Abstract
Study Objectives
While wake duration is a major sleep driver, an important question is if wake quality also contributes to controlling sleep. In particular, we sought to determine whether changes in sensory stimulation affect sleep in Drosophila. As Drosophila rely heavily on their sense of smell, we focused on manipulating olfactory input and the olfactory sensory pathway.
Methods
Sensory deprivation was first performed by removing antennae or applying glue to antennae. We then measured sleep in response to neural activation, via expression of the thermally gated cation channel TRPA1, or inhibition, via expression of the inward rectifying potassium channel KIR2.1, of subpopulations of neurons in the olfactory pathway. Genetically restricting manipulations to adult animals prevented developmental effects.
Results
We find that olfactory deprivation reduces sleep, largely independently of mushroom bodies that integrate olfactory signals for memory consolidation and have previously been implicated in sleep. However, specific neurons in the lateral horn, the other third-order target of olfactory input, affect sleep. Also, activation of inhibitory second-order projection neurons increases sleep. No single neuronal population in the olfactory processing pathway was found to bidirectionally regulate sleep, and reduced sleep in response to olfactory deprivation may be masked by temperature changes.
Conclusions
These findings demonstrate that Drosophila sleep is sensitive to sensory stimulation, and identify novel sleep-regulating neurons in the olfactory circuit. Scaling of signals across the circuit may explain the lack of bidirectional effects when neuronal activity is manipulated. We propose that olfactory inputs act through specific circuit components to modulate sleep in flies.
Keywords: sleep, olfaction, Drosophila
Statement of Significance.
We present a comprehensive characterization of the effects of olfactory signaling on sleep, showing how activation and inhibition of the major neuronal populations in the olfactory processing pathway affect sleep. In doing so, we uncover the inhibitory projection neurons as a major sleep-promoting pathway. Given the complexity of the relationship between olfaction and sleep even in a relatively simple model, such as Drosophila, identifying the role of specific neuronal populations is a crucial first step in understanding how changes in sensory inputs can drive changes in sleep.
Introduction
Although the precise function of sleep remains elusive, it is clear that sleep is restorative, enabling more effective function during waking hours. Loss of sleep impairs cognitive ability and attention and is implicated in major accidents caused by human error [1]. Homeostatic regulation of sleep ensures that an organism gets enough sleep, with increased duration of wake typically resulting in increased drive to sleep [2].
While the duration of wake is a major driver of sleep, an important question is whether the quality of wake is also a factor in controlling sleep. Based upon the synaptic homeostasis model of sleep, which postulates that synapses potentiated during wake are downscaled during sleep [3], the prediction is that the more stimulating the wake-related activity, the more the need for sleep. Indeed, Huber et al. [4] showed that increasing the exploratory behavior of rats during wake increased slow-wave activity, a marker of sleep need, during subsequent sleep. In addition, several studies in Drosophila show that social enrichment increases sleep need [5–8]. While changes in synaptic strength are typically associated with learning processes, there is also some evidence that sensory inputs alone, such as mechanosensory [9, 10], visual [11], and taste stimuli [12], are sufficient to increase sleep even without an operant learning task. However, the extent to which this occurs across different sensory modalities, and the role of neuronal populations downstream of the primary sensory neurons, is still unclear.
The use of small animal models is now providing important insights into sleep regulation and function. Among these, Drosophila is most widely employed, and it allows not only the ability to conduct genetic studies, but also the ability to relate sleep to other behaviors and processes. For instance, research in Drosophila has identified mechanistic links between sleep and learning and memory [5, 13–17], feeding [18–21], aggression [22], and mating [23–27]. Sensory systems, in particular vision and olfaction [28–31], are also well-studied in Drosophila, providing the opportunity to determine how these could impact sleep.
We asked if severing a major sensory conduit to the fly brain, the antenna, would affect Drosophila sleep, and report here that this has lasting effects on sleep duration. We found that genetically silencing olfactory sensory neurons also reduced sleep, while activating inhibitory second-order projection neurons increased sleep. We also identified sleep-regulating properties of the third-order olfactory processing neurons in the lateral horn.
Methods
Flies
The following fly stocks were ordered from the Bloomington Stock Center: MB607B (#68256) [32], 16A06 (#48709) [33], Orco-Gal4 (#23909, referred to as Or83b-Gal4 in the text) [34], and GH146-Gal4 (#30026) [35, 36]. NP6303 and NP6250 lines [37] were ordered from Kyoto Stock Center DGRC. These were subsequently outcrossed into iso31 for five generations unless otherwise indicated. Mz699-Gal4 flies [38] were a gift from Liqun Luo [39] and backcrossed to iso31 for five generations. Stock flies of genotype tubGal80ts/FM7; UAS-kir2.1 were generated by Paula R. Haynes in the white Canton-S genetic background and subsequently outcrossed to iso31 for five generations. The tubGal80ts/FM7; UAS-kir2.1 flies were used in all experiments involving tubGal80ts/+; UAS-kir2.1/+ except for the temperature shift with antennectomy or antennae glue experiments (Supplementary Figure S8) and the Johnston’s Organ silencing experiments with NP6303 and NP6250 (Supplementary Figure S4). Experiments presented in Supplementary Figures S4 and S8A, B instead used flies of genotype tubGal80ts; UAS-kir2.1/TM6B, which were generated and outcrossed into iso31 for five generations by Anna N. King. UAS-dTrpA1(II) was originally a gift from Leslie Griffith [40] and outcrossed into iso31 for seven generations by Daniel J. Cavanaugh [41]. Flies of the genotype 20XUAS-shibts were also outcrossed into iso31 for seven generations by Daniel J. Cavanaugh [42]. All lateral horn lines [43] were ordered from the Janelia Flylight Split-Gal4 Collection and not outcrossed.
Flies were raised on standard cornmeal-molasses medium in a 12 h:12 h light:dark cycle. In experiments that required temperature manipulations, flies were raised at 18°C; otherwise, flies were raised at 25°C. Mated female flies were used for all sleep assays. For flies raised at 25°C, antennae manipulations were performed at 4–6 days (unless otherwise indicated). For flies raised at 18°C, neuronal manipulations were performed at 4–7 days; baseline sleep recordings were also conducted at 18°C.
Single beam sleep experiments
Sleep experiments were conducted using the Drosophila Activity Monitoring (DAM) system (TriKinetics, Waltham, MA). With the exception of experiments conducted in the multibeam activity monitors, the DAM2 monitor was used. Flies were loaded into glass tubes (5 mm diameter and 65 mm length) containing 5% sucrose and 2% agar. Yarn was inserted into the open end to prevent the fly from escaping. DAMFileScan110 (TriKinetics, Waltham, MA) was used to convert monitor data into channel files (counts per minute per channel data). Channel files were converted into sleep using custom software (found at https://github.com/cthsu86/damSleepConverter) written in MATLAB 2014a (Mathworks, Natick, MA). Sleep was defined as any period of time where there is no activity for at least 5 min [44, 45]; the first five minutes are included in computations of bout length and total sleep (i.e. 6 min of inactivity are scored as 6 min of sleep). When computing bouts within a range (such as ZT0 to 12, where ZT refers to Zeitgeiber Time, or lights on), sleep bouts that exceeded the range in question (for instance, bouts that span ZT12) were truncated once they exceeded the specified range. Truncated sleep bouts at these boundaries were treated as complete bouts and included in computations of mean and longest bout lengths. In experiments where data from a baseline day and night are reported, the baseline day refers to either the day or the night immediately preceding sensory or neuronal manipulation. Changes in minutes of sleep, activity index, or sleep parameters were computed by taking the value recorded on the day or night indicated and subtracting the value recorded on the baseline day or night for each individual fly. Activity Index for experiments that were recorded using the single beam monitors was computed as the number of channel counts per minute awake.
Antennae manipulations
Removal of the antennae (antennectomy) was performed between ZT0 and ZT7 under carbon dioxide anesthesia. The distal most part of the antennae (containing the third and fourth antennae segments) was removed using forceps. To glue the antennae, flies were again anesthetized using carbon dioxide. A 0.5 mm stainless steel insect pin (Fine Science Tools, Foster City, CA; Cat. No. 26001-50) was used to coat the entire anterior surface of the fly’s antennae with ultraviolet (UV) curable glue (BONDIC, Aurora, ON). The glue was then illuminated with a UV light for approximately 3 s. UV curable glue was also applied to the control flies, but in a different location (in the center of the top of their head cuticle, between the eyes). In cases where no baseline data is reported, the first-day post antennectomy or post antennae glue represents the day beginning from the first ZT0 timepoint following antennae manipulation (roughly 16–22 h after antennectomy or gluing was performed).
Odor delivery in single beam experiments
To measure sleep while delivering odor, flies were loaded into polycarbonate tubes containing 5% sucrose and 2% agar and one to two 0.030ʺ holes drilled in (PPT5x65D2E, TriKinetics, Waltham, MA); they were inserted into a DAM2 monitor with a gas distribution manifold on one side (TriKinetics, Waltham, MA). For each experiment, 2 mL of fresh odor were diluted to the appropriate concentration (as listed in Supplementary Table S1) no more than 48 h prior to delivery and were stored in 20 mL glass vials (J.G. Finneran Associates, Inc., Vineland, NJ) at 4°C until the stimulation time. Details pertaining to the specific odors used are listed in Supplementary Table S1.
