Abstract
Knowing the hepatic pathological features encountered in patients with chronic hepatitis C (CHC) and the fact that extrahepatic manifestations occur only in people with certain characteristics of the immune system, we tried to evaluate, qualitatively and semi-quantitatively, the liver pathological aspects encountered in 96 patients with CHC, previously untreated with Interferon (naïve), who showed or did not show signs of thyroid disorder (TD), hospitalized in the 2nd Medical Clinic of the Emergency County Hospital, Craiova, Romania, within a period of five years (2007–2012). Following hormonal, immunological, and thyroid ultrasound investigations, 14 (14.58%) of the 96 patients showed signs of TD. The main clinical forms of TD in the studied patients with CHC were autoimmune thyroiditis and subclinical hypothyroidism. In the patients with CHC with TD, we found mild chronic hepatitis in 14.28% of cases, the appearance of moderate chronic hepatitis was found in 71.42% patients, and the appearance of severe chronic hepatitis was found in 14.28% patients, while in the patients with CHC without TD we found chronic mild hepatitis in 62.19% of cases, the appearance of moderate chronic hepatitis was met in 32.92% patients, and the appearance of severe chronic hepatitis was found in 4.87% of patients. Mild and moderate fibrosis were found only in CHC patients without TD in a percentage of 25.6% and 65.85%, respectively, while severe fibrosis was found at 12.19% among CHC patients without TD and 92.85% among CHC patients with TD. The pathological aspect of liver cirrhosis was found only in those with TD (7.14%). In conclusion, the pathological features which define the liver necroinflammatory process, as encountered at the pathological examination in CHC patients with TD are the same as in any active chronic hepatitis, the differences being represented by the higher percentage of the periportal and the preseptal necrosis (piecemeal necrosis), as well as by the higher score of portal inflammation. In addition, the severe hepatic fibrosis and the histopathological appearance of the liver cirrhosis have only defined the cases of CHC with TD.
Keywords: chronic hepatitis C, thyroid disorder, hepatic pathological features, naïve patients
⧉ Introduction
Chronic hepatitis C (CHC) represents a major cause of mortality and morbidity worldwide [1,2]. This disease affects over 71 million of people all over the world and is one of the most frequent causes of hepatic damage, providing the largest number of candidates for liver transplant [3,4]. According to Center for Disease Control and Prevention (CDC), approximately 80% of the persons with acute infection with hepatitis C virus (HCV) will develop CHC. 80–90% of these patients will develop severe hepatic lesions [5]. Persistent viral infection is the consequence of rapid virus replication and continuous spreading from one cell to another, as well as the lack of an adequate immune response following the exposure to the HCV antigens [6]. The evolution of the chronic hepatitis is impermissible and variable depending on the host reactivity on which it was grafted [7]. So, there are patients in which the evolution towards the final stage of cirrhosis is very slow, meanwhile in other patients the evolution towards cirrhosis is realized in less than 10 years [8]. Hepatic biopsy is not necessary for the positive diagnosis but represents the most efficient method for the evaluation of the severity of hepatic damage, for the appreciation of the prognosis and the therapeutic indication, as well as for the exclusion of rare causes of chronic hepatitis, such as alcoholic liver disease and hemochromatosis [9].
Besides the nonspecific pathological changes frequently met in chronic hepatitis (periportal necrosis and inflammation, necrosis and focal intralobular inflammation, portal inflammatory infiltrate, hepatic fibrosis) there are also a number of suggestive but non-pathognomonic aspects for hepatitis C (lymphoid follicles in portal spaces, hepatic steatosis, bile duct lesions and Mallory-like bodies in the hepatocyte cytoplasm) [10]. HCV infection not only affects the liver but also affects other non-hepatic tissues and organs and can be associated with many unknown diseases [11]. Chronic liver disease can induce systolic and diastolic dysfunction, electrophysiological changes (QT prolongation), and hemodynamic conditions in the presence of a cirrhotic cardiomyopathy. All this can improve significantly after liver transplantation [12,13]. Through the extrahepatic manifestations of the disease, CHC contributes to the elevation of the morbidity and the hospitalization cost, developing economic pressure on health insurance systems [14]. Thyroid disorder (TD) is considered one of the most common endocrine disorders in association with HCV infection [15]. Also, autoimmune thyroid disease (AITD) represents the most common TD characterized by the presence of circulating antithyroid antibodies. Autoimmune TDs are a group of different diseases that have in common the presence of increased intensity lymphocytic infiltrate in the thyroid parenchyma and the production of antithyroid antibodies [16]. Thyroid autoimmunity is linked to HCV infection, varying from isolated anti-thyroid autoantibodies to symptomatic autoimmune TDs [17,18]. Hypothyroidism and hyperthyroidism can occur during HCV infection. Hashimoto thyroiditis (HT) is a common TD in patients with HCV infection [14].
Aim
The aim of the study was to establish the hepatic pathological features that may anticipate concomitant TD, in naïve patients with CHC.
⧉ Patients, Materials and Methods
We carried a retrospective clinical study, in which were analyzed the pathological aspects emphasized by liver biopsy (LB) performed in 96 patients with CHC, with or without impaired thyroid who were diagnosed and monitored in 2nd Medical Clinic and Endocrinology Clinic of the Emergency County Hospital, Craiova, Romania, over a period of five years (2007–2012). The diagnosis of CHC was suggested in base of the clinical examination, supported by serological tests (determination of anti-HCV antibodies) and confirmed by virological tests, quantitative HCV ribonucleic acid (RNA) test, and LB puncture. The criteria for including patients in the patient group were the presence of anti-HCV antibodies, detectable titer of HCV-RNA viremia, LB puncture, hormonal tests – thyroid-stimulating hormone (TSH), free thyroxine (FT4) and detection of anti-thyroid peroxidase antibodies (TPOAb) and/or antithyroglobulin antibodies (TgAb) and in selected cases, thyrotropin receptor antibodies (TRAb) or anti-TSH receptor antibodies. The exclusion criteria, represented by the contraindications of LB puncture, were: bleeding disorders [hypoprothrombinemia, with prothrombin index (PI) less than 60%; thrombocytopenia, with platelet count less than 60 000/mm3], hepatic congestion or extrahepatic cholestasis, hepatic hydatid cyst, hepatic suppurative infections (hepatic abscess, acute angiocholitis) and perihepatic suppurations (pulmonary abscess, subdiaphragmatic abscess, subhepatic abscess) and ascites; the presence of mental illness (chronic alcoholism, drug and hepatotoxic dependence) and uncooperative patients. For virological and serological tests, we used the systems Strip Immunoblot Chiron Riba HCV 3.0 CobasAmplicor HCV Assay and 2.0 v methods. The hematological, biochemical, and hormonal tests were performed in the Laboratory of Emergency County Hospital, Craiova, using the Celltac Nihon Kohdens system and Vitros 250 dry chemistry analyzers and Elecsys 2010 (electrochemiluminescence). LB was performed in the patients included in the study group using the percutaneous method using the special Hepafix kit (Braun Melsungen AG). The harvested liver tissue fragments (with dimensions between 10–25 mm/1–1.4 mm) were fixed in formalin and then processed according to the standard protocol practiced in the Laboratory of Pathological Anatomy, Emergency County Hospital, Craiova. The positive diagnostic criteria for TD were altered TSH values and/or the presence of one of the antithyroid antibodies and/or ultrasound (US) thyroid changes. Ultrasonography exam is an essential study of thyroid gland. Thyroid US was performed with a 7.5 MHz linear probe, both in B mode and color Doppler techniques. The statistical analysis was performed using the Microsoft Excel (Microsoft Corp., Redmond, WA, USA), together with the XLSTAT add-on for MS Excel (Addinsoft SARL, Paris, France) and IBM Statistical Package for the Social Sciences (SPSS) Statistics 20.0 (IBM Corporation, Armonk, NY, USA) for data processing. To test the normality of the data, we used the Anderson–Darling test. Because the numerical variables investigated had a normal data distribution, globally or in every studied group, we were allowed to use the parametric statistical tests [e.g., Student’s t-test, analysis of variance (ANOVA)] and the results were summarized as the mean value ± standard deviation (SD). For all statistical tests, p-values less than 0.05 were considered significant. The study was carried out with the consent of the Ethics Commission of University of Medicine and Pharmacy of Craiova, and with obtaining a consent signed by the patient, respecting the fundamental rights of patients.
⧉ Results
In order to highlight the hepatic pathological aspects encountered in patients with CHC who developed TD, we performed a retrospective study to a number of 96 hospitalized patients with CHC not previously treated with Interferon (IFN) (naïve patients). Of the 96 patients, 59 (61.45%) patients were women, and the remaining 37 (38.54%) patients were men. Regarding the age of the patients, the average value of the age of the patients with CHC was 51±24.3 years, with limits between 18 and 70 years. Following hormonal and immunological tests 14 (14.58%) patients showed signs of TD. Of the 96 studied patients, TD assessed by performing TSH and FT4 presented as subclinical hypothyroidism (TSH >4.2 μIU/mL and normal FT4) in 14 (14.58%) patients. In this study, the incidence of antithyroid antibodies (TPOAb and TgAb) in the group of patients with CHC was 14.58%. The averages of demographic and hormonal parameters in the two groups of patients with CHC, with or without TD can be seen in the Table 1.
Table 1.
