Abstract
Pleomorphic sarcoma of the tongue base is an extremely rare pathology finding. Our review of current databases returned fewer than 10 articles available free full text on this subject. We review the current state of art management guidelines for this type of tumor. Our case presented surprisingly a favorable evolution despite the huge dimensions, the tumor type, and associated pathology. The patient received radiation therapy and oncological treatment followed by revision surgery consisting of partial glossectomy for the residual tumor. Histological examination of the operatory specimen showed a residual tumor of pleomorphic sarcoma type, with clear margins. The evolution was without relapse after 18 months.
Keywords: pleomorphic, sarcoma, tongue base, surgery
⧉ Introduction
While searching PubMed database for documentation, using the keywords ‘tongue’, ‘base’, ‘tumor’, ‘carcinoma’, and restricting the research to free full-text articles, published in the last five years in English on human subjects, we retrieve around 70 journal entries. However, if we add the term ‘sarcoma’, we further retrieve fewer than 10 articles meeting all the previously mentioned criteria.
Recent research trends concerning tongue base tumors are focusing on the human papillomavirus (HPV)-related etiology of oropharyngeal squamous carcinoma [1]. Moreover, current protocols should include the standard use of contrast head and neck magnetic resonance imaging (MRI) to prevent the omission of subclinical masses and detect early stages [2]. Tumors in the tongue base can be visualized using ultrasonography or contrast computed tomography (CT) scans [3]. Regarding the surgical approach current state of the art, techniques focus on using transoral robotic surgery in the tongue base [4,5]. Current management guidelines for the tongue base tumors include surgery and oncology treatment, with chemotherapy and radiation therapy [6]. Unfortunately, tumors in the tongue base have a high mortality rate despite current efforts [7]. However, even in cases with a good vital prognosis, there is still the downside of swallowing disorders after glossectomy procedures [8]. Sarcomas at the level of the tongue base are extremely rare [9]. In some cases, this malignancy was associated with acquired immunodeficiency syndrome (AIDS) long treatment side effects [10]. Another case developed an angiosarcoma after radiation therapy at the level of the neck [11]. Sarcomas are very aggressive tumor types regardless of their localization [12].
Aim
Our paper aims is to review the literature on the current principles of diagnosis and therapeutic protocol in sarcomas of the tongue base, to present the aspects of a cured clinical case of bulky basal tongue pleomorphic sarcoma, the advantages of revision surgery after the application of the chemoradiotherapy with curative visa, but especially to detail the importance of correct histological diagnosis on the initial specimen with radical change of the therapeutic strategy, but also on the resection piece to certify the negative margins.
⧉ Case presentation
We present the case of a female patient, 69-year-old, a heavy smoker for 30 years associating chronic alcohol consumption, who develops swallowing progressive disorders during the last three months, with subsequent weight loss around 10 kg.
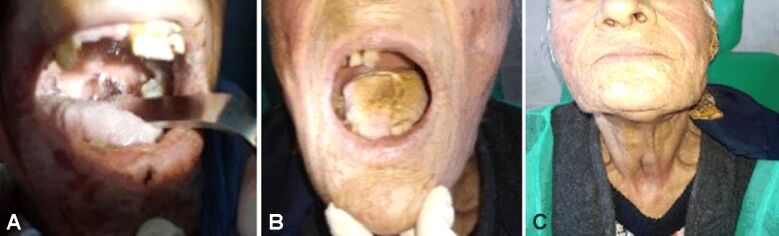
Clinical examination recorded a patient with malnutrition, partial loss of teeth, with a mass in the tongue base overlying the epiglottis and laryngeal vestibule combined with a reduced glottic space around 7 mm. The patient presented small additional lymph nodes enlargement, at the level I–V of the neck (Figure 1). Our clinical suspicion was for lymphoma because of the huge dimensions of the tumor and the presence of adenopathy on both sides of the neck. We performed an emergency tracheostomy and gastrostomy to prepare the patient for the ensuing oncological treatment.
Figure 1.
