Abstract
Study Objectives
Insomnia is common in older adults, and is associated with poor health, including cognitive impairment and cardio-metabolic disease. Although the mechanisms linking insomnia with these comorbidities remain unclear, age-related changes in sleep and autonomic nervous system (ANS) regulation might represent a shared mechanistic pathway. In this study, we assessed the relationship between ANS activity with indices of objective and subjective sleep quality in older adults with insomnia.
Methods
Forty-three adults with chronic insomnia and 16 age-matched healthy sleeper controls were studied. Subjective sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI), objective sleep quality by electroencephalogram spectral components derived from polysomnography, and ANS activity by measuring 24-h plasma cortisol and norepinephrine (NE).
Results
Sleep cycle analysis displayed lower slow oscillatory (SO: 0.5–1.25 Hz) activity in the first cycle in insomnia compared to controls. In insomnia, 24-h cortisol levels were higher and 24-h NE levels were lower than controls. In controls, but not in insomnia, there was a significant interaction between NE level during wake and SO activity levels across the sleep cycles, such that in controls but not in insomnia, NE level during wake was positively associated with the amount of SO activity in the first cycle. In insomnia, lower 24-h NE level and SO activity in the first sleep cycle were associated with poorer subjective sleep quality.
Conclusion
Dysregulation of autonomic activity may be an underlying mechanism that links objective and subjective measures of sleep quality in older adults with insomnia, and potentially contribute to adverse health outcomes.
Keywords: noradrenergic system, sleep slow oscillations, sleep homeostasis, insomnia
Statement of Significance.
Insomnia is a common sleep disorder in the general population, with broad implications for overall quality of life, as well as mental and physical health. Older adults are a population at particular risk for both insomnia and comorbid cardio-metabolic disease and cognitive impairment, yet there is limited understanding of the mechanisms linking these associations. The findings from this study demonstrate a relationship between dysregulation of 24-h noradrenergic activity with subjective sleep quality and objective measures of sleep, as measured by electroencephalogram slow oscillatory activity. These results suggest that autonomic dysregulation may play a role in the expression of subjective and objective sleep quality in insomnia of older adults, and potentially help inform the development of novel therapeutic targets for insomnia.
Introduction
Insomnia disorder is characterized by symptoms of chronic difficulty in sleep initiation and/or maintenance, or poor sleep quality despite an adequate opportunity to sleep, resulting in functional impairment and distress [1]. Insomnia can also have adverse effects on physical health, including impaired cognitive function [2] and increased cardio-metabolic disease (CMD) risk [3]. Older adults are a population at risk for insomnia [4] and comorbid cognitive, cardio-metabolic, and depressive disorders [2, 5, 6]. Improved understanding of the mechanisms linking insomnia with these comorbidities is particularly important because improving wake–sleep function has the potential to modify health-related outcomes.
Changes in sleep architecture have been described in insomnia including, but not limited to, reduction in total sleep duration, increased sleep fragmentation, longer periods of wake after sleep onset (WASO), and reduction in slow-wave sleep (SWS) [7]. Poor sleep quality has been associated with a higher risk for cognitive and cardio-metabolic disorders in experimental and epidemiological studies [8, 9]. In addition, there is evidence that adults with insomnia and objective short sleep duration may be at higher risk for CMD [10–12].
Alterations in spectral quantification of sleep electroencephalogram (EEG) have been reported in young and middle-aged adults with insomnia, including a reduction in the amount of SWA (0.5–4 Hz) [13, 14]. The integrity of SWA is thought to be important for many physiological processes, including autonomic [15] and cognitive function [16] and as such, dysregulation of SWA may represent a mechanistic pathway linking insomnia with cardio-metabolic and cognitive comorbidities. However, a reduction of SWA in insomnia has not been consistently reported in the literature [17, 18], which may in part be due to the lack of more detailed characterization of the spectral components of SWA in some studies. Recent evidence shows that the lower frequency component of SWA, slow oscillatory activity (SO activity: 0.5–1 Hz), rather than the higher frequency component (delta activity: >1–4 Hz), is reduced in some patients with insomnia [19, 20]. There is evidence that SO activity is generated in brain regions that are different from those generating delta activity [21, 22], and shows broader connections with subcortical regions involved in the regulation of sleep and wakefulness, including the locus coeruleus (LC) [21]. SO activity is closely coupled with cardiovascular function [23, 24], and is also important for sleep-dependent memory consolidation [16]. Moreover, in insomnia, both changes in SO activity [20] and markers of altered autonomic activity [25, 26] have been shown to correlate with the severity of subjective sleep disturbance, as measured by Pittsburgh Sleep Quality Index (PSQI). Thus, studying the relationship of specific frequency components of SWA with autonomic function and sleep quality can provide new insights into the link between insomnia and risk for cardio-metabolic and cognitive comorbidities.
Aging is accompanied by changes in sleep macro- and microstructure which may contribute to the high prevalence and severity of sleep complaints in the elderly [27]. With aging, sleep becomes more fragmented and overall shorter in duration [28]. Some prominent age-related changes include increased WASO and lighter nonrapid eye-movement (NREM) sleep stages, N1 and N2, and decreased stage N3 (SWS). In addition, there is a significant decline in SWA across the lifespan [29], which is mainly evident in the first cycle of sleep [30]. Based on this evidence, reduced SWA observed in older adults has been interpreted as a possible indicator of weaker sleep homeostasis [28, 31]. The dynamic changes and in particular the dissipation of SWA across the cycles of sleep are a distinctive feature of sleep homeostasis [32, 33], which have not been systematically described in studies of insomnia. Therefore, studying SWA and its spectral components by sleep cycles can add new insight on sleep homeostatic regulation in older adults with insomnia [28, 31, 32, 34, 35].
Mechanisms linking autonomic activity during the daytime/waking period with objective sleep quality in the following sleep period remain poorly understood in insomnia. One of the proposed mechanisms to explain the association between insomnia and risk for CMD is an overactive sympathetic nervous system, represented by the elevation in the activity of multiple physiological domains (i.e. higher cortisol levels, heart rate, core body temperature) [36]. Notably, these observations mainly originate from studies conducted in young and middle-aged adults with insomnia. However, since aging is accompanied by a significant decline in central wake and alertness promoting neurotransmitters, including norepinephrine (NE) [37], which can alter wake–sleep regulatory mechanisms, it is biologically plausible that the pathways linking sleep quality and autonomic/arousal activity may differ between older and younger adults, as well as among insomnia subtypes.
The overall aim of this study was to assess the relationship between objective measures of sleep microstructure, specifically SO activity and delta activity, with autonomic and arousal-related physiological measures (24-h plasma cortisol and NE), and their relationship with subjective sleep quality (PSQI), in older adults with insomnia and age-matched healthy sleeper controls. The following hypotheses were tested: (1) SO activity will be lower in insomnia than controls, (2) 24-h levels of cortisol and NE will be higher in insomnia than controls, (3) higher cortisol and NE levels during wake will be correlated with lower levels of SO activity in the following sleep period, and (4) in insomnia, higher cortisol and NE levels and lower SO activity will be associated with poorer subjective sleep quality (PSQI).
Methods
Participants
Forty-three adults with chronic insomnia (65 ± 8 years, seven males), and 16 age-matched healthy sleeper controls (68 ± 9 years, four males), participated in two separate parent research studies that employed identical protocols for sleep and autonomic measures, as well as inclusion and exclusion criteria for insomnia and controls. A protocol flow diagram is shown in Figure S1. Participants included in the studies were sedentary men and women, 55 years and older, independent in activities of daily living and without clinical cognitive impairment (mini-mental state exam score ≥ 26).