Demographic and hormonal thyroid parameters in the two groups of patients with CHC, with or without TD
|
Parameter |
Mean values in patients with CHC with TD |
Mean values in patients with CHC without TD |
Statistical test p-value |
|
No. of patients |
14 |
82 |
<0.001* |
|
Men/women |
5/9 |
32/50 |
0.885 |
|
Age [years] |
55.6±26.4 |
46.4±22.2 |
0.078 |
|
TSH [μIU/mL] |
4.86±0.84 |
2.42±0.41 |
<0.001* |
|
FT4 [ng/dL] |
1.21±0.2 |
1.91±0.6 |
0.648 |
CHC: Chronic hepatitis C; FT4: Free thyroxine; TD: Thyroid disorder; TSH: Thyroid-stimulating hormone (thyrotropin). *Highly statistically significant.
The main manifestations and clinical syndromes
The main manifestations and clinical syndromes found in the 96 patients with CHC were: asthenia in 59 (61.45%) patients, vascular purpura in 33 (34.37%) patients, jaundice in 11 (11.45%) patients, xerostomia in 19 (19.79%) patients, articular manifestations in 34 (35.41%) patients, paresthesias in 20 (19.2%) patients, thrombophlebitis in seven (7.29%) patients and hepatomegaly in 50 (52.08%) patients. Incidences of clinical manifestations or syndromes encountered in patients with CHC with or without TD are found in the Table 2.
Table 2.
Incidences of clinical manifestations and syndromes in patients with CHC with or without TD
|
Clinical syndromes |
Patients with TD (14 pts.) |
Patients without TD (84 pts.) |
Statistical significance p-value |
|
Asthenia |
13 pts. (92.85%) |
46 pts. (56.09%) |
0.011 |
|
Vascular purpura |
9 pts. (64.28%) |
24 pts. (29.26%) |
0.049 |
|
Jaundice |
2 pts. (14.28%) |
9 pts. (10.9%) |
0.127 |
|
Xerostomia |
8 pts. (57.14%) |
11 pt. (13.41%) |
0.017 |
|
Arthralgia |
7 pts. (50%) |
27 pts. (32.92%) |
0.231 |
|
Paresthesias |
9 pts. (64.28%) |
11 pts. (13.09%) |
0.005 |
|
Thrombophlebitis |
3 pts. (21.42%) |
4 pts. (4.87%) |
0.291 |
|
Hepatomegaly |
10 pts. (71.42%) |
40 pts (48.78%) |
0.122 |
CHC: Chronic hepatitis C; TD: Thyroid disorder.
Asthenia was found in the most of patients with TD, 13 (92.85%) out of 14 patients, compared to the group of patients without TD, where it was present in only 46 (56.09%) out of 82 patients. Vascular purpura was present in almost two-thirds of patients with TD, nine (64.28%) patients, compared to the group of patients without TD where it was present in only 24 (29.26%) patients. Jaundice was present in two (14.28%) patients with CHC and TD, compared to the group of patients without TD, in which jaundice was present in nine of them (10.97%). Most symptomatic patients with TD had xerostomia, which was present in eight (57.14%) out of 14 patients, compared to the group of patients without TD, 11 (13.41%) out of 82 patients. Joint manifestations were present in half (50%) of patients with TD, and in the group of patients without TD were present at 27 (32.92%) patients. Neurological manifestations (paresthesias) were present in both groups of patients, nine (64.28%) out of 14 patients with TD and only 11 (13.41%) out of 82 patients without TD. Superficial and deep thrombophlebitis were present in three (21.42%) patients with TD and only in four (4.87%) patients without TD. Hepatomegaly was present in the majority (71.42%) of patients with TD, and in approximately half (48.78%) of patients without TD.
The positive diagnosis of CHC
The positive diagnosis of CHC was established based on the serological and virological tests. Anti-HCV antibodies were present in all selected patients (100%), and the virological tests shown a statistically significantly lower mean value (p=0.01) of quantitative viremia (HCV-RNA) in patients with TD compared to the group of patients without TD (1 123 673±225 953.4 copies/mL vs 1 875 432±345 988.3 copies/mL). The mean values of the virological, hematological, and biochemical parameters in the studied patients are summarized in the Table 3.
Table 3.
Mean values of virological, hematological, and biochemical parameters in the two groups of patients with CHC, with or without TD
|
Parameter |
Mean values in patients with CHC with TD |
Mean values in patients with CHC without TD |
Statistical test p-value |
|
HCV-RNA quantitative testing [copies/mL] |
1 123 673±225 953.4 |
1 875 432±345 988.3 |
<0.001* |
|
Hb [g/dL] |
12.14±0.8 |
13.8±0.9 |
0.456 |
|
PLT count [No./mm3] |
158 216.2±24 143.4 |
271.148±29.512 |
0.054 |
|
WBC count [No./mm3] |
6278±1524 |
8454±1624 |
0.061 |
|
ESR [mm/1 h] |
15.7±6.19 |
13.7±4.16 |
0.09 |
|
CRP [mg/dL] |
16.27±7.74 |
8.1±4.28 |
0.16 |
|
IL-6 [ng/L] |
37.08±20.34 |
30.34±19.58 |
0.19 |
|
TNF-α [ng/L] |
54.62±42.93 |
29.88±20.16 |
0.009* |
|
TB [mg/dL] |
1.2±0.5 |
0.7±0.3 |
0.468 |
|
ALP [U/L] |
158±24 |
128±19 |
0.342 |
|
GGT [U/L] |
109±18 |
52±14 |
0.078 |
|
ALT [U/L] |
64±29.6 |
58±28.2 |
0.354 |
|
AST [U/L] |
57.2±26.4 |
49.5±25.4 |
0.378 |
|
PI [%] |
84.2±9.6 |
91.1±11.2 |
0.231 |
|
ALB [g/dL] |
3.6±0.21 |
4.2±0.36 |
0.342 |
|
Υ-gl [g/dL] |
1.92±0.5 |
1.4±0.4 |
0.02* |
ALB: Albumin; ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CHC: Chronic hepatitis C; CRP: C-reactive protein; Υ-gl: Gamma-globulins; GGT: Gamma-glutamyl transferase; ESR: Erythrocyte sedimentation rate; Hb: Hemoglobin; HCV-RNA: Hepatitis C viral load (viremia); IL-6: Interleukin-6; PI: Prothrombin index; PLT: Platelets; RNA: Ribonucleic acid; TB: Total bilirubin; TD: Thyroid disorder; TNF-α: Tumoral necrosis factor-alpha; WBC: White blood cells. *Highly statistically significant
The hematological study
The mean values of hemoglobin (Hb) were 12.14±0.8 g/dL in patients with TD and 13.8±0.9 g/dL in patients without TD. Low values of Hb were found in six (42.85%) patients with TD and in 24 (29.26%) patients without TD.
The platelet count showed a lower mean value in patients with CHC and TD, compared to patients without TD (158 216.2±24143.4/mm3 vs 271.148±29.512/mm3). Thrombocytopenia was found in four (28.57%) patients with TD and in four (4.87%) patients without TD. Regarding the number of white blood cell (WBC) count, patients with CHC and TD showed a lower mean value (6278±1524/mm3) than the one reported in patients with CHC without TD (8454±1624/mm3). Leukopenia was present in only three (21.42%) patients with TD and in two (2.43%) patients without TD.
The systemic inflammation study
The mean value of erythrocyte sedimentation rate (ESR) was higher in patients with CHC with TD (15.7±6.19 mm/1 h) compared to patients with CHC without TD (13.7±4.16 mm/1 h). The mean serum C-reactive protein (CRP) values in patients in both groups were increased: the patients with TD having higher mean value (16.27±7.74 mg/dL) than patients without TD (8.1±4.28 mg/dL). The mean value of serum interleukin-6 (IL-6) was elevated in both groups of patients and was higher in subjects with TD (37.08±20.34 ng/L) compared with patients without TD (30.34±19.58 ng/L). Also, the patients with CHC and TD had higher values (54.62±42.93 ng/L) of tumor necrosis factor-alpha (TNF-α), compared to patients with CHC without TD (29.8±20.16 ng/L).
The biochemical study
The mean value of total bilirubin (TB) in patients with CHC and TD was 1.2±0.5 mg/dL, the percentage of patients with elevated values being 28.57%. Instead, in patients with CHC without TD, the mean value of the TB was 0.7±0.3 mg/dL and only 3.65% of these patients had abnormal values. The mean value of serum alkaline phosphatase (ALP) activity in the 14 patients with TD was 158±24 U/L, with elevated serum activity of ALP in 42.85% of patients, while only 29.26% of patients with CHC without TD had abnormal ALP values, with a mean value of 128±19 U/L. The mean value of serum gamma-glutamyl transferase (GGT) levels in the first group of patients was 109±18 U/L, while in the second group was 52±14 U/L. Elevated GGT values were present in seven (50%) out of 14 patients with CHC and TD, while only 26 (31.7%) out of 82 patients without TD had abnormal values. The mean value of serum alanine aminotransferase (ALT) activity in patients with TD was 64±29.6 U/L, with elevated values of serum ALT level in nine (64.28%) patients, compared with a mean ALT value of 58±28.2 U/L encountered in patients without TD, elevated ALT values being found in only 31 (37.8%) patients. The mean value of serum aspartate aminotransferase (AST) activity in the 14 patients with TD was 57.2±26.4 U/L, increased values of this parameter being recorded in nine (64.28%) patients. The mean value of serum AST activity in the 82 patients without TD was 49.5±25.4 U/L, higher values of AST being present in 31 (37.8%) patients. Analysis of PI values showed a mean value of 84.2±9.6% in the 14 patients with CHC and TD, compared with a mean value of 91.1±11.2% in the 82 patients with CHC without TD. The percentage of patients with low prothrombin time (PT) values (less than 70%) was 21.42% in those with CHC and TD and only 12.19% in those with CHC without TD. The mean values of serum albumin (ALB) from the two groups of patients were 3.6±0.21 g% in the 14 patients with TD and 4.2±0.36 g% in the 82 patients without TD, low values of this parameter being found in two (14.2%) patients with TD, compared to eight (9.7%) patients without TD. In the 14 patients with CHC and TD, the mean value of gamma-globulins (γ-glob) was 1.92±0.5 g%, and the percentage of patients with abnormal values of this parameter was 35.71%, while in the 84 patients with CHC without TD, the mean value of γ-glob was 1.4±0.4 g%, with a percentage of patients with abnormal values of 10.97%.
