Abstract
Background
People with epilepsy (PwE) were concerned about the safety of the novel 2019 Coronavirus Disease (COVID-19) vaccines.
Objective
This study aimed to assess the side effects experienced by PwE following vaccination with COVID-19 vaccines and to identify the causes of vaccine hesitation.
Methods
We administered a questionnaire to PwE, who visited the epilepsy clinic at Ibn Sina Hospital in Kuwait during the first two working weeks of April 2021. It included socio-demographic, epilepsy status, and vaccination data. In addition, we asked those who were not vaccinated yet about the reasons and their plan.
Results
A total of 111 PwE were surveyed, with 82 being vaccinated and 29 being unvaccinated. Out of the 82 vaccinated, 66 (80.5%) reported at least one side effect. Patients who received the Pfizer BioNTech mRNA vaccine (BNT162b2) (first, second dosage); and the Oxford-AstraZenecaa chimpanzee adenovirus-vectored vaccine (ChAdOx1nCoV-19) (first dose) had the following reactions: Pain at the injection site (40%, 67.6%), 43.8%, fatigue (47%, 32.4%), 46.9%, Headache (33.3%, 35.3%), 34.4% and Myalgia (40%, 35%), 50% respectively. Local site effects, including pain (67.6% vs. 40%, p = < 0.001) and redness (26.5% vs 6.7%, p = 0.019), were more statistically significantly after the second dose of BNT162b2 vaccine compared to the first dose of the same vaccine. While there was no significant difference in systemic side effects frequencies between the two doses of the BNT162b2 vaccine. The systemic side effects were more statistically significantly after the first dose of ChAdOx1nCoV-19 compared to the first dose of the BNT162b2 vaccine and those included fever (56.3% vs 13.3%, p = < 0.001), chills (37.5% vs 6.7%, p = < 0.001), myalgia (50% vs 40%, p = < 0.001) and arthralgia (25% vs 6.7%, p = 0.021). The local site reactions were not significantly different between the first doses of both vaccines. Among the subgroup who had vaccine-related side effects, 66.7% were females, 90.9% were 55 or younger, 63.6% were on polytherapy, 74% had side effects for one day or less, and 95% were symptoms free by the end of the first-week post-vaccination. Symptoms were mild in 68% of the patients and moderate in 29.3%. Most patients (93.9%) did not report seizure worsening after vaccination. The relative risk of seizure worsening after the first and second doses of BNT162b2 and the first dose of ChAdOx1nCoV-19 vaccines was 1.027 (95% CI 0.891–1.183), 1.019 (95% CI 0.928-1.119), and 1.026 (95% CI 0.929–1.134) respectively. After the first dose of BNT162b2, one patient reported the development of status epilepticus. Among the non-vaccinated group, 34.9% were still indecisive, while 37.9% rejected the vaccination. Fear of adverse effects (42.9%) and fear of epilepsy worsening (23.8%) were the main reasons for vaccine hesitation.
Conclusions
This study shows that the two vaccines under consideration (BNT162b2 and ChAdOx1nCoV-19) have a good safety profile and a low risk of epilepsy worsening among a cohort of PwE in Kuwait.
Keywords: Epilepsy, COVID-19, Vaccine, Safety, Vaccine hesitancy
1. Introduction
Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) causes COVID-19 [1], which was declared as a pandemic by World Health Organization (WHO) in March 2020 [2]; since then, the entire world is facing unprecedented problems and living conditions.
The ongoing COVID-19 pandemic has had a tremendous impact on public health, and made the need for a safe and effective vaccine a top priority. [3] The vaccination boosts population immunity and lowers illness severity, easing the current health crisis. [4]
Aside from the challenges related to the vaccine's development, production, and distribution, vaccine hesitation because of safety concerns looks to be a significant problem, particularly among persons with preexisting health conditions such as epilepsy. [5,6].
For PwE, breakthrough seizures carry significant consequences, including injuries, impact on driving, and others. [7] Fever, a major adverse effect of the vaccination, is one of the most prevalent triggers of these breakthrough seizures. [8]
The Pfizer-BioNTech mRNA vaccine (BNT162b2) and the Oxford-AstraZenecaa chimp adenovirus-vectored vaccine (ChAdOx1nCoV-19) were both licensed for use in Kuwait for people aged 16 and above during the study time. [9] Vaccination began on December 24, 2020. During this study, there were around 860,000 vaccinated individuals. [10].
Given the lack of real-life experience in patients with chronic disorders such as epilepsy at the start, the recommendations were based on prior vaccine experience and data from the COVID-19 vaccine clinical trials. [11, 12].
This study aimed to determine the safety and tolerability of COVID-19 vaccines among PwE and the causes for vaccine hesitation among non-vaccinated PwE in Kuwait.
2. Methods
2.1. Setting and participants
It is a cross-sectional study designed to assess the safety of the COVID-19 vaccines among PwE by using a questionnaire that includes socio-demographic, epilepsy characteristics, and vaccine-related variables (type, doses, side effects symptoms, duration, severity, and their impact on the seizures).
