Abstract
Several studies report high effectiveness of COVID-19 vaccines against SARS-CoV-2 infection and severe disease, however an important knowledge gap is the vaccine effectiveness against transmission (VET). We present estimates of the VET to household and other close contacts in the Netherlands, from February to May 2021, using contact monitoring data. The secondary attack rate among household contacts was lower for fully vaccinated than unvaccinated index cases (11% vs 31%), with an adjusted VET of 71% (95% confidence interval: 63–77).
Keywords: SARS-CoV-2, transmission, household study, COVID-19, vaccine effectiveness
An important question when making prognoses of the pandemic in the near future and of the need on non-pharmaceutical control measures is to what extent the vaccines reduce the likelihood of transmission from infected vaccinees. Based on routine contact monitoring data, we here estimate the vaccine effectiveness against transmission (VET) and the vaccine effectiveness against infection (VE) among household and other close contacts of confirmed cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the Netherlands, between 1 February and 27 May 2021. The Alpha variant (Phylogenetic Assignment of Named Global Outbreak (Pango) lineage designation B.1.1.7) was the dominant variant in the area at that time.
Source and contact tracing
In the Netherlands, people are encouraged to undergo SARS-CoV-2 testing free of charge when experiencing symptoms or after contact with a confirmed case [1]. Infections confirmed by PCR, loop mediated isothermal amplification (LAMP) or antigen test are notified to the regional Municipal Health Services (MHS), who perform source and contact tracing and contact monitoring [2]. During our study period, household members and other close contacts of confirmed cases needed to quarantine for 10 days post exposure. All close contacts of a confirmed case were encouraged to get tested as soon as possible after exposure. In addition, a test was recommended on the 5th day after last exposure. If negative, contacts could end quarantine. We obtained a pseudonymised minimal contact monitoring dataset from all MHS. Additional data on index cases and contacts who tested positive, including the vaccine received and date of symptom onset, was extracted from the national infectious disease notification registry. Of note, a contact becomes an index case when testing positive, so our study could include some persons both as contact and as index case.
Vaccination status
The time since vaccination of the index case was based on the number of days between vaccination and a date used for statistics (DUFS), which was either the reported date of symptom onset or, if that was unknown, the date of positive test result minus 2 days. For vaccinated contacts, time since vaccination was calculated as the number of days between vaccination and the date of first exposure of the contact to the index case within the infectious period of the index, which is defined during source and contact tracing as 2 days before symptom onset or 2 days before test. Partly vaccinated was defined as having received the first dose of a two-dose coronavirus disease (COVID-19) vaccine, with a time since vaccination of at least 14 days. Fully vaccinated was defined as having completed a two-dose schedule with a time since vaccination of at least 7 days, or the one-dose Janssen (Ad26.COV2-S (recombinant), Janssen-Cilag International NV, Beerse, Belgium) schedule with a time since vaccination of at least 14 days. We included only index cases aged 18 years or older because children were not eligible for vaccination at the time. Contacts aged 0–17 years were included in the VET analyses, but not in the VE analyses. In order to exclude co-primary cases, the household contacts of an index were excluded if the most likely setting of infection of the index was ‘at home’ according to the source tracing interview (excluding 44,676 contacts (15%)). Further, only SARS-CoV-2-positive contacts with a DUFS within 1 to 14 days after the DUFS of the index case were included in the analyses, to reduce misclassification of indexes and secondary cases.
Index cases and contacts
The final dataset contained 253,168 contacts of 113,582 index cases (5,394 persons in our study were both contact and index case). Of the index cases, 622 (0.5%) were fully vaccinated and 2,088 (1.8%) were partly vaccinated. Of the contacts, 5,397 (2.1%) were fully vaccinated and 4,411 were partly vaccinated (1.7%). Characteristics of indexes and contacts are shown in Table 1. We calculated the VET via the secondary attack rate (SAR) among close contacts of confirmed index cases: 1 − (SARvaccinated index/SARunvaccinated index) × 100% [3]. The VE among contacts was calculated as: 1 − (ARvaccinated contacts/ARunvaccinated contacts) × 100%. Both VET and VE were estimated using a binomial generalised linear model. For parameter fitting we used the generalised estimating equations approach with exchangeable correlation structure to account for clustering of contacts belonging to the same index case [4].
