Abstract
Basal cell carcinoma (BCC) is the most frequent human malignancy and at the same time the most frequent periocular malignancy, representing almost 80% of all non-melanoma skin cancers and 90% of eyelid cancers. The study included 50 cases of eyelid BCC, out of which 41 were nodular BCC (NBCC) and nine were infiltrative BCC (IBCC), with various Breslow scores (BS) and primary tumor (pT) category. We analyzed the immunoexpression of matrix metalloproteinases (MMPs) 1 and 13 in the tumoral epithelial component (TEC) and inflammatory stromal component (ISC) of BCC in relation to the two histopathological parameters. The immunoreaction for MMP-1 was identified in 41 (82%) cases and for MMP-13 in 46 (92%) cases both in the TEC and ISC of both types of BCC. The statistical analysis revealed that both collagenases had positive/high scores significantly associated with advanced BS. For MMP-1, there were statistical associations in TEC related to IBCC and high pT category, while MMP-13 only revealed statistical association in ISC with high pT. The presence of collagenase MMP-1 and MMP-13 expression in a high number of cases, both in TEC and ISC, confirms their intervention in the tumor progression and proposes these MMPs as potential targets in antineoplastic therapy.
Keywords: eyelid basal cell carcinoma, MMP-1, MMP-13
⧉ Introduction
The eyelid is the main location of neoplasms in ophthalmology practice, the structures being involved in 5–10% of all types of skin cancer [1,2,3]. BCC is the most frequent human malignancy [4] and it is also the most frequent periocular malignancy, representing almost 80% of all nonmelanoma skin cancers and 90% of eyelid cancers [5,6,7,8,9,10,11,12].
Matrix metalloproteinases (MMPs) are degrading enzymes with an important role in tumor progression through the increase of angiogenesis induces by tumors and the destruction of local tissue architecture and basal membranes to allow tumor invasion and metastasis [13]. Their involvement in tumorigenesis seems to be far more complex than initially considered. Efficient destruction of extracellular matrix (ECM) around the invasive cancer islands involves the interaction between tumor, stromal and inflammatory cells, all of them expressing a distinct set of MMP [13]. Collagenases are the most important secreted proteinases, capable to initiate the native degrading of fibrillar collagen (type I, II, III, V, IX). MMP-1 splits type III collagen, while MMP-8 splits collagen types I and II [14].
Aim
In the present study, we analyzed the expression of MMP-1 and MMP-13 in the tumoral epithelial component (TEC) and inflammatory stromal component (ISC) of nodular BCC (NBCC) and infiltrative BCC (IBCC) in relation with the Breslow score (BS) and primary tumor (pT) category.
⧉ Materials and Methods
The study included 50 cases of eyelid BCC diagnosed in the Laboratory of Pathology, Emergency County Hospital of Craiova, Romania, during 2016–2019. The biological material was represented by fragments of surgical excision from patients hospitalized and operated in the Departments of Plastic Surgery and Ophthalmology of the same Hospital. The material was fixed in 10% neutral buffered formalin and then processed by the paraffin embedding technique and Hematoxylin–Eosin (HE) staining. Lesion classification by the histopathological (HP) type and the depth of tumor invasion (BS and pT category) were done according to the literature recommendations [15,16].
We then performed serial sections on which we performed immunohistochemical analysis using a detection system based on enzyme detection of antigen signal [Labeled Streptavidin–Biotin 2 System–Horseradish Peroxidase (LSAB2–HRP), Dako, code K0675]. For reaction visualization, we used the 3,3’-Diaminobenzidine (DAB, Dako, code 3467) chromogen and for validation, we used external positive (testicle for MMP-1 and liver for MMP-13) and negative controls (by omitting the primary antibody). Antigen recovery was done under microwave for 20 minutes in citrate buffer, pH 6. We assessed the semi-quantitative expression of MMP-1 (clone EP1247y, 1/50 dilution) and MMP-13 (clone ab84594, 1/50 dilution) in TEC and ISC of NBCC and IBCC compared to BS and pT category. We used a reaction quantification system applied independently by two specialists, based on immunostaining intensity noted with 1 (reduced intensity), 2 (moderate intensity) and 3 (high intensity), calculating an average composite score (CS) for each lesion. The absence of the immunoreaction was noted with a score of 0. In case of inconsistency in the intensity of the immunoreactions, the specimens were reviewed, and a consensus was established. The quantification system used was considered optimal to differentiate between positive/negative cases, the positive cases showing differences in relation to the intensity of the reactions and not to the number of labeled cells.