The study included patients with epilepsy diagnosed according to the international league against epilepsy (ILAE) Criteria of both genders, aged 16 years or older, and received the approved COVID-19 vaccines either one or two doses (in the vaccinated group) and at least one week passed after their vaccination. PwE who attended the epilepsy clinic between April 4, 2020, and April 18, 2021, were identified to the study whether they were vaccinated against COVID-19 or not.
Patients with an unconfirmed epilepsy diagnosis and those who refused to take part in the survey were excluded. We also excluded patients with other comorbidities that could have influenced the side effects (one patient only was excluded based on this criterion.). Patients who were mentally ill were also excluded from the study to avoid bias in data collection, as many of the side effects were subjective, and caregivers may not provide accurate answers.
The vaccinated patients were asked if they had any side effects or if their seizures had worsened. The non-vaccinated group was asked about their reasons for not being vaccinated and their vaccination plans.
We stratified the surveyed patients into two groups:
The first group included the vaccinated patients; this group was further subdivided into three sub-groups according to the vaccine type, and the doses received as follows:
-
a)
BNT162b2 vaccine, single dose
-
b)
BNT162b2 vaccine, two doses
-
c)
ChAdOx1nCoV-19 vaccine, one dose
The second dose of the ChAdOx1nCoV-19 vaccine was unavailable in Kuwait at the study time.
The non-vaccinated patients made up the second group, which was studied to identify the causes of the vaccine hesitation among PwE.
2.2. The instruments
-
•The questionnaire was written in English, translated into Arabic, then back-translated into English to ensure accuracy. It was validated after being reviewed by two independent physicians and pretested on ten PwE. The following sections were included in the questionnaire:
-
–Introduction, which included a summary of the study's purpose.
-
–Informed consent (mandatory).
-
–Social and demographic information (age, sex, marital state, and working status).
-
–-Epilepsy-related variables (seizure type, disease duration, number of anti-seizure medications (ASM), seizure frequency, and history of status epilepticus in the three months preceding the vaccination).
-
–-Vaccines- related variables (type, doses, side effect symptoms, duration, severity, and their impact on seizures)
-
–-No information that might identify the patient was gathered.
-
–Patients visiting the epilepsy clinic Ibn Sina hospital, Kuwait, were given the questionnaire by their treating epileptologists.
-
–
-
•
Seizure worsening was defined as an increase in total average monthly seizure frequency, average monthly generalized tonic-clonic seizures (GTCS), new-onset GTCS, or new-onset status epilepticus. [13]
-
•
Vaccine hesitation was defined as a delay in accepting or declining immunization, despite the availability of vaccination services. [14]
-
•
The Food and Drug Administration (FDA) toxicity grading scale was used to grade the adverse effects as Grade 1 (mild), Grade 2 (moderate), Grade 3 (severe), and Grade 4 (serious or life-threatening). [15]
2.3. Outcomes
-
1)
The primary outcome was to determine the frequency, severity, and duration of the vaccination side effects in PwE.
-
2)
The secondary outcomes were to assess the post-vaccination risk of epilepsy worsening and explore vaccine hesitation causes among PwE.
2.4. Ethical approval
Ethical approval was obtained from the research committee at Ibn Sina Hospital, Kuwait.
2.5. Statistical analysis
All statistical tests were executed using the Statistical Package for the Social Sciences(SPSS) version 27.0 (SPSS Inc. Chicago, IL, USA, 2020). Primarily, descriptive statistics were carried out for the demographic variables (gender, age, marital status, and job), epilepsy variables (type, duration, ASMs, seizure frequency, and history of status epilepticus), and COVID-19-vaccine related variables (type, number of doses) and vaccine side effects (type, frequency, duration, severity and their impact on seizures) were represented by frequencies, percentages, means, and standard deviations. Inferential statistics were performed to assess the association between side effects and other variables, including demographic, vaccine, and epilepsy variables using the chi-squared test (X 2), student t-test (t), and Mann Whitney (U) test, with a confidence level of 95% and significance value p ≤ 0.05.
3. Results
-
1)
Demographic characteristics
The study identified 111 individuals (82 vaccinated and 29 non-vaccinated) who visited the epilepsy clinic between April 4, 2021 and April 18, 2021. Ten patients were excluded (eight patients were mentally subnormal, one was unwilling to participate, and one had hypertension). The demographic characteristics of the cohort are shown in Table 1 .
-
1)
Epilepsy
Table 1.
