Key Points
Question
What additional information can we obtain about nonsyndromic asymmetric retinitis pigmentosa (RP) from studying human pathology samples?
Findings
This case report compared clinical imaging with histopathology and sequencing DNA extracted from autopsied eyes of a deceased patient with asymmetric RP. No additional pathogenic variants were found using this approach.
Meaning
This approach may provide a multimodal perspective into functional, cellular, and molecular phenotyping; in this case, an ultrarare presentation of asymmetric RP that can be applied to other rare mendelian diseases.
This case report evaluates whether additional information can be obtained about asymmetric RP from studying clinical imaging and pathology correlates, including pathology samples from autopsied eyes.
Abstract
Importance
Asymmetric retinitis pigmentosa (RP) is a rare presentation of a normally symmetric condition. Histopathologic evidence should be examined to see if this asymmetry extends to the tissue and cellular levels.
Objective
To determine whether additional information can be obtained about asymmetric RP from studying clinical imaging and pathology correlates, including pathology samples from autopsied eyes.
Design, Setting, and Participants
In this case report, clinical and postmortem histopathological characteristics were compared in 2 eyes of a patient in her 50s with asymmetric RP. Individuals with rare mendelian diseases, such as RP, were studied using data from the curated National Eye Institute Eye Pathology collection.
Main Outcome and Measures
Results of clinical evaluation, multimodal retinal imaging, histopathology, and molecular genetic testing in a case of nonsyndromic asymmetric RP using resources from the ocular pathology collection.
Results
Eyes from a deceased patient in her 50s with nonsyndromic asymmetric RP found within the ocular pathology collection were studied. The patient was diagnosed with RP as an adolescent and presented in her 50s to the eye clinic with advanced RP, with the left eye affected much more severely than the right. The patient’s phenotype was studied using in vivo imaging and postmortem histopathology to identify interocular differences in tissue degeneration. Extraction of blood-derived DNA and formalin-fixed paraffin-embedded DNA from autopsied eyes analyzed using next-generation sequencing did not yield a definitive molecular diagnosis nor significant tissue differences.
Conclusions and Relevance
This study demonstrates newly reported histopathological and molecular correlates in asymmetric RP. This report also highlights the relevance of studying previously seen patients and reevaluating their conditions using resources within the ocular pathology collection to gain further insight on their disease.
Introduction
Retinitis pigmentosa (RP) is a group of rare genetic disorders that involves degeneration of retinal photoreceptors leading to peripheral and then central vision loss. Nonsyndromic RP can result from deleterious germline pathogenic variants in any of more than 70 genes involved in the production of proteins found primarily in photoreceptors.1
RP affects rods before cones, presenting with nyctalopia and visual field constriction and later leading to central vision loss.2 Histological analysis shows the outer nuclear layer (ONL) of the retina, consisting of rod and cone photoreceptor nuclei, appearing severely degraded in patients with RP.3 The inner nuclear layer (INL) and the ganglion cell layer (GCL) are fairly well preserved, but many of these cells degenerate later in the disease.
Spectral-domain optical coherence tomography (SD-OCT) can also be used to disclose many changes of the retinal layers in patients with RP. SD-OCT can center on the fovea to analyze the INL and GCL as well as the outer plexiform layer, external limiting membrane (ELM), photoreceptor inner segment–outer segment junction, outer segment–retinal pigment epithelium (RPE) junction, and Bruch membrane. Previous analysis shows that disruption of the ELM and outer segment–RPE is independently associated with a decline in visual acuity, reflecting the functional importance of the RPE and its junction with the photoreceptor layer.4
Asymmetric RP is a rare presentation of a usually symmetric condition.5 Here, we provide clinical, histopathological, and molecular evidence that this asymmetry extends to the tissue and cellular levels.
Methods
Autopsied eyes from a deceased patient in her 50s with nonsyndromic asymmetric RP were studied. These were obtained from an expansive ocular pathology collection established by the National Eye Institute Eye Pathology Directors, Toichiro Kuwabara, MD, PhD, and Chi-Chao Chan, MD. This patient was chosen because this asymmetric case posed potential for novel findings about RP and allowed us to explore the relevance of studying previously seen patients with a different approach. Retrospectively, we investigated the patient’s clinical history as a patient at the National Institutes of Health Clinical Center as well as any available clinical imaging. In conjunction, we conducted postmortem histopathological and genetic analysis using formalin-fixed paraffin-embedded (FFPE) tissue and blood-derived samples. All investigations were conducted in accordance with the principles of the Declaration of Helsinki. This study was approved by the National Institutes of Health institutional review board. The patient consented to data collection with the National Institutes of Health before death.
