Abstract
The fixed orthodontic measures taken induce significant stress to the gingival growth process during arch wire maneuvers of aligning and leveling. We observed, for a period of one to four years, fixed orthodontic devices in 80 human subjects. From these, we selected 44 subjects (22 women and 22 men) where the inflammatory process exhibited following the orthodontic fixed treatment, and with vacuum-formed orthodontic retainers (VFR) succeeding to fixed treatment. Samples were collected from each patient and histological and immunohistochemical (IHC) methodology was made to analyze the cytoarchitecture. Statistics were made after one-way analysis of variance (ANOVA), with the Bonferroni’s correction. The IHC examination performed in the early stage revealed the presence in the inflammatory infiltrate of CD8-type T-lymphocytes, and of dendritic cells in large numbers. The examination performed in the late stage revealed the presence in the inflammatory infiltrate of CD20-type B-lymphocytes, which are mature cells capable of immunoglobulin synthesis, their activation being an important step in the maturation of the antibody response. The stress generated by arch wires in both genders was significantly higher than in the case of VFR. This observation was pointed out also by the cytohistological investigation outcome but was also based on an original scale conceived by our research team, following gingival hyperplasia evaluation. Also, with statistical significance, the comparative obtained values for men (p=0.01) and for women (p=0.001) illustrate clinical observations, allowing to affirm that, in our case, men were more stressed in bearing arch wire devices (AWD) and VFR, in comparison with women.
Keywords: immunohistochemistry, histopathology, gingival overgrowth, orthodontics
⧉ Introduction
The oral cavity is a natural open system. All factors of this medium are interacting and, the medium’s composition fluctuations have direct repercussions on its fixed components. Orthodontic treatments generally have no inflammatory side effects but can sometimes induce excessive gingival growth [gingival overgrowth – GO], manifested as an obvious fibrous and dense structure, dissimilar to the delicate gingival tissue with reddish margins, characteristic for allergic or inflammatory lesions. These observations, however, do not point to a clear in-depth estimate of the origin and histopathology of this process [1,2].
GO induced by orthodontic treatment has traditionally been considered an inflammatory reaction following bacterial plaque buildup due to poor hygiene, but we must also consider the mechanical stress induced by orthodontic retainers [3,4,5].
For this purpose, in the developed adaptive immune process, some special cells were found, such as dendritic cells, which play a vital part, being considered strong antigen-capturing cells and/or antigen-presenting cells (APCs) [6,7,8]. Dendritic cells are associated during immune homeostasis and are sensitive to the stabilization of the disorder of chronic infections, with serious systemic effects [9,10,11].
In common immunoinflammatory disorders, such as periodontal disorders, intracellular pathogens use dendritic cells and prevent decay by evading autophagy [12,13].
Orthodontic devices, fixed and mobile, can induce mechanical stress in the periodontium due to constrains produced by the orthodontic arch wire devices (AWD) and vacuum-formed orthodontic retainers (VFR), during the orthodontic therapy [14]. Orthodontic appliances also often act as retentive factors for plaque biofilm, so strict hygiene is essential throughout orthodontic therapy. Orthodontic therapy is no longer the prerogative of young patients; it can be applied at any age, especially therapy with fixed devices [15,16].
Other periodontal risks in patients with orthodontic appliances are gingival retractions and root resorptions. That is why it is very important to periodically examine these patients and periodically evaluate them during maintenance therapy, for the necessary therapeutic intervention [17].
The present survey initiated from our earlier observations shows that the inflammatory processes were spotted (always) in the same place of gingival tissue from where we gathered samples [18].
Aim
The aim of this research was to evaluate the stress of gingival proliferative processes associated with fixed orthodontic therapy and VFR, mirrored by the cytoarchitecture of gingival tissue using histological and immunohistochemical (IHC) methodologies.
