee W. Wnt5a and Wnt11 as acute respiratory distress syndrome biomarkers for severe acute respiratory syndrome coronavirus 2 patients. Eur. Respir. J. 2020;56 doi: 10.1183/13993003.01531-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deng X., Jin K., Li Y., Gu W., Liu M., Zhou L. Platelet-derived growth factor and transforming growth factor β1 regulate ARDS-associated lung fibrosis through distinct signaling pathways. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015;36:937–946. doi: 10.1159/000430268. [DOI] [PubMed] [Google Scholar]
- 67.Ganeshan K., Johnston L.K., Bryce P.J. TGF-β1 limits the onset of innate lung inflammation by promoting mast cell-derived IL-6. J. Immunol. Baltim. Md. 2013;1950(190):5731–5738. doi: 10.4049/jimmunol.1203362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akbarshahi H., Sam A., Chen C., Rosendahl A.H., Andersson R. Early activation of pulmonary TGF-β1/Smad2 signaling in mice with acute pancreatitis-associated acute lung injury. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/148029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Java A., Apicelli A.J., Liszewski M.K., Coler-Reilly A., Atkinson J.P., Kim A.H., Kulkarni H.S. The complement system in COVID-19: friend and foe? JCI Insight. 2020;5 doi: 10.1172/jci.insight.140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stahel P.F., Barnum S.R. Complement inhibition in coronavirus disease (COVID)-19: a neglected therapeutic option. Front. Immunol. 2020;11:1661. doi: 10.3389/fimmu.2020.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bojkova D., Bechtel M., McLaughlin K.-M., McGreig J.E., Klann K., Bellinghausen C., Rohde G., Jonigk D., Braubach P., Ciesek S., Münch C., Wass M.N., Michaelis M., Cinatl J. Aprotinin inhibits SARS-CoV-2 replication. Cells. 2020;9 doi: 10.3390/cells9112377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ren Z., Wang L., Cui J., Huoc Z., Xue J., Cui H., Mao Q., Yang R. Resveratrol inhibits NF-kB signaling through suppression of p65 and IkappaB kinase activities. Pharm. 2013;68:689–694. [PubMed] [Google Scholar]
- 73.Karunaweera N., Raju R., Gyengesi E., Münch G. Plant polyphenols as inhibitors of NF-ÎoB induced cytokine production—a potential anti-inflammatory treatment for Alzheimer’s disease? Front. Mol. Neurosci. 2015;8 doi: 10.3389/fnmol.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qin J.-D., Cao Z.-H., Li X.-F., Kang X.-L., Xue Y., Li Y.-L., Zhang D., Liu X.-Y., Xue Y.-Z. Effect of ammonium pyrrolidine dithiocarbamate (PDTC) on NF-κB activation and CYP2E1 content of rats with immunological liver injury. Pharm. Biol. 2014;52:1460–1466. doi: 10.3109/13880209.2014.898075. [DOI] [PubMed] [Google Scholar]
- 75.IRCT | Effect of curcumin-piperine supplementation on disease duration, severity and clinical signs, and inflammatory factors in patients with coronavirus (COVID-19): A randomized, double-blind, placebo-controlled clinical trial study, n.d. https://www.irct.ir/trial/47529 (accessed March 1, 2021).
- 76.Hassaniazad M., Inchehsablagh B.R., Kamali H., Tousi A., Eftekhar E., Jaafari M.R., Fathalipour M., Nikoofal-Sahlabadi S., Gouklani H., Alizade H., Nikpoor A.R. The clinical effect of Nano micelles containing curcumin as a therapeutic supplement in patients with COVID-19 and the immune responses balance changes following treatment: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:876. doi: 10.1186/s13063-020-04824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saber-Moghaddam N., Salari S., Hejazi S., Amini M., Taherzadeh Z., Eslami S., Rezayat S.M., Jaafari M.R., Elyasi S. Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease -19 patients: an open label nonrandomized clinical trial. Phytother. Res. 2021:ptr.7004. doi: 10.1002/ptr.7004. [DOI] [PubMed] [Google Scholar]
- 78.IRCT | Evaluation of the effect of Resveratrol on the effectiveness of antiviral drug regimen in patients with COVID-19, n.d. https://en.irct.ir/trial/48330 (accessed March 1, 2021).
