Abstract
Giant cell tumor of bone (GCTB) is a benign neoplasia more frequently encountered in young females. The pathogenic and evolutionary dynamics of the disease is strongly influenced by the presence of depression and cellular mechanisms, especially proinflammatory and immune. Although it is not a malignant tumor, it is often recurrent, which determines a high level of depression, anxiety, and fear of the patients. Cytokine mechanisms, especially through increased tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6), as well as the involvement of the receptor activator of nuclear factor-kappa B (RANK)–RANK ligand (RANK-L) system, can be correlated with the risk of malignancy. Unfavorable evolution is associated with persistent pain, difficulties of movement and body dysmorphic symptoms. The diagnosis is based mainly on histopathological (HP) assessment. The patients can be treated with pharmacological agents (Denosumab), surgery with tumor excision, reconstruction or osteosynthesis, and radiotherapy. Patients with GCTB require HP and imaging evaluations, especially of relapses, to detect the risk of metastasis or malignancy, simultaneously with psychological and psychiatric monitoring to detect depression, addictive behaviors, and suicide risk. It is necessary to evaluate in a multidisciplinary team to avoid unfavorable oncological and psychiatric developments. Through its clinical, HP, and therapeutic features, GCTB has multiple connections with the psychological and psychopathological dimension.
Keywords: giant cell tumor of bone, depression, anxiety, pain, cytokine
⧉ Introduction
Giant cell tumor of bone (GCTB) or osteoclastoma is a benign neoplasia which represents approximately 4–10% of all primary tumors located in the bone system [1,2]. This type of tumor occurs most commonly in young people aged 20–45 years, especially affects the metaphyseal and epiphyseal areas of long bone, such as femur, tibia, radius or humerus. This tumor is more common in women and can be diagnosed in patients over 50 years old [3]. The main symptoms are represented by local chronic pain, swelling, difficulties of movement of the adjacent joint [1, 3,4]. Acute pain is associated with pathological bone fracture, which can occur in 15–20% of cases due to bone destruction or trauma [5]. Persistent pain that accompanies neoplasms (benign or malignant) is a major risk factor for depression [6] and suicidal behavior [7,8].
Neurobiochemical support of depression and suicide risk may be correlated with dopaminergic system dysfunction due to chronic pain [9]. Dopamine deficiency predominates in the mesolimbic system, associating affective comorbidities related to the dysconnectivity of the nucleus accumbens with the frontal cortex and amygdala. These comorbidities associated with chronic pain are mainly represented by depression and vulnerability to addictive behavior, depression can have a prevalence of up to 30% [10]. Before the onset of depression, an increase in anxiety and social stress was observed, which often delays the diagnosis of depressive disorder.
⧉ Diagnosis and treatment options in GCTB
The diagnosis of GCTB consists in radiological evaluation and bone biopsy. If the tumor is recurrent, computed tomography (CT) scan is needed to exclude lung metastases [11,12]. Metastases can occur in 1–9% of cases, most commonly found in the lungs and rarely occur in lymph nodes, skin or even other bones. Histopathological (HP) examination of metastatic nodules showed a benign character, similar to that of the primary tumor [13]. Treatment of the primary tumor consists in surgical resection followed by reconstruction with cement, internal fixing with plate and screws, or bone grafting [14,15,16]. Relapses can be observed in 2.5% to 45% of the cases, depending on the location of the tumor and the surgical method used in the treatment of the primary tumor [17].
The treatment of relapses consists in surgical resection or, if the resection cannot be performed, radiotherapy and embolization. Pharmacological treatment may use bisphosphonates in neoadjuvant settings for targeting osteoclast-like giant cells inducing apoptosis and limiting the progression of the tumor [18,19]. Denosumab is the only drug approved by The United States Food and Drug Administration (FDA), for patients with unresectable, recurrent or metastatic GCTB, or in cases where surgery has a high risk of death [20]. Denosumab is a monoclonal antibody [immunoglobulin G2 (IgG2)] of fully human type, which decreases bone resorption by specific binding to the receptor activator of nuclear factor-kappa B ligand (RANK-L), which inhibits the RANK receptor [5, 21].
⧉ The psycho-pathological dimension in GCTB
Even though GCTBs are benign, they bring an important psychology response associated with the fear of relapse, metastasis or malignant and the presence of pain and difficulties of movement. This fear of recurrence is probably similar to the fear of cancer recurrence, associated with young age, severity of physical symptoms (especially pain) and functional impairments and the presence of stress-inducing factors [22]. In addition to the possibility of recurrence or metastasis of GCTB, the ability of malignancy was highlighted, with a variable rate of 1–11%, an aspect that positions it in a real challenge for the clinician. Aggressive tumor growth and local recurrences are risk factors for metastasis [23], while depressive-anxiety disorders may be considered risk factors for GCTB recurrence.
The association with depression and multiple recurrences seem to favor malignancy, which is why the correct strategies to approach this pathology must be interdisciplinary [24,25,26]. Regardless of the prognosis of GCTB, persistent chronic pain favors the risk of developing suicidal ideation and behavior, but also of depression frequently accompanied by addictive tendencies, especially by dopaminergic deficiency [27,28]. On the other hand, body dysmorphic symptoms and social isolation may occur in GCTB, which can be interpreted by the “interpersonal psychological theory of suicide” as risk factors for suicide [29].
In the treatment of malignant tumors, the approach is interdisciplinary and psychopathological manifestations, especially depressive-anxiety disorders, and stress, are recognized as evolutionary and prognostic risk factors. In the case of benign tumors, this translational strategy is less considered. GCTB highlights, through its clinical, HP and therapeutic particularities, the multiple connections with the psychological and psychopathological dimension. This relationship is amplified by multiple recurrences and surgical bone reconstruction therapies, which can cause body dysmorphic symptoms, but also by persistent pain. These symptoms are risk factors for the development of depressive disorder with suicidal potential. In this context, must be understood the multidisciplinary aspects and the multifactorial mechanisms involved in the therapeutic and recovery process that suggest the imperative need for an interdisciplinary approach. Even if GCTB is benign, it cannot be overlooked that persistent pain, body dysmorphic symptoms, and decreased adaptive coping capacity are evolutionary and prognostic risk factors, which are common to those with malignancies. This similarity raises the suspicion that malignancy could be favored precisely by the multisystemic inflammatory, angiogenetic, and cellular mechanisms favored by depressive disorder.