The Demographic data of the surveyed cohort.
| Variables | BNT162b2 First dose N=15(13.5) | BNT162b2 Second dose N=35(30.6) | ChAdOx1 nCoV-19 First dose N=32 (28.8) | Not vaccinated yet N=29(26.1) | Total |
|---|---|---|---|---|---|
| Gender (N/%) | |||||
| • male | 6(5.4) | 14(12.6) | 13(11.7) | 13(117) | 46(41.4) |
| • female | 9(8.1) | 21(18.9) | 19(17.1) | 16(14.4) | 65(58.6) |
| Age | |||||
| • ≤55 | 14(12.6) | 33(29.7) | 26(23.4) | 28(25.2) | 101(91) |
| • >55 | 1(0.9) | 2(1.8) | 6(5.4) | 19(0.9) | 10(9) |
| • mean/(SD) | 32.93(10.89) | 34.74(12.36) | 35.74(13.86) | 28.69(11.24) | 33.204±12.57 |
| Job | |||||
| • full time | 5(4.5) | 13(11.7) | 14(12.6) | 4(3.6) | 36(32) |
| • part-time | 2(1.9) | 2(1.9) | 5(4.5) | 2(1.9) | 11(9.9) |
| • student | 2(1.9) | 8(7.2) | 5(4.5) | 12(10.8) | 27(24.3) |
| • retired | 1(0.9) | 2(1.9) | 3(2.7) | 3(2.7) | 9(8.1) |
| • not working | 5(4.5) | 9(8.1) | 4(3.6) | 8(7.2) | 26(23.4) |
| • self-employed | 0 | 1(0.9) | 1(0.9) | 0 | 2(1.8) |
| Social status | |||||
| • single | 6(5.4) | 19(17.1) | 15(13.5) | 19(17.1) | 59(53.2) |
| • married | 7(6.3) | 10(9) | 13(11.7) | 6(5.4) | 36(32) |
| • divorced | 2(1.9) | 3(2.7) | 4(3.6) | 3(2.7) | 12(10.8) |
| • widow | 0 | 3(2.7) | 0 | 1(0.9) | 4(3.6) |
The characteristics of the patients’ epilepsy are shown in Table 2 .
-
1)Vaccine side effects
-
A)A total of 66 (80.5%) (12 after the BNT162b2 first dose, 29 after the BNT162b2 second dose, and 25 after the ChAdOx1nCoV-19 first dosage) of the 82 vaccinated patients experienced at least one side effect post-vaccination. The characteristics of this subgroup that experienced vaccine-related side events are shown in Table 3 .
-
A)
Table 2.
Epilepsy characteristics of the surveyed cohort.
| Variables | BNT162b2First dose N=15(13.5) | BNT162b2Second dose N=35(31.5) | ChAdOx1 nCoV-19First dose N=32 (28.8) | Not receive yet N=29(26.1) | Total |
|---|---|---|---|---|---|
| Epilepsy duration | |||||
| • two years or less | 1(0.9) | 6(5.4) | 4(3.2) | 6(5.4) | 18(16.2) |
| • more than two years | 14(12.6) | 28(25.2) | 28(25.2) | 23(20.7) | 93(83.8) |
| Number of ASM | |||||
| • one drug • two drugs • three or more |
8(7.2) 5(4.5) 2(1.9) |
15(13.5) 12(10.8) 8(7.2) |
12(10.8) 13(11.7) 7(6.3) |
19(17.1) 8(7.2) 2(1.9) |
54(48.6) 38(34.2) 19(17.1) |
| Type of epilepsy | |||||
| • generalized tonic-clonic • focal with loss awareness • focal without loss awareness • absence • myoclonus |
3(2.7) 1(0.9) 5(4.5) 6(5.4) 0 |
11(9.9) 7(6.3) 7(6.3) 2(1.9) 8(7.2) |
10(9) 11(9.9) 7(6.3) 1(0.9) 3(2.7) |
11(9.9) 9(8.1) 5(4.5) 1(0.9) 3(2.7) |
35(31.5) 28(25.2) 24(21.6) 10(9) 14(12.6) |
| Number of seizure per month within last 3 months (mean/SD) | 0.733(1.44) | 1.17(1.94) | 2.03(4.07) | 1.89(4.84) | |
| History of status epilepticus | |||||
| • Yes • No |
1 (0.9) 14(12.6) |
3(2.7) 32(28.3) |
3(2.7) 29(26.1) |
7(6.3) 22(19.8) |
14(12.6) 97(87.4) |
Table 3.
Characteristics of the patient who had vaccine-related side effects.
