Abstract
The incidence of cholecystectomy in children has increased considerably since the early 1990s. Management of gallbladder disease in children must include an awareness of choledocholithiasis treatment strategies. Both endoscopic retrograde cholangiopancreatography (ERCP) and common bile duct exploration (open or laparoscopic) are accepted management techniques for choledocholithiasis. Laparoscopic cholecystectomy with preoperative or postoperative ERCP is at least a two-procedure process while cholecystectomy with laparoscopic common bile duct exploration (LCBDE) can provide definitive treatment in a single procedure under one anesthetic. Despite this, the trend over the last decade continues towards less LCBDE utilization in favor of ERCP. This trend has resulted in decreased familiarity with LCBDE by adult and pediatric surgeons and their trainees. Access to the necessary tools and education on the technical aspects can allow for successful single-stage treatment of choledocholithiasis by surgeons during laparoscopic cholecystectomy. This may include a pre-defined stepwise algorithm and understanding of all the equipment and resources necessary to perform a LCBDE. Ultimately, increased understanding of the equipment and procedural steps necessary for LCBDE will result in widened adoption of the technique and thus confer advantages to the patient such as decreased length of stay and fewer required anesthetics.
Keywords: Choledocholithiasis, laparoscopy, cholecystectomy, common bile duct (CBD)
Introduction
The incidence of cholecystectomy in the pediatric population has increased considerably since the early 1990s. This is likely due to increased recognition of gallbladder pathology from widespread use of ultrasonography, a change in physician perception of the disease, and a rise in pediatric obesity (1-3). Comprehensive management of gallbladder disease in children and adolescents must include an awareness of choledocholithiasis treatment strategies. Both endoscopic retrograde cholangiopancreatography (ERCP) and common bile duct (CBD) exploration (open or laparoscopic) are accepted management techniques for choledocholithiasis (4-7). Laparoscopic cholecystectomy (LC) with preoperative or postoperative ERCP is at least a two-procedure process while cholecystectomy with laparoscopic common bile duct exploration (LCBDE) can provide definitive treatment in a single procedure under one anesthetic.
The American Society for Gastrointestinal Endoscopy (ASGE) guidelines advocate for limiting the use of ERCP in suspected choledocholithiasis to only those who are at high risk for CBD stones (8). However, the ASGE criteria (including laboratory values and CBD stone visualization on right upper quadrant (RUQ) ultrasound) has proven unreliable in identifying the patients with CBD stones at time of intervention (9). Thus, if CBD stones are not found to be present during intervention, many patients will have been unnecessarily exposed to the procedural risks associated with ERCP, such as anesthetic harms and pancreatitis (10). Further, the incidence of retained or newly passed CBD stones diagnosed on intraoperative cholangiogram (IOC) during LC after preoperative ERCP is as high as 13% (11). If these newly discovered stones cannot be extracted, then a third procedure with repeat ERCP may be indicated and add increased cost, length of stay (LOS), and anesthetic risk to the patient. As such, there is a clear need for a surgical option that eliminates the need for additional procedures. Randomized control trials comparing ERCP and LC to LCBDE and LC have shown that LCBDE and LC have equal efficacy and safety profiles with an associated decrease in hospital LOS and costs (12-21). Despite this, the trend over the last decade continues towards less LCBDE utilization in favor of ERCP (12). This trend has resulted in decreased familiarity with LCBDE by adult and pediatric surgeons and their trainees. Access to the necessary tools and education on the technical aspects can allow for successful single-stage treatment of choledocholithiasis by surgeons during LC.
LCBDE in children
While management of choledocholithiasis in children with ERCP has been shown to be safe and effective, LCBDEs have also been described (5-7,22). A few studies have specifically compared these two modalities in the typical pediatric surgery population. A study of LC + LCBDE compared to LC + ERCP in 42 children over a one-year period revealed similar operative time for both cohorts with longer LOS (15.7 vs. 6.6 days, P=0.02) and hospital cost ($18,132 vs. $12,735, P<0.01) in those who underwent cholecystectomy with ERCP. There was also no significant difference in morbidity (17). A recent study from the University of Michigan reviewed the management of 81 children with suspected choledocholithiasis. It was found that those patients who underwent primary LC first rather than primary ERCP had decreased LOS. Only a third of patients who first underwent primary cholecystectomy had positive IOC necessitating further intervention (23). Several other studies describe LCBDE in children. However, data is limited compared to the adult population (6,17,24).
Indications for LCBDE
LCBDE is indicated in clinical scenarios in which CBD stones are suspected from preoperative imaging [magnetic resonance cholangiopancreatography (MRCP), ultrasound or CT] or elevated liver function tests (LFTs) and are subsequently confirmed on intraoperative imaging (IOC or ultrasound) (4,25). LCBDE may be particularly advantageous in situations in which ERCP is not easily feasible, such as in patients that have previously had enteric bypasses that prevent endoscopic intervention.
In some instances, sphincter spasm and/or sludge can masquerade as a distal obstruction without a discernible filling defect on IOC. The possibility that the obstruction is more functional than mechanical makes LCBDE preferable due to increased likelihood of clearing the duct with intravenous glucagon administration, duct flushing, or dilation of the sphincter with a balloon. This avoids the ERCP and possible sphincterotomy that would follow. This notion of sphincter spasm at the time of IOC from a functional obstruction that leads to further endoscopic intervention is noteworthy, especially for pediatric providers that place extra emphasis on limiting additional anesthesia events and radiation exposure from fluoroscopy (26). In a previously described cohort of 48 children with suspected choledocholithiasis, ten of 14 had negative findings on preoperative ERCP and twenty-eight of the 34 who had a cholecystectomy first had a negative IOC (22). This discrepancy may be attributed to this spasm/sludge phenomenon causing sphincter spasm or the spontaneous passage of obstructing stones.