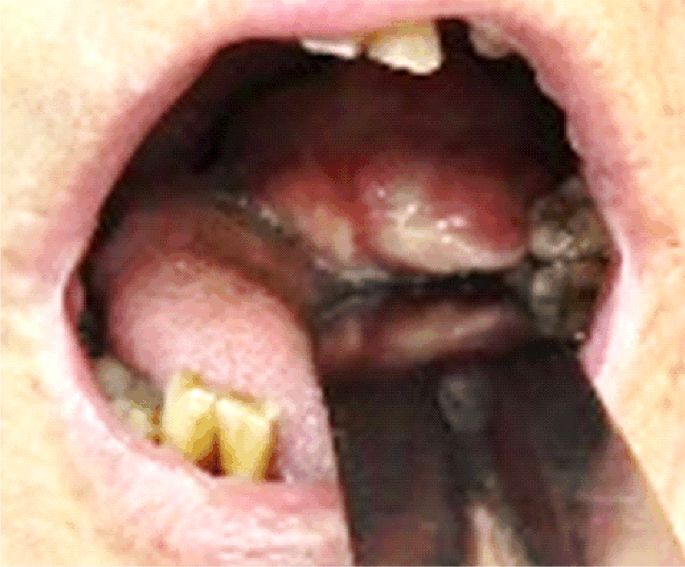
Clinical aspect with the protruding mass in the left tongue base
Neck contrast CT scan showed a tumor expanding from the right side of the tongue base towards the palatine tonsil and larynx measuring 47/44 mm in the axial plane and 58 mm cranially with inhomogeneous inner structure due to necrosis. The mass is partially obstructing the air column. Lymph nodes are present on both sides, the maximum diameter being 21 mm. Moreover, the patient presents bilateral carotid artery atheroma (Figure 2A,2B,2C). Thorax CT scan recorded the absence of tumor masses but confirmed the previous history of the patient contacting tuberculosis with residual calcified nodules. An abdominal CT scan ruled out distant metastasis and focused on multiple abdominal aorta atheroma.
Figure 2.
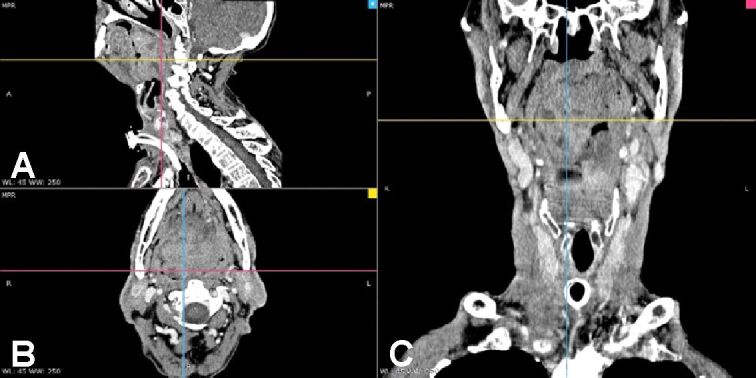
(A–C) Neck CT scan after the emergency tracheostomy showing a huge tumor in the tongue base which obturates the aerodigestive ways. CT: Computed tomography
Under local anesthesia, we performed a biopsy of the mass. The frozen section showed squamous cell carcinoma, but the final pathology result pointed to a pleomorphic sarcoma. The patient was further referred to chemoradiation therapy. The patient underwent chemotherapy combined with radiation therapy and was evaluated in the middle of the oncological therapy and at the end of it. We observe spectacular tumor regression; after four weeks from the end of the therapy, there is only a small persistent mass on the left side of the tongue base less than 3 cm. All lymph nodes regressed. Also, the patient recovered swallowing ability and the gastrostomy is removed. Neck MRI revealed the residual tumor with small dimensions and no local lymph node involvement.
We decided on the opportunity of oncological salvage surgery. A transoral approach was selected for resecting the residual mass. After mounting the mouth opener and displacing the free tongue towards the right for a better view over the tongue base till the inferior pole of the palatine tonsil. After partial glossectomy of the tongue base, the patient benefited from soft tissue reconstruction. At the end of the surgery, we mounted a nasogastric feeding tube.
The fresh-frozen pathology exam described a chronic inflammatory process after irradiation. We removed an oval-shaped white mass with 3.5 cm in diameter. The pathology result shows areas of ulceration, gigantic cells, and associated macrophage changes due to radiation therapy. There was a 1 cm diameter of the remaining tumor cells of pleomorphic sarcoma. The margins of the resected mass were tumor-free (Figures 3,4,5,6,7).
Figure 3.
