Abstract
Background
Syncope has many aetiologies but from a cardiac standpoint, if arrhythmogenic and ischaemic causes are not present, obstructive lesions should be considered. Cardiac spindle cell sarcomas are incredibly rare and difficult to cure.
Case summary
A 62-year-old man presented for exercise stress test and had a syncopal episode on the treadmill. He was found to have a massive mass obstructing the transmitral flow. Patient was taken to the operating room and the mass was resected successfully. Histopathological confirmation revealed the mass to be a cardiac intimal sarcoma. Patient was initiated on a trial regimen of doxorubin, ifosfamide, and mesna.
Discussion
Cardiac intimal sarcomas are aggressive cancers and are difficult to treat; there are no established treatment guidelines. They can lead to obstruction of blood flow through the cardiac chambers. From a cardiac perspective, without arrhythmogenic and ischaemic causes of syncope, obstructive lesions should be considered.
Keywords: Cardiac, Intimal, Sarcoma, Myxoma, Spindle cell, Case report, Echocardiography
Learning points
Syncope in the absence of ischaemic changes and arrhythmias should also be worked up for obstructive cardiac lesions.
Echocardiography is a powerful non-invasive imaging modality that should be utilized early in such patients to provide insight into cardiac structure and valvular function. Timeliness of this is paramount as this can allow earlier escalation of further work up should it be deemed necessary.
Though myxomas are the most common cardiac tumour, large masses with multiple separate sites of attachment within a cardiac chamber—as in our patient—should be concerning for malignancy, whether primary or secondary. Cardiac intimal spindle cell sarcomas are highly aggressive and invasive; there is no curative treatment and no guidelines/protocols or randomized clinical trials to guide treatment.
Introduction
Syncope has many aetiologies but from a cardiac standpoint, if arrhythmogenic and ischaemic causes are not present, obstructive lesions should be considered. In this case report, we delve into an obstructive lesion causing such a presentation. The lesion we focus on is a cardiac sarcoma, specifically a spindle cell sarcoma. Cardiac spindle cell sarcomas are incredibly rare and difficult to cure; we explore the clinical manifestation of this lesion in our patient and the nature of this debilitating disease. The main takeaway from this report for the readers is multifold: (i) to understand that cardiac intimal sarcomas are aggressive cancers and are difficult to treat; (ii) there are no established treatment guidelines; (iii) they can lead to obstruction of blood flow through the cardiac chambers; and (iv) from a cardiac perspective, without arrhythmogenic and ischaemic causes of syncope, obstructive lesions should be considered in the differential.
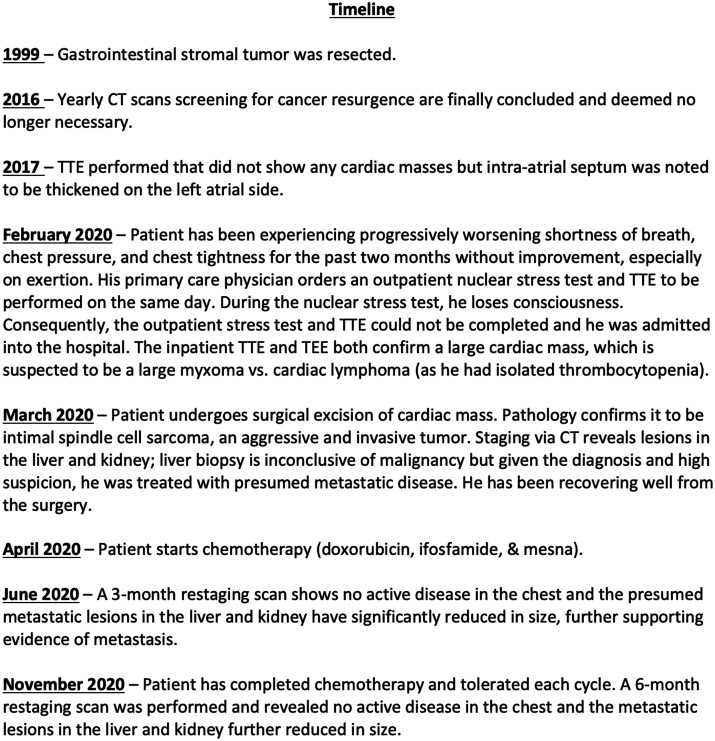
Timeline
Case presentation
A 62-year-old man has a past medical history of low-grade/low-risk gastrointestinal stromal tumour (GIST)—a leiomyosarcoma—treated with excision and small bowel resection with appendectomy in 1999 (now in remission), right and left upper extremity lipomas treated with resection, right lobe thyroidectomy for follicular adenoma, benign prostatic hyperplasia. He presented to his primary care physician (PCP) complaining of a gradual onset of dyspnoea on exertion (DOE), lightheadedness, midsternal chest tightness, and chest pressure/heaviness. The patient denied any other symptoms, including constitutional and B-symptoms.