Participants who endorsed symptoms of insomnia for at least 3 months, associated with functional impairment and/or distress, had a clinical interview with a board-certified sleep medicine clinician, who determined insomnia disorder according to the International Classification of Sleep Disorders 2nd Ed. Criteria [38]. Additional criteria included: (1) sleep efficiency (SE) less than 80% and/or awakening earlier than desired if before 6:00 am, and a total sleep time (TST) of less than 6.5 h (but not less than 5 h) on at least 4 out of 7 days of wrist actigraphy and sleep diary, and (2) PSQI >5 [39]. Inclusion criteria for controls were: (1) no prior or current history of insomnia; (2) SE greater than 80% and TST of 6.5 h, but not more than 8.5 h confirmed by actigraphy and sleep diary for a period of 7 days; (3) PSQI ≤5.
Exclusion criteria for all participants were as follows: (1) history of other sleep disorders, neurological or psychiatric disorders (DSM-IV criteria for any major psychiatric disorder, including mania or alcohol or substance abuse); (2) moderate to severe depression symptoms assessed by Center for Epidemiological Studies Depression (CESD) scale (score > 22) [40] (study 1), or by the Beck Depression Inventory (BDI) scale (score ≥ 16) [41] (study 2); (3) unstable or serious medical conditions; (4) current, or use within the past month, of psychoactive, hypnotic, stimulant, or analgesic medications; (5) shift work or other types of self-imposed irregular sleep schedules; (6) BMI >35 kg/m2; (7) habitual smoking (six or more cigarettes per week) or caffeine consumption of greater than 300 mg per day; (8) apnea-hypopnea index (AHI) ≥15, periodic leg movement arousal index ≥15 by polysomnography (PSG).
Study design
A common aim of the two parent studies was to determine sleep and cardio-metabolic characteristics of older adults with insomnia and short sleep duration. The control groups were age-matched healthy sleepers with normal sleep duration and self-reported good sleep quality. Baseline assessments included daily sleep logs and actigraphy for 7 days, questionnaires related to sleep history, sleep and mood disorders, medication use, and subjective sleep quality (PSQI). Eligible participants stayed in the clinical research unit (CRU) for four nights (night 1 PSG was for adaptation and screening for sleep apnea, followed by three nights with EEG recording). The following procedures were conducted: (1) nightly PSG; (2) three daytime blood pressure (BP) and heart rate (HR) measurements; (3) oral glucose tolerance test on the morning of day 2 of admission; and (4) on the last day (day 4), a 24-h period of serial blood draws [42]. The time of lights off was determined according to their mean habitual bedtime obtained from 7 days of actigraphy and sleep diaries, the week prior to their admission to the CRU.
Depression symptoms
To assess the contribution of depression symptoms as a variable, a standardized common metric of depression severity was calculated for the BDI and CESD measures [43–45].
Subjective sleep quality
PSQI is a 19-item self-rated questionnaire that evaluates sleep over the past month, was used to assess subjective sleep quality. A global PSQI score >5 has shown a sensitivity of ~90% and specificity of ~87% for identification of individuals with poor sleep quality [39].
Wrist actigraphy
Daily rest–activity rhythms were assessed via wrist actigraphy (AW-64 and AWL Actiwatch, Mini Mitter Co. Inc., Bend, OR), and a daily sleep diary upon awakening that included bedtime, waketime, time awake during night and naps, was used to manually set rest intervals within the Actiware software (Mini Mitter Co. Inc., Bend, OR) for the calculation of sleep variables. Devices were set to record every 60 s, and medium sensitivity. Sleep start was defined as the first 10 min period in which no more than one epoch was scored as “mobile.” Sleep end was defined as the last 10 min period in which no more than one epoch was scored as “immobile.” TST was defined as the amount of time between sleep start and sleep end scored as “sleep,” SE was defined as the proportion of sleep between bedtime and waketime, WASO was defined as the time in minutes spent awake after sleep onset and before waketime [42].
Sleep PSG recording
EEG recordings were obtained from central and occipital channels (C3, C4, O1, O2) referenced to the contralateral mastoid (A1, A2). In addition, recordings of left and right electrooculogram, electrocardiogram, and two chin electromyograms were obtained. During the screening PSG, nasal/oral airflow, abdominal and chest respiration, pulse oximetry, and leg electromyogram were also recorded to screen for sleep disorders. The sampling frequency was 250 Hz (Neurofax EEG-1100, Nihon Kohden), with a 70 Hz low-pass filter. Recordings were scored according to the criteria of Rechtschaffen and Kales [46] (the study began prior to the current American Academy of Sleep Medicine (AASM) scoring rules [38]), by an experienced scorer blinded to the participants’ condition (insomnia vs. control). PSG measures were calculated for TST (duration in minutes spent asleep), SE (% of time spent asleep over the entire recording period), time (duration in minutes and % of the time spent asleep) spent in each stage of sleep (N1, N2, N3, REM), sleep onset latency (time from lights off to the first 30 s epoch scored as N1 or N2), WASO (time in minutes spent awake after sleep onset and before lights on), and arousal index (number of arousals lasting at least 3 s in duration per hour of sleep) [47].
EEG power spectral analysis
Electrophysiological recording analyzer software package PRANA (Phitools, France) was used to perform spectral EEG analyses using artifact-free data (left central channel) from nights 2, 3, and 4. An FFT (4 s window, 50% overlap) was applied, and mean power spectral estimates were extracted in 30 s epochs by sleep stage. To be included in the analysis, PSG was required to have less than 20% of NREM data removed due to artifacts.
To determine the contribution of the different frequency bands within SWA, we extracted SO activity (0.5–1.25 Hz) [48], and delta activity (>1.25–4 Hz). Spectral power was also extracted across the following frequency bands including theta (> 4–8.5 Hz), alpha (>8.5–12.5 Hz), sigma (>12.5–15.5 Hz), and beta (>15.5–22 Hz). The averaged spectral power in each frequency band was then calculated for the entire time in NREM sleep. Analysis by a cycle of sleep was performed to examine the time-course of SO activity and delta activity, and the other frequency band changes across the night. Sleep cycles were defined using modified Feinberg and Floyd criteria [49]. The first four sleep cycles were included in the analysis. Average spectral power for each frequency band was calculated for each sleep cycle and normalized to the NREM power of the corresponding frequency band for the entire night.
24-h plasma cortisol and NE
Blood sampling was initiated within 30 min from wake on the last day in the CRU and obtained over a 24-h period at 30 min intervals, using an indwelling catheter. Blood samples were centrifuged immediately after collection and stored at –70oC until further processing. Plasma cortisol was measured using enzyme-linked immunosorbent assay (ELISA, Roche Diagnostics Elecsys Cortisol II) with an intraassay variability of 1.7%. Plasma NE was assayed using high-performance liquid chromatography with an electrochemical detection system (Coulochem Electrode Array System; ESA, Inc.).
Data on 24-h cortisol and NE plasma levels were aligned with the time of lights off. The analysis of mean cortisol and NE levels during wake (i.e. daytime period), included values from the first sample collected after morning awakening until the last one before lights off.
24-h plasma melatonin
Serial plasma melatonin levels were measured using a commercially available radioimmunoassay from IBL International (Catalog No. RE29301). The standard range of sensitivity for this assay is from 3 to 300 pg/mL. Dim light melatonin onset (DLMO) was defined as the clock time of the first value to rise and remain above 2 standard deviations above baseline levels [50].