⧉ Histopathological assessment
From a clinical point of view, the diagnosis of GCTB goes through several stages, the initial one being dominated by pain, motor dysfunction, depression, anxiety, and fear of a possible oncological diagnosis. After surgery for tumor resection, patients experience a false impression of healing, not being psychologically prepared for possible recurrences or risks of metastasis, especially in the lung, or even malignancy. Usually, the initial therapy is based on pharmacological treatment with analgesics, anti-inflammatory drugs and sometimes anxiolytics, without obtaining good quality results. Patients with suspected GCTB are referred to surgery for HP diagnostic evaluation to confirm the diagnosis. The evolution of GCTB is often associated with a favorable prognosis, but may be accompanied by local recurrences, persistent pain that exacerbates depressive disorders, with the occurrence of a possible risk of malignancy.
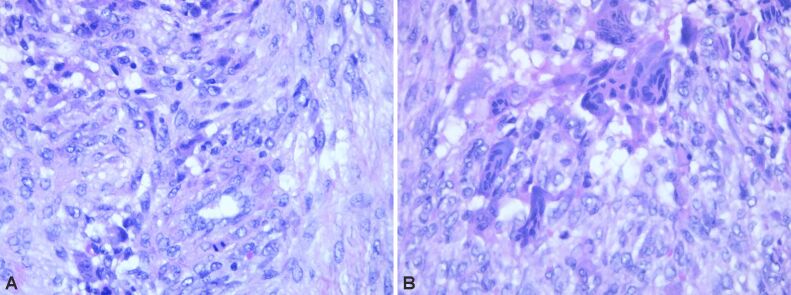
HP examination can most frequently highlight the following disease-specific variants: numerous giant multinucleated cells with osteoclastic aspect, uniformly distributed in a proliferation of mononucleated cells, round-oval and fusiform, with mitotic rate of 7 mitoses/10 high-power fields (HPFs) (×40 objective fields). Nuclear characteristics of the multinucleate cells were similar with the mononucleated ones. No tumor necrosis, but tumor proliferation consists of polygonal or ovoid mononucleated cells, with vesicular nuclei, fusiform cells with long vesicular nuclei, arranged in interlaced beams. Frequent giant multinucleate cells with osteoclastic aspect, variate size, density and position, multiple vesicular nuclei in resemblance with stromal cell’s nuclei. Frequent mitoses, over 10 mitoses/10 HPFs. Hemorrhagic areas, no necrosis, small areas of fibrosis. Tumor proliferation is osteolytic, relatively rare bone lashes, sub periosteal with the invasion of periosteum (Figures 1,2,3,4; Figure 5, A and B).
Figure 1.
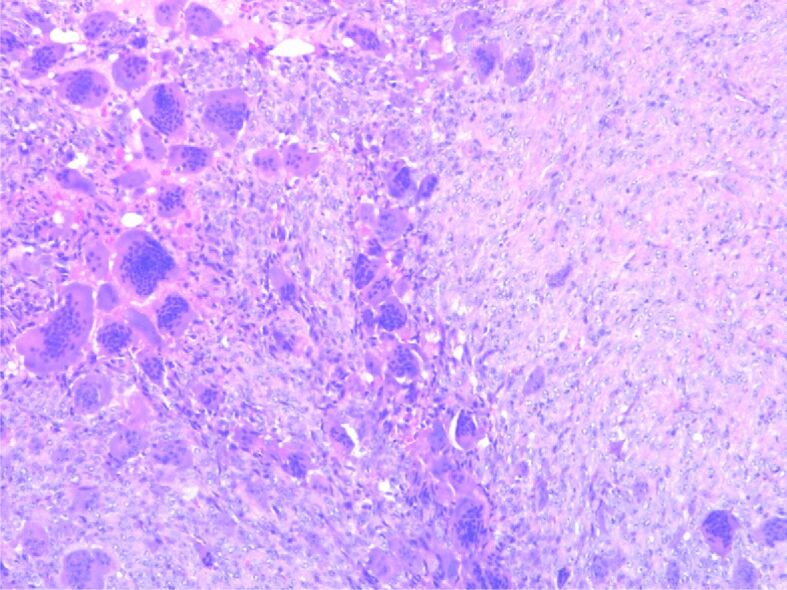
Proliferation of mononuclear cells with sporadic typical mitotic figures, including frequent large-cell nuclei with multiple nuclei (over 20/cell), sporadic intratumoral lymphocytes. Hematoxylin–Eosin (HE) staining, ×100
Figure 2.
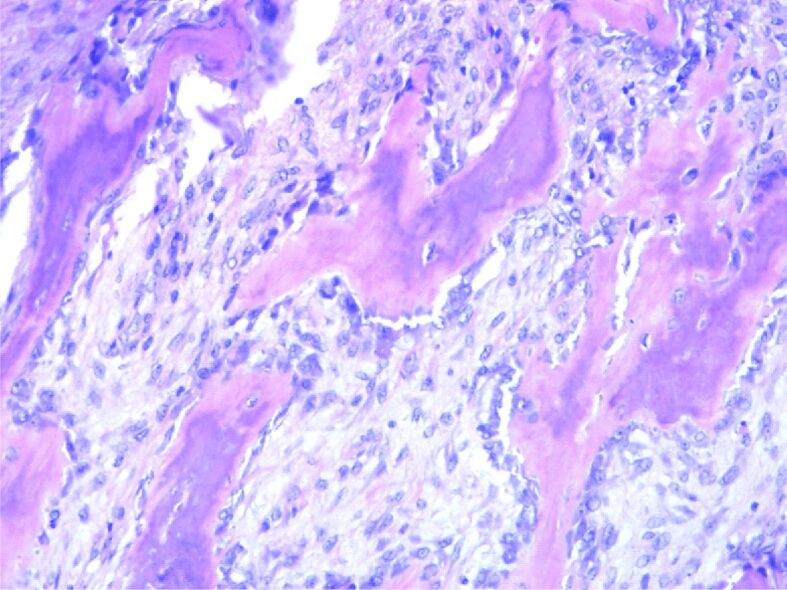
Remnants of bone lashes, marginal to tumor proliferation. HE staining, ×200
Figure 3.