Table 1. Characteristics of COVID-19 index cases (18 years and older), by vaccination status of the index and characteristics of contacts, and by vaccination status of the contact, the Netherlands, 1 February−27 May 2021 (n = 113,582 index cases, n = 253,168 contacts).
| Vaccination status index | Vaccination status contacts | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unvaccinated | Partly vaccinated | Fully vaccinated | Unvaccinated | Partly vaccinated | Fully vaccinated | ||||||||
| n | %a | n | %a | n | %a | n | %a | n | %a | n | %a | ||
| Total | 110,872 | 2,088 | 622 | 243,360 | 4,411 | 5,397 | |||||||
| Number of contacts by type | Household | 139,802 | 56 | 2,032 | 50 | 706 | 55 | 138,095 | 57 | 1,917 | 43 | 2,528 | 47 |
| Other close contacts | 108,041 | 44 | 2,004 | 50 | 583 | 45 | 105,265 | 43 | 2,494 | 57 | 2,869 | 53 | |
| Sex | Female | 56,554 | 51 | 1,325 | 63 | 472 | 76 | 121,183 | 50 | 2,689 | 61 | 4,139 | 77 |
| Male | 54,318 | 49 | 763 | 37 | 150 | 24 | 120,473 | 50 | 1,684 | 38 | 1,216 | 23 | |
| Unknown/ other | 0 | 0 | 0 | 0 | 0 | 0 | 1,704 | 1 | 38 | 1 | 42 | 1 | |
| Age (years) | 0–11 | 0 | 0 | 0 | 0 | 0 | 0 | 42,119 | 17 | 0 | 0 | 0 | 0 |
| 12–17 | 0 | 0 | 0 | 0 | 0 | 0 | 19,770 | 8 | 0 | 0 | 0 | 0 | |
| 18–29 | 31,736 | 29 | 209 | 10 | 122 | 20 | 57,264 | 24 | 437 | 10 | 961 | 18 | |
| 30–49 | 42,142 | 38 | 347 | 17 | 179 | 29 | 54,591 | 22 | 562 | 13 | 1,102 | 20 | |
| 50–74 | 34,383 | 31 | 1,155 | 55 | 194 | 31 | 58,688 | 24 | 2,688 | 61 | 2,280 | 42 | |
| ≥ 75 | 2,611 | 2 | 377 | 18 | 127 | 20 | 4,321 | 2 | 724 | 16 | 1,054 | 20 | |
| Unknown | 0 | 0 | 0 | 0 | 0 | 0 | 6,607 | 3 | 0 | 0 | 0 | 0 | |
| Vaccine received | Vaxzevria | NA | 1,144 | 55 | 35 | 6 | NA | 2,127 | 48 | 407 | 8 | ||
| Comirnaty | NA | 890 | 43 | 530 | 85 | NA | 1,235 | 28 | 3,312 | 61 | |||
| Janssen | NA | 0 | 0 | 21 | 3 | NA | 0 | 0 | 83 | 2 | |||
| Spikevax | NA | 54 | 3 | 36 | 6 | NA | 86 | 2 | 247 | 5 | |||
| Unknown | NA | 0 | 0 | 0 | 0 | NA | 963 | 22 | 1,348 | 25 | |||
| Household compositionb | Couple with children | 12,782 | 15 | 117 | 8 | 61 | 14 | 34,603 | 25 | 126 | 7 | 286 | 11 |
| Couple without children | 33,096 | 40 | 809 | 58 | 181 | 43 | 32,264 | 23 | 908 | 47 | 914 | 36 | |
| Household with > two adults | 21,459 | 26 | 272 | 19 | 112 | 27 | 55,408 | 40 | 648 | 34 | 1,062 | 42 | |
| Other | 16,056 | 19 | 197 | 14 | 68 | 16 | 15,820 | 11 | 235 | 12 | 266 | 11 | |
| Month of notification date of the index case | Feb | 29,953 | 27 | 196 | 9 | 43 | 7 | 62,213 | 26 | 182 | 4 | 374 | 7 |
| Mar | 38,573 | 35 | 435 | 21 | 143 | 23 | 88,116 | 36 | 738 | 17 | 1,571 | 29 | |
| Apr | 20,648 | 19 | 448 | 21 | 151 | 24 | 45,977 | 19 | 919 | 21 | 1,252 | 23 | |
| May | 21,698 | 20 | 1,009 | 48 | 285 | 46 | 47,054 | 19 | 2,572 | 58 | 2,200 | 41 | |
COVID-19: coronavirus disease; NA: not applicable.
a Column percentage.
b Only presented for index cases with household contacts (n=85,210) and for household contacts (n=142,540).