The discovery of changes from the tests of the hepatic mesenchymal activation syndrome
The discovery of changes from the tests of the hepatic mesenchymal activation syndrome, especially in patients with CHC and TD, has supported the involvement of immunological mechanisms in the pathogenesis of hepatic and extrahepatic manifestations encountered in these patients. The mean values of the immunological parameters recorded in the patients with CHC with or without TD are found in the Table 4.
Table 4.
Mean values of immunological parameters recorded in the patients with CHC, with or without TD
|
Parameter |
Mean values in patients with CHC with TD |
Mean values in patients with CHC without TD |
Statistical test p-value |
|
IgG [IU/mL] |
241.2±98.6 |
228.4±16.8 |
0.05 |
|
IgA [IU/mL] |
227.4±94.6 |
206.2±54.2 |
0.05 |
|
IgM [IU/mL] |
208.8±58.4 |
168.6±20.4 |
0.05 |
|
CIC [UF/mL] |
78.4±12.6 |
46.8±9.2 |
0.05 |
|
Se-C3 [mg%] |
44.2±8.4 |
68.2±12.8 |
0.05 |
CHC: Chronic hepatitis C; CIC: Circulating immune complexes; IgA: Immunoglobulin A; IgG: Immunoglobulin G; IgM: Immunoglobulin M; Se-C3: Serum C3 fraction; TD: Thyroid disorder
The analysis of the average values of the immunological parameters in patients with CHC with TD, compared to the average values of the same parameters in patients without TD allowed the following observations. The mean immunoglobulin G (IgG) concentration in patients with CHC and TD was 241.2±98.6 IU/mL, elevated values of this parameter were found in four (28.57%) out of 14 patients. In patients with CHC without TD, the mean IgG value was 228.4±16.8 IU/mL, with elevations of the values in six (7.31%) out of 82 patients. In the group of 14 patients with CHC and TD, four (28.57%) patients had high serum immunoglobulin A (IgA) values, with a mean value of 227.4±94.6 IU/mL, while in the group of 82 patients with CHC without TD only six (7.31%) patients had elevated IgA concentration, with a mean value of 206.2±54.2 IU/mL.
The mean immunoglobulin M (IgM) value in patients with CHC and TD was 208.8±58.4 IU/mL, the increased values being recorded in three (21.42%) out of 14 patients and the mean value of this parameter in patients with CHC without TD was 168.6±20.4 IU/mL, the number of patients with elevated of six (7.31%) out of 82 patients. The mean values of circulating immune complexes (CIC) concentration in the two groups of patients were 78.4±12.6 UF/mL in the group of patients with TD and 46.8±9.2 UF/mL in the group of patients without TD, increased values of this parameter being found in one (7.14%) out of 14 patients with TD, compared to four (4.87%) out of 82 patients without TD. The mean values of the serum C3 fraction (Se-C3) were 44.2±8.4 mg% in the 14 patients with CHC and TD, and 68.2±12.8 mg% in the 82 patients without TD and the percentage of the low values of this parameter was 14.28% in patients with TD and only 4.87% in patients without TD. The prevalence of autoantibodies in the two groups of patients with CHC, with or without TD are summarized in Table 5.
Table 5.
Prevalence of autoantibodies in the two groups of patients with CHC, with or without TD
|
Autoantibodies |
Percentage of patients with autoantibodies present in those with TD |
Percentage of patients with autoantibodies present in those without TD |
Statistical test p-value |
|
CG |
35.71% (5/14 pts.) |
4.87% (4/82 pts.) |
<0.0001* |
|
RF |
42.85% (6/14 pts.) |
7.31% (6/82 pts.) |
<0.0001* |
|
ANA |
28.57% (4/14 pts.) |
8.53% (7/82 pts.) |
<0.0001* |
|
TPOAb |
100% (14/14 pts) |
0% |
<0.0001* |
|
TgAb |
78.57% (11/14 pts.) |
0% |
<0.0001* |
ANA: Antinuclear antibodies; CG: Cryoglobulins; CHC: Chronic hepatitis C; RF: Rheumatoid factors; TD: Thyroid disorder; TgAb: Antithyroglobulin antibodies; TPOAb: Anti-thyroid peroxidase antibodies. *Highly statistically significant
Cryoglobulins (CG) were found in five (35.71%) patients with hepatocellular carcinoma (HCC) and TD, compared with four (4.87%) patients without TD. Rheumatoid factors (RF) were present in six (42.85%) out of 14 patients with TD, compared with six (7.31%) out of 82 patients without TD. Of the 14 patients with CHC and TD, in four (28.57%) patients had positive antinuclear antibodies (ANA), while in the group of patients without TD, only seven (8.53%) patients had ANA.
The positive diagnosis of TD
In the studied group, 14 (14.58%) out of 96 patients with CHC showed TD demonstrated by performing TSH, FT4, thyroid antibodies, and thyroid US. Of the 96 studied patients, TD assessed by performing TSH and FT4, presented as subclinical hypothyroidism (TSH >4.2 μIU/mL and normal FT4) in 14 (14.58%) patients. In this study, the incidence of antithyroid antibodies (TPOAb and TgAb) in the group of patients with CHC was 14.58%. The incidence of TPOAb was 100% in patients with TD, while the incidence of TgAb in the group of patients with TD was 78.57% (Table 5). In the evaluated subjects, the thyroid US had the role to highlight the thyroid morphological changes encountered in patients who presented hormonal or immunological changes. If the mean thyroid volume in patients with CHC and TD was 12.8±1.4 mL, in the patients with CHC without TD it was significantly higher, 15.6±1.5 mL (p<0.05). The incidences of US changes in the studied patients can be found in the Table 6.
Table 6.
Incidence of thyroid US changes in patients in the two groups of patients with CHC, with and without TD
|
US parameter |
Incidence of thyroid US changes in patients with TD |
Incidence of thyroid US changes in patients without TD |
|
|
Thyroid contour |
Regular |
6/14 (42.85%) |
60/82 (73.17%) |
|
Irregular |
8/14 (57.14%) |
22/82 (26.82%) |
|
|
Glandular echogenicity |
Hypoechoic |
8/14 (57.14%) |
9/82 (10.97%) |
|
Normal |
4/14 (28.57%) |
65/82 (79.26%) |
|
|
Hyperechoic |
2/14 (14.28%) |
8/82 (9.75%) |
|
|
Lobe structure |
Homogeneous |
4/14 (28.57%) |
65/82 (79.26%) |
|
Diffuse inhomogeneous |
6/14 (42.85%) |
14/82 (17.07%) |
|
|
Nodular |
4/14 (28.57%) |
3/82 (3.65%) |
|
CHC: Chronic hepatitis C; TD: Thyroid disorder; US: Ultrasound
The thyroid gland contour in patients with CHC and TD was regular in six (42.85%) patients, while the majority, eight (57.14%) patients had an irregular contour. In the group of patients with CHC without TD, the regular contour of the gland predominated, being found in 60 (73.17%) patients, while only 22 (26.82%) patients had an irregular contour. In patients with TD, the normal appearance of the glandular echogenicity was found in four (28.57%) patients, the hypoechoic appearance was found in eight (57.14%) patients, and the hyperechoic appearance was found in only two (14.28%) patients. In patients without TD, the normal appearance of the glandular echogenicity was predominant, being found in 65 (79.26%) patients, the hypoechoic appearance was found in nine (10.97%) patients, and the hyperechoic appearance was found in eight (9.75%) patients. The lobular structure was another important feature highlighted by US, so in the group of patients with CHC and TD the diffuse inhomogeneous appearance of the thyroid structure was found in six (42.85%) patients, the homogeneous appearance and the nodular aspect were found in four (28.75%) patients. In the group of patients without TD, the homogeneous aspect predominated, being found in 65 (79.26%) patients, the diffuse inhomogeneous aspect was found in 14 (17.07%) patients and the nodular aspect was found in only three (3.65%) patients.
Liver histopathological examination
Liver histopathological examination is a valuable criterion for assessing chronicity, but especially for assessing the clinical form of CHC. The description and quantification of pathological lesions is necessary to classify the severity of necroinflammation (grading) and liver fibrosis (staging). The Ishak score is a complex semiquantitative histological score that considers both liver necroinflammatory activity and the degree of liver fibrosis (DLF) (Table 7).
Table 7.