We made the statistical analysis on the results using tests for evaluating the differences between the immunoreaction scores of the lesion categories, respectively χ2 (chi-squared) test, within the Statistical Package for the Social Sciences (SPSS) 10 software. During the study, it were respected the general norms of conduit regarding the research ethics problems and necessities, consisting in the approval of the local Ethics Committee and informed consent of patients.
⧉ Results
HP analysis of the 50 cases of BCC revealed 41 cases of NBCC and nine cases of IBCC, with different BS and pT categories. For NBCC, we found 30 cases in pT1 (BS II six cases, BS III 12 cases and BS IV 12 cases) and 11 cases in pT2 (BS III four cases and BS IV seven cases) and for IBCC, we found three cases in pT1 (BS IV) and six cases in pT2 (BS III two cases and BS IV four cases).
The immunoreaction for MMP-1 was identified in 41 (82%) cases and for MMP-13 in 46 (92%) cases, in both TEC and ISC of both types of BCC. In case of ISC, the inflammatory positive chronic infiltrate was represented mainly by mononuclear cells, respectively lymphocytes, plasma cells, macrophages, fibroblasts. For MMP-1, in both types of BCC, we noticed cytoplasm positivity with CS that varies between TEC and ISC, respectively 0.50–1 in TEC and 1.33–2.75 in ISC. The analysis of MMP-13 expression also indicated cytoplasm positivity in both types of BCC with CS values of 0.33–1.75 for TEC and 0.66–2.50 for ISC (Table 1).
Table 1.
Distribution of CS for MMP-1 and MMP-13 in TEC and ISC of BCC
|
pT category |
BS category |
BCC type |
MMP-1 |
MMP-13 |
||||
|
No. of cases |
CS TEC |
CS ISC |
No. of cases |
CS TEC |
CS ISC |
|||
|
pT1 |
BS II |
NBCC |
3 |
0.50 |
1.33 |
2 |
0.33 |
0.66 |
|
IBCC |
0 |
– |
– |
0 |
– |
– |
||
|
BS III |
NBCC |
8 |
0.66 |
1.66 |
12 |
1.58 |
2.08 |
|
|
IBCC |
0 |
– |
– |
0 |
– |
– |
||
|
BS IV |
NBCC |
12 |
1.00 |
2.50 |
12 |
1.75 |
2.16 |
|
|
IBCC |
3 |
1.00 |
2.00 |
3 |
1.66 |
2.33 |
||
|
pT2 |
BS III |
NBCC |
2 |
0.5 |
1.50 |
4 |
1.50 |
2.00 |
|
IBCC |
2 |
1.00 |
2.50 |
2 |
1.50 |
2.50 |
||
|
BS IV |
NBCC |
7 |
1.00 |
2.71 |
7 |
1.71 |
2.00 |
|
|
IBCC |
4 |
1.00 |
2.75 |
4 |
1.75 |
2.50 |
||
