Abstract
Background
Warfarin is the only approved oral anticoagulant for long-term prophylaxis against valve thrombosis and thromboembolism in patients with mechanical heart valves. To date, apixaban for patients with double (aortic and mitral) mechanical heart valves has not been reported in the literature.
Case summary
We report the case of a 50-year-old female who underwent double (aortic and mitral) mechanical valve replacement in February 2017. Warfarin was prescribed after mechanical valve replacement. However, she complained of side effects of warfarin, including tingling sensation and numbness of legs, urticaria, skin rash, and nausea and voluntarily stopped taking medication. In December 2018, she was admitted to the emergency room due to ongoing chest pain. Coronary angiogram revealed embolic myocardial infarction at the left circumflex coronary artery. Nevertheless, she continued to refuse to take warfarin after anticoagulant therapy for coronary artery embolism. Given the patient’s objection, we prescribed apixaban 5 mg b.i.d. since February 2019. When she was diagnosed with atrial fibrillation in April 2020, no intracardiac thrombosis was confirmed on computed tomography and electrical cardioversion was performed safely. While on apixaban, no evidence of prosthetic valve thrombosis or thrombo-embolic events was observed during a 24-month period.
Conclusion
We report the efficacy and safety of apixaban in a patient with atrial fibrillation and double mechanical heart valves for preventing prosthetic valve thrombus and systemic embolism.
Keywords: Apixaban, Atrial fibrillation, Case report, Mechanical heart valve
Learning points
Non-vitamin K oral anticoagulants (NOACs) are preferred over warfarin for the prevention of stroke or systemic embolism in atrial fibrillation (AF) patients but are contraindicated in patients with mechanical heart valves.
Warfarin is the only recommended anticoagulant for the prevention of thrombosis in patients with AF and mechanical heart valves.
The current case demonstrates the efficacy and safety of apixaban, one of NOACs, in prevention of valve thrombosis, stroke, or systemic embolism in a patient with atrial fibrillation and double (aortic and mitral) mechanical heart valves. The patient did not experience prosthetic valve thrombosis, thrombo-embolic events, or major bleeding while on apixaban over a period of 24 months.
Future studies are needed to prove the efficacy and safety of apixaban or other NOACs in patients with AF and mechanical heart valves.
Introduction
Non-vitamin K oral anticoagulants (NOACs) have demonstrated a favourable balance between efficacy and safety compared with warfarin in patients with non-valvular atrial fibrillation (AF).1 Based on current guidelines, NOACs are recommended in preference to warfarin for stroke prevention in NOACs-eligible AF patients; however, they are contraindicated in patients with mechanical heart valves.2,3
The current report examines the use of apixaban, one of NOACs, in a patient with AF and double mechanical heart valves for the prevention of stroke and systemic embolism.
Timeline
| Date | |
|---|---|
| August 2009 | Aortic and mitral valvuloplasty due to severe rheumatic stenosis of the aortic and mitral valves. |
| February 2017 | Mechanical valve replacement of aortic and mitral valves. |
| March 2017 | Stop visiting the clinic of patient's own volition due to side effects of warfarin including tingling sensation and numbness of legs, urticaria, skin rash, and nausea. |
| December 2018 | Non-ST-elevation myocardial infarction due to coronary artery embolism. Restart warfarin. |
| February 2019 | Patient complaint of side effects of warfarin such as tingling sensation and numbness of legs, urticaria, and skin rash. Refusal to taking warfarin. Start apixaban 5 mg b.i.d. PO |
| April 2020 | Atrial fibrillation was developed. No prosthetic valve thrombosis or intracardiac thrombi on computed tomography. Successful electrical cardioversion was performed without any thrombo-embolic event. |
| Until February 2021 | No development of major bleeding, stroke, or systemic embolism with apixaban |
Case presentation
A 50-year-old woman received open-heart aortic and mitral valvuloplasty due to severe rheumatic stenosis of the aortic and mitral valves 11 years ago (18 August 2009). She had no medical history except for rheumatic heart disease. Eventually, she received open-heart mechanical valve replacement (St. Jude Medical® Mechanical Heart Valve, 17 mm of the aortic valve and 25 mm of mitral valve) due to progressive severe stenosis of aortic and mitral valves (8 February 2017), and was discharged with warfarin. After discharge, she experienced side effects of warfarin including tingling sensation and numbness of legs, urticaria, skin rash, and nausea, and voluntarily stopped taking the medication. Her international normalized ratio (INR) value was measured at around 1.3 at outpatient clinic. We examined any alternative causes of the patient’s symptoms, but we did not find any cause of her symptoms except for warfarin. She gave up visiting the hospital from March 2017 and December 2018.