Consideration for radiation exposure
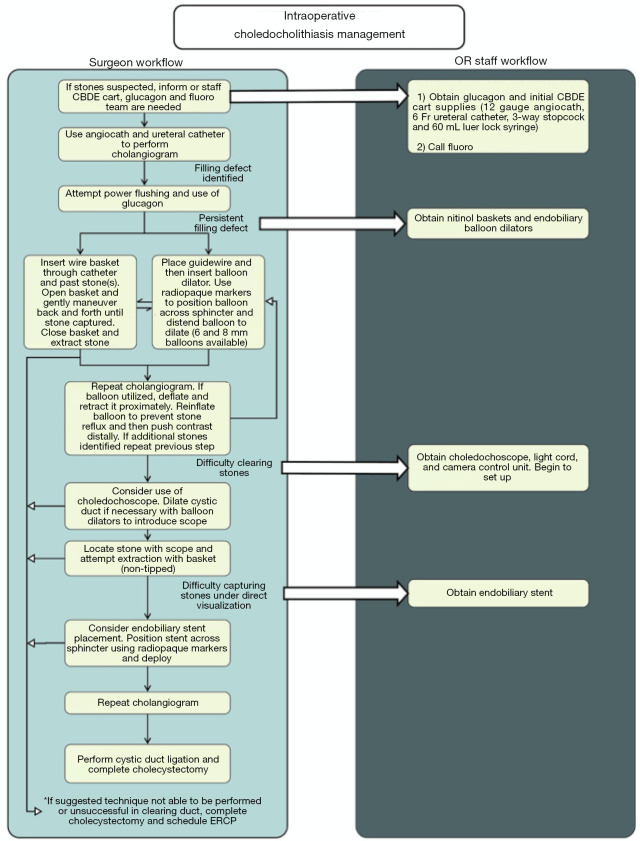
Exposure to radiation with fluoroscopy occurs in both ERCP and LCBDE. Due to the perceived cancer risks associated with medical radiation exposure, special consideration should be given to the use of fluoroscopy in pediatric patients. Children are both more sensitive to the ionizing effect and typically have a longer life span over which they can develop malignancies (27-30). Determining the dose associated with a specific procedure relies on several different factors including exposure settings, length of procedure, age and weight of the patient and scaling factors (28). Often, fluoroscopy time is the chief metric reported. Yet, it is an indirect measure of radiation dose and as such is a poor indicator of exposure (29). Despite the inability to quantify the absorbed dose, surgeons should always utilize dose minimization techniques. Specifically for LCBDE, this principle includes operating the C-arm at the lowest dose setting that still allow for sufficient images, use of spot films with small adjustments rather than continuous fluoroscopy, minimizing the distance between the patient and the detector, and utilizing digital zoom rather than electronic magnification if needed. The use of lead shielding of the patient’s pelvis is also essential and should be part of the nursing portion of the checklist (31,32). Ideally, this protective lead apron would be positioned prior to draping the patient to minimize additional time and disruption to workflow. In comparing the techniques employed for LCBDE, the use of baskets to retrieve stones is likely the most fluoroscopic intensive maneuver as it requires frequent feedback for stone capture. When using any of the available LCBDE techniques, it is important to note that efficiency will decrease fluoroscopy time. One tactic to improve overall operative efficiency is employing a stepwise algorithm. Completing a LCBDE in a defined standardized fashion can improve workflow for the entire operative team (Figure 1). As with any surgical technique, increased experience leads to improved performance and efficiency which will in turn minimize radiation exposure.
Figure 1.
Intraoperative choledocholithiasis management flowsheet. A systematic stepwise algorithm for increased efficiency in the operating room. ERCP, endoscopic retrograde cholangiopancreatography.
Obstacles to adoption of LCBDE
The declining use of LCBDE arises from multiple factors including a paucity of surgical LCBDE training, lack of necessary tools, and the convenience of widely available ERCP (12,20,33). One of the most important factors in increasing adoption is to increase the simplicity of the intervention and minimizing any friction in the process-especially intraoperatively. This efficiency involves a logical, stepwise approach that facilitates the next steps (i.e., being able to perform basket retrieval through same catheter or access platform that the cholangiogram is injected through) and gives preference to employing the easiest methods/cheapest tools early over more expensive supplies and less readily available equipment. This efficiency also involves creating a central location or cart in the operating room complex to store all of the supplies. Making LCBDE easy and “pain free” to perform will ultimately lower the threshold to intervene and drive adoption among surgeons. The lower the threshold, the more attempts surgeons will make and the more experience a surgeon will gain. The end result is increased success with LCBDE and better outcomes for patients (34,35). To this end, training models have been created and validated which can accelerate skill acquisition and result in cost savings (36-40).
Skills necessary to perform LCBDE
In order to perform a LCBDE, a surgeon must:
Have access to all the necessary tools and equipment and an understanding of how to use them;
Be able to adeptly use laparoscopic instruments and guidewires;
Perform an IOC and interpret the produced fluoroscopic image;
-
Understand and be able to perform some or all of the different methods of stone clearance:
“Power flushing” of the CBD;
Fluoroscopic Nitinol Basket deployment and stone retrieval;
“Crush and Flush” of stones using the Nitinol Basket;
Balloon dilation of sphincter with flushing;
Choledochoscopy for Nitinol Basket or Laser lithotripsy deployment;
Endobiliary stent placement.
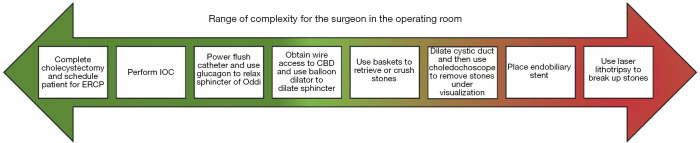
If LCBDE is not technically feasible, alternative options such as open CBD exploration or ERCP may be utilized. Figure 2 displays these interventions on a continuum from easiest to most difficult to perform by the surgeon in the operating room.
Figure 2.
LCBDE complexity progression. Interventions on continuum from easiest to most difficult to perform by the surgeon in the operating room. ERCP, endoscopic retrograde cholangiopancreatography; IOC, intraoperative cholangiogram; CBD, common bile duct; LCBDE, laparoscopic common bile duct exploration.
Technique for performing LCBDE
Successful completion of LCBDEs requires coordination between the surgical team, operating room staff and fluoroscopy. The first step in LCBDE is ensuring the operating room is able to accommodate a C-arm for fluoroscopy and confirming that the operating room staff will have all the necessary supplies. A complete list of these supplies can be found in Table 1. Optimal set up also includes the capability of displaying the fluoroscopy images on the operating room screens and orienting the equipment and monitors (even a serial connection to one of the overhead monitors) to maximize the ergonomics of surgeon and intervention (Figure 3) (41,42). This is a seemingly minor point, but efficiency and success are predicated on creating the most favorable working conditions possible. Additionally, consideration must be given to where the second laparoscopic tower would be located for choledochoscopy, if required.
Table 1. Complete list of supplies needed to perform a LBCDE.
| Equipment necessary to complete LCBDE | Details |
|---|---|
| Intraoperative cholangiogram | |
| Fluoroscopy | – |
| 12-Fr Angiocath | – |
| 6–8-Fr open ended ureteral catheter | – |
| Tuohy Borst Connector | – |
| 60-mL Luer Lock Syringes | One containing saline and one with 50/50 saline-contrast mix |
| 3-way stopcock | – |
| Extension Tubing | – |
| Glucagon, 1 to 2 mg | Given intravenously by anesthesia provider to relax sphincter of Oddi |
| Clips or Olsen-Reddick Clamp | To secure ureteral catheter at ductotomy |
| Stone capture with Nitinol Baskets | |
| Fluoroscopy and IOC setup | – |
| Stone retrieval baskets | Tipped baskets are more easily visualized on fluoroscopy but can damage the choledochoscope |
| Balloon dilation of sphincter of Oddi | |
| 0.035" floppy-tipped guidewire ×150 cm | Balloons are advanced into CBD via Seldinger technique |
| Balloon dilators | 6 or 8 Fr ×75 cm; 40, 80 and 100 mm are common lengths of the balloons |
| Rotational inflation device | – |
| Choledochoscopy | |
| Flexible choledochoscope | With a working channel in order to introduce stone retrieval baskets |
| 0.035" floppy-tipped guidewire ×150 cm | The scope can be advanced into CBD via Seldinger technique |
| Second video monitor tower with a light source | Allows for simultaneous endoscopic and laparoscopic views during choledochoscopy |
| Pressurized bag of saline with tubing | To allow for visualization during choledochoscopy in the same fashion as cystoscopy |
| Atraumatic laparoscopic grasper | For intra-abdominal manipulation of the choledochoscope |
| Nitinol stone retrieval baskets | Advanced through the working channel |
| Balloon dilators | Used to dilate cystic duct if necessary to accommodate scope |
| Additional optional equipment | |
| Laser lithotripter | To fragment stones |
| Endobiliary stent | May be placed over wire across sphincter of Oddi if obstruction not relieved by other measures. ERCP then would be need to be scheduled postoperatively |
| Laparoscopic ligating loop | To close cystic duct after manipulation/exploration |
LCBDE, laparoscopic common bile duct exploration; CBD, common bile duct; ERCP, endoscopic retrograde cholangiopancreatography; IOC, intraoperative cholangiogram.