BP and HR during wake
BP and HR were measured by a nurse using an ambulatory monitoring device (Accutracker II, Suntech Medical Instruments) on the nondominant arm, with participants in a seated position. Measurements were obtained at least 3 times/day during the waking period, but the exact timing varied between the parent protocols. The average of BP and HR across the days spent in the CRU was calculated for each participant. Average daily BP and HR are presented to characterize clinical features of cardiovascular health.
Statistical analysis
Statistical analyses were carried out in R [51]. Comparison between insomnia and control groups for non-repeated measures (demographics, baseline actigraphy characteristics, PSQI, PSG, and EEG spectral power averaged across the night) were performed using t-tests or nonparametric and Wilcoxon rank-sum tests as necessary due to significant non-normality of the observations. Normality assumptions for pairwise differences were checked using “Shapiro test.” For repeated measures (including 24-h time course profiles of plasma cortisol and NE, and EEG power throughout sleep cycles), a generalized estimating equation (GEE) model with exchangeable within-subject covariance was used to account for correlations within subjects. p-values were adjusted using the Bonferroni–Dunn method to account for the multiplicity of tests across sleep cycles and EEG spectral bands.
In models for 24-h cortisol and NE profiles, time was treated as a third-order polynomial to capture nonlinearities. Robust standard errors of the model parameters were computed using the methods of Diggle et al. [52], which has been shown to yield consistent estimates even when the within-subject covariance matrix is misspecified. GEE models were estimated using the R geepack library [53]. Within each group, insomnia and controls, GEE models were used to test the interaction between average cortisol and NE levels, and PSQI with EEG spectral power levels across the cycles of sleep. Within each group, nonparametric Spearman rank correlation coefficients were computed to analyze the relationship between average cortisol and NE levels, EEG spectral power averaged across the night, and PSQI. To adjust for depression symptoms, the common metric score (depression score) was used in the analyses as a continuous variable. Sensitivity analyses of different sleep durations in the insomnia group were conducted using the mean of 7 days of actigraphy at screening. Data are presented as mean ± standard deviation (SD) unless otherwise specified. All statistical tests are two-sided.
For plasma cortisol and NE, participants missing >25% of the time points were excluded. For cortisol data, three participants with insomnia met this criterion due to poor sample quality and were removed from the analysis. As a result, 43 participants with insomnia and 16 controls were included in the analyses on 24-h NE profiles and 40 participants with insomnia and 16 controls were included in the analysis of 24-h cortisol profiles.
Analyses of PSG and EEG spectral measures were conducted on data averaged across nights 2, 3, and 4. For the associations with daytime plasma cortisol and NE levels, EEG data from night 4 was used because it was concomitant with 24-h blood sampling. PSG and EEG spectral data from nights 2 and 3 included 43 participants with insomnia and 16 controls. PSG and EEG spectral data on night 4 included 37 participants with insomnia and 16 controls, as data of six participants with insomnia were excluded due to technical issues (more than 20% of the EEG recording had artifacts). Sleep characteristics derived from actigraphy or PSG averaged from nights 2 and 3 did not differ between individuals with missing EEG data on night 4 from those with EEG data, nor from the entire insomnia group (p > 0.05, nonparametric tests).
Results
Characteristics of participants with insomnia and controls
The main demographic and clinical features of 43 participants with insomnia and 16 controls are shown in Table 1. There were no significant differences between the groups for age, sex, BMI, activity levels, mean BP and HR values during wake, and DLMO.
Table 1.
Demographic and clinical features of participants with insomnia and controls
| Controls, n = 16 | Insomnia, n = 43 | p value | |
|---|---|---|---|
| Age (years) | 68.1 ± 9.2 | 65.2 ± 7.6 | 0.318b |
| Gender F (%) | 75% | 84% | 0.697c |
| BMI (kg/m2) | 26.0 ± 3.1 | 26.3 ± 3.9 | 0.815a |
| SBP (mmHg) | 128.6 ± 20.5 | 127.6 ± 11.9 | 0.918a |
| DBP (mmHg) | 74.0 ± 9.6 | 74.4 ± 9.1 | 0.739a |
| HR (bpm) | 69.5 ± 11.4 | 69.8 ± 7.8 | 0.867a |
| Average daily activity count (a.u.) | 278880.1 ± 73333.4 | 269323.9 ± 90010.9 | 0.732b |
| AHI | 3.8 ± 4.5 | 4.9 ± 4.4 | 0.426a |
| ESS | 6.1 ± 2.8 | 7.0 ± 4.2 | 0.425a |
| DLMO (h:min) | 19:50 ± 4:17 | 19:06 ± 4:06 | 0.560b |
Values are shown as mean ± SD. p values are obtained using (a) two-sided t-test; (b) nonparametric two-sided Wilcoxon rank-sum test; (c) chi-squared test.
AHI, apnea–hypopnea index at screening; BMI, body max index; DBP, diastolic blood pressure; DLMO, dim light melatonin onset was defined as clock time of plasma melatonin onset (2 standard deviations above baseline level); ESS, Epworth sleepiness scale administered upon admission to CRU; HR, heart rate; PSQI, Pittsburgh Sleep Quality Index; SBP, systolic blood pressure.
Median (25th, 75th percentiles) score for the BDI scale was 4.5 (1, 7) in controls and 8.5 (6, 12) in insomnia. Median (25th, 75th percentiles) score for the CESD scale was 2 (0, 11) in controls and 5 (3, 11) in insomnia. The comparison between insomnia and the control group after the conversion of CESD and BDI scores into a standardized metric, showed a higher score in the insomnia group (50.8 ± 8.8) compared to controls (43.9 ± 8.9; p = 0.02).
Subjective sleep quality
Higher PSQI scores, indicating greater subjective sleep disturbance, were observed in the insomnia group (11.0 ± 2.8) compared to controls (2.4 ± 1.6, p < 0.001), reflecting the inclusion criteria. Analysis of PSQI subscales (Figure S2) showed significantly higher subs-cores in participants with insomnia compared to controls (p < 0.001 adjusted for multiple comparisons) in all domains except for “medication use,” as sleep medication use was an exclusion criterion in the study.
Objective sleep characteristics
Wrist actigraphy
The mean (± SD) for measures of home wrist actigraphy obtained at screening in participants with insomnia and controls are provided in Table 2. As expected, based on the inclusion criteria, the screening actigraphy showed a shorter TST in insomnia compared to controls, as well as lower SE and a greater amount of WASO.
Table 2.
Summary sleep characteristics for insomnia and controls, from 7 days of home wrist actigraphy at screening and from laboratory-based polysomnography
| Controls, n = 16 | Insomnia, n = 43 | p value | |
|---|---|---|---|
| Screening actigraphy | |||
| Total sleep time (h)/median (25th, 75th percentiles) | 6.9 ± 0.7/6.9 (6.6, 7.1) | 6.1 ± 1.0/6.2 (5.3, 6.8) | 0.010 a |
| Sleep efficiency (%) | 85.5 ± 5.5 | 80.9 ± 7.8 | 0.006 b |
| Wake after sleep onset (min) | 46.5 ± 15.9 | 58.9 ± 23.7 | 0.053a |
| In lab. polysomnography (nights 2, 3, and 4 averaged) | |||
| Total recording time (min) | 476.2 ± 27.7 | 477.4 ± 38.9 | 0.874a |
| Total sleep time (min) | 477.6 ± 52.8 | 347.0 ± 65.5 | 0.030 a |
| Sleep efficiency (%) | 79.5 ± 9.8 | 72.9 ± 12.1 | 0.011 b |
| Sleep onset latency (min) | 17.9 ± 16.6 | 14.3 ± 19.4 | 0.358b |
| Wake after sleep onset (min) | 82.8 ± 38.9 | 114.3 ± 51.7 | 0.005 a |
| N1 sleep (min) | 47.9 ± 26.8 | 40.7± 17.4 | 0.105b |
| N2 sleep (min) | 185.3 ± 45.5 | 185.5 ± 52.6 | 0.963a |
| Slow wave sleep (min) | 58.5 ± 45.3 | 50.1 ± 34.9 | 0.309b |
| REM sleep (min) | 69.4 ± 29.3 | 50.1 ± 34.9 | 0.309a |
| N1 (%) | 14.2 ± 8.4 | 12.1 ± 5.5 | 0.132b |
| N2 (%) | 50.7 ± 8.0 | 53.1 ± 9.7 | 0.219a |
| SWS (%) | 15.4 ± 10.7 | 14.5 ± 9.9 | 0.677a |
| REM sleep (%) | 18.9 ± 6.8 | 20.2 ± 6.4 | 0.332a |
| Arousal index (n/h) | 14.8 ± 6.6 | 19.1 ± 8.0 | 0.010 b |
N1, sleep stage 1; N2, sleep stage 2; REM, rapid eye movement sleep. Values are shown as mean ± SD. p values are obtained using (a) two-sided t-test; (b) two-sided nonparametric Wilcoxon rank-sum test.