| Variable | Patient-reported Side effect N= 66 (N/ %) | test of significance | p |
|---|---|---|---|
| Age: | |||
| • Mean± SD • Median (min-max) |
34.758±11.906 33.5 (16-67) |
U=1157.0 | 0.049* |
| Gender: | |||
| • Male • Female |
22(33.3) 44(66.7) |
X2 =4.41 | 0.036* |
| Social status: | |||
| • Single • Married • Divorced • Widow |
30(45.5) 24(36.6) 9(13.6) 3(4.5) |
X2 =4.28 | 0.232 |
| Job: | |||
| • Full time • Part-time • Student • Retired • Jobless • Self-employed |
27(40.9) 5(7.6) 11(16.7) 6(9.1) 16(24.2) 1(1.5) |
X2 =8.860 | 0.155 |
| Epilepsy duration: | |||
| • Two years or less • More than two years |
7(10.6) 59(89.4) |
t=1.731 | 0.086 |
| Epilepsy type: | |||
| • Generalized tonic-clonic • Focal with loss of awareness • Focal without loss of awareness • Absence • myoclonus |
20(30.3) 14(21.2) 15(22.7) 8(12.1) 9(13.6) |
X2=3.850 | 0.517 |
| ASMs: | |||
| • monotherapy • polytherapy |
24(36.4) 42(63.6) |
t=2.918 | 0.004* |
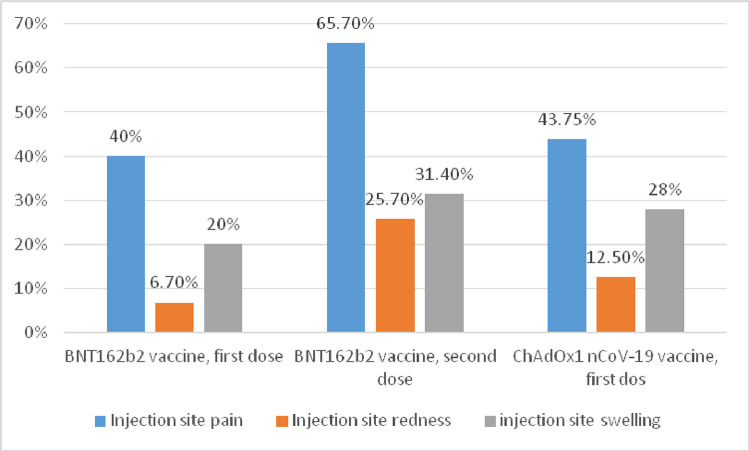
The frequencies of local side effects experienced after each vaccine are demonstrated in Fig. 1 .
Fig. 1.
Local side effects after different vaccines.
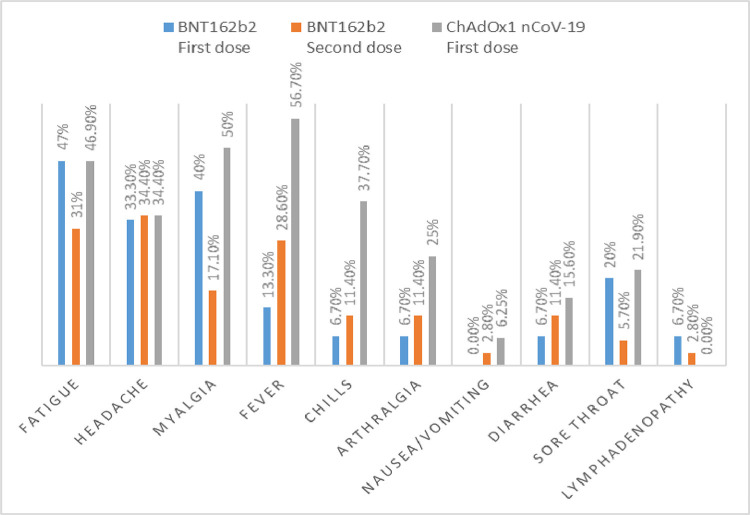
The frequencies of systemic side effects experienced after each vaccine are shown in Fig. 2 .
-
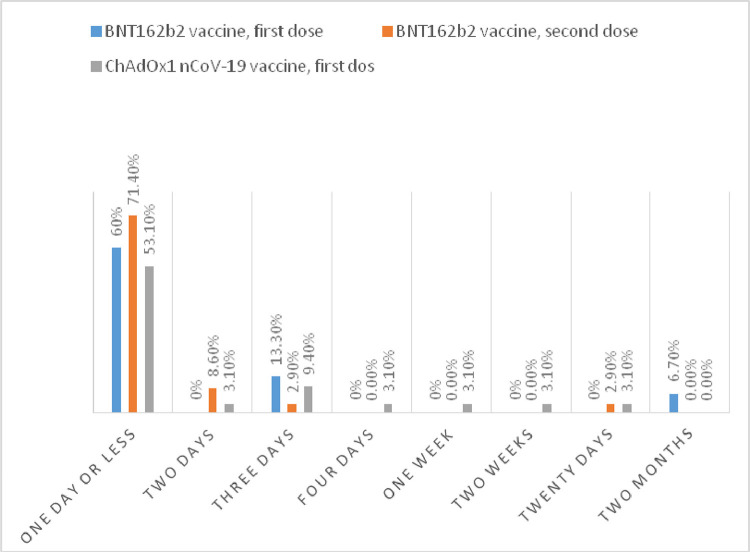
A)The duration of the symptoms in most of our patients was one day or less. Ninety-five percent of patients who suffered vaccine-related adverse effects were symptom free by the end of the first week after vaccination, as shown in Fig. 3 .
-
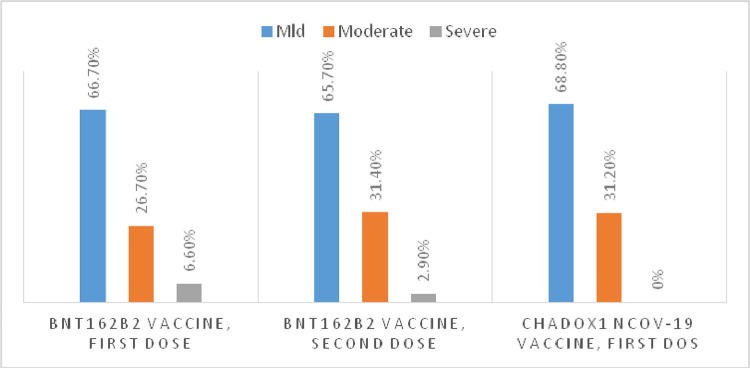
B)In nearly two-thirds of the patients who suffered vaccine-related side effects, the symptoms were mild. None of the patients was hospitalized because of serious side effects, as shown in Fig. 4 .