Vaccine effectiveness against transmission
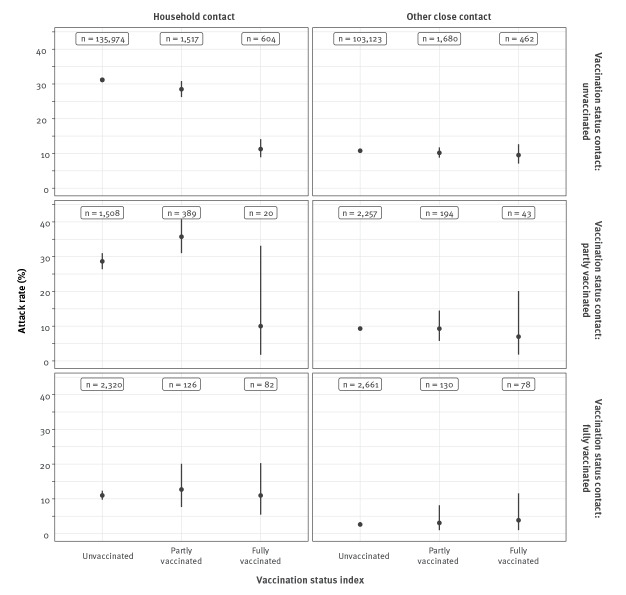
The Figure shows the crude attack rates among contacts, in relation to the vaccination status of both index and contact. The SAR was 31% among household contacts of unvaccinated index cases and 11% among household contacts of fully vaccinated index cases (Table 2). Adjusting for age of the index and contact, vaccination status of the contact and month of notification date of the index case, the VET to household contacts after full vaccination was 71% (95% confidence interval (CI): 63 to 77). The VET to other close contacts was much lower (22%; 95% CI: −5 to 43), probably because of the larger risk of the contact being infected through another source (i.e. misclassification of the index case). Stratified by vaccine received by the index case, VET values were estimated at 58% for Vaxzevria (ChAdOx1-S; AstraZeneca, Cambridge, United Kingdom), 70% for Comirnaty (BNT162b2; BioNTech/Pfizer, Mainz, Germany/New York, United States (US)), 88% for Spikevax (mRNA-1273, Moderna, Cambridge, US) and 77% for the Janssen vaccine. For all vaccines with a two-dose schedule, the adjusted VET (aVET) after one dose was considerably lower than after two doses: 15% for Vaxzevria, 26% for Comirnaty and 51% for Spikevax (Table 2).
Figure.
Crude attack rate of SARS-CoV-2 among contacts, by vaccination status of the index (left to right) and vaccination status of the contact (top to bottom), the Netherlands, 1 February−27 May 2021 (n = 113,582 index cases, n = 253,168 contacts)
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
The n subtotals in the panels indicate the number of unvaccinated/partly vaccinated/fully vaccinated household and other close contacts of unvaccinated/partly vaccinated/fully vaccinated index cases. For example, n=604 in the upper left panel denotes that fully vaccinated index cases had 604 unvaccinated household contacts.
Table 2. Secondary attack rate of SARS-CoV-2 by vaccination status of the index case (≥ 18 years) and vaccine effectiveness against transmissiona, crude and adjusted for age group of the index caseb and contactc and for vaccination statusd of contacts and month of notification date of the index case, the Netherlands, 1 February−27 May 2021 (n = 113,582 index cases, n = 253,168 contacts).