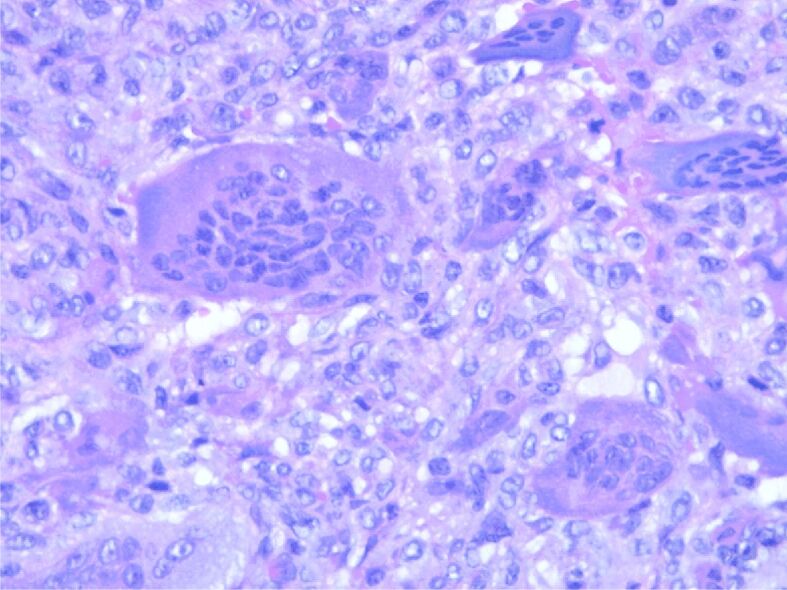
Tumor proliferation with multiple nucleated cells with more than 50 nuclei. HE staining, ×400
Figure 4.
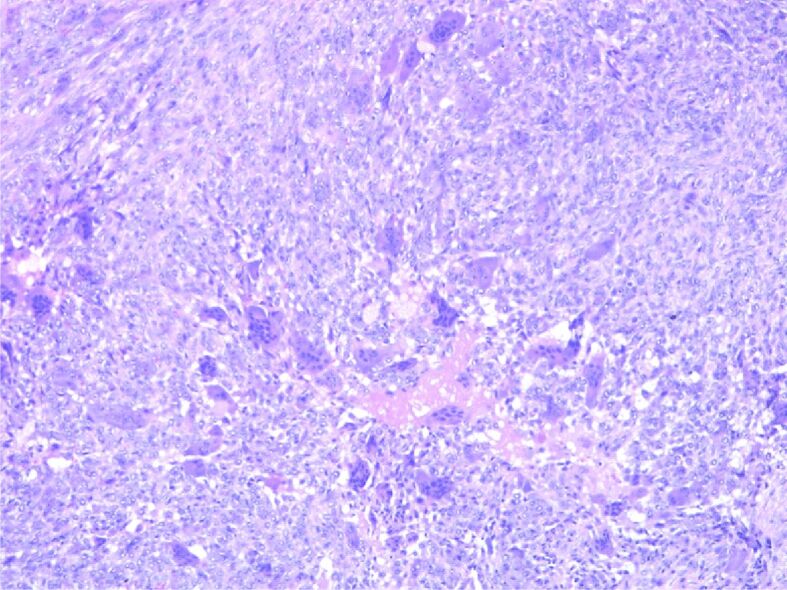
Bone tumor detail with mononuclear cells and multinucleated cells. HE staining, ×100
Figure 5.
(A) Bone tumor detail with typical mitosis in mononuclear cells. (B) Bone tumor detail with sporadic mitosis in mononuclear cells. HE staining: (A and B) ×400
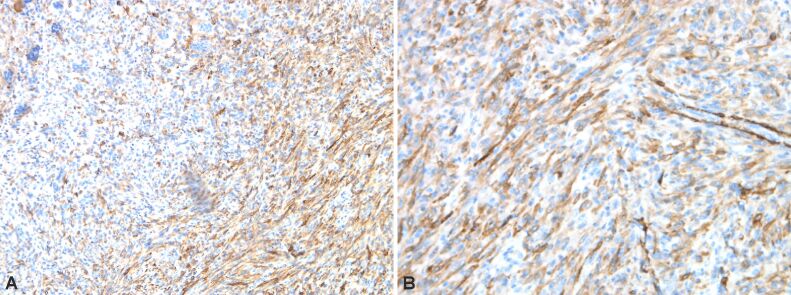
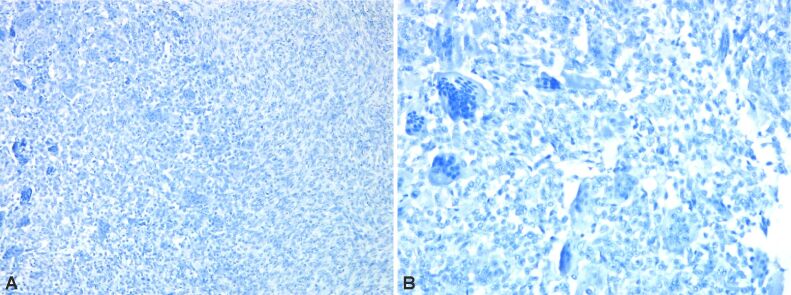
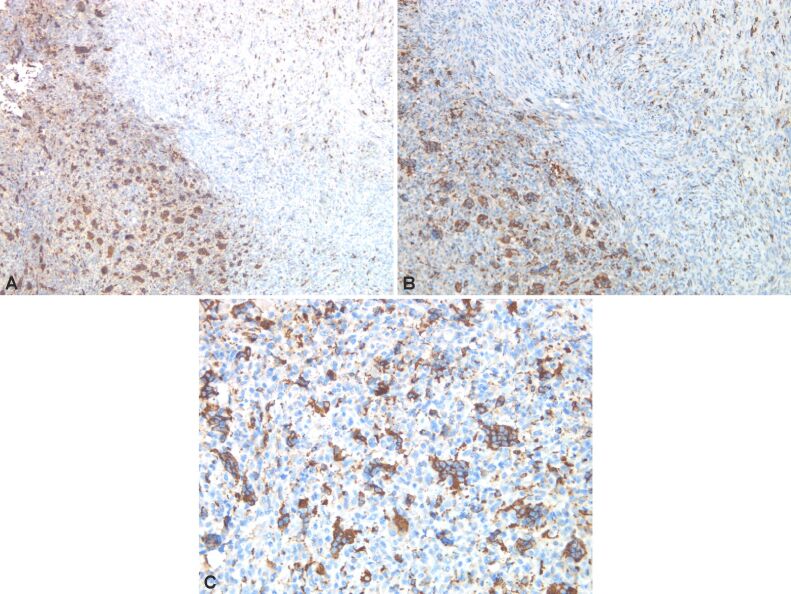
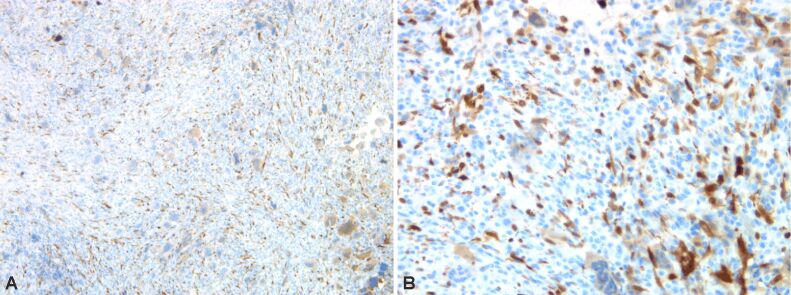
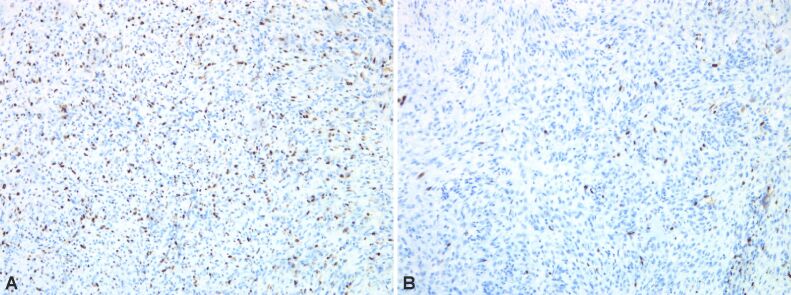
Immunostaining highlights giant multinucleate cells positive for cluster of differentiation 68 (CD68), fusiform cells positive for alpha-smooth muscle actin (α-SMA), cytokeratin (CK) AE1/AE3 negative (no epithelial cells), p53 protein positive in 30% of the fusiform cellular nuclei, Ki67 index positive 10% in fusiform component (Figures 6 and 7, A and B; Figure 8A,8B,8C; Figures 9 and 10, A and B).