- 79.M.M. MD, Randomized Double-Blind Placebo-Controlled Proof-of-Concept Trial of a Plant Polyphenol for the Outpatient Treatment of Mild Coronavirus Disease (COVID-19), clinicaltrials.gov, 2020. https://clinicaltrials.gov/ct2/show/NCT04400890 (accessed February 28, 2021).
- 80.Miller S.C., Huang R., Sakamuru S., Shukla S.J., Attene-Ramos M.S., Shinn P., Van Leer D., Leister W., Austin C.P., Xia M. Identification of known drugs that act as inhibitors of NF-κB signaling and their mechanism of action. Biochem. Pharmacol. 2010;79:1272–1280. doi: 10.1016/j.bcp.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin Z., Wu D., Huang L., Jiang C., Pan T., Kang X., Pan J. Nobiletin inhibits IL-1β-induced inflammation in chondrocytes via suppression of NF-κB signaling and attenuates osteoarthritis in mice. Front. Pharmacol. 2019;10:570. doi: 10.3389/fphar.2019.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grimes J.M., Grimes K.V. p38 MAPK inhibition: a promising therapeutic approach for COVID-19. J. Mol. Cell. Cardiol. 2020;144:63–65. doi: 10.1016/j.yjmcc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fulcrum Therapeutics, A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of the Safety and Efficacy of Losmapimod in Adult Subjects With COVID-19 (LOSVID STUDY), clinicaltrials.gov, 2021. https://clinicaltrials.gov/ct2/show/results/NCT04511819 (accessed February 28, 2021).
- 84.Watson A., Madsen J., Clark H.W. SP-A and SP-D: dual functioning immune molecules with antiviral and immunomodulatory properties. Front. Immunol. 2021;11 doi: 10.3389/fimmu.2020.622598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bollag W.B., Gonzales J.N. Phosphatidylglycerol and surfactant: a potential treatment for COVID-19? Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.University Hospital Southampton NHS Foundation Trust, A Clinical Trial of Nebulized Surfactant for the Treatment of Moderate to Severe COVID-19, clinicaltrials.gov, 2020. https://clinicaltrials.gov/ct2/show/NCT04362059 (accessed February 28, 2021).
- 87.L.L.C. Biosurf, Open-label Trial to Assess the Efficacy and Safety of Inhalation Use of the Approved Drug Surfactant-BL (Biosurf LLC, Russia) as a Part of Complex Therapy of Acute Respiratory Distress Syndrome (ARDS) in Patients With SARS-CoV-2 Coronavirus Infection (COVID-19), clinicaltrials.gov, 2020. https://clinicaltrials.gov/ct2/show/NCT04568018 (accessed February 28, 2021).
- 88.Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L., Huang L., Meng F., Huang L., Wang N., Zhou X., Luo H., Mao Z., Chen X., Xie J., Liu J., Cheng H., Zhao J., Huang G., Wang W., Zhou J. Ruxolitinib in treatment of severe coronavirus disease, (COVID-19): a multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 2019;146(2020):137–146.e3. doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.A Study of Baricitinib (LY3009104) in Participants With COVID-19 - Tabular View - ClinicalTrials.gov, n.d. https://clinicaltrials.gov/ct2/show/record/NCT04421027 (accessed March 2, 2021).