⧉ Patients,Materials and Methods
This study was endorsed by the Ethics Committee of the Victor Babeş University of Medicine and Pharmacy, Timişoara, Romania (Certificate No. 17/26.06.2018), and all patients included gave their written consent. The study group consisted in healthy patients, without any acute or systemic disorders; none of the patients had taken any medication that would impact their periodontal condition during the study period. The orthodontic treatment was accomplished in the Clinic of the Department of Orthodontics, Faculty of Dental Medicine, Victor Babeş University of Medicine and Pharmacy, Timişoara, and in a specialized private Dental Office from Timişoara.
The studied subjects
From 80 initially monitored human subjects, 44 patients, aged 18–45 years (22 women and 22 men) were selected and included. All subjects displayed varying degrees of inflammation generated using AWD for aligning and leveling, and afterwards, of VFR, that followed AWD in orthodontic therapy. The patients suffered from GO, affecting 1/3 or 2/3 of each patient’s clinical tooth crown.
Applied criteria
Exclusion criteria – we excluded patients who had been administered, within the last month, by their attending physician, overall treatment with anti-infective, anti-inflammatory, antiepileptic medication, and/or calcium antagonists.
Inclusion criteria – we included patients who did not suffer from any disorders that could be related to periodontal lesions with or without GO [e.g., diabetes, blood disorders, human immunodeficiency virus (HIV) infection, hypovitaminosis, etc.]. All the patients included in our study had appropriate oral hygiene, which was initially assessed using the classical simplified oral hygiene indices (IH-S) [19], the gingival index (GI) [20], and the Eastman interdental bleeding index (EIBI) [21], showed a normal gingival condition. Also, sampling of thickened gum sections was not performed during the women’s period.
Orthodontic examination
All the participants were diagnosed with initial dentomaxillary irregularities and were treated with fixed orthodontic devices with brackets for a period of one to four years. Following this, a Roth prescription – Mini 2000 brackets was used (Ormco, Germany), with a slot size of 0.022 inch. During alignment, 0.012–0.016-inch × 0.022-inch nickel–titanium arch wires were used (Ormco, Germany) and for correction, we opted for Sentalloy (Super Elastic Nickel Titanium Alloy, Dentsply Sirona, USA). After the fixed treatment, for maintaining the results, we used the Essix VFRs (Dentsply Sirona, USA) for a three-month period.
Tissue sampling
The patients went through oral prophylaxis programs and were instructed to maintain adequate oral hygiene by brushing at home (or at work). Samples were taken from each patient in the same gingival area. The technique used is a specific dental technique consisting in taking two tissue samples: one being taken in the early stages of inflammation, which coincided with the use of leveling and alignment of the arch and the other, corresponding to the late stages of the injury, which coincided with using VFR.
The first moment of tissue sampling was considered in the fixed orthodontic treatment, when the patient accused maximum gingival pain associated with the inflammatory process and the final tissue sampling was accomplished at three months after the appliance of VFR devices. The gum was cleaned and disinfected with a sterile compress imbued with 70% ethanol and sprayed with 10% Lidocaine.
Interdental papillae (hyperplastic/hypertrophic) were excised with a trimmer with ceramic cutting edges, especially devised only for soft tissues (Precicut DFS-Diamon, Germany) similar to a filler cutter/enamel/bone, which acts at very high speeds friction grip (FG). No water or saline was used for cooling. The ceramic cutter (trimmer) was introduced into a handpiece with an operating speed of 300 000 rpm.
At such high speeds, the contact between the tip of the ceramic tool and the tissue causes heat. At that time, the tissue will coagulate rapidly leading to hemostasis, without damaging the tissue. Also, the recovery time was shorter than if the papilla would bleed, and one of the great advantages is that no healthy tissue was lost.
Tissue samples preparation for histological examination
Fragments of gingival mucosa with an area of about 2–3 mm2 were collected, and after 48 hours 10% neutral buffered formalin solution fixation they were embedded in paraffin, using the standard histopathological protocol. The biological material was then sectioned at the microtome, obtaining serial sections with a thickness of 4 μm that were stained with Hematoxylin–Eosin (HE).