To deliver the odor, a BOYU Aquatic Air Pump S200 (Guongdong Boyu Group Co., Ltd., Guangdong, China) set to its maximum airflow of 4 L/min was used. The air pumps were connected such that air would flow first through a vial of water (to humidify the air) and next through a vial containing the odor. Flies in the control group received air that would flow through a vial of water (to humidify the air); in cases where the odor was diluted in paraffin oil, a vial of paraffin oil was added in lieu of a vial containing the odor.
After two full days of recording (one to allow the flies to acclimate and a second day to record as the baseline day), odor was delivered for 2 s out of every 20 s between the hours of ZT0.5 and ZT11.5. To control the timing of odor delivery, either an LC4 Light Controller (TriKinetics, Waltham, MA) or a Multifunctional Infinite Cycle Programmable Plug-in Digital Timer Switch with 3-Prong Outlet for Appliances, Energy-Saving Timer (Nearpow, Beaverton, OR). Odor vials were connected at the start of the stimulation period (ZT0.5) and removed at the end of the stimulation period (ZT11.5). Both the control and the odor groups received identical air stimulation.
Multibeam sleep and odor experiments
The MB5 MultiBeam Activity Monitor (TriKinetics, Waltham, MA) was used to obtain high-resolution activity measurements (using infrared beams placed every 3 mm along the length of the tube). Flies were loaded into glass tubes 5 mm diameter and 80 mm length containing 5% sucrose and 2% agar. Yarn was inserted into the open end of the tube to prevent the flies from escaping. To analyze sleep, channel files containing the “Movement” parameter [46], which is the number of times flies move from one beam to another, were exported using the DAMFileScan110 software. The same MATLAB code as above was then used to compute time asleep, where sleep was then defined as any time period of at least five minutes without movement [46]. Activity Index for experiments recorded using the multibeam monitors is computed as the number of movements between beams per minute awake.
For odor experiments in the multibeam monitors, four small pieces of polyethylene tubing with an inner diameter of 0.045ʺ (Fisher Scientific, Waltham, MA; Cat. No. 14-170-12E) were inserted into the open end of the glass tube in lieu of yarn. To release air pressure during odor delivery, polycarbonate tubes with one to two 0.030ʺ holes drilled (PPT5x65D2E, TriKinetics, Waltham, MA) were connected, using 3/16ʺ inner diameter tubing (Fisher Scientific; Cat. No. 14-171-215), to the glass tube containing the fly. This was then attached to a five-port manifold (Cole-Parmer, Vernon Hills, IL; Cat. No. EW-30600–43) so that air could be diverted to five tubes at once.
Exposing flies to the odor of other flies was assayed using the multibeam monitors and was delivered without an external air stream. For this experiment, we prepared locomotor tubes in which two female flies were housed for 2 days with the standard 5% sucrose 2% agar solution and subsequently removed. Experimental flies, following a baseline recording period, were transferred between 0 and 1.5 h after lights on (ZT0 and ZT1.5) to the locomotor tubes that previously contained two females, and then transferred to a clean tube within 1.5 h before lights off (ZT10.5–12). Control flies were transferred to clean tubes at each timepoint.
Hydroxyurea treatment
The protocol for hydroxyurea ablation of the mushroom bodies was based on that of Sweeney et al. [47]. Hydroxyurea (Fisher Scientific; Cat. No. AAA1083103) was stored in a stock solution of 50 mg/mL in the dark at room temperature. A yeast paste was prepared by mixing 1:1 yeast to water (volume to volume) then microwaving briefly. Yeast paste was then diluted 1:3 in either hydroxyurea stock solution (ablation media) or water (for the “No Hydroxyurea” control media). Female flies (2–5 days old) were then placed in egg-laying chambers that contained a petri dish of grape egg-laying medium (100 mL H2O, 1 mL ethanol, 0.5 mL acetic acid, 10 mL grape juice). After the female flies were allowed to lay eggs, for 22 h, they were then discarded. Larvae were collected from the plates once an hour (for the next 5 h) to ensure they were within one hour of hatching. Once collected, larvae were transferred to ablation media or the no hydroxyurea control media, where they remained for 4 h, after which they were washed with water and then transferred to standard cornmeal-molasses food and allowed to develop normally.
Statistical analysis
All graphs and statistics generated using GraphPad Prism 8.4.2. Line plots showing sleep per 30 min bin are plotted with mean and standard error measure (SEMs). Box and whisker plots use boxes to represent median and interquartile ranges and whiskers to represent points within 2.5 times the interquartile range from the median. Points beyond that range are shown as solid circles.
Results
Loss of antennal input reduces sleep in flies
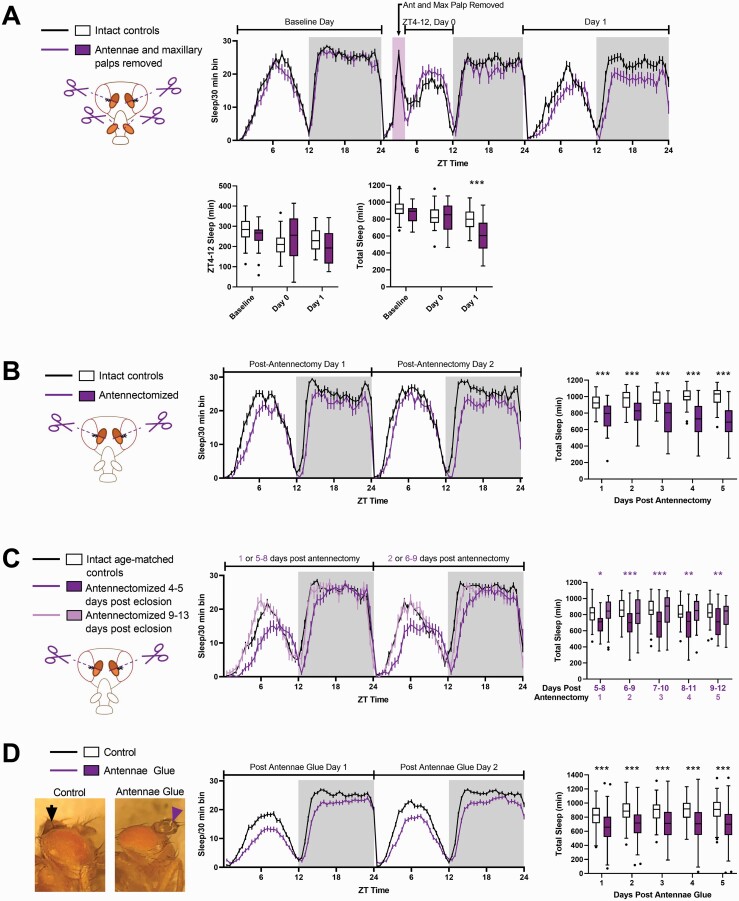
We first sought to determine whether physical manipulation of sensory organs was sufficient to induce a change in sleep. We began by removing both the antennae and maxillary palps of the antennae 4–6 days posteclosion. We found that immediately following antennae and maxillary palp removal (day 0), flies exhibited a slight but not significant increase in sleep (p = 0.0904), presumably in response to the injury as described previously in the literature [48] (Figure 1A). This value was significant if sleep from Zeitgeber Time (ZT) 5–12 (ZT0 = lights on; ZT12 = lights off) was compared between intact controls and flies with their antennae and maxillary palps removed (p = 0.0306, data not shown). However, we also found that flies slept significantly less on the first full day (day 1, beginning at ZT0) following antennae and maxillary palp removal (Figure 1A). This significant reduction in sleep was also observed in flies in which only antennae (rather than both their antennae and maxillary palps) were removed (Figure 1B). Decreased sleep was accompanied by a decrease in the longest sleep bout length and a successively increasing latency to sleep at night (following lights off) (Supplementary Figure S1A). Activity index, which represents the amount of locomotor activity per waking minute, was also higher in antennectomized flies (relative to intact controls) during both the day and the night (Supplementary Figure S1A). Sleep loss was still observed up to 9 days postantennectomy (Figure 1C), although antennectomized flies did not show consistent differences in sleep bout length at 9–13 days of age (Supplementary Figure S1B). Instead, the decrease in sleep in older antennectomized flies appeared to be driven primarily by an increased latency to sleep. Interestingly, we found that flies that were antennectomized 9–13 days posteclosion (rather than 4–5 days posteclosion) did not show loss in total sleep (i.e. summed over the 24 h day), but had significantly lower nighttime sleep starting 2 days postantennectomy (Figure 1C and Supplementary Figure S1B).
Figure 1.
