Abstract
The skin is the largest organ of the human body, and its dysfunction is related to many diseases. There is a need to find new potential effective therapies for some skin conditions such as inflammatory diseases, wound healing, or hair restoration. Mesenchymal stromal cell (MSC)-conditioned medium (CM) provides a potential opportunity in the treatment of skin disease. Thus, the objective of this review is to evaluate the uses of MSC-CM for treating skin diseases in both animal and human models. A systematic review was conducted regarding the use of MSC-CM for treating skin conditions. One hundred one studies were analyzed. MSC-CM was evaluated in wound healing (55), hypertrophic scars (9), flap reperfusion (4), hair restoration (15), skin rejuvenation (15), and inflammatory skin diseases (3). MSC-CM was obtained from different MSC sources, mainly adipose tissue, bone marrow, and umbilical cord blood. MSC-CM was tested intravenously, intraperitoneally, subcutaneously, intradermally or intralesionally injected or topically applied. MSC-CM was used in both animals and humans. MSC-CM improved wound healing, hair restoration, skin rejuvenation, atopic dermatitis, and psoriasis in both animals and humans. MSC-CM also decreased hypertrophic scars and flap ischemia in animal models. In conclusion, MSC-CM is a promising therapy for skin conditions. Further studies are needed to corroborate safety and effectiveness and to standardize CM manufacturing.
Keywords: advanced therapy, conditioned medium, dermatology, mesenchymal stem cells, stem cells
Introduction
Mesenchymal stromal cells (MSCs) are a type of multipotent adult stem cells that have the potential to proliferate, self-regenerate, and differentiate into multiple cell lineages (Dominici et al., 2006). They can be isolated from several sources such as bone marrow (BM-MSCs), adipose tissue (AT-MSCs), umbilical cord (UC-MSCs), amnion, placenta, or dental pulp (Bogatcheva and Coleman, 2019). MSCs are considered to be one of the most promising therapeutic options in cell therapy and tissue engineering, as they can be used for treating skin, cardiovascular, hematological, neurological, bone, and cartilage diseases (Wong et al., 2015; Kim et al., 2017; Hu et al., 2018; Martinez-Lopez et al., 2020).
It has been observed that the beneficial effects of MSCs are due not only to their multipotent ability but also to their secreted cytokines and growth factors (Caplan, 2017). Cell-free preparations have several advantages over cell therapy, as they can be obtained more easily and more economically and can be manufactured, packaged, and transported straightforwardly (Yang et al., 2021). Moreover, cell-free preparations do not have adverse events associated with cell administration such as rejection, tumorigenic, thrombogenic, ossification, or calcification risk (Bogatcheva and Coleman, 2019; Yang et al., 2021). The risk of malignant transformation of MSCs is a great concern, as MSCs therapy involves ex vivo production and expansion of cell lines, although the spontaneous malignant transformation of human MSCs has not been completely proved and there are many studies that have demonstrated that MSCs, even after physical and chemical stress, undergo senescence rather than become tumorigenic (Caplan et al., 2019). MSCs activate the host innate immune systems and the coagulation, increasing the expression of procoagulant tissue factor and demonstrating a procoagulant effect after MSC contact with blood in in vitro investigations. Infusion reactions and thromboembolism have been reported when using intravascular MSC products. A possible proposed solution to this problem is diluting or treating the MSCs with tissue factor pathway-blocking reagents. Moreover, hemocompatibility testing and optimal product delivery are important for designing safer MSC therapies. Other experimental intravascular therapies, such as islets, hepatocytes, and products derived from MSCs, could also improve MSC safety (Moll et al., 2019). The activation of the immune response could also lead to rejection. There are many investigations that focus on strategies to evade immune recognition, such as human leukocyte antigen (HLA)-matched cells or pharmaceutical immunosuppression (Moll et al., 2019, 2020). Cell-free preparation could help to reduce this risk of malignant transformation, thrombogenic risk, and rejection.
The molecules secreted by stromal cells are referred to the stromal cell secretome and include proteins, microRNA, growth factors, antioxidants, proteasomes, and exosomes (Maguire, 2013). The stromal cell culture media that comprise the secretome are known as the conditioned media (CM), and they are considered to be an abundant resource of paracrine factors (Pawitan, 2014; Mizukami and Yagihashi, 2016). The paracrine factors secreted in vitro include vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF-1), IGF−2, and stromal cell-derived factor 1 (SDF−1) (Ratajczak et al., 2012; Deng et al., 2018). The administration of these factors to the site of an injured organ increases its metabolic activity and oxygen supply and remodels the extracellular matrix (Ratajczak et al., 2012).
The skin is the largest organ of the human body, and its dysfunction is linked to several diseases (Montero-Vilchez et al., 2021). MSCs provide a supply of new cells for epidermal homeostasis, hair cycling, and repairing injured tissue (Kim et al., 2017; Guo et al., 2020). MSCs-CM also provide a potential opportunity in the treatment of skin disease, and there is increased evidence justifying its use for the treatment of cutaneous conditions such as wound healing, hair growth, inflammatory skin diseases, or skin rejuvenation. Thus, the objective of this review is to evaluate the use of MSC-CM for treating skin diseases in both in animals and humans.
Materials and Methods
Search Strategy
A literature search was performed using Medline, Scopus, Embase, and ClinicalTrials.gov from conception to October 2020, following PRISMA Guidelines (Supplementary Material). The following search terms were used: [(MSC) OR (Mesenchymal Stem Cell) OR (Mesenchymal Stromal Cell) OR (Multipotent Stem Cell) OR (Multipotent Stromal Cell) OR (Stem Cell)] AND [(Conditioned Medium) OR (Conditioned Culture Media)] AND [(skin) OR (dermatology)].
Inclusion and Exclusion Criteria
The search was limited to (i) human or animal data, (ii) in vivo studies, (iii) using MSC-CM for skin conditions, and (iv) articles written in English or Spanish.
All types of epidemiological studies (clinical trials, cohort studies, case–control studies, and cross-sectional studies) regarding MSC-CM use for skin conditions were included and analyzed. Reviews, guidelines, protocols, and conference abstracts were excluded.
Study Selection
Two researchers (TMV and AML) independently reviewed the titles and abstracts of the articles obtained in the first search to assess relevant studies. The full texts of all articles meeting the inclusion criteria were reviewed, and their bibliographic references were checked for additional sources. The articles considered relevant by both researchers were included in the analysis. Disagreements about inclusion or exclusion of articles were discussed until a consensus was reached. If no consensus was reached, resolution was achieved by discussion with a third researcher (SAS).
Variables
The variables assessed were MSC source, passage number and percentage of confluence, model tested, treatment group, route of administration, sample size, assessment, outcomes, and adverse events.
Results
Our literature search identified 1,422 references, 757 after removing duplicates. After the title and abstract were screened, 197 records underwent full-text review. A total of 96 records were excluded because they did not investigate MSC-CM treatment in vivo skin conditions. Other reasons for exclusion along with the flowchart are shown in Figure 1. Ultimately, 101 studies met the eligible criteria.
FIGURE 1.
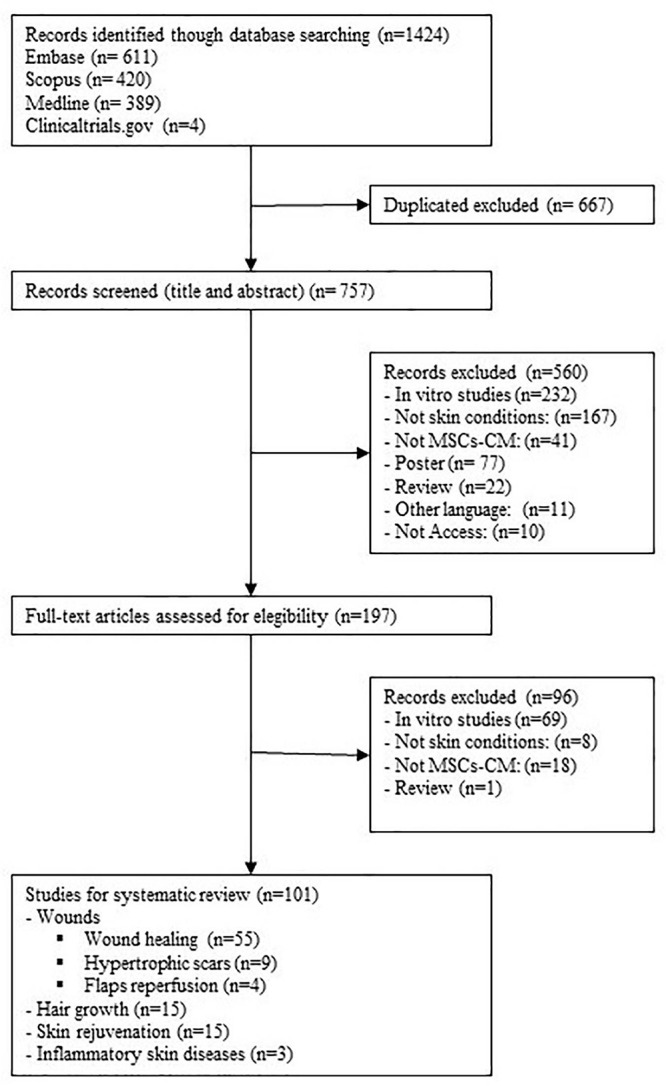
Flow diagram of the study selection process.
Mesenchymal stromal cell-CM has been tested in several skin conditions: wound healing, hypertrophic scars, flap and graft reperfusion, hair restoration, aesthetic applications, and cutaneous inflammatory diseases.
Wounds
Preclinical Studies
Fifty-three studies evaluated the effects of MSC-CM on wound healing, 58.49% (31/53) evaluated outcomes in non-diabetic wounds, and 35.85% (19/53) in diabetic wounds, including three studies that evaluated wound healing in both diabetic and non-diabetic animals (Fong et al., 2014; Tam et al., 2014; Raj et al., 2019). Moreover MSC-CM has also been tested on burn wounds (5.66%, 3/53), infected wounds (3.77%, 2/53), and radiation-induced wounds (1.89%, 1/53).
Non-diabetic wounds
Thirty-one studies evaluated the potential of MSC-CM for treating non-diabetic wounds (Table 1). To obtain CM, cells were mainly isolated from human tissues (74.19%, 23/31): from adipose tissue (Cho et al., 2010; Heo et al., 2011; Deng et al., 2017; Yuan et al., 2018; Chen et al., 2021) (21.74%, 5/23), amnion (Yoon et al., 2010; Jun et al., 2014; Park et al., 2018; He et al., 2020) (17.34%, 4/23), umbilical cord blood (Lee et al., 2011; Dong et al., 2017; Raj et al., 2019; Rong et al., 2019) (17.34%, 4/23), bone marrow (Tamari et al., 2013; Chen et al., 2014; Ahangar et al., 2020) (13.04%, 3/23), and Wharton’s jelly (Fong et al., 2014; Tam et al., 2014; Sabzevari et al., 2020) (13.04%, 3/23). Human dental pulp (Zhou et al., 2020) and skin (Robert et al., 2019) were also used. Moreover, murine, swine, caprine, canine, deer, and rabbit tissues were also employed (Chen et al., 2008; Templin et al., 2009; Sun et al., 2014; Mehanna et al., 2015; Du et al., 2017; Payushina et al., 2018; Rong et al., 2019; Joseph et al., 2020). CM was collected from MSCs between passages 1 and 12 at 50–100% confluence. The most common model was murine (96.77%, 30/31), although a swine model was also used (Joseph et al., 2020). Regarding the route of administration, MSC-CM was mainly used topically (51.61%, 16/31), applied to the wounds or around them in cream, hydrogel, or membranes (Heo et al., 2011; Lee et al., 2011; Chen et al., 2014; Jun et al., 2014; Sun et al., 2014; Mehanna et al., 2015; Park et al., 2018; Joseph et al., 2020). MSC-CM was also injected (41.94%, 13/31), mainly subcutaneously (25.81%, 8/31) (Templin et al., 2009; Lee et al., 2011; Deng et al., 2017; He et al., 2020; Zhou et al., 2020) but also intradermally (Cho et al., 2010) and intraperitoneally (Fong et al., 2014). Subcutaneously injected and topically applied concomitant MSC-CM was also tested (Chen et al., 2008; Yoon et al., 2010). Topical application might be more effective than subcutaneous injections (Lee et al., 2011).
TABLE 1.
Studies regarding mesenchymal stromal cell-conditioned medium for treating wounds in animal models.
| MSC source | Method of tissue extraction | MSC characterization | Preparation of MSC-CM | Model | Groups of treatments and via of administration | Follow-up (days) | Assessment | Main outcome | Other outcomes | |
| Chen et al., 2021 | Human subcutaneous adipose tissue samples | Liposuction | Flow cytometry (CD29+, CD90+, CD105+, CD31−, CD34−, CD45−). Osteogenic and adipogenic differentiation | MSCs of passage 3 at 90% confluence were used. CMs at different dilutions (100, 50, and 25%) were collected. Electrospun fibrous scaffolds formed by emulsion electrospinning; an ADSC-CM loaded micro-nano polylactic acid (PLA) electrospun fiber (MPF) was developed | Murine. Full-thickness excisional wounds, 15 mm × 15 mm, on dorsal surface | Wounds were covered with an electrospun membrane - Control - MPF alone - MPF loaded with 100% AT-MSC-CM - MPF loaded with 50% AT-MSC-CM - MPF loaded with 25% ATMSC-CM (n = 3/group) |
15 | Macroscopic appearance (photography), histology (HE, MT), IHC, qRT-PCR | MPF loaded with MSC-CM accelerated wound healing and prevented abnormal scar formation, promoting angiogenesis without adversely affecting epidermal cells | MPF loaded with MSC-CM inhibited ECM deposition, including Col I and Col III, and decreased α-SMA expression, showing an inhibitory effect on fibroblast differentiation. Groups with less collagen deposition and smaller scar area showed more VEGF expression and a faster healing rate. The wound area of MPF loaded with 100% AT-MSC-CM was only 34.9% of that of the control group |
| Zhou et al., 2020 | Human dental pulp isolated from periodontally compromised teeth (P-DP-MSCs) and healthy teeth (H-DP-MSCs) | Teeth extracting | Flow cytometry (CD90+, CD105+, CD146+, CD31−, CD34−, CD45−). Osteogenic, adipogenic, and chondrogenic differentiation | MSCs of passage 4 at 90% confluence were used. CM was collected and, it was used to obtain EVs | Murine. Full-thickness excisional wounds, on the dorsal surface | 100 μl subcutaneously injected at 4 sites around the wound (25 μl per site) - Control - P-DP-MSC-CM EVs - H-DP-MSC- CM EVs (n = 10/group) |
14 | Macroscopic appearance (photography), microscopic appearance (newly formed vessels), histology (HE), IHC (VEGF, CD31) | Wound closure was markedly accelerated by DP-MSC-CM EVs and P-DP-MSC-CM EVs. P-DP-MSC-CM EV group closed faster than H-DP-MSC-CM EV group (p < 0.05) | DP-MSC-CM extracellular vesicle-treated wounds had a lower level of scar formation and enhanced vessel formation in the wound sites. No adverse events were observed |
| Sabzevari et al., 2020 | Wharton’s jelly from human umbilical cords | Cesarean section | Flow cytometry (CD44+, CD73+, CD90+, CD105+, CD14−, CD31−, CD34−, CD45−, HLA-DR−). Osteogenic, adipogenic, and chondrogenic differentiation | MSC of passages 4–6 at 70% confluence were used. WJ-MSCs were transfected with a recombinant construct encoding hCAP-18/LL-37 gene. Next, the CM of the transfected cells (LL-37-MSCs) was harvested, and its concentrate was formulated in a sodium alginate (SA)/gelatine (G) hydrogel | Murine. Full-thickness circular excision wound, 20 mm, below the skull | The hydrogel was placed on the wound and covered by a wound dressing - PBS (control) - SA/G-PBS group (the SA/G-2/8 hydrogel contained only PBS) - SA/G-V-CM group (the SA/G-2/8 hydrogel contained only CM vehicle) - SA/G-LL-37-CM group (the SA/G-2/8 hydrogel contained LL-37-CM) (n = 3/group) |
21 | Macroscopic appearance (photography), histology (HE, MT), qRT-PCR | WJ-MSC-CM hydrogel effectively accelerated wound contraction and promoted wound healing, even more in the SA/G-LL-37-CM group | Blood vessels were higher in SA/G-LL-37-CM group, showing the early angiogenesis profile with higher VEGF-A levels. This group also showed the best collagen arrangement, thickness, and density |
| Joseph et al., 2020 | Caprine, canine, and guinea pig bone marrow | Aspiration needle from the iliac crest | Flow cytometry (CD 73+, CD90+, CD 105+, CD34−). Chondrogenic, osteogenic, and adipogenic differentiation | MSCs of passage 3 at 80% confluence were used. CM was collected and then lyophilized by the freeze−drying method and was vacuum sealed and stored at 4°C. A serum−free DMEM medium was processed for use as a control | Guinea pig. Full-thickness excisional skin wounds, 20 mm × 20 mm, on the dorsum on either side of the midline | 100 μl of formulated respective CM was applied topically with sterile applicator over the wounds one per week - Control (laminin gel+DMEM) - Canine CM (laminin gel+canine MSC−CM) - Caprine CM (laminin gel+caprine MSC−CM) |
28 | Macroscopic appearance (photography), histology (HE) | MSC−CM accelerates excision wound healing compared with control (p < 0.05) | Surface epithelium, neovascularization, and collagen depositions improved more in MSC-CM-treated group than in controls (p < 0.05). Allogeneic and xenogenic application of CM significantly improved wound healing quality, with minimal scar formation. Better healing rate was observed in the allogeneic group |
| - Guinea pig CM (laminin gel+guinea pig MSC−CM) (n = 6/group) | ||||||||||
| He et al., 2020 | Human amnion | Amniocentesis | Flow cytometry (CD73, CD45, CD31, CD105, CD44, CD34, CD90, CD29, SSEA-3, SSEA-4, EP-CAM, HLA-DR). Chondrogenic, osteogenic, and adipogenic differentiation | MSCs of passage 2 at 90% confluence were used. CM was collected | Murine. Full-thickness excisional skin wounds, 10 mm, on the dorsum | 50 μl subcutaneous injected into the surrounding tissues of the wound bed at four sites - PBS (control) - MSC-CM - Murine LOXL2 (5 μg, Sino Biologicals, China) (n = 4/group) |
12 | Macroscopic appearance (photography), histology (HE, TC) | Wound sizes were significantly reduced in the LOXL2 and MSC-CM groups compared with controls (p < 0.05) | MSC-CM and LOXL2 enhanced wound healing. Epidermis of the MSC-CM and LOXL2 group mice resembled normal skin and the keratinocytes were well organized and tightly arranged. These treatments also significantly reduced fibrosis and improved keratinocyte proliferation. No adverse events were reported |
| Ahangar et al., 2020 | Human bone marrow | Aspiration | − | MSCs of passage 1 at 70% confluence were used. CM was collected | Murine. Full-thickness excisional skin wounds, 6 mm2, on dorsal surface | 100 μl was injected at four sites into the margins of each wound MSC-CM - DMEM (n = 8/group) |
14 | Macroscopic appearance (photography), histology (HE, TC), IHC, qRT-PCR | MSC-CM improved wound healing, showing a significant reduction in average wound area and wound width compared with DMEM-treated group (p < 0.05) | At day 7 post-wounding, wounds in MSC-CM-treated mice were 90% re-epithelialized compared with 78% in control group. MSC-CM also decreased inflammatory response, increased endothelial cell number and angiogenesis (showed in an increased numbers of CD31-positive |
| endothelial cells), and increased both collagen I and III expression | ||||||||||
| Rong et al., 2019 | ASC: sticks of antlers from sika deer hU-MSC: human umbilical cords | hU-MSC: Cesarean sections | − | MSCs of passage 3 at 80% confluence were used. CM was collected | Murine. Full-thickness skin excisional wounds, 8 mm in diameter, on the dorsal surface | MSC-CM was mixed with the hydrogel. 200 μl of each group was pipetted onto the wound every 2 days - DMEM - EGF - MSC-CM - ASC-CM (n = 8/group) |
16 | Photography, histology (HE), IHC | ASC-CM group accelerated wound healing compared with other treatments. At 16 days, wounds in ASC-CM group were completely closed, while other groups showed different sizes of unhealed wounds (DMEM, 6.14 ± 4.1 mm2; EGF, 1.79 ± 3.2 mm2; hU-MSC-CM, 0.61 ± 2.3 mm2, p < 0.05) | ASC-CM group had the thickest dermis containing the highest number of cutaneous appendages and the highest vessel numbers. The ASC-CM treatment significantly upregulated the expression ratios of Col3A1/Col1A2, TGF-β3/TGF-β1, MMP1/TIMP1 and MMP3/TIMP1 |
| Robert et al., 2019 | Human skin | Face-lifting | − | MSCs of passage 6 at 90% confluence were used. CM was collected | Murine. Full-thickness excisional wound, 6 mm in diameter, on dorsum surface | CM was directly applied to the wounds or embedded within hydrogels - Control, no treatment (n = 12) - Carrageenan hydrogel (n = 9) - Carrageenan hydrogel embedded with MSC-CM (n = 10) - Polyvinyl alcohol hydrogel (n = 10) - Polyvinyl alcohol hydrogel-embedded with MSC-CM (n = 11) - Only MSC-CM (n = 8) |
14 | Macroscopic appearance (photography), histology (HE) | All groups showed successfully repaired and closed wounds, although the animals treated with CM embedded in PVA (or only with PVA) displayed slightly larger wounds (p > 0.05) | Improvements in wound closure were not observed, but MSC-CM increased angiogenesis, independently of the hydrogel used |
| Raj et al., 2019 | Human umbilical cord | Umbilical cord dissection | Flow cytometry (CD13+, CD29+, CD44+, CD90+, CD10−, CD14−, CD34−, CD117−) | MSCs were transduced with a lentiviral vector (green fluorescence protein tagged). MSCs of passage 3–4 were used. CM was collected. Wound dressing patches: impregnated of aloe verapolycaprolactone (AV/PCL) nanoscaffolds with hWJSCs or hWJSC−CM were also created | Murine. Full-thickness excisional wound, 8 mm, on the back | 100 μl of: - PBS with 1 × 106 MSCs - MSC-CM - PBS with 1 × 106 fibroblast - Fibroblast-CM - UCM - PBS with 1 × 106 MSCs+AV/PCL - MSC-CM+AV/PCL - PBS with 1 × 106 fibroblast + AV/PCL - Fibroblast-CM+AV/PCL - PBS+AV/PCL - Untreated group (n = 9/group) |
28 | Macroscopic appearance (photography), histology (HE), IHC, qRT−PCR | The dermal thickness of both MSCs+AV/PCL (290.55 μm) and MSC−CM+AV/PCL (338.3 μm) treatment groups was significantly greater than that of the controls (PBS+AV/PCL, 193.51 μm; UCM, 266.55 μm; fibroblast+AV/PCL, 235.29 μm; fibroblast−CM+ AV/PCL, 227.31 μm) | CD31 marker showed strong positive signals in the MSC-CM group and in the MSCs group compared with untreated controls |
