Abstract
Background
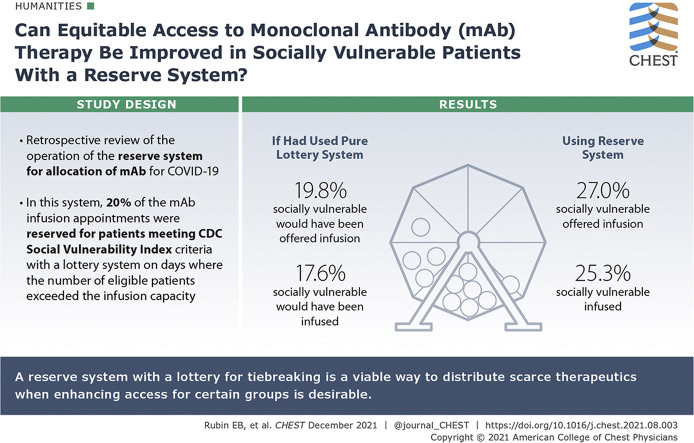
In fall 2020, the Food and Drug Administration issued emergency use authorization for monoclonal antibody (mAb) therapies for outpatients with COVID-19. The Commonwealth of Massachusetts issued guidance outlining the use of a reserve system with a lottery for allocation of mAbs in the event of scarcity that would prioritize socially vulnerable patients for 20% of the infusion slots. The Mass General Brigham health system subsequently implemented such a reserve system.
Research Question
Can a reserve system be deployed successfully in a large health system in a way that promotes equitable access to mAb therapy among socially vulnerable patients with COVID-19?
Study Design and Methods
We conducted a retrospective review of the operation of the reserve system for allocation of mAb therapies to identify how referrals moved through the allocation process and what proportion of patients who were offered and received mAb therapies were socially vulnerable.
Results
Notwithstanding multiple operational challenges, the reserve system for allocation of mAb therapy worked as intended to enhance the number of socially vulnerable patients who were offered and received mAb therapy. A significantly higher proportion of patients offered mAb therapy were socially vulnerable (27.0%) than would have been the case if the infusion appointments had been allocated using a pure lottery system without a vulnerable reserve (19.8%), and a significantly higher proportion of patient who received infusions were socially vulnerable (25.3%) than would have been the case if the infusion appointments had been allocated using a pure lottery system (17.6%)
Interpretation
Our health system experience demonstrates that a reserve system with a lottery for tiebreaking is a viable way to distribute scarce therapeutics when enhancing access for certain groups is desirable.
Key Words: allocation, COVID-19, ethics, lottery, monoclonal antibodies
Abbreviations: EUA, emergency use authorization; mAb, monoclonal antibody; MGB, Mass General Brigham; SVI, Social Vulnerability Index
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 2005
In November 2020, the Food and Drug Administration issued emergency use authorizations (EUAs) for two monoclonal antibodies (mAbs)—bamlanivimab and the dual-antibody cocktail casirivimab plus imdevimab—for the treatment of outpatients with mild to moderate COVID-19 at high risk of developing severe disease.1 , 2 The EUAs authorized use of the mAbs within 10 days of symptom onset for patients with one or more of several risk factors including age ≥ 65 years and BMI ≥ 35 kg/m2.
The initial EUAs for bamlanivimab and casirivimab plus imdevimab were based on modest data from phase 2 clinical trials that suggested that mAb therapy might reduce hospitalization, ED visits, or other medical visits in patients with mild to moderate COVID-19 if given early in the course of the disease.3 , 4 Phase 3 trial data supporting the benefits of mAb therapy subsequently were documented in press releases.5 , 6 The Food and Drug Administration issued an EUA for the dual antibody cocktail bamlanivimab plus etesevimab on February 9, 2021.7
The mAbs, which are delivered by IV infusion, initially were distributed by the federal government directly to states. It was unclear at the outset to what extent supply—both product and infusing capacity—would be sufficient to satisfy demand for mAb therapy. The prospect of scarcity, along with the many logistical challenges of patients with a contagious disease reaching infusion sites, led to concern that patients who enjoyed socioeconomic advantages and easy access to health care would receive mAbs disproportionately. This was of particular concern given the disproportionate impact of COVID-19 on communities of color and other socially vulnerable populations.8
In this setting, the Commonwealth of Massachusetts issued guidance detailing how mAbs should be allocated in the event of scarcity9 with a stated goal of promoting equitable access for socially vulnerable patients. The guidance specified that patients who met age criteria, BMI criteria, or both should be prioritized over patients who met other EUA criteria. It further provided that patients with a Centers for Disease Control and Prevention Social Vulnerability Index (SVI)10 of more than the 50th percentile, patients who lived in towns or cities with the highest quartile of COVID-19 incidence in the Commonwealth, or both should receive priority for 20% of the infusion spots over and above their share from the remaining 80% (an over-and-above reserve). The Massachusetts guidance further specified that, if there were more patients within a given priority group than available infusion slots, a lottery should decide which patients within the priority group were assigned the slots.