-
A)
-
1)
A comparison of the prevalence of the side effects between the first and second BNT162b2 vaccination doses groups:
Fig. 2.
systemic side effects of different Vaccines.
Fig. 3.
the duration of the vaccine side effects symptoms.
Fig. 4.
the severity of the vaccine side effects symptoms.
Injection site pain (67.6% vs. 40%, p = 0.001) and redness (26.5% vs. 6.7%, p = 0.019) were more statistically significantly after the second dose of BNT162b2 vaccination. There was no significant difference in systemic side effects between the two doses, as shown in Table 4 .
-
1)
A comparison of the side effects frequency between the first BNT162b2 vaccination dose and the first ChAdOx1nCoV-19 vaccine dose groups:
Table 4.
Comparing in side effects between the two BNT162b2 vaccination doses.
| Variables | BNT162b2First doseN=15(13.5) | BNT162b2Second doseN=35(30.6) | X2 | P |
|---|---|---|---|---|
| Local side effects | ||||
| • Injection site pain/tenderness • Injection site swelling • Injection site redness |
6(40) 3(20) 1(6.7) |
23(67.6) 11(32.4) 9(26.5) |
17.149 3.770 7.981 |
<0.001* 0.152 0.019* |
| Systemic side effects | ||||
| • Fever • Chills • Fatigue • Headache • Myalgia • Arthralgia • Lymphadenopathy • Sore throat • Nausea/vomiting • Diarrhea |
2(13.3) 1(6.7) 7(47) 5(33.3) 6(40) 1(6.7) 1(6.7) 3(20) 0 1(6.7) |
10(29.4) 4(11.8) 11(32.4) 12(35.3) 6(17.6) 4(11.8) 1(2.9) 2(5.9) 1(2.9) 4(11.8) |
1.659 2.166 2.879 3.715 2.981 0.488 3.346 5.728 0.497 0.392 |
0.436 0.339 0.237 0.156 0.225 0.783 0.188 0.057 0.639 0.822 |
Systemic side effects including fever (56.3% vs. 13.3%, p = 0.001), chills (37.5% vs. 6.7%, p = 0.001), myalgia (50% vs. 40%, p = 0.001), and arthralgia (25% vs. 6.7 %, p = 0.021) were more statistically significantly after the first dose of ChAdOx1nCoV-19 vaccine than after the first dose of BNT162b.
In terms of local side effects, there was no significant difference between the two doses, according to Table 5 .
-
1)
Impact of the vaccination on epilepsy
Table 5.
Comparing the side effects of the BNT162b2 vaccine's first dose to the first dose of ChAdOx1nCoV-19.
| Variables | BNT162b2First doseN=15(13.5) | ChAdOx1 nCoV-19First doseN=32 (28.8) | X2 | P1 |
|---|---|---|---|---|
Local side effects
|
6(40) 3(20) 1(6.7) |
14(43.8) 9(28.1) 4(12.5) |
0.560 1.359 0.604 |
0.756 0.459 0.739 |
Systemic side effects
|
2(13.3) 1(6.7) 7(47) 5(33.3) 6(40) 1(6.7) 1(6.7) 3(20) 0 1(6.7) |
18(56.3) 12(37.5) 15(46.9) 11(34.4) 16(50) 8(25) 0 2(6.25) 2(6.25) 5(15.6) |
19.501 17.063 11.382 3.336 20.673 7.680 2.614 5.857 2.264 2.199 |
<0.001* <0.001* 0.06 0.189 <0.001* 0.021* 0.271 0.053 0.322 0.333 |
Five patients (6.1%) of the vaccinated group had seizure worsening. They were ≤55 years old, have had epilepsy for more than two years, and were on polytherapy. Four are females, and four are single. (Table 6 )
Table 6.
Impact of the vaccines on epilepsy.
| Variables | BNT162b2First doseN=15(13.5) | BNT162b2Second doseN=35(30.6) | ChAdOx1 nCoV-19First doseN=32 (28.8) | p |
|---|---|---|---|---|
Seizure worsening:
|
1 (20) 14 (18.1) 1.027 0.891-1.183 |
2(40) 33(42.9) 1.019 0.928-1.119 |
2(40) 30(39) 1.026 0.929-1.134 |
0.505 |
Status epilepticus:
|
1(100) 14(17.3) 1.060 0.925-1.216 |
0 35(43.2) |
0 32(39.5) |
0.253 |
The relative risks of the seizure worsening after the vaccination were minimal, with no significant differences between the three groups p = 0.505, as shown in Table 6
One patient reported having status epilepticus after receiving the first dose of BNT162b2vaccine, with a relative incidence of 1.060 (95% CI 0.925–1.216). This patient was 30 years old, female with epilepsy for more than two years, on polytherapy. She also had status epilepticus within the last three months before the vaccination. She had moderate myalgia and fever for three days post-vaccination. Her neurological examination and MRI brain were normal.