Physical manipulations of the antennae (antennectomy and antennae glue) result in sleep loss in young flies. (A) Top panel shows the amount of sleep per 30 min bin on the day preceding, on the day of, and on the day following antennectomy. The pink rectangle represents the time during which flies were removed from the DAM system and had their antennae and maxillary palps removed. Sleep during lights on immediately following antennae and maxillary palp removal (day 0, ZT4–12) was slightly but not significantly greater than that of intact controls (bottom left panel). Total sleep on the first day following antennae clip was significantly reduced, however (bottom right panel). Intact control flies (n = 32). Antennectomized flies (with maxillary palps removed, n = 30). *p < 0.05, **p < 0.01, ***p < 0.001 by Mann–Whitney test. (B) Left panel shows amount of sleep per 30 min bins during the first two full days following antennectomy (starting with ZT0 the day following antennectomy) of flies 4–5 days old. Right panels show amount of sleep per day following antennectomy. Intact control flies (n = 33) and antennectomized flies (n = 32). *p < 0.05, **p < 0.01, ***p < 0.001 by Mann–Whitney test .(C) Flies antennectomized at 9–13 days old (n = 48, light-lavender) do not show sleep loss on the first day following antennectomy, relative to age-matched controls (n = 54, black lines in top panel and white boxes in bottom panel). Left panel shows the amount of sleep per 30 min bins of flies antennectomized at 4–5 days old (n = 38, dark purple), flies antennectomized at 9–13 days old (n = 48, light-lavender), and intact age-matched controls (10–14 days old at the time indicated as “1 or 5–8 postantennectomy,” black). Dark purple asterisks indicate significant differences between the intact controls (n = 54) and flies antennectomized 4–7 days before loading (n = 38). Light lavender asterisks indicate significant comparisons between intact controls and flies antennectomized on the day of loading (n = 48). *p < 0.05, **p < 0.01, ***p < 0.001 by Kruskal–Wallis test with Dunn’s multiple comparison test. (D) Far left panel shows examples of flies with their antennae glued (purple arrowheads) and control flies with glue in the top center cuticle (black arrowheads). Middle panel shows the amount of sleep per 30 min bin during the first two full days following antennae glue. Far right panel show the difference between day (left panel) and night (right panel) sleep in control flies (n = 124) versus those with their antennae glued (n = 136). *p < 0.05, **p < 0.01, ***p < 0.001 by Mann–Whitney test.
To test the effect of sensory deprivation without injuring the fly, we next tested how covering the fly’s antennae with UV curable glue could affect sleep (Figure 1D). UV curable glue was also applied to the control flies, but in a different location (in the center of the top of their head cuticle, between the eyes). We found that covering the fly’s antennae with glue caused sleep loss that, as with antennectomy, was observable on the day following sensory deprivation, and accompanied by a decrease in longest sleep bout length and an increase in latency to sleep at night (Supplementary Figure S1C). Unlike antennectomized flies, however, activity index in flies with glued antennae was not greater than that of controls (Supplementary Figure S1C). This suggests that the decrease in sleep is not the result of increased locomotion.
Loss of olfactory input reduces sleep
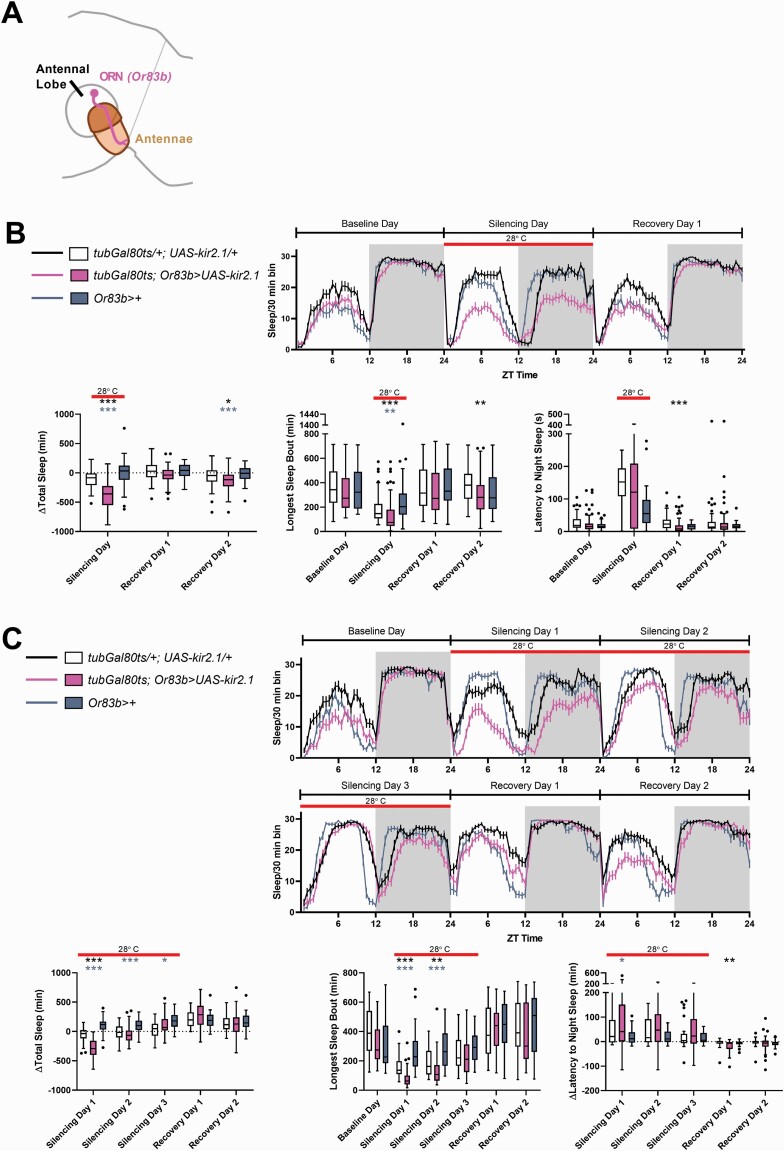
Because antennae are used in multiple modalities of sensory detection, we asked whether silencing neurons associated with a specific sensory modality would also cause a decrease in sleep. We used the Or83b-Gal4 driver to silence all olfactory receptor neurons (ORNs) [34], which relay information from the antennae to the antennae lobe of the fly brain (Figure 2A), and restricted silencing to the adult stage by including temperature-sensitive tub-Gal80 (the TARGET system) [49, 50] to prevent Gal4 expression during development. At the restrictive temperature, silencing of ORNs via expression of the inward rectifying potassium channel Kir2.1 [51] resulted in a significant decrease in both daytime and nighttime sleep relative to controls (Figure 2B). Experimental animals also showed a small reduction in sleep at the permissive temperature (baseline) (Supplementary Figure S2), but differences from controls were evident even when differences in sleep at the permissive temperature were subtracted (Figure 2B). This indicates that sleep loss produced by silencing of ORNs is caused by loss of adult function and does not just reflect a developmental deficit. This decrease in sleep amount was accompanied by a decrease in the longest sleep bout duration and did not result from increased activity; in fact, we observed a decrease in activity index (activity counts per waking minute) during the night (Figure 2B and Supplementary Figure S2B). There was also no significant change in sleep latency as a consequence of ORN silencing (Figure 2B).
Figure 2.
Silencing ORNs also decreases sleep. (A) Schematic illustrating the first order olfactory sensory neurons (ORNs) carrying information from the antennae to fly’s brain via the antennae lobe. ORNs are labeled using Or83b-Gal4. (B) Top panel shows the amount of sleep during the baseline, silencing, and recovery days in 30 min bins over the 24 h day. The red bar (in all panels) indicates the time period during which the temperature was raised to 28°C, thereby inactivating the GAL80 repressor and silencing the Or83b+ neurons. Flies with ORNs silenced have reduced sleep (bottom left) and decreased bout length (bottom middle). Change in sleep relative to baseline was plotted because of significant differences between the three genotypes at baseline (at the permissive temperature) (Supplementary Figure S2B). *p < 0.05, **p < 0.01, ***p < 0.001 by Kruskal–Wallis test with Dunn’s multiple comparison test between the experimental genotype (tubGal80ts; Or83b>UAS-kir2.1) and each of the controls. Black asterisks indicate significant comparisons between control flies of the genotype tubGal80ts/+; UAS-kir2.1/+ (n = 45) and experimental flies of the genotype tubGal80ts; Or83b>UAS-kir2.1 (n = 61). Blue-grey asterisks indicate significant differences between control flies of the genotype Or83b>+ (n = 45) and experimental flies of the genotype tubGal80ts; Or83b>UAS-kir2.1. (C) Extending the time at the restrictive temperature did not increase the magnitude of sleep loss; instead, experimental flies (tubGal80ts; Or83b>UAS-kir2.1, pink, n = 37) only showed significant differences relative to both genetic controls on the first day of silencing (bottom left). Change in sleep relative to baseline was plotted because of significant differences between the three genotypes at baseline (at the permissive temperature, see Supplementary Figure S2C). Black asterisks indicate significant comparisons between control flies of the genotype tubGal80ts/+; UAS-kir2.1/+ (n = 33) and experimental flies of the genotype tubGal80ts; Or83b>UAS-kir2.1. Blue–gray asterisks indicate significant differences between control flies of the genotype Or83b>+ (n = 34) and experimental flies of the genotype tubGal80ts; Or83b>UAS-kir2.1.