Polysomnography
Sleep macrostructure
Averaged PSG data from nights 2, 3, and 4, showed significantly lower TST and SE, higher WASO and higher arousal index in participants with insomnia compared to controls (Table 2).
Sleep microstructure
EEG spectral activity averaged across the night
There were no differences between insomnia and controls in the average total amount of SO activity (insomnia: 188.8 ± 115.1 µV2, controls: 162.4 ± 105.4 µV2; p = 0.17), or delta activity (insomnia: 92.3 ± 48.4 µV2, controls: 78.4 ± 41.7 µV2; p = 0.15), and results did not change after adjusting for sex and depression score (p = 0.57 and p = 0.56, respectively). No between-group differences were observed in the other frequency bands (Table S1).
EEG spectral activity by cycles of sleep
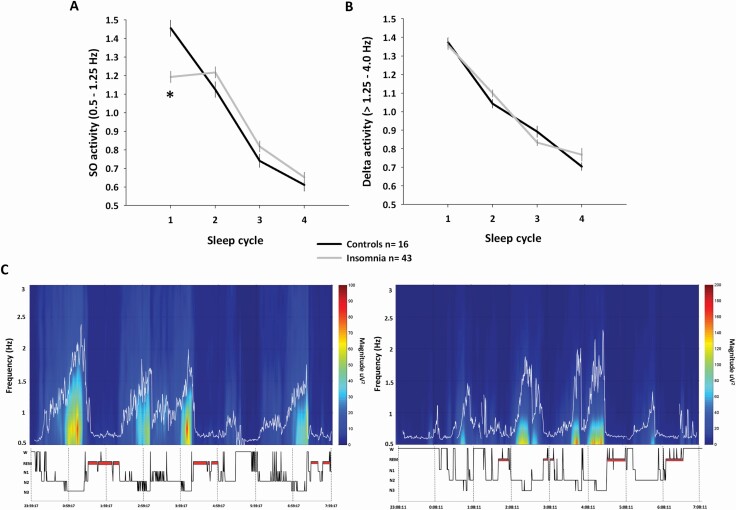
The duration of sleep cycles was not different between insomnia and the control group (all sleep cycles p > 0.1). GEE analysis of SO activity levels across the cycles of sleep showed a group × cycle interaction effect (p < 0.0001), characterized by reduced SO activity levels in the first sleep cycle in participants with insomnia compared to controls (p < 0.0001; Figure 1A, C). There was no significant difference in delta activity levels across the cycles of sleep between participants with insomnia and controls (group × cycle interaction p = 0.16; Figure 1B). No other group differences were found across the cycles of sleep for any other frequency band (Figure S3).
Figure 1.
Slow oscillatory activity (SO: 0.5–1.25 Hz) and delta activity (>1.25–4 Hz) changes across the cycles of sleep in insomnia and controls. Data are averaged from nights 2, 3, and 4. (A) Lower SO activity levels in the first sleep cycle characterized participants with insomnia compared to controls (p < 0.0001, adjusted for multiple comparisons). (B) No significant differences were observed in delta activity levels across the sleep cycles between the two groups. Spectral power in each frequency band, in each cycle of sleep, was normalized to the NREM power averaged across the night, for that specific frequency. Bars represent standard error of the mean. (C) Individual spectrogram focused on lower EEG frequencies (0.5–3 Hz; Y-axis) obtained from left EEG central derivation in one control subject (left panel, female, 64-year-old) and one participant with insomnia (right panel, female, 64-year-old). Large power values are shown in warm colors. Time is shown on X-axis in parallel with the hypnogram. The figure highlights the different distribution of SO activity across the night, characterized by its significant reduction in the first cycle of sleep in participant with insomnia.
Sex and depression score introduced in the analyses as covariates did not affect the between-group differences in SO activity (group × cycle interaction p < 0.0001), and delta activity (group × cycle interaction p = 0.16).
Autonomic measures
24-h plasma cortisol levels
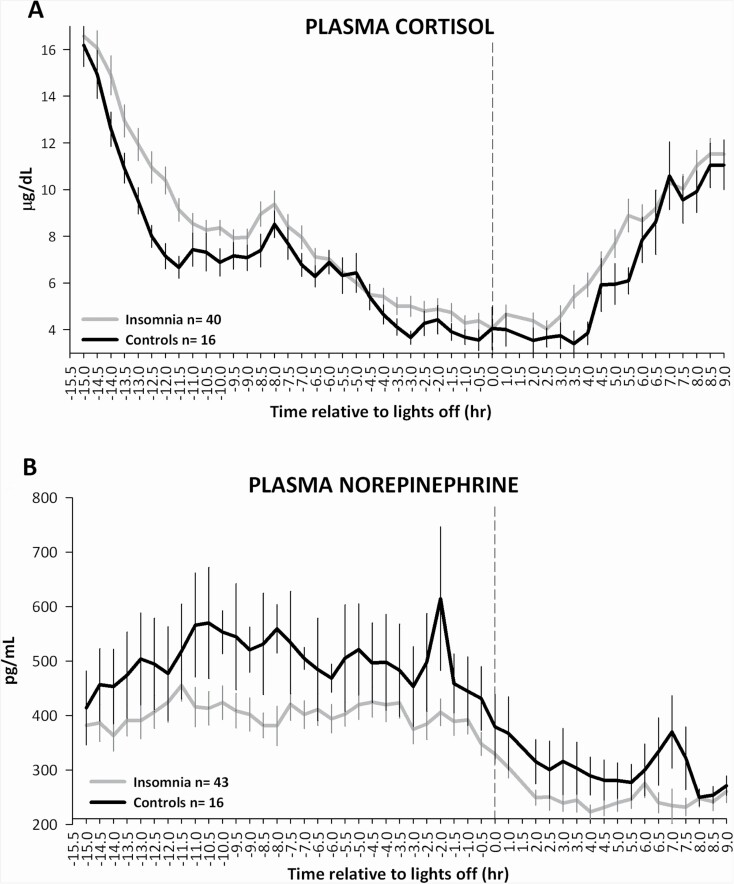
GEE analysis showed that 24-h plasma cortisol levels (Figure 2A) were significantly higher in participants with insomnia (8.17 ± 1.78 µg/dL) compared to controls (7.09 ± 1.12 µg/dL; group p < 0.001), across the 24-h period (group × time interaction p = 0.55). After adjusting for sex and depression score, this result remained significant (group p < 0.0001).