Figure 6.
(A and B) Bone tumor with positive immunoreaction to α-SMA in mononucleate cells. Anti-α-SMA antibody immunomarking: (A) ×100; (B) ×200. α-SMA: Alpha-smooth muscle actin
Figure 7.
(A and B) Bone tumor with negative immunoreaction to desmin. Anti-desmin antibody immunomarking: (A) ×100; (B) ×200
Figure 8.
(A–C) Bone tumor with positive CD68 immunomarker in multicellular cells and dispersed mononuclear cells. Anti-CD68 antibody immunomarking: (A) ×50; (B) ×100; (C) ×200. CD68: Cluster of differentiation 68
Figure 9.
(A and B) Bone tumor with p16 cytoplasmic immunoreaction in multinucleated cells; cytoplasmic and nuclear immunoreaction in dispersed mononuclear cells. Anti-p16 antibody immunomarking: (A) ×50; (B) ×200
Figure 10.
(A) Bone tumor with Ki67 immunoreaction in rare mononuclear tumor cells dispersed and immunoreaction in frequent inflammatory cells. (B) Bone tumor with Ki67 immunoreaction in dispersed mononuclear tumor cells and rare inflammatory cells. Anti-Ki67 antibody immunomarking: (A and B) ×100
⧉ Multifactorial model of psychiatric disorders in GCTB
GCTB tumor, due to the constant presence of pain generated by the disease but also by resection and reconstruction surgery, frequently associates severe depression, which can be present even in 85% of patients with chronic pain [30]. Reconstructive surgeries are associated with increased distress due to excessive pain and alteration of body image perception. This rises the anxiety levels and the risk of suicidal behavior [8, 31]. In psychiatric disorders of bone tumors, it is important to choose a proper antidepressant therapy, that would not increase prolactin levels, these being involved in bone loss [32,33], but it would be efficient in obtaining a stable remission of depression and anxiety, incomplete remission favoring immune system dysfunction [34] (Figure 11).
Figure 11.
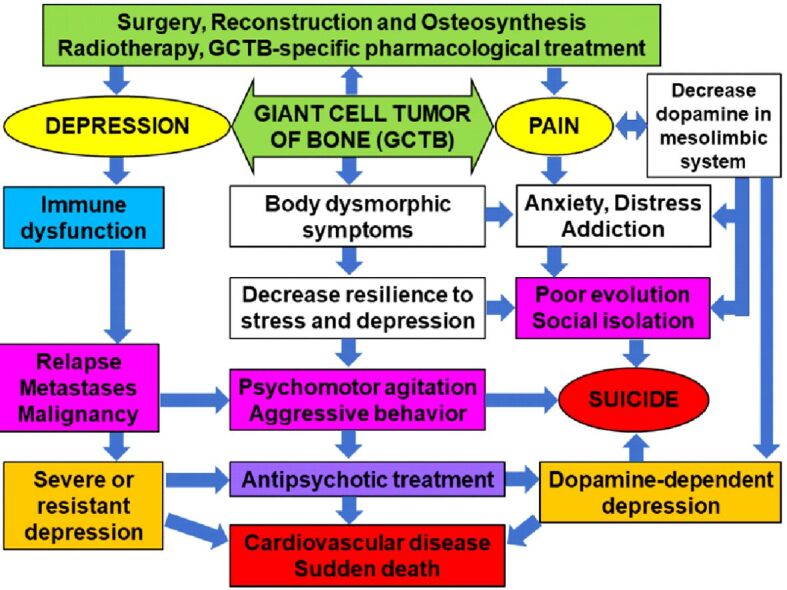
Multifactorial theoretical model of psychiatric disorders in patients with giant cell tumor of bone (GCTB)
The association of psychological factors of stress, especially of depression, favors the increase of cytokine-type proinflammatory factors, such as tumor necrosis factor alpha (TNFα), interleukin-6 (IL-6) and IL-1β [35]. Thus, IL-6 can become a marker for predicting the severe evolution of depression, especially in the context of a particular physical symptomatology (body dysmorphic symptoms). This symptomatology is more common in young women, being an important factor in decreasing compliance with therapy and psychometric assessments, but also a factor in increasing the therapeutic resistance of depression, which may favor oncogenesis processes. On the other hand, the relationship between the presence of adipose tissue of CD68, IL-6 and TNFα with obesity and resistance to insulin treatment was highlighted [36].
Simultaneous increase in these factors may be a risk of predicting the occurrence of psychotic disorders, persistent pain, and cognitive impairment. The use of antipsychotics may lower the level of CD68, involved in inflammatory processes. The use in GCTB of antipsychotics for the control of chronic pain, treatment-resistant depression, and psychotic symptoms, may bring predominantly symptomatic benefits [37].