- 90.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., Marconi V.C., Ruiz-Palacios G.M., Hsieh L., Kline S., Tapson V., Iovine N.M., Jain M.K., Sweeney D.A., El Sahly H.M., Branche A.R., Regalado Pineda J., Lye D.C., Sandkovsky U., Luetkemeyer A.F., Cohen S.H., Finberg R.W., Jackson P.E.H., Taiwo B., Paules C.I., Arguinchona H., Erdmann N., Ahuja N., Frank M., Oh M., Kim E.-S., Tan S.Y., Mularski R.A., Nielsen H., Ponce P.O., Taylor B.S., Larson L., Rouphael N.G., Saklawi Y., Cantos V.D., Ko E.R., Engemann J.J., Amin A.N., Watanabe M., Billings J., Elie M.-C., Davey R.T., Burgess T.H., Ferreira J., Green M., Makowski M., Cardoso A., de Bono S., Bonnett T., Proschan M., Deye G.A., Dempsey W., Nayak S.U., Dodd L.E., Beigel J.H. Baricitinib plus remdesivir for hospitalized adults with Covid-19, N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2031994. NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hospital Israelita Albert Einstein, A Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-design Trial of Tofacitinib in Hospitalized Participants With COVID-19 Pneumonia, clinicaltrials.gov, 2021. https://clinicaltrials.gov/ct2/show/study/NCT04469114 (accessed February 28, 2021).
- 92.I.M. Sechenov First Moscow State Medical University, Efficacy and Safety of Tofacitinib in Patients With COVID-19 Pneumonia, clinicaltrials.gov, 2021. https://clinicaltrials.gov/ct2/show/study/NCT04750317 (accessed February 28, 2021).
- 93.Yale University, Investigation of Tofacitinib to Mitigate the Impact of COVID-19 (I-TOMIC) in Moderate SARS-CoV-2 (MODERATE I-TOMIC), clinicaltrials.gov, 2021. https://clinicaltrials.gov/ct2/show/NCT04415151 (accessed February 28, 2021).
- 94.Philipps University Marburg Medical Center, Ruxolitinib for Treatment of Covid-19 Induced Lung Injury ARDS A Single-arm, Open-label, Proof of Concept Study, clinicaltrials.gov, 2020. https://clinicaltrials.gov/ct2/show/study/NCT04359290 (accessed March 1, 2021).
- 95.P.D. med A. Hochhaus, A Phase-II Clinical Trial for First Line Treatment of Stage II/III Covid-19 Patients to Treat Hyperinflammation, clinicaltrials.gov, 2021. https://clinicaltrials.gov/ct2/show/NCT04338958 (accessed March 1, 2021).
- 96.Cheng X., Xu X., Chen D., Zhao F., Wang W. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed. Pharmacother. 2019;110:473–481. doi: 10.1016/j.biopha.2018.11.082. [DOI] [PubMed] [Google Scholar]
- 97.Voronkov A., Krauss S. Wnt/beta-catenin signaling and small molecule inhibitors. Curr. Pharm. Des. 2013;19:634–664. doi: 10.2174/138161213804581837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jung Y.-S., Park J.-I. Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp. Mol. Med. 2020;52:183–191. doi: 10.1038/s12276-020-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Akhurst R.J. Targeting TGF-β signaling for therapeutic gain. Cold Spring Harb. Perspect. Biol. 2017;9 doi: 10.1101/cshperspect.a022301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Horiuchi T., Tsukamoto H. Complement-targeted therapy: development of C5- and C5a-targeted inhibition. Inflamm. Regen. 2016;36:11. doi: 10.1186/s41232-016-0013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jayne D.R.W., Bruchfeld A.N., Harper L., Schaier M., Venning M.C., Hamilton P., Burst V., Grundmann F., Jadoul M., Szombati I., Tesař V., Segelmark M., Potarca A., Schall T.J., Bekker P. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J. Am. Soc. Nephrol. 2017;28:2756–2767. doi: 10.1681/ASN.2016111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao S., Cui Z., Zhao M. The complement C3a and C3a receptor pathway in kidney diseases. Front. Immunol. 2020;11:1875. doi: 10.3389/fimmu.2020.01875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alexion Pharmaceuticals, SOLIRIS® (Eculizumab) for the Treatment of Participants With Coronavirus Disease 2019 (COVID 19) – An Expanded Access Program for Hospital-based Emergency Treatment, clinicaltrials.gov, 2021. https://clinicaltrials.gov/ct2/show/NCT04355494 (accessed March 18, 2021).