Given his DOE and chest pressure, his PCP ordered a nuclear exercise stress test and a transthoracic echocardiogram (TTE) to be performed as an outpatient, both on the same day. During the stress test, he exercised according to the Bruce protocol for 6 min and 33 s achieving a work level of 5.7 metabolic equivalents. His resting blood pressure was 138/90 mmHg and rose to 150/90 mmHg. His heart rate rose from 67 beats per min (b.p.m.) to 130 b.p.m. He developed DOE, diaphoresis and lost consciousness for approximately 10 s prior to receiving the radioactive tracer injection. He was resuscitated with intravenous fluids and blood glucose level was 100 mg/dL at that time. His stress electrocardiogram showed 0.5-mm ST depressions in the inferolateral leads with no evidence of ST elevations or ventricular arrhythmias. He was immediately admitted to the hospital under the care of our team for further evaluation. Bloodwork on admission only showed that he was thrombocytopenic with a platelet count of 40 000/µL. Physical exam only revealed a diastolic murmur.
His TTE revealed normal left ventricular function with no regional wall motion abnormalities, but there was a large mass in the left atrial (LA) side of the mitral valve, measuring 7.4 × 3.5 cm which was obstructing the transmitral flow during diastole as well as another non-mobile mass attached to the region of the fossa ovalis measuring 3.3 × 2.3 cm (Figure 1A and B). There were no other abnormalities noted. Interestingly, the patient had a prior TTE in 2017 that did not show any clear intracardiac masses; however, it was noted that the intra-atrial septum is thickened on the LA side.
Figure 1.
(A) Transthoracic echocardiogram apical four-chamber view showing the mass in the left atrium. (B) Left atrial mass prolapsing and obstructing the trans-mitral diastolic flow.
For further characterization of the mass, the patient underwent transoesophageal echocardiogram (TOE) that confirmed TTE reports with at least two large oblong multilobular mobile masses with heterogeneous echogenicity and hypoechoic areas (vacuolated portions) that are broad based and originate from LA septal wall and LA free wall; there was also a possible attachment to the pulmonary vein (Figures 2 and 3; Videos 1–3). At its greatest dimension on TOE, the mass measured 6.4 × 3.46 cm. To definitively rule out coronary artery disease (CAD) as a cause of his chest pressure and discomfort in the setting of syncope, the patient also had a coronary angiogram done that showed subtotal occlusion of the mid left anterior descending artery (LAD) with Grade 1 collaterals from the right coronary artery and no other obstructive CAD. The LAD lesion was left for medical therapy since it looked chronic with collaterals and less likely to explain his presentation. Also, with the newly discovered intracardiac mass, he may need surgical intervention which might be challenging while on dual antiplatelet therapy should a stent be placed. The patient was due to undergo a cardiac magnetic resonance imaging (MRI) but due to his anxiety and claustrophobia, he could not tolerate the MRI despite multiple attempts with anti-anxiety medications.
Figure 2.
(A) Transoesophageal echocardiogram mid-oesophageal long-axis view of the mass in the left atrium. (B) Left atrial mass prolapses into the ventricle and obstructs the trans-mitral diastolic flow.
Figure 3.
Transoesophageal echocardiogram short-axis aortic valve view. The mass and its size can be clearly seen taking up the atrial chamber.
The differential diagnosis for the cardiac mass at that time was atrial myxoma, primary cardiac lymphoma, primary cardiac sarcoma, and metastatic mass from another source. Given his isolated thrombocytopenia in the presence of a cardiac mass, cardiac lymphoma was under serious consideration. Resurgence of his GIST after being in remission for nearly 20 years was thought to be unlikely but was also considered along with a primary malignancy elsewhere in the body with metastasis to the heart.
He underwent cardiac surgery with cardiopulmonary bypass and excision of three LA masses; cryoablation; excision of mass within the inferior pulmonary vein orifice; repair of the atrial septum; inspection of the mitral valve; and LA tear repair. The tumour measured 8.6 cm in its greatest dimension, grossly (Figure 4A and B). The pathology showed malignant spindle sarcoma with heterogeneous differentiation confirming intimal sarcoma with Ki-67 proliferation index 25%, Fluorescence in situ hybridization (FISH) study positive for MDM2 gene amplification and negative signal for SYT gene (Figure 5A and B).