Figure 2.
24-h profiles of (A) plasma cortisol and (B) plasma norepinephrine (NE), in insomnia and controls. Participants with insomnia showed higher 24-h plasma cortisol levels compared to controls (p < 0.001, GEE model), and lower 24-h plasma NE levels compared to controls (group p < 0.001, GEE model). Blood samples were obtained every 30 minutes starting approximately 30 min after the morning awakening. Data were analyzed after alignment with lights off (dotted line). Bars represent standard error of the mean.
24-h plasma NE levels
GEE analysis showed that 24-h plasma NE levels (Figure 2B) were significantly lower in participants with insomnia (350 ± 112 pg/mL) compared to controls (432 ± 107 pg/mL, group p < 0.001), across the 24-h period (group × time interaction p = 0.27). After adjusting for sex and depression score, this result remained significant (group p < 0.0001).
Relationship between autonomic measures and EEG microstructure
Relationship between cortisol levels during wake with SO activity and delta activity in the following sleep period
In either insomnia or controls, there were not significant correlations between mean cortisol levels during wake and the average total amount of SO activity (insomnia: R = – 0.29, p = 0.13; controls: R = 0.11, p = 0.82) and delta activity (insomnia: R = –0.29, p = 0.13; controls: R = 0.11, p = 0.82).
GEE analysis did not show significant interactions between mean plasma cortisol levels during wake and levels of SO activity or delta activity across the cycles of sleep in either participants with insomnia (SO activity: p = 0.34; delta activity: p = 0.42), or controls (SO activity: p = 0.45; delta activity: p = 0.66).
Relationship between NE levels during wake with SO activity and delta activity in the following sleep period
In either insomnia or controls, there were not significant correlations between mean NE levels during wake and the average total amount of SO activity (insomnia: R = –0.16, p = 0.50; controls: R = –0.24, p = 0.29), and delta activity (insomnia: R = –0.16, p = 0.50; controls: R = –0.24, p = 0.28).
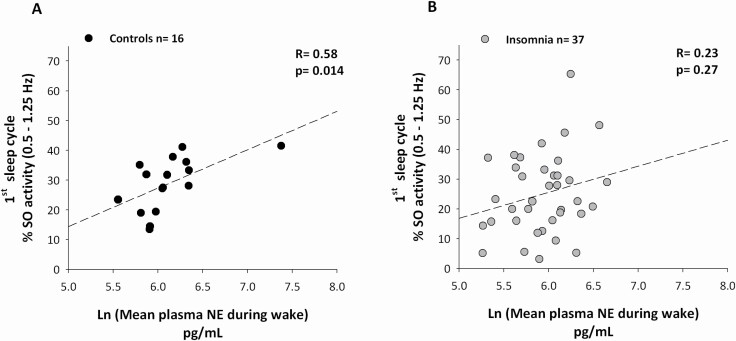
GEE analysis showed a significant interaction between mean NE levels during wake and levels of SO activity across the cycles in controls (p = 0.017), but not in insomnia (p = 0.21). Post hoc analysis indicated that a significant positive correlation between higher mean NE levels during wake with higher SO activity levels in the first cycle of sleep was present in controls (Figure 3A, R = 0.58, p = 0.014), but not participants with insomnia (Figure 3B, R = 0.23, p = 0.26).
Figure 3.
Higher plasma norepinephrine (NE) levels during wake correlate with greater Slow Oscillatory activity (SO: 0.5–1.25 Hz) in the first cycle of sleep in controls but not in insomnia. In controls, a significant positive correlation was present between mean plasma NE levels during wake (data Ln transformed) and the amount of SO activity in the first cycle of sleep (A), that was not present in participants with insomnia (B). Mean plasma NE levels during wake included values from the first sample collected after the morning awakening until the last one before lights off. The amount of SO activity in the first cycle of sleep is expressed as % of the entire night NREM SO power. Correlations are nonparametric.
GEE analysis did not show significant interactions between mean NE levels during wake and delta activity levels across the cycles of sleep in either participants with insomnia (p = 0.09) or controls (p = 0.94).
Relationship between autonomic measures, EEG microstructure, and subjective sleep quality
Relationship between mean 24-h cortisol and NE levels with PSQI in insomnia
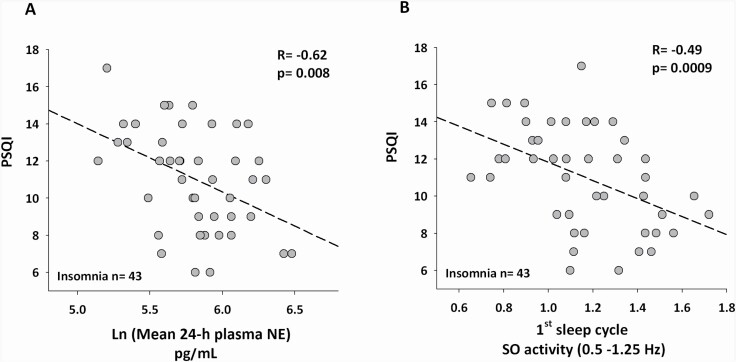
There was no significant correlation between mean 24-h cortisol levels and PSQI scores (R = –0.11, p = 0.32). There was a significant correlation between lower mean 24-h plasma NE levels with higher PSQI scores (Figure 4A, R = –0.62, p = 0.008). GEE analysis showed a significant inverse relationship between NE levels and PSQI, such that for every –16.56 pg/mL decrease in NE there was a unit increase in PSQI score (p < 0.001).
Figure 4.
Lower 24-h plasma norepinephrine (NE) and lower slow oscillatory activity (SO: 0.5–1.25 Hz) in the first cycle of sleep correlate with greater subjective sleep disturbance (PSQI) in insomnia. Spearman rank correlations showing significant negative associations between (A) lower mean 24-h plasma NE (data Ln transformed), and (B) lower SO activity in the first cycle of sleep, with higher Pittsburg Sleep Quality Index (PSQI) scores, in participants with insomnia. Data on SO activity in the first cycle of sleep are averaged from nights 2, 3, and 4.
Relationship between SO activity and delta activity with PSQI in insomnia
There were no significant correlations between the average total amount of SO activity and delta activity with PSQI (R = –0.08, p = 0.11 and R = –0.15, p = 0.10, respectively).
GEE analysis showed a significant interaction between SO activity levels across the cycles of sleep with PSQI (p = 0.0003), which was not observed for delta activity (p = 0.82). Within SO activity, post-hoc analysis indicated that the reduction in SO activity levels in the first sleep cycle was associated with higher PSQI scores (SO activity R = –0.49, p = 0.0009, Figure 4B).
Sensitivity analyses of sleep duration in insomnia
Sensitivity analyses were conducted to assess the potential effect of sleep duration on SO activity levels across the cycles of sleep, 24-h cortisol and NE levels. Within the insomnia group, for all sleep durations tested (≤5, ≤6, >5, or > 6 h), the same direction of effect was observed for all the variables, such that SO activity in the first sleep cycle and 24-h NE levels were lower, and 24-h cortisol levels were higher, in insomnia vs. controls (Table S2).
Relationship between mean 24-h cortisol and NE levels
We explored whether the association between the 24-h levels of plasma cortisol and NE, which are interrelated and expected to exhibit similar physiological responses, differed between participants with insomnia and controls. A significant positive correlation was observed between mean 24-h cortisol and plasma NE levels in the control group (R = 0.52, p = 0.046; Figure S4A), but not in the insomnia group (R = 0.13, p = 0.59; Figure S4B).