The 20% reserve size for socially vulnerable patients in the Massachusetts guidance was based on data suggesting that people from a census tract with SVI of more than the 50th percentile accounted for 60% of COVID-19 cases in Massachusetts. Assuming 50% of entrants into the system would have an SVI of more than the 50th percentile, in an idealized system, an over-and-above reserve of 20% for socially vulnerable patients would have resulted in 60% of infusion capacity going to those patients (50% of 80% of the infusion slots, plus 100% of 20% of the infusion slots), thereby ensuring allocation of mAbs proportional to the burden of disease. We sought to determine whether a reserve system could be deployed successfully in a large health system and whether doing so would promote equitable access to mAb therapy among socially vulnerable patients.
Methods
In December 2020, the Mass General Brigham (MGB) health system implemented a centralized lottery system for the allocation of mAb infusions to outpatients with COVID-19. Given anticipated scarcity of mAb infusion capacity, MGB initially limited distribution of mAbs to patients ≥ 65 years of age and patients ≥ 18 years of age with a BMI of ≥ 35 kg/m2. Trial participants had been infused within 72 h of a test with positive results being performed,3 , 4 and evidence suggested the benefit of mAb therapy is greater before antibody formation.4 MGB therefore required infusion within 4 days of a test with positive results being performed as well as within 10 days of symptom onset, an approach that was more restrictive than the EUAs.
At the start of the process in early December 2020, referrals for mAbs were generated automatically for all adult outpatients with positive SARS-CoV-2 test results in the MGB system who had been designated as symptomatic on their test order and were ≥ 65 years of age, had a registered BMI of ≥ 35 mg/m2, or both. The process started with automatic referrals for several reasons, including the prospect that referral bias could favor patients with privileged access to health care, concern about limited knowledge of mAb therapy among clinicians treating patients with COVID-19, and the sense that it would be challenging for clinicians to incorporate discussion and prescription of mAb therapy into their practice in a timely manner.
The referrals were screened to determine whether the patient’s time window for therapy would still be open on the date of the next infusion (which typically was the following day, with the exception of referrals placed on weekends) and to eliminate patients who were not eligible based on chart review (eg, patients who had been admitted to the hospital since demonstrating positive test results). On days when the number of eligible patients exceeded the infusion capacity for the following day, eligible patients whose time window would still be open were entered into a lottery for the available infusion appointments. The lottery generated a list of patients who would be guaranteed infusion slots for the following day, and a wait list of patients in case all of the available slots were not taken by those who were guaranteed slots.
Twenty percent of infusion appointments on any given day were reserved for vulnerable patients (termed vulnerable slots), and the remainder were available to all referred patients regardless of SVI or town of residence (termed open slots). Consistent with the intended over-and-above implementation of the reserve system, the open slots were assigned first in order of lottery number. The vulnerable slots then were assigned, with first priority for those slots going to patients living in zip codes with an average SVI in the top quartile for the Commonwealth of Massachusetts, then to those living in towns within the highest quartile of COVID-19 incidence in Massachusetts. Only patients residing in Massachusetts were eligible to be prioritized for the vulnerable slots.
The reserve for vulnerable patients was a soft reserve, meaning that if there were not enough patients in either the high SVI or high incidence town categories to fill the vulnerable slots, those slots were allocated to patients who were next in line by overall lottery number. This was done to avoid unused capacity for a therapy that is time sensitive and requires significant infrastructure to provide. After the lottery had been run, dedicated, primarily multilingual, clinicians who had been trained to discuss the therapies with patients called patients to verify eligibility and to engage in a shared decision-making conversation to determine whether the patient would like to receive an infusion.