In patients with GCTB and chronic pain, complex mechanisms and stress-favored cardiovascular effects may exacerbate the risk of severe cardiovascular effects following prolonged QT interval. The QT interval may be increased by hypocalcemia induced by denosumab treatment [38]. In this context, antipsychotic treatment has a major contraindication in patients with hypocalcemia and requires specific cardiological monitoring, because it increases the risk of sudden death by multiple cellular mechanisms. The vulnerability of patients with GCTB and distress favors the activation of the hypothalamic–pituitary–adrenal (HPA) axis, with the symmetrical disturbance of the functionality of the autonomous system. This dysautonomia is dominated by sympathomimetic hyperactivity, which increases the risk of cardiovascular and cerebrovascular events [39]. In addition to prolonging the QT interval, hypocalcemia may also be a risk factor for exacerbation of depressive-anxiety disorder, cognitive impairment, extrapyramidal symptoms, dysautonomia, seizures [40,41].
The risk of suicide in cancer is frequently associated with body dysmorphic syndrome, which determines a permanent stigma and a decrease in adaptive coping capacity by aberrant perception of social exclusion and loss of self-esteem (colostomy, amputation of a limb, breast resection, colon resection). The same situation was observed in solid brain tumors (glioblastoma or astrocytoma), in which chronic hyperalgesia is a vulnerability factor for suicidal behavior due to reduced therapeutic response to chemotherapy and significant neurological, psychiatric, and cognitive sequelae, following radical surgical techniques. Suicide risk is estimated at 62% in glioblastoma [42], and 15% in astrocytoma [43].
In these circumstances, as well as in conditions of GCTB malignancy, which can cause limb amputation [44], the use of alternative therapeutic strategies could improve the prognosis by reducing suicidal risk behaviors. These alternative therapies are based on pain control by stopping the action of hyperglutamatergy, following astroglia activations, while for brain tumor cells the use of compounds with specific proapoptotic action is discussed [45,46,47].
The risk of malignancy can also occur in the case of another benign tumor, giant condyloma Buschke–Löwenstein tumor, being associated with depression, social isolation, and a negative psychiatric prognosis [48]. The risk of malignancy also occurs in meningiomas, which are 92.8% benign on HP examination [49]. Long-term survival of patients with meningioma raises complex therapeutic management problems due to depression [50] and cognitive deficit [51]. On the other hand, these psychiatric disorders can occur even before the diagnosis of brain tumor.
⧉ Psychopharmacological precautions in GCTB
The pharmacological perspective of the standardized treatment in oncological pathology requires a personalization according to the complex characteristics of each case, from a HP, immunohistochemical (IHC), psychological and psychopharmacological point of view. The use of antipsychotics as an adjuvant medication may be useful in limiting the evolution of glioblastomas, and those that act by inhibiting N-methyl-D-aspartate (NMDA) receptors simultaneously have antidepressant effects [52,53].
The psychological perspective requires careful monitoring and early psychiatric therapeutic intervention, from the early stages of GCTB, to correct depression and reduce suicidal potential, caused by diagnostic uncertainty, but also in advanced stages, with multiple recurrences that induce body dysmorphic symptoms. The high levels of depression, anxiety and stress can be correlated with the risk of recurrence, surgical reintervention and the possibility of metastasis or malignancy.
If for the attending physician, patients with GCTB are considered with a favorable evolution since the tumor is benign, the prognosis of the disease, viewed from the perspective of biological psychiatry and psychopharmacology, highlights multiple conditions of evolutionary risk. Chronic pain, difficulties in movement, functional and structural change of self-image (body dysmorphic symptoms) increase anxiety, social stress, and depression. These factors may favor through the multisystemic mechanisms of depression the increase of the risk of malignancy, but especially of the number of recurrences that require reconstruction techniques through new surgeries.
From our point of view, chronic pain is the major point of interest because it determines the reduction and dysfunction of the dopamine system, being considered that the persistent painful syndrome is accompanied by a “hypodopaminergic state” [54,55]. The involvement of low dopamine levels in central pain has been reported in Parkinson’s disease (PD), associated with altered nigrostriatal system and non-motor structures, which may precede by years the onset of motor symptoms in PD. Hyperalgesia is accompanied by depression and autonomic nervous system dysfunction especially by orthostatic intolerance syndrome [56].
Dopamine deficiency-induced depressive disorder is generally underestimated in psychopharmacology, and therapy with pro-serotonergic antidepressants, such as selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants causes worsening dopaminergic deficiency and a lack of therapeutic response. This type of depression is a common psychopharmacological form, responsible for the therapeutic resistance to nondopaminergic antidepressants, especially in women [57].
Dopamine-dependent depression is associated with altered dopaminergic circuits involved in the brain reward system [58]. Biological psychiatry can define depression as the chronic inability of the individual to obtain a satisfactory reward. This hypothesis is objectified by involving the dysconnectivity of the nucleus accumbens and the caudate nucleus [59]. Dopamine deficiency in the nucleus accumbens is an important biological vulnerability for the development of addictive behaviors. The dysconnectivity of reward dopaminergic circuits may be caused by the predominant dopamine deficiency at the presynaptic level [60], this mechanism being correlated with depression and cognitive dysfunction. The alteration of the efficiency of dopaminergic transmission at meso-limbic level, present in chronic pain, is characterized by an increased suicidal risk behavior. This risk may be preceded by sleep disorders resistant to hypnotics or benzodiazepines therapies, and which have a specific clinical pattern with multiple nocturnal awakenings. The risk of suicidal behavior due to dopaminergic deficiency is amplified by addictive behavior, especially by excessive alcohol consumption, by voluntary drug intoxications with analgesics, antidepressants or benzodiazepines or by Internet addiction [10, 61,62].
The decrease of the adaptive coping capacities can be favored by the alteration of the microglial system, especially from the nucleus accumbens, which releases massive glutamate. Hyperglutamatergy amplifies suicidal ideation and catastrophic anticipation of disease progression in 30% of patients with chronic pain [28]. The psychological problems associated with recurrent tumors, such as depression, anxiety, fear, and stress, should not be neglected, as they could lead to a poor prognosis and low quality of life [63].
Early diagnosis and treatment of psychiatric symptoms associated with bone tumors could reduce the risk of suicide and significantly prolong the patient’s life. Psychotropic therapy should avoid the use of pro-serotonergic antidepressants, which decrease dopamine levels and increase the phenomenon of angiogenesis by increasing vascular endothelial growth factor (VEGF), with the risk of metastasis. Serotonin is an angiogenesis factor that amplifies tumor vascularity more than VEGF [64,65]. On the other hand, the excess of serotonin and antipsychotic medication lowers the level of dopamine with increased prolactin levels. Hyperprolactinemia is a factor involved in the risk of developing breast cancer in women [66] and prostate cancer in men [67].