Discussion
The findings in this study provide further support for a link between alterations in sleep microstructure and autonomic dysregulation in older adults with insomnia and short sleep duration. The insomnia group showed lower levels of SO activity (the slower frequency component of SWA), higher 24-h plasma cortisol levels, and lower 24-h plasma NE levels compared to healthy sleeper controls. In the control group, NE levels during the waking period were positively associated with the levels of SO activity in the first cycle of sleep, but interestingly, this association was not present in the insomnia group. Furthermore, within the insomnia group, lower 24-h plasma NE levels and lower SO activity in the first sleep cycle correlated with poorer subjective sleep quality (PSQI).
Although sleep EEG spectral activity of all frequency bands averaged across the night did not differentiate participants with insomnia from healthy sleepers, analysis by sleep cycles revealed lower SO activity in the first cycle in those with insomnia. This specific observation raises the possibility of an alteration in homeostatic sleep regulation in this older insomnia group with short sleep duration. In healthy people, SO activity in the first cycle of sleep has been shown to proportionally increase as a function of time awake [54], but much less is known within the context of insomnia in older adults. Lower amounts of SO activity in the first cycle of sleep have been reported in older adults compared to younger individuals [55], and are associated with a reduction in the volume of the medial prefrontal cortex (mPFC), a key anatomical area for the generation of sleep slow waves [56]. This age-related decline in SO activity may further impact sleep homeostatic regulation [57], particularly in those with insomnia and short sleep duration. The specificity to SO activity in the first cycle of sleep might explain the mixed results reported in prior studies examining SWA in insomnia disorder and controls [20, 58–61]. Most of these studies did not report specific changes in SWA by cycle of sleep, and were conducted in young and middle-aged adults. However, consistent with our finding, lower SWA in the first cycle of sleep has been reported both in young adults with insomnia [13], and also in response to sleep deprivation in those with insomnia [14].
While higher cortisol levels have not been consistently replicated in insomnia, we found a small, but higher 24-h plasma cortisol level (~1.1 μg/dL) in the insomnia group compared to healthy sleepers. A similar magnitude of difference has previously been reported in insomnia vs. controls [62]. Also, there is evidence that elevated levels of cortisol of similar magnitude (~ 0.76–1.6 μg/dL) [63–65], including the age-related increase in mean cortisol levels (~0.35 μg/dL per decade) [66, 67], are associated with alterations in glucose metabolism and cardiovascular function. Together, these data support the postulate that chronic activation of the hypothalamic–pituitary–adrenal (HPA) axis in some patients with insomnia, such as those with short sleep duration, may contribute to increased CMD risk [68].
The lower 24-h plasma NE levels in the insomnia group compared to controls was unexpected. Our initial hypothesis was based on findings in younger adults with insomnia [69, 70], such that we anticipated that NE levels would be higher in the insomnia group compared to controls. Similar to cortisol levels, prior studies do not consistently show indices of heightened autonomic activation in patients with insomnia compared to controls [71–76]. In our sample of older adults with insomnia and short sleep duration, it is possible that NE levels may partially reflect compensatory changes to the age-related decline in noradrenergic function [37], and thus NE levels in older adults may not represent an accurate estimation of sympathetic activity [77]. Although speculative, it is plausible that the normal age-related decline in noradrenergic activity [37], coupled with alterations in wake-sleep regulation, may further decrease daytime NE activity regulation in older adults with insomnia.
A novel finding from this study is that NE levels during the waking period positively correlated with the amount of SO activity in the first sleep cycle in the control group, but not in the insomnia group. A possible explanation for this unexpected finding comes from animal models showing that an intact NE system is important for the homeostatic regulation of sleep SO activity [78, 79]. The LC in the brainstem is the primary source of NE in the brain and its activity closely mirrors the sleep–wake cycle, with high rates of activity during wakefulness and lower activity during sleep [80]. To investigate the causal relationship between an intact LC–NE system and the homeostatic regulation of sleep, Cirelli et al. [79] destroyed LC-NE neurons in rats and found that this intervention resulted in the selective reduction of sleep SO activity (0.5–1.5 Hz) at the beginning of the sleep period, which was even more pronounced after exposure to sleep deprivation. It is important to note that we examined plasma NE, and therefore cannot infer that the results reflect the central noradrenergic activity. However, there is evidence that NE levels in cerebrospinal fluid and plasma are correlated under normal physiological conditions and in neuropsychiatric diseases [81–83], as well as following pharmacologic suppression of the central NE system with an α 2 agonist [84, 85], suggesting that a common mechanism links NE secretion in the central nervous system and peripheral nervous system [86, 87]. Whether such a relationship is present in insomnia is unknown.
In the insomnia group, the relationship between poorer subjective sleep quality (PSQI) and EEG microarchitecture was specific to lower SO activity levels in the first cycle of sleep. This result is consistent with findings from a prior study [20] showing that lower SO activity across the entire night in middle-aged insomniacs vs. controls was associated with higher PSQI scores. Interestingly, in older adults (>66 years), the severity of sleep disturbance indexed by PSQI [88] correlates with the degree of atrophy of the mPFC, an anatomic location important for the regulation of SO activity. In addition to SO activity, lower 24-h NE levels were associated with higher PSQI score, indicating a potential connection between noradrenergic activity and subjective sleep quality, and potentially worth exploring as a biomarker in insomnia research.
However, we did not find a correlation between 24-h cortisol levels with PSQI. A similar finding was observed in a study of postmenopausal women in whom no difference in the diurnal pattern of salivary cortisol between good versus poor sleepers (by PSQI) was seen, but a relationship with subjective sleep latency, sleep duration, and sleep efficiency was present [89]. Moreover, in our study, in insomnia not only was their lack of association between cortisol levels and subjective sleep quality, but also between cortisol levels and SO activity. Together, these data support the notion of a dynamic and complex interplay between different sleep dimensions and the HPA axis.
Another interesting and unexpected finding was the lack of correlation between 24-h cortisol and NE levels in the insomnia group. Typically, the circuits involved in cortisol and NE release are heavily intertwined in the adaptive responses to daily physical or psychobiological stressors [90]. The uncoupling of the noradrenergic–HPA axis shown here in insomnia has been previously described in panic disorder [91]. While speculative, this finding may be explained by alterations of the integrity of the noradrenergic system, resulting in atypical HPA responses [92, 93]. To our knowledge, the dissociation between cortisol and NE levels has not been explored in prior studies of insomnia and deserves further investigation.
While this study provides important insight into potential biological mechanisms of insomnia, there are also limitations. First, because of the older age and high representation of female participants in this study, the association between plasma NE and SO activity may not be generalizable to males and younger age groups with insomnia. Furthermore, although we adjusted for sex in the analyses, the study was not sufficiently powered to address sex differences, which are known to affect sleep architecture and autonomic function. Second, due to the lack of a comparison group of older adults without insomnia, but with similar sleep duration as the insomnia group, we cannot directly address the potential effect of short sleep duration on our findings. However, in the sensitivity analyses of different sleep durations within the insomnia group, the findings of lower SO activity in the first sleep cycle and 24-h NE levels and higher 24-h cortisol levels, did not change. Third, the possibility of type I and type II errors in the findings on EEG frequency changes across the cycles of sleep cannot be excluded. Fourth, the difference in timing of BP and HR measurements in the two parent studies precluded our ability to examine concomitant changes between BP and HR with cortisol and NE levels. Fifth, our findings do not address the directionality of the association linking the NE system and sleep SO homeostatic regulation. Lastly, it is possible that those with insomnia have lower SO activity in the first cycle of sleep, which is not a result of intrinsic neurobiological dysregulation of sleep homeostasis.