BCC: Basal cell carcinoma; BS: Breslow score; CS: Composite score; IBCC: Infiltrative basal cell carcinoma; ISC: Inflammatory stromal component; MMP: Matrix metalloproteinase; NBCC: Nodular basal cell carcinoma; pT: Primary tumor; TEC: Tumoral epithelial component
MMP-1 immunoexpression
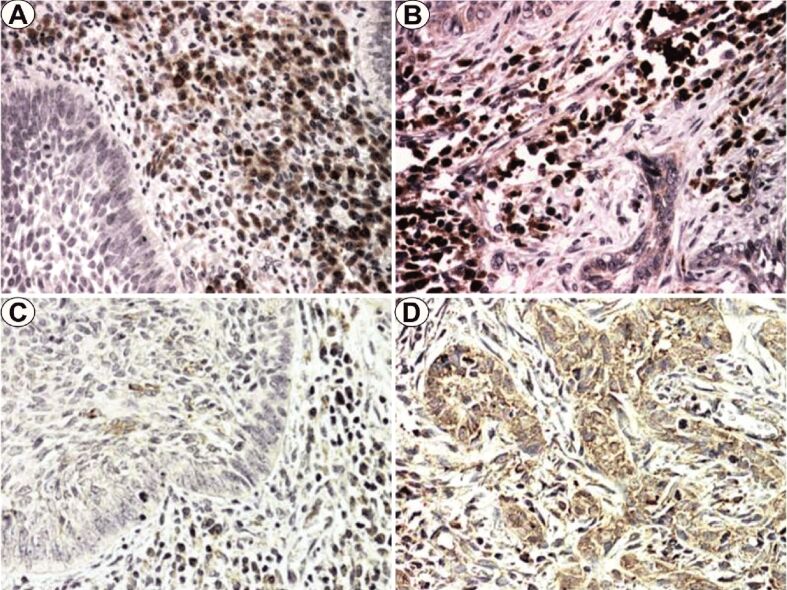
MMP-1 immunoexpression analysis in NBCC indicated a moderate/high reaction intensity for ISC and low intensity for TEC (Figure 1A). In the case of TEC, immunoreaction positivity was identified especially in cells on the bounds of tumor cell islands. For ISC, the positivity of the immunoreaction was identified especially in the cells of the peritumoral inflammatory infiltrate.
Figure 1.
(A) NBCC, BS IV; (B) IBCC, BS IV; (C) NBCC, BS III; (D) IBCC, BS III. Anti-MMP-1 antibody immunostaining: (A and B) ×200. Anti-MMP-13 antibody immunostaining: (C and D) ×200. BS: Breslow score; IBCC: Infiltrative basal cell carcinoma; MMP: Matrix metalloproteinase; NBCC: Nodular basal cell carcinoma
In IBCC, the CS was low in TEC and high in ISC (Figure 1B). In the case of TEC, the positivity of the immunoreaction was diffusely identified in the tumor cell cords. For ISC, the immunoreaction was identified in the cells of the intratumoral inflammatory infiltrate.
MMP-13 immunoexpression
MMP-13 immunoexpression analysis in NBCC indicated low/moderate CS values in TEC and moderate values in ISC with random distribution on the neoplastic islands and in the intratumoral inflammatory cells (Figure 1C).
For IBCC, we also found mostly moderate CS values independent of BS, also with random distribution in the tumor and ISC (Figure 1D).
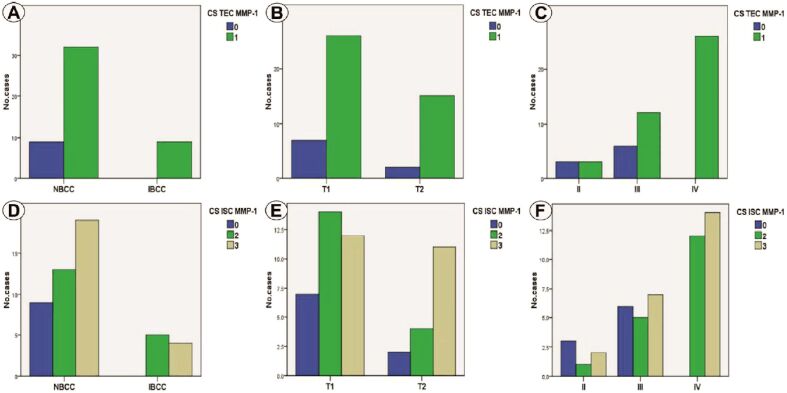
Statistical analysis of MMP-1 immunoexpression in TEC indicated the association of high CS with IBCC (p=0.183, χ2 test), high pT category (p=0.699, χ2 test) and advanced BS (p=0.002, χ2 test), with statistically significant association only for the last parameter (Figure 2A,2B,2C). For MMP-1 immunoexpression in ISC, we determined a non-significant statistically relation of IBCC (p=0.207, χ2 test) of pT2 category (p=0.163, χ2 test) with high CS (Figure 2, D and E). By comparison, high BS was significantly associated with high MMP-1 CS (p=0.012, χ2 test) (Figure 2F).