Interestingly, in conditions of depression and stress, there is an amplification of TNFα, and the RANK–RANK-L–osteoprotegerin cytokine system, considered TNF-related proteins, is involved in bone tumors as well as breast and prostate cancer [68,69]. HP and IHC assessment must be correlated with a preventive attitude towards psychiatric risks. Based on these arguments, we recommend the therapeutic approach of these patients in a multidisciplinary team.
⧉ Conclusions
Patients with GCTB need a HP evaluation, especially of relapses, to detect the risk of metastasis or malignancy, simultaneously with psychological and psychiatric monitoring to assess depression, addictive behaviors, and suicide risk. This type of benign tumor has a risk of malignancy that can be increased by depression, anxiety, and stress through complex mechanisms. Chronic pain and body dysmorphic symptoms amplify depression through dopamine-lowering mechanisms at the mesolimbic level, diminishing the mechanisms of reward and adaptive coping, thus increasing the risk of addictive and suicidal behavior. A personalized pharmacological management is required associated with an adequate psychological training of the patient regarding the chronic pain. GCTB, after HP diagnosis, require an interdisciplinary therapeutic approach, including psychiatric, to improve treatment adherence and compliance, evolution and prognosis, and the patient’s quality of life.
Conflict of interest
The authors declare that they have no conflict of interests.
References
- 1.Mavrogenis AF, Igoumenou VG, Megaloikonomos PD, Panagopoulos GN, Papagelopoulos PJ, Soucacos PN. Giant cell tumor of bone revisited. SICOT J. 2017;3:54–54. doi: 10.1051/sicotj/2017041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sobti A, Agrawal P, Agarwala S, Agarwal M. Giant cell tumor of bone - an overview. Arch Bone Jt Surg. 2016;4(1):2–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Reid R, Banerjee S, Sciot R. Giant cell tumours. In: Fletcher CDM, Unni KK, Mertens F, editors. Pathology and genetics of tumours of soft tissue and bone World Health Organization (WHO) Classification of Tumors. Lyon France: International Agency for Research on Cancer (IARC) Press; 2002. pp. 309–312. [Google Scholar]
- 4.Turcotte RE. Giant cell tumor of bone. Orthop Clin North Am. 2006;37(1):35–51. doi: 10.1016/j.ocl.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 5.van der Heijden L, Dijkstra PDS, van de Sande MAJ, Kroep JR, Nout RA, van Rijswijk CSP, Bovée JVMG, Hogendoorn PCW, Gelderblom H. The clinical approach toward giant cell tumor of bone. Oncologist. 2014;19(5):550–561. doi: 10.1634/theoncologist.2013-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maletic V, DeMuri B. Chronic pain and depression: understanding 2 culprits in common. Curr Psychiatry. 2016;15(2):40–44,52,54. https://cdn.mdedge.com/files/s3fs-public/issues/articles/0216CP_Maletic_Web_01.pdf [Google Scholar]
- 7.Tang NKY, Crane C. Suicidality in chronic pain: a review of the prevalence, risk factors and psychological links. Psychol Med. 2006;36(5):575–586. doi: 10.1017/S0033291705006859. [DOI] [PubMed] [Google Scholar]
- 8.Hassett AL, Aquino JK, Ilgen MA. The risk of suicide mortality in chronic pain patients. Curr Pain Headache Rep. 2014;18(8):436–436. doi: 10.1007/s11916-014-0436-1. [DOI] [PubMed] [Google Scholar]
- 9.Jääskeläinen SK, Rinne JO, Forssell H, Tenovuo O, Kaasinen V, Sonninen P, Bergman J. Role of the dopaminergic system in chronic pain - a fluorodopa-PET study. Pain. 2001;90(3):257–260. doi: 10.1016/S0304-3959(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 10.Serafini RA, Pryce KD, Zachariou V. The mesolimbic dopamine system in chronic pain and associated affective comorbidities. Biol Psychiatry. 2020;87(1):64–73. doi: 10.1016/j.biopsych.2019.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira HM, Marchiori E, Severo A. Magnetic resonance imaging aspects of giant-cell tumours of bone. J Med Imaging Radiat Oncol. 2014;58(6):674–678. doi: 10.1111/1754-9485.12249. [DOI] [PubMed] [Google Scholar]
- 12.Cavanna L, Biasini C, Monfredo M, Maniscalco P, Mori M. Giant cell tumor of bone. Oncologist. 2014;19(11):1207–1207. doi: 10.1634/theoncologist.2014-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viswanathan S, Jambhekar NA. Metastatic giant cell tumor of bone: are there associated factors and best treatment modalities. Clin Orthop Relat Res. 2010;468(3):827–833. doi: 10.1007/s11999-009-0966-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreinhöfer KE, Rydholm A, Bauer HC, Kreicbergs A. Giant-cell tumours with fracture at diagnosis. Curettage and acrylic cementing in ten cases. J Bone Joint Surg Br. 1995;77(2):189–193. [PubMed] [Google Scholar]
- 15.Puthoor D, Iype W. Giant cell tumor: curettage and bone grafting. Indian J Orthop. 2007;41(2):121–123. doi: 10.4103/0019-5413.32042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He H, Zeng H, Luo W, Liu Y, Zhang C, Liu Q. Surgical treatment options for giant cell tumors of bone around the knee joint: extended curettage or segmental resection. Front Oncol. 2019;9:946–946. doi: 10.3389/fonc.2019.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L, Jin J, Hu A, Xiong J, Wang D, Sun Q, Wang S. Soft tissue recurrence of giant cell tumor of the bone: prevalence and radiographic features. J Bone Oncol. 2017;9:10–14. doi: 10.1016/j.jbo.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishisho T, Hanaoka N, Miyagi R, Sakai T, Toki S, Takahashi M, Kenji E, Yasui N, Sairyo K. Local administration of zoledronic acid for giant cell tumor of bone. Orthopedics. 2015;38(1):e25–e30. doi: 10.3928/01477447-20150105-56. [DOI] [PubMed] [Google Scholar]
- 19.Cheng YY, Huang L, Lee KM, Xu JK, Zheng MH, Kumta SM. Bisphosphonates induce apoptosis of stromal tumor cells in giant cell tumor of bone. Calcif Tissue Int. 2004;75(1):71–77. doi: 10.1007/s00223-004-0120-2. [DOI] [PubMed] [Google Scholar]