- 104.Assistance Publique – Hôpitaux de Paris, CORIMUNO-ANA: Trial Evaluating Efficacy Of Anakinra In Patients With Covid-19 Infection, Nested In The CORIMUNO-19, clinicaltrials.gov, 2021. https://clinicaltrials.gov/ct2/show/study/NCT04341584 (accessed February 28, 2021).
- 105.Hamad Medical Corporation, Efficacy of Anakinra in the Management of Patients With COVID-19 Infection in Qatar: A Randomized Clinical Trial, clinicaltrials.gov, 2021. https://clinicaltrials.gov/ct2/show/NCT04643678 (accessed February 28, 2021).
- 106.National Cancer Institute, Naples, Multicenter Study on the Efficacy and Tolerability of Tocilizumab in the Treatment of Patients With COVID-19 Pneumonia, clinicaltrials.gov, 2020. https://clinicaltrials.gov/ct2/show/study/NCT04317092 (accessed February 28, 2021).
- 107.Rosas I.O., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., Skiest D., Aziz M.S., Cooper N., Douglas I.S., Savic S., Youngstein T., Del Sorbo L., Cubillo Gracian A., De La Zerda D.J., Ustianowski A., Bao M., Dimonaco S., Graham E., Matharu B., Spotswood H., Tsai L., Malhotra A. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pegylated Interferon – α2b With SARSCoV- 2 (COVID-19) – Tabular View – ClinicalTrials.gov, n.d. https://clinicaltrials.gov/ct2/show/record/NCT04480138 (accessed March 2, 2021).
- 109.Stanford University, A Phase 2 Randomized, Single-Blind Study of a Single Dose of Peginterferon Lambda-1a (Lambda) Compared With Placebo in Outpatients With Mild COVID-19, clinicaltrials.gov, 2020. https://clinicaltrials.gov/ct2/show/study/NCT04331899 (accessed February 28, 2021).
- 110.Feld J.J., Kandel C., Biondi M.J., Kozak R.A., Zahoor M.A., Lemieux C., Borgia S.M., Boggild A.K., Powis J., McCready J., Tan D.H.S., Chan T., Coburn B., Kumar D., Humar A., Chan A., O’Neil B., Noureldin S., Booth J., Hong R., Smookler D., Aleyadeh W., Patel A., Barber B., Casey J., Hiebert R., Mistry H., Choong I., Hislop C., Santer D.M., Lorne Tyrrell D., Glenn J.S., Gehring A.J., Janssen H.L.A., Hansen B.E. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir. Med. 2021 doi: 10.1016/S2213-2600(20)30566-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strope J.D., PharmD C.H.C., Figg W.D. TMPRSS2: potential biomarker for COVID-19 outcomes. J. Clin. Pharmacol. 2020;60:801–807. doi: 10.1002/jcph.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yamamoto M., Kiso M., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Takeda M., Kinoshita N., Ohmagari N., Gohda J., Semba K., Matsuda Z., Kawaguchi Y., Kawaoka Y., Inoue J. The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses. 2020;12:629. doi: 10.3390/v12060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.CAMOVID : Evaluation of Efficacy and Safety of Camostat Mesylate for the Treatment of SARS-CoV-2 Infection – COVID-19 in Ambulatory Adult Patients – Tabular View – ClinicalTrials.gov, n.d. https://clinicaltrials.gov/ct2/show/record/NCT04608266 (accessed March 22, 2021).
- 114.University of Aarhus, The Impact of Camostat Mesilate on COVID-19 Infection: An Investigator-initiated Randomized, Placebo-controlled, Phase IIa Trial, clinicaltrials.gov, 2021. https://clinicaltrials.gov/ct2/show/study/NCT04321096 (accessed March 18, 2021).
- 115.The Potential of Oral Camostat in Early COVID-19 Disease in an Ambulatory Setting to Reduce Viral Load and Disease Burden – Tabular View – ClinicalTrials.gov, n.d. https://clinicaltrials.gov/ct2/show/record/NCT04625114 (accessed March 22, 2021).