| Analysis | Unvaccinated index - SAR | Partly vaccinated index - SAR | Partly vaccinated index - crude VET (95% CI) | Partly vaccinated index - adjusted VET (95% CI) | Fully vaccinated index - SAR | Fully vaccinated index - crude VET (95% CI) | Fully vaccinated index - adjusted VET (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Total | % | Positive | Total | % | Positive | Total | % | |||||
| Household contacts | 43,069 | 139,802 | 31 | 587 | 2,032 | 29 | 9 (−1 to 17) | 21 (12 to 28) | 79 | 706 | 11 | 72 (65 to 78) | 71 (63 to 77) |
| Household contacts - unvaccinated only | 42,382 | 135,974 | 31 | 432 | 1,517 | 28 | 12 (1 to 21) | 23 (14 to 32) | 68 | 604 | 11 | 73 (64 to 79) | 73 (65 to 79) |
| Other close contacts | 11,395 | 108,041 | 11 | 193 | 2,004 | 10 | 11 (−4 to 23) | 22 (9 to 33) | 50 | 583 | 9 | 22 (−5 to 42) | 22 (−5 to 43) |
| Other close contacts - unvaccinated only | 11,115 | 103,123 | 11 | 171 | 1,680 | 10 | 7 (−9 to 21) | 22 (8 to 34) | 44 | 462 | 10 | 14 (−18 to 37) | 24 (−5 to 45) |
| Household contacts - Vaxzevria | 43,069 | 139,802 | 31 | 364 | 1,306 | 28 | 13 (1 to 23) | 15 (4 to 26) | 5 | 39 | NP | 67 (16 to 87) | 58 (−12 to 84) |
| Household contacts - Comirnaty | 43,069 | 139,802 | 31 | 211 | 663 | 32 | −4 (−22 to 12) | 26 (12 to 37) | 70 | 596 | 12 | 71 (62 to 77) | 70 (61 to 77) |
| Household contacts - Spikevax | 43,069 | 139,802 | 31 | 12 | 63 | NP | 46 (−3 to 71) | 51 (8 to 74) | 2 | 40 | NP | 88 (51 to 97) | 88 (50 to 97) |
| Household contacts - Janssen | 43,069 | 139,802 | 31 | NA |
NA | NA | 2 | 31 | NP | 85 (37 to 96) | 77 (6 to 94) | ||
CI: confidence interval; GEE: generalised estimating equations; NA: not applicable; NP: not presented; SAR: secondary attack rate; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; VET: vaccine effectiveness against transmission.
a VET estimated by GEE with exchangeable correlation structure.
b Age groups index cases: 18–29, 30–49, 50–74, ≥75 years.
c Age groups contacts: 0–17, 18–59, ≥60 years.
d Vaccination status of contacts: unvaccinated, partly or fully vaccinated.
Partly vaccinated was defined as having received the first dose of a two-dose schedule at least 14 days before onset of symptoms. Fully vaccinated was defined as having completed a two-dose schedule at least 7 days or the one-dose Janssen schedule at least 14 days before symptom onset. Only percentages based on denominators above 100 are presented, please note the VET estimates based on smaller numbers are less precise.
Vaccine effectiveness among contacts
The adjusted VE (aVE) for fully vaccinated household contacts of confirmed cases was estimated at 75% (95% CI: 72 to 78) and for fully vaccinated other close contacts at 79% (95% CI: 74 to 83) (Table 3). Stratified by the vaccine received by the contact, aVE was 87% for Vaxzevria, 65% for Comirnaty, 91% for Spikevax and 12% for Janssen’s vaccine. Note that the estimate for the Janssen vaccine was based on only 44 vaccinated contacts, with a median time since vaccination of 21 days. The proportion of vaccinated contacts with unknown vaccine manufacturer was large (Table 1), which reduces the power of the analyses stratified by vaccine.
Table 3. Attack rate among close contacts (≥ 18 years) of confirmed SARS-CoV-2 infected index cases, and vaccine effectiveness against infectiona, crude and adjusted for age group of the index caseb and contactc and for vaccination status of indexd, and month of notification date of the index case, the Netherlands, 1 February−27 May 2021 (n =184,672 contacts).