| Im et al., 2019 | Human (type is not specified) | Purchased from Lonza (Basel, Switzerland) | − | MSCs of passages 6–12 were used. CM from the MSCs treated with or without gold–iron nanoparticles was collected | Murine. Full-thickness excisional wound, 20 mm × 20 mm, on the back | Daily injection of CM (200 μl/wound) was for 4 days - MSC-CM passage 6 - MSC-CM passage 12 - MSC-CM passage 12 with gold–iron nanoparticles (n = 4/group) |
14 | Macroscopic appearance (photography), histology (HE), IHC, qRT-PCR | All wounds were almost closed with similar appearance in all groups | Increased CD31 expression was observed in MSC- CM passage 12 with gold–iron nanoparticles group compared with the passage 12 without gold–iron nano- particles. SM-α expression did not exhibit differences between groups. The relative amount of involucrin and laminin was higher in MSC-CM passage 6 and MSC-CM passage 12 with gold–iron nanoparticles compared with MSC-CM passage 12 |
| Yuan et al., 2018 | Human adipose tissue | − | Flow cytometry (CD49+, CD73+, CD90+, CD105+, CD34−). Adipogenic and chondrogenic differentiation | MSCs of passages 2–5 were used. Cells were cultured in the presence of the profibrogenic cytokine TGF-β1. CM was collected | Murine. Full-thickness skin wounds, 6 mm punch, on the back | Wounds were applied with 0, 10, 50, and 100% MSC-CM* or left untreated (n = 7/group) | 14 | Macroscopic appearance (photography) | MSC-CM treatment significantly accelerated wound healing (p < 0.05) | Compared with the non-treated wounds and 0% MSC-CM group, 100% MSC-CM treatment accelerated wound healing on the seventh day after wounding (ratio of untreated wound area: 100% in untreated group, 100% in 0% MSC-CM, and 50% in 100% MSC-CM) |
| Payushina et al., 2018 | Murine bone marrow from tibia and femur | − | Flow cytometry (CD73+, CD90+). Osteogenic and adipogenic differentiation | MSCs of passage 2 were used. CM was collected | Murine. Full-thickness skin wounds, 20 mm in diameter, on the back | 100 μl MSC-CM or control CM was injected into the wound bed each other day five times (n = not specified) | 14 | Clinical examination, histology | MSC-CM group showed greater number of blood vessels (53.20 ± 2.58 blood vessels per field of view) compared with controls (37.20 ± 4.73), p < 0.05 | MSC-CM group showed less intensive inflammation and more complete epithelialization compared with controls |
| Park et al., 2018 | Human amniotic fluid | Amniocentesis | Flow cytometry (CD13+, CD29+, CD44+, CD71+, CD90+, CD120a+, CD31−, CD106−, CD15−, CD33−, CD34−, CD45−). Adipogenic, osteogenic, and chondrogenic differentiation | MSCs of passage 12 at 70–100% confluence were used. They were supplemented with selenium and bFGF. CM was collected | Murine. Full-thickness skin wounds, 8 mm, side of the midline | Vehicle, CM supplemented with selenium (−/s), CM supplemented with bFGF (b/−), or CM supplemented with bot selenium and bFGF (b/s), was topically applied to the induced wounds in a volume of 20 μl (n = 10/group) | 11 | Macroscopic appearance (photography), histology (HE, 3,3′-diaminobenzidine tetrahydrochloride staining), IHC | Treatment with CM (b/s) resulted in complete wound closure, while not completed closure was observed in the other groups | The CM (−/s) group exhibited better recovery than the CM (b/−) group. The CM (b/s) group showed the thickest epidermis region and the highest expression of involucrin. Smad2, AKT-MEK1/2-ERK, and NFκB signaling pathways were more effectively activated by CM (−/s) than by CM (b/−), and their |
| highest activation was seen when treated with CM (b/s) | ||||||||||
| Tarcisia et al., 2017 | Adipose tissue | − | Flow cytometry positive for (CD73+, CD90+, CD34−). Osteogenic, chondrogenic, and adipogenic differentiation | MSCs of passage 3 were used. CM was then collected | Murine. Full-thickness excisional skin wounds, 20 mm long and 5 mm depth, on the back | The four cuts of each rat were treated randomly with MSC-CM (100%), complete culture medium, basal medium, and without treatment (control). The treatment was only done once after the rat skin injury (n = 30/group) | 28 | Macroscopic appearance (photography), histology (HE, MT). | Wounds treated with MSC-CM showed improvement in wound healing process | MSC-CM showed greater collagen density, angiogenesis, ratio, and length of epithelialization than the other groups (p < 0.05) |
| Tarcisia et al., 2017 | Adipose tissue | − | Flow cytometry positive for (CD73+, CD90+, CD34−). Osteogenic, chondrogenic, and adipogenic differentiation | MSCs of passage 3 were used. CM was then collected | Murine. Full-thickness excisional skin wounds, 20 mm long and 5 mm depth, on the back | The four cuts of each rat were treated randomly with MSC-CM (100%), complete culture medium, basal medium, and without treatment (control). The treatment was only done once after the rat skin injury (n = 30/group) | 28 | Macroscopic appearance (photography), histology (HE, MT). | Wounds treated with MSC-CM showed improvement in wound healing process | MSC-CM showed greater collagen density, angiogenesis, ratio, and length of epithelialization than the other groups (p < 0.05) |
| Lee et al., 2017 | Adipose tissue | − | − | Ell3 expression was suppressed using siRNA transfection in MSCs. CM harvested from MSCs transfected with siNS or siEll3 was collected | Murine. Full-thickness excisional skin wound, back | 100 μl of CM prepared from siNS- or siEll3-transfected MSCs or serum-free media was applied to the wound (n = not specified) | 7 | Macroscopic appearance (photography), histology (HE), IHC. | Skin wounds treated with siEll3 CM recovered to a lesser extent than those treated with serum-free media | siEll3 CM could not enhance the wound healing rate, whereas siNS CM significantly promoted wound repair |
| Du et al., 2017 | Rabbit bone marrow | − | − | MSCs of passage 2 were used. MSCs were cultured in normoxic (Nc) and chemical hypoxic conditioning by adding CoCl2 (Cc). Decellularization was conducted and extracellular matrices (ECM) cell-free were constructed | Murine. Full-thickness excisional skin wound, 7 mm, on the back | No treated group (control), mice treated with Nc-ECM sheets or Cc-ECM sheets (n = 16/group) | 7 | Macroscopic appearance (photography), histology (HE, MT, picrosirius red) | All the Cc-ECM-treated wounds completely healed on day 7, while Nc-ECM-treated wounds healed about 85.0 ± 8.6%, and non-treated wounds only healed 69.8 ± 9.6% | No inflammatory signs or visible indication of necrosis or other adverse events were observed |
| Dong et al., 2017 | Human umbilical cord | − | − | MSCs were transduced with human Wnt7a cDNA retroviral vector. MSCs of passage 3 at 50–60% confluence were used. Passage-3 MSCs were. CM from MSCs (MSC-CM) and from MSC with Wnt7a (Wnt-CM) was collected | Murine. Full-thickness excisional wound, 10 mm, on the back | 100 μl of Wnt-CM, MSC-CM, or DMEM was subcutaneously injected at multiple points into the wound area (n = 3/group) | 14 | Macroscopic appearance (photography), histology (HE, MT) | Wnt-CM significantly enhanced the closure rates in comparison with MSC-CM and DMEM (91.5 vs. 76.3 vs. 65.1%, respectively) | Wnt-CM accelerated migration of HFs into the wound area, had a thicker epidermis with more organized cell layers, and showed regeneration of more hair follicles. Increased expression of the α-SMA, collagen I, and collagen III was also observed in Wnt-CM group |
| Dong et al., 2017 | Human adipose tissues | − | − | Coleman adipose tissue was mechanically emulsified to obtain ECM/SVF-gel. For SVF preparation, the Coleman adipose tissue was digested. Supernatant from ECM/SVF-gel (gel-CM), Coleman adipose tissue (adi-CM), and SVF (SVF-CM) culture were collected to obtain CM | Murine. Full-thickness excisional skin wound, 8 mm, on the back | 100 ml PBS (control), gel-CM, adi-CM, or SVF-CM was injected into the wounds (n = 15/group) | 14 | Macroscopic appearance (photography), histology (HE, MT), ELISA | CM treatments resulted in a significant upregulation of collagen production compared with controls, revealing that the Gel-CM-group had the highest production of collagen | Higher expression of bFGF, EGF, and TGF-b in Gel-CM was observed |
| Mehanna et al., 2015 | Murine bone marrow | Needle flushing | Flow cytometry (CD44+, CD45−) | MSCs of passage 3 were used, and CM was collected | Murine. Full-thickness square-shaped skin wound (35 mm × 35 mm), on the back | Topical application - Untreated group (control) - Fibrin glue only group - Fibrin+MSCs group - Fibrin +MSC-CM group (n = 13/group) |
35 | Macroscopic appearance (photography), functional parameters (TEWL, SCH, tensile strength assessment), histology (HE, MT), IHC | Wounds’ size in fibrin+MSCs and fibrin+MSC-CM groups showed significant decrease in comparison with only fibrin and controls | CD68+ macrophages infiltrating granulation tissue were considerably higher in fibrin+MSC and fibrin+CM groups. SCH and tensile strength were higher, while TEWL was lower in both fibrin+MSC and fibrin+CM than in only fibrin and control group |
| Tam et al., 2014 | Wharton’s jelly from human umbilical cord | Cutting umbilical cords | Flow cytometry (CD13+, CD29+, CD44+, CD90+, CD10−, CD14−, CD34, CD117) | MSCs of passage 3–4 were used. It was a constructed wound dressing patch made up of an aloe vera−PCL (AV/PCL) nanoscaffold impregnated with WJ-MSCs or its CM | Murine. Full-thickness skin wounds, 8 mm, on dorsal region | - MSCs+AV/PC - MSC-CM+AV/PCL - Fibroblast+ AV/PCL - Fibroblast-CM+AV/PCL - PBS+ AV/PCL - Untreated (n = 9/group) |
28 | Macroscopic appearance (photography), histology (HE and MT), IF, WB, qRT-PCR | MSCs+AV/PCL and MSC-CM+AV/PCL treatment arms showed faster wound closure compared with other groups (p < 0.05) | MSCs+AV/PCL and MSC-CM+AV/PCL groups showed higher numbers of sebaceous glands, hair follicles, cellularity, and vasculature compared with other groups |
| Sun et al., 2014 | Murine abdominal subcutaneous adipose tissue | Incision | Flow cytometry (CD29+, CD90+, CD105+, CD34−). Osteogenic and adipogenic differentiation | MSCs of passage 3 at 90% confluence were used. Hypoxic microenvironment was generated using a disposable oxygen-absorbed and CO2 generator. CM was collected | Murine. Full-thickness skin incisions, 30 mm in diameter, on the back | Topical application - Concentrated hypoxic MSC-CM - Hypoxic MSC-CM - Serum medium (n = 10/group) |
21 | Macroscopic appearance (photography), histology (HE) | The average healing time was lower in concentrated hypoxic MSC-CM than in hypoxic MSC-CM and control group (16.2 ± 0.98 vs. 17.7 ± 0.78 vs. 21.3 ± 1.10 days) | Wound closure after treatment with concentrated hypoxic MSC-CM showed well-organized epidermis, thick cuticular layer, and increased collagen content than the other groups |
| Jun et al., 2014 | Human amniotic fluid | Amniocentesis | Immunofluorescence. Adipogenic and osteogenic differentiation | MSCs at 70% confluence were used. Cells were cultured in normoxic (nor) and hypoxic (hypo) conditions. CM was collected | Murine. Full-thickness excisional skin wound, 2 mm full thickness, on each side of the midline | 100 μl topically applied - MSC-hypoCM - MSC-norCM - DMEM (n = 10/group) |
5 | Macroscopic appearance (photography), histology (HE), IHC | MSC-hypoCM significantly accelerated wound closure compared with DMEM and MSC-norCM groups (p < 0.05) | The skin structure of wounds treated by MSC-hypoCM was more similar to normal skin structure. TGF-β/SMAD2 and PI3K/AKT signal pathways were upregulated in AF-MSC-hypoCM |
| Fong et al., 2014 | Wharton’s jelly from human umbilical cord | Full-term delivery | Immunofluorescence (CD10+, CD13+, CD29+, CD44+, CD90+) | MSCs of passages 3–4 at 80% confluence were used. CM was then collected | Murine. Full-thickness excisional skin wound, 8 mm, on dorsum surface | 100 μl injected intraperitoneally - MSCs - MSC-CM - Fibroblast - Fibroblast-CM - UCM (n = 9/group) |
28 | Macroscopic appearance (photography), histology (HE), IHC | MSC and MSC-CM healing rates were greater compared with controls (p < 0.05) | Wounds treated with MSCs and MSC-CMs showed greater re-epithelialization, vascularity, cellular density, sebaceous gland, and hair follicle numbers compared with controls |
| Chen et al., 2014 | Human bone marrow | − | − | MSCs of passages 3–4 at 80% confluence were used. MSCs were cultured and expanded under normoxic or hypoxic conditions. CM was collected from the normoxic and hypoxic MSCs to yield norCM and hypoCM | Murine. Full-thickness excisional skin wounds, 18 mm, on dorsal surface | 100 μl of treatment topically applied to skin wounds and covered with dressings daily for the first 4 days - MSC-norCM - MSC-hypoCM - Vehicle (control) (n = 16/group) |
14 | Macroscopic appearance (photography), IHC, IF | HypoCM-treated mice showed smaller wound area compared with norCM groups and vehicle control (26.42 ± 62.48 vs. 37.92 ± 62.44 vs. 45.00 ± 61.97%, respectively) | MSC-hypoCM accelerated wound closure compared with norCM and vehicle control |
| Tamari et al., 2013 | Human bone marrow | − | − | MSCs purchased from LONZA | Murine. Full-thickness excisional skin wound, 8 mm, on the midline on the back | Injection administered at 4 spots around the wound - PBS - MSCs - MSC-CM (n = not specified) |
14 | Macroscopic appearance (photography), histology, ELISA | Epithelialization rate was higher in MSC and MSC-CM group compared with controls: non-epithelialized wound area after treatment was 42.64 ± 5.36% in the control group, 4.90 ± 2.36% in MSC group, and 5.74 ± 2.85% in MSC-CM group | MSC and MSC-CM accelerated wound healing. HA production in MSC and MSC-CM group was increased compared with controls |
| Lee et al., 2011 | hCB: human cord blood hESC: human embryo | − | Fluorescence-activated cell sorter (CD133+, KDR+) | MSCs of passages 5–8 at 80 confluence were used. CM was collected | Murine. Full-thickness skin wound, 12 mm, on dorsal surface | 200 μl of CM was subcutaneously injected around the wound site or applied topically - hESC-CM - hCB-CM - Control medium (n = 10/group) |
21 | Macroscopic appearance (photography), histology | Wound healing rate was higher in groups treated with CM (90, 70, and 40% in the hESC-CM, hCB-CM, and vehicle medium, respectively) | Wound closure rate in hESC-CM group was higher when it was used topically instead of subcutaneously |
| Heo et al., 2011 | Human adipose tissue | Elective surgery | − | MSCs of passages 2–5 were used. CM was collected. Immunoprecipitation of TNF-α was also used, and CM implemented TNF-α was collected | Murine. Full-thickness excisional skin wounds, 8 mm, on the dorsal surface | 20 μl topically applied on the wound bed daily - PBS - MSC-CM - MSC-CM with TNF-α (n = 8/group) |
12 | Macroscopic appearance (photography), histology | MSC-CM with TNF-α accelerated wound closure compared with PBS MSC-CM | Number of blood vessel was the highest in MSC-CM with TNF-α group |
| Yoon et al., 2010 | Human amniotic fluid | Amniocentesis | Immunofluorescence (CD13+, CD29+, CD44+). Osteogenic, adipogenic, and chondrogenic differentiation | MSCs of passage 3 at 70% confluence were used. CM was collected | Murine. Full-thickness excisional skin wound, 2 mm, on each side of the midline. | 100 μl subcutaneous injection around the wound and topically applied on the wound bed | 8 | Macroscopic appearance (photography), histology (HE), IHC | MSC-CM accelerated wound closure compared with control | No difference in skin structure was observed between groups |
| - Control medium | ||||||||||
| - MSC-CM (n = 10/group) | ||||||||||
| Cho et al., 2010 | Human subcutaneous adipose tissue | Liposuction | Flow cytometry (CD90+, CD49d−) | MSCs of passage. TGF-β1-treated MSC-CM or non-treated MSC-CM was collected | Murine. Circular full-thickness skin wounds, 4-mm diameter, on the back | Intradermal injections (0.05 ml/point × 4 points) into the wound base twice per week | 10 | Macroscopic appearance (photography), histology (HE) | Wound size was reduced in both groups | TGF-β1-treated MSC-CM accelerated wound healing compared with MSC-CM |
| - Bactroban oint with MSC-CM - Bactroban oint with TGF-β1-treated MSC-CM (n = 3/group) |
||||||||||
| Templin et al., 2009 | Murine bone marrow | − | − | Retroviral gene transfer into lin- cells was performed, and DK mix cells were created. Cells and CM were then collected | Murine. Full-thickness skin wounds, 5-mm diameter, on dorsal surface | 200 μl subcutaneously injected around the wound at 8 different sites - MSC-CM - MSCs - PBS (n = 8/group) |
13 | Macroscopic appearance (photography), | MSC and MCS-CM accelerated wound healing compared with PBS (area non-epithelialized after follow-up: MSC = 25.9 ± 2.6%, MSC-CM = 25.1 ± 1.5%, PBS = 48.3 ± 4.7%) | Capillary density was higher in MSC-CM and MSC groups |
| Chen et al., 2008 | Murine bone marrow from femurs and tibia | − | q | MSCs in passage 3 at 80% confluence under hypoxic conditions were used | Murine. Full-thickness skin wounds, 6-mm diameter, on each side of midline | 100 ml (80 ml for subcutaneous injection around the wound and 20 ml for topical application on the wound bed) - MSC-CM - Fibroblast-CM - Vehicle (n = 5/group) |
14 | Macroscopic appearance (photography), histology (HE), IHC | MSC-CM significantly accelerated wound closure compared with fibroblast-CM or vehicle (wound closure percentage: 61 vs. 53 vs. 51%, respectively) | MSC-CM increased cell recruitment. |
ASCs, antler stem cells; AT, Adipose tissue-derived; bFGF, basic fibroblast growth factor; AF, amniotic fluid; BM, bone marrow; CM, conditioned medium; DMEM, Dulbecco’s modified Eagle medium; DP, dental pulp; ECM, extracellular matrix; EGF, epidermal growth factor; ELISA, enzyme-linked immunosorbent assay; EVs, extracellular vesicles; Exos, exosomes; HE, hematoxylin and eosin; hCB, human cord blood; hESC, human embryonic stem cell; IHC, immunohistochemistry; IF, immunofluorescence; MPF, micro-nano polylactic acid electrospun fiber; MSCs, mesenchymal stromal cells; MT, Masson’s trichrome; PBM, photobiomodulation; PBS, phosphate-buffered saline; qRT-PCR, real-time quantitative polymerase chain reaction; SVF, stromal vascular fraction; UCM, unconditioned medium; WB, Western blotting; WJ, Wharton’s jelly. *0, 10, 50, and 100% MSC-CM refers to the dilutions of the conditioned medium in DMEM/F12 with 2% FBS.
MSC-CM was tested for treating wounds in 421 animals. The mean wound size was 11.65 mm (from 2 to 35 mm), and the mean follow-up was 16.48 days (from 5 to 35 days). Wounds were assessed mainly by macroscopic appearance, histology, immunohistochemistry, and qRT-PCR. Outcomes showed that MSC-CM had better results in terms of wound closure, re-epithelialization, and vascularization than non-treated groups or other treatment groups (phosphate-buffered saline (PBS), hydrogel without cells, Dulbecco’s modified Eagle medium (DMEM), and unconditioned medium). Moreover, some research compared the effects between MSCs and MSC-CM with similar results. Topically applied MSCs and their CM via fibrin vehicle showed similar wound healing rates (Mehanna et al., 2015). Healing rates, vascularity, and cellular density were similar between MSCs and MSC-CM injected intraperitoneally (Fong et al., 2014). Injections of MSCs or MSC-CM around the wound showed similar results in wound healing (Templin et al., 2009; Tamari et al., 2013) (non-epithelialized area after 14 days’ follow-up: 25.9 ± 2.6% in MSCs and 25.1 ± 1.5% in the MSC-CM group).
Different MSC sources and CM delivery were also compared. Allogeneic MSC-CM showed better healing rate than xenogeneic MSC-CM treatment (Joseph et al., 2020). The wound closure rate was higher in animals treated with carrageenan-embedded CM than mice treated with polyvinyl alcohol-embedded CM (Robert et al., 2019). Moreover, CM supplemented with selenium and basic fibroblast growth factor (bFGF) showed better results in wound healing than CM alone or CM only supplemented with bFGF or selenium, as complete wound closure after 11 days’ follow-up was only observed in CM supplemented with both growth factors (Park et al., 2018). Hypoxic conditions also improved wound healing, accelerating wound closure (Chen et al., 2014; Jun et al., 2014; Sun et al., 2014; Du et al., 2017). TNF-α- and TFG-β1-implemented CM also accelerated wound healing as compared with non-supplemented MSC-CM (Cho et al., 2010; Heo et al., 2011). CM derived from Wnt7a-transduced MSCs also showed higher closures rates than did the MSC-CM group (Dong et al., 2017). No adverse events were reported in these studies.