- 116.A Trial Looking at the Use of Camostat to Reduce Progression of Symptoms of Coronavirus (COVID-19) in People Who Have Tested Positive But Are Able to Stay at Home - Tabular View - ClinicalTrials.gov, n.d. https://clinicaltrials.gov/ct2/show/record/NCT04455815 (accessed March 22, 2021).
- 117.Heinrich-Heine University, Duesseldorf, Reconvalescent Plasma/Camostat Mesylate Early in Sars-CoV-2 Q-PCR (COVID-19) Positive High-risk Individuals, clinicaltrials.gov, 2021. https://clinicaltrials.gov/ct2/show/results/NCT04681430 (accessed March 18, 2021).
- 118.Zhang Y., Li D., Jiang Q., Cao S., Sun H., Chai Y., Li X., Ren T., Yang R., Feng F., Li B.-A., Zhao Q. Novel ADAM-17 inhibitor ZLDI-8 enhances the in vitro and in vivo chemotherapeutic effects of Sorafenib on hepatocellular carcinoma cells. Cell Death Dis. 2018;9:743. doi: 10.1038/s41419-018-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moss M.L., Minond D. Recent advances in ADAM17 research: a promising target for cancer and inflammation. Mediators Inflamm. 2017;2017:1–21. doi: 10.1155/2017/9673537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu T., Luo S., Libby P., Shi G.-P. Cathepsin L-selective inhibitors: a potentially promising treatment for COVID-19 patients. Pharmacol. Ther. 2020;213 doi: 10.1016/j.pharmthera.2020.107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.IRCT | Study of the Efficacy of Teicoplanin on mortality rate of patients with COVID-19 Infection: A randomized clinical trial, n.d. https://en.irct.ir/trial/46814 (accessed March 22, 2021).
- 122.Sun D., Li H., Lu X.-X., Xiao H., Ren J., Zhang F.-R., Liu Z.-S. Clinical features of severe pediatric patients with coronavirus disease, in Wuhan: a single center’s observational study. World J. Pediatr. 2019;16(2020):251–259. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gao Y., Li T., Han M., Li X., Wu D., Xu Y., Zhu Y., Liu Y., Wang X., Wang L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shimizu M. In: Cytokine Storm Syndr. Cron R.Q., Behrens E.M., editors. Springer International Publishing; Cham: 2019. Clinical features of cytokine storm syndrome; pp. 31–41. [DOI] [Google Scholar]
- 125.Dewan S.M.R., Osaka M., Deushi M., Yoshida M. Complement C5a-triggered differentiated HL-60 stimulates migration of THP-1 monocytic leukocytes via secretion of CCL2. FEBS Open Bio. 2021;11:1374–1381. doi: 10.1002/2211-5463.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carter M.J., Fish M., Jennings A., Doores K.J., Wellman P., Seow J., Acors S., Graham C., Timms E., Kenny J., Neil S., Malim M.H., Tibby S.M., Shankar-Hari M. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 2020;26:1701–1707. doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 127.Ronconi G. SARS-CoV-2, which induces COVID-19, causes Kawasaki-like disease in children: role of pro-inflammatory and anti-inflammatory cytokines. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/EDITORIAL-RONCONI-E-59. [DOI] [PubMed] [Google Scholar]
- 128.Conti P. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/Editorial-Conti-2. [DOI] [PubMed] [Google Scholar]
- 129.Hojyo S., Uchida M., Tanaka K., Hasebe R., Tanaka Y., Murakami M., Hirano T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., Zheng M., Sundaram B., Banoth B., Malireddi R.K.S., Schreiner P., Neale G., Vogel P., Webby R., Jonsson C.B., Kanneganti T.-D. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Immunology. 2020 doi: 10.1101/2020.10.29.361048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luo W., Li Y.-X., Jiang L.-J., Chen Q., Wang T., Ye D.-W. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol. Sci. 2020;41:531–543. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Spinelli F.R., Conti F., Gadina M. HiJAKing SARS-CoV-2? The potential role of JAK inhibitors in the management of COVID-19. Sci. Immunol. 2020;5:eabc5367. doi: 10.1126/sciimmunol.abc5367. [DOI] [PubMed] [Google Scholar]