In December 2018, the patient returned to the emergency room due to ongoing chest pain. On physical examinations, there were no abnormal murmurs and neurologic deficits. Electrocardiography did not show typical findings of ST-elevation myocardial infarction. Patient’s cardiac rhythm was sinus. However, laboratory cardiac muscle enzyme levels were increased, with a creatine kinase-MB level of 191.4 ng/mL (reference ∼6.6 ng/mL) and a high sensitive troponin I level of 39 171 ng/L (reference ∼16.1 ng/L). INR level was measured as 1.16 (reference 0.87–1.2). So, we performed emergent coronary angiography and revealed a floating thrombus in the distal left circumflex artery (Figure 1A and Video 1). We thought that the lesion was an embolic thrombus and performed continuous intravenous heparin and oral warfarin treatment. A follow-up coronary angiography 2 days later showed decreased thrombus burden (Figure 1B and Video 2). We decided to continue anticoagulation with warfarin because coronary blood flow was good despite of remnant thrombus. Additionally, her chest pain was resolved after anticoagulation treatment with heparin and warfarin. Fortunately, transthoracic and transoesophageal echocardiography showed normal left ventricular systolic function without regional wall motion abnormality and no prosthetic valve thrombus or intracardiac thrombus. The patient was discharged from the hospital under warfarin 4.5 mg PO with INR of 2.3. However, she did not take warfarin consistently because of its side effects including tingling sensation and numbness of legs, urticaria, and skin rash. When she visited the outpatient clinic, her INR value was 1.2, and she strongly refused warfarin treatment. Given the patient’s non-compliance due to the side effects from warfarin, we prescribed apixaban 5 mg b.i.d. beginning in February 2019. After taking apixaban, she did not complain about previous side effects of warfarin furthermore.
Figure 1.
Emergent coronary angiography (A) and follow-up coronary angiography (B). Emergent coronary angiography (A) revealed a large floating thrombus (red arrow) in the distal bifurcation area of the left circumflex artery. Follow-up coronary angiography (B) showed decreased thrombus burden (red arrow) 2 days later.
In April 2020, the patient visited the emergency room again due to chest discomfort. Atrial fibrillation with rapid ventricular response was confirmed and persisted. We performed cardiac computed tomography to check for intracardiac thrombus and confirmed there were no thrombus in coronary arteries (Figure 2) and no evidence of intracardiac thrombus or valvular thrombus (Figure 3 and Video 3). Electrical cardioversion was performed with biphasic 150 J, and sinus rhythm was restored immediately (Figure 4). Dronedarone was prescribed to maintain sinus rhythm.
Figure 2.
Computed tomographic coronary angiography. There was no coronary thrombus present in the right coronary artery (A), the left anterior descending artery (B), or the left circumflex artery (C).
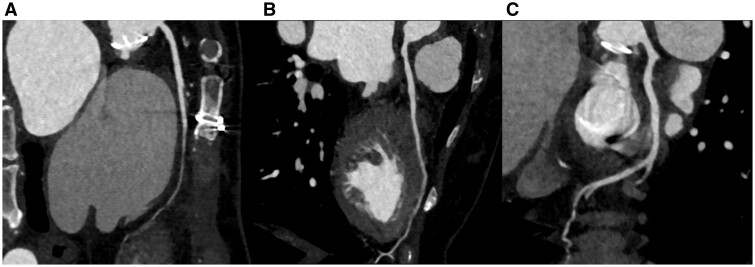
Figure 3.