Figure 2.
(A) Distribution of cases depending on CS TEC MMP-1 and tumor type; (B) Distribution of cases depending on CS TEC MMP-1 and pT category; (C) Distribution of cases depending on CS TEC MMP-1 and BS; (D) Distribution of cases depending on CS ISC MMP-1 and tumor type; (E) Distribution of cases depending on CS ISC MMP-1 and pT category; (F) Distribution of cases depending on CS ISC MMP-1 and BS. BS: Breslow score; CS: Composite score; ISC: Inflammatory stromal component; MMP-1: Matrix metalloproteinase-1; pT: Primary tumor; TEC: Tumoral epithelial component
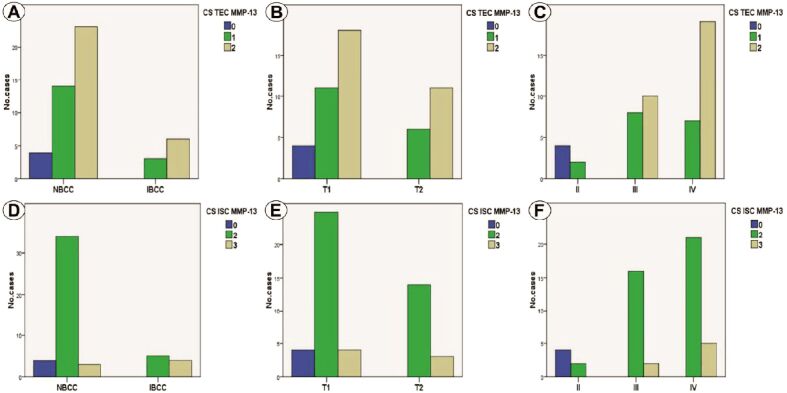
MMP-13 immunoreaction analysis in TEC indicated a relation between positive/moderate CS with IBCC (p=0.600, χ2 test) and pT2 category (p=0.321, χ2 test), without statistically significance (Figure 3, A and B). For BS, we determined statistically significant differences, moderate values being associated only with BS III and IV (p<0.001, χ2 test) (Figure 3C). In ICS, moderate values of CS were associated with NBCC, for IBCC exiting moderate/high values, which was a statistically significant association (p=0.012, χ2 test) (Figure 3D). In the case of pT category, we found moderate CS in pT1 tumors and moderate/high CS in pT2 tumors, but the correlation was not statistically significant (p=0.306, χ2 test) (Figure 3E). Moderate/high MMP-13 immunoexpression was associated with BS III and IV, which was a statistically significant relation (p<0.001, χ2 test) (Figure 3F).
Figure 3.
(A) Distribution of cases depending on CS TEC MMP-13 and tumor type; (B) Distribution of cases depending on CS TEC MMP-13 and pT category; (C) Distribution of cases depending on CS TEC MMP-13 and BS; (D) Distribution of cases depending on CS ISC MMP-13 and tumor type; (E) Distribution of cases depending on CS ISC MMP-13 and pT category; (F) Distribution of cases depending on CS ISC MMP-13 and BS. BS: Breslow score; CS: Composite score; ISC: Inflammatory stromal component; MMP-13: Matrix metalloproteinase-13; pT: Primary tumor; TEC: Tumoral epithelial component
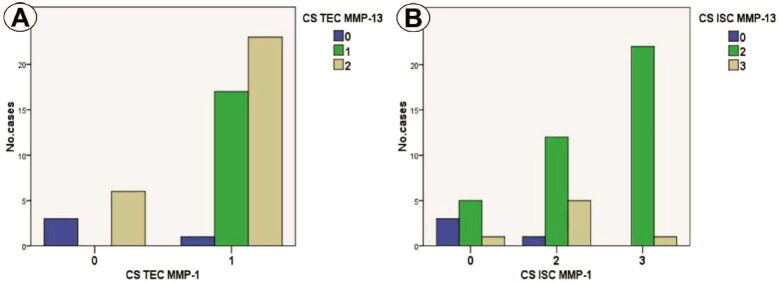
MMP-1 and MMP-13 immunoexpression analysis indicated a significant association of the two immunomarkers in the sense of positive/high CS of MMP-1 and high CS of MMP-13 in TEC (p=0.002, χ2 test) and ICS (p=0.005, χ2 test) (Figure 4, A and B).