Despite the significant sleep loss in response to ORN silencing, there was no increase in sleep (relative to the controls) once the flies were returned to the permissive temperature. This lack of rebound sleep suggests that the reduced sleep in response to olfactory deprivation did not lead to accumulation of sleep drive; thus it likely reflects reduced sleep need rather than reduced sleep ability. We found that silencing ORNs using temporally restricted UAS-kir2.1 decreased sleep in both iso31 (Figure 2, Supplementary Figure S2B and S2C) and white Canton-S genetic backgrounds (Supplementary Figure S2D), suggesting that loss of sleep in response to olfactory deprivation is independent of background. Because we observed long-term sleep loss as a consequence of antennectomy and antennae glue, we next wanted to test whether silencing the ORNs for several days would result in a persistent sleep loss. Surprisingly, we found that while experimental flies with ORNs silenced showed a sleep loss on the first day of neuronal silencing (upon exposure to the restrictive temperature), prolonged exposure to the restrictive temperature gradually increased their sleep until change in total sleep amount (relative to baseline) was comparable to genetic controls (Figure 2C and Supplementary Figure S2C).
We also tested whether other sensory modalities mediated by antennae affect sleep. To silence wind-responsive neurons in the Johnston’s Organ of the antennae (JO-CE) we used NP6250-Gal4 [37] and, as with Or83b-Gal4, we coupled it with tub-Gal80ts to restrict the manipulation to adults. Silencing wind-responsive neurons did not reduce sleep (Supplementary Figure S3A). To silence the sound-responsive neurons, we used the NP6305-Gal4 driver to target the Johnston’s organ zone A neurons in addition to JO-CE neurons. We found that adult-specific silencing of JO-A and JO-CE actually increased daytime sleep, although the difference was not significantly greater than both control genotypes (Supplementary Figure S3B). Together these data indicate that sleep-reducing effects of antenna removal/glue, particularly the reduction in longest sleep bout length within the first few nights, are due to loss of olfactory sensory neurons.
Activation of ORNs does not increase sleep
Having established that silencing the ORNs results in a decrease in sleep, we asked whether activating the same neurons would result in an increase in sleep. We first used Or83b-Gal4 to drive UAS-dTrpA1, a temperature-activated cation channel that increases neuronal activity at high temperatures [40]. Stimulating the ORNs in this fashion did not cause an increase in sleep, regardless of whether the neurons were stimulated during the day or during the night (Supplementary Figure S4). In fact, daytime activation produced a small sleep loss, which did not persist or trigger a rebound on subsequent days.
We considered three possible explanations for the contradiction between silencing versus activating the ORNs. The first possibility is that TrpA1 activation is unable to replicate an ethologically relevant firing pattern, either because it activates the neurons beyond their normal physiological range or because it does not replicate the temporal dynamics of firing typically evoked by odors [52]. The second possibility is that different ORNs have different effects on sleep; thus activation of all ORNs simultaneously results in contradictory signals that interfere with the effects of individual ORNs. Finally, as normalization at the ORN-Projection neuron synapse scales with the total activity of the ORN population [29, 53], it is possible that increases in the activity of all ORNs are normalized and therefore undetected by downstream neurons.
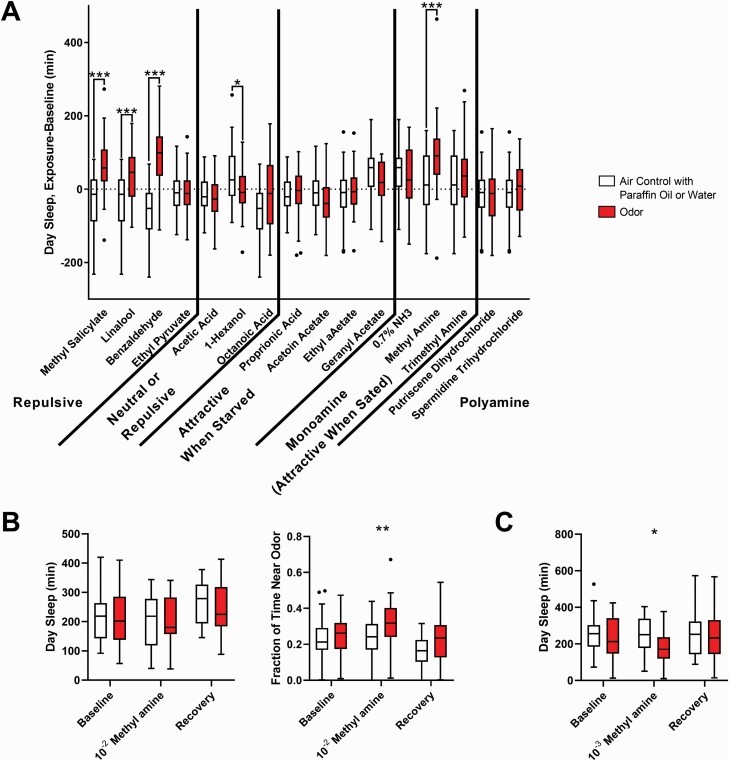
To stimulate the ORNs in a more physiologically relevant manner, we measured the change in sleep in response to daytime exposure to a panel of 16 different odors (Figure 3A). Daytime exposure (from ZT0.5 to ZT11.5) was used rather than nighttime exposure because we theorized that too much stimulation at night would disrupt their sleep; this was supported by our Or83b-Gal4>UAS-dTrpA1 experiments, which showed that flies exhibited a more dramatic sleep loss when their ORNs were activated at night rather than during the day (Supplementary Figure S4). Odors were delivered via air pump across 32 locomotor tubes (125 mL/min to each tube) in 2-s pulses every 20 s. Control flies received paraffin oil or humidified air delivered at the same flow rate and during the same time of day.
Figure 3.
Olfactory stimulation via odor delivery did not increase sleep. (A) In the single beam assay, several odors appeared to cause an increase in sleep when flies were exposed to them (during the day). Flies were on 5% sucrose, 2% agar medium regardless of whether or not odor valence was dependent on satiety or starvation. Concentrations and number of flies in Supplementary Table S1. Sleep is reported as the difference in sleep from ZT0 to ZT12 on the exposure day. (B) No increase in sleep in response to methyl amine at 10–2 was observed when sleep was measured in multibeam activity monitors. Instead, flies increased the amount of time spent in the quarter of the tube closest to the odor (beams 14, 15, 16, and 17). Data from control flies, which received the same air puff delivered over water instead an odorant, shown in white (n = 30 on baseline and methyl amine/air puff day, n = 16 on recovery day). Flies exposed to methyl amine shown in red (n = 29 on baseline and methyl amine day, n = 15 on recovery day). Day sleep and fraction of time spent near odor computed from ZT0 to ZT12. (C) Changing the concentration of methyl amine from 10–2 to 10–3 caused a decrease in sleep rather than an increase in sleep when measured in the multibeam activity monitors (n = 24 for both groups). For all panels, flies exposed to the air control flies shown in white, flies exposed to the test odorant shown in red. *p < 0.05, **p < 0.01, ***p < 0.001 by Mann–Whitney test comparing the odor to the in cohort control.
The initial screen of several odors suggested that aversive odors such as benzaldehyde, linalool, and methyl salicylate [54] caused an increase in sleep, as did methylamine, an odorant that is attractive to sated flies (Figure 3A) [55]. In follow-up experiments, we focused on benzaldehyde (as a representative of an aversive odorant) and methylamine (an attractive odorant), but did not see reliable increases in daytime sleep with benzaldehyde (Supplementary Figure S5A). There did appear to be an increase in sleep in response to methylamine (Supplementary Figure S5B), but because our standard assay infers sleep based upon lack of activity in a single beam break monitor, apparent sleep increases could result from either preference for a specific location (side of the infrared beam) rather than decreased locomotion. Thus, we repeated the experiments with methylamine while measuring sleep using the multi-beam assay. In this higher resolution assay, beams are placed at 17 locations 3 mm apart along the length of the locomotor tube, allowing more precise monitoring of the fly’s activity and even its location. When sleep was measured using the higher resolution multibeam system rather than the classical single-beam system, we found that methylamine exposure did not cause an increase in sleep. Instead, methylamine, an attractive odor, increased the fraction of time flies spent in the quarter of the locomotor tube (12 cm) closest to the odor source (Figure 3B). Thus, the apparent increase in sleep in the single beam assay likely resulted from flies dwelling near the source of the attractive odor and failing to cross the beam in the center of the tube.
We also tested whether flies increase their sleep in response to daytime exposure to the odor of other flies. For this experiment, we prepared locomotor tubes in which two female flies were housed for 2 days and subsequently removed. Following a baseline recording period, experimental flies, which were also female, were transferred between 1 and 1.5 h after lights on (ZT0 and ZT1.5) to the locomotor tubes that previously contained two females, and then transferred to a clean tube 1–1.5 h before lights off (ZT10.5–12). Control flies were transferred to clean tubes at each timepoint. We found that exposing flies to the odor of conspecifics actually decreased rather than increased sleep at the time of exposure, and did not increase sleep the following night or the subsequent day (Supplementary Figure S5C).