Figure 3.
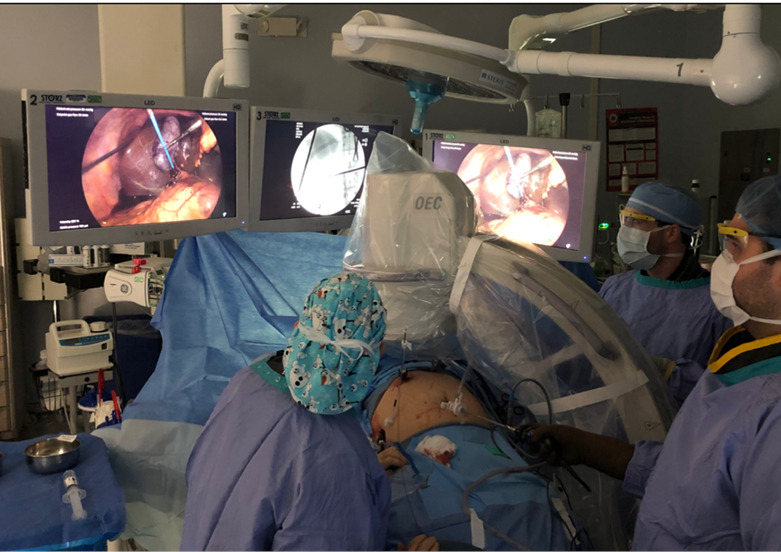
Optimizing efficiency in the operating room when performing LCBDE. Operating room set up during LCBDE that maximizes ergonomics with fluoroscopy images displayed on OR screens. LCBDE, laparoscopic common bile duct exploration; OR, operating room.
After the critical view of safety has been obtained, proceeding with CBD exploration requires transabdominal placement of 12-gauge angiocath or other style of introducer to accommodate the cholangiogram catheter (Figure 4). Alternatively, if an additional port is placed, laparoscopic cholangiogram forceps can be used to both introduce the cholangiogram catheter and secure it in place. An additional 5th port may also be particularly advantageous because strategic positioning of the port will allow for a more favorable angle of insertion and direct instrumentation of the ductotomy. The trocar can guide tools such as the choledochoscope into the cystic duct with minimal to no manipulation.
Figure 4.
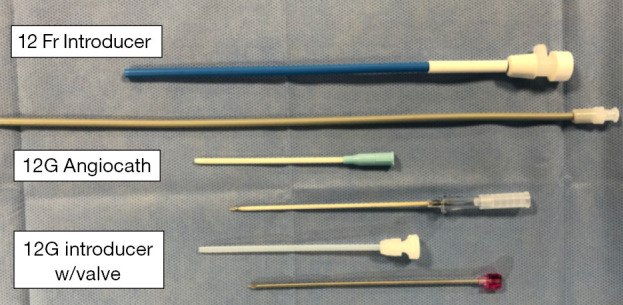
Examples of various introducers that can be utilized during LBCDE. LCBDE, laparoscopic common bile duct exploration.
A ductotomy is then made in the cystic duct with laparoscopic scissors. A transcholedochal approach can also be used, but requires intracorporeal suturing of the CBD for closure of the ductotomy. In contrast, the cystic duct stump can be closed with clips or an endoloop. Therefore, the transcystic approach may be technically less difficult to perform in comparison to the transcholedochal approach (43-45).
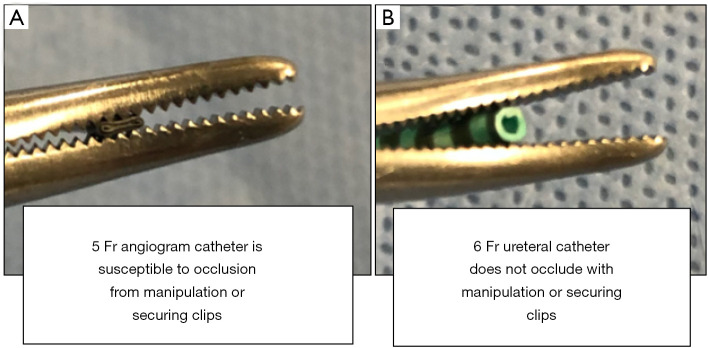
After the cholangiogram catheter is positioned into the duct, it must be secured in place by a clip. Specifically, use of a 6–8-Fr open-ended ureteral catheter to perform the cholangiogram rather than a 5-Fr angiogram catheter prevents occlusion with manipulation or securing clips (Figure 5). The ureteral/angiogram catheter should be connected to a setup that allows for flushing of contrast and saline to perform an IOC. The setup depicted in Figure 6 using a Tuohy-Borst connector, extension tubing with a 3-way stopcock and attached syringes containing both a 50/50 saline-contrast mix and saline allows for the IOC to be performed in addition to flushing and threading of a 0.035” guidewire through one system. It should be noted that backing the ureteral catheter out of the aperture on the back end of Tuohy-Borst connector is necessary to successfully thread the wire in this setup (Figure 7). These small alterations in the setup will contribute significantly to procedural efficiency.
Figure 5.
Angiogram catheters are susceptible to occlusion with manipulation or use of clips when performing an intraoperative cholangiogram. By contrast, a ureteral catheter does not occlude with manipulation or securing clips, thus allowing for free flow of contrast and saline. This will also allow for various other interventions to be utilized through the ureteral catheter such as the threading of a wire or use of a basket.
Figure 6.
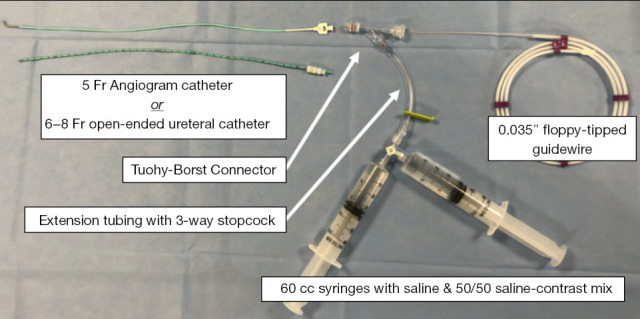
Initial setup for performing an intraoperative cholangiogram. Use of a Tuohy-Borst connector, extension tubing with a 3-way stopcock and syringes with saline and 50/50 saline-contrast mix along with either a 5-Fr angiogram catheter or 6–8-Fr open-ended ureteral catheter allows for flushing and subsequent threading of a guidewire without any alteration in setup or additional steps.