Figure 4.
(A) Distribution of CS for MMP-1 and MMP-13 in TEC; (B) Distribution of CS for MMP-1 and MMP-13 in ISC. CS: Composite score; ISC: Inflammatory stromal component; MMP: Matrix metalloproteinase; TEC: Tumoral epithelial component
⧉ Discussions
MMPs are degrading enzymes, responsible for the destruction of tissues in a series of pathologies including cancer, their expression being induced by inflammatory cytokines at the stromal level [17] but also by tumor cells (autocrine and paracrine mechanisms). They are involved in tumor progression by increasing tumor induced angiogenesis and the destruction of local tissue architecture and basal membranes to allow tumor invasion and metastasis [13]. The proteins are coded by a gene family that play role in the development of normal tissues and their remodeling and repair [18,19].
Varani et al. reported that from the MMP family, MMP-1 is the most abundantly expressed in different skin lesions [20]. In BCC, MMP-1 immunoexpression, unlike other collagenases, such as MMP-3, MMP-11, MMP-2, and MMP-9, is mainly located in stromal cells around malignant cells [20,21,22,23].
In the present study, the immunoreaction of MMP-1 was identified in the cytoplasm in 82% of the investigated cases, both in tumor epithelium and in stromal inflammatory cells of both types of NBCC and IBCC BCC. Statistical analysis of MMP-1 immunoexpression in TEC indicated the association of high CS with IBCC, pT category and advanced BS, with statistically significant correlation only for BS. For ISC, statistical analysis of MMP-1 immunoexpression showed an association statistically non-significant of IBCC and pT2 tumors with high CS. By comparison, high BS was significantly associated with high CS.
One study in the literature revealed increased MMP-1 immunoexpression in peritumoral tissue compared to distant epidermal tissue [24]. In another study, different varieties of BCC also expressed MMP-1 in all investigated cases [25]. Shaheen et al. noticed that the immunoexpression of MMP-1 in peritumor area of BCC is significantly higher than inside the tumor, surgical safety margins and control tissue [26]. A significantly higher immunoexpression of MMP-1 was determined in peritumoral areas of undifferentiated BCC compared to differentiated BCC, leading the authors to conclude that MMP-1 can have a role in tumor progression among stromal cells in the peritumoral area around the tumor cell islands [26].
The loss of palisade arrangement in BCC was correlated with MMP-1 immunoexpression in stromal cells [27]. Moreover, it was determined that MMP-1 immunoexpression in tumor and stromal cells are markers that are significantly associated with tumor invasiveness and they can be useful in evaluating the invasiveness through analysis of this marker’s expression [27]. Similarly, Vanjaka-Rogošić et al. suggest that MMP-1 immunoexpression in tumor cells, along with MMP-9 immunoexpression in stromal cells and the absence of E-cadherin are associated with morpheaphorm and recurring BCC [28].
MMP-13 is involved in the degradation of ECM and its immunoexpression is associated with malignant transformation in skin carcinogenesis [29,30]. One study reported in most BCC cases reduced immunostaining for MMP-13 both in the stroma and tumor epithelium, while in normal epithelium around the tumors the immunostaining was intense [20]. In another study, all BCC cases were variably positive for MMP-13, with the most reactive being the metatypical subtype (50% of the cases), especially in areas with squamous differentiation [31]. In the same study, the micronodular subtype was less reactive for MMP-13, its expression being limited to a few tumor cells [31].