Within the context of the existing literature, these findings suggest a plausible physiological connection between noradrenergic activity during wake and the homeostatic regulation of sleep SO activity, and that dysregulation in the pathway linking the NE system with sleep SO activity may be involved in the pathophysiology of insomnia and the severity of perceived sleep disturbance in older adults. Due to its wide-spread connections to multiple brain areas and peripheral tissues, the integrity of the central NE system is essential for brain function [94] and the health of multiple physiological systems [95]. As such, dysregulation of the NE system may contribute to health comorbidities in insomnia. Future studies to replicate these findings, and powered to assess the role of sex and race in the various insomnia phenotypes, including younger adults, and those with insomnia without short sleep duration are needed.
Supplementary Material
Acknowledgments
We gratefully acknowledge the cooperation of the Northwestern Clinical Core and its Participants. We also thank Harry Whitmore for scoring polysomnography recordings, Natalie Pace, Nicole Bostic, and Rosemary Ortiz for helping with data collection, and Dr. Lisa Wolfe, Dr. Brandon Lu, and Dr. Kelly Baron for helping with clinical interviews during the screening process. We thank Dr. Jeffrey N. Savas for reviewing the manuscript and providing feedback.
Disclosure Statements
Financial disclosure: This work was supported by P01 AG11412 (PPG), UL1TR001422 (Northwestern University Clinical and Translational Sciences Institute/CRU).
Non-financial disclosure: none.
References
- 1. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed., Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2. Irwin MR, et al. Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol. 2019;18(3):296–306. [DOI] [PubMed] [Google Scholar]
- 3. Javaheri S, et al. Insomnia and risk of cardiovascular disease. Chest. 2017;152(2):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ford ES, Cunningham TJ, Giles WH, Croft JB. Trends in insomnia and excessive daytime sleepiness among US adults from 2002 to 2012. Sleep Med. 2015;16(3):372–378. doi: 10.1016/j.sleep.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel D, et al. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perlis ML, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104–113. [DOI] [PubMed] [Google Scholar]
- 7. Baglioni C, et al. Sleep changes in the disorder of insomnia: a meta-analysis of polysomnographic studies. Sleep Med Rev. 2014;18(3):195–213. [DOI] [PubMed] [Google Scholar]
- 8. Cappuccio FP, et al. Sleep and cardio-metabolic disease. Curr Cardiol Rep. 2017;19(11):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knutson KL, et al. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34(5):1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vgontzas AN, et al. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17(4):241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bertisch SM, Pollock BD, Mittleman MA, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: sleep Heart Health Study. Sleep. 2018;41(6). doi: 10.1093/sleep/zsy047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vgontzas AN, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33(9):1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Merica H, et al. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10(5):1826–1834. [DOI] [PubMed] [Google Scholar]
- 14. Besset A, et al. Homeostatic process and sleep spindles in patients with sleep-maintenance insomnia: effect of partial (21 h) sleep deprivation. Electroencephalogr Clin Neurophysiol. 1998;107(2):122–132. [DOI] [PubMed] [Google Scholar]
- 15. Dijk DJ. Slow-wave sleep, diabetes, and the sympathetic nervous system. Proc Natl Acad Sci USA. 2008;105(4): 1107–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rasch B, et al. About sleep’s role in memory. Physiol Rev. 2013;93(2):681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bastien CH, et al. Sleep EEG power spectra, insomnia, and chronic use of benzodiazepines. Sleep. 2003;26(3):313–317. [DOI] [PubMed] [Google Scholar]
- 18. Spiegelhalder K, et al. Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol Psychol. 2012;91(3):329–333. [DOI] [PubMed] [Google Scholar]
- 19. Hogan SE, et al. Slow-oscillation activity is reduced and high frequency activity is elevated in older adults with insomnia. J Clin Sleep Med. 2020;16(9):1445–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neu D, et al. Slow wave sleep in the chronically fatigued: power spectra distribution patterns in chronic fatigue syndrome and primary insomnia. Clin Neurophysiol. 2015;126(10):1926–1933. [DOI] [PubMed] [Google Scholar]
- 21. Dang-Vu TT, et al. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci USA. 2008;105(39):15160–15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steriade M, et al. Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993;13(8):3266–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nikulin VV, et al. Monochromatic ultra-slow (~0.1 Hz) oscillations in the human electroencephalogram and their relation to hemodynamics. Neuroimage. 2014;97:71–80. [DOI] [PubMed] [Google Scholar]
- 24. Pfurtscheller G, et al. Brain-heart communication: evidence for “central pacemaker” oscillations with a dominant frequency at 0.1 Hz in the cingulum. Clin Neurophysiol. 2017;128(1):183–193. [DOI] [PubMed] [Google Scholar]
- 25. Xia L, et al. Alterations in hypothalamus-pituitary-adrenal/thyroid axes and gonadotropin-releasing hormone in the patients with primary insomnia: a clinical research. PLoS One. 2013;8(8):e71065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Zambotti M, et al. Nighttime cardiac sympathetic hyper-activation in young primary insomniacs. Clin Auton Res. 2013;23(1):49–56. [DOI] [PubMed] [Google Scholar]
- 27. Foley DJ, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–432. [DOI] [PubMed] [Google Scholar]
- 28. Mander BA, et al. Sleep and human aging. Neuron. 2017;94(1):19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mander BA, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16(3):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carrier J, et al. Sleep slow wave changes during the middle years of life. Eur J Neurosci. 2011;33(4):758–766. [DOI] [PubMed] [Google Scholar]
- 31. Dijk DJ, et al. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516 (Pt 2):611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borbély AA, et al. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14(6):557–568. [DOI] [PubMed] [Google Scholar]
- 33. Robillard R, et al. Topography of homeostatic sleep pressure dissipation across the night in young and middle-aged men and women. J Sleep Res. 2010;19(3):455–465. [DOI] [PubMed] [Google Scholar]
- 34. Gilberg MAT. The dynamics of the first sleep cycle. Sleep. 1991;14(2):147–154. [PubMed] [Google Scholar]
- 35. Munch M, et al. Is homeostatic sleep regulation under low sleep pressure modified by age? Sleep. 2007;30(6):781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vargas I, Nguyen AM, Muench A, Bastien CH, Ellis JG, Perlis ML. Acute and chronic insomnia: what has time and/or hyperarousal got to do with it? Brain Sci. 2020;10(2). doi: 10.3390/brainsci10020071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mather M. How arousal-related neurotransmitter systems compensate for age-related decline. In: Gutchess, A., Thomas, AE, eds. The Cambridge Handbook of Cognitive Aging: A Life Course Perspective. Cambridge, England: Cambridge University Press; 2019. [Google Scholar]
- 38. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 2nd ed., Darien, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 39. Buysse DJ, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 40. Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385. [Google Scholar]
- 41. Beck AT, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 42. Reid KJ, et al. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 2010;11(9):934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fischer HF, et al. www.common-metrics.org: a web application to estimate scores from different patient-reported outcome measures on a common scale. BMC Med Res Methodol. 2016;16(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wahl I, et al. Standardization of depression measurement: a common metric was developed for 11 self-report depression measures. J Clin Epidemiol. 2014;67(1):73–86. [DOI] [PubMed] [Google Scholar]