Early experience with running the lottery before patient engagement revealed that a large number of patients declined the therapy after being offered it, were deemed ineligible once contacted, or wished to discuss the therapy with a trusted clinician. The process subsequently was changed to allow clinicians to enter referrals for their own patients once they established patient interest (termed manual referrals). For a period, both automatic and manual referrals were entered into the lottery. During that period, manual referrals for patients already identified as interested in the therapy took priority over automatic referrals to optimize use of infusion capacity. Ultimately, with increasing evidence base for benefit of mAb therapy and awareness of the therapy by individual treating clinicians, the transition was made to accepting only manual referrals for entry into the lottery.
We reviewed the data generated by implementation of the reserve system. Pearson χ 2 test was used to compare the proportion of patients with high SVI in various categories (eg, patients who received infusion vs patients who did not).
Results
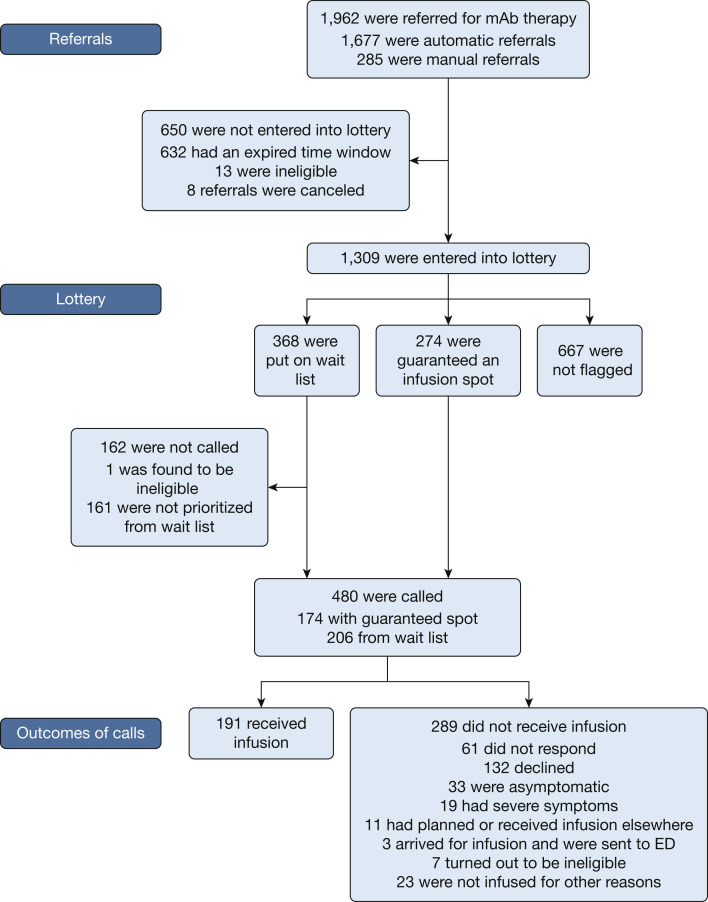
From December 8, 2020, through February 28, 2021, a total of 1,962 patients were referred for consideration of mAb therapy. The flow of the referrals through the process is outlined in Figure 1 . One thousand six hundred seventy-seven patients were referred via automatic referral and 285 were referred via manual referral. Of the 1,962 potential lottery entrants, 632 (32.2%) were not entered into the lottery because the time window for treatment would have expired by the time of the next available infusion. Eight referrals (0.4%) were canceled before they could be placed in the lottery, and 14 patients (0.7%) were deemed ineligible by criteria other than time window.
Figure 1.
Flowchart showing referrals through the mAb reserve lottery system. mAb = monoclonal antibody.
A total of 1,309 patients (66.7%) were entered into the lottery. Of those, a total of 274 (20.9%) were guaranteed infusion spots, of which 220 (80.3%) were assigned as open slots and 54 (19.7%) were assigned as vulnerable slots. Three hundred sixty-eight lottery entrants (28.1%) were put on a wait list to be called if those offered guaranteed appointments could not be reached, declined, or otherwise were not signed up for infusion; the wait list was determined based on lottery number, and the number of people on the wait list was adjusted based on what we learned about rate of acceptance among those who were guaranteed spots. Six hundred sixty-seven lottery entrants (51.0%) were not flagged, that is, they were neither assigned a guaranteed slot nor put on a wait list.