Excitatory and inhibitory projection neurons show differential effects on sleep
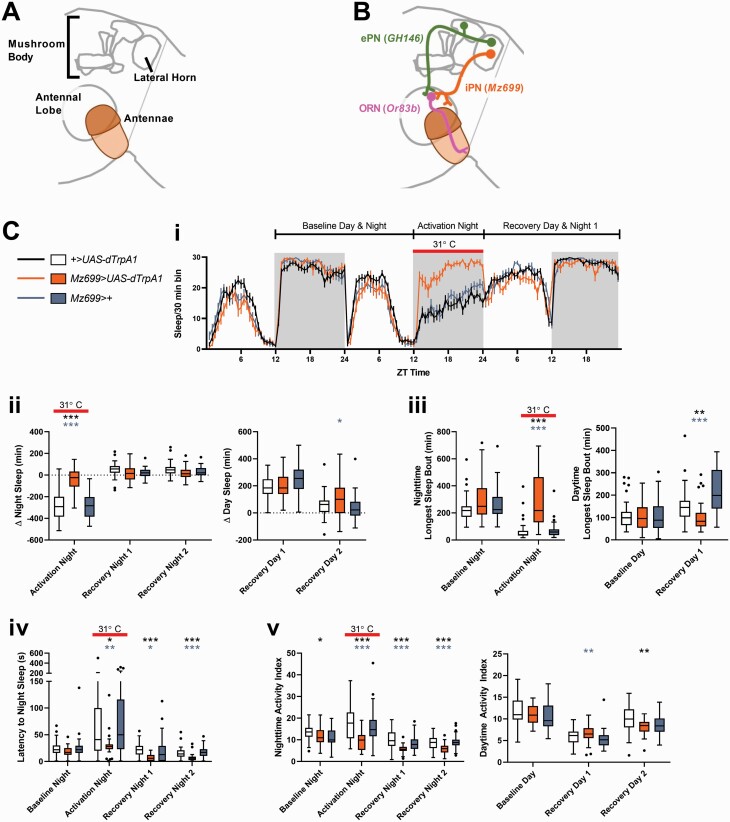
Because we were unable to elicit an increase in sleep with the presentation of odors, we returned to examining neuronal activation, using thermogenetic methods. To circumvent the problem of homeostatic normalization at the ORN level, we focused instead on activating the second-order projection neurons (PNs) that innervate the antennal lobe. There are two populations of PNs that carry information from the antennae lobe to other parts of the brain—the excitatory PNs (ePNs), which carry information to both the mushroom body and the lateral horn, and the inhibitory projection neurons (iPNs), which carry information just to the lateral horn (Figure 4A,B).
Figure 4.
Activating second order inhibitory projection neurons at night increases sleep. (A) Schematic illustrating the antennae and the three major neuropil involved in olfactory processing—the antennae lobe, the mushroom body, and the lateral horn. (B) Schematic illustrating the anatomical relationships between the ORNs (pink), the ePNs (green), and iPNs (orange) and the respective neuropil they innervate. In subsequent figures, only the relevant neuronal population will be colored, with pink representing populations that are silenced via UAS-kir2.1 and orange representing populations that are activated by UAS-dTrpA1. (C) Activating secondary order inhibitory projection neurons at night increases sleep. Panel (i) shows the amount of sleep during the baseline, experimental, and recovery days in 30 min bins over the 24 h day as reported by multibeam monitors. The time at which the temperature was raised from 18°C to 31°C, which activates iPNs in the experimental genotype Mz699>UAS-dTrpA1 (shown in orange), is indicated in all panels with the solid red line. Panel (ii) shows the difference between sleep on the night iPNs were activated and the night before (left) and the difference in sleep daytime sleep between the day following activation and the preceding day (immediately before activation). Panel (iii) shows the longest sleep bout observed during the baseline night (the night preceding activation) and the activation night (during which the temperature was raised to 31°C), and the longest sleep bout observed during the baseline day and the recovery day. Panel (iv) shows the latency to sleep at night. Panel (v) shows the nighttime and daytime activity index. *p < 0.05, **p < 0.01, ***p < 0.001 by Kruskal–Wallis test with Dunn’s multiple comparison test between the experimental genotype (Mz699>UAS-dTrpA1) and each of the controls. Black asterisks indicate significant comparisons between control flies of the genotype +>UAS-dTrpA1 (n = 43) and experimental flies of the genotype Mz699>UAS-dTrpA1 (n = 30). Blue–gray asterisks indicate significant differences between control flies of the genotype Mz699>+ (n = 37) and experimental flies (Mz699>UAS-dTrpA1).
We first activated ePNs using GH146-Gal4>UAS-dTrpA1 [35, 36]. Activating ePNs at night did not change sleep in single beam DAM monitors (Supplementary Figure S6B). In contrast, activating ePNs during the day appeared to increase sleep in the single beam monitors (Supplementary Figure S6C), but the higher resolution multibeam monitors indicated a decrease in activity index rather than an increase in sleep (Figure S6D).
We next activated the iPNs using Mz699-Gal4>UAS-dTrpA1 [38]. We first tested daytime (ZT0–12) activation of iPNs using the single beam (Supplementary Figure S7B) and subsequently the multibeam (Supplementary Figure S7C) DAM system. As with the ePNs, an apparent sleep increase with the single beam system was not supported by multibeam data (Supplementary Figure S7A and B). In contrast, activating the iPNs at night dramatically increased sleep when tested using both the single and multibeam system (Figure 4 and Supplementary Figure S7D), primarily through an increase in bout length. Interestingly, although the increased sleep only occurred during the time of activation, there was a decrease in locomotor activity that occurred not only while the iPNs were activated but continued for two subsequent nights (Figure 4C, v). Latency to nighttime sleep was also decreased on the activation night as well as the subsequent recovery nights, even though total minutes of sleep had returned to normal (Figure 4C, iv).
At higher temperatures flies typically reduce nighttime sleep and sleep more during the day [56]. As expected, activation of iPNs at night reduced sleep in control flies, resulting in sleep rebound the following day (Figure 4C, ii). Surprisingly, this apparent rebound was also evident in flies in which iPNs had been activated, even though these flies slept significantly more at night, suggesting a high sleep need. Although the minutes of rebound sleep were comparable between experimental flies and genetic controls (particularly when baseline differences in sleep were subtracted), experimental flies showed a significant decrease in sleep bout length, suggesting that, in spite of their high sleep need, they did not consolidate their rebound sleep well (Figure 4C, iii).
We next asked whether we could combine activation of iPNs with physical means of olfactory deprivation. However, we found that effects of physical manipulations (antennectomy or antennae gluing) on sleep were sensitive to temperature, making it difficult to couple these with activation/silencing experiments that require temperature shifts. For instance, antenna gluing or antennectomy did not reduce sleep at 18oC (Supplementary Figure S8A and B); in fact, the gluing resulted in a sleep increase. Also, gluing antennae at the start of a shift to 28°C resulted in a sleep increase the same day and no change on subsequent days (Supplementary Figure S8C).
Although activating iPNs will increase sleep, the converse is not true: silencing the iPNs is not sufficient to significantly decrease sleep relative to controls (Supplementary Figure S9A). This suggested the possibility that the loss in sleep observed when the ORNs were silenced was mediated through a different population of neurons. Because both the ePNs and iPNs receive inputs from the ORNs, we next tested whether silencing ePNs could reduce sleep. However, silencing ePNs also had no effect on sleep (Supplementary Figure S9B). Thus, while activating one population (namely the iPNs) is sufficient to increase sleep, sleep loss might require a reduction of neuronal activity in both populations of second-order projection neurons.
Some lateral horn output neurons affect sleep
We next sought to identify neurons downstream from the PNs that might be required to translate olfactory signals to sleep. Information from the antennal lobe is relayed to two parts of the brain, the lateral horn and the Kenyon Cells of the mushroom body [30]. Our experiments with the ePNs and iPNs suggested that olfaction drives sleep primarily through the iPNs which, unlike the ePNs, project only to the lateral horn (and not to the mushroom body). In support of this, we found that flies in which hydroxyurea had been used to ablate the α/β and α′/β′ lobes of the mushroom body [57] still exhibited sleep loss in response to antennectomy (Supplementary Figure S10A) and antennae glue (Supplementary Figure S10B). We also tested the role of the γ-lobe, which is partially spared in the hydroxyurea treatment, and found that silencing these neurons did not induce sleep loss regardless of whether we used a general γ-lobe driver [33] (Supplementary Figure S10C) or one specific for the γ-dorsal Kenyon cells (Supplementary Figure S10D), which were previously shown to increase sleep upon activation and prevent rebound sleep when silenced [58].