Computed tomographic coronary angiography. Left atrial enlargement was shown, but no thrombus was seen in the left atrium (A) or the left atrial appendage (B). Modified sagittal view indicated well-functioning mechanical aortic and mitral valves without thrombus (C, D).
Figure 4.
Electrical cardioversion. Atrial fibrillation was terminated by external electrical cardioversion with biphasic 150 J. Sinus rhythm was restored immediately.
After discharge, the patient was compliant and continued with apixaban and dronedarone as reported through the outpatient clinic. We followed-up the patient every 3 months, and planned to check blood test including complete blood count and blood chemistry every 6 months and transthoracic echocardiography every 1 year. At July 2020, follow-up transthoracic echocardiography showed well-functioning mechanical heart valves and no evidence of prosthetic valve thrombosis (Supplementary material online, Videos S4 and S5). Sinus rhythm was maintained, and there is no evidence of the development of major bleeding, stroke, or systemic embolism as of the time of this report (February 2021).
Discussion
In this case report, we introduced a case of off-label use of apixaban due to refusal to take warfarin in patient with mechanical heart valves and AF. The patient received successful external electrical cardioversion and did not experience major bleeding, stroke, or systemic embolic events during apixaban use.
In patients with mechanical heart valves, the incidence of obstructive prosthetic valve thrombosis varies between 0.3 and 1.3% patient-years, and the risk of thrombo-embolic complications, including systemic emboli, is 0.7 and 6.0% patient-years.4
The mechanism of prosthetic valve thrombosis is complex. Thrombus formation on mechanical heart valves is traditionally explained by the principle of Virchow’s triad including surface characteristics of the prosthesis, blood flow, and characteristics of the blood constituents of the patient.5 Surface factors include incomplete prosthesis endothelialization, leaflet damage or deterioration, stent fracture, or prosthesis malposition. Haemodynamic or haemostatic factors include low cardiac output, anatomical prosthesis position, prosthetic haemodynamic profile, hyperviscosity, hypercoagulable state, significant tissue injury, heparin-induced thrombocytopenia, suboptimal anticoagulation, or platelet reactivity.6
Based on current guidelines, warfarin is recommended for the prevention of prosthetic valve thrombosis.2,3 A previous meta-analysis reported the efficacy of warfarin in reducing the incidence of major embolism in patients with mechanical heart valves.7 They found an incidence of major embolism according to different antithrombotic strategies as follows: 4 per 100 patient-years in the absence of antithrombotic therapy, 2.2 per 100 patient-years in antiplatelet therapy, and 1 per 100 patient-years in warfarin therapy.7 Findings in this meta-analysis are strengthened given the large size of the study populations (13 088 patients) and long-term follow-up periods (53 647 patient-years).
Non-vitamin K oral anticoagulants are preferred over warfarin for the prevention of stroke or systemic embolism in AF patients but are contraindicated in patients with mechanical heart valves.2,3 Dabigatran, an oral direct thrombin inhibitor, has shown to be both safe and efficacious for stroke prevention compared with warfarin in AF patients.8 Based on these positive results, dabigatran has been tested to investigate the efficacy and safety compared to warfarin in patients with mechanical heart valves.9 However, the trial was terminated prematurely as the use of dabigatran was associated with increased rates of thrombo-embolic and bleeding complications compared with warfarin.9 The negative results of this study prompted black-box warnings against the use of dabigatran and other direct oral anticoagulants in patients with mechanical heart valves.6 Consequently, current guidelines for AF patients prohibit the use of NOACs but recommend warfarin in patients with mechanical heart valves.2,3 Blood-contacting medical devices induce clotting by activating the contact system and triggering intrinsic pathways: factor XII absorbed to the surface undergoes autoactivation, and the resulting factor XIIa converts prekallikrein to kallikrein, initiating coagulation and thrombin generation.10 By triggering this intrinsic pathway, mechanical heart valves induce the generation of thrombin in concentrations that overwhelm those of dabigatran, which inhibits thrombin in a 1:1 manner.6 In contrast, warfarin attenuates factor Xa and thrombin by reducing the functional levels of factor IX, factor X, and factor II, thereby generating minimal clotting.6 The failure of dabigatran to prevent clotting in patients with mechanical heart valves is explained by these potential pharmacodynamic mechanisms.