All of the 274 patients who were guaranteed slots and 206 of 368 patients on the wait list were called, for a total of 480 patients called. The number of wait list patients called on a given day was a function of both how many of the guaranteed slots were not filled and how much capacity the system had to make phone calls on any given day. Of those patients who were called, 132 (27.5%) declined, 33 (6.9%) were deemed ineligible by virtue of being asymptomatic, 19 (4.0%) were deemed ineligible by virtue of having severe symptoms, 11 (2.3%) had been or were planning to be infused elsewhere, 61 (12.7%) could not be reached, and 191 were infused (39.8% of those called and 9.7% of total referred patients). The demographic characteristics of the 1,962 identified potential lottery entrants, those who were called, those who received infusions, and those who declined infusions are detailed in Table 1 .
Table 1.
Demographic Characteristics of All Patients Referred for mAb Therapy and Subgroups of Referred Patients
| Characteristics | All Referred (N = 1,962) | Offered Infusion (n = 480) | Infused (n = 191) | Patient Declined (n = 132) |
|---|---|---|---|---|
| Age, y | 64.4 | 64.3 | 65.9 | 61.9 |
| Female sex | 1119 (57.0) | 289 (60.3) | 111 (58.1) | 89 (67.9) |
| Race | ||||
| White | 1582 (80.6) | 375 (78.3) | 151 (79.1) | 101 (76.5) |
| Black | 113 (5.76) | 35 (7.31) | 17 (8.90) | 7 (5.30) |
| Asian | 35 (1.78) | 7 (1.46) | 3 (1.57) | 1 (0.76) |
| Hispanic or Latino | 26 (1.33) | 4 (0.84) | 0 | 1 (0.76) |
| Other | 104 (5.30) | 31 (6.47) | 11 (5.76) | 12 (9.09) |
| Unknown/declined | 94 (4.79) | 24 (49.0) | 7 (3.66) | 9 (6.82) |
| Ethnicity | ||||
| Hispanic or Latino | 154 (7.85) | 41 (8.56) | 12 (6.28) | 16 (12.1) |
| Not Hispanic or Latino | 1298 (66.2) | 299 (62.4) | 124 (64.9) | 71 (53.8) |
| Other | 39 (1.99) | 12 (2.92) | 7 (3.66) | 2 (1.52) |
| Unknown/declined | 508 (24.0) | 125 (26.1) | 45 (25.1) | 43 (32.6) |
| Preferred language English | 1728 (88.1) | 414 (86.4) | 169 (88.5) | 112 (85.5) |
| Massachusetts resident | 1822 (92.9) | 445 (92.7) | 182 (95.3) | 125 (94.7) |
Data are presented as No. (%) or mean. mAb = monoclonal antibody.
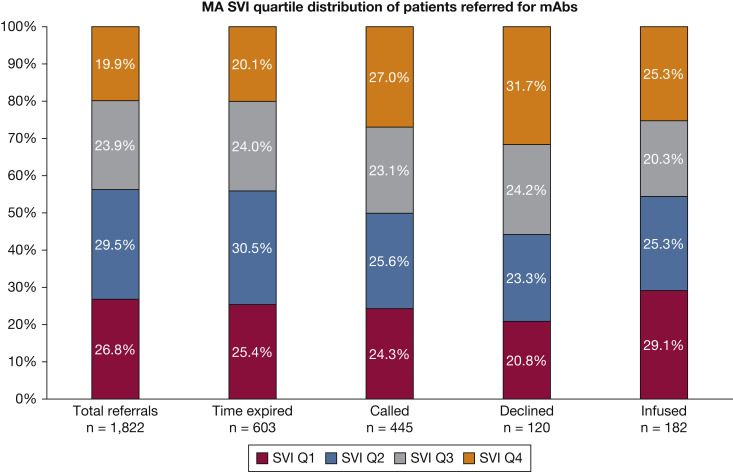
Figure 2 depicts the distribution by Massachusetts SVI quartile of different groups of patients: all patients referred for mAb therapy, patients whose time window expired before entry into the lottery, patients who were called to be offered infusion slots, patients who declined infusion, and patients who received infusion. For this analysis, we included only patients from Massachusetts (n = 1,822). Of these, 19.9% of all referred patients from Massachusetts, 20.1% of the patients whose time window expired before entry into the lottery, 27.0% of patients who were called to be offered an infusion appointment, 31.7 (%) of those who were offered and declined infusion, and 25.3% of those who received an infusion were in the top quartile of SVI for the Commonwealth of Massachusetts (termed high SVI patient).