- 20.Lewin J, Thomas D. Denosumab: a new treatment option for giant cell tumor of bone. Drugs Today (Barc) 2013;49(11):693–700. doi: 10.1358/dot.2013.49.11.2064725. [DOI] [PubMed] [Google Scholar]
- 21.Singh AS, Chawla NS, Chawla SP. Giant-cell tumor of bone: treatment options and role of denosumab. Biologics. 2015;9:69–74. doi: 10.2147/BTT.S57359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simard S, Thewes B, Humphris G, Dixon M, Hayden C, Mireskandari S, Ozakinci G. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv. 2013;7(3):300–322. doi: 10.1007/s11764-013-0272-z. [DOI] [PubMed] [Google Scholar]
- 23.Bertoni F, Present D, Sudanese A, Baldini N, Bacchini P, Campanacci M. Giant-cell tumor of bone with pulmonary metastases. Six case reports and a review of the literature. Clin Orthop Relat Res. 1988;(237):275–285. [PubMed] [Google Scholar]
- 24.Palmerini E, Picci P, Reichardt P, Downey G. Malignancy in giant cell tumor of bone: a review of the literature. Technol Cancer Res Treat. 2019;18:1533033819840000–1533033819840000. doi: 10.1177/1533033819840000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.López-Pousa A, Martín Broto J, Garrido T, Vázquez J. Giant cell tumour of bone: new treatments in development. Clin Transl Oncol. 2015;17(6):419–430. doi: 10.1007/s12094-014-1268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Chan CM, Gong L, Bui MM, Han G, Letson GD, Yang Y, Niu X. Malignancy in giant cell tumor of bone in the extremities. J Bone Oncol. 2020;26:100334–100334. doi: 10.1016/j.jbo.2020.100334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belujon P, Grace AA. Dopamine system dysregulation in major depressive disorders. Int J Neuropsychopharmacol. 2017;20(12):1036–1046. doi: 10.1093/ijnp/pyx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards RR, Smith MT, Kudel I, Haythornthwaite J. Pain-related catastrophizing as a risk factor for suicidal ideation in chronic pain. Pain. 2006;126(1-3):272–279. doi: 10.1016/j.pain.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Hooley JM, Franklin JC, Nock MK. Chronic pain and suicide: understanding the association. Curr Pain Headache Rep. 2014;18(8):435–435. doi: 10.1007/s11916-014-0435-2. [DOI] [PubMed] [Google Scholar]
- 30.Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371–9724371. doi: 10.1155/2017/9724371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fauske L, Lorem G, Grov EK, Bondevik H. Changes in the body image of bone sarcoma survivors following surgical treatment - a qualitative study. J Surg Oncol. 2016;113(2):229–234. doi: 10.1002/jso.24138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park YM. Serum prolactin levels in patients with major depressive disorder receiving selective serotonin-reuptake inhibitor monotherapy for 3 months: a prospective study. Psychiatry Investig. 2017;14(3):368–371. doi: 10.4306/pi.2017.14.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naidoo U, Goff DC, Klibanski A. Hyperprolactinemia and bone mineral density: the potential impact of antipsychotic agents. Psychoneuroendocrinology. 2003;28(Suppl 2):97–108. doi: 10.1016/s0306-4530(02)00129-4. [DOI] [PubMed] [Google Scholar]
- 34.Sotelo JL, Nemeroff CB. Depression as a systemic disease. Pers Med Psychiatr. 2017;1-2:11–25. [Google Scholar]
- 35.Ghoneim MM, O’Hara MW. Depression and postoperative complications: an overview. BMC Surg. 2016;16:5–5. doi: 10.1186/s12893-016-0120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, Ranganathan G, Peterson CA, McGehee RE, Kern PA. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54(8):2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 37.Bahramabadi R, Samadi M, Vakilian A, Jafari E, Fathollahi MS, Arababadi MK. Evaluation of the effects of antipsychotic drugs on the expression of CD68 on the peripheral blood monocytes of Alzheimer patients with psychotic symptoms. Life Sci. 2017;179:73–79. doi: 10.1016/j.lfs.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 38.Laskowski LK, Goldfarb DS, Howland MA, Kavcsak K, Lugassy DM, Smith SW. A RANKL wrinkle: denosumab-induced hypocalcemia. J Med Toxicol. 2016;12(3):305–308. doi: 10.1007/s13181-016-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dehelean L, Marinescu I, Stovicek PO, Andor M. Cardiovascular anomalies and evolutionary risk factors in schizophrenia – multifactorial approach. Rom J Morphol Embryol. 2019;60(4):1105–1113. [PubMed] [Google Scholar]
- 40.Mehta S, Mehta S. Hypocalcemia masquerading as schizophreniform disorder. Indian J Psychol Med. 2016;38(5):463–465. doi: 10.4103/0253-7176.191386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fong J, Khan A. Hypocalcemia: updates in diagnosis and management for primary care. Can Fam Physician. 2012;58(2):158–162. [PMC free article] [PubMed] [Google Scholar]
- 42.Saad AM, Elmatboly AM, Gad MM, Al-Husseini MJ, Jazieh KA, Alzuabi MA, Alfaar AS. Association of brain cancer with risk of suicide. JAMA Netw Open. 2020;3(5):e203862–e203862. doi: 10.1001/jamanetworkopen.2020.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costanza A, Zenga F, Rudà R, Amerio A, Aguglia A, Serafini G, Amore M, Bondolfi G, Berardelli I, Nguyen KD. Suicidality in patients with brain tumors: a brief literature review with clinical exemplar. Medicina (Kaunas) 2020;56(12):725–725. doi: 10.3390/medicina56120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mastboom MJL, Verspoor FGM, Gelderblom H, van de Sande MAJ. Limb amputation after multiple treatments of tenosynovial giant cell tumour: series of 4 Dutch cases. Case Rep Orthop. 2017;2017:7402570–7402570. doi: 10.1155/2017/7402570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mármol I, Jiménez-Moreno N, Ancín-Azpilicueta C, Osada J, Cerrada E, Rodríguez-Yoldi MJ. A combination of Rosa canina extracts and gold complex favors apoptosis of Caco-2 cells by increasing oxidative stress and mitochondrial dysfunction. Antioxidants (Basel) 2019;9(1):17–17. doi: 10.3390/antiox9010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tătăranu LG, Georgescu AM, Buteică SA, Siloşi I, Mogoşanu GD, Purcaru ŞO, Alexandru O, Stovicek OP, Brînduşa C, Doşa M, Taisescu CI, Dricu A. Ligustrum vulgare hydroalcoholic extract induces apoptotic cell death in human primary brain tumour cells. Farmacia. 2017;65(5):766–771. https://farmaciajournal.com/wp-content/uploads/2017-05-art-18-Tataranu_Buteica_Dricu_02_766-771.pdf [Google Scholar]