The system of semi-quantitative assessment of the liver morphopathological lesions elaborated by Ishak (1995)
|
Necroinflammatory activity |
Fibrosis |
||||||||
|
Periportal or preseptal interface hepatitis (piecemeal necrosis) |
Score |
Confluent necrosis |
Score |
Focal (spotty) lytic necrosis apoptosis and focal inflammation |
Score |
Portal inflammation |
Score |
Fibrosis |
Score |
|
Absent |
0 |
Absent |
0 |
Absent |
0 |
Absent |
0 |
No fibrosis |
0 |
|
Mild (focal, few portal areas) |
1 |
Focal confluent necrosis |
1 |
One focus or less per ×10 objective |
1 |
Mild, some or all portal areas |
1 |
Fibrous expansion of some portal areas, with or without short fibrous septa |
1 |
|
Mild (focal, few portal areas) |
2 |
Zone 3 necrosis in some areas |
2 |
Two to four foci per ×10 objective |
2 |
Moderate, some or all portal areas |
2 |
Fibrous expansion of most portal areas, with or without short fibrous septa |
2 |
|
Moderate (continuous around <50% of tracts or septa) |
3 |
Zone 3 necrosis in most areas |
3 |
Five to 10 foci per ×10 objective |
3 |
Moderate/marked, all portal areas |
3 |
Fibrous expansion of most portal areas, with occasional portal to portal bridging |
3 |
|
Severe (continuous around >50% of tracts or septa) |
4 |
Zone 3 necrosis + occasional portal-central (P-C) bridging |
4 |
More than 10 foci per ×10 objective |
4 |
Marked, all portal areas |
4 |
Fibrous expansion of most portal areas, with marked bridging (portal to portal as well as portal to central) |
4 |
|
|
|
Zone 3 necrosis + multiple P-C bridging |
5 |
|
|
|
|
Marked bridging with occasional nodules (incomplete cirrhosis) |
5 |
|
|
|
Panacinar or multiacinar necrosis |
6 |
|
|
|
|
Cirrhosis, probable or definite |
6 |
Histological activity index (HAI) represents the sum of scores: periportal or preseptal necrosis (piecemeal necrosis), confluent necrosis (bridging necrosis), focal or spotty necrosis, and portal inflammation. Depending on the sum of the values of the scores of the four histological parameters that define necroinflammation, chronic hepatitis was classified into: mild chronic hepatitis (HAI: 1–6), moderate chronic hepatitis (HAI: 7–10), and severe chronic hepatitis (HAI: 11–16). In mild chronic hepatitis, there is only a discrete portal inflammation, with respect to the limiting plaque and without fibrosis lesions. In moderate chronic hepatitis, there is a portal and periportal inflammatory infiltrate, with inter- and intralobular extension, achieving the appearance of piecemeal necrosis. In severe chronic hepatitis, necrosis becomes confluent, achieving the appearance of bridge necrosis (bridging necrosis) or multilocular and is associated with the presence of fibrous septa extending into the liver lobe. DLF, that expresses the size and extent of fibrous septa, is an important histological parameter in assessing the severity of chronic hepatitis. Liver fibrosis assessed by the Ishak score can be classified into: mild fibrosis (0–1), moderate fibrosis (2), severe fibrosis (3–4), and cirrhosis (5–6). In patients with CHC studied, the mean value of score of HAI was 7.04±2.2, while the mean value of score of liver fibrosis (grade of fibrosis) was 2.68±0.7. The mean values of the scores of the parameters of necroinflammation and hepatic fibrosis evaluated by the Ishak score following LB in the patients with CHC with or without TD are given in the Table 8.
Table 8.
Average of pathological parameters scores researched by liver biopsy puncture
|
Parameters |
Mean values in patients with CHC with TD |
Mean values in patients with CHC without TD |
Statistical test p-value |
|
Histological activity index |
8.68±2.30 |
5.4±2.11 |
0.01 |
|
Piecemeal necrosis score |
2.88±0.76 |
1.64±0.77 |
0.05 |
|
Confluent necrosis score |
1.29±0.95 |
0.63±0.56 |
0.003* |
|
Focal necrosis score |
2.33±0.56 |
1.5±0.56 |
0.05 |
|
Portal inflammation score |
2.51±0.71 |
1.43±0.66 |
0.05 |
|
Grade fibrosis score |
3.6±0.78 |
1.76±0.62 |
0.0002* |
CHC: Chronic hepatitis C; TD: Thyroid disorder. *Highly statistically significant
The mean HAI was statistically significant (p=0.01) higher in patients with CHC with TD (8.68±2.3), compared with patients without TD (5.4±2.11). The mean value of periportal and preseptal necrosis score was higher (p=0.05) in patients with CHC with TD (2.88±0.76), compared with patients with CHC without TD (1.64±0.77). The mean value of portal inflammation score was statistically significant (p=0.05) higher in patients with CHC with TD (2.51±0.71), compared to patients with CHC without TD (1.43±0.66). The mean value of the confluent necrosis score was statistically significantly (p=0.003) higher in patients with CHC with TD (1.29±0.95), compared to patients with CHC without TD (0.63±0.56). The mean value of the focal necrosis score was higher in patients with CHC with TD (2.33±0.56) compared to patients with CHC without TD (1.5±0.56) (Figure 1).
Figure 1.
Nonspecific pathological features observed in patients with CHC: (A) Feature of piecemeal necrosis, with periportal and preseptal inflammation; (B) Aspect of portal inflammation (an inflammatory infiltrate consisting of lymphocytes, plasmocytes and polymorphonuclears inside the portal tracts); (C) Feature of bridging necrosis, when the hepatic necrosis becomes confluent or multilocular, and is associated with the presence of fibrous septa extending into the liver lobe; (D) Aspect of focal necrosis with nonspecific pathological alterations. HE staining: (A) ×100; (B–D) ×200. CHC: Chronic hepatitis C; HE: Hematoxylin–Eosin
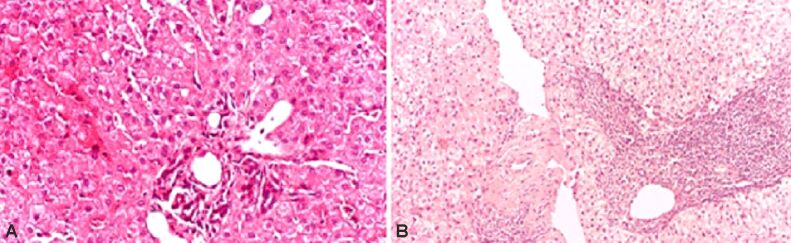
We found mild chronic hepatitis in two (14.28%) of the 14 patients with CHC and TD, the appearance of moderate chronic hepatitis in 10 (71.42%), and that of severe chronic hepatitis in two (14.28%) patients (Figure 2).
Figure 2.
Pathological features encountered in patients with CHC: (A) Histological aspect of CHC with mild activity – pathological aspect frequently encountered in patients with CHC without TD; (B) Histological aspect of CHC with severe activity – pathological aspect frequently encountered in patients with CHC with TD. HE staining: (A and B) ×100. CHC: Chronic hepatitis C; HE: Hematoxylin–Eosin; TD: Thyroid disorder
On the other hand, we identified mild chronic hepatitis in 51 of the 82 (62.19%) patients with CHC that did not have TD, the appearance of moderate chronic hepatitis in 27 (32.92%) patients and the appearance of severe chronic hepatitis in four (4.87%) patients. In the studied patients with CHC, the mean value of the degree of hepatic fibrosis (DHF) score was statistically significantly higher (p=0.0002) in patients with CHC with TD (3.6±0.78), compared to patients with CHC without TD (1.76±0.62). Mild and moderate fibrosis were found only in patients with CHC without TD in 21 (25.6%) patients and respectively, in 54 (65.85%) patients out of 82 patients, while severe fibrosis was found in 10 (12.19%) patients out of 82 among patients with CHC without TD and in 13 (92.85%) patients out of 14 among patients with CHC with TD. The pathological aspect of liver cirrhosis was found only in those with TD (7.14%) (Figure 3).
Figure 3.
Pathological features of CHC associated with hepatic fibrosis: (A) CHC with mild fibrosis, aspect frequently showed in patients without TD; (B) CHC with severe fibrosis, feature frequently showed in patients with TD. Masson’s trichrome staining: (A and B) ×200. CHC: Chronic hepatitis C; TD: Thyroid disorder
⧉ Discussions
Following hormonal, immunological, and thyroid US investigations of the 96 patients, 14 (14.58%) showed signs and changes of TD. Among the patients with CHC with TD, the most, nine (64.28%) were women, same fact observed in the group of CHC patients without TD, 50 (60.97%) being women. Regarding the age of the patients, the average value of the age of the patients with TD was 55.6±26.4 years (22–70 years), higher than the average age value of the patients without TD (46.4±22.2 years; 18–69 years). The prevalence of positive anti-thyroid antibodies and hypothyroidism in women is substantially higher (14.7% and 10.5%, respectively, compared to 0% in men, p<0.01) and is greatly related to the increasing age (p<0.01) in a study performed in Spain [19].