For immunohistochemistry studies, the sections of biological material were taken on poly-L-lysine slides to increase the adhesion of the biological material to the slide, then the working protocol of polymeric amplification with Horseradish Peroxidase (HRP) was applied. For the detection of the IHC reaction, we used 3,3’-Diaminobenzidine (DAB), and the contrast of the nuclei was performed with Mayer’s Hematoxylin.
In our study, we used antibodies: anti-cluster of differentiation (CD) 45RO (monoclonal mouse anti-human CD45R0, UCHL1 clone, 1/100 dilution, Dako) to highlight lymphocytes; anti-CD68 (monoclonal mouse anti-human CD68, KP1 clone, 1/100 dilution, Dako) for selective highlighting of macrophages; anti-CD3 (monoclonal mouse anti-human CD3, F7.2.38 clone, 1/50 dilution, Dako) for the study of T-lymphocytes; anti-CD20 (monoclonal mouse anti-human CD20cy, L26 clone, 1/50 dilution, Dako) for the study of B-lymphocytes and anti-CD34 (monoclonal mouse anti-human CD34 Class II, QBEnd 10 clone, 1/100 dilution, Dako).
Statistical analysis
The values we obtained were expressed as mean ± standard error of the mean (SEM), and for assessing the difference between groups, one-way analysis of variance (ANOVA) with the Bonferroni’s correction tests were utilized, with statistical differences ranging between the following values: *p<0.05, **p<0.01 and ***p<0.001, respectively. The software used was GraphPad Prism 6.0 for Windows (GraphPad Software, San Diego, USA).
⧉ Results
Clinical observations
The stress generated by arch wires, in all studied cases, and in both genders was significantly higher than in the case of the employment of VFR. This observation was pointed out also by the cytohistological investigation outcome but was also based on an original scale conceived by our research team, following gingival hyperplasia evaluation in the subjects included in this study. For a clear inspection, we displaced the orthodontic devices when photos were made. The images revealed what we identified also as cytoarchitecture in the histological study.
Figures 1 and 2 show images from different male subjects during AWD and VFR therapy.
Figure 1.
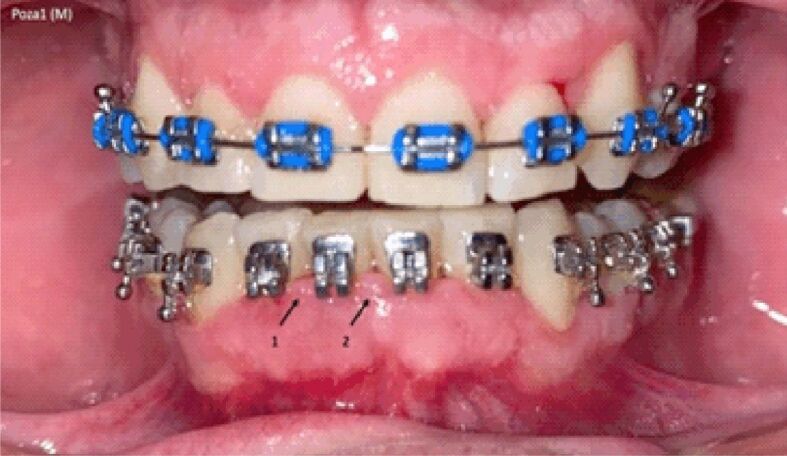
Image of gingival mirror from a male subject during arch wire devices (AWD) therapy
Figure 2.