In the present study, the cytoplasm immunostaining for MMP-13 was identified in 92% of the cases, both in the tumor epithelium and stromal inflammatory cells, regardless of invasion depth. The analysis of MMP-13 immunoreactions in TEC indicated a relation between positive/moderate cases with IBCC and pT2 category, but the associations were not statistically significant. In the case of BS, we determined there are statistically significant differences, moderate values being associated only with BS III/IV. In ICS, moderate values were associated with NBCC and for IBCC there are only moderate/high, this correlation being statistically significant. For pT category, we predominantly found moderate CS in T1 tumors and moderate and high CS in T2 tumors, but the association was not statistically significant. In a study conducted by Zlatarova et al. noticed a statistically significant correlation between high tissue inhibitor of metalloproteinase-1 (TIMP-1) in tumor and/or stromal cells and MMP-13 immunoexpression [32]. MMP-13 immunoexpression is not limited to tumor cells, because its expression is regulated by stromal cells around tumors including fibroblasts, inflammatory cells, and endothelial cells. In the context of these results further studies to investigate the immunoexpression of MMPs in relation with the type of associated inflammatory cells and angiogenesis or other biomolecular mechanisms involved in the progression of eyelid BCCs should be undertaken.
⧉ Conclusions
The presence of collagenases MMP-1 and MMP-13 immunoexpression in a high number of cases, both in TEC and ICS confirms the intervention of the investigated MMPs in tumor progression. Both collagenases indicated positive/high scores that were statistically significantly associated with advanced BS. For MMP-1, statistical associations were present in TEC reported to IBCC and high pT, while for MMP-13, this aspect was present in ICS only compared to high pT. Reaction positivity for MMP-1 and MMP-13 in peritumoral inflammatory cells indicates the role of inflammation in the tumor progression modulation. Given their role in tumor development, both collagenases can be potential targets in cancer therapy.
Conflict of interest
The authors declare that they have no conflict of interests.
References
- 1.Rossato LA, Carneiro RC, de Macedo EMS, de Lima PP, Miyazaki AA, Matayoshi S. Diagnosis of aggressive subtypes of eyelid basal cell carcinoma by 2-mm punch biopsy: prospective and comparative study. Rev Col Bras Cir. 2016;43(4):262–269. doi: 10.1590/0100-69912016004008. [DOI] [PubMed] [Google Scholar]
- 2.Deprez M, Uffer S. Clinicopathological features of eyelid skin tumors. A retrospective study of 5504 cases and review of literature. Am J Dermatopathol. 2009;31(3):256–262. doi: 10.1097/DAD.0b013e3181961861. [DOI] [PubMed] [Google Scholar]
- 3.Allali J, D’Hermies F, Renard G. Basal cell carcinomas of the eyelids. Ophthalmologica. 2005;219(2):57–71. doi: 10.1159/000083263. [DOI] [PubMed] [Google Scholar]
- 4.Lacour JP. Carcinogenesis of basal cell carcinomas: genetics and molecular mechanisms. Br J Dermatol. 2002;146(Suppl 61):17–19. doi: 10.1046/j.1365-2133.146.s61.5.x. [DOI] [PubMed] [Google Scholar]
- 5.Mantese SAO, Berbert ALCV, Gomides MDA, Rocha A. Basal cell carcinoma - analysis of 300 cases observed in Uberlândia - MG, Brazil. An Bras Dermatol. 2006;81(2):136–142. [Google Scholar]