Diabetic wounds
Nineteen studies evaluated the effects of MSC-CM for treating diabetic wounds (Table 2). To obtain CM, cells were mainly isolated from human tissues (89.47%, 17/19): from umbilical cord blood (Kim et al., 2010; Kusindarta et al., 2016; Chen Z. et al., 2018; Raj et al., 2019; Zhang S. et al., 2020) (29.41%, 5/17), bone marrow (Pouriran et al., 2016; Amini et al., 2018; Bagheri et al., 2018; Saheli et al., 2020) (23.53%, 4/17), adipose tissue (Deng et al., 2019; De Gregorio et al., 2020) (11.76%, 2/17), and Wharton’s jelly (Fong et al., 2014; Tam et al., 2014) (11.76%, 2/17). Human hair follicles (Ma et al., 2015), menstrual blood (Dalirfardouei et al., 2019), and urine (Chen C. Y. et al., 2018) were also used. Moreover, murine (Li T. et al., 2019) and swine (Irons et al., 2018) adipose tissues were employed. CM was collected from MSCs between passages 1 and 12 at 50–100% confluence. The most common model was murine (94.12%, 16/17), although a swine model was also used (Irons et al., 2018). Regarding the route of administration, MSC-CM was injected (78.95%, 15/19) mainly intraperitoneally (26.31%, 5/19) (Fong et al., 2014; Pouriran et al., 2016; Amini et al., 2018; Bagheri et al., 2018; Saheli et al., 2020), subcutaneously (15.79%, 3/19) (Chen C. Y. et al., 2018; Li T. et al., 2019; Zhang S. et al., 2020), intradermally (15.79%, 3/19) (Kim et al., 2010; Dalirfardouei et al., 2019; Deng et al., 2019), or intravenously (5.26%, 1/19) (De Gregorio et al., 2020). MSC-CM was also applied topically (21.05%, 4/19) to the wounds or around them in creams, hydrogels, or membranes (Kusindarta et al., 2016; Chen Z. et al., 2018; Irons et al., 2018).
TABLE 2.
Studies regarding mesenchymal stromal cell-conditioned medium for treating diabetic wounds in animal models.
| MSC source | Method of tissue extraction | MSC characterization | Preparation of MSC-CM | Model | Groups of treatments and via of administration | Follow-up (days) | Assessment | Main outcome | Other outcomes | |
| Zhang S. et al., 2020 | Human umbilical cords | Full-term delivered infants | Flow cytometry (CD105+, CD73+, CD90+, CD166+, CD54+, CD13+, CD45−, CD34−, CD14−, CD19−, CD117−, HLA-DR−) | MSCs of passages 3–4 at 80% confluence were used. CM was collected | Murine. Diabetic model. Full-thickness excisional skin wounds, 800 mm2, on the back | 150 μl subcutaneously injected around the wounds at six injection sites (25 μl per site) every day for three consecutive days - Control (non-CM) - FB - UC-MSC - UC-MSC-CM (n = 12/group) |
14 | Macroscopic appearance (photography), histology (HE), IHC, qRT-PCR | UC-MSC and UC-MSC-CM treatment accelerated wound healing rate. The wound area of UC-MSC and UC-MSC-CM was ≈15% reduced compared with controls | UC-MSC and UC-MSC-CM increased the percentage of M2 macrophages in the local wounds and the levels of anti-inflammatory cytokines, IL-10, and VEGF and significantly decreased the levels of proinflammatory cytokines, IL-1β, TNF-α, and IL-6 |
| Saheli et al., 2020 | Human bone marrow | − | Flow cytometry (CD73+, CD90+, CD105+, CD34−, CD45−). Osteogenic and adipogenic differentiation | MSCs of passage 4 at 80% were used. CM was then collected | Murine. Diabetic model. Full-thickness excisional skin wounds, 20 mm long, on the chest proximal part intraperitoneal | 50-fold concentrated DMEM or CM twice at 12 and 24 h after wounding intraperitoneally - Control (no treatment) - Placebo - hBM-MSC-CM (n = 6/group) |
15 | Macroscopic appearance, histology (HE, MT), qRT-PCR | MSC-CM accelerated diabetic wound closure (67% wound closure in CM group vs. 33% in placebo group, p < 0.05) | MSC-CM treatment leads to upregulation of EGF and bFGF genes and higher cell viability/proliferation and migration |
| De Gregorio et al., 2020 | Human subcutaneous adipose tissue samples from abdominal region | Liposuction | Flow cytometry (CD29+, CD13+, CD105+, CD73+, CD90+, CD235a−, CD31−, CD45−). Adipogenic and osteogenic differentiation | MSCs of passage 3 at 70% confluence were used. Cells were supplemented with 400 μM DFX (preconditioned MSCs) or with saline (vehicle) as non-preconditioned MSCs. CM was then collected | Murine. Diabetic mice. Full-thickness skin (2.5 mm × 3.5 mm) surgically removed from the dorsal surface of both feet, mimicking a foot ulcer | Intravenous administration of 50 μl of CM every 2 weeks - CM derived from DFX-preconditioned MSCs - CM derived from non-preconditioned MSCs - Vehicle (n = 6/group) |
14 | Macroscopic appearance (photography), histology (HE, MT), qRT-PCR, proteomic analysis | MSC-CM accelerated wound healing. The wound area of MSC-CM was ≈20% reduced compared with controls at day 7 | MSC-CM derived from DFX-preconditioned MSCs had a more potent effect in recovering the skin vasculature |
| Bian et al., 2020 | Human placenta | Cesarean section births | Flow cytometry (CD73+, CD90+, CD105+, CD19−, CD34−, CD45−, HLA-DR−). Osteogenic, adipogenic, and chondrogenic differentiation | MSCs of passages 3–7 were used. CM was collected, and EVs were obtained | Murine. Diabetic model. Full-thickness excisional wounds, 16 mm, on the back | 100 μl was injected around the wounds at 4 sites (25 μl per site) - MSC-EVs - PBS (n = 5/group) |
28 | Photography, histology (HE, MT), IHC | MSC-EVs significantly accelerated wound healing. The narrowest scar widths were observed at day 14 post-wounding (2.41 ± 0.24 mm in MSC-EVs group vs. 3.87 ± 0.60 mm in PBS group) | CXCR4, p21 PCNA, and α-SMA were upregulated in the MSC-EV group |
| Raj et al., 2019 | Human umbilical cord | Umbilical cord dissection | Flow cytometry (CD13+, CD29+, CD44+, CD90+, CD10−, CD14−, CD34−, CD117−) | MSCs were transduced with a lentiviral vector (green fluorescence protein tagged). MSCs of passages 3–4 were used. CM was collected. Wound dressing patches: impregnated with aloe verapolycaprolactone (AV/PCL) nanoscaffolds with hWJSCs or hWJSC−CM were also created | Murine. Diabetic model. Full-thickness excisional wounds, 6 mm, on the back | 100 μl of: - PBS with 1 × 106 MSCs - MSC-CM - UCM - PBS with 1 × 106 MSCs+AV/PCL - MSC-CM+AV/PCL - UCM+ AV/PCL - Untreated group (n = 12/group) |
28 | Macroscopic appearance (photography), histology (HE), IHC, qRT−PCR | Thickness of the epidermis and dermis was significantly greater in both MSCs and MSC-CMs without AV/PCL compared with their controls without AV/PCL | AV/PCL groups showed an earlier re-epithelialization and increases in thickness of dermis and epidermis, cellularity, vasculature, and hair follicle numbers |
| Li T. et al., 2019 | Murine adipose tissue | − | Flow cytometry and oil red O staining (CD29+, CD90, CD45−) | MSCs of passages 3–5 were used. They were cultured on various matrices (tissue culture plates (TCP), pure three-dimensional-printed | Murine. Diabetic murine model. Full-thickness skin defects, 7 mm diameter | 200 μl of each solution was used (150 μl injected subcutaneously around the defect and 50 μl smeared onto the wound bed) - DMEM (control) |
14 | Macroscopic appearance (photography), histology (HE, MT), IF | All groups improved wound healing. The highest wound-healing rate was observed in DOPA-BC-CM group. Remaining wound area at | The newly formed capillary network around the excisional regions was the most intense in the DOPA-BC-CM group. Higher levels of CD31 and a higher amount of |
| bioceramic (BC), and polydopamine-modified BC scaffolds (DOPA-BC), and each CM was collected | - MSC-CMs derived from TCP - MSC-CMs derived from BC - MSC-CMs derived from DOPA-BC (n = 4/group) |
day 14 was 7.1 ± 3.4% in DOPA-BC-CM group, ∼15.2 ± 6.6% in BC-CM, ∼21.2 ± 11.3% in TCP-CM, and ∼31.8 ± 7.2% in DMEM group | collagen deposition were also observed in this group | |||||||
| Deng et al., 2019 | Human adipose tissue | Liposuction | − | SVF gel was prepared, and its CM was collected (Gel-CM). CM from MSCs was also collected (MSC-CM) | Murine. Diabetic model. Full- thickness excisional wound, 20-mm diameter, on the back | 100 μl was administered intradermally every 2 days: - Gel-CM - MSC-CM - PBS (n = 18/group) |
14 | Macroscopic appearance (photography), histology (HE), ELISA | The wound size in all groups was reduced. Wound-healing rate in the Gel-CM-treated group was significantly higher than that in the MSC-CM group (p < 0.05) | Gel-CM-treated rats exhibited complete re-epithelialization of the wound, while MSC-CM did not. Number of capillaries in the Gel-CM-treated group was higher in MSC-CM |
| Dalirfardouei et al., 2019 | Human menstrual blood | Collecting menstrual blood from healthy women | Flow cytometry (CD29+, CD44+, CD90+, CD34−, CD45−, CD117−, HLA−DR-). Adipogenic and osteogenic differentiation | MSCs of passages 4–6 at 70–80% confluence were used. CM was collected, and exosomes were isolated | Murine. Diabetic mice models. Full-thickness excisional wound including the panniculus carnosus, 8 mm, on the back | 100 μl was intradermally injected around the wound - PBS - MSCs - Exos-MSCs (n = 6/group) |
14 | Macroscopic appearance (photography), histology (HE, MT), IHC, qRT-PCR | Increased wound closure was observed in Exo-group compared with the control or MSC group. At day 12, wound closure was 84.34 ± 7.00% in Exo-MSCs, 46.4 ± 8.5% in MSCs, and 43.78 ± 6.95% in controls | Microvessel density was significantly higher in the Exo-group compared with the other two groups. Size of scar tissues significantly decreased in themice treated with MSCs and their exosomes compared with control group. A major reduction in the granulation tissue cellularity was observed in mice treated with exosomes compared with the cell group |
| Irons et al., 2018 | Adipose tissue from pigs’ gluteal regions | Wound incision | − | MSCs at 90% were used. CM was collected | Pigs. Diabetic pigs. Full-thickness skin wounds, 50 mm circular, on the back | -Injection of low-dose MSCs - Injection of high-dose MSCs - Injection of low-dose EC/MSCs - Injection of high-dose EC/MSCs - 2 ml of MSC-CM topically applied every 3 days - 2 ml of EC-CM topically applied every 3 days - 2 ml of serum-free medium topically applied every 3 days (control) (n = 7/group) |
28 | Macroscopic appearance (photography), histology (HE) | Wounds treated with MSCs and MSC-CMs displayed a significant increase in the percentage of wound closure compared with controls (p < 0.05) | Decreases in the acute inflammation scores were observed in wounds treated with MSCs and MSC-CMs compared with controls |
| Chen Z. et al., 2018 | Human umbilical cord | Umbilical cords sections | Electron microscopy (CD63+, CD81+) | MSCs of passages 3–5 at 100% confluence were used. CM was collected, and Exos were obtained. PF-127 hydrogel was mixed with Exos, and PF-127 composite (MSC-Exos/PF-127) was obtained | Murine. Diabetic rat model. Full-thickness skin wounds, 10 mm circular, on the back | Treatment was used topically - 100 μg MSC-Exos dissolved in 100 μl Pluronic F127 hydrogel (24%) - 100 μg MSC-Exos dissolved in 100 μl PBS - 100 μl PF-127 hydrogel (24%) - 100 μl PBS (control) (n = 6/group) |
14 | Macroscopic appearance (photography), histology (HE), IHC, qRT-PCR, IF | Wound area was significantly smaller in the MSC-Exos/PF-127 group than in the other groups. Wounds in the MSC-Exos/PF-127 group were almost completely healed at day 14, while the wound healing rates in the MSC-Exos, PF-127 hydrogel, and control groups were 8.95, 14.52, and 23.09%, respectively | New hair was only evident in the MSC-Exos/PF-127 group. Number of blood vessels was higher in the MSC-Exos/PF-127 and MSC-Exos groups than in the PF-127 hydrogel or control group |
| Chen C. Y. et al., 2018 | Human urine samples | Urine sample collection | Flow cytometry, and electron microscopy (CD29+, CD44+, CD73+, CD90+, CD34−, CD45−). Osteogenic, adipogenic, and chondrogenic differentiation | MSCs of passages 2–6 at 80–90% confluence were used. CM was collected, and Exos were created. Lentivirus shRNAs were transfected | Murine. Diabetic rat model. Full-thickness skin wounds, 6 mm, on upper back | Treatment was subcutaneously injected around the wounds at 4 injection sites (25 μl per site) - 100 μl PBS - 200 μg MSC-Exos in 100 μl PBS - 200 μg MSC-Exos without DMBT1 in 100 μl PBS (n = 8/group) |
12 | Macroscopic appearance (photography), histology (HE, MT), IHC, qRT-PCR, IF | Faster wound closure was observed in MSC-Exos group compared with controls and MSC-Exos without DMBT1 | Higher rate of re-epithelialization, lower level of scar formation, and higher number of newly formed blood vessels were observed in MSC-Exos group compared with controls and MSC-Exos without DMBT1 |
| Bagheri et al., 2018 | Human bone marrow | Aspiration | Flow cytometry (CD73+, CD90+, CD105+, CD34−, CD45−) | MSCs of passage 4 at 80% confluence were used. CM was collected | Murine. Diabetic rat model. Full-thickness skin wounds, 12 mm, on upper back | PBM was administered once daily, 6 days per week. CM was administered at days 0 and 1 intraperitoneally - DMEM vehicle (control) - MSC-CM - PBM - PMB+MSC-CM (n = 18/group) |
15 | Stereological methods, tensiometric examination | MSC-CM and PBM+MSC-CM increased the tensiometric properties compared with DMEM and PBM | MSC-CM, PBM, and PBM+MSC-CM groups showed a significant decrease in the three types of mast cells and in the total number of mast cells compared with controls |
| Amini et al., 2018 | Human bone marrow | Aspiration | Flow cytometry (CD73+, CD90+, CD105+, CD34−, CD45−) | MSCs of passage 4 at 80% confluence were used. CM was collected | Murine. Diabetic rat model. Full-thickness skin wounds, 12 mm, on upper thoracic and lumbar regions | PBM was administered once daily, 6 days per week. CM was administered at days 0 and 1 intraperitoneally - DMEM vehicle (control) - MSC-CM - PBM - PMB+MSC-CM (n = 18/group) |
15 | Stereological methods, tensiometric examination, qRT-PCR | All treated groups significantly enhanced wound healing compared with controls. The extent of healing was significantly greater in the CM+PBM group | Number of fibroblast and epidermal cells, the lengths of blood vessels, and bFGF and SDF-1α expression were significantly higher in the CM+PBM group |
| Pouriran et al., 2016 | Human bone marrow | Aspiration | Flow cytometry (CD105+, CD90+, CD73+, CD34−, CD45−) | MSCs of passage 4 at 80% confluence were used. CM was then collected | Murine. Diabetic rat model. Full-thickness skin wounds, 12 mm, on the thoracic and lumbar regions | PWLLLT was administered once daily, 6 days per week. MSC-CM was administered twice intraperitoneally - Non-treated - MSC-CM - PWLLLT - MSC-CM+PWLLLT (n = 7/group) - Cream containing 1 ml MSC-CM in ratio 10 g cream base - Povidone iodine (control) (n = 6/group) |
15 | Macroscopic appearance (photography), biomechanical examination | PWLLLT and MSC-CM, alone or in combination, improved biomechanical parameters in the wound | PWLLLT was more effective compared with MSC-CM |
| Kusindarta et al., 2016 | Human umbilical cord | − | − | MSCs of passage 4 at 60% confluence were used. CM was then collected | Murine. Diabetic rat model. Full-thickness skin wounds, 7 mm, on the left side of the body | Topical application twice daily | 9 | Macroscopic appearance (photography), histology (HE) | MSC-CM induced faster re-epithelialization than other groups | MSC-CM promoted increasing density of collagen fiber and stimulated hair follicle and muscle regeneration greater than the other groups |
| Ma et al., 2015 | Human hair follicle | Dissection from excess scalp tissue discarded after surgery | Flow cytometry (CD105+, CD29+, CD49b+, CD49d+, CD73+, CD271+, GD2+, CD90−, CD44−, CD34−, CD45−). Adipogenic, osteogenic and chondrogenic differentiation | MSCs of passage 1 at 80–90% confluence were used. CM was then collected | Murine. Diabetic rat model. Full-thickness skin wound, 6 mm, on dorsal surface | 100 ml injected into each wound - DMEM - Normal fibroblast-CM - HF-MSC-CM (n = 3/group) |
24 | Macroscopic appearance (photography), histology (HE) | HF-MSC-CM accelerated wound healing compared with the other groups. The average number of days to complete wound closure in the group administered with HF-MSC-CM was 18.7 days compared with | The epidermal thickness of the HF-MSC-CM-treated wound sites was significantly higher than the other groups |
| 22.3 days in the group treated with fibroblast-CM and 24 days in DMEM group | ||||||||||
| Tam et al., 2014 | Wharton’s jelly from human umbilical cord | Cutting umbilical cords | Flow cytometry (CD13+, CD29+, CD44+, CD90+, CD10−, CD14−, CD34, CD117) | MSCs of passage 3–4 were used. It was constructed wound dressing patch made up of an aloe vera−PCL (AV/PCL) nanoscaffold impregnated with WJ-MSCs or its CM | Murine. Full-thickness skin wounds, 6 mm, on dorsal region | - MSCs+AV/PCL - MSC-CM+AV/PCL - UCM + AV/PCL (n = 12/group) |
28 | Macroscopic appearance (photography), histology (HE and MT), IF, WB, qRT-PCR | MSCs+AV/PCL and MSC-CM+AV/PCL groups showed faster wound closure compared with other groups | MSCs+AV/PCL and MSC-CM+AV/PCL groups showed increased numbers of sebaceous glands and hair follicles and greater cellularity and vasculature compared with other groups |
| Fong et al., 2014 | Wharton’s jelly from human umbilical cord | Full-term delivery | Immunofluorescence (CD10+, CD13+, CD29+, CD44+, CD90+) | MSCs of passages 3–4 at 80% confluence were used. CM was then collected | Murine. Full-thickness excisional skin wound, 6 mm, on dorsum | 100 μl injected intraperitoneally - MSCs - MSC-CM - UCM (n = 12/group) |
28 | Macroscopic appearance (photography), histology (HE), IHC | MSC and MSC-CM healing rates were greater compared with controls | MSCs and MSC-CMs showed greater re-epithelialization, increased vascularity, cellular density, sebaceous gland, and hair follicle numbers compared with controls |
| Kim et al., 2010 | Human umbilical cord | Not specified (CD34+, CD31+, KDR+, Tie2+) | Cells were cultured, and CM was then collected | Murine. Diabetic rats. Full-thickness excisional wounds, 5 mm, on dorso-lateral area | Intradermal injections injected at three different intact dermis site near the wound - MSC - MSC-CM - PBS (n = 6/group) |
12 | Macroscopic appearance (photography), histology (HE) | MSC-CM and MSC promoted wound healing greater than controls | MSC-CM and MSC groups showed greater increases in neovascularization compared with controls. The effect of MSC and MSC-CM improving wound healing was similar |
AT, adipose tissue-derived; bFGF, basic fibroblast growth factor; AF, amniotic fluid; BM, bone marrow; CM, conditioned medium; DFX, deferoxamine; DMEM, Dulbecco’s modified Eagle medium; DP, dental pulp; EC, endothelial-differentiated; ECM, extracellular matrix; EGF, epidermal growth factor; ELISA, enzyme-linked immunosorbent assay; EVs, extracellular vesicles; Exos; exosomes; HE, hematoxylin and eosin; hCB, human cord blood; hESC, human embryonic stem cell; IHC, immunohistochemistry; IF, immunofluorescence; MPF, micro-nano polylactic acid electrospun fiber; MSCs, mesenchymal stromal cells; MT, Masson’s trichrome; PBM, photobiomodulation; PBS, phosphate-buffered saline; qRT-PCR, real-time quantitative polymerase chain reaction; SVF, stromal vascular fraction; UCM, unconditioned medium; WB, Western blotting; WJ, Wharton’s jelly.
MSC-CM has been tested on 256 animals with diabetic wounds. The mean wound size was 13.47 mm (from 3.5 to 50 mm), and the mean follow-up was 18.83 days (from 9 to 28 days). Wounds were assessed mainly by macroscopic appearance, histology, immunohistochemistry, and qRT-PCR. MSC-CM had better results in terms of wound closure, re-epithelialization, and vessel formation than non-treated groups or other treatment groups (PBS, hydrogel without cells, DMEM, unconditioned medium, or povidone iodine). Moreover, some studies compared the effect between cells and its CM with similar results (Kim et al., 2010; Fong et al., 2014; Tam et al., 2014; Irons et al., 2018; Dalirfardouei et al., 2019; Zhang S. et al., 2020). MSC-CM was also used to heal wounds in combination with laser therapy, showing that the combined treatment enhanced wound healing compared with laser or MSC-CM alone (Pouriran et al., 2016; Amini et al., 2018; Bagheri et al., 2018).
Different ways of CM implementation have been also evaluated. CM supplemented with deferoxamine, a hypoxic mimetic agent, had a more potent effect on recovering skin vasculatures than non-supplemented CM (De Gregorio et al., 2020). DMBT1 expression also improves wound healing (Chen C. Y. et al., 2018). Moreover, CM cultured on polydopamine-modified three-dimensional-printed bioceramic (DOPA-BC) scaffolds showed a higher healing rate than CM cultured on tissue culture plates (TCPs) or pure three-dimensional-printed bioceramic (pure BC) (Li T. et al., 2019). After 14 days’ follow-up, the remaining wound area was 7.1 ± 3.4% in the DOPA-BC-CM group, 15.2 ± 6.6% in the BC-CM group, and 21.2 ± 11.3% in TCP-CM group (Li T. et al., 2019).
Other wounds
MSC-CM was also evaluated in second- and third-degree burn wounds (Aryan et al., 2019; Li J. Y. et al., 2019; Zhou et al., 2019), radiation-induced wounds (Sun et al., 2019), and infected wounds (Fridoni et al., 2019; Kouhkheil et al., 2019; Table 3). Cells were isolated from human tissues: bone marrow (Aryan et al., 2019; Fridoni et al., 2019; Kouhkheil et al., 2019), umbilical cord (Zhou et al., 2019), Wharton’s jelly (Sun et al., 2019), and placenta (Li J. Y. et al., 2019). MSCs from passages 3 to 8 at 60–80% confluence were used. All tested models were murine.
TABLE 3.