Clinical manifestations in chronic HCV infection
The clinical manifestations present in chronic HCV infection include nonspecific symptoms, such as asthenia, nausea, abdominal or musculoskeletal pain, weight loss, and neuropsychiatric symptoms, including depression, and irritability [20]. The analysis of incidences of the main clinical manifestations found in the patients with CHC and TD compared to the patients with CHC without TD allowed the following discussions. Asthenia was found in many patients with TD, 13 (92.85%) out of 14 patients, compared to the group of patients without TD, where it was present in only 46 (56.09%) out of 82 patients. The incidence of asthenia was statistically significantly higher (p=0.011) in patients with CHC and TD, compared with those without TD. In a study of 1614 patients, asthenia was present in 53% of patients, of which 17% had marked asthenia, which affected their daily activity. The prevalence of asthenia was higher in women over 50 years of age. There was no significant association between asthenia and viral load or genotype, alcohol consumption, TD and CG levels. Asthenia has also been associated with arthralgia, myalgia, paresthesias, sicca syndrome and pruritus [21]. The same behavior was observed in vascular purpura, the incidence being significantly higher (p=0.049) in patients with TD compared to those without TD. A close association between mixed cryoglobulinemia and HCV infection has been reported in the literature. Thus, a clinical study performed on a group of 62 patients, which aimed to highlight the cutaneous manifestations of patients with mixed cryoglobulinemia, depending on the presence or absence of anti-HCV antibodies, showed that anti-HCV antibodies were present in 32 (56.45%) patients [22]. Jaundice was present in two (14.28%) patients with CHC and TD, compared to the group of patients without TD, in which jaundice was present in nine (10.97%) of them. CHC may be associated with hepatic cytolysis syndrome, which may associate jaundice as an evident clinical manifestation. Most often, cytolysis is asymptomatic and, rarely, causes liver failure [23]. The incidence of xerostomia was statistically significantly (p=0.017) higher in patients with CHC with TD compared to the group of patients without TD. A clinical study performed in 65 patients, which investigated the prevalence of HCV-RNA in the saliva and salivary glands of patients with HCC, but also the possible association of viral infection with xerostomia, hyposalivation and sialadenitis, showed that xerostomia was found in 23 (35.4%) out of 65 patients, hyposalivation in 13 (20%) out of 65 patients and sialadenitis (confirmed by histopathological evidence) in 31 (47.7%) out of 65 patients. Although xerostomia, hyposalivation, and sialadenitis are common in patients with CHC, the study did not confirm the association of HCV-RNA with these manifestations. However, an indirect role of HCV through autoimmune mechanisms in the pathogenesis of these diseases cannot be ruled out [24]. Joint manifestations were present in half (50%) of patients with TD, and in the group of patients without TD were present at 27 (32.92%) patients. The incidence of joint manifestations was documented in a study performed on a number of 1614 patients with CHC, in which the incidence of extrahepatic manifestations was evaluated. At least one extrahepatic manifestation was present in 1202 (74%) patients, and joint manifestations were present in 23% of patients [25]. Neurological manifestations (paresthesias) were present in both groups of patients, nine (64.28%) out of 14 patients with TD and only 11 (13.41%) out of 82 patients without TD. In patients with CHC, a wide variety of motor, sensitive, and mixed mono- and polyneuropathy have been described. The most common neurological manifestations were reported in patients with mixed cryoglobulinemia in the context of CHC, with a prevalence of 86% of cases [26]. Peripheral motor neuropathy was found in 30% of patients with mixed cryoglobulinemia in CHC infection. Such neuropathy is the consequence of nerve changes caused by ischemia, secondary to vasculitis of small vessels or necrotizing arteritis of medium vessels. It should be noted that neuropathy has been reported in patients with CHC, but without cryoglobulinemia, with a lower prevalence of only 9% [26]. Patients with CHC and TD had a higher incidence of superficial and deep thrombophlebitis compared to patients with CHC without TD, but statistically non-significant. A study based on the collection of data on the association of CHC and venous thromboembolism showed that the risk of thromboembolism is significant in patients with CHC compared to healthy subjects [27]. The mechanisms of the association of CHC with venous thromboembolism are unknown. The first theory is related to chronic inflammation through inflammatory cytokines, and the second theory incriminates the presence of autoantibodies. A recent study showed that up to 6% of patients with CHC have anti-phospholipid antibodies, antibodies that generate a state of hypercoagulability. High levels of CG are also associated with hyperviscosity, another factor that predisposes to thrombosis [28]. Hepatomegaly was present in the majority (71.42%) of patients with TD, and in approximately half (48.78%) of patients without TD. CHC is known to cause damage to the liver, such as inflammation, fibrosis, and cirrhosis of the liver, but also a number of extrahepatic manifestations that include the circulatory, immune and peripheral nervous system. The systemic immune response affects liver cells, causing cell death, inflammation, and hepatomegaly. The fiber-elastic sheath around the liver is relaxed due to inflammation causing pain and tenderness in the right-upper quadrant. Hepatomegaly was found in 60% of patients with CHC [29].
The mean values of the virological and hematological parameters
The analysis of the mean values of the virological and hematological parameters in patients with CHC with TD, compared to the mean values of the same parameters in patients without TD allowed the following observations. The mean viremia value was statistically significantly (p=0.001) lower in patients with CHC and TD, compared to patients with CHC without TD. A Taiwanese study showed that the pretreatment HCV-RNA level was significantly lower in patients who developed TD than in those who did not develop TD [30]. Patients with CHC and TD had a lower mean Hb value, statistically non-significant, compared to patients without TD. Although anemia associated to HCV infection is often related to treatment with Peginterferon and Ribavirin, nevertheless this hematological feature was found in treatment-naïve patients. Two thirds of the patients receiving dual antiviral therapy (IFN and Ribavirin) have associated anemia, and the dose reduction influences the virological response. Although Ribavirin is the main cause of hemolytic anemia, there have also been documented cases in patients treated with IFN-α. Pegylated IFN can cause bone marrow suppression which contributes to anemia [31]. The mean platelet count was statistically significantly lower in patients with TD compared to patients without TD. One retrospective study [32], that included 89 patients with CHC, 50 patients with chronic hepatitis, 18 patients with cirrhosis and 21 patients diagnosed with HCC, which aimed to evaluate the factors that predispose to thrombocytopenia showed that: thrombocytopenia appears and worsens with the progression of the disease, splenomegaly and plasma activity of the von Willebrand factor are correlated with thrombocytopenia, splenomegaly appears to be the main factor correlated with the decrease of platelets, although there are cases of thrombocytopenia without splenomegaly; the activity of the von Willebrand factor is inversely proportional to the platelet count. Soluble thrombomodulin, a marker of endothelial dysfunction, increases proportionally with the progression of liver fibrosis. Thrombomodulin is correlated proportionally with von Willebrand factor and inversely proportional to platelet count. The results of the study showed that endothelial dysfunction is also involved in the production of thrombocytopenia in patients with CHC [32]. The same behavior was present in the case of WBC count, their mean value in patients with CHC and TD was significantly lower compared to patients with CHC without TD. Leukopenia is common in patients with HCV infection who receive antiviral therapy and requires the reduction of the therapeutic doses of Peginterferon. In a study made in India, severe neutropenia (<500/μL) was present in more than 10% of patients treated with Peginterferon with or without Ribavirin. Another mechanism involved in the occurrence of leukopenia is represented by hypersplenism. Usually, drug induced leukopenia is not associated with a severe form of the disease and responds to therapeutic dose reduction as well as the use of WBC growth factors [33].
The mean values of systemic inflammation parameters
Analysis of mean values of systemic inflammation parameters showed similar behavior, ESR showed a significantly higher mean value in patients with CHC and TD, compared to patients without TD, while CRP showed a higher mean value but statistically non-significant at patients with CHC and TD, than patients with CHC without TD. In a study conducted in France, on two groups of patients, the first group of patients with confirmed HCV infection and rheumatic involvement, and the second group of patients with HCV infection, without rheumatic involvement, the mean value of ESR in the first group was 18.7±8.4 mm/1 h (6–40 mm/1 h), compared to the relative normal values ESR and CRP in the patients from the second group [34]. The mean values of serum CRP in the patients from the two groups were increased, the patients with TD having higher mean values (16.27±7.74 mg/dL) than the patients without TD (8.1±4.28 mg/dL). CRP values were analyzed in an American study of 5511 subjects in which about 2% had HCV infection. In the group of HCV-positive patients, the mean CRP value was 0.12±0.08 mg/dL, which was half of the mean value in HCV-negative subjects (mean value of 0.24±0.02 mg/dL) [35]. The mean value of IL-6 was increased in both patient groups, higher in patients with CHC and TD than those without TD, without statistically significant differences. IL-6 can be used for early detection of HCV infection, and those with high IL-6 levels seem to have a harder time responding to antiviral treatment [36]. TNF-α recorded statistically significant mean values (p=0.009) higher in patients with CHC and TD, compared to patients without TD. TNF-α is a particular cytokine involved in HCV infections, which activates fibroblasts and attracts them to the inflammatory site [36]. Antonelli et al. conducted a study that demonstrated a significant increase in IL-6 and TNF-α levels in patients with TD and mixed cryoglobulinemia who had chronic HCV infection [37]. Serum values of IL-6 and TNF-α were analyzed in several groups of patients, with the first group including 41 patients with chronic HCV infection and mixed cryoglobulinemia, the second group with 41 patients with HCV infection, mixed cryoglobulinemia and autoimmune thyroiditis, 20 patients with autoimmune thyroiditis and 41 control subjects. The results showed a significant increase in IL-6 in patients from the second group (an average value of 15.8 ng/L, with limits between 0.5 and 781 ng/L) compared to patients from the first group (a value average of 8.1 ng/L with limits between 0.7 and 651 ng/L) and compared to patients with autoimmune thyroiditis or control subjects. Also, the mean value of TNF-α was significantly higher in the subjects from the second group (11.2 ng/L), compared to the average value of the patients from the first group (9.9 ng/L) [37].