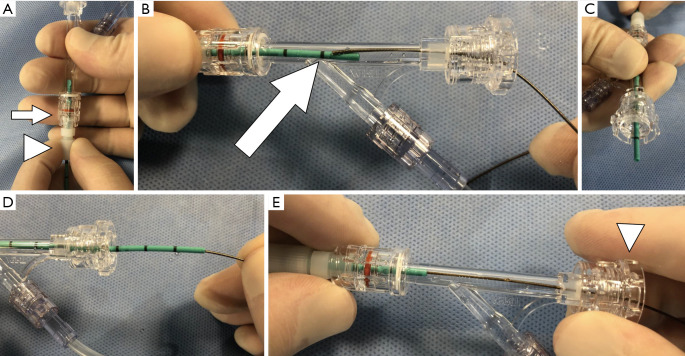
Figure 7.
Wire management through the Tuohy-Borst connector. After ureteral catheter is secured to the Tuohy-Borst connector (A), if the wire is attempted to be advanced inside the connector (B), it will be difficult to direct the wire into the lumen of the catheter. In order to successfully tread a 0.035" floppy tipped guidewire into the catheter through the Tuohy-Borst connector, the catheter should be backed out through the distal port (C). This allows for easy advancement of the wire (D) and once this has been accomplished, the catheter can be re-secured in its prior position (E).
If a filling defect is then noted on the IOC (Figure 8), 1–2 mg of glucagon can be intravenously administered (46-52). Note that the single milligram of glucagon is just as effective as 2 mg (47). Flushing can be performed after a few minutes once the glucagon has taken effect in an attempt to clear the duct. If a filling defect persists, the surgeon has several options outlined below:
Figure 8.
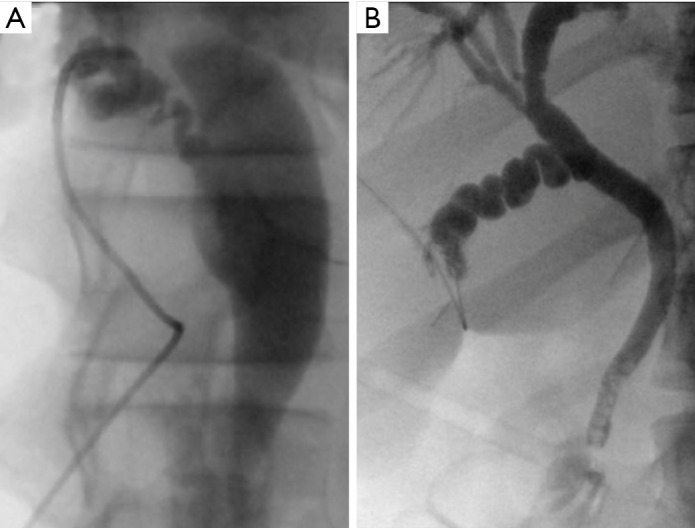
Intraoperative cholangiograms using ureteral stents. LCBDE was not technically possible in these two cases because of the very tortuous cystic ducts. There are distal CBD filling defects. The use of hydrophilic guidewires can often help navigate difficult cystic duct anatomy. LCBDE, laparoscopic common bile duct exploration; CBD, common bile duct.
Basket extraction
A wire basket can be threaded down the cholangiogram catheter in an attempt to capture stones under the fluoroscopic guidance (4,53-55). Examples of these baskets in our described setup are depicted in Figure 9. Captured stones may be removed in whole from the duct or the surgeon may employ the technique of “crush and flush”. Often, the captured stones are relatively soft and can be intentionally or unintentionally crushed or “sliced through” during basket closure. If the calculi are able to sufficiently break into smaller pieces, they then can be flushed through the sphincter of Oddi and into the duodenum. Very careful attention to completion cholangiogram is needed to confirm the fragmented stones were able to be cleared. These baskets can be used not only under fluoroscopic guidance, but also under direct visualization with use of the choledochoscope. The baskets can be passed through the working channel of the choledochoscope.
Figure 9.
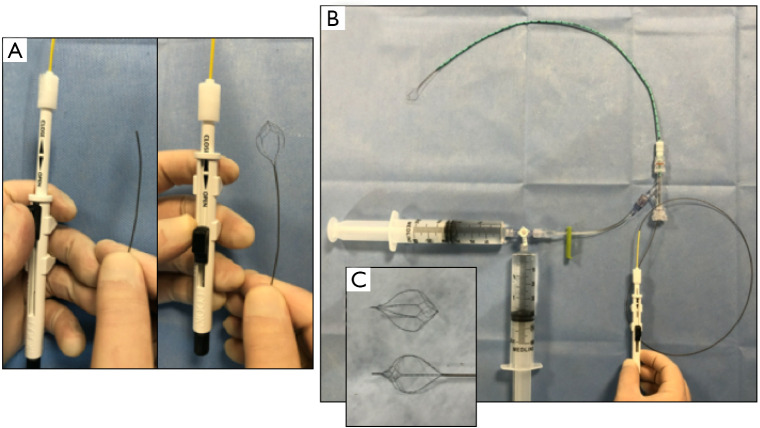
Example of nitinol baskets. Advancing and retracting the switch on the basket handpiece opens and closes the baskets distally (A). If the setup in Figure 6 is utilized, the baskets can be threaded through the Tuohy-Borst connector immediately after filling defect is noted on intraoperative cholangiogram without any additional alterations to the setup (B). Nitinol baskets are available as tipped or non-tipped. The tipped baskets are more easily visualized with fluoroscopy but will damage choledochoscopes and are more prone to duct mucosal injury (C).
Balloon dilation
Balloon catheters are used to both dilate the cystic duct to allow entry with choledochoscope and/or enlarge the sphincter of Oddi to allow for stone passage (Figure 10A,B) (56-59). These balloon dilators are advanced over a wire. As such, the first step to employing this technique is placement of a guidewire into the CBD through the cholangiogram catheter. It is necessary to use fluoroscopy to ensure the floppy-tipped wire is in the duodenum. This helps to prevent mispositioning of the catheter and loss of wire access in the duct when manipulating the balloon catheter. Selection of the balloon should take into account the diameter of the CBD. In general, it is safe to dilate the sphincter up to the diameter of the CBD (e.g., 6–10 mm). Other practical issues of proper balloon selection include overall length (75 cm is a standard working length for a non-compliant balloon catheter that works best) and the length of the actual balloon (40, 80, 100 mm are common lengths). The 40-mm balloon length offers the most versatility when working within the confines of the duodenum and navigating the cystic-common duct junction.
Figure 10.

Balloon dilation in LCBDE. (A) LBCDE supplies needed for sphincter dilation including balloon dilators, a rotation inflation device and a 0.035" guidewire. (B) Setup for use of balloon dilator including a rotational inflation device and a guidewire. Prior to advancement of the balloon dilator over the wire, it is necessary to ensure the floppy-tipped wire is in the duodenum by use of fluoroscopy. The balloon dilator should be inflated to the manufacturer’s specification noted on the balloon packaging. LCBDE, laparoscopic common bile duct exploration.
When positioning the balloon for sphincter dilation, it is helpful to advance it until the entirety of the balloon is in the duodenum and then inflate to the manufacturer’s specifications using a rotational inflation device (the inflation pressure to achieve the defined balloon profile diameter is found on the balloon packaging). When pulling back gently, the balloon will hub against the sphincter and allow for better visual and tactile feedback regarding its position. Also during fluoroscopy, either markers on the dilator or use of a 50/50 contrast/saline mix for balloon inflation can assist with positioning. Once hubbed against the sphincter, the balloon should be deflated slightly and then retracted so that it straddles the sphincter. After appropriate positioning, the balloon may be blown back up to full inflation pressure and held there for 3–5 minutes.