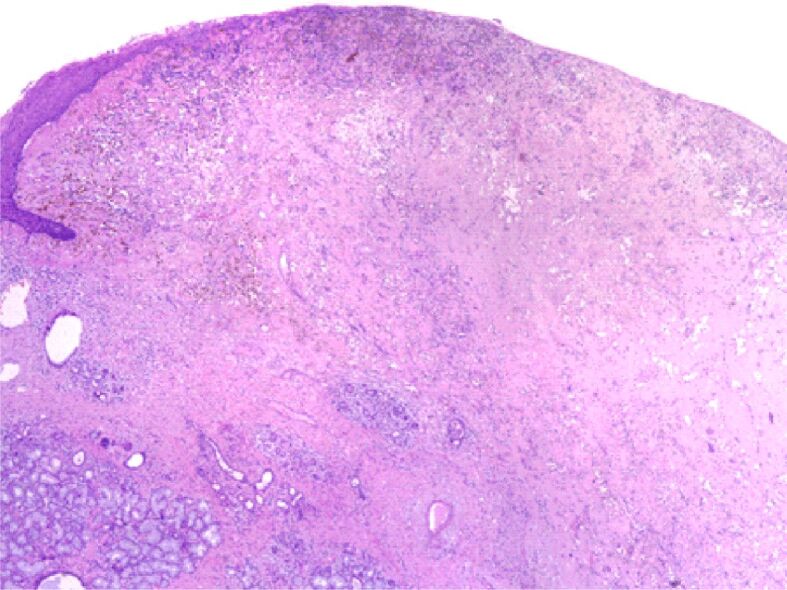
Overall appearance: ulcerated tumor of the tongue (lining epithelial cells remaining in the upper left corner of the image); in the lower-left corner, we can see exocrine glands salivary with a remarkable abundance of macrophages with hemosiderin. Hematoxylin–Eosin (HE) staining, ×40
Figure 4.
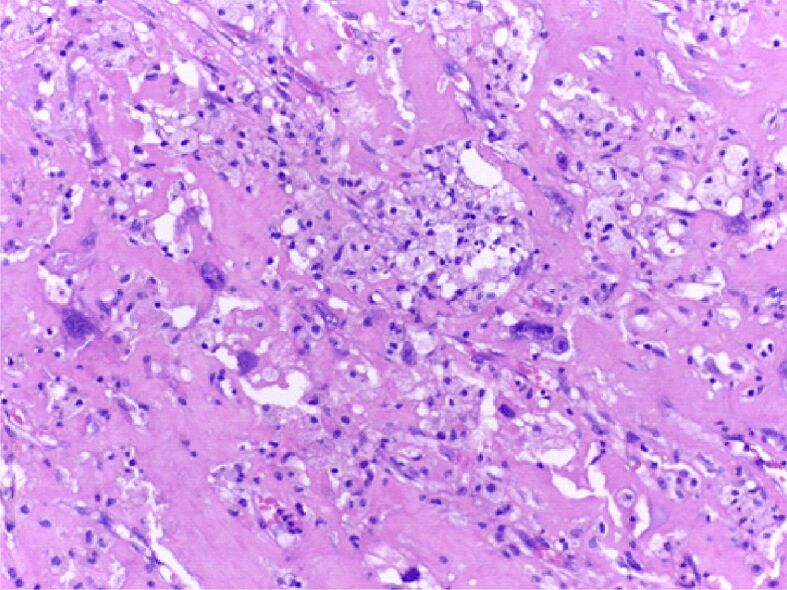
Detail of Figure 3 for the appearance of pleomorphic sarcoma: sarcomatous tumor component in the atmosphere of lipoblasts has large tachychromatic nuclei, with atypical mitoses. HE staining, ×200
Figure 5.