The patient’s eyes were donated for research. DNA was extracted from FFPE ocular tissues of the autopsied eyes (InviMag FFPE DNA kit/IG [Invitek Molecular]). Clinical testing was performed as described.6 Imaging was done using color photography, short-wavelength fundus autofluorescence (Topcon) and SD-OCT (Zeiss). Molecular diagnostic testing of a blood sample was performed through the National Institutes of Health eyeGENE program using an inherited retinal dystrophies sequencing panel with copy number variant detection by a commercial laboratory. The DNA samples prepared from blood and both autopsied eyes were captured using a custom next-generation sequencing panel for 731 genes implicated in eye development or disease (eTable in the Supplement), followed by Illumina sequencing.7
Results
The patient presented for a last clinical visit at the National Institutes of Health Clinical Center and subsequently died. This patient was diagnosed with RP as an adolescent and lost vision in the left eye in her late 30s. On presentation, best-corrected visual acuity was 20/32 + 1 OD with no light perception OS at the most recent clinical visit (in her 50s). Goldmann visual field testing revealed severely constricted isopters with all target sizes (V4e less than 10°). Color vision in the right eye was within normal range using the Farnsworth D-15 test. Family history was unremarkable for any eye disease. The patient’s phenotype was studied using multimodal retinal imaging and postmortem histopathology to identify the interocular differences in retinal degeneration.
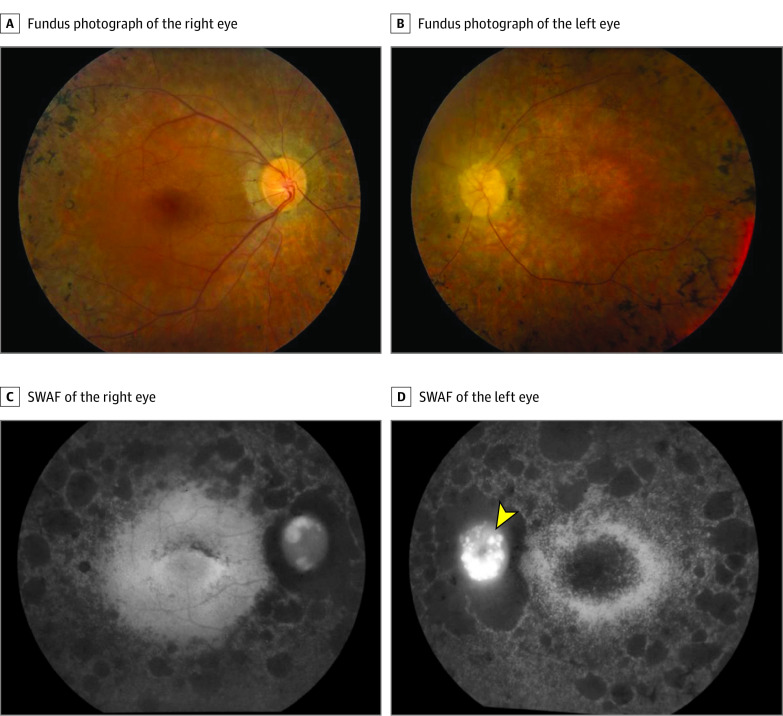
Fundus examination showed clinical findings consistent with typical RP (Figure 1), including retinal atrophy that was worse in the left eye than in the right eye with numerous bone spicules (eFigure 1 in the Supplement), severe retinal vascular attenuation, and optic nerve pallor. SD-OCT images of both eyes also demonstrated asymmetric findings, with more widespread foveal atrophic changes (ONL and ELM) in the left eye (eFigure 2 in the Supplement).
Figure 1. In Vivo Imaging of the Patient Shows Typical Signs of Retinitis Pigmentosa, With Degenerative Changes More Advanced in the Right Eye Compared With the Left Eye.
Fundus photography and short-wavelength fundus autofluorescence (SWAF) show severe retinal vascular attenuation, optic disc pallor, and retinal atrophy with bone spicules in both eyes. The fovea is globally hypoautofluorescent in the left eye (D), whereas there are speckled patches of hypofluoresence in an arc superior to the fovea in the right eye (C).