Following the dilation of the sphincter, the balloon is deflated and pulled back so that it now straddles the cystic duct-CBD junction. The retracted balloon is then reinflated to seal the CBD. The wire is removed so that a cholangiogram can be performed through the same wire lumen. Sealing the CBD with the balloon allows for a more pressurized system through which to flush debris and stones forward and will prevent unintended flushing of stones proximally into the hepatic ducts. This technique is depicted in a stepwise fashion in Figure 11.
Figure 11.
Fluoroscopic images depicting balloon dilator use. (A) Filling defect is noted in the distal CBD without the passage of contrast into the duodenum. (B) A floppy-tipped wire is advanced past the stone into the duodenum. (C) A balloon dilator is advanced over the wire by Seldinger technique and is pictured straddling the sphincter of Oddi. The markers on the proximal and distal ends of the balloon can be seen with fluoroscopy. After properly positioned, the balloon is inflated per the manufacturer’s specifications and is held there for 3–5 minutes. (D) For completion cholangiogram, the balloon is then deflated and retracted proximally within the CBD. The balloon is then reinflated to seal the CBD. This allows for a cholangiogram to be performed through the balloon and prevents debris from being flushed proximally into the hepatic ducts. CBD, common bile duct.
Choledochoscopy
Choledochoscopy allows for direct visualization of stones while attempting removal (4,53,60,61). The choledochoscope can be advanced into the CBD either over a wire by Seldinger technique or by direct instrumentation through the ductotomy. Introduction of the choledochoscope into the cystic duct may require dilation of the duct with a balloon dilator. As a general rule, avoid dilating the cystic duct or sphincter more than the diameter of the CBD (52,62). If the choledochoscope requires intra-abdominal manipulation, an atraumatic laparoscopic grasper must be used to ensure the scope is not damaged. Use of a choledochoscope requires a second video monitor system so that both the endoscopic and laparoscopic views can be seen simultaneously. A pressurized bag of saline must also be connected to the choledochoscope to allow for visualization in the same fashion as genitourinary (GU) irrigation for cystoscopy. After the stones have been located with the scope, baskets are advanced through its working channel to permit attempted stone removal under direct visualization. Once again, a completion cholangiogram should be performed to ensure stone clearance.
Endobiliary stent placement
If wire access can be obtained into the duodenum but all other stone clearance techniques have failed or are only partially successful, then placement of an endobiliary stent versus completing the cholecystectomy and post-operative ERCP should be considered. Management of CBD stones with endobiliary stents has been shown to be safe and effective (63-65). Similar to the balloon dilators, these endobiliary stents can be passed over a wire and advanced into place by identifying the associated markers on fluoroscopy. The markers should be positioned on either side of the sphincter and will allow for drainage of the ductal system as a temporizing measure. The patient will still require ERCP with sphincterotomy and removal of the stent, but with appropriate drainage of the biliary system, this can be performed on an outpatient basis if necessary. Placement of a stent during LCBDE can also aid the endoscopist during postoperative ERCP, especially in pediatric patients with a diminutive papilla that may be hard to identify and cannulate from the intestinal lumen.
Laser lithotripsy
An additional adjunct to the use of the choledochoscope is laser lithotripsy (43,66). Once stones are identified under direct visualization, the laser is passed through the working channel of the scope and is used to fragment the calculi. After the stones have been broken up, the pieces are removed either by flushing into the duodenum or with nitinol basket retrieval. In most hospitals, special credentialing for the use of lasers is required. Partnering with Urology colleagues may be advantageous. Most laser wires are designed to fit in the working channel of small cystoscopes and choledochoscopes.
Tips, tricks and troubleshooting potential problems
Mastery of any surgical technique requires instruction and repetition. When beginning to perform LCBDEs, inexperience with the described techniques can generate frustrations. Listed below are “tips and tricks” to help circumvent anticipated difficulties:
Performing an IOC with a catheter large enough to accommodate wires, balloons, and baskets allows the surgeon to immediately take the next step after a filling defect is noted. Success with LCBDE first starts with reducing the threshold for taking the next step. This is the rationale for using ureteral catheters as the IOC catheter at the outset.
When using wires, close approximation of an introducer to the ductotomy (or even entering into the cystic duct) will allow for the wire to be advanced and manipulated without inducing undue tension in the wire causing a “spring back effect” and losing wire access into the CBD. Losing wire access to the CBD is a frustrating occurrence that can be mitigated by this simple maneuver. Avoiding simple problems that add to the overall time of the procedure and surgeon frustration level will aid adoption and success rate of LCBDE.
Tortuous cystic ducts can be more readily accessed with a hydrophilic guidewire that is delivered into the ductotomy with the ureteral catheter (Figure 8).
Using the Touhy-Borst system allows for flushing of the catheter and removal of all the air in the system after the wire is removed. This will prevent air bubbles from entering the CBD system that may be confused for filling defects.
After successful flushing or retrieval of stones, the vigilance for retained stones should be heightened as they can be missed on completion IOC if they are pushed into hepatic duct during repeated flushing. In order to not miss these calculi, special attention should be given to the proximal portion of the ductal system during the final cholangiogram.
When performing a transcystic LCBDE, use of an endoloop on the cystic duct stump should be considered when completing the cholecystectomy if the duct cannot be cleared.
If numerous stones are encountered as seen in Figure 12 or several unsuccessful attempts have been made, it may be best to abandon LCBDE and perform completion cholecystectomy with postoperative ERCP. The corollary to this statement is that some debris can likely be left behind with little untoward effect if the main obstruction or large stone is diminished or significantly broken up. In this scenario, close follow up is a reasonable option in favor of a reflexive call for postoperative ERCP because the natural history of smaller stones is to spontaneously pass with little complication or downside (22,26,67).
Use intermittent low volume installation of contrast solution into the CBD during nitinol basket retrieval attempts. The benefit is an accentuation of the stone as a filling defect that can be otherwise hard to see-especially if the contrast media from earlier cholangiogram runs has been diluted by repeated flushing attempts with saline.
Take a stepwise approach and do the quick maneuvers first. With an array of strategies and tools for LCBDE at the surgeon’s disposal, the temptation may be to instrument the CBD at the first indication that contrast isn’t passing through the sphincter of Oddi. Utilizing the larger 5–6-Fr catheters, the surgeon can apply much more force and deliver a large stream of contrast that may succeed in breaking a spasm after the administration of glucagon. Sometimes the simplest moves are the most effective because not all that inhibits flushing is a stone.
Figure 12.
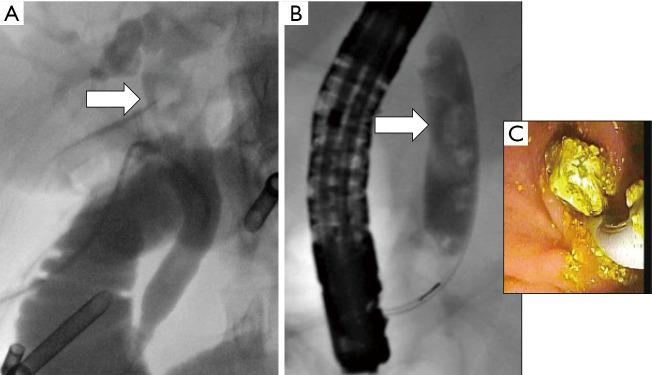
Considerations for abandoning LCBDE. (A) Stones found in proximal CBD that are difficult to access with the described techniques. (B) Numerous filling defects which will complicate successful clearance by LCBDE. (C) Intraluminal view of stone extraction during ERCP. LCBDE, laparoscopic common bile duct exploration; CBD, common bile duct; ERCP, endoscopic retrograde cholangiopancreatography.