Figure 4.
(A) Gross specimen of the left atrial mass after surgical excision against a ruler. (B) Gross specimen dissected against a ruler.
Figure 5.
(A and B) Histological images of sarcoma using haematoxylin and eosin stain. (A) Myxoid areas are the predominant histology and spindle cells can be seen. (B) A chondrosarcomatous area of the sarcoma.
Given his anxiety and claustrophobia, he only underwent staging via computed tomography (CT) of chest, abdomen, and pelvis after surgical confirmation of malignancy and to limit the number of times he would endure the scans in discomfort. The CT of the chest, abdomen, and pelvis revealed no residual cardiac mass, but numerous heterogeneously hypodense lesions within the liver and kidney were noted concerning for Stage IV disease. He subsequently underwent imaging-guided liver biopsy by the interventional radiology team to confirm metastasis; however, pathology of the liver lesion was inconclusive for malignancy. After significant discussion with his oncology team, he was to be started on adjuvant chemotherapy using combination approach of (doxorubicin, ifosfamide, and mesna) for four to six cycles. Despite the liver biopsy result, given the aggressive nature of the diagnosis and high suspicion of metastasis, he was planned to be treated as though he had metastatic disease since this would not alter his current chemotherapy regimen.
He tolerated his chemotherapy cycles well despite experiencing fatigue and lethargy. On his 3-month and 6-month staging scans, there was no evidence of active disease in the heart and the liver and kidney lesions significantly decreased in size only to further serve as evidence of metastasis and not as isolated cysts in those organs. With his excellent response to the treatment regimen, the oncology team is hopeful of a positive prognosis and will continue to monitor him to ensure there is no recurrence of disease in the chest and treat the metastatic lesions.
Discussion
The incidence of primary cardiac tumours is only 0.001–0.03%, mostly found during autopsy.1 Among the primary cardiac tumours, 75% are benign and 25% of are malignant. Amongst the malignant tumours, majority are composed of a variety of sarcomas with the remainder consisting of lymphomas and mesothelial origin.1 Cardiac sarcomas include angiosarcoma (37%, most common), undifferentiated sarcoma (24%), malignant fibrous histiocytoma (11–24%), leiomyosarcoma (9%), osteosarcoma (3–9%), and 1–2% reported cases are intimal (spindle cell) sarcoma, liposarcoma, synovial sarcoma, hemangiopericytoma, and rhabdomyosarcoma.1–6 Cardiac spindle cell sarcomas are the least reported; they are incredibly rare, and patients experience non-specific symptoms.6,7 Imaging modalities consisting of TTE, TOE, CT scans, and MRIs can be quite useful in diagnosing these individuals; CT and MRI can provide an idea of the extent of the disease beyond the heart compared to TTE and TOEs. However, by the time, these patients are subjected to these various imaging modalities, they tend to already be symptomatic given the growth of the tumour and typically, these are presumed to be a myxoma until surgery is performed and pathological analysis reveals the aggressive and invasive nature of the disease.6,7
Cardiac spindle cell sarcomas tend to involve large vessels since they are of mesenchymal origin.6 Their highly aggressive and infiltrating nature allows them to metastasize easily; thus, these patients tend to have a very poor prognosis with a mean survival of 3–12 months.3,6 Symptoms and effects of cardiac sarcomas rely upon a variety of factors such as location within the heart, degree of invasion, size and extent of the tumour, and rate of growth.6 Patients can experience positional symptoms of chest pressure, chest discomfort, orthopnoea, dyspnoea, and syncope with our patient experiencing all these except for orthopnea.7 Despite the fact that malignancy promotes a hypercoagulable state, tumours within the heart can also promote clot formation due to disruption of blood flow and promotion of stasis, leading to an elevated risk of embolic events occurring in these patients, with the brain being the most common site of involvement.7 Surgical resection tends to show the greatest symptomatic benefit and increased life expectancy (2× increase) in these patients but complete resection with negative margins is only possible in <50% of patients.6 The inherent aggressive nature of spindle cell sarcomas allows for local and systemic recurrence despite surgical and chemotherapeutic intervention.