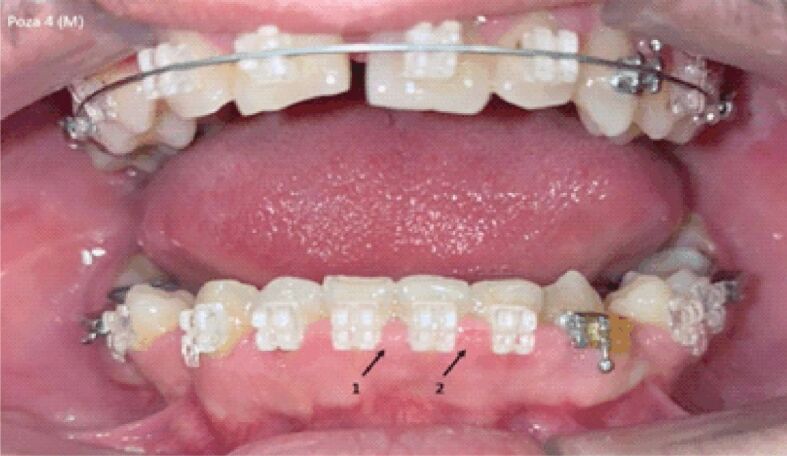
Image of gingival mirror from a male subject during vacuum-formed retainers (VFR) therapy
Tissues cytoarchitecture
GO induced by the orthodontic treatment that we applied appeared as firm and pink enlargements of the tissue in the gingival status, without bleeding tendency. Histological examination of the inflamed gum, in our patients, presented a well-structured and thickened epithelium, with elongated and very thick papillae inserted in the fibrous connective tissue. At the same time, analyzing the tissue, we observed an area strongly infiltrated with chronic inflammatory cells full of plasma cells, lymphocytes, and fewer dilated blood vessels.
Male subjects
The histological investigation in men subjected to arch wires presented nucleated epithelial cells from the superficial layer of the gingival mucosa, with pyknotic and elongated nuclei, which indicate the installation of parakeratosis. Also, certain epithelial cells from the stratum granulosum were enlarged. However, the predominant cytohistological aspect was highlighted by the presence of inflammatory infiltrate, whose cells have invaded the subjacent connective tissue and perivascular and parabasal locations. In the connective tissue, the collagen fibers were thicker and grouped, forming boundless or fascicles. The presence of this infiltrate is a defense reaction designed to anticipate, intercept, and protect the area against the causative agent.
Vasodilator phenomena at the level of the lamina propria induced massive diapedesis, making possible the appearance of leukocytes. Inflammatory leukocyte infiltrate usually has neutrophils, lymphocytes, and monocytes.
Neutrophils are the first leukocytes to move to the site of inflammation or infection, being the fastest cells in locomotion, and have a function of micro phagocytes.
T-lymphocytes recognize the self and non-self proteins of the body in which they are found, and macrophages, the mature form of monocytes, are the secondary line of immune defense. In some cases, the inflammatory cells cross and invade the epithelium.
In the case of VFR, in male subjects, the inflammatory features were mildly expressed, even if they were still present. Thus, the lamina propria maintained the inflammatory infiltrate, localized especially around the blood vessels, and the vascular dilatations. Under the stratum basale, the collagen fibers become thinner, giving a rarefied appearance of this zone.
Female subjects
In women subjected to arch wires, the cytoarchitectural aspects presented the same elements, as in the case of men, but mentioning the fact that the leukocyte infiltrate was not limited perivascularly but, on the contrary, was diffuse, extending throughout the connective tissue of the lamina propria. Also, in the connective tissue the presence of small, newly formed blood vessels was found. In addition, there were no changes in the epithelium of the gingival mucosa. In the diffuse leukocyte infiltrate, we also found the presence of plasma cells, which are antigenically stimulated B-lymphocytes, involved in the synthesis of antibodies. In women with VFR, the cytoarchitecture presented the same elements as in the case of male subjects, but were expressed much more moderately, with slight diffuse leukocyte infiltrate, without structural changes in the epithelium. All these cytoarchitectural observations are indicative of a better supportability of these devices by the women subjects.
Figures 3,4,5,6,7,8 present the most important cytoarchitecture modifications of gingival epithelium found in our study.
Figure 3.