Figure 2.
Bar graph showing the MA SVI quartile distribution of all patients referred for mAb therapy and subgroups of referred patients. MA = Massachusetts; mAb = monoclonal antibody; Q = quartile; SVI = Social Vulnerability Index.
The proportion of high SVI patients in the group that was called was significantly higher than the proportion of high SVI patients in the group that was not called (27.0% vs 17.6%; P < .001), and the proportion of high SVI patients in the group that declined infusion was significantly higher than the proportion of high SVI patients in the remainder of the group that was referred (31.7% vs 19.0%; P = .001). The proportion of high SVI patients in the group that received infusion was higher than the proportion of high SVI patients in the group that did not receive infusion, but the difference did not meet statistical significance (25.3% vs 19.3%; P = .05). The proportion of high SVI patients in the group that had an expired time window was not significantly different from the proportion of high SVI patients in the group that did not have an expired time window (20.1% vs 19.8%; P = .89).
Had we operated a pure lottery with no reserve for socially vulnerable patients and all other factors had remained constant, 19.8% of patients offered therapy (n = 88) would have been in the top SVI quartile, as opposed to 27.0% (n = 120) in our actual population, and 17.6% of infused patients (n = 32) would have been in the top SVI quartile, as opposed to 25.3% (n = 46) in the actual population.
Discussion
Multiple systems for allocating scarce resources allow for the prioritization of individuals with certain defined characteristics, and many of these systems have been used or proposed as ways to improve equitable distribution of resources during the COVID-19 pandemic. These include weighted lottery systems,11 point systems that add or deduct points from a priority score based on the existence of certain characteristics,12 and reserve systems. A reserve system with specific reserve categories and mechanisms for tiebreaking within categories (eg, lottery) is a useful tool to ensure that a certain amount of a good that is being allocated is distributed to patients with certain characteristics. Such a system allows the incorporation of multiple different ethical principles and priorities into an allocation framework.13 If it is known with reasonable certainty what proportion of entrants into the system will have a given characteristic (eg, social vulnerability, age within a certain range, essential worker status), it is possible to adjust the size of the reserve to accomplish a specific outcome. Reserve systems have been advocated for the administration of COVID-19 vaccines14, 15, 16 and in some states have been adopted at the community, but not individual, level.17 , 18 To our knowledge, the system we describe is the first instance of a reserve system being used to allocate scarce resources at the individual level during a pandemic.
A reserve system with lottery for tiebreaking within categories can be straightforward to operate if few or no steps exist between the assignment of lottery spots and the distribution of the good. This could be true, for example, of allocation of antiviral medications to inpatients with COVID-19. In the case of mAb therapies, multiple factors could—and often did—interrupt the trajectory between allocation and distribution. These included the complexity of administering infusion therapy, the time-sensitive nature of the therapy, the relative paucity of evidence for the therapy at the time the mAb program started, and the dynamic nature of COVID-19. The conversations with patients about a therapy that held promise, but did not yet have strong evidence to support its efficacy and had not been formally approved by the Food and Drug Administration, often were challenging and time-consuming. It proved difficult or impossible to reach many patients identified for allocation. Others declined therapy after it was offered and discussed, or had become either too well or too sick to be candidates for the therapy by the time they were reached.
Several additional challenges were encountered in operationalizing the lottery for mAbs. One of the key challenges was determining when to enter patients into the lottery in relationship to ascertainment of their interest in receiving the therapy. Restricting entry to patients already identified by individual clinicians as interested ran the risk that the entrant pool would be affected significantly by referral bias. However, it was highly resource intensive to call patients who had been allocated a place without prior affirmation of interest, because uptake using this approach was fairly low. Conversely, it was challenging for clinicians to have a shared decision-making conversation about the therapy and simultaneously to explain that the therapy might not be available to the patient if demand were to exceed supply. Given that allocating medical therapy by lottery is a departure from usual practice, some resistance to having to explain a lottery system to patients ensued.