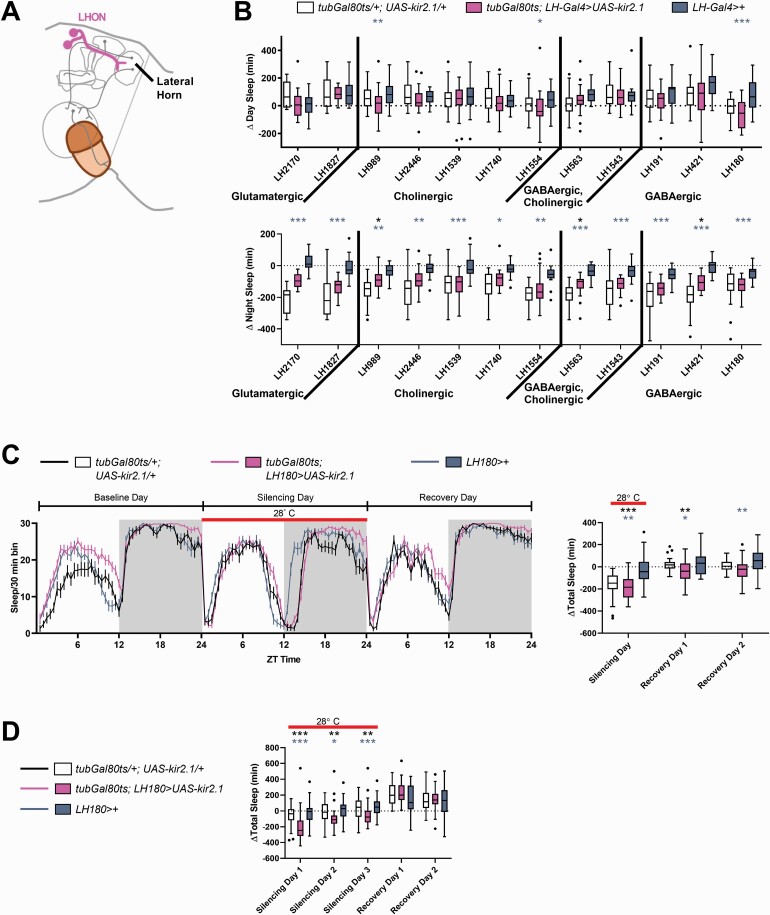
As the MB did not appear to drive changes in sleep in response to sensory input, we focused on the lateral horn output neurons (LHONs). We silenced different groups of LHONs using drivers that labeled at least five neurons in each hemisphere. In total, twelve LHON lines were tested, representing approximately 36% of previously described LHONs (85 of 239 neurons) [43] (Figure 5B). In the initial screen, three split-Gal4s, LH180, LH989, and LH1554, caused a decrease in daytime sleep when silenced, but only one, LH180, caused a significant reduction in sleep in subsequent assays (Figure 5C and Supplementary Figure S11A, B). Exposing the flies to three days at the restrictive temperature (Figure 5D) did not strengthen the phenotype, as was the case when silencing ORNs. Activating LH180 neurons during the day (Supplementary Figure S12B) or the night (Supplementary Figure S12C) did not result in an increase in sleep.
Figure 5.
Some lateral horn output neurons affect sleep. (A) Lateral Horn output neurons (LHONs, pink) carry information from the lateral horn to different parts of the brain. (B) For the lateral horn (LH) Gal4 drivers listed, the change in day sleep (ZT0–12) and night sleep (ZT12–24) was computed by subtracting the minutes of sleep on the day or night when the neurons labeled were silenced from the minutes of sleep on the day or night preceding the silencing. Control flies (tubGal80ts/+; UAS-kir2.1/+ and LH-Gal4) are plotted adjacent to experimental flies from the same cohort. LH-Gal4 refers to the GAL4 drivers listed underneath each bar representing change in sleep for the experimental flies (pink). Refer to Supplementary Table S2 for the sample sizes. (C) Silencing LH180-Gal4 neurons decreases sleep. Left panel shows the amount of sleep during the baseline, silencing, and recovery days in 30 min bins over the 24 h day. The red bar (in all panels) indicates the time period during which the temperature was raised to 28°C, thereby inactivating the GAL80 repressor and silencing the LH180-Gal4 neurons. Subtracting baseline differences in sleep showed that experimental flies of the genotype tubGal80ts; LH180>UAS-kir2.1 (n = 41) had significantly less sleep than controls (right). Change in sleep relative to baseline was plotted because of significant differences between the three genotypes at the permissive temperature (data not shown). For control flies of the genotype tubGal80ts/+; UAS-kir2.1/+ and LH180>+, n = 32 and 40, respectively. (D) Extending the time at the restrictive temperature did not increase the magnitude of sleep loss although experimental flies (tubGal80ts; LH180>UAS-kir2.1, pink, n = 33) showed significant differences relative to both genetic controls on all 3 days of silencing. Change in sleep relative to baseline was plotted because of significant differences between the three genotypes at the permissive temperature (data not shown). For control flies of the genotype tubGal80ts/+; UAS-kir2.1/+ and LH180>+, n = 33 and 34 respectively. For all panels, *p < 0.05, **p < 0.01, ***p < 0.001 by Kruskal–Wallis test with Dunn’s multiple comparison test between the experimental genotype and its genetic controls. Black asterisks indicate significant comparisons between control flies of the genotype tubGal80ts/+; UAS-kir2.1/+ (n = 33) and experimental flies. Blue–gray asterisks indicate significant differences between the Gal4 control and the experimental flies.
Discussion
We show here that reducing olfactory inputs is sufficient to drive sleep loss in flies, with more rapid effects evident in young animals (4–5 days posteclosion). While activating ORNs or their second-order ePNs is not sufficient to increase sleep, activating the second-order iPNs at night does increase sleep. These findings suggest that sensory inputs typically associated with wake contribute to normal daily sleep amounts.
In the mushroom body, output neurons (MBONs) that encode a positive valence are typically sleep-promoting while neurons that encode a negative valence are typically wake promoting [59]. Consistent with these ideas, we find that Mz699+ iPNs, whose GABAergic release is necessary for attraction towards most odors [54], also promote sleep when activated. However, there are some caveats to this interpretation. First, in addition to iPNs, Mz699 also labels ventrolateral protocerebrum (vlPr) neurons, which respond to aversive odors and do not innervate the antennal lobe at all [54]. Second, approximately 6 out of the 50 iPNs are labeled by GH146 [60, 61], which is referred to here and in other studies as an excitatory PN driver. We surmise that the six iPNs labeled by GH146 do not contribute significantly towards regulating sleep. Third, silencing Mz699+ neurons did not cause a sleep loss, which suggests that sleep loss in response to olfactory deprivation (antennae glue or silencing through Or83b-Gal4) is not mediated, at least not exclusively, through Mz699+ neurons. Nevertheless, the finding that projection neurons involved in attraction are sleep-promoting [54, 62] is in line with findings in the MB.
While we were able to identify one LHON population that reduced sleep when silenced, the effect of silencing these neurons was much less than the effect of silencing ORNs. We speculate that because our screen covered less than half of the LHON neurons, there are likely additional LHON neurons that are necessary for relaying the reduction in sleep, and that the effects would be stronger if multiple neuronal populations were silenced at once.
While we have shown through three different methods that olfactory deprivation leads to a loss in sleep, physical manipulations appeared somewhat inconsistent when performed under different conditions, such as in the presence of temperature shifts (Supplementary Figure S8A–C) or physical stressors in the early larval stages (hydroxyurea experiments, Supplementary Figure S9A, B). Thus, genetic strategies, which we have demonstrated here to be effective in two different genetic backgrounds (Figure 2, and Supplementary Figures S2, and S3), are highly recommended over physical manipulations for studying sensory deprivation. However, even genetic strategies are subject to some temperature interaction—although silencing of ORNS was effective at reducing sleep the first day, sleep loss was not maintained under prolonged exposure to restrictive temperatures, in contrast to the prolonged effects of physical antennae manipulations in flies maintained at 25°C. On the other hand, sleep loss persisted, but did not increase in magnitude, during prolonged silencing of LH180 LHONs. Complex interactions between both temperature and sleep [56, 63, 64] and temperature and olfaction [65] have also been reported by others in the literature. Moreover, neurons responsive to temperature have been reported in the antennae and the lateral horn [66, 67].
The reason for a lack of a sleep-promoting effect from activation of ORNs is unclear, but as stated earlier, there are a few possible explanations related in part to the properties of the activating TrpA1 channel Thus, while TrpA1 activation has been used to characterize many other sleep-promoting populations such as the dorsal fan-shaped body [15, 68], the ellipsoid body [69], and the mushroom body [58, 59], it may not be the ideal strategy for testing the effects of activating neurons in a sensory pathway.