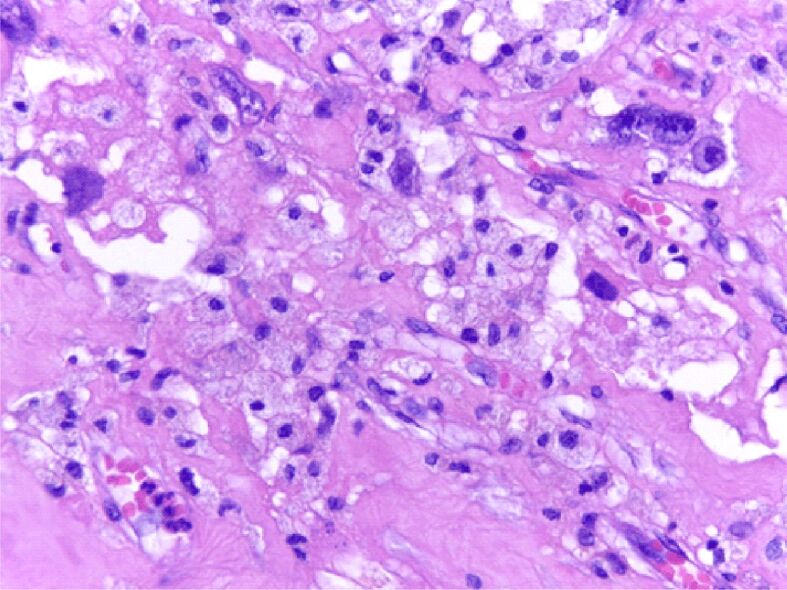
Detail of Figure 4 for the appearance of pleomorphic sarcoma: the tumor component has large tachychromatic nuclei, monstrous or with atypical mitoses in the atmosphere of lipoblasts; the vessels are newly-formed with prominent cores. HE staining, ×400
Figure 6.
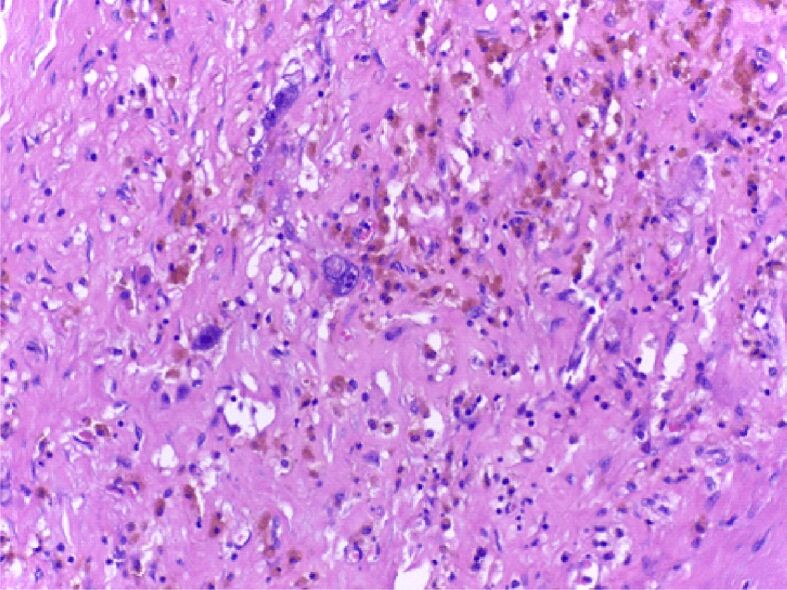
Details of Figure 3: pleomorphic malignant cells have around them macrophages with hemosiderin, predominantly upper right. HE staining, ×200)
Figure 7.
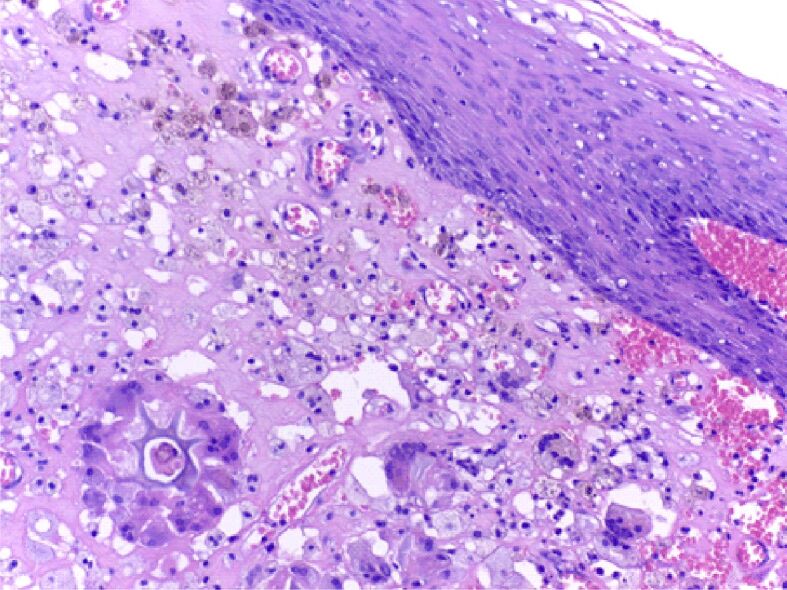
Detail from the periphery of the tongue sarcomatous tumor: in the atmosphere of pleomorphic lipoblasts, multinucleated giant macrophage cells are identified (in the lower part of the image), including around a foreign body (lower left); macrophages loaded with hemosiderin (upper left). HE staining, ×200)
The surgical healing was uneventful for the next 10 days followed by the removal of nasogastric feeding tube, with the progressive recovery of swallowing and removal of the tracheostomy tube in the next 21 days (Figure 8A,8B,8C).