-
1)
Non-vaccinated group: (N = 29)
The causes of non-vaccination and the most common reasons for vaccine hesitation are shown in Table 7 .
Table 7.
Non-vaccination and vaccine hesitation causes.
| Variable | N(%) |
|---|---|
Causes of non-vaccination (N=29)
|
11 (37.9) 8(27.6) 10(34.5) |
Causes of vaccine hesitation(N=21)
|
9(42.9) 4 (19) 5 (23.8) 3 (14.3) |
4. Discussion
This cross-sectional study shows that the two COVID-19 vaccines under consideration (the BNT162b2 vaccine and the ChAdOx1nCoV-19 vaccine) are safe and tolerable in the short term among PwE. Most of the side effects are mild, brief, and moderately frequent. Furthermore, they had a minor impact on seizure worsening.
The majority of our cohort was 55 years old or younger, with a mean age of 33.207 ± 12.517, which was younger than the study populations of clinical trials and other real-world studies. [16], [17], [18], [19], [20]
In our cohort, the incidence of injection site pain in patients who received the first dose of the BNT162b2 vaccine (40%) was lower than the result from the clinical trial (71 percent -83%) [20] but higher than community-based study results (30 percent) [17].
The incidence of systemic side effects in the same group was very similar to those reported in the clinical trial; for example, the incidence of fatigue, myalgia, and headache is 47%, 40%, and 33.3%, respectively, while the incidences of the same side effects in the clinical trial were (34–47%), (14–21%), and (25–42%), respectively [20]. Our cohort had these adverse effects more frequently than the United Kingdom (UK) Community study, where only about a quarter of the participants reported fatigue or headache. [17].
Injection site pain was reported by more than two-thirds of our patients (67.6%) who received the second dosage of the BNT162b2 vaccine, which is consistent with the clinical trial's findings (66–78%). [20].
Fatigue and headache affected 32.4% and 35.3% of the same group, respectively. These figures were lower than the clinical trial's rates of fatigue (53–59%) and headache (39–52%). [20] In a large UK community study, a lower incidence of fatigue (15%) was identified following the BNT162b2 second dose. [17]
Our findings were less prevalent than those of a Czech study that looked at the prevalence of adverse effects among healthcare personnel in the Czech Republic after receiving the BNT162b2, one, and two doses. The following were their findings: injection site pain (89.8%), fatigue (62.2%), headache (45.6%), muscular pain (37.1%), and chills (37.1%). (33.9%). [18]
The most often reported side effects in the group that got the first dose of the ChAdOx1 nCoV-19 vaccine were injection site pain (43.8%), myalgia (50%), fatigue (46.9%), and headache (43.8%). The incidence of both local and systemic side effects was lower in this group than in the ChAdOx1nCoV-19 clinical trial, where the most common side effects after the first dose were injection site pain (67%), tenderness (83%), fatigue (70%), headache (68%), malaise (61%), and muscle ache (60%). [16,19]
In the large UK community-based study that evaluated the side effects after the first dose of ChAdOx1nCoV-19, Local side effects (58.7% (104282 of 177655) were higher than our results. On the other hand, the Systemic side effects (33.7% (116473 of 345280) were lower than ours. [17]
Most individuals' symptoms lasted one day or less. Ninety-five percent of our patients who experienced vaccine-related side effects were symptom free by the end of the first week post-vaccination.
Other studies and vaccination clinical trials have found similar findings. In the BNT162b2 vaccine clinical trial, systemic side effects, such as fever and chills, were noted within the first one to two days after immunization and quickly resolved. [20] In the ChAdOx1nCoV-19 trial, the severity and intensity of local and systemic side effects were highest on the first day of vaccination. [16,19]
Real-world data were shown in two studies: The Czech Study, in which the side effects lasted for one day (45.1%) or three days (35.8%) after the vaccination. [18] The other study, conducted in the United Kingdom, found that the most common side effects occurred within the first 24 h of vaccination and lasted an average of 1.01 days. In the same study, 89.3% of ChAdOx1nCoV-19 vaccination recipients reported no systemic symptoms after three days, and 98.3% reported none after one week. [17]
Most of the individuals' symptoms were mild to moderate in severity. None of the patients was admitted to the hospital because of serious side effects. This finding is consistent with finding in the BNT162b2 clinical trial, where most participants had mild-to-moderate pain in the injection site within the first week of vaccination. [20]
Also, the majority of the participants in the ChAdOx1nCoV-19 clinical trial experienced mild to moderate and self-limiting side effects.