Postoperative management of patients
The success rate of complete CBD stone clearance with LCBDE ranges from 74% to 97% (16,19,68). As such, the surgeon must be aware that a clean-appearing cholangiogram may be misleading and fail to reveal retained stones that require further intervention. Often, these stones are inadvertently flushed proximally into the right and left hepatic ducts and are not detected on final cholangiogram and no concerns arise until elevation in routine postoperative LFTs are noted. Yet, even these LFTs can be misleading as down trending LFTs only carry a 49% accuracy in predicting stone passage (9). One study specifically evaluating the usefulness of postoperative LFTs in predicting retained calculi status post LBCDE found no difference in laboratory trends in those who had successful LCBDE and those who had persistent choledocholithiasis. 75% of the patients who had persistent choledocholithiasis had improved LFTs while many with duct clearance had worsening LFTs postoperatively (69). Ultimately, trending labs in the postoperative period is likely unreliable. Patients should be advanced back to a regular diet and activity as would be typical of a standard postoperative LC patient.
Conclusions
Successful completion of LCBDE not only requires surgical skill but also coordination between the surgeon, operating room staff and fluoroscopy. Thoughtful organization and systematization of the procedure will allow for improved efficiency and success. This includes a pre-defined stepwise algorithm and an understanding of all the equipment and resources necessary to perform a LCBDE. Some of the described techniques include completion and interpretation of an IOC, power flushing of the CBD, fluoroscopic nitinol basket deployment and stone retrieval, “crush and flush” of stones using the nitinol basket, balloon dilation of the sphincter of Oddi with subsequent flushing, use of choledochoscopy for nitinol basket or laser lithotripsy deployment, and endobiliary stent placement. Ultimately, increased understanding of the equipment and procedural steps necessary for LCBDE will result in widened adoption of the technique and thus better outcomes for patients.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Eduardo Perez, Samir Pandya, and Matthew S. Clifton) for the series “Current Topics in Pediatric General Surgery” published in Translational Gastroenterology and Hepatology. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tgh-20-172). The series “Current Topics in Pediatric General Surgery” was commissioned by the editorial office without any funding or sponsorship. MEB reports that she assisted in designing a laparoscopic common bile duct exploration curriculum for the general surgery residents at Wake Forest Baptist Medical Center. In order to facilitate technical practice, an unrestricted educational grant was obtained from Cook that provided the supplies. The authors have no other conflicts of interest to declare.
References
- 1.Waldhausen JH, Benjamin DR. Cholecystectomy is becoming an increasingly common operation in children. Am J Surg 1999;177:364-7. 10.1016/S0002-9610(99)00063-X [DOI] [PubMed] [Google Scholar]
- 2.Kaechele V, Wabitsch M, Thiere D, et al. Prevalence of gallbladder stone disease in obese children and adolescents: influence of the degree of obesity, sex, and pubertal development. J Pediatr Gastroenterol Nutr 2006;42:66-70. 10.1097/01.mpg.0000187816.31213.06 [DOI] [PubMed] [Google Scholar]
- 3.Poffenberger CM, Gausche-Hill M, Ngai S, et al. Cholelithiasis and its complications in children and adolescents: update and case discussion. Pediatr Emerg Care 2012;28:68-76; quiz 77-78. 10.1097/PEC.0b013e31823f5b1e [DOI] [PubMed] [Google Scholar]
- 4.Fitzgibbons RJ, Jr, Gardner GC. Laparoscopic Surgeon and the Common Bile Duct. World J Surg 2001;25:1317-24. 10.1007/s00268-001-0117-1 [DOI] [PubMed] [Google Scholar]
- 5.Green JA, Scheeres DE, Conrad HA, et al. Pediatric ERCP in a multidisciplinary community setting: experience with a fellowship-trained general surgeon. Surg Endosc 2007;21:2187-92. 10.1007/s00464-007-9334-5 [DOI] [PubMed] [Google Scholar]
- 6.Newman KD, Powell DM, Holcomb GW. The management of choledocholithiasis in children in the era of laparoscopic cholecystectomy. J Pediatr Surg 1997;32:1116-9. 10.1016/S0022-3468(97)90411-5 [DOI] [PubMed] [Google Scholar]
- 7.Rocca R, Castellino F, Daperno M, et al. Therapeutic ERCP in paediatric patients. Dig Liver Dis 2005;37:357-62. 10.1016/j.dld.2004.09.030 [DOI] [PubMed] [Google Scholar]
- 8.Maple JT, Ben-Menachem T, Anderson MA, et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc 2010;71:1-9. 10.1016/j.gie.2009.09.041 [DOI] [PubMed] [Google Scholar]
- 9.Suarez AL, LaBarre NT, Cotton PB, et al. An assessment of existing risk stratification guidelines for the evaluation of patients with suspected choledocholithiasis. Surg Endosc 2016;30:4613-8. 10.1007/s00464-016-4799-8 [DOI] [PubMed] [Google Scholar]
- 10.Tranter SE, Thompson MH. Comparison of endoscopic sphincterotomy and laparoscopic exploration of the common bile duct. Br J Surg 2002;89:1495-504. 10.1046/j.1365-2168.2002.02291.x [DOI] [PubMed] [Google Scholar]
- 11.Pierce RA, Jonnalagadda S, Spitler JA, et al. Incidence of residual choledocholithiasis detected by intraoperative cholangiography at the time of laparoscopic cholecystectomy in patients having undergone preoperative ERCP. Surg Endosc 2008;22:2365-72. 10.1007/s00464-008-9785-3 [DOI] [PubMed] [Google Scholar]
- 12.Wandling MW, Hungness ES, Pavey ES, et al. Nationwide Assessment of Trends in Choledocholithiasis Management in the United States From 1998 to 2013. JAMA Surg 2016;151:1125-30. 10.1001/jamasurg.2016.2059 [DOI] [PubMed] [Google Scholar]
- 13.Bansal VK, Misra MC, Rajan K, et al. Single-stage laparoscopic common bile duct exploration and cholecystectomy versus two-stage endoscopic stone extraction followed by laparoscopic cholecystectomy for patients with concomitant gallbladder stones and common bile duct stones: a randomized controlled trial. Surg Endosc 2014;28:875-85. 10.1007/s00464-013-3237-4 [DOI] [PubMed] [Google Scholar]
- 14.Cuschieri A, Lezoche E, Morino M, et al. E.A.E.S. multicenter prospective randomized trial comparing two-stage vs single-stage management of patients with gallstone disease and ductal calculi. Surg Endosc 1999;13:952-7. 10.1007/s004649901145 [DOI] [PubMed] [Google Scholar]