Demand for the therapy fluctuated from day to day and it was often unnecessary to run a lottery on a given day. In this setting, it was challenging to determine whether each patient should have a single chance at the lottery, or whether patients should be permitted to stay in the lottery for as many days as they were eligible to receive the therapy. We identified conflict between the goals of ensuring that access did not depend significantly on when in the course of illness the patient was tested or received results (both of which may be affected by social vulnerability), maximizing benefit by infusing patients as early in their illness as possible, and optimizing use of capacity in a system with a fixed minimum resource commitment. Delivery of mAbs to patients with COVID-19 required dedicated staff and space, both of which otherwise might have been used to provide infusions to patients with other medical problems, and leaving set-aside capacity unused was highly undesirable.
The fact that eligibility for and interest in mAb therapy in an individual patient could fluctuate from day to day based on severity of symptoms or other factors posed additional difficulties. Even after the move toward manual referrals and relying on treating clinicians to refer patients after discussing the therapy, patients often changed their minds or felt better or were sicker by the time they were called to schedule an infusion.
One of the key decisions in operating a reserve system for the mAbs was determining whether to offer the therapeutics reserved for vulnerable patients to nonvulnerable patients in the event that vulnerable patients were not identified to take advantage of them. Ideally, such a circumstance would exert pressure on those operating the system to find a way to address the root causes of the failure to reach more vulnerable patients. In our health system, for example, a relative dearth of testing in socially vulnerable populations within the system might have been a culprit.19 Nevertheless, where the potential value of the therapy to those who were eligible for it diminished by the day, it seemed preferable to use capacity fully while concurrently addressing causes of disparity.
Interpretation
Notwithstanding significant challenges, the reserve system implemented in our health system for allocation of mAb therapy worked as intended to enhance the number of socially vulnerable patients who were offered the therapy. A significantly higher proportion of socially vulnerable patients were offered mAb therapy than would have been if the infusion appointments had been allocated using a pure lottery system without a vulnerable reserve. The intended enhancement of the pool of vulnerable patients who actually received mAb therapy was counterbalanced to some extent by the disproportionate number of vulnerable patients who declined therapy, but even fewer socially vulnerable patients would have received the therapy if the lottery system had not included a vulnerable reserve.
The MGB experience demonstrated that a reserve system with a lottery for tiebreaking is a viable way to distribute scarce therapeutics when enhancing access for certain groups is desirable. It also highlighted some of the real-world challenges associated with operationalizing such a system in an outpatient setting, particularly in the context of a dynamic disease and a time-sensitive therapy with evolving evidence base. Although enhancing capacity to meet demand for beneficial therapies should be a priority in pandemics and other disaster situations, it is critical to continue to plan for times when that it is not feasible. Future work should focus on the desirability and feasibility of reserve systems for allocating scarce resources.
Take-home Points.
Study Question: Can a reserve system be deployed successfully in a large health system in a way that promotes equitable access to monoclonal antibody (mAb) therapy among socially vulnerable patients with COVID-19?
Results: Notwithstanding multiple operational challenges, a reserve system for allocation of mAb therapy worked as intended to enhance the number of socially vulnerable patients who were offered and received mAb therapy.
Interpretation: Our health system experience demonstrated that a reserve system with a lottery for tiebreaking is a viable way to distribute scarce therapeutics when enhancing access for certain groups is desirable.
Acknowledgments
Author contributions: E. R. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. E. R. contributed to study concept and design; and collection, management, analysis, and interpretation of the data: E. R., S. L. D.-P., S. P. H., I. L., A. R. L., P. P., T. S., and U. U. drafted or critically revised the manuscript for important intellectual content.
Financial/nonfinancial disclosures: None declared.
Other contributions: The authors thank the many people at MGB for their tireless work in helping to implement the allocation framework described herein, including Melanie Cassamas, MHA, Lisa Dutton, MSN, Aleah Carvotta, RN, and Juliana Cuomo, RN.