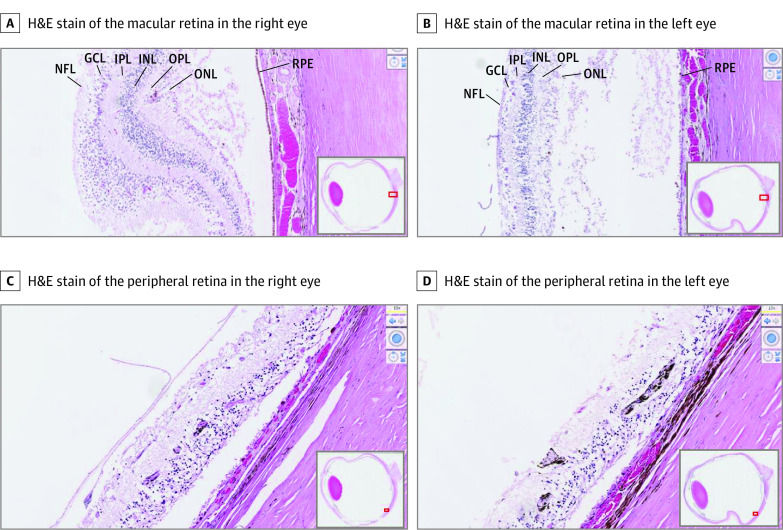
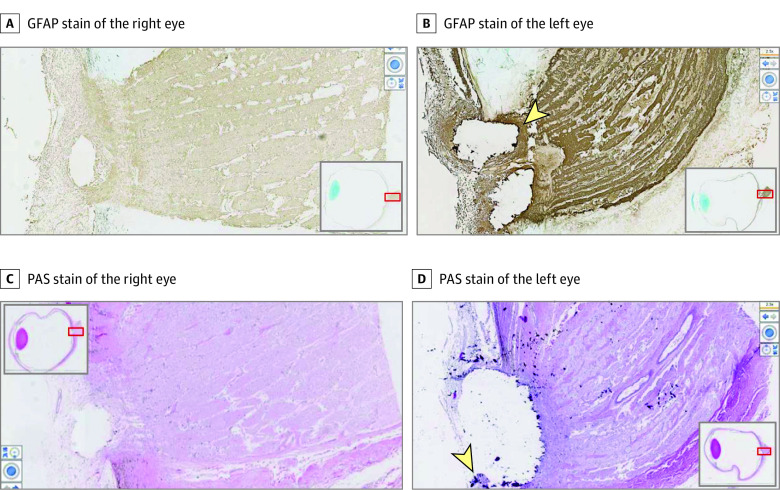
Postmortem microscopic examination of the central and peripheral retinas showed atrophy of the INL and ONL, RPE hyperplasia and hypoplasia, depigmentation, and RPE migration into the perivascular area of the inner retina in both eyes (Figure 2). As the retina degenerates, the number of rows of photoreceptor nuclei decreases, allowing use of ONL thickness as a marker of severity.8 Within the macula, 1 to 3 cell thickness of the ONL and outer plexiform layer are preserved in the right eye compared with only a few spotty residues of individual photoreceptor nuclei identified in the left eye. Further studies showed more severe photoreceptor loss and attenuation of layers of the retina in the left eye (Figure 2). Periodic acid-Schiff–positive corpora amylacea were noted in the nerve fiber and optic nerve in both eyes and in the INL of the left eye. Therefore, the combination of histology and OCT corroborates more advanced degeneration in all layers of the retina and optic nerve in the left eye. Independent of the asymmetric retinal degeneration, optic disc drusen were noted in both eyes and were larger in the left eye than in the right eye (Figure 3), with disruption of the cribriform layer in the left eye.
Figure 2. In Vitro Histopathological Studies Confirm More Advanced Degeneration of Retina in the Left Eye Than the Right Eye.
Representative hematoxylin-eosin (H&E)–stained histology of the right and left eyes taken at ×100 original magnification shows loss of photoreceptors and attenuation of layers of the retina to be more advanced in the left eye, continuing to the extent of the ganglion cell layer (GCL). This is evident both in the central (A and B) and peripheral (C and D) retina. Along with retinal atrophy, retinal pigment deposits are particularly evident in the peripheral retina in the left eye. INL indicates inner nuclear layer; IPL, inner plexiform layer; NFL, nerve fiber layer; ONL, outer nuclear layer; OPL, outer plexiform layer; RPE, retinal pigment epithelium.
Figure 3. In Vitro Histopathological Studies Demonstrate Greater Optic Disc Drusen in the Left Eye Than the Right Eye.
Images were taken at a magnification of ×25. Additional images of right and left eyes reveal artifact of where optic disc drusen once were. Glial fibrillary acidic protein (GFAP) staining shows gliosis at the optic nerve head (B), a corollary of the extent of retinal atrophy. There appear to be fragments of positive periodic acid–Schiff (PAS)–stained material at the edge of the gap in panel D (arrowhead), representative of remaining drusen.
To determine whether asymmetric RP in this case is because of segmental mosaicism, DNA from paraffin-embedded ocular tissue from each eye was extracted, sequenced, and compared with blood-derived DNA. Next-generation sequencing of 731 genes associated with inherited eye conditions9 did not detect any differences in genetic variants observed in each eye and blood or evidence of somatic mosaicism. A clear molecular diagnosis was not established.