We were also unable to change sleep levels by exposure to attractive or aversive odors, but categorizing odorants in this manner may be an oversimplification for several reasons. Previous research has shown that even among attractive odors, many different locomotor programs are elicited [70]; thus a reasonable assumption is that not all attractive or aversive odors have the same effect on sleep. Moreover, most ethologically relevant odors in the environment are found in mixtures, and changing either the components, the relative concentrations, or the frequency of delivery of these mixtures dramatically changes the neuronal activity of the ORNs and the PNs [52, 71–74]. The odorants tested in this study were probably insufficient to represent the diverse number of ethologically relevant parameters that likely contribute to the olfactory system’s effect on sleep. This raises the question of what control flies were smelling that led to more sleep than olfactory-deprived flies. While food is one possible source of smell, flies in this assay were on a diet consisting of sucrose and agar, which is not a very odorous food source. An alternate possible source of smell is the smell of other flies, since air and presumably odors can still flow in and out of the locomotor tubes even when the flies are individually housed. Although we saw a sleep decrease when flies were exposed to the odor of two females, this may not be comparable to the experience of a fly surrounded by other moving flies in an activity monitor. Constantly computing changes in the environment as the fly moves about may be a key factor in the role of olfactory stimuli in increasing sleep.
The difference between single and multibeam data we report here underscores the importance of scoring any increased sleep phenotype at a higher resolution. While the single beam data line up well with video monitoring or multibeam assays for most sleep studies [46, 75–77], in particular for a short-sleep phenotype, an apparent higher sleep phenotype could result from either a decrease in locomotion, as shown by our ePN activation data, or a preference for a specific part of the tube, as shown when the fly spent more time near the source of an attractive odor.
Previous work has shown that antennectomized flies show an increase in sleep immediately after injury before returning to baseline levels in the subsequent days [48]. Similarly, we observed a slight (though not significant) sleep gain on the day of injury (Figure 1A), but sleep loss on subsequent days. This sleep loss, rather than a return to baseline levels is likely the result of a younger age of antennectomy (4–5 days posteclosion rather than 6–9 days). In support of this, we found that flies antennectomized at 9–13 days of age (Figure 1C) did not show a significant decrease in total sleep (although they did show a decrease in night sleep two days post antennectomy). The younger age of deprivation may also account for the sleep loss we observed when Or83b+ neurons were silenced, whereas previous work in the literature reported no change [48]. These findings are consistent with the idea that young animals display more plasticity in their response to changes in sensory input [27, 78–82]. Overall, through genetic silencing, the prolonged recording post antennectomy, and covering the antennae with glue, our findings indicate that reduction of olfactory input causes a sleep decrease, which, in the case of antennectomy, may be masked by a response to injury during the first 24–48 h.
We propose that sensory inputs are important determinants of sleep quantity and quality. While the most straightforward model posits that increased stimulation during wake promotes sleep for synaptic downscaling or for restoring metabolic homeostasis [2, 3], we find that blocking some sensory inputs may actually increase sleep (specifically sound and wind sensing neurons in Johnston’s organ zones A, C, and E). Thus, effects may differ depending on the sensory modality and even within a single sensory modality, as suggested by our odor delivery experiments.
While some studies in vertebrates have related olfaction to sleep, the complexity of the olfactory system, especially in vertebrate models, has hindered a comprehensive and mechanistic understanding of this relationship. There is some evidence that slow-wave oscillations in the olfactory bulb can drive similar oscillations in the cortex [83], but the relevant neuronal populations or the role of olfactory stimuli (as opposed to respiration) are not understood. Another link between the olfactory system and sleep comes from the analysis of adenosine receptors. Activation of adenosine A2A receptors in the olfactory bulb will suppress rapid eye movement (REM) sleep without sacrificing non-REM sleep [84], but activation of the same receptors in the downstream olfactory tubercle will promote NREM sleep [85].
The data we present here, which represent a comprehensive characterization of how manipulations of first-, second-, and third-order neurons in the olfactory processing pathway affect sleep, underscore the complexity of the relationship between olfaction and sleep even in the relatively simple Drosophila model. We observe that some but not all GABAergic populations play a role in sleep, as evidenced by the LHON data. We also find that different populations play a role at different times of day—activating iPNs only significantly increases sleep at night, while silencing LH180 neurons only decreases sleep during the day. Moreover, there is no single neuronal population in the olfactory processing pathway that can bidirectionally regulate sleep when manipulated through thermogenetic means. Thus, the data we present here provide important details that lay the groundwork for determining the mechanism through which sensory information, especially olfactory information, drives sleep through different neuronal populations at different times of the day.
Supplementary Material
Acknowledgments
We would like to thank members of the Sehgal Lab for valuable discussion. We would also like to thank Dr Christine Dubowy, Kiet Luu, Anna Kolesnik, Joy Shon, and Mae Zhang for their assistance with experiments, and Dr Joseph L. Bedont and Dr Paula R. Haynes for their assistance troubleshooting the analysis scripts. We would also like to thank Dr Liqun Luo for providing us with the Mz699-Gal4 line.
Disclosure Statement
Financial disclosure: This was not an industry-supported study. Dr. Hsu was supported by a National Institutes of Health training grant for Age Related Neurodegenerative Diseases (NIH T32AG00255). Dr. Sehgal is an Investigator of the Howard Hughes Medical Institute.
Non-financial disclosure: The authors report no non-financial conflicts of interest.
Data Availability
The data underlying this article will be shared on a reasonable request to the corresponding author.
References
- 1. Colten HR, et al. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington (DC): National Academies Press; 2006. doi: 10.17226/11617 [DOI] [PubMed] [Google Scholar]
- 2. Allada R, et al. Molecular mechanisms of sleep homeostasis in flies and mammals. Cold Spring Harb Perspect Biol. 2017. doi: 10.1101/cshperspect.a027730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tononi G, et al. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81(1):12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huber R, et al. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep. 2007;30(2):129–139. [DOI] [PubMed] [Google Scholar]
- 5. Ganguly-Fitzgerald I, et al. Waking experience affects sleep need in Drosophila. Science. 2006;313(5794):1775–1781. [DOI] [PubMed] [Google Scholar]
- 6. Bushey D, et al. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332(6037):1576–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donlea JM, et al. Genetic rescue of functional senescence in synaptic and behavioral plasticity. Sleep. 2014;37(9):1427–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown MK, et al. Reduced sleep during social isolation leads to cellular stress and induction of the unfolded protein response. Sleep. 2017;40(7). doi: 10.1093/sleep/zsx095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lone SR, et al. Mechanosensory stimulation via Nanchung expressing neurons can induce daytime sleep in Drosophila bioRxiv. 2019:829861. doi: 10.1101/829861, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kompotis K, et al. Rocking promotes sleep in mice through rhythmic stimulation of the vestibular system article rocking promotes sleep in mice through rhythmic stimulation of the vestibular system. Curr Biol. 2018; 29(3): 392– 401. doi: 10.1016/j.cub.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 11. Kirszenblat L, et al. Visual experience drives sleep need in Drosophila. Sleep. 2019;42(7). doi: 10.1093/sleep/zsz102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hasegawa T, et al. Sweetness induces sleep through gustatory signalling independent of nutritional value in a starved fruit fly. Sci Rep. 2017;7(1):14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haynes PR, et al. A single pair of neurons links sleep to memory consolidation in Drosophila melanogaster. Elife. 2015;4:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dag U, et al. Neuronal reactivation during post-learning sleep consolidates long-term memory in drosophila. Elife. 2019; 8:e42786: 1– 23. doi: 10.7554/eLife.42786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donlea JM, et al. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science. 2011;332(6037):1571–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seugnet L, et al. Identifying sleep regulatory genes using a Drosophila model of insomnia. J Neurosci. 2009;29(22):7148–7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dissel S, et al. Sleep restores behavioral plasticity to Drosophila mutants. Curr Biol. 2015;25(10):1270–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murphy KR, et al. Postprandial sleep mechanics in Drosophila. Elife. 2016; 5: e19334. doi: 10.7554/eLife.19334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Masek P, et al. Altered regulation of sleep and feeding contributes to starvation resistance in Drosophila melanogaster. J Exp Biol. 2014;217(Pt 17):3122–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen J, et al. Allatostatin a signalling in drosophila regulates feeding and sleep and is modulated by PDF. PLoS Genet. 12(9): e1006346: 1– 33. doi: 10.1371/journal.pgen.1006346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yurgel ME, et al. A single pair of leucokinin neurons are modulated by feeding state and regulate sleep-metabolism interactions. PLoS Biol. 2019;17(2):e2006409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kayser MS, et al. Sleep deprivation suppresses aggression in Drosophila. Elife. 2015;4:e07643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Machado DR, et al. Identification of octopaminergic neurons that modulate sleep suppression by male sex drive. Elife. 2017; 6:e23130: 1– 21. doi: 10.7554/eLife.23130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beckwith EJ, et al. Regulation of sleep homeostasis by sexual arousal. Elife. 2017; 6:e27445: 1– 19. doi: 10.7554/eLife.27445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen D, et al. Genetic and neuronal mechanisms governing the sex-specific interaction between sleep and sexual behaviors in Drosophila. Nat Commun. 2017;8(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang W, et al. Hierarchical control of drosophila sleep, courtship, and feeding behaviors by male-specific P1 neurons. Neurosci Bull. 2018; 34(6): 1105– 1110. doi: 10.1007/s12264-018-0281-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kayser MS, et al. A critical period of sleep for development of courtship circuitry and behavior in Drosophila. Science. 2014;344(6181):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Q, et al. Aversion and attraction through olfaction. Curr Biol. 2015;25(3):R120–R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wilson RI. Early olfactory processing in Drosophila: mechanisms and principles. Annu Rev Neurosci. 2013;36:217–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frechter S, et al. Functional and anatomical specificity in a higher olfactory centre. Elife. 2019; 8:e44590: 139. doi: 10.7554/eLife.44590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paulk A, et al. Vision in Drosophila: seeing the world through a model’s eyes. Annu Rev Entomol. 2013;58:313–332. [DOI] [PubMed] [Google Scholar]