The mean values of biochemical parameters
Patients with CHC and TD had slightly higher mean TB values than patients with CHC without TD. An Egyptian study performed on a group of 20 patients diagnosed with HCV showed hyperbilirubinemia in 35% of the patients with CHC [38]. The average value of serum ALP activity was higher in patients with CHC and TD compared to those without TD. Another Egyptian study performed on a group of 154 patients diagnosed with chronic HCV infection showed a mean ALP value of 81.65±38.4 U/L in non-cirrhotic patients (n=124), while the patients with liver cirrhosis had the mean value of ALP statistically significantly (p=0.004) higher, 111±42.5 U/L [39]. The mean value of serum GGT levels was statistically significantly higher in the group of patients with CHC and TD, compared to the group of patients with CHC without TD. A Japanese study that compared the mean values of hematological, biochemical, and immunological parameters obtained in chronic HCV carriers with persistently normal ALT values and in patients with CHC, found statistically significant differences in platelet count, ALT, AST and GGT between chronic HCV carriers with persistent ALT levels and patients with CHC [40]. The enzymes of hepatic cytolytic syndrome showed a similar behavior, the average values of serum aminotransferases activities were increased but statistically non-significant in patients with CHC and TD, compared to patients with CHC without TD. An Egyptian study, which analyzed the biochemical parameters values in patients with chronic HCV infection compared to patients without CHC showed an increase of serum ALT and AST activities at 85% and 100% in patients with CHC [38]. As for regarding the PI, it registered lower values, but statistically non-significant in patients with TD compared to patients with CHC without TD. An Ukrainian study performed on several groups of patients with chronic HCV infection, but with different degrees of liver fibrosis at the biopsy, showed statistically significant differences between mean values of PT in patients with CHC without liver fibrosis, compared to patients with severe liver fibrosis and control patients, emphasizing that the PT is a faithful marker of liver failure and therefore a prognostic marker of chronic liver disease, which is why PT is included in the calculation of the prognostic indices of chronic liver disease [Child-Pugh and model for end-stage liver disease (MELD) score] [41]. A similar behavior was shown by serum ALB, with a lower mean value but statistically non-significant in patients with CHC and TD compared to patients with CHC without TD. In an Egyptian study performed on a group of 150 patients diagnosed with CHC, the mean value of serum ALB was statistically significantly higher (p=0.004) in patients with CHC (5.803±0.789 g%), compared with the mean value of serum ALB recorded in the 30 patients with liver cirrhosis related to HCV infection (4.1±0.42%) [39]. γ-Glob showed statistically significant mean values (p=0.02) higher in patients with CHC and TD, compared to the group of patients without TD.
The average values of the immunological parameters
The analysis of the average values of the immunological parameters in patients with CHC with TD, compared to the average values of the same parameters in patients without TD allowed the following discussions. Patients with CHC with TD had higher mean values of IgG, IgA, IgM, CIC concentrations and lower average values of Se-C3 compared to the mean values of these parameters in patients without TD. In a Kuwait study performed on a group of 100 patients with CHC, the mean values for serum IgG was 18.4 g/L (with limits between 4–39 g/L), serum IgA was 2.9 g/L (with limits between 0.09 and 8 g/L), and serum IgM was 1.6 g/L (with limits between 0.14 and 6.08 g/L) [42]. In a study conducted on 54 patients with CHC, 15 asymptomatic carriers of HCV and 54 healthy subjects, Chinese researchers have shown that 96.3% of patients with CHC had altered CIC values [43]. In an American study performed on a group of 12 patients with CHC, in which both the concentration of Se-C3 and the concentration of messenger RNA (mRNA) encoding the synthesis of Se-C3 from liver fragments obtained at LB were analyzed, low mean values for both Se-C3 and C3 mRNA levels were observed, suggesting that the third component of human complement (C3) plays a central role in innate immune function as its activation is required to trigger classical as well as alternative complement pathways [44]. In patients with CHC and TD, the prevalence of CG, RF, and ANA were higher than in patients with CHC without TD. In a study conducted in Italy on a group of 343 patients with CHC, CG were identified in 163 (47%) of patients, with a mean level of 173±142 mg/L, of which 80% were type III CG [45]. In a study performed on a group of 965 patients with CHC, 60 of them 6.3% had RF in the serum [46]. An international, multicenter study conducted simultaneously in three different countries (Sweden, England, and Italy) showed a different prevalence of ANA (4.4% vs 10.3% vs 10.3%) depending on the geographical location, but not depending on the duration of the infection, age of infection, current age, infection pathway, viral genotype, stage of fibrosis and the degree of liver inflammation [47].
The positive diagnosis of TD
The positive diagnosis of TD was established based on hormonal, immunological tests, and thyroid US. In the 96 patients studied, TD assessed by performing TSH and FT4, was represented by subclinical hypothyroidism (TSH >4.2 μIU/mL and normal FT4) in 14 (14.58%) patients. Worth mentioning is the fact that we did not encounter cases of hyperthyroidism or TRAb. However, there are studies in which hyperthyroidism has been reported in patients with CHC [48]. More studies have shown that hypothyroidism is induced by immune mechanisms rather than directly triggered by infection with HCV. This can include the destruction of tolerance to self-antigens and the subsequent activation of self-reactivity [11]. The correlation between HCV infection and anti-thyroid antibodies prevalence is well known. Anti-thyroid antibodies were identified in the serum of subjects infected with HCV before IFN-α therapy [49]. In a study of a group of 190 patients infected with HCV, 51 (26.8%) patients had TPOAb [50]. In our study, the incidence of antithyroid antibodies (TPOAb and TgAb) was 14.58% in the group of patients with CHC. TPOAb were present in 14 (100%) patients in the group of patients with TD, while the incidence of TgAb in the group of patients with CHC was 11.45%, 11 out of 96 patients. Among patients with TD, TgAb was present in 11 (78.57%) patients, while no patient in the group of patients without TD had TgAb. In a study conducted on a group of 72 people with CHC, of which 43 men and 29 women, no men were TgAb positive, but nine (31%) out of 29 women had high autoantibody titers [49]. In the evaluated subjects, the thyroid US had the role to highlight the thyroid morphological changes encountered in patients who presented hormonal or immunological changes. If the average thyroid volume in patients with CHC and TD was 12.8±1.4 mL, in patients with CHC without TD it was significantly higher, 15.6±1.5 mL (p<0.05). The US presentation of lymphocytic thyroiditis can differ, probably reflecting the stage and intensity of the patient’s condition. Reduced echogenicity was found in 54.54% of patients and diffuse inhomogeneous lobe structure in 45.45% of the patients with thyroid dysfunction. Some patients have a diffusely heterogeneous, hypoechoic gland [51]. US reveals diffuse or multifocal echo loss as a signal of many thyroiditis types. This echo reduction may be related to increased thyroid blood circulation, increased thyroid follicle cells, reduced colloid production, or infiltration of lymphocytes [52]. In the Doppler color and Strength tests, the blood supply of the whole gland increases [53]. As a result of this finding, as the echo increases, the echogenicity of the gland decreases. High blood vessels indicate diffuse thyroiditis [53]. Moreover, the thyroid is sometimes heterogeneous. Heterogeneity can maintain if chronic thyroiditis develops (fibrosis may occur inside the gland, resulting in a coarse echotexture) [54]. While unspecific, a diffuse hypoechoic pattern in a patient with lymphocytic thyroiditis is associated with diffuse lymphocyte and fibrosis replacement of the gland and is highly predictive of either the presence or potential development of hypothyroidism [54].
The average values of the liver histological parameters
The analysis of the average values of the liver histological parameters in patients with CHC with TD, compared to the average values of the same parameters in patients without TD allowed the following observations. In patients with CHC with TD the mean value HAI was significant higher (p=0.01), compared with patients without TD. Between the scores that define the hepatic necroinflammatory activity, only the scores of portal inflammation and that of confluent necrosis were statistically correlated (p=0.05) with the presence of TD in the patients with CHC studied. In another study, we showed that liver portal inflammation correlates with DLF, which leads us to believe that patients with significant portal inflammation will also develop significant fibrosis [55]. In addition, there are studies which have mentioned that in patients with CHC a high viral titer acts synergistically with steatosis, to accelerating the progression of liver disease [56]. In patients with HCC with TD, aspects of moderate and severe chronic hepatitis predominated, while in patients with HCC without TD, those who dominated were those with mild and moderate chronic hepatitis.
The average DHF score was statistically significantly higher (p=0.0002) in patients with CHC with TD, compared to patients with CHC without TD. Mild and moderate fibrosis were the pathological forms of fibrosis found especially in patients with CHC without TD, while severe and very severe fibrosis were the pathological forms of fibrosis found especially in patients with CHC and TD. The pathological aspect of liver cirrhosis was found only in those with TD (7.14%). A Taiwanese study showed that TD was more common in patients with higher liver fibrosis before IFN treatment [27, 57]. Inflammation and fibrosis evaluation represent key elements in investigating the liver diseases [58,59]. Thus, DHF, correctly assessed only by LB puncture, may represent a semi-quantitative histological parameter that allows the differentiation of the categories of patients with CHC with or without TD.
⧉ Conclusions
TD was present in 14.58% of cases of CHC not treated with IFN-α (naïve patients). The main clinical forms of manifestation of TD in the studied patients with CHC were autoimmune thyroiditis and subclinical hypothyroidism. The hepatic pathological aspects which define the necroinflammatory process, encountered at the morphopathological examination in patients with CHC with TD, are the same as in any active chronic hepatitis, the differences being represented only by the higher percentage of periportal and preseptal necrosis (piecemeal necrosis), but also by the higher score of portal inflammation. Severe hepatic fibrosis and the pathological appearance of liver cirrhosis have defined only cases of CHC with TD.