| Analysis | Unvaccinated contacts - AR | Partly vaccinated contacts - AR | Partly vaccinated contacts - crude VE (95% CI) | Partly vaccinated contacts - adjusted VE (95% CI) | Fully vaccinated contacts - AR | Fully vaccinated contacts - crude VE (95% CI) | Fully vaccinated contacts - adjusted VE (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Total | % | Positive | Total | % | Positive | Total | % | |||||
| Household contacts | 32,086 | 91,528 | 35 | 573 | 1,917 | 30 | 21 (13 to 28) | 23 (14 to 30) | 280 | 2,528 | 11 | 76 (73 to 79) | 75 (72 to 78) |
| Household contacts - only unvaccinated indexes | 31,694 | 90,066 | 35 | 432 | 1,508 | 29 | 25 (17 to 33) | 26 (17 to 34) | 255 | 2,320 | 11 | 77 (74 to 79) | 76 (73 to 79) |
| Other close contacts | 9,883 | 83,336 | 12 | 231 | 2,494 | 9 | 24 (13 to 34) | 28 (17 to 37) | 77 | 2,869 | 3 | 79 (74 to 83) | 79 (74 to 83) |
| Other close contacts - only unvaccinated indexes | 9,699 | 81,666 | 12 | 210 | 2,257 | 9 | 24 (12 to 34) | 27 (15 to 37) | 70 | 2,661 | 3 | 79 (74 to 84) | 80 (74 to 84) |
| Household contacts - Vaxzevria | 32,086 | 91,528 | 35 | 356 | 1,052 | 34 | 5 (−8 to 16) | 2 (−11 to 14) | 11 | 186 | 6 | 88 (78 to 93) | 87 (77 to 93) |
| Household contacts - Comirnaty | 32,086 | 91,528 | 35 | 194 | 467 | 42 | −30 (−56 to −8) | −18 (−43 to 2) | 242 | 1,605 | 15 | 67 (62 to 71) | 65 (60 to 70) |
| Household contacts - Spikevax | 32,086 | 91,528 | 35 | 11 | 48 | NP | 45 (−5 to 71) | 33 (−27 to 64) | 4 | 121 | 3 | 93 (83 to 97) | 91 (79 to 97) |
| Household contacts - Janssen | 32,086 | 91,528 | 35 | NA | NA | NA | 11 | 44 | NP | 37 (−21 to 67) | 12 (−71 to 54) | ||
AR: attack rate; CI: confidence interval; GEE: generalised estimating equations; NA: not applicable; NP: not presented; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; VE: vaccine effectiveness.
a VE estimated by GEE with exchangeable correlation structure.
b Age groups index cases: 18–29, 30–49, 50–74, ≥75 years.
c Age groups contacts: 0–17, 18–59, ≥60 years.
d Vaccination status of contacts: unvaccinated, partly or fully vaccinated.
Only contacts 18 years and older were included. Partly vaccinated was defined as having received the first dose of a two-dose schedule at least 14 days before onset of symptoms. Fully vaccinated was defined as having completed a two-dose schedule at least 7 days or the one-dose Janssen schedule at least 14 days before symptom onset. Only percentages based on denominators above 100 are presented, please note the VE estimates based on smaller numbers are less precise.
Discussion
We estimated a VET of 71% among household contacts of fully vaccinated index cases. Harris et al. found a VET of 40–50% for unvaccinated households contacts [5]. That study mostly included partly vaccinated index cases. A study among household contacts of healthcare workers by Shah et al. found a 30% (after the first dose) and 64% (after the second dose) reduction in the risk of confirmed SARS-CoV-2 infection among household members of vaccinated healthcare workers, however these healthcare workers were not confirmed as index cases [6].
Martínez-Baz et al. recently estimated VE among 20,961 close contacts of confirmed cases in Spain [7]. In that study, the VE for two doses of Comirnaty was 65% (95% CI: 56 to 73) against infection, which is in agreement with our finding of 65% VE for this vaccine. These estimates are lower than VE from other observational post-marketing studies. Possibly, the VE against infection is lower when there is high and prolonged exposure to SARS-CoV-2, which is likely for household contacts of confirmed cases [8]. Of note, Martínez-Baz et al. showed an aVE for Comirnaty of 94% (95% CI: 60 to 99) against hospitalisation among close contacts of confirmed cases.
As our study used data not primarily collected for research purposes, it has some important limitations. Our data do not contain information on negative tests among contacts, therefore we do not know if contacts did not get infected or did not seek testing. However, it is likely that close contacts were tested regardless of vaccination status, as the quarantine period for close contacts at the time was reduced from 10 to 5 days when tested negative on day 5 after exposure. For contacts who tested positive, data on vaccination status were more complete because missing data could be supplemented from the notifications. We explored whether this differential completeness influenced our results by excluding all index cases with any household contact with unknown vaccination status or date of vaccination, and the results were the same (data not shown).
Although we tried to minimise misclassification of indexes and contacts by excluding index cases infected at home and contacts with symptom onset before or at the same time as the index, it is plausible that in some instances, the transmission route was reversed or transmission occurred though another source (especially for non-household contacts). If some contacts of vaccinated index cases were infected through other sources, our VET is an underestimation. In addition, we do not have reliable data on the symptoms of the included index cases. Because our analysis on household contacts was restricted to notified index cases not infected at home, probably most of these index cases sought testing because they had symptoms. Symptomatic cases may have been misclassified as index cases in a household, where in reality an asymptomatic household member was the source of transmission to the supposed index case and a third household member. If vaccinees are more likely to be asymptomatic, this source of misclassification may result in an overestimation of the VET.