- 15.Rogers SJ, Cello JP, Horn JK, et al. Prospective randomized trial of LC+LCBDE vs ERCP/S+LC for common bile duct stone disease. Arch Surg 2010;145:28-33. 10.1001/archsurg.2009.226 [DOI] [PubMed] [Google Scholar]
- 16.Dasari BVM, Tan CJ, Gurusamy KS, et al. Surgical versus endoscopic treatment of bile duct stones. Cochrane Database Syst Rev 2013;(12):CD003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Short SS, Frykman PK, Nguyen N, et al. Laparoscopic common bile duct exploration in children is associated with decreased cost and length of stay: results of a two-center analysis. J Pediatr Surg 2013;48:215-20. 10.1016/j.jpedsurg.2012.10.041 [DOI] [PubMed] [Google Scholar]
- 18.Topal B, Vromman K, Aerts R, et al. Hospital cost categories of one-stage versus two-stage management of common bile duct stones. Surg Endosc 2010;24:413-6. 10.1007/s00464-009-0594-0 [DOI] [PubMed] [Google Scholar]
- 19.Mattila A, Mrena J, Kellokumpu I. Cost-analysis and effectiveness of one-stage laparoscopic versus two-stage endolaparoscopic management of cholecystocholedocholithiasis: a retrospective cohort study. BMC Surg 2017;17:79. 10.1186/s12893-017-0274-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulose BK, Arbogast PG, Holzman MD. National analysis of in-hospital resource utilization in choledocholithiasis management using propensity scores. Surg Endosc 2006;20:186-90. 10.1007/s00464-005-0235-1 [DOI] [PubMed] [Google Scholar]
- 21.Schroeppel TJ, Lambert PJ, Mathiason MA, et al. An economic analysis of hospital charges for choledocholithiasis by different treatment strategies. Am Surg 2007;73:472-7. 10.1177/000313480707300511 [DOI] [PubMed] [Google Scholar]
- 22.Mah D, Wales P, Njere I, et al. Management of suspected common bile duct stones in children: role of selective intraoperative cholangiogram and endoscopic retrograde cholangiopancreatography. J Pediatr Surg 2004;39:808-12; discussion 808-12. 10.1016/j.jpedsurg.2004.02.019 [DOI] [PubMed] [Google Scholar]
- 23.Overman RE, Hsieh LB, Thomas TT, et al. Pediatric Laparoscopic Common Bile Duct Exploration: An Opportunity to Decrease ERCP Complications. J Surg Res 2019;242:318-22. 10.1016/j.jss.2019.04.072 [DOI] [PubMed] [Google Scholar]
- 24.Hill SJ, Wulkan ML, Parker PM, et al. Management of the pediatric patient with choledocholithiasis in an era of advanced minimally invasive techniques. J Laparoendosc Adv Surg Tech A 2014;24:38-42. 10.1089/lap.2013.0306 [DOI] [PubMed] [Google Scholar]
- 25.Keeling NJ, Menzies D, Motson RW. Laparoscopic exploration of the common bile duct. Surg Endosc 1999;13:109-12. 10.1007/s004649900916 [DOI] [PubMed] [Google Scholar]
- 26.Wesdorp I, Bosman D, de Graaff A, et al. Clinical presentations and predisposing factors of cholelithiasis and sludge in children. J Pediatr Gastroenterol Nutr 2000;31:411-7. 10.1097/00005176-200010000-00015 [DOI] [PubMed] [Google Scholar]
- 27.Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol 2006;36:121-5. 10.1007/s00247-006-0191-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linet MS, Kim KP, Rajaraman P. Children’s exposure to diagnostic medical radiation and cancer risk: epidemiologic and dosimetric considerations. Pediatr Radiol 2009;39 Suppl 1:S4-26. 10.1007/s00247-008-1026-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stecker MS, Balter S, Towbin RB, et al. Guidelines for patient radiation dose management. J Vasc Interv Radiol 2009;20:S263-73. 10.1016/j.jvir.2009.04.037 [DOI] [PubMed] [Google Scholar]
- 30.National Research Council (U.S.). Committee to Assess Health Risks from Exposure to Low Level of Ionizing Radiation Staff, Board on Radiation Effects Research Staff, Division on Earth and Life Studies Staff, National Research Council, National Academy of Sciences. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII, Phase 2. Washington, D.C., UNITED STATES: National Academies Press; 2006. Accessed February 18, 2020. Available online: http://ebookcentral.proquest.com/lib/wfu/detail.action?docID=3378060
- 31.Sidhu M, Coley BD, Goske MJ, et al. Image Gently, Step Lightly: increasing radiation dose awareness in pediatric interventional radiology. Pediatr Radiol 2009;39:1135-8. 10.1007/s00247-009-1392-5 [DOI] [PubMed] [Google Scholar]
- 32.Geise RA, Eubig C, Franz S, et al. Report No. 058 - Managing the Use of Fluoroscopy in Medical Institutions (1998). Available online: 10.37206/57 10.37206/57 [DOI]
- 33.Berci G, Hunter J, Morgenstern L, et al. Laparoscopic cholecystectomy: first, do no harm; second, take care of bile duct stones. Surg Endosc 2013;27:1051-4. 10.1007/s00464-012-2767-5 [DOI] [PubMed] [Google Scholar]
- 34.Zhu JG, Han W, Guo W, et al. Learning curve and outcome of laparoscopic transcystic common bile duct exploration for choledocholithiasis. Br J Surg 2015;102:1691-7. 10.1002/bjs.9922 [DOI] [PubMed] [Google Scholar]
- 35.Herrero A, Philippe C, Guillon F, et al. Does the surgeon's experience influence the outcome of laparoscopic treatment of common bile duct stones? Surg Endosc 2013;27:176-80. 10.1007/s00464-012-2416-z [DOI] [PubMed] [Google Scholar]
- 36.Schwab B, Teitelbaum EN, Barsuk JH, et al. Single-stage laparoscopic management of choledocholithiasis: An analysis after implementation of a mastery learning resident curriculum. Surgery 2018;163:503-8. 10.1016/j.surg.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 37.Santos BF, Reif TJ, Soper NJ, et al. Development and evaluation of a laparoscopic common bile duct exploration simulator and procedural rating scale. Surg Endosc 2012;26:2403-15. 10.1007/s00464-012-2213-8 [DOI] [PubMed] [Google Scholar]
- 38.Sánchez A, Rodríguez O, Benítez G, et al. Development of a training model for laparoscopic common bile duct exploration. JSLS 2010;14:41-7. 10.4293/108680810X12674612014464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sánchez A, Otaño N, Rodríguez O, et al. Laparoscopic common bile duct exploration four-task training model: construct validity. JSLS 2012;16:10-5. 10.4293/108680812X13291597715709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teitelbaum EN, Soper NJ, Santos BF, et al. A simulator-based resident curriculum for laparoscopic common bile duct exploration. Surgery 2014;156:880-7, 890-3. 10.1016/j.surg.2014.06.020 [DOI] [PubMed] [Google Scholar]