Conflict of interest
The authors declare that they have no conflict of interests.
Acknowledgments
Acknowledgments
Viorel Biciuşcă and Mihaela Popescu equally contributed to the manuscript.
References
- 1.Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Abu-Raddad LJ, Assadi R, Bhala N, Cowie B, Forouzanfour MH, Groeger J, Hanafiah KM, Jacobsen KH, James SL, MacLachlan J, Malekzadeh R, Martin NK, Mokdad AA, Mokdad AH, Murray CJL, Plass D, Rana S, Rein DB, Richardus JH, Sanabria J, Saylan M, Shahraz S, So S, Vlassov VV, Weiderpass E, Wiersma ST, Younis M, Yu C, El Sayed Zaki M, Cooke GS. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388(10049):1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popescu NL, Predescu OI, Badea O, Pirici I, Pantiş C, Busuioc CJ, Cotoi BV, Mogoantă L. The process of liver fibrosis in chronic hepatitis C - histological and immunohistochemical study. Rom J Morphol Embryol. 2018;59(4):1121–1126. [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Geneva: WHO; 2016. Global health sector strategy on viral hepatitis, 2016–2021. Towards ending viral hepatitis. Document No. WHO/HIV/2016.06.https://apps.who.int/iris/handle/10665/246177 [Google Scholar]
- 4.Bhamidimarri KR, Satapathy SK, Martin P. Hepatitis C virus and liver transplantation. Gastroenterol Hepatol (N Y) 2017;13(4):214–220. [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). Hepatitis C questions and answers for the public. CDC, Viral Hepatitis, Hepatitis C Information. https://www.cdc.gov/hepatitis/hcv/cfaq.htm.
- 6.Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3(2):47–52. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin North Am. 2015;44(4):717–734. doi: 10.1016/j.gtc.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keshav S, Trivedi P. Viral hepatitis. In: Davey P, Sprigings D, editors. Diagnosis and treatment in internal medicine. Oxford UK: Oxford University Press; 2018. pp. 700–708. [Google Scholar]
- 9.Sanai FM, Keeffe EB. Liver biopsy for histological assessment: the case against. Saudi J Gastroenterol. 2010;16(2):124–132. doi: 10.4103/1319-3767.61244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrescu F, Petrescu OI, Taisescu CI, Comănescu MV, Forţofoiu MC, Predescu IO, Roşu AF, Gheonea C, Biciuşcă V. Histopathological aspects described in patients with chronic hepatitis C. Rom J Morphol Embryol. 2015;56(2):439–444. [PubMed] [Google Scholar]
- 11.Shen Y, Wang XL, Xie JP, Shao JG, Lu YH, Zhang S, Qin G. Thyroid disturbance in patients with chronic hepatitis C infection: a systematic review and meta-analysis. J Gastrointestin Liver Dis. 2016;25(2):227–234. doi: 10.15403/jgld.2014.1121.252.chc. [DOI] [PubMed] [Google Scholar]
- 12.Ţieranu E, Donoiu I, Istrătoaie O, Ţieranu LM, Gheonea DI, Ciurea T, Ghenea AE, Ungureanu A. Electric and hemodynamic effects of beta-blockers in patients with liver cirrhosis. Farmacia. 2020;68(5):843–848. [Google Scholar]
- 13.Ţieranu EN, Donoiu I, Istrătoaie O, Găman AE, Ţieranu ML, Ţieranu CG, Gheonea DI, Ciurea T. Rare case of single coronary artery in a patient with liver cirrhosis. Rom J Morphol Embryol. 2017;58(4):1505–1508. [PubMed] [Google Scholar]
- 14.Behzadifar M, Gorji HA, Rezapour A, Behzadifar M, Bragazzi NL. The role of insurance providers in supporting treatment and management of hepatitis C patients. BMC Health Serv Res. 2019;19(1):25–25. doi: 10.1186/s12913-019-3869-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferri C, Colaci M, Fallahi P, Ferrari SM, Antonelli A, Giuggioli D. Thyroid involvement in hepatitis C virus-infected patients with/without mixed cryoglobulinemia. Front Endocrinol (Lausanne) 2017;8:159–159. doi: 10.3389/fendo.2017.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med. 1996;335(2):99–107. doi: 10.1056/NEJM199607113350206. [DOI] [PubMed] [Google Scholar]
- 17.Pateron D, Hartmann DJ, Duclos-Vallée JC, Jouanolle H, Beaugrand M. Latent autoimmune thyroid disease in patients with chronic HCV hepatitis. J Hepatol. 1993;17(3):417–419. doi: 10.1016/s0168-8278(05)80228-4. [DOI] [PubMed] [Google Scholar]
- 18.Ploix C, Verber S, Chevallier-Queyron P, Ritter J, Bousset G, Monier JC, Fabien N. Hepatitis C virus infection is frequently associated with high titers of anti-thyroid antibodies. Int J Immunopathol Pharmacol. 1999;12(3):121–126. [PubMed] [Google Scholar]
- 19.Marazuela M, García-Buey L, González-Fernández B, García-Monzón C, Arranz A, Borque MJ, Moreno-Otero R. Thyroid autoimmune disorders in patients with chronic hepatitis C before and during interferon-alpha therapy. Clin Endocrinol (Oxf) 1996;44(6):635–642. doi: 10.1046/j.1365-2265.1996.751768.x. [DOI] [PubMed] [Google Scholar]
- 20.Tang L, Marcell L, Kottilil S. Systemic manifestations of hepatitis C infection. Infect Agent Cancer. 2016;11:29–29. doi: 10.1186/s13027-016-0076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poynard T, Cacoub P, Ratziu V, Myers RP, Dezailles MH, Mercadier A, Ghillani P, Charlotte F, Piette JC, Moussalli J, Multivirc Group Fatigue in patients with chronic hepatitis C. J Viral Hepat. 2002;9(4):295–303. doi: 10.1046/j.1365-2893.2002.00364.x. [DOI] [PubMed] [Google Scholar]
- 22.Dupin N, Chosidow O, Lunel F, Cacoub P, Musset L, Cresta P, Frangeul L, Piette JC, Godeau P, Opolon P, Frances C. Essential mixed cryoglobulinemia. A comparative study of dermatologic manifestations in patients infected or noninfected with hepatitis C virus. Arch Dermatol. 1995;131(10):1124–1127. doi: 10.1001/archderm.131.10.1124. [DOI] [PubMed] [Google Scholar]
- 23.Modi AA, Liang TJ. Hepatitis C: a clinical review. Oral Dis. 2008;14(1):10–14. doi: 10.1111/j.1601-0825.2007.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grossmann SMC, Teixeira R, Oliveira GC, Gleber-Netto FO, Araújo FMG, Araújo FM, Carmo MAV. Xerostomia, hyposalivation and sialadenitis in patients with chronic hepatitis C are not associated with the detection of HCV RNA in saliva or salivary glands. J Clin Pathol. 2010;63(11):1002–1007. doi: 10.1136/jcp.2010.080036. [DOI] [PubMed] [Google Scholar]
- 25.Cacoub P, Poynard T, Ghillani P, Charlotte F, Olivi M, Piette JC, Opolon P. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment virus C. Arthritis Rheum. 1999;42(10):2204–2212. doi: 10.1002/1529-0131(199910)42:10<2204::AID-ANR24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 26.Adinolfi LE, Nevola R, Lus G, Restivo L, Guerrera B, Romano C, Zampino R, Rinaldi L, Sellitto A, Giordano M, Marrone A. Chronic hepatitis C virus infection and neurological and psychiatric disorders: an overview. World J Gastroenterol. 2015;21(8):2269–2280. doi: 10.3748/wjg.v21.i8.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Ungprasert P. Hepatitis C virus infection and risk of venous thromboembolism: a systematic review and meta-analysis. Ann Hepatol. 2017;16(4):514–520. doi: 10.5604/01.3001.0010.0279. [DOI] [PubMed] [Google Scholar]
- 28.Ramos-Casals M, Muñoz S, Medina F, Jara LJ, Rosas J, Calvo-Alen J, Brito-Zerón P, Forns X, Sánchez-Tapias JM, HISPAMEC Study Group Systemic autoimmune diseases in patients with hepatitis C virus infection: characterization of 1020 cases (The HISPAMEC Registry) J Rheumatol. 2009;36(7):1442–1448. doi: 10.3899/jrheum.080874. [DOI] [PubMed] [Google Scholar]
- 29.Pierce JM. HepatitisC.net. Health Union; 2015. Chronic phase of hepatitis C.https://hepatitisc.net/living/chronic-phase [Google Scholar]
- 30.Hsieh MC, Yu ML, Chuang WL, Shin SJ, Dai CY, Chen SC, Lin ZY, Hsieh MY, Liu JF, Wang LY, Chang WY. Virologic factors related to interferon-alpha-induced thyroid dysfunction in patients with chronic hepatitis C. Eur J Endocrinol. 2000;142(5):431–437. doi: 10.1530/eje.0.1420431. [DOI] [PubMed] [Google Scholar]
- 31.Kedia S, Bhatt VR, Rajan SK, Tandra PK, El Behery RA, Akhtari M. Benign and malignant hematological manifestations of chronic hepatitis C virus infection. Int J Prev Med. 2014;5(Suppl 3):S179–S192. [PMC free article] [PubMed] [Google Scholar]