Figure 8.
(A–C) Clinical aspect of the oral cavity, oropharynx, and neck at 21 days after surgery with surgical healing, without the feeding tube and tracheostomy tube
The patient was checked at three, six, and 12 months. Control MRI at eight weeks after surgery reveals the absence of the tumor with healing in the posterior third of the left side of the tongue with small contrast uptake regions due to inflammation. Moreover, there were no enlarged lymph nodes in the neck (Figure 9, A and B).
Figure 9.
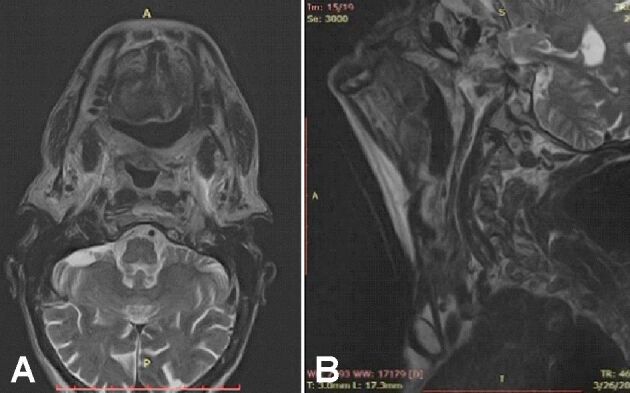
(A and B) Neck MRI at eight weeks after surgery with no local relapse. MRI: Magnetic resonance imaging
The patient was uncompliant to the oncological survey and after 18 months, she was lost to control.
⧉ Discussions
Undifferentiated pleomorphic sarcoma (UPS) formerly known as malignant fibrous histiocytoma (MFH) is known as an aggressive form of head and neck sarcoma, with a reserved prognosis. Head and neck localization is relatively rare (3–10% of all cases) and there are few cases described in the literature. The most common UPS in neck staples are described with localization in soft tissues (40%), nasosinusal (20–46%), in the craniofacial bones (10%), and only 10% in the oral cavity and oropharynx. Incidence of lymphatic metastases is of 10%, with a range of 4–17%, the lung being the most common site for distant dissemination, followed by bone (10%) and liver (1%) [13].
The average age of reported cases is between 50 and 70 years, and the ratio of men:women is 2–3. In the oropharyngeal location, the clinical picture consists of swallowing disorders at the onset, which may associate respiratory disorders such as inspiratory dyspnea with the tumor increase in volume. Metastatic cervical lymphadenopathy is rare at first presentation [13].
CT imaging may suggest UPS if a voluminous, multilobed mass with a cervical muscle-like density is present, calcifications in 5–20% of cases, center with low density due to tumor necrosis or the presence of a myxoid material, with heterogeneous loading at the administration of the contrast substance. MRI shows a heterogeneous signal in all sequences, sometimes with areas of intratumorally hemorrhage [14].
UPS histopathology includes marked nuclear pleomorphism, intense mitotic activity, and areas of necrosis. Although the histogenesis of this type of neoplasm remains debated, it is most commonly viewed as a primitive pleomorphic sarcoma with partial fibroblastic and histiocytic differentiation, reflected by the production of collagen and intratumorally phagocytosis [15].