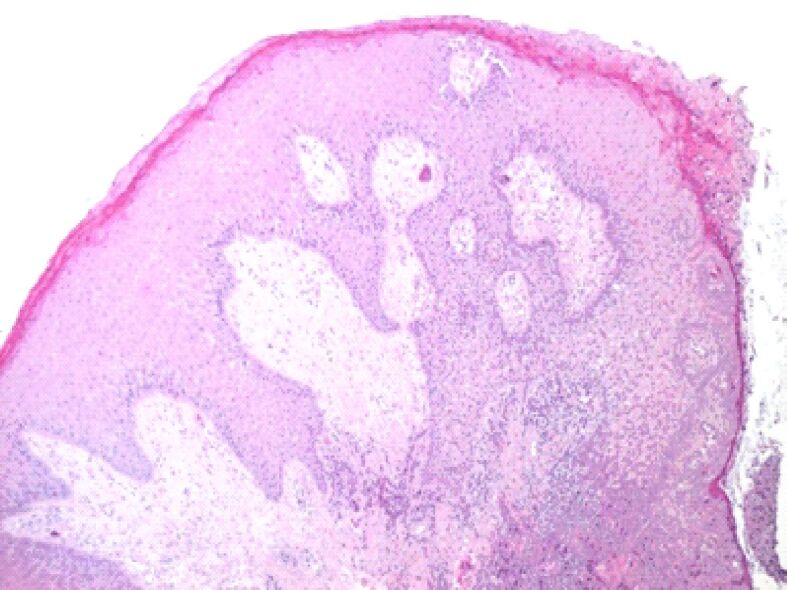
Microscopic image of the gingival mucosa showing its overall hypertrophy. On the surface, there is a thickened gingival epithelium, with deep epithelial ridges, acanthosis, and hyperkeratosis in the superficial layers. HE staining, ×40
Figure 4.
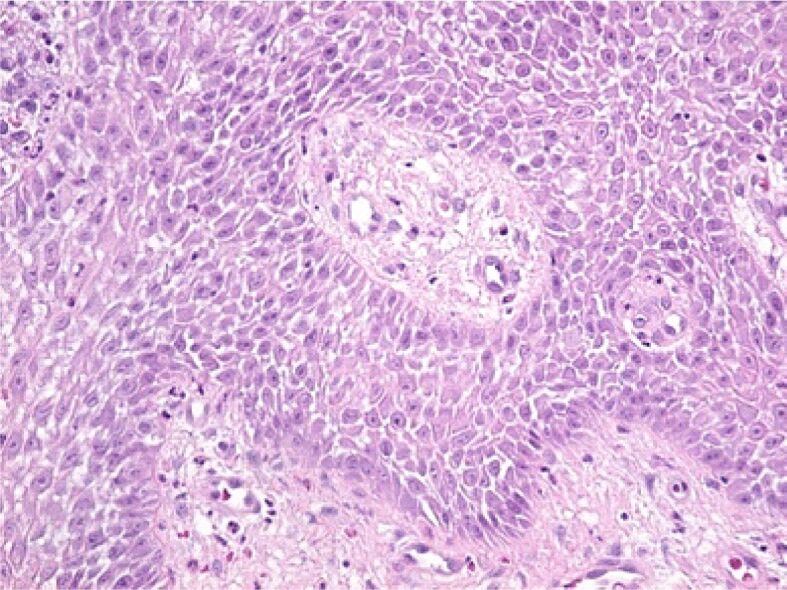
In the deep areas of the gingival epithelium, the widening of the intercellular spaces was noticed with the highlighting of the intercellular “spines” (desmosomes) because of an edema present both in the epithelium and at the level of the lamina propria (gingival chorion). HE staining, ×200
Figure 5.