- 45. Moshe I, et al. Three decades of internet- and computer-based interventions for the treatment of depression: protocol for a systematic review and meta-analysis. JMIR Res Protoc. 2020;9(3):e14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rechtschaffen AKA. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. BIS/BRI, UCLA. 1968. [DOI] [PubMed] [Google Scholar]
- 47. Iber C, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 48. Staresina BP, et al. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci. 2015;18(11):1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Feinberg I, et al. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16(3):283–291. [DOI] [PubMed] [Google Scholar]
- 50. Voultsios A, et al. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12(5):457–466. [DOI] [PubMed] [Google Scholar]
- 51. R Core Team. R: A Language and Environment for Statistical Computing. R foundation for statistical computing; Vienna, Austria, 2016. [Google Scholar]
- 52. Diggle P, et al. Analysis of Longitudinal Data. Oxford Statistical Science Series. 2nd ed.New York, NY: Oxford University Press; 2013. [Google Scholar]
- 53. Ulrich H, et al. The R package geepack for generalized estimating equations. J Stat Softw. 2006;15(2):1–11. [Google Scholar]
- 54. Campbell IG, et al. Homeostatic behavior of fast Fourier transform power in very low frequency non-rapid eye movement human electroencephalogram. Neuroscience. 2006;140(4):1395–1399. [DOI] [PubMed] [Google Scholar]
- 55. Dubé J, et al. Cortical thinning explains changes in sleep slow waves during adulthood. J Neurosci. 2015;35(20):7795–7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Murphy M, et al. Source modeling sleep slow waves. Proc Natl Acad Sci USA. 2009;106(5):1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Klerman EB, et al. Age-related reduction in the maximal capacity for sleep–implications for insomnia. Curr Biol. 2008;18(15):1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. St-Jean G, et al. REM and NREM power spectral analysis on two consecutive nights in psychophysiological and paradoxical insomnia sufferers. Int J Psychophysiol. 2013;89(2):181–194. [DOI] [PubMed] [Google Scholar]
- 59. Staner L, et al. Sleep microstructure around sleep onset differentiates major depressive insomnia from primary insomnia. J Sleep Res. 2003;12(4):319–330. [DOI] [PubMed] [Google Scholar]
- 60. Lamarche CH, et al. Electrophysiological changes during the sleep onset period of psychophysiological insomniacs, psychiatric insomniacs, and normal sleepers. Sleep. 1997;20(9):724–733. [PubMed] [Google Scholar]
- 61. Krystal AD, et al. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25(6):630–640. [PubMed] [Google Scholar]
- 62. Vgontzas AN, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2014;86(8):3787–3794. [DOI] [PubMed] [Google Scholar]
- 63. Sbardella E, et al. Cardiovascular features of possible autonomous cortisol secretion in patients with adrenal incidentalomas. Eur J Endocrinol. 2018;178(5):501–511. [DOI] [PubMed] [Google Scholar]
- 64. Androulakis II, et al. Letter to the editor response. J Clin Endocrinol Metab. 2014;99(11):L3. [DOI] [PubMed] [Google Scholar]
- 65. Plat L, et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab. 1999;84(9):3082–3092. [DOI] [PubMed] [Google Scholar]
- 66. Roelfsema F, et al. Impact of age, sex and body mass index on cortisol secretion in 143 healthy adults. Endocr Connect. 2017;6(7):500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yiallouris A, et al. Adrenal aging and its implications on stress responsiveness in humans. Front Endocrinol (Lausanne). 2019;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Walker BR, et al. Independent effects of obesity and cortisol in predicting cardiovascular risk factors in men and women. J Intern Med. 2000;247(2):198–204. [DOI] [PubMed] [Google Scholar]
- 69. Irwin M, et al. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav Immun. 2003;17(5):365–372. [DOI] [PubMed] [Google Scholar]
- 70. Vgontzas AN, et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45(1):21–31. [DOI] [PubMed] [Google Scholar]
- 71. Seelig E, et al. Concentrations of the stress hormone copeptin increase upon hypoglycaemia in patients with type 1 diabetes dependent of hypoglycaemia awareness. PLoS One. 2013;8(8):e72876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jurysta F, et al. The impact of chronic primary insomnia on the heart rate–EEG variability link. Clin Neurophysiol. 2009;120(6):1054–1060. [DOI] [PubMed] [Google Scholar]
- 73. Spiegelhalder K, et al. Heart rate and heart rate variability in subjectively reported insomnia. J Sleep Res. 2011;20(1 Pt 2):137–145. [DOI] [PubMed] [Google Scholar]
- 74. Stepanski EJ, et al. Heart rate changes in chronic insomnia. Stress Med. 1994;10:261–266. [Google Scholar]
- 75. Dodds KL, et al. Heart rate variability in insomnia patients: a critical review of the literature. Sleep Med Rev. 2017;33:88–100. [DOI] [PubMed] [Google Scholar]
- 76. Floam S, et al. Sleep characteristics as predictor variables of stress systems markers in insomnia disorder. J Sleep Res. 2015;24(3):296–304. [DOI] [PubMed] [Google Scholar]
- 77. Seals DR, et al. Human ageing and the sympathoadrenal system. J Physiol. 2000;528(Pt 3):407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim Y, et al. Sleep allostasis in chronic sleep restriction: the role of the norepinephrine system. Brain Res. 2013;1531:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cirelli C, et al. Locus ceruleus control of slow-wave homeostasis. J Neurosci. 2005;25(18):4503–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Aston-Jones G, et al. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1(8):876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ziegler MG, et al. Relationship between norepinephrine in blood and cerebrospinal fluid in the presence of a blood-cerebrospinal fluid barrier for norepinephrine. J Neurochem. 1977;28(3):677–679. [DOI] [PubMed] [Google Scholar]
- 82. Vlachakis ND, et al. Catecholamines and their major metabolites in plasma and cerebrospinal fluid of man. Brain Res. 1981;229(1):67–74. [DOI] [PubMed] [Google Scholar]
- 83. Raskind MA, et al. Norepinephrine and MHPG levels in CSF and plasma in Alzheimer’s disease. Arch Gen Psychiatry. 1984;41(4):343–346. [DOI] [PubMed] [Google Scholar]
- 84. Martin PR, et al. Effects of clonidine on central and peripheral catecholamine metabolism. Clin Pharmacol Ther. 1984;35(3):322–327. [DOI] [PubMed] [Google Scholar]
- 85. Sullivan PA, et al. Effects of clonidine on central and peripheral nerve tone in primary hypertension. Hypertension. 1986;8(7):611–617. [DOI] [PubMed] [Google Scholar]
- 86. Maas JW. Relationships between central nervous system noradrenergic function and plasma and urinary concentrations of norepinephrine metabolites. Adv Biochem Psychopharmacol. 1984;39:45–55. [PubMed] [Google Scholar]
- 87. Woods JH, ed. Neurobiology of Cerebrospinal Fluid. 1st ed.New York, NY: Plenum Press; 1980. [Google Scholar]
- 88. Sexton CE, et al. Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology. 2014;83(11):967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Huang T, et al. Habitual sleep quality and diurnal rhythms of salivary cortisol and dehydroepiandrosterone in postmenopausal women. Psychoneuroendocrinology. 2017;84:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Axelrod J, et al. Stress hormones: their interaction and regulation. Science. 1984;224(4648):452–459. [DOI] [PubMed] [Google Scholar]
- 91. Coplan JD, et al. Uncoupling of the noradrenergic-hypothalamic-pituitary-adrenal axis in panic disorder patients. Neuropsychopharmacology. 1995;13(1):65–73. [DOI] [PubMed] [Google Scholar]
- 92. Hermans EJ, et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334(6059):1151–1153. [DOI] [PubMed] [Google Scholar]
- 93. Radley JJ, et al. Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. J Neurosci. 2008;28(22):5806–5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mather M, et al. The locus coeruleus: essential for maintaining cognitive function and the aging brain. Trends Cogn Sci. 2016;20(3):214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. O’Donnell J, et al. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem Res. 2012;37(11):2496–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.