Surgery remains the first therapeutic option in most sarcomas, given their radioresistant nature. Prognostic factors are tumor grade, histological subtype, size, depth, and distant metastases at first presentation. Most sarcomas are high-grade lesions and are associated with a high risk of local recurrence. Head and neck UPS have a poor prognosis due to significant impairment of the airway, vascular and nervous structures that frequently require tracheostomy and gastrostomy. Survival at five years is on average 48% significantly lower than for other locations such as the trunk and extremities (77%) [16].
The clinical case we presented is a rare localization of UPS, more precisely at the level of the tongue base. The first presentation of the patient was for inspiratory dyspnea that required tracheostomy and for swallowing disorders, without cervical lymphadenopathy. After the clinical examination, our first suspicion was of a lymphoma derived from the tongue base tonsil. Other carcinomas were also possible given the history of the patient for alcohol and tobacco consumption. This diagnosis was a surprise after the initial biopsy under local anesthesia, which raised the suspicion of carcinoma. Because of the huge dimensions of the tumor, the surgery was not an initial option. Therefore, the patient underwent concomitant chemotherapy and radiation therapy. The case had unexpectedly a favorable outcome. This is because it was a low-grade sarcoma otherwise resistant to radiation therapy. The revision surgery was successful given the possibility of preserving a 0.5 cm circumferential border in healthy tissue. There are still active debates about these surgical margins in tongue carcinoma surgery. The tongue is an organ with extensive lymphatics and well-vascularized presenting a high risk of metastasis. In the tongue base also is exceedingly difficult to preserve the lateral wall of the pharynx and pathology extending there is not recommended for surgery. Essentially, in deciding to operate in the tongue base is the preservation of tongue mobility and normal tongue protrusion to have a good deglutition to prevent aspiration into the respiratory way.
Given the fact that after chemoradiotherapy the mass had a submucous situation, we opted for direct surgical removal instead of biopsy prone to false-negative results of chronic inflammation due to radiation therapy. This patient recorded full recovery with the removal of gastrostomy and tracheostomy tubes.
The peculiarities of the case consisted in the large dimensions at the presentation that required a tracheotomy, the discrepancy between the extemporaneous histological examination and the paraffin examination, the good response to curative radiotherapy, and the possibility of performing a revision surgery with negative edges. Also, the fact that the patient was a heavy smoker adds an additional risk factor in the evolution and yet it is worth noting the favorable evolution with clinical healing and the lack of recurrence at 18 months.
⧉ Conclusions
Tongue base sarcoma is an extremely rare tumor without a precise standardization of the therapeutic protocol. We must keep in mind this option when we see a huge tumor in the base of the tongue. In our case, we have had the suspicion of lymphoma after clinical examination, the frozen sections were with squamous cell carcinoma and finally, the paraffin examination showed pleomorphic sarcoma. Tracheostomy and gastrostomy are mandatory before any kind of treatment. The treatment should be tailored according to the histology type. Sometimes pathology result is difficult to be obtained due to the pleomorphic nature of the tumor. Salvage surgery should be considered only on the condition of obtaining free margins of resection.
Conflict of interest
The authors declare that they have no conflict of interests.
Acknowledgments
Acknowledgments
We would like to thank to Department of Pathology, Emergency University Hospital of Bucharest, Romania, for their availability in diagnosis of the case and the collection of our data.