- 41.Berci G, Shore JM, Hamlin JA, et al. Operative fluoroscopy and cholangiography. The use of modern radiologic technics during surgery. Am J Surg 1978;135:32. 10.1016/0002-9610(78)90005-3 [DOI] [PubMed] [Google Scholar]
- 42.MacFadyen BV. Intraoperative cholangiography: past, present, and future. Surg Endosc 2006;20 Suppl 2:S436-40. 10.1007/s00464-006-0053-0 [DOI] [PubMed] [Google Scholar]
- 43.Fang L, Wang J, Dai WC, et al. Laparoscopic transcystic common bile duct exploration: surgical indications and procedure strategies. Surg Endosc 2018;32:4742-8. 10.1007/s00464-018-6195-z [DOI] [PubMed] [Google Scholar]
- 44.Strömberg C, Nilsson M, Leijonmarck CE. Stone clearance and risk factors for failure in laparoscopic transcystic exploration of the common bile duct. Surg Endosc 2008;22:1194-9. 10.1007/s00464-007-9448-9 [DOI] [PubMed] [Google Scholar]
- 45.Pang L, Zhang Y, Wang Y, et al. Transcystic versus traditional laparoscopic common bile duct exploration: its advantages and a meta-analysis. Surg Endosc 2018;32:4363-76. 10.1007/s00464-018-6286-x [DOI] [PubMed] [Google Scholar]
- 46.Ponce J, Garrigues V, Pertejo V, et al. Effect of intravenous glucagon and glucagon-(1–21)-peptide on motor activity of sphincter of Oddi in humans. Dig Dis Sci 1989;34:61-4. 10.1007/BF01536155 [DOI] [PubMed] [Google Scholar]
- 47.Evans AF, Whitehouse GH. Further experience with glucagon enhanced cholangiography. Clin Radiol 1980;31:663. 10.1016/S0009-9260(80)80015-8 [DOI] [PubMed] [Google Scholar]
- 48.Jones RM, Coultas RJ, Pollard BJ, et al. Reversal of Biliary Sphincter Spasm with Low dose Glucagon during Operative Cholangiography. Anaesth Intensive Care 1983;11:174-5. 10.1177/0310057X8301100218 [DOI] [PubMed] [Google Scholar]
- 49.Jones RM, Fiddian-Green R, Knight PR. Narcotic-Induced Choledochoduodenal Sphincter Spasm Reversed by Glucagon. Anesth Analg 1980;59:946–7. 10.1213/00000539-198012000-00011 [DOI] [PubMed] [Google Scholar]
- 50.Mahmud S, McGlinchey I, Kasem H, et al. Radiological treatment of retained bile duct stones following recent surgery using glucagons. Surg Endosc 2001;15:1359-60. 10.1007/s00464-001-4207-9 [DOI] [PubMed] [Google Scholar]
- 51.Bordley J, 4th, Olson JE. The use of glucagon in operative cholangiography. Surg Gynecol Obstet 1979;149:583-4. [PubMed] [Google Scholar]
- 52.Hungness ES, Soper NJ. Management of common bile duct stones. J Gastrointest Surg 2006;10:612-9. 10.1016/j.gassur.2005.08.015 [DOI] [PubMed] [Google Scholar]
- 53.Lyass S, Phillips EH. Laparoscopic transcystic duct common bile duct exploration. Surg Endosc 2006;20:S441-5. 10.1007/s00464-006-0029-0 [DOI] [PubMed] [Google Scholar]
- 54.DePaula AL, Hashiba K, Bafutto M. Laparoscopic management of choledocholithiasis. Surg Endosc 1994;8:1399-403. 10.1007/BF00187344 [DOI] [PubMed] [Google Scholar]
- 55.Paganini AM, Guerrieri M, Sarnari J, et al. Thirteen years’ experience with laparoscopic transcystic common bile duct exploration for stones. Surg Endosc 2007;21:34-40. 10.1007/s00464-005-0286-3 [DOI] [PubMed] [Google Scholar]
- 56.Carroll BJ, Phillips EH, Chandra M, et al. Laparoscopic transcystic duct balloon dilatation of the sphincter of Oddi. Surg Endosc 1993;7:514-7. 10.1007/BF00316692 [DOI] [PubMed] [Google Scholar]
- 57.Fujisaki S, Nezu T, Miyake H, et al. Laparoscopic treatment for common bile duct stones by transcystic papilla balloon dilatation technique. Surg Endosc 1999;13:824-6. 10.1007/s004649901111 [DOI] [PubMed] [Google Scholar]
- 58.Masoni L, Mari FS, Pietropaolo V, et al. Laparoscopic Treatment for Unsuspected Common Bile Duct Stones by Transcystic Sphincter of Oddi Pneumatic Balloon Dilation and Pressure-Washing Technique. World J Surg 2013;37:1258-62. 10.1007/s00268-013-1992-y [DOI] [PubMed] [Google Scholar]
- 59.Sjer AEB, Boland DM, van Rijn PJJ, et al. A decade of washing out common bile duct stones with papillary balloon dilatation as a one-stage procedure during laparoscopic cholecystectomy. Surg Endosc 2010;24:2226-30. 10.1007/s00464-010-0937-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Topal B, Aerts R, Penninckx F. Laparoscopic common bile duct stone clearance with flexible choledochoscopy. Surg Endosc 2007;21:2317-21. 10.1007/s00464-007-9577-1 [DOI] [PubMed] [Google Scholar]
- 61.Phillips E, Berci G, Barber K, et al. The Role of Choledochoscopy: The Eternal Problem of How to Remove a CBD Stone. Surg Innov 2015;22:540-5. 10.1177/1553350615594444 [DOI] [PubMed] [Google Scholar]
- 62.Hunter JG, Soper NJ. Laparoscopic management of bile duct stones. Surg Clin North Am 1992;72:1077. 10.1016/S0039-6109(16)45833-0 [DOI] [PubMed] [Google Scholar]
- 63.Choudhuri G, Sharma B, Saraswat V, et al. Biliary stenting for management of common bile duct stones. J Gastroenterol Hepatol 1998;13:594-7. 10.1111/j.1440-1746.1998.tb00696.x [DOI] [PubMed] [Google Scholar]
- 64.Gomez D, Cox MR. Laparoscopic Transcystic Stenting and Postoperative ERCP for the Management of Common Bile Duct Stones at Laparoscopic Cholecystectomy: Ann Surg 2018;267:e86-8. 10.1097/SLA.0000000000002426 [DOI] [PubMed] [Google Scholar]
- 65.Dietrich A, Alvarez F, Resio N, et al. Laparoscopic management of common bile duct stones: transpapillary stenting or external biliary drainage? JSLS 2014;18:e2014.00277. [DOI] [PMC free article] [PubMed]
- 66.Xia HT, Liu Y, Jiang H, et al. A novel laparoscopic transcystic approach using an ultrathin choledochoscope and holmium laser lithotripsy in the management of cholecystocholedocholithiasis: An appraisal of their safety and efficacy. Am J Surg 2018;215:631-5. 10.1016/j.amjsurg.2017.05.020 [DOI] [PubMed] [Google Scholar]
- 67.Collins C, Maguire D, Ireland A, et al. A Prospective Study of Common Bile Duct Calculi in Patients Undergoing Laparoscopic Cholecystectomy. Ann Surg 2004;239:28-33. 10.1097/01.sla.0000103069.00170.9c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alexakis N, Connor S. Meta-analysis of one- vs. two-stage laparoscopic/endoscopic management of common bile duct stones. HPB 2012;14:254-9. 10.1111/j.1477-2574.2012.00439.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wewelwala C, Cashin P, Berry R, et al. Usefulness of early post-operative liver function test monitoring after laparoscopic common bile duct exploration. ANZ J Surg 2017;87:925-9. 10.1111/ans.13217 [DOI] [PubMed] [Google Scholar]