Studies regarding mesenchymal stromal cell-conditioned medium for treating other types of wounds in animal models.
| MSC source | Method of tissue extraction | MSC characterization | Preparation of MSC-CM | Model | Groups of treatments and via of administration | Follow-up (days) | Assessment | Main outcomes | Other outcomes | |
| Zhou et al., 2019 | Human umbilical cords | Umbilical cord section from full-term healthy fetuses that were born via cesarean section | Flow cytometry (CD29+, CD44+, CD73+, CD90+, CD105+, CD34−, CD45, CD31−, CD271−) | MSCs of passages 3–8 at 70–80% confluence were used. CM was collected. The thermosensitive MSC-CM/hydrogel was prepared by mixing the precooled MSC-CM, chitosan/β-GP, and collagen solutions at a ratio of 1:2:1 on ice | Murine. Third-degree burned mice, 15 mm | Wound was covered and changed twice daily - Unconditioned medium (UM) - MSC-CM group - UM/hydrogel - MSC-CM/hydrogel (n = 18/group) |
28 | Macroscopic appearance (photography), histology (HE), IHC | Application of the MSC-CM/hydrogel shortened healing time. The average healing time in MSC-CM/hydrogel group was approximately 5 days shorter than in UM group and shorter than MSC-CM and UM/hydrogel groups | MSC-CM/hydrogel limited the area of inflammation; enhanced re-epithelialization; promoted the formation of high-quality, well-vascularized granulation tissue; and attenuated the formation of fibrotic and hypertrophic scar tissue |
| Sun et al., 2019 | Wharton’s jelly from human umbilical cords | Section from umbilical cords | − | MSCs of passage 3 at 60% confluence were used. CM was collected | Murine. Radiation-induced skin injury rat model, 20 mm × 20 mm | 200 ml of hydrogel was pipetted onto the radiation wound every 2 days - Non-treated - EGF - MSC-CM (n = 12/group) |
56 | Macroscopic appearance (photography), histology (HE), IHC | MSC-CM significantly accelerated wound closure and enhanced the wound healing quality. The great difference was observed at day 42, with a relative wound size of the MSC-CM group 2.63-fold smaller than the EGF group and 3.38-fold smaller than the non-treated group (0.8 ± 0.29, 2.1 ± 0.37, 2.7 ± 0.34 mm2, respectively) | α-SMA, Ki-67 expression, and the number of vessels/HPF were increased in MSC-CM group |
| Li J. Y. et al., 2019 | Human fetal placenta | Placenta section | Flow cytometry adipogenic and qRT-PCR (CD29+, CD73+, CD105+, CD90+, CD34−, CD45−, CD133−). Adipogenic and osteogenic differentiation | MSCs of passages 3–7 at 80% confluence were used. CM was collected | Murine. Second-degree burn injury model. Back skin of mice was injured with 80°C water for 100 s to create a 10-mm diameter wound | 200 μl of the treatments was subcutaneously injected near the wound at four sites - PBS containing 2 × 106 MSCs - MSC-CM - PBS - DMEM (n = 5/group) |
21 | Macroscopic appearance (photography), histology (HE), IF | MSCs and MSC-CMs promoted wound healing compared with PBS and DMEM | High levels of new blood vessels and tubular structures were observed in the MSC and MSC-CM groups |
| Kouhkheil et al., 2019 | Human bone marrow | Aspiration | Flow cytometry | MSCs of passage 4 at 80% confluence were used. CM was collected | Murine. MRSA rats infected. Full-thickness excisional wound, 15 mm, on the back | PBM was administered once daily, 6 days per week. 50 μl of the 10-fold CM was administered from day 0 until day 3 - Control - PBM - MSC-CM - PBM+MSC-CM (n = 18/group) |
15 | Clinical observation, microbiological, tensiometric, and stereological analyses | There was a significant decrease in colony-forming units in PBM+MSC-CM and PBM groups compared with controls | PBM+MSC-CM, PBM, and MSC-CM groups significantly increased wound strength compared with the control group. The PBM+MSC-CM and PBM groups had more stable MCs, less significant degranulated and disintegrated MCs, and less significant total number of MCs compared with the control group |
| Fridoni et al., 2019 | Human bone marrow | Aspiration | Flow cytometry (CD105+, CD90+, CD73+, CD34−, CD45−) | MSCs of passage 4 were used. CM was collected | Murine. MRSA diabetic rats infected. Full−thickness wound, 15-mm diameter round, on the back | PBM was administered once daily, 6 days per week. 500 μl of the 10-fold CM was injected intraperitoneally daily from day 0 until day 3 - Control group - PBM - MSC-CM - PBM+MSC-CM (n = 18/group) |
15 | Histology (HE), IHC | PBM+MSC-CM hastened wound healing process | PBM+MSC-CM, MSC-CM, and PBM groups showed a decrease in the number of neutrophils and macrophages and an increase in the number of fibroblasts and angiogenesis compared with those of the control group |
| Aryan et al., 2019 | Human bone marrow | − | Flow cytometry (CD73+, CD90+, CD105+, CD45−) | MSCs of passages 3–4 at 80–90% confluence were used. CM was collected | Murine. Second-degree burns (induced from boiling water), 30 mm × 30 mm | - Not treated rats (control) - 0.5 ml of DMEM injected intraperitoneally every other day - 1% topical silver sulfadiazine cream daily - 0.5 ml of MSC-CM injected intraperitoneally every other day (n = 5/group) |
28 | Macroscopic appearance (photography), histology (HE, MT), IHC | Wound closure area was significantly increased in the MSC-CM and sulfadiazine groups | There was a reduction in the volume of the epidermis and dermis in the burn wound of the control, DMEM, and sulfadiazine groups compared with the MSC-CM group |
CM, conditioned medium; DMEM, Dulbecco’s modified Eagle medium; ECM, extracellular matrix; EGF, epidermal growth factor; HE, hematoxylin and eosin; IHC, immunohistochemistry; IF, immunofluorescence; MRSA, methicillin-resistant Staphylococcus aureus; MSCs, mesenchymal stromal cells; MT, Masson’s trichrome; PBM, photobiomodulation; PBS, phosphate-buffered saline; qRT-PCR, real-time quantitative polymerase chain reaction.
Twenty-eight mice with burn wounds were treated with MSC-CM with a mean follow-up of 25.67 days (from 21 to 28 days). MSC-CM was tested using subcutaneous (Li J. Y. et al., 2019) or intraperitoneal (Aryan et al., 2019) injections or topically applied (Zhou et al., 2019). In burn wounds, MSC-CM showed faster wound healing, increased re-epithelialization, and vascularization compared with controls (unconditioned medium, PBS, or DMEM).
Moreover, 72 mice infected with methicillin-resistant Staphylococcus aureus were treated with MSC-CM or MSC-CM plus photobiomodulation (PBM). MSC-CM plus PBM decreased colony-forming units and the number of inflammatory cells (Fridoni et al., 2019; Kouhkheil et al., 2019).
Twelve mice with radiation-induced skin injuries were also treated with MSC-CM. Topical application of MSC-CM in hydrogel accelerated wound closure and enhanced the wound healing quality (Sun et al., 2019).
Combined therapies using mesenchymal stromal cell–conditioned medium
Five studies evaluated the effects of MSC-CM combined with PBM or pulsed wave low-level laser therapy (PWLLLT) for treating diabetic wounds and infected wounds (Pouriran et al., 2016; Amini et al., 2018; Bagheri et al., 2018; Fridoni et al., 2019; Kouhkheil et al., 2019) (Supplementary Table 1). Regarding diabetic wounds, the results are controversial (Pouriran et al., 2016; Amini et al., 2018; Bagheri et al., 2018). Two studies showed that MSC-CM, PBM, and the combined therapy improved wound healing as compared with control group (Pouriran et al., 2016; Bagheri et al., 2018). Moreover, it was found that the extent of healing was significantly greater in the MSC-CM+PBM group (Amini et al., 2018). On the other hand, it was showed that PWLLLT and MSC-CM, alone or in combination, improved biomechanical parameters in the wound but that PWLLLT was more effective compared with MSC-CM (Pouriran et al., 2016). Concerning infected wounds, it was observed that both PBM+MSC-CM and PBM groups decreased colony-forming units and hastened wound healing process as compared with controls, while it did not happen when using MSC-CM alone (Fridoni et al., 2019; Kouhkheil et al., 2019).
Clinical Studies
Only one clinical study evaluated the effects of MSC-CM on wound healing (Table 4). This research assessed the use of MSC-CM derived from human amniotic membrane for treating chronic plantar ulcers in leprosy. The mean age of the patients was 52.12 ± 1.33 years, the mean ulcer duration was 1.41 ± 0.36 years, and the mean ulcer size at baseline was 2.64 ± 0.5 cm2 with a depth lower than 0.5 cm. Sixty-six patients were divided into groups to receive MSC-CM, MSC-CM+vitamin C, or MSC-CM+vitamin E topically applied every 3 days for 8 weeks. All groups improved wound healing, with MSC-CM+vitamin E being the most effective treatment. Wound size was reduced by 1.7 ± 1.05 vs. 2.01 ± 1.19 vs. 2.84 ± 1.67 cm2; and depth was decreased by 0.35 ± 0.14 vs. 0.25 ± 0.11 vs. 0.27 ± 0.15 cm in MSC-CM, MSC-CM+vitamin C, and MSC-CM+vitamin E groups, respectively. No adverse events were reported (Prakoeswa et al., 2018).
TABLE 4.
Studies regarding mesenchymal stromal cell-conditioned medium for treating wounds in human.
| MSC source | Method of tissue extraction | Indication | Study type | Age (years) | Sex (male:female) | Groups of treatments and via of administration | Follow-up (days) | Assessment | Main outcome | |
| Prakoeswa et al., 2018 | Human amniotic fluid | Amniocentesis | Chronic plantar ulcer in leprosy | Randomized controlled trial | 52.18 ± 1.33 | 5:6 | Topical application every 3 days - MSC-CM - MSC-CM+vitamin C - MSC-CM+vitamin E (n = 22/group) |
56 | Macroscopic appearance (photography), spectrophotometric | All groups reduced wound size and depth. Healing percentage increased in all groups. Size reduction was 1.7 ± 1.05 vs. 2.01 ± 1.19 vs. 2.84 ± 1.67 cm2, and depth was decreased 0.35 ± 0.14 vs. 0.25 ± 0.11 vs. 0.27 ± 0.15 cm in MSC-CM, MSC-CM+vitamin C, and MSC-CM+vitamin E group, respectively. No adverse events were reported |
CM, conditioned medium; MSC, mesenchymal stromal cells.
Hypertrophic and Keloid Scars
Preclinical Studies
Nine studies evaluated the potential of MSC-CM in the treatment of hypertrophic scars or avoiding scar formation (Wu et al., 2015; Zhang et al., 2015; Du et al., 2016a, b; Li et al., 2016; Choi et al., 2017; Liu et al., 2018; Arjunan et al., 2020; Hu et al., 2020; Table 5). To obtain CM, MSCs were mainly isolated from human tissues: adipose tissue (Li et al., 2016; Choi et al., 2017; Liu et al., 2018) (33.33%, 3/9), umbilical cord blood (Arjunan et al., 2020), placenta (Du et al., 2016a), and bone marrow (Wu et al., 2015). Murine bone marrow (Hu et al., 2020), murine placenta (Du et al., 2016b), and rabbit adipose tissue (Zhang et al., 2015) were also used. CM was collected from MSCs between passages 3 and 13 at 70–90% confluence. The most common model was murine (88.89%, 8/9), although a rabbit model was also used (Zhang et al., 2015). CM was used mainly by subcutaneous injection (66.66%, 6/9) (Wu et al., 2015; Du et al., 2016a, b; Li et al., 2016; Liu et al., 2018; Hu et al., 2020). Intralesional (Zhang et al., 2015; Arjunan et al., 2020) and intravenous (Choi et al., 2017) injections were also used. MSC-CM was tested in 97 animals for treating hypertrophic scar and keloids or preventing scar formation. The mean follow-up time was 23 days. Scars were assessed mainly by macroscopic appearance, histology, immunohistochemistry, and qRT-PCR.
TABLE 5.
Studies regarding mesenchymal stromal cell-conditioned medium for treating hypertrophic scars in animal models.
| MSC source | Method of tissue extraction | MSC characterization | MSC treatment | Model | Groups of treatments and via of administration | Follow-up (days) | Assessment | Main outcome | Other outcomes | |
| Arjunan et al., 2020 | Human umbilical cords | Dissection | Flow cytometry and IF (Tra-1–60, Tra-1–81, SSEA-1, SSEA-4, Oct-4 and alkaline phosphatase, CD105, CD90, CD44) | MSCs at 70–89% confluence were used. CM was then collected | Murine. Keloid xenograft SCID mouse model, 3-6 mm, limbs | 50 μl intralesional injected - Placebo - HSF-CM - MSC- CM (n = 9/group) |
30 | Macroscopic appearance | MSC-CM group showed greater keloid reduction | A reduction in keloid tumor volumes (12.04 ± 3.69 vs. 54.65 ± 8.97 vs. 71.78 ± 20.67 mm) and weights (26.50 ± 6.38 vs. 76.70 ± 9.58 vs. 73.70 ± 12.12 mg) in the WJ-MSC-CM group compared with HSF-CM and untreated group were observed |
| Hu et al., 2020 | Murine bone marrow | Needle aspiration from tibia and femur | Flow cytometry (CD150+/CD74+). Osteogenic, adipogenic, chondrogenic differentiation | MSCs of passages 8–13 at 70% confluence. CM was then collected | Murine. Human HS-buried null mouse model, 6 mm, back | 200 μl subcutaneously injected every 7 days - DMEM - Botox - MSC-CM - MSC-CM+Botox (n = 4/group) |
28 | Macroscopic appearance, histology, IF, collagen deposition assay, fibroblast apoptosis assay (caspase-7 staining), qRT-PCR, WB | HS was the most reduced in MSC-CM+Botox group compared with the other groups. Scar weight was reduced up to 70% in MSC-CM+Botox, up to 80% in MSC-CM, up to 81% in botox, and up to 91% in control group | Collagen fiber deposition was eased and well-arranged after all treatments, except control group. α-SMA expression was lower in the combined regimen, MSC-CM, and botox than in DMEM group |
| Liu et al., 2018 | Human subcutaneous adipose tissue | Surgical excision of redundant tissue from surgical operations | Flow cytometry (CD105+/CD90+/ CD34−/CD45−/ CD19−). Adipogenic and osteogenic differentiation | MSCs of passages 3–4 at 80% confluence were used. CM was then collected | Murine. Keloid xenograft, 10 mm, back | 200 μl injected subcutaneously into each keloid xenograft every week - Untreated - DMEM - MSC-CM (n = 4/group) |
28 | Macroscopic appearance, histology, IHC, BrdU proliferation assay (bromodeoxyuridine/5-bromo-2′-deoxyuridine) (ELISA), qRT-PCR, contraction assay, phosphatidylserine apoptosis assay, antibody-based array | MSC-CM had a greater effect in scar reduction than control group. MSC-CM group decreased HS weight, by 34% compared with untreated group and by 23% compared with DMEM group | MSC-CM reduced the proportion of both cellularity/inflammatory cells and blood vessel density |
| Choi et al., 2017 | Human subcutaneous adipose tissue | Cesarean section | − | MSCs of passage 3 at 70–80% confluence were used. CM was then collected, and exosomes were created | Murine. Full-thickness wound scar, 20 mm × 15 mm, on dorsal surface | 200 μl intravenously injected - PBS - Exos-free CM - Exos-MSCs (n = 6/group) |
21 | Macroscopic appearance (photography), histology (HE, MT, picrosirius red), IHC, qRT-PCR | MSC-Exos treatment attenuated the thickness of the dermal layer and the length of the scar | The surface of the epidermis was more flattened, and collagen in the dermis was well distributed with less crosslinking in MSC-Exos group. MSC-Exos reduced collagen deposition, mitigated scar formation, and increased the ratio of collagen III |
| Li et al., 2016 | Human adipose tissue | Liposuction | Flow cytometry (CD73+/CD90+/ CD34−/CD14−), adipogenic and osteogenic differentiation | MSCs of passages 3–5 at 80–90% confluence were used. CM was then collected | Murine. Full-thickness excisional wound, 10 mm, back | 1,000 μl subcutaneously injected into each scar at four points - DMEM - MSC-CM (n = 6/group) |
14 | Macroscopic appearance (photography), histology (HE and Masson trichrome staining), immunohistochemistry, qRT-PCR, WB | Reduced scar formation and fibrosis were observed in MSC-CM group | MSC-CM decreased the expression of Col1, Col3, and α-SMA |
| Du et al., 2016a | Murine placenta | Placenta collected | Flow cytometry (CD29+, CD31−, CD34−, CD44+, CD45− and HLA-DR−) | MSCs of passage 3 were used. Normoxic CM, hypoxic CM, and hypoxic plus inhibitor CM (HIF-1α) were collected | Murine. Hypertrophic scald mouse model, 20 mm, back | 100 μl injected subcutaneously - Control - Normoxic MSC-CM - Hypoxic MSC-CM - HIF-1a inhibitor+hypoxic MSC-CM (n = 10/group) |
15 | Macroscopic appearance, histology (HE), WB | MSC-CM reduced scar formation | MSC-CM attenuated inflammatory responses and decreased the deposition of collagens |
| Du et al., 2016b | Human placenta | Placenta collected | Flow cytometry and immunofluorescence positive for CD73+, CD90+, CD105+, CD34−, CD45−, HLA-DR− | MSCs of passages 3–6 at 80–90% confluence were used. Normoxic CM and hypoxic CM were collected | Murine. Hypertrophic scald mouse model, 20 mm, back | 100 μl injected subcutaneously - Normal medium - Normoxic MSC-CM - Hypoxic MSC-CM (n = 10/group) |
15 | Macroscopic appearance (photography), contracture rate histology (HE), Trypan blue staining, qRT-PCR, WB, ELISA, wound healing assay | Hypoxic MSC-CM reduced scar formation and decreased wound size compared with normal medium and normoxic MSC-CM | Decreased levels of TGF-β1 and collagen I were observed using hypoxic MSC-CM. Hypoxic MSC-CM also inhibited the proliferation and migration of skin fibroblasts |
| Wu et al., 2015 | Human bone marrow | − | Flow cytometry (CD14, CD34, CD45, CD44, and CD73) | MSCs of passages 5–10 at 80% confluence were used. CM was then collected | Murine. Skin fibrosis model. Hypertrophic skin, almost 2-fold greater thickness than normal skin, back. | 100 μl injected subcutaneously into the lesion every - Placebo - MSC-CM - BM-MSC-CM with TGF-β3 blocked (n = 5/group) |
21 | Macroscopic appearance, histology (HE), IHC, cellular proliferation (Ki-67) | MSC-CM decreased skin fibrosis | MSC-CM decreased the fibroblast viability, reduced skin dermal thickness, and inhibited cells proliferation. Moreover, MSC-CM-treated group exhibited significantly fewer proliferating cells compared with MSC-CM with TGF-β3-blocked |
| Zhang et al., 2015 | Rabbit adipose tissue | Surgical excision of inguinal fat pad | Flow cytometry (CD73+/CD90+/ CD34−/CD14−). Adipogenic and osteogenic differentiation | MSCs of passage 3 at 80–90% confluence were used. CM was then collected | Rabbit. Full-thickness wound scar, 10 mm, ear | 200 μl intralesionally injected into center of each scar - Untreated (n = 4) - MSCs (n = 4) - MSC-CM (n = 4) - DMEM (n = 12) |
35 | Macroscopic appearance, histology (HE, MT), IHC, qRT-PCR, gene expression, ultrasonography | MSCs and MSC-CMs decreased scar hypertrophy and led to scar with a more normal appearance | MSCs and MSC-CM decreased α-smooth muscle actin and collagen type I. MSC-CM was less effective than MSCs |
BM, bone marrow; CM, conditioned medium; DMEM, Dulbecco’s modified Eagle medium; ELISA, enzyme-linked immunosorbent assay; Exos, exosomes; HE, hematoxylin and eosin; HS, hypertrophic scar; HSF, human skin fibroblast; IF, immunofluorescence; IHC, immunohistochemistry; MSCs, mesenchymal stromal cells; MT, Masson’s trichrome; qRT-PCR, real-time quantitative polymerase chain reaction; WB, Western blotting; WJ, Wharton’s jelly.
Mesenchymal stromal cell-CM had better results than non-treated groups or other treatments groups (PBS, DMEM, and unconditioned medium) in terms of tumor volumes, tumor weight, collagen fiber deposition, and skin fibrosis. CM derived from MSCs cultured in hypoxic conditions showed greater effects on scar formation than cultured in normoxic conditions (Du et al., 2016a, b). Moreover, the MSC-CM-treated group exhibited significantly fewer proliferating cells than did MSC-CM with TGF-β3 blocked, showing that TGF-β3 may be responsible for parts of the antifibrotic effect of the MSC-CM (Wu et al., 2015). The combination of MSC-CM and botulinum toxin type A (botox) had a greater effect when treating human hypertrophic scars than did botox alone, MSC-CM alone, or DMEM. Scar weight was reduced by 21, 11, or 10% more compared with DMEM, MSC-CM, or botox, respectively (Hu et al., 2020). Although both MSCs and MSC-CMs reduced hypertrophic scars, MSC-CM might be more effective than MSCs (Zhang et al., 2015). The Scar Elevation Index was reduced in both MSC- and MSC-CM-treated groups compared with internal controls (44.04 and 32.48%, p < 0.01, respectively), and it was significantly lower in the MSC-treated group than in the MSC-CM-treated group (p < 0.01) (Zhang et al., 2015).
Clinical Studies
No clinical study specifically evaluated the effects of MSC-CM on hypertrophic scar formation. Nevertheless, the effect of MSC-CM has been tested on acne scars in combination with laser therapy, showing that combined therapy had a greater effect on reducing acne scars than laser alone (Zhou et al., 2016; Abdel-Maguid et al., 2019; El-Domyati et al., 2019; Park C. S. et al., 2019). These studies are further developed in the Skin Rejuvenation section.
Flap and Graft Reperfusion
Preclinical Studies
Four studies evaluated the effects of MSC-CM for treating ischemic wounds or flaps (Mirabella et al., 2012; Lee S. M. et al., 2014; Cooper et al., 2018; Pu et al., 2019; Table 6). MSCs were isolated from human adipose tissue (Lee S. M. et al., 2014; Cooper et al., 2018; Pu et al., 2019) or human amniotic fluid (Mirabella et al., 2012). CM was collected from MSCs between passages 3 and 8 at 70–90% confluence. All the models employed were murine.
TABLE 6.