References
- 1.Morbini P, Alberizzi P, Ferrario G, Capello G, De Silvestri A, Pedrazzoli P, Tinelli C, Benazzo M. The evolving landscape of human papillomavirus-related oropharyngeal squamous cell carcinoma at a single institution in Northern Italy. Acta Otorhinolaryngol Ital. 2019;39(1):9–17. doi: 10.14639/0392-100X-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghavan K, Wen KW, Small EJ, Ha P, Flavell RR. Incidentally detected oropharyngeal squamous cell carcinoma on 18F-Fluciclovine PET/CT. Clin Nucl Med. 2019;44(5):e367–e369. doi: 10.1097/RLU.0000000000002507. [DOI] [PubMed] [Google Scholar]
- 3.Vrinceanu D, Dumitru M, Cergan R, Anghel AG, Costache A, Patrascu ET, Sarafoleanu CC. Correlations between ultrasonography performed by the ENT specialist and pathologic findings in the management of three cases with thyroglossal duct cyst. Med Ultrason. 2018;20(4):524–526. doi: 10.11152/mu-1422. [DOI] [PubMed] [Google Scholar]
- 4.Chung J, Bender-Heine A, Lambert HW. Improving exposure for transoral oropharyngeal surgery with the floor of the mouth window: a cadaveric feasibility study. J Otolaryngol Head Neck Surg. 2019;48(1):62–62. doi: 10.1186/s40463-019-0383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meccariello G, Montevecchi F, D’Agostino G, Iannella G, Calpona S, Parisi E, Costantini M, Cammaroto G, Gobbi R, Firinu E, Sgarzani R, Nestola D, Bellini C, De Vito A, Amadori E, Vicini C. Transoral robotic surgery for the management of oropharyngeal carcinomas: a 9-year institutional experience. Acta Otorhinolaryngol Ital. 2019;39(2):75–83. doi: 10.14639/0392-100X-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldeira PC, Bonardi MJF, Pantuzzo ERM, Soares JMA, Soto AML, Aguiar MCF, Sousa AA. Advanced carcinoma of the oropharynx: survival analysis comparing two treatment modalities. Braz Oral Res. 2020;34:e032–e032. doi: 10.1590/1807-3107bor-2020.vol34.0032. [DOI] [PubMed] [Google Scholar]
- 7.Cosetti-Olivera ML, da Cunha AR, Prass TS, Martins MAT, Hugo FN, Martins MD. Mortality due to oral and oropharyngeal cancer in Uruguay from 1997 to 2014. J Appl Oral Sci. 2019;28:e20190166–e20190166. doi: 10.1590/1678-7757-2019-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen DW, Wang T, Shey-Sen Ni J, Sandulache VC, Graboyes EM, Worley M, Hornig JD, Skoner JM, Day TA, Huang AT. Prognostic factors associated with achieving total oral diet after glossectomy with microvascular free tissue transfer reconstruction. Oral Oncol. 2019;92:59–66. doi: 10.1016/j.oraloncology.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyizoba-Ebozue Z, Burton C, Prestwich RJD. Histiocytic sarcoma of the base of tongue treated with radical radiotherapy: a case report and review of the literature. Clin Transl Radiat Oncol. 2020;21:66–68. doi: 10.1016/j.ctro.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS-defining & non-AIDS defining malignancies in North India. Indian J Med Res. 2016;143(Suppl 1):S129–S135. doi: 10.4103/0971-5916.191813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ball SL, Kwong FNK, Young F, Robson AK. Pharyngeal angiosarcoma following multimodal treatment for oropharyngeal squamous cell carcinoma. Ann R Coll Surg Engl. 2014;96(2):e5–e6. doi: 10.1308/003588414X13814021676792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonseca AS, Azevedo ACA, Magalhães FM, de Andrade NA. Synovial sarcoma in head and neck: a case report. Int Arch Otorhinolaryngol. 2014;18(1):87–89. doi: 10.1055/s-0033-1361081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boccalatte LA, Gómez NL, Yanzon A, Mazzaro EL, Cayol F, Figari MF. Head and neck tumors: management of primary undifferentiated pleomorphic sarcoma. Iran J Otorhinolaryngol. 2019;31(107):335–342. doi: 10.22038/ijorl.2019.30195.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lombardi N, Varoni EM, Bazzacchi R, Moneghini L, Lodi G. Secondary undifferentiated pleomorphic sarcoma of the mandible in a HIV patient who underwent radiotherapy for oral carcinoma. Spec Care Dentist. 2021 doi: 10.1111/scd.12574. [DOI] [PubMed] [Google Scholar]
- 15.da Cruz NFS, Matsuno CA, da Cruz SFS, de Miranda AP, Vital Filho J. Undifferentiated pleomorphic sarcoma of the orbital region: a case report. Arq Bras Oftalmol. 2018;81(2):153–156. doi: 10.5935/0004-2749.20180033. [DOI] [PubMed] [Google Scholar]
- 16.Lee K, Song JS, Kim JE, Kim W, Song SY, Lee MH, Chung HW, Cho KJ, Lee JS, Ahn JH. The clinical outcomes of undifferentiated pleomorphic sarcoma (UPS): a single-centre experience of two decades with the assessment of PD-L1 expressions. Eur J Surg Oncol. 2020;46(7):1287–1293. doi: 10.1016/j.ejso.2020.02.029. [DOI] [PubMed] [Google Scholar]