- 133.Chua R.L., Lukassen S., Trump S., Hennig B.P., Wendisch D., Pott F., Debnath O., Thürmann L., Kurth F., Völker M.T., Kazmierski J., Timmermann B., Twardziok S., Schneider S., Machleidt F., Müller-Redetzky H., Maier M., Krannich A., Schmidt S., Balzer F., Liebig J., Loske J., Suttorp N., Eils J., Ishaque N., Liebert U.G., von Kalle C., Hocke A., Witzenrath M., Goffinet C., Drosten C., Laudi S., Lehmann I., Conrad C., Sander L.-E., Eils R. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 2020;38:970–979. doi: 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- 134.Smits S.L., de Lang A., van den Brand J.M.A., Leijten L.M., van IJcken W.F., Eijkemans M.J.C., van Amerongen G., Kuiken T., Andeweg A.C., Osterhaus A.D.M.E., Haagmans B.L. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee J.S., Park S., Jeong H.W., Ahn J.Y., Choi S.J., Lee H., Choi B., Nam S.K., Sa M., Kwon J.-S., Jeong S.J., Lee H.K., Park S.H., Park S.-H., Choi J.Y., Kim S.-H., Jung I., Shin E.-C. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci. Immunol. 2020;5:eabd1554. doi: 10.1126/sciimmunol.abd1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sarzi-Puttini P., Giorgi V., Sirotti S., Marotto D., Ardizzone S., Rizzardini G., Antinori S., Galli M. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin. Exp. Rheumatol. 2020;38:337–342. [PubMed] [Google Scholar]
- 137.Heimfarth L., Serafini M.R., Martins-Filho P.R., Quintans J. de S.S., Quintans-Júnior L.J. Drug repurposing and cytokine management in response to COVID-19: a review. Int. Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tanaka T., Kamiyama T., Daikoku T., Takahashi K., Nomura N., Kurokawa M., Shiraki K. T-705 (Favipiravir) suppresses tumor necrosis factor α production in response to influenza virus infection: a beneficial feature of T-705 as an anti-influenza drug. Acta Virol. 2017;61:48–55. doi: 10.4149/av_2017_01_48. [DOI] [PubMed] [Google Scholar]
- 139.Meng Z., Wang T., Chen L., Chen X., Li L., Qin X., Li H., Luo J. An experimental trial of recombinant human interferon alpha nasal drops to prevent COVID-19 in medical staff in an epidemic area. Infect. Dis. (except HIV/AIDS) 2020 doi: 10.1101/2020.04.11.20061473. [DOI] [PubMed] [Google Scholar]
- 140.Curreli S., Romerio F., Secchiero P., Zella D. IFN- α 2b increases interleukin-10 expression in primary activated human CD8 + T cells. J. Interferon Cytokine Res. 2002;22:1167–1173. doi: 10.1089/10799900260475678. [DOI] [PubMed] [Google Scholar]
- 141.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2019;382(2020):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li L., He X., Wang X., Sun Y., Wu G., Fang H., Wang C., Luo P., Xia Z. Ruxolitinib protects lipopolysaccharide (LPS)-induced sepsis through inhibition of nitric oxide production in mice. Ann. Transl. Med. 2020;8 doi: 10.21037/atm-20-2972. 546-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Virtanen A.T., Haikarainen T., Raivola J., Silvennoinen O. Selective JAKinibs: prospects in inflammatory and autoimmune diseases. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2019;33:15–32. doi: 10.1007/s40259-019-00333-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hossen M.S., Barek M.A., Jahan N., Islam M.S. A review on current repurposing drugs for the treatment of COVID-19: reality and challenges. SN Compr. Clin. Med. 2020;2:1777–1789. doi: 10.1007/s42399-020-00485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]