Apixaban has been shown to be superior to warfarin in preventing stroke or systemic embolism, bleeding, and mortality in non-valvular AF patients.11 The current case demonstrates the efficacy and safety of apixaban to prevent valve thrombosis or systemic embolism in a patient with a mechanical heart valve. The patient has not experienced a stroke or systemic embolism during a 24-month period while taking apixaban and has safely received electrical cardioversion for AF. Apixaban is a factor Xa inhibitor and each molecule of factor Xa generates 1000 molecules of thrombin. Therefore, a factor Xa inhibitor may be better than dabigatran for preventing thrombosis on mechanical heart valves due to upstream inhibition at the level of factor Xa.6 Furthermore, in a heterotopic mechanical aortic valve porcine model, post-mortem valve thrombosis weight was lowest in the apixaban infusion group compared with no anticoagulation, oral warfarin, and oral apixaban groups.12 Ongoing randomized trials are needed to demonstrate the efficacy and safety of apixaban vs. warfarin in patients with a mechanical aortic heart valve.13
Conclusion
In conclusion, we report the efficacy and safety of apixaban in a patient with AF and double mechanical heart valves for preventing prosthetic valve thrombus and systemic embolism.
Lead author biography

I am a cardiologist, especially for arrhythmia and electrophysiology. I am working in Konkuk University Medical Center, Seoul, South Korea.
Ethics statement
This study was approved by the Institutional Review Board of the Konkuk University Medical Center (protocol no. KUMC 2020-12-042). Informed consent that allows the publication of clinical data was obtained from the patient.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD. et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. ; Writing Group Members. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2019;16:e66–e93. [DOI] [PubMed] [Google Scholar]
- 3. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C. et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 4. Roudaut R, Serri K, Lafitte S.. Thrombosis of prosthetic heart valves: diagnosis and therapeutic considerations. Heart 2007;93:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolberg AS, Aleman MM, Leiderman K, Machlus KR.. Procoagulant activity in hemostasis and thrombosis: Virchow's triad revisited. Anesth Analg 2012;114:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dangas GD, Weitz JI, Giustino G, Makkar R, Mehran R.. Prosthetic heart valve thrombosis. J Am Coll Cardiol 2016;68:2670–2689. [DOI] [PubMed] [Google Scholar]
- 7. Cannegieter SC, Rosendaal FR, Briet E.. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994;89:635–641. [DOI] [PubMed] [Google Scholar]
- 8. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A. et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 9. Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, et al. ; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013;369:1206–1214. [DOI] [PubMed] [Google Scholar]
- 10. Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI.. Medical device-induced thrombosis: what causes it and how can we prevent it? J Thromb Haemost 2015;13 Suppl 1:S72–81. [DOI] [PubMed] [Google Scholar]
- 11. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M. et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 12. Lester PA, Coleman DM, Diaz JA, Jackson TO, Hawley AE, Mathues AR. et al. Apixaban versus warfarin for mechanical heart valve thromboprophylaxis in a swine aortic heterotopic valve model. Arterioscler Thromb Vasc Biol 2017;37:942–948. [DOI] [PubMed] [Google Scholar]
- 13. Jawitz OK, Wang TY, Lopes RD, Chavez A, Boyer B, Kim H. et al. Rationale and design of PROACT Xa: a randomized, multicenter, open-label, clinical trial to evaluate the efficacy and safety of apixaban versus warfarin in patients with a mechanical On-X Aortic Heart Valve. Am Heart J 2020;227:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.