As the Alpha variant of SARS-CoV-2 dominated during the study period, an important question is to what extent these VET and VE estimates hold in the context of the Delta variant (Pango lineage designation B.1.617.2) which is now dominant in the Netherlands. Also, further research is needed to determine whether the observed differences between the different vaccines are due to the small sample size or have real public health relevance. We will prospectively monitor both VET and VE among household contacts over the next months to address these questions.
Conclusion
Our study showed that the COVID-19 vaccines not only protect the vaccinee against SARS-CoV-2 infection, but also offer protection against transmission to close contacts after completing the full schedule. This finding underscores the importance of full vaccination of close contacts of vulnerable persons.
Acknowledgements
The authors would like to thank all source and contact tracing personnel at the 25 Municipal Health Services (GGDen) who have been invaluable for control and surveillance of the COVID-19 epidemic in the Netherlands, and who collected these important data. Funding: This work was funded by the Ministry of Health, Welfare and Sports.
Conflict of interest: None declared.
Authors’ contributions: Study design: Brechje de Gier, Stijn Andeweg, Jan van de Kassteele, Mirjam Knol. Data collection, management and quality control: Rosa Joosten, Ronald ter Schegget, Stijn Andeweg, Naomi Smorenburg, the RIVM COVID-19 surveillance and epidemiology team. Data analysis: Brechje de Gier, Stijn Andeweg. Interpretation of the data: Brechje de Gier, Stijn Andeweg, Susan Hahné, Susan van den Hof, Hester de Melker, Mirjam Knol. Manuscript draft: Brechje de Gier. All authors critically revised the manuscript and approved the final manuscript.
References
- 1. de Gier B, de Oliveira Bressane Lima P, van Gaalen RD, de Boer PT, Alblas J, Ruijten M, et al. Occupation- and age-associated risk of SARS-CoV-2 test positivity, the Netherlands, June to October 2020. Euro Surveill. 2020;25(50):2001884. 10.2807/1560-7917.ES.2020.25.50.2001884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Infectious Disease Control. Protocol bron- en contactonderzoek COVID-19. [Protocol source and contact tracing]. Bilthoven: Rijksinstituut voor Volksgezondheid en Milieu; 8 July 2021. Dutch. Available from: https://lci.rivm.nl/COVID-19-bco
- 3. Snijders BEP, van Lier A, van de Kassteele J, Fanoy EB, Ruijs WLM, Hulshof F, et al. Mumps vaccine effectiveness in primary schools and households, the Netherlands, 2008. Vaccine. 2012;30(19):2999-3002. 10.1016/j.vaccine.2012.02.035 [DOI] [PubMed] [Google Scholar]
- 4. Halloran ME, Préziosi M-P, Chu H. Estimating vaccine efficacy from secondary attack rates. J Am Stat Assoc. 2003;98(461):38-46. 10.1198/016214503388619076 [DOI] [Google Scholar]
- 5.Harris RJ, Hall JA, Zaidi A, Andrews NJ, Dunbar JK, Dabrera G. Impact of vaccination on household transmission of SARS-COV-2 in England. London: Knowledge Hub; 2021. Available from: https://khub.net/documents/135939561/390853656/Impact+of+vaccination+on+household+transmission+of+SARS-COV-2+in+England.pdf/35bf4bb1-6ade-d3eb-a39e-9c9b25a8122a?t=1619551571214 [DOI] [PMC free article] [PubMed]
- 6.Shah AS, Gribben C, Bishop J, Hanlon P, Caldwell D, Wood R, et al. Effect of vaccination on transmission of COVID-19: an observational study in healthcare workers and their households. medRxiv. 2021:2021.03.11.21253275.
- 7. Martínez-Baz I, Miqueleiz A, Casado I, Navascués A, Trobajo-Sanmartín C, Burgui C, et al. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection and hospitalisation, Navarre, Spain, January to April 2021. Euro Surveill. 2021;26(21):2100438. 10.2807/1560-7917.ES.2021.26.21.2100438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Halloran ME, Haber M, Longini IM, Jr, Struchiner CJ. Direct and indirect effects in vaccine efficacy and effectiveness. Am J Epidemiol. 1991;133(4):323-31. 10.1093/oxfordjournals.aje.a115884 [DOI] [PubMed] [Google Scholar]