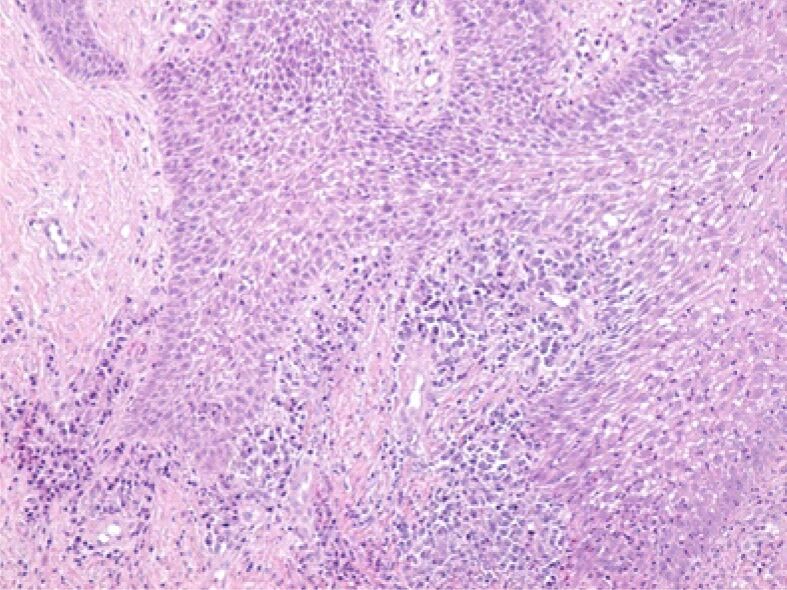
Image of abundant inflammatory infiltrate in the papillae of the lamina propria. HE staining, ×100
Figure 6.
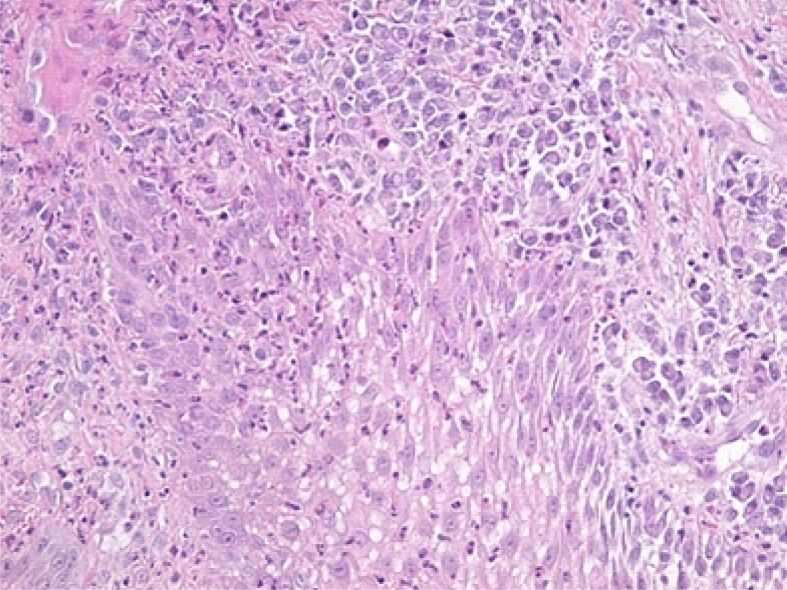
Microscopic image from the deep area of the gingival epithelium showing the presence of an intraepithelial edema and infiltration with lymphocytes and neutrophilic granulocytes. HE staining, ×200
Figure 7.