Studies regarding mesenchymal stromal cell-conditioned medium for improving flaps reperfusion in animal models.
| MSC source | Method of tissue extraction | MSC characterization | MSC treatment | Model | Groups of treatments and via of administration | Follow-up (days) | Assessment | Main outcome | |
| Pu et al., 2019 | Human abdominal adipose tissue | Liposuction | Flow cytometry and IHC (CD34, CD45, CD73, CD90, CD105). Adipogenic, osteogenic, chondrogenic differentiation | MSCs of passages 3–8 at 70–80% confluence were used. CM was then collected | Murine. Ischemia/reperfusion flap model by clamping the flap (ischemia) and then releasing it (reperfusion) | Treatment was applied to the subcutaneous layer between the flap and its bed and at the proximal, middle, and distal parts of the skin flap - Saline injections - MSC-CM (n = 6/group) |
5 | Macroscopic appearance (photography), histology (HE, MT), IHC, qRT-PCR | MSC-CM treatment attenuated the necrotic area and reversed the detrimental proliferation effect induced by I/R compared with saline |
| Cooper et al., 2018 | Human adipose tissue | Elective surgery | − | MSCs of at 70–90% confluence were used. CM was then collected. EVs were isolated by centrifugation | Murine. Full-thickness excisional wounds were created in the center of a 105 mm × 35 mm flap (ischemic wounds) | 20 μl topically applied daily - MSC-CM - Control media (n = 6/group) |
21 | Macroscopic appearance (photography) | MSC-CM accelerated closure of ischemic wounds. In MSC-CM-treated group, the wound area reduction was 65% compared with only 15% wound closure for the control |
| Lee S. M. et al., 2014 | Human subcutaneous adipose tissue | − | Flow cytometry (CD29+, CD44+, CD73+, CD90+, CD105+, CD34−, CD45−, CD14−) | MSCs of passage 3 at 80% confluence were used | Murine. Full-thickness skin graft, 30 mm × 30 mm, back | Intravenously injected - PBS - MSCs - MSC-CM (n = 12/group) |
30 | Macroscopic appearance (photography), histology (HE), IHC | MSCs and MSC-CM increased skin allograft survival compared with control (23.9 ± 2.0, 19.6 ± 2.4, and 9.3 ± 1.4 days, respectively), without differences between these both groups |
| Mirabella et al., 2012 | Human amniotic fluid | Amniocentesis | − | MSCs of passage 6 at 80% confluence. Lyophilized gelatine film membranes were prepared by using MSC-CM or saline as control | Murine. Ischemia/reperfusion epigastric flap model. Flap 70 mm × 70 mm with vasculature ligated and then opened | A membrane with MSC-CM or saline was positioned under the elevated skin flap (n = 5/group) | 7 | Macroscopic appearance (photography), histology | Perfusion was increased in flaps treated with MSC-CM compared with controls. Necrosis was decreased in the MSC-CM group |
CM, conditioned medium; EVs, extracellular vesicles; HE, hematoxylin and eosin; IHC, immunohistochemistry; I/R, ischemia/reperfusion; IF, immunofluorescence; MSCs, mesenchymal stromal cells; MT, Masson’s trichrome; PBS, phosphate-buffered saline; qRT-PCR, real-time quantitative polymerase chain reaction.
Two studies tested MSC-CM in ischemia/reperfusion flap models by clamping the flap and then releasing it. Pu et al. evaluated the effects of MSC-CM applied to the subcutaneous layer between the flap and its bed and at the proximal, middle, and distal parts as compared with saline (n = 6/group) (Pu et al., 2019). A porcine skin-derived gelatine membrane soaked in either MSC-CM or saline was also tested (n = 5/group) (Mirabella et al., 2012). Both studies observed that the MSC-CM treatment group enhanced skin flap recovery, attenuated the necrotic area, increased hair growth, and stimulated angiogenesis as compared with controls (Mirabella et al., 2012; Pu et al., 2019).
MSC-CM were also applied topically on flap ischemic wounds in six mice. It was observed that MSC-CM accelerated the closure of flap ischemic wounds by 50% as compared with controls (Cooper et al., 2018). MSC-CM was also tested on skin allografts, intravenously injected, and compared with placebo and MSCs. MSCs and MSC-CMs increased skin allograft survival as compared with the control (23.9 ± 2.0, 19.6 ± 2.4, and 9.3 ± 1.4 days, respectively), without any differences between MSCs and MSC-CMs (Lee S. M. et al., 2014).
There are no studies at the clinical level.
Hair Restoration
Preclinical Studies
Ten studies evaluated the effect of MSC-CM on hair growth at the preclinical level (Park et al., 2010; Won et al., 2010, 2015; Dong et al., 2014; Shim, 2015; Choi et al., 2019; Gunawardena et al., 2019a; Park J. et al., 2019; Ou et al., 2020; Xiao et al., 2020; Table 7). MSCs were isolated mainly from human hair follicles (Won et al., 2010; Won et al., 2015; Gunawardena et al., 2019a; Ou et al., 2020) (40%, 4/10) and human adipose tissue (Park et al., 2010; Choi et al., 2019; Xiao et al., 2020) (30%, 3/10). Other tissues used were human amniotic fluid (Park J. et al., 2019), human deciduous teeth (Gunawardena et al., 2019a), human dermal cells (Shim, 2015), and murine bone marrow (Dong et al., 2014). CM was collected from MSCs between passages 2 and 6 at 50–100% confluence. All studies employed murine models.
TABLE 7.
Studies regarding mesenchymal stromal cell-conditioned medium for hair restoration in animal models.
| MSC source | Method of tissue extraction | MSC characterization | MSC treatment | Model | Groups of treatments and via of administration | Follow-up (days) | Assessment | Main outcome | Other outcomes | |
| Xiao et al., 2020 | Human adipose tissue | Fat grafting | IF (alkaline phosphatase+ and β-catenin+) | MSCs of passage 3 were used. CM from MSCs and from ECM/SVF-gel was collected | Murine. Back hair shaved with clippers and then completely removed using hair remover cream | Injected subcutaneously in the dorsal skin once per week - PBS (control) - ECM/ADSVC-CM - ADSVC-CM (n = 10/group) |
21 | Macroscopic appearance (skin darkening, hair growth score, gross observation of the inner skin of the hair-regrowth), IHC (CD31), WB | Both CMs enhanced hair growth. CM from ECM/SVF had a stronger ability to stimulate hair growth. 95–100% hair regeneration to full length was observed in the ECM/SVF group and 70–75% in the ADSVC-CM group | Both CM promoted dermal papilla cells and bulge cell proliferation, neovascularization, and anagen induction. Growth factor levels (VEGF, bFGF, PDGF, KGF) increased in CM-treated mice, even more than in ECM/ADSVC-CM group |
| Ou et al., 2020 | Donor hair follicles from the adult occipital scalp | Biopsy sample of hair follicles | − | MSCs at 80% confluence were used. CM was then collected | Murine. Left and right body of each rat shaved as close as possible to the skin | Gel topically applied - Vehicle (control) - MSC-CM (n = 4/group) |
15 | Macroscopic appearance (photography), histology (HE) | No significant differences in hair growth were observed between MSC-CM and control group | MSC-CM-treated group showed a darker area of the skin and bigger diffusion. Histologically, the hair follicle cycling was enlarged in the MSC-CM-treated group |
| Park J. et al., 2019 | Human amniotic fluid | Amniocentesis performed for fetal karyotyping between 16- and 20-week gestation | IF and quantification (CD13+, CD29+, and CD44+). Osteogenic, adipogenic, and chondrogenic differentiation | MSCs of passage 3 at 70–80% confluence were used. CM was then collected | Murine. Plucking the dorsal skin of mice in the telogen phase of the hair cycle, inducing the second anagen stage | 50 μl topically applied - MSC-CM - MSC-Nanog-CM - Minoxidil 2% (control) (n = 3/group) |
10 | Macroscopic appearance (photography), histology (HE), IHC (ALP, CK15), qRT-PCR, WB (ALP, LEF1, and Versican) | The dorsal skin of MSC-Nanog-CM and minoxidil-treated mice was fully darkened, while bare spots were observed in MSC-CM group | MSC-Nanog-CM accelerated the telogen-to-anagen transition in HFs and increased HF density to a greater extent than MSC-CM. The expression of DP and HF stem cell markers and genes related to hair induction were higher in MSC-Nanog-CM than in AF-MSC-CM. Paracrine factors contained in MSC-Nanog-CM |
| upregulated the expression of hair induction genes and accelerated hair regeneration | ||||||||||
| Gunawardena et al., 2019 | hDP-MSCs: human deciduous teeth hHF-MSCs: human donor hair follicles | hDP-MSCs: teeth extraction hHF-MSCs: scalp biopsy | Flow cytometry (CD73+, CD90+, CD105+). Adipogenic, chondrogenic, osteogenic differentiation | SHED of passages 2–5 and HFSCs of passage 1 at 80% confluence were used. CM was then collected | Murine. Dorsal region shaved with clippers | 100 μl subcutaneously injected at the dorsal region every 3 days - hDP-MSCs -CM (n = 9) - hHF-MSC-CM (n = 9) - STK2 media, control (n = 3) - No treatment, control (n = 2) |
60 | Macroscopic appearance (photography) | hDP-MSC-CM-treated group showed a faster stimulation of hair growth in comparison with hHF-MSC-CM. The visual observance for the appearance of dark patches indicating the transition from telogen to anagen stage by hDP-MSC-CM ranged from 8 to 12 days while hHF-MSC-CM ranged from 12 to 15 days | VEGF-A and HGF were overexpressed in hDP-MSC-CM and hHF-MSC-CM group. No adverse events were reported |
| Choi et al., 2019 | Human adipose subcutaneous fat | Liposuction | − | MSCs were of passages 4–6 were used. Cells were seeded and treated with HB-EGF. CM was then collected. | Murine. Dorsal region shaved with clippers | Subcutaneously injected - ADSC-CM - ADSC-HB-EGF- CM (n = 4/group) |
17 | Macroscopic appearance (photography), histology (HE), IHC, qRT-PCR | MSC-CM and MSC-HB-EGF-CM promoted hair growth. MSC-HB-EGF-CM showed higher increase in hair weight | MSC-HB-EGF-CM more rapidly induced telogen-to-anagen hair cycling and showed higher number of mature hair follicles. Expression levels of several growth factors in the MSC-HB-EGF-CM were upregulated compared with MSC-CM group |
| Won et al., 2015 | Donor hair follicles | CELLnTEC | Flow cytometry (integrin a6 and CD71) | MSCs were obtained from CELLnTEC. MSCs were used | Murine. Dorsal side shaved with a clipper and electric shaver | Subcutaneously injected every 2 days - PBS (control) |
14 | WB | MSC-CM-treated group increased hair growth compared | MSC-CM enhanced the proliferation of HFs. The level of |
| at 100% confluence. CM was then collected | - MSC-CM (n = 5/group) | with controls | phosphorylated AKT and phosphorylated ERK1/2 was significantly increased after MSC-CM treatment | |||||||
| Shim, 2015 | Human dermal cells | − | Flow cytometry. Adipogenic, chondrogenic, osteogenic differentiation | MSCs of passage 2 were used. CM was then collected | Murine. Dorsal region shaved with a clipper | 100 μl topically applied daily - MSC-CM or - Minoxidil - Non-treated group (n = 6/group) |
35 | Visual scoring of hair growth | MSC-CM increased hair regeneration. After the follow-up, hair weight was around 20, 40, and 80 mg in control, minoxidil, and MSC-CM group, respectively | MSC-CM increased promoted early telogen-to-anagen phase conversion of hair follicles compared with minoxidil treated group and non-treated mice |
| Dong et al., 2014 | Murine bone marrow from the femur | Needle aspiration | Flow cytometry (CD29+, CD44+, CD73+, CD14−, CD34, CD45, CD133−). Adipogenic, chondrogenic, osteogenic differentiation | MSCs were transfected with murine Wnt1a cDNA retroviral vector. MSCs and Wnt1a-MSCs at 50–60% confluence were used. CM was collected from MSCs and Wnt1a-MSC- | Murine. Dorsal region shaved with a clipper | 100 ml intradermally injected - Wnt-CM - MSC-CM - DMEM (control) (n = 3/group) |
14 | Macroscopic appearance (photography), histology (HE), IHC, qRT-PCR | Both Wnt-CM and MSC-CM promoted the hair follicle cycling more than control group | Wnt-CM induces hair to enter earlier anagen of the hair cycle. ALP expression was enhanced in both MSC-CM and Wnt-CM group. More Ki67-positive cells were observed in Wnt-CM-treated mice. Both MSC-CM and Wnt-CM upregulated the hair induction-related genes |
| Won et al., 2010 | Human hair follicles | Dissection | IHC (alpha smooth muscle actin, alkaline phosphatase) | MSCs were transfected with plasmid carrying SV40T antigen and c-myc gene and neomycin-resistant gene. MSCs of passages 3–4 were used. CM was collected | Murine | Topical application - MSC-CM - DMEM (n = 3/group) |
21 | Macroscopic appearance (photography) | MSC-CM enhanced hair growth. Hair weight was 2- to 5-fold higher in MSC-CM compared with control group | MSC-CM accelerated hair growth |
| Park et al., 2010 | Human subcutaneous adipose tissue | Liposuction | − | MSCs of passages 4–5 were used. MSCs were cultured in nomoxic and hypoxic conditions. CM was then collected | Murine | Subcutaneously injected - Normoxic MSC-CM (n = 5) - Hypoxic MSC-CM (n = 5) - Control (n = 3) |
56 | Macroscopic appearance (photography) | MSC-CM increased hair regeneration compared with control | MSC-CM induced the anagen phase. Hair regeneration growth factors significantly increased by hypoxia |
ADSCs, adipose-derived stem cells; ADSVCs, adipose-derived stromal vascular cells; BM, bone marrow; DP, dermal papilla; hDPs, human dermal papilla cells; ECM, extracellular matrix; HF, hair follicle; hFDPs, human follicle dermal papilla cells; hHF-MSCs, human hair follicle mesenchymal stromal cells; hDSPC, human dermal stem/progenitor cell; hDP-MSC, human dental pulp mesenchymal stromal cells; IF, immunofluorescence IHC, immunohistochemistry; KSCs, keratinocyte stem/progenitor cells; MSCs, mesenchymal stromal cells, PBS, phosphate-buffered saline; SHED, dental pulp stem cells obtained from human deciduous teeth; SVF, stromal vascular fraction; WB, Western blotting.
Mesenchymal stromal cell-CM was used subcutaneously (Park et al., 2010; Won et al., 2015; Choi et al., 2019; Gunawardena et al., 2019a; Xiao et al., 2020) (50%, 5/10) or intradermally (Dong et al., 2014) injected or topically applied (Won et al., 2010; Shim, 2015; Park J. et al., 2019; Ou et al., 2020) (40%, 4/10). MSC-CM was tested on 86 animals with shaved regions. The mean follow-up time was 26.3 days (from 10 to 60 days). Hair growth improvement was assessed mainly by macroscopic appearance (skin darkening and hair weight) or histology. It was found that MSCs enhanced hair growth compared with control group (PBS, minoxidil, or DMEM). Hair weight in the MSC-CM group increased by 40 mg as compared with that in the non-treated groups and by 20 mg as compared with that in the minoxidil group (Shim, 2015). MSC-CM stimulated hair growth by promoting dermal papilla cell proliferation, accelerating telogen-to-anagen transition, and promoting neovascularization.
The overexpression of a reprogramming factor in the CM, Nanog, improved hair induction even more than MSC-CM without Nanog (Park J. et al., 2019). Moreover, it was observed that CM collected from human deciduous teeth accelerated hair growth more than CM derived from hair follicle cells. The transition from telogen to anagen stage for teeth-MSC-CM was between 8 and 12 days, while that at hair follicle-MSC-CM ranged from 12 to 15 days (Gunawardena et al., 2019a). CM enrichment with heparin binding-epidermal growth factor-like growth factor (HB-EGF) showed a greater effect in promoting hair growth compared with non-supplemented CM (Choi et al., 2019). Furthermore, CM collected from MSCs cultured in hypoxic conditions increased hair regeneration growth factors (Park et al., 2010). No adverse events were reported when using MSC-CM in hair restoration.
Clinical Studies
Four clinical studies evaluated the effects of MSC-CM on hair growth (Shin et al., 2015; Lee et al., 2020; Narita et al., 2020; Oh et al., 2020; Table 8). To obtain CM, cells were isolated from human adipose tissue (Shin et al., 2015; Lee et al., 2020; Narita et al., 2020) (75%) and human umbilical cord blood (Oh et al., 2020) (25%). All studies evaluated the effect of MSC-CM in androgenetic alopecia (AGA). MSC-CM was tested on 100 patients, both men and women, with an age range of 23–74 years. Two observational studies used intradermal injections of human AT-MSC-CM and observed that hair density increased by 17.3 hairs/cm2 and hair thickness increased by 6.5 μm as compared with baseline (Shin et al., 2015; Narita et al., 2020). A clinical trial also evaluated the effects of human UC-MSC-CM enriched with TGF-1 and LiCl applied topically twice daily for 112 days. It was observed that MSC-CM increased hair density by 14.24%, hair thickness by 28.19%, and hair growth by 19.54% as compared with a placebo (Oh et al., 2020). Moreover, MSC-CM treatment was also evaluated in patients who underwent non-ablative fractional laser treatment for AGA. It was found that concomitant treatment (MSC-CM+laser) had a greater effect than laser treatment only. The MSC-CM group had greater hair density (102.1 ± 4.09 vs. 89.3 ± 3.79/cm2) and gross hair volume (2 ± 0.13 vs. 1.2 ± 0.19, evaluated by a 7-point global improvement score) than the placebo group after 12 weeks of treatment administration (Lee et al., 2020). No serious adverse events were reported in these studies.
TABLE 8.
Studies regarding mesenchymal stromal cell-conditioned medium for hair restoration in humans.
| MSC source | Method of tissue extraction | MSC characterization | MSC treatment | Indication | Study type | Age (years) | Sex (male: female) | Groups of treatments and via of administration | Follow-up (days) | Assessment | Main outcomes | |
| Oh et al., 2020 | Human umbilical cord | Umbilical cord section | Flow cytometry (CD14, CD45, HLA-DR, PE-conjugated human CD73, CD166, BD, CD90, and CD105) | MSCs of passaged 5 at 60–70% confluence were used. CM enriched with TGF-1 and LiCl (primed-MSC-CM). CM was collected without pretreatment with TGF-1 and LiCl to act as the control | Healthy adults diagnosed with AGA (males: Type II according to the modified Norwood–Hamilton classification, women: Ludwig classification Type I) | Double blind placebo-controlled clinical trial | 46.9 (range 33–55) | 1:30 | 5% CM topically applied twice daily - Primed-MSC-CM (n = 16) - Placebo (n = 14) |
112 | Hair density and diameter (phototrichogram), hair density (hair count/cm2, counting the total number of hairs in the target area), hair thickness (mm) and hair growth rate (mm/day), rate of hair growth | Primed-MSC-CM improved androgenetic alopecia. Hair density increased by 14.24% in primed-MSC-CM group, while no improvement was observed in placebo group. Hair thickness increased by 28.19%, and hair growth rate increased by 19.54% in primed-MSC-CM-treated group. Primed-CM significantly increased the viability of DPCs |
| Narita et al., 2020 | Human subcutaneous adipose tissues | Liposuction | Flow cytometry (CD73+/CD90+/ CD34−/CD14−). Adipogenic and osteogenic differentiation | MSCs of passage 4 were used. Cells were cultured under hypoxia conditions. CM was then collected | Patients with AGA | Prospective observational study | Range 23–74 | 21:19 | Intradermal injection of ADSC-CM every month (n = 40) | 180 | Trichograms, physiological examinations (TEWL, SCH lipid level), ultrasonogray (dermal thickness and echogenicity), histology (HE, Elastica Masson–Goldner staining, Sirius red/fast green staining) | Hair density and anagen hair rate increased significantly compared with baseline. TEWL increased, while SCH and lipid level showed no obvious changes. Dermal thickness and dermal echogenicity increased significantly |
| Lee et al., 2020 | Human subcutaneous adipose tissues | Liposuction | Flow cytometry (CD73+/CD90+/ CD34−/CD14−). Adipogenic and osteogenic differentiation | MSCs are cultured with hydrogel, and the CM is collected, after being filtered using 0.22-mm filters. Then, it is added to a solution, and SCM2-Black3 is obtained | Patients with AGA | Double-blinded, randomized placebo-controlled study | 46.6 (range 20–61) | 1:1 | Before CM application, a single session of treatment was performed at the first visit and weekly single-pass self-applications of microneedle stamps. Topically applied once per week - MSC-CM - Normal saline (placebo) (n = 30) |
84 | Macroscopic appearance (photography), phototrichograms (hair density), gross hair volume improvement, investigator’s improvement (measured by questionnaire response) | MSC-CM group had significantly higher hair density than placebo (102.1 ± 4.09 vs. 89.3 ± 3.79/cm2). The gross hair volume of the MSC-CM group was also significantly higher (2 ± 0.13 vs. 1.2 ± 0.19/cm2). Investigator’s improvement was similar in both groups. No adverse effects associated with ADSC-CM were reported |
| Shin et al., 2015 | Human subcutaneous adipose tissues | Liposuction | Flow cytometry (CD73+/CD90+/ CD34−/CD14−), Adipogenic and osteogenic differentiation | MSCs of passage 4 were used. Cells were cultured under hypoxia conditions. CM was then collected | Patients with AGA, female pattern hair loss | Retrospective observational study. | 41.9 ± 13.4 (range 22–69) | 0:27 | Intradermal injection of MSC-CM every week (n = 27) | 84 | Patients’ medical records and phototrichographic images (hair density and thickness) | Hair density increased from 17.3 hairs/cm2, and hair thickness increased by 6.5 μm compared with baseline. None of the patients reported severe adverse events. The only inconvenience reported was the pricking of the needles during application |
AGA, androgenetic alopecia; CM, conditioned medium; DPC, dermal papilla cells; HE, hematoxylin and eosin; IHC, immunohistochemistry; IF, immunofluorescence; MSCs, mesenchymal stromal cells; MT, Masson’s trichrome; PBS, phosphate-buffered saline; qRT-PCR, real-time quantitative polymerase chain reaction; SCH, stratum corneum hydration; TEWL, transepidermal water loss.
Skin Rejuvenation
Preclinical Studies
Three studies evaluated the effect of MSC-CM in 36 UV-induced photoaged mice (Ueda and Nishino, 2010; Kwon et al., 2016; Zhang K. et al., 2020; Table 9). MSCs were isolated from human umbilical cord (33.33%, 1/3) (Zhang K. et al., 2020), human bone marrow (33.33%, 1/3) (Kwon et al., 2016), and human dental pulp (33.33%, 1/3) (Ueda and Nishino, 2010). The mean follow-up was 35 days (from 14 to 35). MSC-CM reduced the level of wrinkles, improved transepidermal water loss (TEWL) and stratum corneum hydration (SCH) values, and increased collagen fibers than did vehicle or PBS. Nevertheless, the effect of MSC-CM on improving wrinkles was lower than that of MSCs (Ueda and Nishino, 2010).