- 32.Osada M, Kaneko M, Sakamoto M, Endoh M, Takigawa K, Suzuki-Inoue K, Inoue O, Satoh K, Enomoto N, Yatomi Y, Ozaki Y. Causes of thrombocytopenia in chronic hepatitis C viral infection. Clin Appl Thromb Hemost. 2012;18(3):272–280. doi: 10.1177/1076029611429124. [DOI] [PubMed] [Google Scholar]
- 33.Kedia S, Goyal R, Mangla V, Kumar A, S S, Das P, Pal S, Sahni P, Acharya SK. Splenectomy in cirrhosis with hypersplenism: improvement in cytopenias, Child’s status and institution of specific treatment for hepatitis C with success. Ann Hepatol. 2012;11(6):921–929. [PubMed] [Google Scholar]
- 34.Nissen MJ, Fontanges E, Allam Y, Zoulim F, Trépo C, Miossec P. Rheumatological manifestations of hepatitis C: incidence in a rheumatology and non-rheumatology setting and the effect of methotrexate and interferon. Rheumatology (Oxford) 2005;44(8):10161020–10161020. doi: 10.1093/rheumatology/keh668. [DOI] [PubMed] [Google Scholar]
- 35.Bhuiyan AR, Mitra AK, Ogungbe O, Kabir N. Association of HCV infection with C-reactive protein: National Health and Nutrition Examination Survey (NHANES), 2009–2010. Diseases. 2019;7(1):25–25. doi: 10.3390/diseases7010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boldeanu MV, Siloşi I, Bărbulescu AL, Sandu RE, Geormăneanu C, Pădureanu V, Popescu-Drigă MV, Poenariu IS, Siloşi CA, Ungureanu AM, Dijmărescu AL, Boldeanu L. Host immune response in chronic hepatitis C infection: involvement of cytokines and inflammasomes. Rom J Morphol Embryol. 2020;61(1):33–43. doi: 10.47162/RJME.61.1.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antonelli A, Ferri C, Ferrari SM, Di Domenicantonio A, Ferrari P, Pupilli C, Nicolini A, Zignego AL, Marchi S, Fallahi P. The presence of autoimmune thyroiditis in mixed cryoglobulinemia patients is associated with high levels of circulating interleukin-6, but not of tumor necrosis factor-alpha. Clin Exp Rheumatol. 2011;29(1 Suppl 64):S17–S22. [PubMed] [Google Scholar]
- 38.Wahib AA, Seif El Nasr MS, Mangoud AM, El Shazly AM, Morsy ATA. The liver function profile in PCR-RNA Egyptian HCV-patients and normal controls. J Egypt Soc Parasitol. 2005;35(2):451–466. [PubMed] [Google Scholar]
- 39.Fouad SA, Esmat S, Omran D, Rashid L, Kobaisi MH. Non-invasive assessment of hepatic fibrosis in Egyptian patients with chronic hepatitis C virus infection. World J Gastroenterol. 2012;18(23):2988–2994. doi: 10.3748/wjg.v18.i23.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imakiire K, Uto H, Sato Y, Sasaki F, Mawatari S, Ido A, Shimoda K, Hayashi K, Stuver SO, Ito Y, Okanoue T, Tsubouchi H. Difference in serum complement component C4a levels between hepatitis C virus carriers with persistently normal alanine aminotransferase levels or chronic hepatitis C. Mol Med Rep. 2012;6(2):259–264. doi: 10.3892/mmr.2012.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sajjadieh M, Viunytska L. Prothrombin time in patients with and without fibrotic chronic liver disease. Internet J Pathol. 2008;8(1):1–5. https://ispub.com/IJPA/8/1/12789 [Google Scholar]
- 42.Al-Shemmari SH, Siddique I, Hassan F, Nkansa-Dwamena D, El-Naga HA, Ameen R. Monoclonal gammopathy among patients with chronic hepatitis C infection. Med Princ Pract. 2004;13(2):88–90. doi: 10.1159/000075635. [DOI] [PubMed] [Google Scholar]
- 43.Tsai JF, Jeng JE, Chang WY, Ho MS, Lin ZY, Tsai JH. Circulating immune complexes in chronic hepatitis C. J Med Virol. 1995;46(1):12–17. doi: 10.1002/jmv.1890460104. [DOI] [PubMed] [Google Scholar]
- 44.Mazumdar B, Kim H, Meyer K, Bose SK, Di Bisceglie AM, Ray RB, Ray R. Hepatitis C virus proteins inhibit C3 complement production. J Virol. 2012;86(4):2221–2228. doi: 10.1128/JVI.06577-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viganò M, Lampertico P, Rumi MG, Folli C, Maggioni L, Morabito A, Del Ninno E, Cicardi M, Colombo M. Natural history and clinical impact of cryoglobulins in chronic hepatitis C: 10-year prospective study of 343 patients. Gastroenterology. 2007;133(3):835–842. doi: 10.1053/j.gastro.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 46.Antonescu C, Mayerat C, Mantegani A, Frei PC, Spertini F, Tissot JD. Hepatitis C virus (HCV) infection: serum rheumatoid factor activity and HCV genotype correlate with cryoglobulin clonality. Blood. 1998;92(9):3486–3487. [PubMed] [Google Scholar]
- 47.Yee LJ, Kelleher P, Goldin RD, Marshall S, Thomas HC, Alberti A, Chiaramonte M, Braconier JH, Hall AJ, Thursz MR. Antinuclear antibodies (ANA) in chronic hepatitis C virus infection: correlates of positivity and clinical relevance. J Viral Hepat. 2004;11(5):459–464. doi: 10.1111/j.1365-2893.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 48.Antonelli A, Ferri C, Pampana A, Fallahi P, Nesti C, Pasquini M, Marchi S, Ferrannini E. Thyroid disorders in chronic hepatitis C. Am J Med. 2004;117(1):10–13. doi: 10.1016/j.amjmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 49.Tran A, Quaranta JF, Benzaken S, Thiers V, Chau HT, Hastier P, Regnier D, Dreyfus G, Pradier C, Sadoul JL, Hebutern X, Rampa P. High prevalence of thyroid autoantibodies in a prospective series of patients with chronic hepatitis C before interferon therapy. Hepatology. 1993;18(2):253–257. [PubMed] [Google Scholar]
- 50.Sheikh M, Doi SAR, Sinan T, Al-Shoumer KAS. Technical observations on the assessment of thyroid volume by palpation and ultrasonography. J Ultrasound Med. 2004;23(2):261–266. doi: 10.7863/jum.2004.23.2.261. [DOI] [PubMed] [Google Scholar]
- 51.Langer JE, Khan A, Nisenbaum HL, Baloch ZW, Horii SC, Coleman BG, Mandel SJ. Sonographic appearance of focal thyroiditis. AJR Am J Roentgenol. 2001;176(3):751–754. doi: 10.2214/ajr.176.3.1760751. [DOI] [PubMed] [Google Scholar]
- 52.Singhal AA. Imaging of the thyroid. Ultrasound in diffuse thyroid disorders autoimmune thyroiditis. In: Kapre ML, editor. Thyroid surgery: principles and practice. Boca Raton USA: CRC Press; 2020. pp. 120–142. [Google Scholar]
- 53.Shah C, Johnson PT. SonoWorld; 2021. Diffuse thyroiditis.https://sonoworld.com/CaseDetails/Diffuse_thyroiditis.aspx?CaseId=50 [Google Scholar]
- 54.Marcocci C, Vitti P, Cetani F, Catalano F, Concetti R, Pinchera A. Thyroid ultrasonography helps to identify patients with diffuse lymphocytic thyroiditis who are prone to develop hypothyroidism. J Clin Endocrinol Metab. 1991;72(1):209–213. doi: 10.1210/jcem-72-1-209. [DOI] [PubMed] [Google Scholar]
- 55.Petrescu IO, Biciuşcă V, Taisescu CI, Alexandru DO, Taisescu O, Comănescu MV, Petrescu F, Popescu IAS, Traşcă DM, Forţofoiu MC, Siloşi CA, Forţofoiu M. Histological factors that predict the liver fibrosis in patients with chronic hepatitis C. Rom J Morphol Embryol. 2016;57(2 Suppl):759–765. [PubMed] [Google Scholar]
- 56.Adinolfi LE, Utili R, Andreana A, Tripodi MF, Marracino M, Gambardella M, Giordano M, Ruggiero G. Serum HCV RNA levels correlate with histological liver damage and concur with steatosis in progression of chronic hepatitis C. Dig Dis Sci. 2001;46(8):1677–1683. doi: 10.1023/a:1010697319589. [DOI] [PubMed] [Google Scholar]
- 57.Şerbănescu MS, Pleşea IE. A hardware approach for histological and histopathological digital image stain normalization. Rom J Morphol Embryol. 2015;56(2 Suppl):735–741. [PubMed] [Google Scholar]
- 58.Popescu M, Popescu IAS, Stanciu M, Cazacu SM, Ianoşi NG, Comănescu MV, Singer CE, Neagoe CD. Non-alcoholic fatty liver disease – clinical and histopathological aspects. Rom J Morphol Embryol. 2016;57(4):1295–1302. [PubMed] [Google Scholar]
- 59.Găman AE, Ungureanu AM, Turculeanu A, Gheonea DI, Drocaş AI, Mitroi G, Dobriţoiu M, Comănescu MV, Stănculescu AD, Cioboată R, Jieanu CF, Tomescu PI. The impact of liver steatosis on early and sustained treatment response in chronic hepatitis C patients. Rom J Morphol Embryol. 2017;58(1):107–113. [PubMed] [Google Scholar]