When the frequency of vaccine side effects was compared between patients who received the first and second doses of BNT162b2 vaccine, local side effects such as injection site pain (67.6% vs. 40%, p = 0.001) and redness (26.5% vs. 6.7%, p = 0.019) were more statistically significantly after the second dose. There was no significant difference in systemic side effects frequencies between the two doses.
Systemic side effects such as fever (56.3% vs. 13.3%, p = 0.001), chills (37.5% vs. 6.7%, p = 0.001), myalgia (50% vs. 40%, p = 0.001), and arthralgia (25% vs. 6.7%, p = 0.021) were statistically significantly higher after the first dose of ChAdOx1nCoV-19 vaccine than after the first dose of BBNT162b2 vaccine. In terms of local side effects, there was no notable difference between the two doses.
These findings were supported by real-world data. [17] In the large real-world study in the UK, individuals who received the BNT162b2 vaccine had more local side effects after the second dose than after the first dose. In contrast, those who received the ChAdOx1nCoV-19 vaccine had more systemic adverse effects than those who received the BNT162b2 vaccine. [17]
Side-effects were more common in women than in men, in patients aged 55 years or younger, and in patients receiving polytherapy. These findings are consistent with the clinical trials and real-world data. [16], [17], [18], [19], [20]
Post-vaccination, most of our cohort (93.9%) did not experience any seizure worsening. The relative risk of seizure worsening was low in the three vaccination groups.
The majority of patients who experienced seizures worsening after vaccination are young female patients who have had epilepsy for more than two years and were on polytherapy.
One 30-year-old female patient receiving polytherapy for epilepsy for more than two years and had status epilepticus within the three months before vaccination reported having status epilepticus after getting the first dose of the BNT162b2 vaccine. The calculated relative incidence was low.
Our results were much lower than those of Barlow et al., who found a significantly increased risk of seizures on day one of receiving of diphtheria, tetanus, and whole-cell pertussis (DTR) vaccine (relative risk 5.70; 95% CI 1.98–16.42) and 8–14 days after the receiving of the measles, mumps, and rubella (MMR) vaccine (relative risk 2.82;95% CI 1.44–5.55). [21]
The risk of the seizure in our cohort was slightly higher than the risk in another study investigating the risk of epileptic seizures after vaccination with A/H1N1 2009 influenza vaccine (Pandemrix); in which 859 (out of 373 398) participants got epileptic seizures. There was a very low increased risk of seizures in PwE (1.01, 95% confidence interval 0.74 to 1.39) during the first week after vaccination. In the following three weeks, there was no increase in risk for them (1.00, 0.83 to 1.21). [22]
Many studies supported the safety of different vaccines in patients with idiopathic or symptomatic epilepsy. Huang et al., for example, used risk-interval cohort and self-controlled case series analysis to compare the incidence of seizures in the risk and control periods. They found no evidence of an increased risk of seizures within three days of receiving diphtheria, tetanus, and acellular pertussis (DTaP) vaccine in early childhood. At an older age, there was no mention of seizures or epilepsy. [23]
Shorvon and Berg did a thorough literature review and concluded that the risk of vaccine-induced encephalopathy and/or epilepsy is exceedingly low. [24] Gold et al. reviewed 421 Australian children, concluded that revaccinating children who had immunization-related side effects seemed safe. [25]
According to the literature, the risk of a febrile seizure was one in 19,496 vaccinations, while the risk of an afebrile seizure was one in 76,133 vaccinations. [12]
In our cohort, more than a third (37.9%) of the non-vaccinated group were undecided, while nearly a third (34.9%) had decided against it. The chief causes for their apprehension were the concern of vaccination side effects (42.9%) and fear of epilepsy worsening (23.8%). Because of previous SARS-CoV-2 infection, a tiny percentage of our cohort believed they were ineligible for vaccination. For them, the fault of this theory was well-explained.
These findings were consistent with those of a Lithuanian study, which found that concern about vaccination safety and efficacy and fear of epilepsy worsening were the two most common causes for vaccine hesitation among PwE. [6]
5. Recommendation
-
1-
More extensive and long-term studies are required to report on the long-term safety of the different vaccines.
-
2-
Good explanation and clarification of facts to patients by providing evidence-based information on the vaccine's safety, helping them to make the best vaccination decision possible.
-
3-
We proposed that future research should include subgroups such as mentally ill patients and those with other comorbidities.
6. Conclusion
This study shows that the two vaccines under consideration (the BNT162b2 vaccine and the ChAdOx1 nCoV-19 vaccine) have a good safety profile. Most of the COVID-19 vaccine side effects reported by PwE are mild, moderately frequent, and transient. The risk of seizure worsening was minimal. Nonetheless, for some apprehensive individuals, the safety concern and fear of epilepsy worsening are the major impediments to immunization.
7. Strength of this study
This study is the first to examine the safety of COVID-19 vaccines and the causes of vaccine hesitation among PwE.
8. Limitations
-
a-
Patients’ self-reporting of their side effects may result in recall bias.
-
b-
The patients reported their side effects at different duration after the vaccination. The exact period was not systematically collected for many patients, so this point was not included in this study.
-
c-
Our sample size is relatively small.