TABLE 9.
Studies regarding mesenchymal stromal cell-conditioned medium for skin rejuvenation in animal models.
| MSC source | Method of tissue extraction | MSC characterization | MSC treatment | Model | Groups of treatments and via of administration | Follow-up (days) | Assessment | Main outcomes | |
| Zhang K. et al., 2020 | Human umbilical cord | Umbilical cord dissection | Western blotting (CD9+, CD63+, CD81+, and calnexin−) | MSC-CMs of passage 6 at 80–90% confluence were used. CM was collected, and Exos were obtained | Murine. Photoaged mouse model | - Control (non-photoaged mouse) - SHS-PBS - Exos - SHS-Exos (n = 6/group) |
14 | Macroscopic appearance, microscopic appearance (microwrinkles analysis), histology, and qRT-PCR | SHS-Exos reduced microwrinkles, alleviated histopathological changes, and promoted the expression of extracellular matrix constituents, while Exos alone produced weaker effects |
| Kwon et al., 2016 | Human bone marrow from the posterior iliac crest | Aspiration | Flow cytometry (CD73+, CD105+, CD14−, CD34−, CD45−) | MSCs of passage 6 at 80% confluence were used and cultured in hypoxic conditions. CM was then collected | Murine. UVB-irradiated mice model | 200 μl of treatment topically applied three times a week - Vehicle solution (polyethylene glycol:ethanol) - Adenosine 0.04% - MSC-CM 1% - MSC-CM 10% (n = 8/group) |
56 | Macroscopic appearance (photography, visual skin grading), tape stripping, biophysical measurements (TEWL and hydration), histology (HE, IHC) | The MSC-CM group exhibited significantly reduced levels of total wrinkle area compared with the vehicle-treated group. TEWL levels decreased while hydration increased in MSC-CM |
| Ueda and Nishino, 2010 | Human dental pulp tissue | Healthy permanent deciduous teeth extraction | − | MSCs of passage 1 to 3 were used, and CM was collected | Murine. Wrinkled mice induced by UVB | Subcutaneous injections - MSC-CM - MSCs - PBS (n = 8/group) |
35 | Macroscopic appearance (photography, histology (HE) | MSCs and MSC-CM decreased wrinkles more than the PBS group. The greatest effect was observed in MSC group. Measurement of the dermal thickness showed significant increases in MSC and MSC-CM groups |
CM, conditioned medium; Exos, exosomes; HE, hematoxylin and eosin; IHC, immunohistochemistry; MSCs, mesenchymal stromal cells; MT, Masson’s trichrome; PBS, phosphate-buffered saline; qRT-PCR, real-time quantitative polymerase chain reaction; SHSs, sponge Haliclona sp. spicules; TEWL, transepidermal water loss.
Clinical Studies
Twelve studies evaluated the effect of MSC-CM on skin wrinkles (Seo et al., 2013; Zhou et al., 2013; Lee S. M. et al., 2014; Xu et al., 2016; Kim et al., 2018; Prakoeswa et al., 2019; El-Domyati et al., 2020; Kim J. et al., 2020) and acne scars (Zhou et al., 2016; Abdel-Maguid et al., 2019; El-Domyati et al., 2019; Park C. S. et al., 2019; Table 10). MSCs were isolated from human tissues: adipose tissue (Zhou et al., 2013, 2016; Xu et al., 2016; Park C. S. et al., 2019) (30.77%, 4/13), amniotic fluid (Abdel-Maguid et al., 2019; El-Domyati et al., 2019, 2020; Prakoeswa et al., 2019) (30.77%, 4/13), umbilical cord blood (Kim et al., 2018; Kim J. et al., 2020) (15.38%, 2/13), human embryos (Seo et al., 2013; Lee S. M. et al., 2014) (15.38%, 2/13), and the placenta (Xu et al., 2016).
TABLE 10.
Studies regarding mesenchymal stromal cell-conditioned medium for skin rejuvenation in humans.
| MSC source | Method of tissue extraction | Type of study | Indication and population | Groups of treatments and via of administration | Follow-up (days) | Assessment | Main outcome | Other outcomes | |
| El-Domyati et al., 2020 | Human amniotic fluid | − | Prospective observational study | Volunteers with facial aging 49.9 ± 5.59 years 3:7 (M:F) | 1 ml of MSC-CM was topically applied to the treated group - Skin needling (control) - Skin needling+MSC-CM (n = 10) |
30 | Clinical examination, photographs, histology (HE, MT, Orcein stains) | The percentage of improvement was higher in the MSC-CM group compared with controls (65.40 ± 11.34 vs. 38.60 ± 9.02; p < 0.001) | Remodeling of the dermal structures was observed mainly on the combined side. Epidermal thickness increased on both treated sides |
| Kim J. et al., 2020 | Human umbilical cord | Umbilical cord dissection | Randomized, investigator−blinded, prospective, split−face comparison study | Patient with large pores or wrinkles on the face that underwent laser resurfacing (42.2 years, range 25–56) | Topical application twice a day - MSC−CM cream (control) - MSC−CM cream and serum (n = 23) |
21 | Macroscopic appearance (photography, area of microcrusts), skin biophysical parameters (TEWL, SCH, erythema) | The percentage of the total microcrust area was significantly smaller in the MSC−CM cream and serum group than in the cream group (2.70 ± 0.56 vs. 3.13 ± 0.76, p < 0.05) | A slight increase in SCH values were observed in both groups without changes in TEWL. Patient satisfaction was similar in both groups. No adverse events were reported related to MSC-CM application |
| Abdel-Maguid et al., 2019 | Human amniotic fluid | − | Prospective randomized split-face study | Patients with atrophic acne scars | - Group I (n = 17). FxCR+topical MSC-CM on one side of the face or FxCR plus saline on the other side - Group II (n = 16). FxCR+ topical PRP on one side of the face or FxCR+ topical MSCs on the other side |
90 | Macroscopic appearance (photography), histology (HE, MT), qRT-PCR | In both groups, scars improved after treatment. No significant difference in clinical improvement of acne scars was observed between the FxCR+MSC-CM and FxCR, while better and faster improvement was detected on FxCR+PRP side compared with FxCR +MSC-CM side | All patients developed transient erythema and mild edema without differences between groups. Dermal collagen was increased and procollagen type I gene was upregulated in both FCL/PRP and FCL/SC-CM sides compared with FCL only sides |
| El-Domyati et al., 2019 | Human amniotic fluid | − | Prospective observational study | Patients with atrophic acne scars. | Topical application after five sessions of microneedling with dermaroller - 1 ml of MSCs−CM - Non-treated (n = 10) |
90 | Clinical examination, histology, histometric analysis | There was a significant increase in the improvement percentage of acne scars on the MSC−CM-treated side (65.40 ± 11.34 vs. 38.60 ± 9.02) | Improvement of character of collagen and elastic fibers was noticed, especially on MSC−CM side. Significant increase in epidermal thickness on both sides of face was detected. Erythema and slight edema appeared on both cheek sides |
| Park C. S. et al., 2019 | Human adipose tissue | − | Prospective randomized split-face study | Patients with atrophic acne scars | Topical application twice a day after laser treatment - 80% MSC-CM+20% HA - HA (n = 15) |
60 | Scar volume and erythema were objectively evaluated using an Antera 3DVR CS | Scar volume was reduced by 23.5% in MSC-CM side vs. 15.0% in control side, and the volume of the skin pores was reduced by 37.6% in MSC-CM side vs. 15.9% in control side | The erythema increase was lower in MSC-CM side (2.8% vs. vs. 3.1%) |
| Prakoeswa et al., 2019 | Human amniotic membrane | − | Randomized, matching pair, clinical trial | Healthy women with clinical photoageing (50.31 ± 5.1 years) | 3 ml topically applied every 2 weeks after microneedling - Normal saline (control) - MSC-CM (n = 48) |
56 | Macroscopic appearance (photography, Glogau scale) | MSC-CM group showed better improvements in pore and wrinkle | Skin tone did not improve in either of the groups |
| Kim et al., 2018 | Human umbilical cord | − | Prospective observational study | Healthy women with face wrinkles (range 18–55 years) | 10% MSC-CMs in cream base topically applied daily on face skin (n = 22) | 28 | Macroscopic appearance (photography), ultrasound | Dermal density was increased by 2.46% compared before treatment | Wrinkles of eye-end area were decreased after the treatment. No irritation, stinging, or any adverse reactions were observed |
| Xu et al., 2016 | Human adipose tissue and placenta | − | Prospective randomized clinical trial | Healthy volunteer | Intradermal injections - AT-MSC-CM+HA - P-MSC-CM+HA - HA (control) (n = 6/group) |
15 | Biophysical measurements | Erythema, melanin, elasticity, TEWL, and hydration showed improvement in hAD-MSC-CM and hP-MSC-CM compared with the control group | Only the melanin index of the hAD-MSC-CM group was significantly lower than that of the hP-MSC-CM group |
| Zhou et al., 2016 | Human subcutaneous adipose tissue | Liposuction | Prospective observational study | Patients with facial wrinkles and patients with atrophic acne scars. 36.4 years (range 24–50) 5:6 male:female | 3 ml topical application after FxCR - MSC-CM - DMEM (n = 9 with facial wrinkles n = 13 with atrophic acne scars) |
90 | Macroscopic appearance (photography), subjective satisfaction scale, biophysical measurements (erythema, melanin, TEWL, elasticity, skin surface roughness, hydration), and histology (HE, MT, Gomori’s aldehyde fuchsine staining) | Subjective satisfaction (2.35 ± 0.69 vs. 2.08 ± 0.76, p < 0.05) and objective clinical assessment (2.78 ± 0.45 vs. 1.89 ± 0.60, p < 0.05) were higher in MSC-CM group than DMEM | Elasticity and hydration were significantly higher in MSC-CM side, while TEWL and roughness were lower. Increased dermal collagen and elastin density were found in MSC-CM side. No adverse events were reported in the study |
| Lee H. J. et al., 2014 | Human embryo | Prospective randomized controlled observer-blind split face study | Healthy individuals with face wrinkles (51.6 years, range 41–64) | Treatment every 2 weeks - Microneedling (control) - Microneedling + 1.5 ml of MSC-CM (n = 25) |
84 | Macroscopic appearance (photography), biophysical parameters (erythema, melanin, elasticity) | Overall satisfaction was higher in MSC-CM group than in controls (3.25 ± 1.26 vs. 2.72 ± 1.45) and also objective clinical improvement (1.92 ± 0.42 vs. 1.49 ± 0.48) | Erythema, melanin, and elasticity improved more in MSC-CM group. No serious adverse events were observed; only mild pain, erythema, and desquamation were found | |
| Seo et al., 2013 | Human embryo | − | Prospective randomized controlled, investigator-blinded, split-face study | Healthy volunteers. 53.8 ± 3.21 years, range 41–64 | - Microneedling fractional radiofrequency (control) - Microneedling fractional radiofrequency+MSC-CM (n = 15) |
28 | Macroscopic appearance (photography), biophysical parameters (SCH, erythema, melanin, wrinkles, elasticity), histology (HE), IHC | More improvements of wrinkles and overall skin appearance were observed in combined treatment compared with microneedling alone (2.06 ± 0.70 for radiofrequency and 2.20 ± 0.68 for the combined treatment, p < 0.05) | Patients’ overall satisfaction scores were higher in combined treatment (2.35 ± 0.42 vs. 2.00 ± 0.65). SCH showed a greater increase in the combined treatment. Similar decreases in erythema and melanin index were observed in both groups. No serious adverse events were reported |
| Zhou et al., 2013 | Human subcutaneous adipose tissue samples | Liposuction | Prospective observational study | Healthy volunteers. 24–33 years 5:14 male:female | Topical application after laser treatment: - FxCR 8 mJ, with MSC-CM - FxCR 8 mJ with DMEM - FxCR 16 mJ with MSC-CM - FxCR 8 mJ with DMEM (n = 19) |
21 | Macroscopic appearance (photography), biophysical parameters (TEWL, erythema, melanin, elasticity), histology (HE, MT, Gomori’s aldehyde fuchsine staining), qT-PCR | The MSC-CM-treated side shows less erythema and less pigmentation | MSC-CM side also showed a greater reduction of TEWL. There were no differences in elasticity parameters. The mRNA of type III procollagen in MSC-CM-treated group was 2.6 times that of control. No adverse events were reported |
AT, adipose tissue-derived; CM, conditioned medium; DMEM, Dulbecco’s modified Eagle medium; FxCR, fractional carbon dioxide laser resurfacing; HA, hyaluronic acid; HE, hematoxylin and eosin; hCB, human cord blood; hESC, human embryonic stem cell; IHC, immunohistochemistry; MSCs, mesenchymal stromal cells; MT, Masson’s trichrome; P-MSCs, placenta-derived mesenchymal stromal cells; qRT-PCR, real-time quantitative polymerase chain reaction; PRP, platelet-rich plasma.
Two hundred six healthy volunteers were treated with MSC-CM for anti-aging therapies (Seo et al., 2013; Zhou et al., 2013; Lee S. M. et al., 2014; Xu et al., 2016; Kim et al., 2018; Prakoeswa et al., 2019; El-Domyati et al., 2020; Kim J. et al., 2020). This research included women between 24 and 64 years. The studies evaluated the effects of microneedling, radiofrequency, or laser therapy alone compared with combined treatment with MSC-CM. They observed that combined therapy showed better improvement in macroscopic appearance (less pores and wrinkles), biophysical parameters (increased SCH and decreased TEWL, erythema, and melanin), and histology (increased dermal density) and had higher patient satisfaction scores. Similar results were found for the application of CM derived from human adipose tissue and human placenta, showing only a greater decrease in melanin index in the AT-MSC-CM group (Xu et al., 2016). The application of human UC-MSC-CM containing serum and cream on patients who underwent fractional laser therapy showed less microcrusts than the application of UC-MSC-CM only containing cream (Kim J. et al., 2020). No serious adverse events were observed in these reports.
Moreover, MSC-CM was also tested on acne scars (Zhou et al., 2016; Abdel-Maguid et al., 2019; El-Domyati et al., 2019; Park C. S. et al., 2019), suggesting that combined therapy (laser+MSC-CM) may increase the regenerative effects of fractional laser. Four studies evaluated the therapeutic potential in 71 patients with a mean follow-up of 82.5 days (range 60–90). After laser treatment, topical application of MSC-CM showed greater scar volume reduction than did with the control, by 23.5% on the MSC-CM side vs. 15.0% on the control side (Park C. S. et al., 2019). Furthermore, the MSC-CM-treated side improved subjective satisfaction and clinical assessment, showing better TEWL, SCH, elasticity, roughness, and collagen density than did the controls (laser alone or DMEM). Nevertheless, laser therapy+MSC-CM showed lower clinical improvement in acne scars than the combination of laser+platelet-rich plasma (Abdel-Maguid et al., 2019). Adverse events reported were erythema and edema, linked more to laser therapy than MSC-CM treatment.
Inflammatory Skin Diseases
Preclinical Studies
One study evaluated the effect of CM derived from murine adipose tissue for treating atopic dermatitis (AD) (Park H. S. et al., 2019; Table 11). Mice were followed up 33 days after AD lesions had developed and treated with subcutaneous injections every 3 days with saline, MSC, MSC-CM, or 2.5% cortisone lotion (n = 6/group). A higher AD severity index decrease was observed in groups treated with MSC, MSC-CM, or cortisone, as compared with the saline group. Moreover, the severity index (assessed by SCORAD) was lower in the MSC-CM group treated with MSCs or cortisone. Skin thickness decreases were similar between MSC-CM, MSC, and cortisone, and all greater than in the saline group. Likewise, mast cell infiltration was reduced in MSC-CM, MSC, and cortisone groups. Th2 expression, measured in optical densities, was lower in the MSC-CM groups than in the MSC, cortisone, or saline group (1.58 ± 1.84 vs. 3.79 ± 2.08 vs. 4.10 ± 3.32 vs. 19.19 ± 5.54). Ig E levels were slightly reduced in mice that were treated with MSC, MSC-CM, or cortisone. IL-4 was also reduced in the group treated with MSC-CM (Park H. S. et al., 2019).
TABLE 11.
Studies regarding mesenchymal stromal cell- conditioned medium for treating inflammatory skin diseases.
| MSC source | Method of tissue extraction | MSC characterization | MSC treatment | Model and patients | Groups of treatments and via of administration | Follow-up (days) | Assessment | Main outcome | Other outcomes | |
| Animals | ||||||||||
| Park H. S. et al., 2019 | Murine adipose tissue from inguinal region | Inguinal incision | Flow cytometry (CD45−, CD29+, CD105+, CD73+, CD34−, CD44+, and CD90+) | MSCs of passage 1 at 70% confluence were used. CM was then collected | Murine. AD model. 2,4-dinitrochlorobenzene was applied on the shaved dorsal skin of every mouse for 7.5 weeks to induce AD-like skin lesions | 200 μl subcutaneously injected every 3 days (on days 13, 16, and 19) - Saline - MSCs - MSC-CM - 2.5% cortisone lotion (n = 6/group) |
33 | Macroscopic appearance (photography, SCORAD), histology (HE), IHC | AD severity index was significantly lower in groups treated with MSC, MSC-CM, or cortisone, compared with saline group (p < 0.001) | Skin thickness decreases were similar between MSC, MSC-CM, and cortisone and all greater than placebo. Mast cell infiltration was reduced in the MSC-treated group (40.20 ± 9.44 cells, p < 0.001), MSC−CM (47.33 ± 13.13 cells, p < 0.001), or cortisone (51.00 ± 21.46 cells, p < 0.001) compared with saline (134.00 ± 7.42 cells). Thymic stromal lymphopoietin expression level in saline group was higher (11.7 ± 1.69) than in MSC group (5.07 ± 0.76, p < 0.001), MSC−CM (4.52 ± 1.33, p < 0.001), or cortisone group (6.72 ± 1.66, p < 0.001). CD45 expression was elevated in saline−treated group (1.68 ± 0.29), but it was reduced in MSC group (0.38 ± 0.18, p < 0.001), MSC−CM (0.19 ± 0.17, p < 0.001), or cortisone (0.37 ± 0.15, p < 0.001). Moreover, TH2 expression levels were lower in MSC-treated mice (3.79 ± 2.08, p < 0.001), MSC−CM (1.58 ± 1.84, p < 0.001), or cortisone (4.10 ± 3.32, p < 0.001) compared with saline group (19.19 ± 5.54). CXCL9 expression levels were lower in MSC group |
| (4.49 ± 1.25, p < 0.01), MSC−CM (8.78 ± 0.51, p < 0.01), or cortisone (15.83 ± 3.94, p < 0.05) compared with saline group (41.2 ± 4.15). CCL20 expression was higher in saline−treated group (61.18 ± 1.97), but it was reduced by MSCs (23.99 ± 2.46, p < 0.001), MSC−CM (25.98 ± 3.19, p < 0.001), or cortisone (28.87 ± 3.1, p < 0.001). IgE levels were slightly reduced in mice that were treated with MSC, MSC-CM, or cortisone. Tissue levels of IFN−γ were reduced MSCs (66.20 ± 14.51 pg/ml, p < 0.001) or MSC−CM (36.27 ± 3.26 pg/ml, p < 0.001) compared with saline (117.14 ± 45.84 pg/ml). Similarly, serum levels of IL−33 were lower in MSC group (32.20 ± 3.04 pg/ml; p < 0.001) and MSC−CM group (42.83 ± 6.07 pg/ml, p < 0.001) than in saline group (64.06 ± 8.58 pg/ml). IL-4 level in the MSC-CM group (30.03 ± 8.39 pg/ml, p < 0.001) was decreased compared with saline (51.97 ± 2.24 pg/ml). Serum IL−13 level was decreased in MSCs−treated group (0.27 ± 0.03 pg/ml, | ||||||||||
| Human | ||||||||||
| Kim Y. J. et al., 2020 | Human umbilical cord | Umbilical cord dissection | Flow cytometry (CD24+, CD29+, CD44+, CD73+, CD90+, CD105+, CD10−, CD14−, CD31−, CD34−, CD45−, CD62−, CD133−, HLA-DR−). Osteogenic, adipogenic, and chondrogenic differentiation | MSCs of passage 6 were used. CM was collected | Prospective observational study Patients with atopic dermatitis patients. Age 24.68 ± 4.32 Sex 15:13 male:female | MSC-CM containing cream bases were applied twice a day to patients’ lesion and non-lesion skin (n = 28) | 28 | Skin biophysical parameters (TEWL, SCH) | MSC-CM improved skin barrier functions | p < 0.05) compared with saline group (3.68 ± 1.40 pg/ml) SCH increased and TEWL decreased on both the lesion and non-involved skin after MSC-CM. SCH increased by 15.67 and 14.49 AU on the lesion and non-involved skin, respectively. TEWL decreased 15.09 AU and 3.08 AU on the lesion and non-involved skin, respectively |
| Seetharaman et al., 2019 | Human adipose tissue from the waist area | Lipoaspiration | − | MSCs of passage at 90% confluence were used. CM was then collected | Case report. Patient with scalp psoriasis. 38 years Male | MSC-CM was topically applied on the lesions once a day | 30 | Clinical examination (PSSI) | Psoriatic plaques were reduced after MSC-CM treatment | PSSI score reduced from 28 to 0 and diseases regression continued for 6 months of follow-up |
AD, atopic dermatitis; CM, conditioned medium; HE, hematoxylin and eosin; IHC, immunohistochemistry; MSCs, mesenchymal stromal cell; PSSI, Psoriasis Scalp Severity Index; SCORAD, SCORing Atopic Dermatitis Index; SCH, stratum corneum hydration, TEWL, transepidermal water loss.
Clinical Studies
One study evaluated the effects of MSC-CM for treating AD (Kim Y. J. et al., 2020), and one case report showed the outcomes in a patient with scalp psoriasis treated with MSC-CM (Seetharaman et al., 2019; Table 11).
Twenty-eight patients (15 males and 13 females; mean age 24.68 ± 4.32 years) were treated with human UC-MSC-CM in cream bases for 4 weeks. MSC-CM was applied on both eczematous lesions and non-involved skin twice a day. After treatment, SCH increased by 15.67 arbitrary units (AU), a relative unit of measurement to show the ratio of amount of some quantities to a predetermined reference measurement, on eczematous lesions and 14.49 AU on non-involved skin. Moreover, TEWL decreased by 15.09 g⋅m−2⋅h−1 AU on eczematous lesions and 3.08 g⋅m−2⋅h−1 on non-involved skin (Kim Y. J. et al., 2020). In agreement with preclinical studies, these data showed that cosmetics that contained MSC-CM improved the skin barrier function and could be an effective treatment for AD patients.