Our patient had denied any constitutional and B-symptoms (nights sweats, chills, etc.) and during his hospitalization, he only had isolated thrombocytopenia. The haematology/oncology team was concerned after pathological confirmation of a rare cardiac intimal sarcoma as there will be great difficulty in achieving a curative treatment regimen. A point to note is that our patient’s mass had multiple points of attachment. Though myxomas are the most common cardiac tumour, large masses with multiple separate sites of attachment—as in our patient—within a cardiac chamber should be concerning for malignancy, whether primary or secondary. Intimal (spindle cell) sarcomas have a poor prognosis because of their aggressive nature, diagnostic delay, therapeutic difficulty, and high metastatic potential.3,5 It most commonly involves the large arteries (pulmonary artery more than aorta), pulmonary valves, and right ventricular system and tends to be seen in the fourth decade of life. Clinical presentation depends on the location within the heart, tumour size, viability, rate of growth, and invasiveness. The most common metastasis site is the lung but can also involve lymph nodes, bone, liver, brain, bowel, spleen, adrenal glands, pleura, diaphragm, kidneys, thyroid, and skin.8 Patients would have to undergo further workup and staging to assess metastatic burden and extent of the disease. Again, with the aggressive and invasive nature of this disease, the mean survival in affected individuals is between 3 and 12 months.3,6
Due to the rarity of these tumours and given the highly aggressive nature of cardiac intimal sarcomas, there has been no consensus on chemotherapy.6 There are no established clinical trials for treatment and there are no definitive treatment guidelines or protocols. Our patient underwent surgical excision of the tumour given how large it was and because he was symptomatic due to obstruction of flow. Cryoablation was performed for areas where proceeding with surgical excision was too high risk for perforation, significant bleeding, and damaging the atrioventricular conduction system. With the aggressive nature of the tumour and after discussion with the oncological team, adjuvant chemotherapy with doxorubicin, ifosfamide, and mesna was deemed to be the best approach to attempt remission. Again, without established guidelines, the risks and benefits of surgical excision in conjunction with adjuvant chemotherapy was discussed in complete detail with our patient. For such cases, ultimately, treatment would have to be discussed with the oncologist but the mainstay of management would involve resection of tumour with negative margins followed by chemotherapy and palliative radiation therapy.8–10
Conclusion
Syncope during stress testing in the absence of ischaemic changes or ventricular arrhythmia should alert the clinician to obstructive cardiac lesions, such as a large myxoma or sarcoma. A timely echocardiogram is necessary prior to proceeding with further testing to exclude intracardiac masses, outflow obstruction or severe valvular heart disease. Cardiac spindle cell sarcomas are rare and highly aggressive tumours with poor prognosis. Treatment involves surgical excision with possible adjuvant chemotherapy but currently, there are no guidelines or clinical trials that exist to guide therapy. Though myxomas are the most common primary cardiac tumour, large masses with multiple separate sites of attachment within a cardiac chamber should be concerning for malignancy, whether primary or secondary.
Lead author biography

Mahin Rehman is a resident physician in the USA and will be doing his Fellowship in Cardio-Oncology at Memorial Sloan Kettering Cancer Center in New York City. As an internal medicine resident, he has had an interest in cardiology and aspires to enter a cardiovascular fellowship after completing his cardio-oncology fellowship. His interests lie with cardiac imaging, cardio-oncology, and heart failure.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1.Amano J, Nakayama J, Yoshimura Y, Ikeda U.. Clinical classification of cardiovascular tumors and tumor-like lesions, and its incidences. Gen Thorac Cardiovasc Surg 2013;61:435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T.. Cardiac tumours: diagnosis and management. Lancet Oncol 2005;6:219–228. [DOI] [PubMed] [Google Scholar]
- 3.Modi A, Lipnevicius A, Moorjani N, Haw M.. Prolonged survival with left atrial spindle cell sarcoma. Interact Cardiovasc Thorac Surg 2009;8:703–704. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Hsieh T, Salehi A.. Recurrent cardiac intimal (Spindle cell) sarcoma of the left atrium. J Cardiothorac Vasc Anesth 2013;27:103–107. [DOI] [PubMed] [Google Scholar]
- 5.Cho GJ, Kim HJ, Kang JS.. Primary cardiac sarcoma in pregnancy: a case report. J Korean Med Sci 2006;21:940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muturi A, Kotecha V, Ruturi J, Muhinga M, Waweru W, High-grade spindle cell sarcoma of the heart: a case report and review of literature. J Cardiothorac Surg 2015;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta N, Desai A, Shivdasani B, Suryawanshi S, Mehta AB, Behranwala A. et al. Left atrial spindle cell sarcoma—case report. Indian Heart J 2012;64:416–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke AP, Virmani R.. Osteosarcomas of the heart. Am J Surg Pathol 1991;15:289–295. [DOI] [PubMed] [Google Scholar]
- 9.Chitwood WR Jr. Cardiac neoplasms: current diagnosis, pathology, and therapy. J Card Surg 1988;3:119–154. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A. Primary cardiac sarcomas. Expert Rev Cardiovasc Ther 2008;6:1295–1297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.