-
d-
We surveyed the patients who visited the clinic, which may have resulted in bias due to the absence of the patients with serious side effects.
-
e-
In this study, we excluded the patients with other comorbidities and mentally subnormal patients. As this study was conducted a little early during the vaccine campaign, we only identified one patient with hypertension. We had difficulty obtaining the precise answer for some questions for mentally subnormal patients, as many symptoms were subjective, and we couldn't rely on caregiver answers.
Authors’ contributions
FA: design and conceptualized the study and wrote the initial draft. SFA, JA, KJA: major role in methods creation, statistical analysis, and interpretation. JA, AMH: major role in the acquisition of data. MA: interpreted the data and revised the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Ethical considerations
Ethical approval was obtained from the research committee at Ibn Sina Hospital, Kuwait.
Funding
This study received no funds.
Availability of data and materials
The data of this study are available on request to the corresponding author.
Declaration of Competing Interest
All authors disclose no conflict of interest related to this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.seizure.2021.08.001.
Appendix. Supplementary materials
References
- 1.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. COVID-19 Pandemic 2020, 11 mARCH [Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid- 19—11-march-2020.
- 3.Gostin LO, Salmon DA, Larson HJJJ. Mandating COVID-19 vaccines. 2021;325(6):532-3. [DOI] [PubMed]
- 4.Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. 2008;86:140-6. [DOI] [PMC free article] [PubMed]
- 5.Vergara RJD, Sarmiento PJD, Lagman JDNJJoph. Building public trust: a response to COVID-19 vaccine hesitancy predicament. 2021. [DOI] [PMC free article] [PubMed]
- 6.Puteikis K, Mameniškienė RJIjoer, health p. Factors Associated with COVID-19 Vaccine Hesitancy among People with Epilepsy in Lithuania. 2021;18(8):4374. [DOI] [PMC free article] [PubMed]
- 7.Al-Kattan M, Afifi L, Shamloul R, Mostafa EEDJTEJoN. Assessment of precipitating factors of breakthrough seizures in epileptic patients. Psychiatry, Neurosurg. 2015;52(3):165. [Google Scholar]
- 8.Chowdhury RN, Hasan MH, Rahman KM, Dev SR, Amin MA, Miah T. Precipitating factor of seizure in epilepsy: experience in a tertiary care hospital. Mymensingh Med J. 2014;23(1):56–61. [PubMed] [Google Scholar]
- 9.tracker C-v. Vaccine tracker 2021, June 11 [Available from: https://covid19.trackvaccines.org/country/kuwait/.
- 10.kKuwait M. Kuwait COVID-19 Vaccine 2021 [Available from: https://ourworldindata.org/covid-vaccinations?country=OWID_WRL.
- 11.Kim JH, Marks F, Clemens JDJNm. Looking beyond COVID-19 vaccine phase 3 trials. 2021;27(2):205-11. [DOI] [PubMed]
- 12.Pruna D, Balestri P, Zamponi N, Grosso S, Gobbi G, Romeo A, et al. Epilepsy and vaccinations: Italian guidelines. 2013;54:13-22. [DOI] [PubMed]
- 13.Sarkis RA, Jehi L, Bingaman W, Najm IMJE. Seizure worsening and its predictors after epilepsy surgery. 2012;53(10):1731-8. [DOI] [PubMed]
- 14.MacDonald NE. Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015;33(34):4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Food U, Food DAJ, Drug administration UDoH, Services H. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. 2007.
- 16.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. 2020;396(10249):467-78. [DOI] [PMC free article] [PubMed]
- 17.Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. 2021. [DOI] [PMC free article] [PubMed]
- 18.Riad A, Pokorná A, Attia S, Klugarová J, Koščík M, Klugar MJJoCM. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. 2021;10(7):1428. [DOI] [PMC free article] [PubMed]
- 19.Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet (London, England) 2021;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. 2020;383(27):2603-15. [DOI] [PMC free article] [PubMed]
- 21.Barlow WE, Davis RL, Glasser JW, Rhodes PH, Thompson RS, Mullooly JP, et al. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. 2001;345(9):656-61. [DOI] [PubMed]
- 22.Arnheim-Dahlström L, Hällgren J, Weibull CE, Sparén PJB. Risk of presentation to hospital with epileptic seizures after vaccination with monovalent AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine (Pandemrix): self controlled case series study. 2012;345. [DOI] [PMC free article] [PubMed]
- 23.Huang W-T, Gargiullo PM, Broder KR, Weintraub ES, Iskander JK, Klein NP, et al. Lack of association between acellular pertussis vaccine and seizures in early childhood. 2010;126(2):263-9. [DOI] [PubMed]
- 24.Shorvon S, Berg AJE. Pertussis vaccination and epilepsy—an erratic history, new research and the mismatch between science and social policy. 2008;49(2):219-25. [DOI] [PubMed]
- 25.Gold M, Goodwin H, Botham S, Burgess M, Nash M, Kempe AJAodic. Re-vaccination of 421 children with a past history of an adverse vaccine reaction in a special immunisation service. 2000;83(2):128-31. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this study are available on request to the corresponding author.