Discussion
To our knowledge, this is the first report of histopathological and molecular examinations of a case of asymmetric RP. Asymmetry in bilateral RP is a rare expression of a rare disease. From our clinical record, the patient had reached end-point blindness in the left eye long before vision was lost in the right eye. We are unsure whether the onset of the disease was asymmetric, although by the patient’s report, there was still useful vision in the right eye and no vision in the left eye for 10 to 13 years prior to death.
Histopathological correlates suggest that differences in degenerative rates and potential onset of disease extend to the cellular level for neural retinal tissues. One hypothesis is that the asymmetric macular atrophy could be partly caused by the asymmetrically severe optic disc drusen causing secondary degeneration of the GCL in the macula. Somatic mosaicism is another possibility for differences in tissue-level progression of degeneration, as proposed for Coats disease.10 While we found no support for this mechanism among a large number of genes associated with critical ocular functions, we also did not find a primary RP variation or disease-modifying variants in blood or ocular tissue. Therefore, we acknowledge that our results provide no evidence for or against mosaicism. Other possibilities include noncoding variation, DNA methylation, or environmental factors, although these were not assayed here.
Limitations
This study had limitations. The amount and fragment sizes of the DNA obtained from the paraffin extraction of the autopsied eyes limited our ability to detect very low-level somatic mosaicism by next generation sequencing.
Conclusions
Our study involves a detailed histopathological examination of tissue samples of the patient’s eyes as well as analysis of DNA extracted from the autopsied eyes themselves. Using this approach, we attempted to elucidate somatic vs germline mutations in disease pathogenesis, although we did not identify any somatic variants. Expanded use of this approach may permit a better understanding of asymmetric RP and other rare mendelian diseases from curated pathology collections.
eTable. Gene variants detected in blood samples.
eFigure 1. In vivo gross pathology highlights bony spicules characteristic of RP.
eFigure 2. Further in vivo imaging of the patient supports asymmetric findings.
References
- 1.RetNet . Summaries of genes and loci causing retinal diseases. Accessed May 21, 2021. https://sph.uth.edu/retnet/sum-dis.htm
- 2.Gupta MP, Herzlich AA, Sauer T, Chan CC. Retinal anatomy and pathology. Dev Ophthalmol. 2016;55:7-17. doi: 10.1159/000431128 [DOI] [PubMed] [Google Scholar]
- 3.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368(9549):1795-1809. doi: 10.1016/S0140-6736(06)69740-7 [DOI] [PubMed] [Google Scholar]
- 4.Battaglia Parodi M, La Spina C, Triolo G, et al. Correlation of SD-OCT findings and visual function in patients with retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2016;254(7):1275-1279. doi: 10.1007/s00417-015-3185-x [DOI] [PubMed] [Google Scholar]
- 5.Massof RW, Finkelstein D, Starr SJ, Kenyon KR, Fleischman JA, Maumenee IH. Bilateral symmetry of vision disorders in typical retinitis pigmentosa. Br J Ophthalmol. 1979;63(2):90-96. doi: 10.1136/bjo.63.2.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Shepard MJ, Zhang C, et al. Deletion of the von Hippel-Lindau gene in hemangioblasts causes hemangioblastoma-like lesions in murine retina. Cancer Res. 2018;78(5):1266-1274. doi: 10.1158/0008-5472.CAN-17-1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrackova A, Vasinek M, Sedlarikova L, et al. Standardization of sequencing coverage depth in NGS: recommendation for detection of clonal and subclonal mutations in cancer diagnostics. Front Oncol. 2019;9:851. doi: 10.3389/fonc.2019.00851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chui TY, Song H, Clark CA, Papay JA, Burns SA, Elsner AE. Cone photoreceptor packing density and the outer nuclear layer thickness in healthy subjects. Invest Ophthalmol Vis Sci. 2012;53(7):3545-3553. doi: 10.1167/iovs.11-8694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasov L, Guan B, Ullah E, et al. Novel TMEM98, MFRP, PRSS56 variants in a large United States high hyperopia and nanophthalmos cohort. Sci Rep. 2020;10(1):19986. doi: 10.1038/s41598-020-76725-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black GC, Perveen R, Bonshek R, et al. Coats’ disease of the retina (unilateral retinal telangiectasis) caused by somatic mutation in the NDP gene: a role for norrin in retinal angiogenesis. Hum Mol Genet. 1999;8(11):2031-2035. doi: 10.1093/hmg/8.11.2031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Gene variants detected in blood samples.
eFigure 1. In vivo gross pathology highlights bony spicules characteristic of RP.
eFigure 2. Further in vivo imaging of the patient supports asymmetric findings.