- 47.Lamy S, Muhire É, Annabi B. Antiproliferative efficacy of elderberries and elderflowers (Sambucus canadensis) on glioma and brain endothelial cells under normoxic and hypoxic conditions. J Funct Food. 2018;40:164–179. [Google Scholar]
- 48.Ciobanu AM, Popa C, Marcu M, Ciobanu CF. Psychotic depression due to giant condyloma Buschke–Löwenstein tumors. Rom J Morphol Embryol. 2014;55(1):189–195. [PubMed] [Google Scholar]
- 49.Barnholtz-Sloan JS, Kruchko C. Meningiomas: causes and risk factors. Neurosurg Focus. 2007;23(4):E2–E2. doi: 10.3171/FOC-07/10/E2. [DOI] [PubMed] [Google Scholar]
- 50.Ciobanu AM, Lisievici MGh, Coman TC, Ciubotaru GhV, Drăghia A, Drăghia Fl, Ciucu AA. Giant wing sphenoid meningioma with principal manifestation depression. Rom J Morphol Embryol. 2009;50(4):713–717. [PubMed] [Google Scholar]
- 51.Saha R, Jakhar K, Kumar R. Sphenoid wing meningioma presenting as cognitive impairment. Shanghai Arch Psychiatry. 2016;28(3):173–176. doi: 10.11919/j.issn.1002-0829.215142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JK, Nam DH, Lee J. Repurposing antipsychotics as glioblastoma therapeutics: potentials and challenges. Oncol Lett. 2016;11(2):1281–1286. doi: 10.3892/ol.2016.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L, Hanahan D. Hijacking the neuronal NMDAR signaling circuit to promote tumor growth and invasion. Cell. 2013;153(1):86–100. doi: 10.1016/j.cell.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 54.Loggia ML, Berna C, Kim J, Cahalan CM, Gollub RL, Wasan AD, Harris RE, Edwards RR, Napadow V. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. 2014;66(1):203–212. doi: 10.1002/art.38191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hipólito L, Wilson-Poe A, Campos-Jurado Y, Zhong E, Gonzalez-Romero J, Virag L, Whittington R, Comer SD, Carlton SM, Walker BM, Bruchas MR, Morón JA. Inflammatory pain promotes increased opioid self-administration: role of dysregulated ventral tegmental area μ opioid receptors. J Neurosci. 2015;35(35):12217–12231. doi: 10.1523/JNEUROSCI.1053-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coon EA, Laughlin RS. Burning mouth syndrome in Parkinson’s disease: dopamine as cure or cause. J Headache Pain. 2012;13(3):255–257. doi: 10.1007/s10194-012-0421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marinescu I, Vasiliu O, Vasile D. Translational approaches in treatment-resistant depression based on animal model. Rom J Morphol Embryol. 2018;59(3):955–964. [PubMed] [Google Scholar]
- 58.Naranjo CA, Tremblay LK, Busto UE. The role of the brain reward system in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(4):781–823. doi: 10.1016/s0278-5846(01)00156-7. [DOI] [PubMed] [Google Scholar]
- 59.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jarcho JM, Mayer EA, Jiang ZK, Feier NA, London ED. Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. Pain. 2012;153(4):744–754. doi: 10.1016/j.pain.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng YS, Tseng PT, Lin PY, Chen TY, Stubbs B, Carvalho AF, Wu CK, Chen YW, Wu MK. Internet addiction and its relationship with suicidal behaviors: a meta-analysis of multinational observational studies. J Clin Psychiatry. 2018;79(4):17r11761–17r11761. doi: 10.4088/JCP.17r11761. [DOI] [PubMed] [Google Scholar]
- 62.Błachnio A, Przepiórka A, Gorbaniuk O, Benvenuti M, Ciobanu AM, Senol-Durak E, Durak M, Giannakos MN, Mazzoni E, Pappas IO, Popa C, Seidman G, Wu AMS, Yu S, Ben-Ezra M. Cultural correlates of Internet addiction. Cyberpsychol Behav Soc Netw. 2019;22(4):258–263. doi: 10.1089/cyber.2018.0667. [DOI] [PubMed] [Google Scholar]
- 63.Jidveian Popescu M, Slabaru A, Marinescu I, Popescu A, Popa-Velea O, Ciobanu AM. Difficulties in histopathology diagnosis and treatment of depressive disorder of breast cancer in male – clinical case presentation. Rom J Morphol Embryol. 2019;60(3):1031–1037. [PubMed] [Google Scholar]
- 64.Zamani A, Qu Z. Serotonin activates angiogenic phosphorylation signaling in human endothelial cells. FEBS Lett. 2012;586(16):2360–2365. doi: 10.1016/j.febslet.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 65.Peters MAM, Meijer C, Fehrmann RSN, Walenkamp AME, Kema IP, de Vries EGE, Hollema H, Oosting SF. Serotonin and dopamine receptor expression in solid tumours including rare cancers. Pathol Oncol Res. 2020;26(3):1539–1547. doi: 10.1007/s12253-019-00734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang M, Wu X, Chai F, Zhang Y, Jiang J. Plasma prolactin and breast cancer risk: a meta-analysis. Sci Rep. 2016;6:25998–25998. doi: 10.1038/srep25998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Porcaro AB, Ghimenton C, Petrozziello A, Migliorini F, Romano M, Sava T, Caruso B, Cocco C, Antoniolli SZ, Lacola V, Rubilotta E, Monaco C, Comunale L. Investigative clinical study on prostate cancer. Part VIII: Prolactin hormone and the pituitary–testicular–prostate axis at the time of initial diagnosis and subsequent cluster selection of the patient population after radical prostatectomy. Anticancer Res. 2012;32(4):1499–1506. [PubMed] [Google Scholar]
- 68.Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R, Hojilla CV, Komnenovic V, Kong YY, Schreiber M, Dixon SJ, Sims SM, Khokha R, Wada T, Penninger JM. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440(7084):692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 69.Sisay M, Mengistu G, Edessa D. The RANK/RANKL/OPG system in tumorigenesis and metastasis of cancer stem cell: potential targets for anticancer therapy. Onco Targets Ther. 2017;10:3801–3810. doi: 10.2147/OTT.S135867. [DOI] [PMC free article] [PubMed] [Google Scholar]