One study also evaluated the clinical efficacy of MSC-CM derived from human adipose tissue for treating psoriasis scalp. A 38-year-old male was treated topically with MSC-CM on the lesions once a day. After 1-month follow-up, the number of psoriatic plaques was reduced and Psoriasis Scalp Severity Index (PSSI) changed from 28 to 0. Moreover, no relapse or adverse events were observed after 6 months’ follow-up (Seetharaman et al., 2019).
Discussion
MSC-CM may be an alternative, safe, and easily delivered therapy for several skin conditions, but evidence in clinical studies is limited. They have been mainly tested in hair restoration (Shin et al., 2015; Lee et al., 2020; Narita et al., 2020; Oh et al., 2020) and skin rejuvenation (Abdel-Maguid et al., 2019; El-Domyati et al., 2019; Kim J. et al., 2020), with promising results. Preclinical studies are mainly focused on wound healing (Shin et al., 2015; Lee et al., 2020; Narita et al., 2020; Oh et al., 2020), showing high rates in wound closure. MSC-CM may also be an effective treatment for decreasing hypertrophic scars (Arjunan et al., 2020; Hu et al., 2020), improving flap reperfusion (Mirabella et al., 2012; Lee S. M. et al., 2014; Cooper et al., 2018; Pu et al., 2019), and treating psoriasis (Seetharaman et al., 2019) and AD (Park H. S. et al., 2019; Kim Y. J. et al., 2020). Nevertheless, there was significant variability between studies in the cell source, cell treatment, method of delivery, disease model used to assess efficacy, ways of assessment, and outcome evaluation. This review provides a useful summary regarding the use of MSC-CM for skin conditions and highlights the need to standardize manufacturing processes and characterize CM. The main findings of this review are summarized in Figure 2.
FIGURE 2.
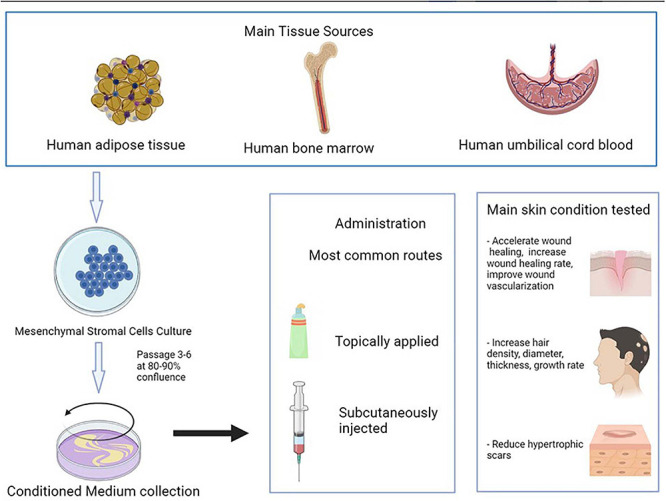
Iconographic summary of this review’s main findings.
It is important to characterize the composition of CM, but there is a lack of evidence in this aspect. Some studies showed that CM contained high concentrations of growth factors and cytokines associated with angiogenesis and endothelial cell migration such as EGF, tissue inhibitors of metalloproteinase-1, or IGF-binding protein-7 (De Gregorio et al., 2020; Kim Y. J. et al., 2020). The properties of MSC-CM vary depending on the cell source (Sagaradze et al., 2019). More than 10 sources of MSCs were used to treat skin conditions. MSCs were mainly isolated from human adipose tissue, human bone marrow, and human umbilical cord blood. Another important aspect is the timing of the collection of CM from the cells and concentration steps carried out for obtaining CM (Im et al., 2019). Most studies used cells from passages 3 to 6 at 80–90% confluence. The secretome of cells may also vary depending on the age of the cells (Maguire, 2013). Furthermore, the same type of cells is reported to secrete different levels of paracrine factors depending on the culture condition (Park et al., 2010) (hypoxic vs. normoxic) and the scaffold type (Li T. et al., 2019) (monolayer vs. 3D cultures). Hypoxic treatment is one of the most frequently used ways to improve CM, as hypoxic stress reduces oxygen and improves cellular functions (Du et al., 2017). CM collected from MSCs cultured under hypoxic conditions improved the cells’ capacities of proliferation and self-renewal (Chen et al., 2014; Jun et al., 2014; Du et al., 2016a, 2017). Determining the levels of paracrine factors secreted by MSCs at different passages with different conditions may also provide knowledge about the best stromal cell growth stage for obtaining a specific group of paracrine factors. Furthermore, MSC-CM should be considered both an alternative therapy and a synergic therapy for improving skin conditions, as MSC-CM combined with the conventional therapy has been proved to have better results than MSC-CM or the conventional therapy alone (Pouriran et al., 2016; Amini et al., 2018; Bagheri et al., 2018; Fridoni et al., 2019; Kouhkheil et al., 2019). In fact, some studies regarding wound healing in diabetic and infected wounds showed that PBM+MSC-CM application accelerated the process of wound healing, while it did not happen when using MSC-CM alone (Pouriran et al., 2016; Fridoni et al., 2019; Kouhkheil et al., 2019).
Several routes of administration have been also tested. Topical application and subcutaneous injection of CM were the most common routes of administration, and they are also the least invasive ways, appearing as promising routes for future research on MSC-CM.
No adverse events were reported to be related to MSC-CM in either preclinical or clinical trials. This is one of the main advantages of using MSC-CM instead of CM, as adverse events related to cells such as hyperimmunogenicity or tumorigenicity can be avoided (Gunawardena et al., 2019b). Only three studies evaluated differences between MSCs and MSC-CM, showing similar results between them (Tamari et al., 2013; Park H. S. et al., 2019; Zhang S. et al., 2020). Similar effectiveness was observed between UC-MSCs and UC-MSC-CM for accelerating wound healing rate. The wound area of UC-MSC and UC-MSC-CM was ≈15% reduced as compared with controls without differences in UC-MSC and UC-MSC-CM (Park H. S. et al., 2019; Zhang S. et al., 2020). Other research also showed similar epithelialization rate when using MSC or MSC-CM (Tamari et al., 2013). Moreover, it was observed that the effect of autologous AT-MSCs and AT-MSC-CM was similar for treating AD in mouse and higher than in the control group (Park H. S. et al., 2019).
It is also important to differentiate MSC-CM from culture media only. MSC-CM is the one in which MSCs have been grown for a period of time and with some requirements, and the culture media only is the media in which we grow the MSC-CM but has not been exposed to the cells. The culture media were used as control in some studies to prove the effectiveness of MSC-CM in wound healing (Jun et al., 2014; Deng et al., 2019; Rong et al., 2019). Higher wound healing rates and faster healing were observed with MSC-CM treatment than DMEM. Moreover, it was observed that hypertrophic scar reduction was higher using MSC-CM than DMEM (Zhang et al., 2015; Liu et al., 2018; Hu et al., 2020). It was also found that the topical application of MSC-CM after laser treatment showed less erythema and less pigmentation than the application of DMEM (Zhou et al., 2013, 2016). This might mean that the effect observed of MSC-CM in these diseases is due to the secretome and is not being caused by the culture media alone.
One of the limitations of MSC-CM is that, to translate its use to patients, it is necessary to know the exact composition of each CM and that its use validation should be conducted for every disease it is to be used for. Moreover, CM needs to be given more frequently than MSC, as the half-lives of cytokines and growth factors are mostly shorter than those of stromal cells, which may survive for rather long periods (Pawitan, 2014). Further research is needed to determine the components of MSC-CM that improves skin diseases. Moreover, regulatory requirements for manufacturing, standardization, and quality control of MSC-CM regarding MSC origin, isolation methods, and culture conditions are necessary to establish the safety and efficacy profiles of these products (Park H. S. et al., 2019).
Although there are some reviews regarding MSC-CM (Pawitan, 2014; Mizukami and Yagihashi, 2016; Bogatcheva and Coleman, 2019), they include little information about cutaneous disease. Pawitan et al. included some studies where MSC-CM was used for skin repair and alopecia. Bogatcheva and Coleman only included two articles where MSC-CM was used for skin diseases, one on treating dermatitis and the other on burns. Mizukami and Yagihashi reviewed the status of preclinical studies on stromal cell therapy for diabetic polyneuropathy, but they focused on MSCs and did not include information on other diseases. As far as we know, here, we review the use of MSC-CM in all skin conditions reported in the literature for the first time.
In conclusion, MSC-CM is a promising therapy for skin conditions. Studies on animals showed important rates of wound closure after MSC-CM treatment and clinical studies showed good results for skin rejuvenation. Further studies are needed to corroborate safety and effectiveness and to standardize CM manufacturing.
Author Contributions
TM-V and SA-S contributed to conceptualization, formal analysis, investigation, and writing – original draft preparation. TM-V, ÁS-S, and SA-S contributed to methodology. AM-L contributed to software. TM-V, AM-L, and SA-S contributed to validation and visualization. MQ-V contributed to resources. TM-V, ÁS-S, MS-D, and MQ-V contributed to data curation. TM-V, SA-S, ÁS-S, and AM-L contributed to writing – review and editing. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
- AD
atopic dermatitis
- AGA
androgenetic alopecia
- AT
adipose tissue
- BM
bone marrow
- Botox
botulinum toxin type A
- CM
conditioned medium
- DMEM
Dulbecco’s modified Eagle medium
- ECM
extracellular matrix
- EV
extracellular vesicles
- Exos
exosomes
- HB-EGF
heparin binding-epidermal growth factor-like growth factor
- HDFs
human dermal fibroblasts
- HGF
hepatocyte growth factor
- IGF
insulin-like growth factor
- IL
interleukin
- Men
menstrual blood
- MSCs
mesenchymal stromal cells
- PBS
phosphate-buffered saline
- PSSI
Psoriasis Scalp Severity Index
- qRT-PCR
real-time quantitative polymerase chain reaction
- SCH
stratum corneum hydration (SCH)
- SCORAD
SCORing Atopic Dermatitis Index
- SHSs
Haliclona sp. spicules
- SDF − 1
stromal cell-derived factor 1
- TEWL
transepidermal water loss
- TGF- β
transforming growth factor β
- Th2
lymphocyte T helper type 2
- TNF- α
tumor necrosis factor α
- UC
umbilical cord
- VEGF
vascular endothelial growth factor.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.654210/full#supplementary-material
References
- Abdel-Maguid E. M., Awad S. M., Hassan Y. S., El-Mokhtar M. A., El-Deek H. E., Mekkawy M. M. (2019). Efficacy of stem cell-conditioned medium vs. platelet-rich plasma as an adjuvant to ablative fractional CO2 laser resurfacing for atrophic post-acne scars: a split-face clinical trial. J. Dermatolog. Treat. 32 242–249. 10.1080/09546634.2019.1630701 [DOI] [PubMed] [Google Scholar]
- Ahangar P., Mills S. J., Smith L. E., Strudwick X. L., Ting A. E., Vaes B., et al. (2020). Human multipotent adult progenitor cell-conditioned medium improves wound healing through modulating inflammation and angiogenesis in mice. Stem Cell Res. Ther. 11:299. 10.1186/s13287-020-01819-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini A., Pouriran R., Abdollahifar M. A., Abbaszadeh H. A., Ghoreishi S. K., Chien S., et al. (2018). Stereological and molecular studies on the combined effects of photobiomodulation and human bone marrow mesenchymal stem cell conditioned medium on wound healing in diabetic rats. J. Photochem. Photobiol. B 182 42–51. 10.1016/j.jphotobiol.2018.03.010 [DOI] [PubMed] [Google Scholar]
- Arjunan S., Gan S. U., Choolani M., Raj V., Lim J., Biswas A., et al. (2020). Inhibition of growth of Asian keloid cells with human umbilical cord Wharton’s jelly stem cell-conditioned medium. Stem Cell Res. Ther. 11:78. 10.1186/s13287-020-01609-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryan A., Bayat M., Bonakdar S., Taheri S., Haghparast N., Bagheri M., et al. (2019). Human bone marrow mesenchymal stem cell conditioned medium promotes wound healing in deep second-degree burns in male rats. Cells Tissues Organs. 206 317–329. 10.1159/000501651 [DOI] [PubMed] [Google Scholar]
- Bagheri M., Amini A., Abdollahifar M. A., Ghoreishi S. K., Piryaei A., Pouriran R., et al. (2018). Effects of photobiomodulation on degranulation and number of mast cells and wound strength in skin wound healing of streptozotocin-induced diabetic rats. Photomed Laser Surg. 36 415–423. 10.1089/pho.2018.4453 [DOI] [PubMed] [Google Scholar]
- Bian X., Li B., Yang J., Ma K., Sun M., Zhang C., et al. (2020). Regenerative and protective effects of dMSC-sEVs on high-glucose-induced senescent fibroblasts by suppressing RAGE pathway and activating Smad pathway. Stem Cell Res. Ther. 11:166. 10.1186/s13287-020-01681-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogatcheva N. V., Coleman M. E. (2019). Conditioned medium of mesenchymal stromal cells: a new class of therapeutics. Biochemistry (Mosc) 84 1375–1389. 10.1134/S0006297919110129 [DOI] [PubMed] [Google Scholar]
- Caplan A. I. (2017). Mesenchymal stem cells: time to change the name! Stem Cells Transl. Med. 6 1445–1451. 10.1002/sctm.17-0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan H., Olson S. D., Kumar A., George M., Prabhakara K. S., Wenzel P., et al. (2019). Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front. Immunol. 10:1645. 10.3389/fimmu.2019.01645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Rao S. S., Ren L., Hu X. K., Tan Y. J., Hu Y., et al. (2018). Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics 8 1607–1623. 10.7150/thno.22958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Cheng L., Wang Z., Zhang J., Mao X., Liu Z., et al. (2021). Conditioned medium-electrospun fiber biomaterials for skin regeneration. Bioact Mater. 6 361–374. 10.1016/j.bioactmat.2020.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Tredget E. E., Wu P. Y., Wu Y. (2008). Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3:e1886. 10.1371/journal.pone.0001886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Xu Y., Zhao J., Zhang Z., Yang R., Xie J., et al. (2014). Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One 9:e96161. 10.1371/journal.pone.0096161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Pan D., Hu S., Yang J., Zhou L., Shen J. (2018). The combination of exosomes derived from umbilical cord mesenchymal stem cells and Pluronic F-127 promotes the proliferation and migration of vascular endothelial cells. Diabetes Metabolism Res. Rev. 34 24–25. 10.1002/dmrr.3079 [DOI] [Google Scholar]
- Cho J. W., Kang M. C., Lee K. S. (2010). TGF-β1-treated ADSCs-CM promotes expression of type I collagen and MMP-1, migration of human skin fibroblasts, and wound healing in vitro and in vivo. Int. J. Mol. Med. 26 901–906. 10.3892/ijmm_00000540 [DOI] [PubMed] [Google Scholar]
- Choi N., Kim W. S., Oh S. H., Sung J. H. (2019). HB-EGF improves the hair regenerative potential of adipose-derived stem cells via ROS Generation and Hck Phosphorylation. Int. J. Mol. Sci. 21:122. 10.3390/ijms21010122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. J., Lee K. S., Yeom S. H., Cho Y. W. (2017). Exosomes secreted by human adipose-derived stem cells regulate the expression of collagen synthesis-related genes in human dermal fibroblasts. J. Extracell. Vesicles 6:141. 10.1080/20013078.2017.1310414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. R., Wang C., Patel R., Trujillo A., Patel N. A., Prather J., et al. (2018). Human adipose-derived stem cell conditioned media and exosomes containing MALAT1 promote human dermal fibroblast migration and ischemic wound healing. Adv. Wound Care 7 299–308. 10.1089/wound.2017.0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalirfardouei R., Jamialahmadi K., Jafarian A. H., Mahdipour E. (2019). Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J. Tissue Eng. Regener. Med. 13 555–568. 10.1002/term.2799 [DOI] [PubMed] [Google Scholar]
- De Gregorio C., Contador D., Diáz D., Cárcamo C., Santapau D., Lobos-Gonzalez L., et al. (2020). Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res. Ther. 11:168. 10.1186/s13287-020-01680-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C., He Y., Feng J., Dong Z., Yao Y., Lu F. (2019). Conditioned medium from 3D culture system of stromal vascular fraction cells accelerates wound healing in diabetic rats. Regen. Med. 14 925–937. 10.2217/rme-2018-0083 [DOI] [PubMed] [Google Scholar]
- Deng C., He Y., Feng J., Dong Z., Yao Y., Mok H., et al. (2017). Extracellular matrix/stromal vascular fraction gel conditioned medium accelerates wound healing in a murine model. Wound Repair Regen. 25 923–932. 10.1111/wrr.12602 [DOI] [PubMed] [Google Scholar]
- Deng H., Sun C., Sun Y., Li H., Yang L., Wu D., et al. (2018). Lipid, Protein, and MicroRNA composition within mesenchymal stem cell-derived exosomes. Cell Reprogram 20 178–186. 10.1089/cell.2017.0047 [DOI] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8 315–317. 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- Dong L., Hao H., Liu J., Ti D., Tong C., Hou Q., et al. (2017). A conditioned medium of umbilical cord mesenchymal stem cells overexpressing Wnt7a promotes wound repair and regeneration of hair follicles in mice. Stem Cells Int. 2017:3738071. 10.1155/2017/3738071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Hao H., Xia L., Liu J., Ti D., Tong C., et al. (2014). Treatment of MSCs with Wnt1a-conditioned medium activates DP cells and promotes hair follicle regrowth. Sci. Rep. 4:5432. 10.1038/srep05432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H. C., Jiang L., Geng W. X., Li J., Zhang R., Dang J. G., et al. (2017). Growth Factor-Reinforced ECM fabricated from chemically hypoxic msc sheet with improved in vivo wound repair activity. Biomed. Res. Int. 2017:2578017. 10.1155/2017/2578017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Lv R., Yang X., Cheng S., Ma T., Xu J. (2016a). Hypoxic conditioned medium of placenta-derived mesenchymal stem cells protects against scar formation. Life Sci. 149 51–57. 10.1016/j.lfs.2016.02.050 [DOI] [PubMed] [Google Scholar]
- Du L., Lv R., Yang X., Cheng S., Xu J., Ma T. (2016b). Hypoxia enhances the protective effects of placenta-derived mesenchymal stem cells against scar formation through hypoxia-inducible factor-1α. Biotechnol. Lett. 38 931–939. 10.1007/s10529-016-2067-6 [DOI] [PubMed] [Google Scholar]
- El-Domyati M., Moftah N. H., Nasif G. A., Ameen S. W., Ibrahim M. R., Ragaie M. H. (2020). Facial rejuvenation using stem cell conditioned media combined with skin needling: a split-face comparative study. J. Cosmetic Dermatol. 19 2404–2410. 10.1111/jocd.13594 [DOI] [PubMed] [Google Scholar]
- El-Domyati M., Moftah N. H., Nasif G. A., Ragaie M. H., Ibrahim M. R., Ameen S. W. (2019). Amniotic fluid-derived mesenchymal stem cell products combined with microneedling for acne scars: a split-face clinical, histological, and histometric study. J. Cosmetic Dermatol. 18 1300–1306. 10.1111/jocd.13039 [DOI] [PubMed] [Google Scholar]
- Fong C. Y., Tam K., Cheyyatraivendran S., Gan S. U., Gauthaman K., Armugam A., et al. (2014). Human Wharton’s jelly stem cells and its conditioned medium enhance healing of excisional and diabetic wounds. J. Cell Biochem. 115 290–302. 10.1002/jcb.24661 [DOI] [PubMed] [Google Scholar]
- Fridoni M., Kouhkheil R., Abdollhifar M. A., Amini A., Ghatrehsamani M., Ghoreishi S. K., et al. (2019). Improvement in infected wound healing in type 1 diabetic rat by the synergistic effect of photobiomodulation therapy and conditioned medium. J. Cell Biochem. 120 9906–9916. 10.1002/jcb.28273 [DOI] [PubMed] [Google Scholar]
- Gunawardena T. N. A., Masoudian Z., Rahman M. T., Ramasamy T. S., Ramanathan A., Abu Kasim N. H. (2019a). Dental derived stem cell conditioned media for hair growth stimulation. PLoS One 14:e0216003. 10.1371/journal.pone.0216003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena T. N. A., Rahman M. T., Abdullah B. J. J., Abu Kasim N. H. (2019b). Conditioned media derived from mesenchymal stem cell cultures: the next generation for regenerative medicine. J. Tissue Eng. Regen. Med. 13 569–586. 10.1002/term.2806 [DOI] [PubMed] [Google Scholar]
- Guo S., Wang T., Zhang S., Chen P., Cao Z., Lian W., et al. (2020). Adipose-derived stem cell-conditioned medium protects fibroblasts at different senescent degrees from UVB irradiation damages. Mol. Cell Biochem. 463 67–78. 10.1007/s11010-019-03630-8 [DOI] [PubMed] [Google Scholar]
- He D., Zhao F., Jiang H., Kang Y., Song Y., Lin X., et al. (2020). LOXL2 from human amniotic mesenchymal stem cells accelerates wound epithelialization by promoting differentiation and migration of keratinocytes. Aging (Albany NY) 12 12960–12986. 10.18632/aging.103384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo S. C., Jeon E. S., I, Lee H., Kim H. S., Kim M. B., Kim J. H. (2011). Tumor necrosis factor-α-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J. Invest. Dermatol. 131 1559–1567. 10.1038/jid.2011.64 [DOI] [PubMed] [Google Scholar]
- Hu C. H., Tseng Y. W., Lee C. W., Chiou C. Y., Chuang S. S., Yang J. Y., et al. (2020). Combination of mesenchymal stem cell-conditioned medium and botulinum toxin type A for treating human hypertrophic scars. J. Plast Reconstr. Aesthet Surg. 73 516–527. 10.1016/j.bjps.2019.07.010 [DOI] [PubMed] [Google Scholar]
- Hu L., Yin C., Zhao F., Ali A., Ma J., Qian A. (2018). Mesenchymal stem cells: cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. Int. J. Mol. Sci. 19:360. 10.3390/ijms19020360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im G. B., Kim Y. H., Kim Y. J., Kim S. W., Jung E., Jeong G. J., et al. (2019). Enhancing the wound healing effect of conditioned medium collected from mesenchymal stem cells with high passage number using bioreducible nanoparticles. Int. J. Mol. Sci. 20:4835. 10.3390/ijms20194835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons R. F., Cahill K. W., Rattigan D. A., Marcotte J. H., Fromer M. W., Chang S., et al. (2018). Acceleration of diabetic wound healing with adipose-derived stem cells, endothelial-differentiated stem cells, and topical conditioned medium therapy in a swine model. J. Vasc. Surg. 68 115s–125s. 10.1016/j.jvs.2018.01.065 [DOI] [PubMed] [Google Scholar]