- 6.Raasch BA, Buettner PG, Garbe C. Basal cell carcinoma: histological classification and body-site distribution. Br J Dermatol. 2006;155(2):401–407. doi: 10.1111/j.1365-2133.2006.07234.x. [DOI] [PubMed] [Google Scholar]
- 7.Wong CSM, Strange RC, Lear JT. Basal cell carcinoma. BMJ. 2003;327(7418):794–798. doi: 10.1136/bmj.327.7418.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soysal HG, Soysal E, Markoç F, Ardiç F. Basal cell carcinoma of the eyelids and periorbital region in a Turkish population. Ophthal Plast Reconstr Surg. 2008;24(3):201–206. doi: 10.1097/IOP.0b013e31816d954d. [DOI] [PubMed] [Google Scholar]
- 9.Haws AL, Rojano R, Tahan SR, Phung TL. Accuracy of biopsy sampling for subtyping basal cell carcinoma. J Am Acad Dermatol. 2012;66(1):106–111. doi: 10.1016/j.jaad.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee S, Moore S, Kumar B. Punch biopsy in the management of periocular basal cell carcinomas. Orbit. 2004;23(2):87–92. doi: 10.1080/01676830490501497. [DOI] [PubMed] [Google Scholar]
- 11.Wang CJ, Zhang HN, Wu H, Shi X, Xie JJ, He JJ, He JJ, Kook KH, Lee SY, Ye J. Clinicopathologic features and prognostic factors of malignant eyelid tumors. Int J Ophthalmol. 2013;6(4):442–447. doi: 10.3980/j.issn.2222-3959.2013.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu XL, Li B, Sun XL, Li LQ, Ren RJ, Gao F, Jonas JB. Eyelid neoplasms in the Beijing Tongren Eye Centre between 1997 and 2006. Ophthalmic Surg Lasers Imaging. 2008;39(5):367–372. doi: 10.3928/15428877-20080901-18. [DOI] [PubMed] [Google Scholar]
- 13.Kerkelä E, Saarialho-Kere U. Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp Dermatol. 2003;12(2):109–125. doi: 10.1034/j.1600-0625.2003.120201.x. [DOI] [PubMed] [Google Scholar]
- 14.Kähäri VM, Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol. 1997;6(5):199–213. doi: 10.1111/j.1600-0625.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 15.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR, editors. American Joint Committee on Cancer (AJCC) Cancer Staging Manual. 8. Springer International Publishing; 2017. pp. 523–523. [Google Scholar]
- 16.Elder DE, Massi D, Scolyer RA, Willemze R, editors. World Health Organization (WHO) Classification of skin tumours. 4. Lyon, France: International Agency for Research on Cancer (IARC) Press; 2018. pp. 7–14. [Google Scholar]
- 17.Coussens LM, Werb Z. Matrix metalloproteinase expression and the development of cancer. Chem Biol. 1996;3(11):895–904. doi: 10.1016/s1074-5521(96)90178-7. [DOI] [PubMed] [Google Scholar]
- 18.Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994;125(3):681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue M, Kratz G, Haegerstrand A, Ståhle-Bäckdahl M. Collagenase expression is rapidly induced in wound-edge keratinocytes after acute injury inhuman skin, persists during healing, and stops at re-epithelialization. J Invest Dermatol. 1995;104(4):479–483. doi: 10.1111/1523-1747.ep12605917. [DOI] [PubMed] [Google Scholar]
- 20.Varani J, Hattori Y, Chi Y, Schmidt T, Perone P, Zeigler ME, Fader DJ, Johnson TM. Collagenolytic and gelatinolytic matrix metalloproteinases and their inhibitors in basal cell carcinoma of skin: comparison with normal skin. Br J Cancer. 2000;82(3):657–665. doi: 10.1054/bjoc.1999.0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez Suárez ML, González Vázquez LO, Barbón García JJ, Vázquez Rojo J, Lamelas Suárez-Pola ML, Vizoso Piñeiro FJ. Collagenase-3 (MMP-13) expression in epithelial cancers of the eyelids] Arch Soc Esp Oftalmol. 2004;79(6):281–288. doi: 10.4321/s0365-66912004000600006. [DOI] [PubMed] [Google Scholar]