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
References
- 1.Food and Drug Administration Letter to Eli Lilly and Company, March 2, 2021. Food and Drug Administration website. https://www.fda.gov/media/143602/download
- 2.Food and Drug Administration Letter to Regeneron Pharmaceuticals, Inc., July 30, 2021. Regeneron Pharmaceuticals, Inc., website. https://www.regeneron.com/sites/default/files/treatment-covid19-eua-fda-letter.pdf
- 3.Chen P., Nirula A., Heller B., et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinreich D.M., Sivapalasingam S., Norton T., et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eli Lilly and Company Lilly’s bamlanivimab and etesevimab together reduced hospitalizations and death in phase 3 trial for early COVID-19. March 10, 2021. Eli Lilly and Company website. https://investor.lilly.com/news-releases/news-release-details/lillys-bamlanivimab-and-etesevimab-together-reduced
- 6.Regeneron Pharmaceuticals, Inc Phase 3 trial shows REGEN-COV™ (casirivimab with imdevimab) antibody cocktail reduced hospitalization or death by 70% in non-hospitalized COVID-19 patients. March 23, 2021. Regeneron Pharmaceuticals, Inc., website. https://investor.regeneron.com/news-releases/news-release-details/phase-3-trial-shows-regen-covtm-casirivimab-imdevimab-antibody
- 7.Food and Drug Administration Letter to Eli Lilly and Company, February 25, 2021. Food and Drug Administration website. https://www.fda.gov/media/145801/download
- 8.Goldstein R.H., Walensky R.P. The challenges ahead with monoclonal antibodies: from authorization to access. JAMA. 2020;324(21):2151–2152. doi: 10.1001/jama.2020.21872. [DOI] [PubMed] [Google Scholar]
- 9.Cooke M., Madoff L. Memorandum to: healthcare facilities infusing COVID-19 antibodies therapeutics. April 13, 2021. State of Massachusetts website. https://www.mass.gov/doc/guidance-for-allocation-of-covid-19-monoclonal-antibody-therapeutics-in-non-hospital-settings/download
- 10.Agency for Toxic Substances and Disease Registry, Centers for Disease Control and Prevention CDC/ATSDR social vulnerability index. Centers for Disease Control and Prevention website. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- 11.White D.B., Schmidhofer M., McCreary E., et al. A model hospital policy for fair allocation of scarce medications to treat COVID-19. May 28, 2020. University of Pittsburgh website. https://ccm.pitt.edu/node/1133
- 12.White D.B., Lo B. Mitigating inequities and saving lives with ICU triage during the COVID-19 pandemic. Am J Respir Crit Care Med. 2021;203(3):287–295. doi: 10.1164/rccm.202010-3809CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sönmez T., Pathak P.A., Unver M.U., Persad G., Truog R.D., White D.B. Categorized priority systems: a new tool for fairly allocating scarce medical resources in the face of profound social inequities. Chest. 2021;159(3):1294–1299. doi: 10.1016/j.chest.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pathak P.A., Sonmez T., Unver M.U., Yenmez B.M. National Bureau of Economic Research; 2020. Leaving No Ethical Value Behind: Triage Protocol Design for Pandemic Rationing. [Google Scholar]
- 15.Persad G., Peek M.E., Emanuel E.J. Fairly prioritizing groups for access to COVID-19 vaccines. JAMA. 2020;324(16):1601–1602. doi: 10.1001/jama.2020.18513. [DOI] [PubMed] [Google Scholar]
- 16.National Academies of Sciences, Engineering, and Medicine. Framework for equitable allocation of COVID-19 vaccine. 2020. [PubMed]
- 17.State of Connecticut, Department of Health Correspondence, February 28, 2021. https://647302b3-84b9-4f64-9c36-948d9371c62f.usrfiles.com/ugd/647302_f26f4ac1c2c6453186d433da6a86a9d8.pdf. Accessed May 4, 2021.
- 18.Office of Governor Gavin Newsom, State of California California leads with public health and vaccine equity to safely and sustainably reopen. March 4, 20221. State of California website. https://www.gov.ca.gov/2021/03/04/california-leads-with-public-health-and-vaccine-equity-to-safely-and-sustainably-reopen/
- 19.Dryden-Peterson S., Velasquez G.E., Stopka T.J., Davey S., Lockman S., Ojikutu B. Disparities in SARS-CoV-2 testing in Massachusetts during the Covid-19 pandemic. JAMA Netw Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2020.37067. [DOI] [PMC free article] [PubMed] [Google Scholar]