- Joseph A., Baiju I., Bhat I. A., Pandey S., Bharti M., Verma M., et al. (2020). Mesenchymal stem cell-conditioned media: a novel alternative of stem cell therapy for quality wound healing. J. Cell. Physiol. 235 5555–5569. 10.1002/jcp.29486 [DOI] [PubMed] [Google Scholar]
- Jun E. K., Zhang Q., Yoon B. S., Moon J. H., Lee G., Park G., et al. (2014). Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt pathways. Int. J. Mol Sci. 15 605–628. 10.3390/ijms15010605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kim B., Kim S., Lee Y. I., Kim J., Lee J. H. (2020). The effect of human umbilical cord blood–derived mesenchymal stem cell media containing serum on recovery after laser treatment: a double-blinded, randomized, split-face controlled study. J. Cosmetic Dermatol. 19 651–656. 10.1111/jocd.13063 [DOI] [PubMed] [Google Scholar]
- Kim J. Y., Song S. H., Kim K. L., Ko J. J., Im J. E., Yie S. W., et al. (2010). Human cord blood-derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing. Cell Transplant. 19 1635–1644. 10.3727/096368910X516637 [DOI] [PubMed] [Google Scholar]
- Kim K. H., Blasco-Morente G., Cuende N., Arias-Santiago S. (2017). Mesenchymal stromal cells: properties and role in management of cutaneous diseases. J. Eur. Acad Dermatol. Venereol. 31 414–423. 10.1111/jdv.13934 [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Ahn H. J., Lee S. H., Lee M. H., Kang K. S. (2020). Effects of conditioned media from human umbilical cord blood-derived mesenchymal stem cells in the skin immune response. Biomed. Pharmacother. 118:109131. 10.1016/j.biopha.2020.110789 [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Seo D. H., Lee S. H., Lee S. H., An G. H., Ahn H. J., et al. (2018). Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem. Biophys. Rep. 16 96–102. 10.1016/j.bbrep.2018.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouhkheil R., Fridoni M., Abdollhifar M. A., Amini A., Bayat S., Ghoreishi S. K., et al. (2019). Impact of photobiomodulation and condition medium on mast cell counts, degranulation, and wound strength in infected skin wound healing of diabetic rats. Photobiomodul Photomed Laser Surg. 37 706–714. 10.1089/photob.2019.4691 [DOI] [PubMed] [Google Scholar]
- Kusindarta D. L., Wihadmadyatami H., Fibrianto Y. H., Nugroho W. S., Susetya H., Musana D. K., et al. (2016). Human umbilical mesenchymal stem cells conditioned medium promote primary wound healing regeneration. Vet. World 9 605–610. 10.14202/vetworld.2016.605-610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T. R., Oh C. T., Choi E. J., Kim S. R., Jang Y. J., Ko E. J., et al. (2016). Conditioned medium from human bone marrow-derived mesenchymal stem cells promotes skin moisturization and effacement of wrinkles in UVB-irradiated SKH-1 hairless mice. Photodermatol. Photoimmunol. Photomed 32 120–128. 10.1111/phpp.12224 [DOI] [PubMed] [Google Scholar]
- Lee H. J., Lee E. G., Kang S., Sung J. H., Chung H. M., Kim D. H. (2014). Efficacy of microneedling plus human stem cell conditioned medium for skin rejuvenation: a randomized, controlled, blinded split-face study. Ann. Dermatol. 26 584–591. 10.5021/ad.2014.26.5.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Oh N., Park K. S. (2017). Ell3 modulates the wound healing activity of conditioned medium of adipose-derived stem cells. Dev. Reprod. 21 335–342. 10.12717/dr.2017.21.3.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. J., Kim J., Lee K. I., Shin J. M., Chae J. I., Chung H. M. (2011). Enhancement of wound healing by secretory factors of endothelial precursor cells derived from human embryonic stem cells. Cytotherapy 13 165–178. 10.3109/14653249.2010.512632 [DOI] [PubMed] [Google Scholar]
- Lee S. M., Lee S. C., Kim S. J. (2014). Contribution of human adipose tissue-derived stem cells and the secretome to the skin allograft survival in mice. J. Surg. Res. 188 280–289. 10.1016/j.jss.2013.10.063 [DOI] [PubMed] [Google Scholar]
- Lee Y. I., Kim J., Kim J., Park S., Lee J. H. (2020). The effect of conditioned media from human adipocyte-derived mesenchymal stem cells on androgenetic alopecia after nonablative fractional laser treatment. Dermatol. Surg. 46 1698–1704. 10.1097/DSS.0000000000002518 [DOI] [PubMed] [Google Scholar]
- Li J. Y., Ren K. K., Zhang W. J., Xiao L., Wu H. Y., Liu Q. Y., et al. (2019). Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res. Ther. 10:247. 10.1186/s13287-019-1366-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Ma H., Ma H., Ma Z., Qiang L., Yang Z., et al. (2019). Mussel-Inspired nanostructures potentiate the immunomodulatory properties and angiogenesis of mesenchymal stem cells. ACS Appl. Mater. Interfaces 11 17134–17146. 10.1021/acsami.8b22017 [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang W., Gao J., Liu J., Wang H., Li J., et al. (2016). Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res. Ther. 7:102. 10.1186/s13287-016-0356-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Ren J., Su L., Cheng S., Zhou J., Ye X., et al. (2018). Human adipose tissue-derived stem cells inhibit the activity of keloid fibroblasts and fibrosis in a keloid model by paracrine signaling. Burns 44 370–385. 10.1016/j.burns.2017.08.017 [DOI] [PubMed] [Google Scholar]
- Ma D., Kua J. E. H., Lim W. K., Lee S. T., Chua A. W. C. (2015). In vitro characterization of human hair follicle dermal sheath mesenchymal stromal cells and their potential in enhancing diabetic wound healing. Cytotherapy 17 1036–1051. 10.1016/j.jcyt.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Maguire G. (2013). Stem cell therapy without the cells. Commun. Integr. Biol. 6:e26631. 10.4161/cib.26631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lopez A., Montero-Vilchez T., Sierra-Sanchez A., Molina-Leyva A., Arias-Santiago S. (2020). Advanced medical therapies in the management of non-scarring alopecia: areata and androgenic alopecia. Int. J. Mol. Sci. 21:8390. 10.3390/ijms21218390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehanna R. A., Nabil I., Attia N., Bary A. A., Razek K. A., Ahmed T. A., et al. (2015). The effect of bone marrow-derived mesenchymal stem cells and their conditioned media topically delivered in fibrin glue on chronic wound healing in rats. Biomed. Res. Int. 2015:846062. 10.1155/2015/846062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella T., Hartinger J., Lorandi C., Gentili C., van Griensven M., Cancedda R. (2012). Proangiogenic soluble factors from amniotic fluid stem cells mediate the recruitment of endothelial progenitors in a model of ischemic fasciocutaneous flap. Stem Cells Dev. 21 2179–2188. 10.1089/scd.2011.0639 [DOI] [PubMed] [Google Scholar]
- Mizukami H., Yagihashi S. (2016). Is stem cell transplantation ready for prime time in diabetic polyneuropathy? Curr. Diab. Rep. 16:86. 10.1007/s11892-016-0776-9 [DOI] [PubMed] [Google Scholar]
- Moll G., Ankrum J. A., Kamhieh-Milz J., Bieback K., Ringden O., Volk H. D., et al. (2019). Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol. Med. 25 149–163. 10.1016/j.molmed.2018.12.006 [DOI] [PubMed] [Google Scholar]
- Moll G., Hoogduijn M. J., Ankrum J. A. (2020). Editorial: safety, efficacy and mechanisms of action of mesenchymal stem cell therapies. Front. Immunol. 11:243. 10.3389/fimmu.2020.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Vilchez T., Segura-Fernandez-Nogueras M. V., Perez-Rodriguez I., Soler-Gongora M., Martinez-Lopez A., Fernandez-Gonzalez A., et al. (2021). Skin barrier function in psoriasis and atopic dermatitis: transepidermal water loss and temperature as useful tools to assess disease severity. J. Clin. Med. 10:359. 10.3390/jcm10020359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K., Fukuoka H., Sekiyama T., Suga H., Harii K. (2020). Sequential scalp assessment in hair regeneration therapy using an adipose-derived stem cell-conditioned medium. Dermatol. Surg. 46 819–825. 10.1097/dss.0000000000002128 [DOI] [PubMed] [Google Scholar]
- Oh H. A., Kwak J., Kim B. J., Jin H. J., Park W. S., Choi S. J., et al. (2020). Migration inhibitory factor in conditioned medium from human umbilical cord blood-derived mesenchymal stromal cells stimulates hair growth. Cells 9:1344. 10.3390/cells9061344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou K. L., Kuo Y. W., Wu C. Y., Huang B. H., Pai F. T., Chou H. H., et al. (2020). The potential of a hair follicle mesenchymal stem cell-conditioned medium for wound healing and hair follicle regeneration. Appl. Sci. (Switzerland) 10:2646. 10.3390/APP10082646 [DOI] [Google Scholar]
- Park B. S., Kim W. S., Choi J. S., Kim H. K., Won J. H., Ohkubo F., et al. (2010). Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed. Res. 31 27–34. 10.2220/biomedres.31.27 [DOI] [PubMed] [Google Scholar]
- Park C. S., Park J. H., Kim C. R., Lee J. H. (2019). Objective analysis of volume restoration in atrophic acne scars and skin pores: a split study using human stem cell-conditioned media. J. Dermatol. Treat. 32 73–77. 10.1080/09546634.2019.1628915 [DOI] [PubMed] [Google Scholar]
- Park H. S., Son H. Y., Choi M. H., Son Y., Kim S., Hong H. S., et al. (2019). Adipose-derived stem cells attenuate atopic dermatitis-like skin lesions in NC/Nga mice. Exp. Dermatol. 28 300–307. 10.1111/exd.13895 [DOI] [PubMed] [Google Scholar]
- Park J., Jun E. K., Son D., Hong W., Jang J., Yun W., et al. (2019). Overexpression of Nanog in amniotic fluid-derived mesenchymal stem cells accelerates dermal papilla cell activity and promotes hair follicle regeneration. Exp. Mol. Med. 51:72. 10.1038/s12276-019-0266-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Lee J. H., Yoon B. S., Jun E. K., Lee G., I, Kim Y., et al. (2018). Additive effect of bFGF and selenium on expansion and paracrine action of human amniotic fluid-derived mesenchymal stem cells. Stem Cell Res. Ther. 9:293. 10.1186/s13287-018-1058-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawitan J. A. (2014). Prospect of stem cell conditioned medium in regenerative medicine. Biomed. Res. Int. 2014:965849. 10.1155/2014/965849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payushina O. V., Butorina N. N., Sheveleva O. N., Domaratskaya E. I. (2018). Effect of mesenchymal stromal cells and conditioned media on healing of skin wound. Bull. Exp. Biol. Med. 165 572–575. 10.1007/s10517-018-4215-6 [DOI] [PubMed] [Google Scholar]
- Pouriran R., Piryaei A., Mostafavinia A., Zandpazandi S., Hendudari F., Amini A., et al. (2016). The effect of combined pulsed wave low-level laser therapy and human bone marrow mesenchymal stem cell-conditioned medium on open skin wound healing in diabetic rats. Photomed Laser Surg. 34 345–354. 10.1089/pho.2015.4020 [DOI] [PubMed] [Google Scholar]
- Prakoeswa C. R. S., Natallya F. R., Harnindya D., Thohiroh A., Oktaviyanti R. N., Pratiwi K. D., et al. (2018). The efficacy of topical human amniotic membrane-mesenchymal stem cell-conditioned medium (hAMMSC-CM) and a mixture of topical hAMMSC-CM + vitamin C and hAMMSC-CM + vitamin E on chronic plantar ulcers in leprosy:a randomized control trial. J. Dermatol. Treat. 29 835–840. 10.1080/09546634.2018.1467541 [DOI] [PubMed] [Google Scholar]
- Prakoeswa C. R. S., Pratiwi F. D., Herwanto N., Citrashanty I., Indramaya D. M., Murtiastutik D., et al. (2019). The effects of amniotic membrane stem cell-conditioned medium on photoaging. J. Dermatol. Treat. 30 478–482. 10.1080/09546634.2018.1530438 [DOI] [PubMed] [Google Scholar]
- Pu C. M., Chen Y. C., Chen Y. C., Lee T. L., Peng Y. S., Chen S. H., et al. (2019). Interleukin-6 from adipose-derived stem cells promotes tissue repair by the increase of cell proliferation and hair follicles in ischemia/reperfusion-treated skin flaps. Mediators Inflamm. 2019:2343867. 10.1155/2019/2343867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V., Claudine S., Subramanian A., Tam K., Biswas A., Bongso A., et al. (2019). Histological, immunohistochemical, and genomic evaluation of excisional and diabetic wounds treated with human Wharton’s jelly stem cells with and without a nanocarrier. J. Cell. Biochem. 120 11222–11240. 10.1002/jcb.28398 [DOI] [PubMed] [Google Scholar]
- Ratajczak M. Z., Kucia M., Jadczyk T., Greco N. J., Wojakowski W., Tendera M., et al. (2012). Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia 26 1166–1173. 10.1038/leu.2011.389 [DOI] [PubMed] [Google Scholar]
- Robert A. W., Azevedo Gomes F., Rode M. P., Marques da Silva M., Veleirinho M., Maraschin M., et al. (2019). The skin regeneration potential of a pro-angiogenic secretome from human skin-derived multipotent stromal cells. J. Tissue Eng. 10:2041731419833391. 10.1177/2041731419833391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong X., Chu W., Zhang H., Wang Y., Qi X., Zhang G., et al. (2019). Antler stem cell-conditioned medium stimulates regenerative wound healing in rats. Stem Cell Res. Ther. 10:326. 10.1186/s13287-019-1457-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabzevari R., Roushandeh A. M., Mehdipour A., Alini M., Roudkenar M. H. (2020). SA/G hydrogel containing hCAP-18/LL-37-engineered WJ-MSCs-derived conditioned medium promoted wound healing in rat model of excision injury. Life Sci. 261:118381. 10.1016/j.lfs.2020.118381 [DOI] [PubMed] [Google Scholar]
- Sagaradze G., Grigorieva O., Nimiritsky P., Basalova N., Kalinina N., Akopyan Z., et al. (2019). Conditioned medium from human mesenchymal stromal cells: towards the clinical translation. Int. J. Mol. Sci. 20:1656. 10.3390/ijms20071656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheli M., Bayat M., Ganji R., Hendudari F., Kheirjou R., Pakzad M., et al. (2020). Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Arch. Dermatol. Res. 312 325–336. 10.1007/s00403-019-02016-6 [DOI] [PubMed] [Google Scholar]
- Seetharaman R., Mahmood A., Kshatriya P., Patel D., Srivastava A. (2019). Mesenchymal stem cell conditioned media ameliorate psoriasis vulgaris: a case study. Case Rep. Dermatol. Med. 2019:8309103. 10.1155/2019/8309103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo K. Y., Kim D. H., Lee S. E., Yoon M. S., Lee H. J. (2013). Skin rejuvenation by microneedle fractional radiofrequency and a human stem cell conditioned medium in Asian skin: a randomized controlled investigator blinded split-face study. J. Cosmet Laser Ther. 15 25–33. 10.3109/14764172.2012.748201 [DOI] [PubMed] [Google Scholar]
- Shim J. H. (2015). Hair growth-promoting effect of human dermal stem/progenitor cell-derived conditioned medium. Tissue Eng. Regen. Med. 12 268–275. 10.1007/s13770-015-0012-8 [DOI] [Google Scholar]
- Shin H., Ryu H. H., Kwon O., Park B. S., Jo S. J. (2015). Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss: a retrospective case series study. Int. J. Dermatol. 54 730–735. 10.1111/ijd.12650 [DOI] [PubMed] [Google Scholar]
- Sun B., Guo S., Xu F., Wang B., Liu X., Zhang Y., et al. (2014). Concentrated hypoxia-preconditioned adipose mesenchymal stem cell-conditioned medium improves wounds healing in full-thickness skin defect model. Int. Sch. Res. Notices 2014:652713. 10.1155/2014/652713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zhang Y., Song X., Zhu J., Zhu Q. (2019). The healing effects of conditioned medium derived from mesenchymal stem cells on radiation-induced skin wounds in rats. Cell Transplant 28 105–115. 10.1177/0963689718807410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam K., Cheyyatraviendran S., Venugopal J., Biswas A., Choolani M., Ramakrishna S., et al. (2014). A nanoscaffold impregnated with human wharton’s jelly stem cells or its secretions improves healing of wounds. J. Cell. Biochem. 115 794–803. 10.1002/jcb.24723 [DOI] [PubMed] [Google Scholar]
- Tamari M., Nishino Y., Yamamoto N., Ueda M. (2013). Acceleration of wound healing with stem cell-derived growth factors. Int. J. Oral. Maxillofac. Implants 28 e369–e375. 10.11607/jomi.te17 [DOI] [PubMed] [Google Scholar]
- Tarcisia T., Damayanti L., Antarianto R. D., Moenadjat Y., Pawitan J. A. (2017). Adipose derived stem cell conditioned medium effect on proliferation phase of wound healing in sprague dawley rat. Med. J. Indonesia 26 239–245. 10.13181/mji.v26i4.1755 [DOI] [Google Scholar]
- Templin C., Grote K., Schledzewski K., Ghadri J. R., Schnabel S., Napp L. C., et al. (2009). Ex vivo expanded haematopoietic progenitor cells improve dermal wound healing by paracrine mechanisms. Exp. Dermatol. 18 445–453. 10.1111/j.1600-0625.2008.00809.x [DOI] [PubMed] [Google Scholar]
- Ueda M., Nishino Y. (2010). Cell-based cytokine therapy for skin rejuvenation. J. Craniofac. Surg. 21 1861–1866. 10.1097/SCS.0b013e3181f43f0a [DOI] [PubMed] [Google Scholar]
- Won C. H., Choi S. J., Kwon O. S., Park W. S., Kang Y. J., Yoo H. G., et al. (2010). The establishment and characterization of immortalized human dermal papilla cells and their hair growth promoting effects. J. Dermatol. Sci. 60 196–198. 10.1016/j.jdermsci.2010.08.015 [DOI] [PubMed] [Google Scholar]
- Won C. H., Jeong Y. M., Kang S., Koo T. S., Park S. H., Park K. Y., et al. (2015). Hair-growth-promoting effect of conditioned medium of high integrin α6 and low CD 71 (α6bri/CD71dim) positive keratinocyte cells. Int. J. Mol. Sci. 16 4379–4391. 10.3390/ijms16034379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. P., Rowley J. E., Redpath A. N., Tilman J. D., Fellous T. G., Johnson J. R. (2015). Pericytes, mesenchymal stem cells and their contributions to tissue repair. Pharmacol. Ther. 151 107–120. 10.1016/j.pharmthera.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Wu Y., Peng Y., Gao D., Feng C., Yuan X., Li H., et al. (2015). Mesenchymal stem cells suppress fibroblast proliferation and reduce skin fibrosis through a TGF-β3-dependent activation. Int. J. Lower Extremity Wounds 14 50–62. 10.1177/1534734614568373 [DOI] [PubMed] [Google Scholar]
- Xiao S., Deng Y., Mo X., Liu Z., Wang D., Deng C., et al. (2020). Promotion of hair growth by conditioned medium from extracellular matrix/stromal vascular fraction gel in C57BL/6 Mice. Stem Cells Int. 2020:9054514. 10.1155/2020/9054514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Guo S., Wei C., Li H., Chen L., Yin C., et al. (2016). The comparison of adipose stem cell and placental stem cell in secretion characteristics and in facial antiaging. Stem Cells Int. 2016:7315830. 10.1155/2016/7315830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. Y., Chang P. Y., Chen J. Y., Wu B. S., Yang A. H., Lee O. K. (2021). Adipose-derived mesenchymal stem cells attenuate dialysis-induced peritoneal fibrosis by modulating macrophage polarization via interleukin-6. Stem Cell Res. Ther. 12:193. 10.1186/s13287-021-02270-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon B. S., Moon J. H., Jun E. K., Kim J., Maeng I., Kim J. S., et al. (2010). Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev. 19 887–902. 10.1089/scd.2009.0138 [DOI] [PubMed] [Google Scholar]
- Yuan B., Broadbent J. A., Huan J., Yang H. (2018). The effects of adipose stem cell-conditioned media on fibrogenesis of dermal fibroblasts stimulated by transforming growth factor-β1. J. Burn Care Res. 39 129–140. 10.1097/bcr.0000000000000558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Yu L., Li F. R., Li X., Wang Z., Zou X., et al. (2020). Topical application of exosomes derived from human umbilical cord mesenchymal stem cells in combination with sponge spicules for treatment of photoaging. Int. J. Nanomed. 15 2859–2872. 10.2147/ijn.S249751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Liu L. N., Yong Q., Deng J. C., Cao W. G. (2015). Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res. Ther. 6:145. 10.1186/s13287-015-0133-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Chen L., Zhang G., Zhang B. (2020). Umbilical cord-matrix stem cells induce the functional restoration of vascular endothelial cells and enhance skin wound healing in diabetic mice via the polarized macrophages. Stem Cell Res. Ther. 11:39. 10.1186/s13287-020-1561-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B. R., Xu Y., Guo S. L., Xu Y., Wang Y., Zhu F., et al. (2013). The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. Biomed Res. Int. 2013:519126. 10.1155/2013/519126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B. R., Zhang T., Bin Jameel A. A., Xu Y., Xu Y., Guo S. L., et al. (2016). The efficacy of conditioned media of adipose-derived stem cells combined with ablative carbon dioxide fractional resurfacing for atrophic acne scars and skin rejuvenation. J. Cosmet Laser Ther. 18 138–148. 10.3109/14764172.2015.1114638 [DOI] [PubMed] [Google Scholar]
- Zhou H., Li X., Yin Y., He X. T., An Y., Tian B. M., et al. (2020). The proangiogenic effects of extracellular vesicles secreted by dental pulp stem cells derived from periodontally compromised teeth. Stem Cell Res. Ther. 11:110. 10.1186/s13287-020-01614-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Li X., Zhang B., Shi Q., Li D., Ju X. (2019). A Human umbilical cord mesenchymal stem cell-conditioned medium/chitosan/collagen/β-glycerophosphate thermosensitive hydrogel promotes burn injury healing in mice. Biomed Res. Int. 2019:5768285. 10.1155/2019/5768285 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