- 32. Aso Y, et al. The neuronal architecture of the mushroom body provides a logic for associative learning. Elife. 2014;3:e04577. doi: 10.7554/eLife.04577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohn R, et al. Coordinated and compartmentalized neuromodulation shapes sensory processing in Drosophila. Cell. 2015;163(7):1742–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Couto A, et al. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15(17):1535–1547. [DOI] [PubMed] [Google Scholar]
- 35. Jefferis GS, et al. Target neuron prespecification in the olfactory map of Drosophila. Nature. 2001;414(6860):204–208. [DOI] [PubMed] [Google Scholar]
- 36. Stocker RF, et al. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32(5):443–456. [DOI] [PubMed] [Google Scholar]
- 37. Yorozu S, et al. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature. 2009;458(7235):201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ito K, et al. GAL4-responsive UAS-tau as a tool for studying the anatomy and development of the Drosophila central nervous system. Cell Tissue Res. 1997;290(1):1–10. [DOI] [PubMed] [Google Scholar]
- 39. Liang L, et al. GABAergic projection neurons route selective olfactory inputs to specific higher-order neurons. Neuron. 2013;79(5):917–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pulver SR, et al. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol. 2009;101(6): 3075–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cavanaugh DJ, et al. Identification of a circadian output circuit for rest: activity rhythms in Drosophila. Cell. 2014;157(3):689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cavanaugh DJ, et al. The drosophila circadian clock gates sleep through time-of-day dependent modulation of sleep-promoting neurons. Sleep. 2016;39(2):345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dolan MJ, et al. Neurogenetic dissection of the drosophila lateral horn reveals major outputs, diverse behavioural functions, and interactions with the mushroom body. Elife. 2019; 8:e43079: 1– 45. doi: 10.7554/eLife.43079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shaw PJ, et al. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287(5459):1834–1837. [DOI] [PubMed] [Google Scholar]
- 45. Hendricks JC, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000; 25(1): 129– 138. doi: 10.1016/S0896-6273(00)80877-6 [DOI] [PubMed] [Google Scholar]
- 46. Garbe DS, et al. Context-specific comparison of sleep acquisition systems in Drosophila. Biol Open. 2015;4(11): 1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sweeney ST, et al. Hydroxyurea ablation of mushroom bodies in Drosophila. Cold Spring Harb Protoc. 2012;2012(2):231–234. doi: 10.1101/pdb.prot067777 [DOI] [PubMed] [Google Scholar]
- 48. Singh P, et al. Bidirectional regulation of sleep and synapse pruning after neural injury. Curr Biol. 2020;30(6):1063–1076.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McGuire SE, et al. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004(220):pl6. [DOI] [PubMed] [Google Scholar]
- 50. McGuire SE, et al. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302(5651):1765–1768. [DOI] [PubMed] [Google Scholar]
- 51. Paradis S, et al. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001. doi: 10.1016/S0896-6273(01)00326-9 [DOI] [PubMed] [Google Scholar]
- 52. Su CY, et al. Temporal coding of odor mixtures in an olfactory receptor neuron. Proc Natl Acad Sci U S A. 2011;108(12):5075–5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Olsen SR, et al. Divisive normalization in olfactory population codes. Neuron. 2010;66(2):287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Strutz A, et al. Decoding odor quality and intensity in the Drosophila brain. Elife. 2014;3:e04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Min S, et al. Dedicated olfactory neurons mediating attraction behavior to ammonia and amines in Drosophila. Proc Natl Acad Sci U S A. 2013;110(14):E1321–E1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Parisky KM, et al. Reorganization of sleep by temperature in drosophila requires light, the homeostat, and the circadian clock. Curr Biol. 2016;26(7):882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Douglas Armstrong J, et al. Metamorphosis of the mushroom bodies; large-scale rearrangements of the neural substrates for associative learning and memory in Drosophila. Learn Mem. 1998; 5(1-2): 102– 114. doi: 10.1101/lm.5.1.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sitaraman D, et al. Propagation of homeostatic sleep signals by segregated synaptic microcircuits of the drosophila mushroom body. Curr Biol. 2015;25(22):2915–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aso Y, et al. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. Elife. 2014;3:e04580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wilson RI, et al. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25(40):9069–9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lai SL, et al. Clonal analysis of Drosophila antennal lobe neurons: diverse neuronal architectures in the lateral neuroblast lineage. Development. 2008;135(17):2883–2893. [DOI] [PubMed] [Google Scholar]
- 62. Knaden M, et al. Spatial representation of odorant valence in an insect brain. Cell Rep. 2012;1(4):392–399. [DOI] [PubMed] [Google Scholar]
- 63. Kim JH, et al. The voltage-gated potassium channel Shaker promotes sleep via thermosensitive GABA transmission. Commun Biol. 2020; 3(174): 1– 13. doi: 10.1038/s42003-020-0902-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lamaze A, et al. Regulation of sleep plasticity by a thermo-sensitive circuit in Drosophila. Sci Rep. 2017;7:40304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Riveron J, et al. The effect of environmental temperature on olfactory perception in Drosophila melanogaster. J Insect Physiol. 2009;55(10):943–951. [DOI] [PubMed] [Google Scholar]
- 66. Frank DD, et al. Temperature representation in the Drosophila brain. Nature. 2015;519(7543):358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Frank DD, et al. Early integration of temperature and humidity stimuli in the drosophila brain. Curr Biol. 2017;27(15):2381–2388.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ni JD, et al. Differential regulation of the drosophila sleep homeostat by circadian and arousal inputs. Elife. 2019; 8:e40487: 1– 27. doi: 10.7554/eLife.40487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu S, et al. Sleep drive is encoded by neural plastic changes in a dedicated circuit. Cell. 2016; 165: 1347– 1360. doi: 10.1016/j.cell.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jung SH, et al. Odor-identity dependent motor programs underlie behavioral responses to odors. Elife. 2015; 4:e11092: 1– 31. doi: 10.7554/eLife.11092.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martelli C, et al. Intensity invariant dynamics and odor-specific latencies in olfactory receptor neuron response. J Neurosci. 2013;33(15):6285–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Getahun MN, et al. Temporal response dynamics of Drosophila olfactory sensory neurons depends on receptor type and response polarity. Front Cell Neurosci. 2012;6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mohamed AAM, et al. Odor mixtures of opposing valence unveil inter-glomerular crosstalk in the Drosophila antennal lobe. Nat Commun. 2019;10(1):1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hallem EA, et al. Coding of odors by a receptor repertoire. Cell. 2006;125(1):143–160. [DOI] [PubMed] [Google Scholar]
- 75. Gilestro GF. Video tracking and analysis of sleep in Drosophila melanogaster. Nat Protoc. 2012;7(5):995–1007. [DOI] [PubMed] [Google Scholar]
- 76. Faville R, et al. How deeply does your mutant sleep? Probing arousal to better understand sleep defects in Drosophila. Sci Rep. 2015;5:8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Donelson NC, et al. High-resolution positional tracking for long-term analysis of Drosophila sleep and locomotion using the “tracker” program. PLoS One. 2012;7(5):e37250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Levelt CN, et al. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012;35:309–330. [DOI] [PubMed] [Google Scholar]
- 79. Ma L, et al. A developmental switch of axon targeting in the continuously regenerating mouse olfactory system. Science 2014;344(6180):194–197. [DOI] [PubMed] [Google Scholar]
- 80. Tsai L, et al. A critical period defined by axon-targeting mechanisms in the murine olfactory bulb. Science 344(6180):1947–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chodankar A, et al. Glomerulus-selective regulation of a critical period for interneuron plasticity in the Drosophila antennal lobe. J Neurosci. 2020;40(29):5549–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Golovin RM, et al. Activity-dependent remodeling of drosophila olfactory sensory neuron brain innervation during an early-life critical period. J Neurosci. 2019;39(16):2995–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fontanini A, et al. Slow-waves in the olfactory system: an olfactory perspective on cortical rhythms. Trends Neurosci. 2006;29(8):429–437. [DOI] [PubMed] [Google Scholar]
- 84. Wang YQ, et al. Adenosine A2A receptors in the olfactory bulb suppress rapid eye movement sleep in rodents. Brain Struct Funct. 2017; 222: 1351– 1366. doi: 10.1007/s00429-016-1281-2 [DOI] [PubMed] [Google Scholar]
- 85. Li R, et al. Activation of adenosine A2A receptors in the olfactory tubercle promotes sleep in rodents. Neuropharmacology. 2020; 168(107923): 1– 13. doi: 10.1016/j.neuropharm.2019.107923 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on a reasonable request to the corresponding author.