- 22.Thewes M, Worret WI, Engst R, Ring J. Stromelysin-3 (ST-3): immunohistochemical characterization of the matrix metalloproteinase (MMP)-11 in benign and malignant skin tumours and other skin disorders. Clin Exp Dermatol. 1999;24(2):122–126. doi: 10.1046/j.1365-2230.1999.00431.x. [DOI] [PubMed] [Google Scholar]
- 23.Pyke C, Ralfkiaer E, Huhtala P, Hurskainen T, Danø K, Tryggvason K. Localization of messenger RNA for Mr 72,000 and 92,000 type IV collagenases in human skin cancers by in situ hybridization. Cancer Res. 1992;52(5):1336–1341. [PubMed] [Google Scholar]
- 24.Monhian N, Jewett BS, Baker SR, Varani J. Matrix metalloproteinase expression in normal skin associated with basal cell carcinoma and in distal skin from the same patients. Arch Facial Plast Surg. 2005;7(4):238–243. doi: 10.1001/archfaci.7.4.238. [DOI] [PubMed] [Google Scholar]
- 25.Kuznetsova EV, Snarskaya ES, Zavalishina LE, Tkachenko SB. Immunohistochemical study of the specific features of expression of matrix metalloproteinases 1, 9 in the photoaged skin, the foci of actinic keratosis and basal cell carcinoma] Arkh Patol. 2016;78(6):17–22. doi: 10.17116/patol201678617-22. [DOI] [PubMed] [Google Scholar]
- 26.Shaheen MA, Asaad MK, Elmasry AI, Zaki MSE, El Hefnawy NG. Comparison of matrix metalloproteinase-1 expression in tumor-associated and nontumor-associated skin in patients with basal cell carcinoma. J Egypt Women Dermatol Soc. 2018;15(1):23–29. [Google Scholar]
- 27.Son KD, Kim TJ, Lee YS, Park GS, Han KT, Lim JS, Kang CS. Comparative analysis of immunohistochemical markers with invasiveness and histologic differentiation in squamous cell carcinoma and basal cell carcinoma of the skin. J Surg Oncol. 2008;97(7):615–620. doi: 10.1002/jso.21006. [DOI] [PubMed] [Google Scholar]
- 28.Vanjaka-Rogošić L, Puizina-Ivić N, Mirić L, Rogošić V, Kuzmić-Prusac I, Babić MS, Vuković D, Mardešić S. Matrix metalloproteinases and E-cadherin immunoreactivity in different basal cell carcinoma histological types. Acta Histochem. 2014;116(5):688–693. doi: 10.1016/j.acthis.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Boyd S, Tolvanen K, Virolainen S, Kuivanen T, Kyllönen L, Saarialho-Kere U. Differential expression of stromal MMP-1, MMP-9 and TIMP-1 in basal cell carcinomas of immunosuppressed patients and controls. Virchows Arch. 2008;452(1):83–90. doi: 10.1007/s00428-007-0526-0. [DOI] [PubMed] [Google Scholar]
- 30.Chu CY, Cha ST, Chang CC, Hsiao CH, Tan CT, Lu YC, Jee SH, Kuo ML. Involvement of matrix metalloproteinase-13 in stromal-cell-derived factor 1α-directed invasion of human basal cell carcinoma cells. Oncogene. 2007;26(17):2491–2501. doi: 10.1038/sj.onc.1210040. [DOI] [PubMed] [Google Scholar]
- 31.Ciurea ME, Cernea D, Georgescu CC, Cotoi OS, Pătraşcu V, Pârvănescu H, Popa D, Pârvănescu V, Ciurea RN, Mercuţ R. Expression of CXCR4, MMP-13 and β-catenin in different histological subtypes of facial basal cell carcinoma. Rom J Morphol Embryol. 2013;54(4):939–951. [PubMed] [Google Scholar]
- 32.Zlatarova ZI, Softova EB, Dokova KG, Messmer EM. Expression of matrix metalloproteinase-1, -9, -13, and tissue inhibitor of metalloproteinases-1 in basal cell carcinomas of the eyelid. Graefes Arch Clin Exp Ophthalmol. 2012;250(3):425–431. doi: 10.1007/s00417-011-1810-x. [DOI] [PubMed] [Google Scholar]