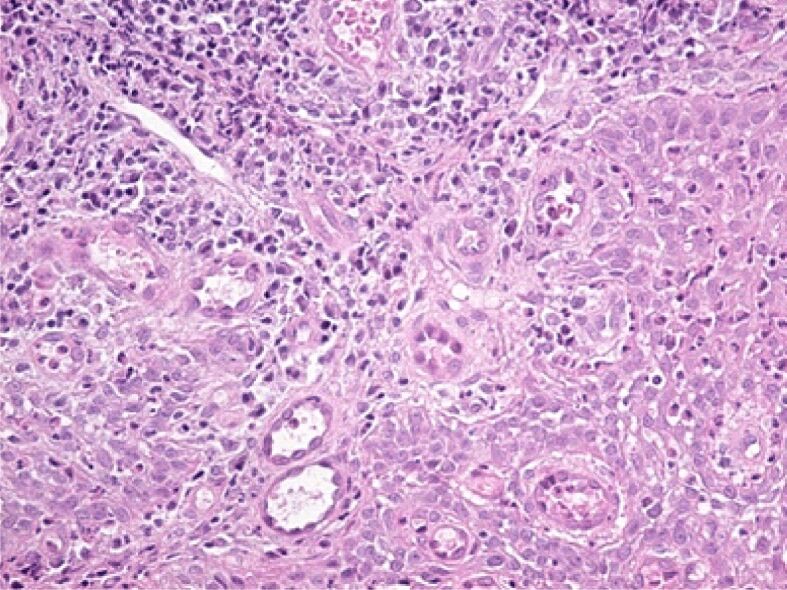
Image of papilla from the lamina propria, with numerous vessels of angiogenesis, congested and moderately inflammatory infiltrate, consisting mainly of lymphocytes. HE staining, ×200
Figure 8.
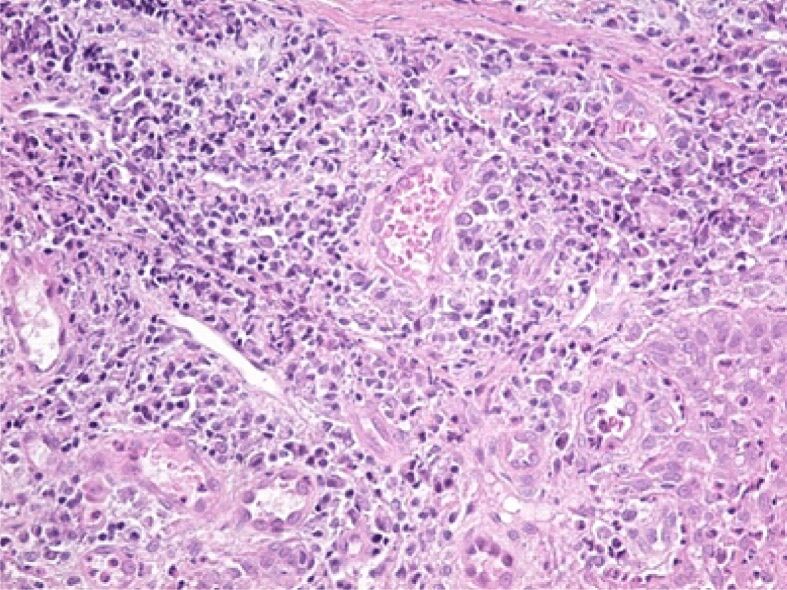
Abundant inflammatory infiltrate in an area of the lamina propria, associated with vascular congestion. HE staining, ×200
Immunohistochemistry
Early stages (arch wires)
The IHC examination performed in the early stage revealed the presence in the inflammatory infiltrate of CD8-type T-lymphocytes, and dendritic cells in large numbers. Dendritic cells are mobile, APCs capable of synthesizing cytokines, along with T-lymphocytes. Also, the dendritic cells can express the major histocompatibility complex (MHC) Class II molecules, having thus a co-stimulating activity.
Cytokines will induce the synthesis of neuropeptides that will cause vasodilatation in blood vessels located in connective tissue. In response, there is diapedesis of neutrophils and the appearance of mobile macrophages, mast cells, which can induce histamine dilation of blood vessels and plasma cells.
T-lymphocytes were classified into two categories, depending on the expression on the surface of the cell membrane of either the CD4+ molecule or the CD8+ molecule. CD4+ lymphocytes are considered helper T-lymphocytes involved in the synthesis of many chemicals, such as cytokines, interleukins, interferon-gamma, and others, that increase the cell-mediated immune response. CD8+ lymphocytes or toxic lymphocytes are immune effectors involved in the synthesis of cytokines, similar to those produced by helper T-lymphocytes.
Late stages (vacuum-formed orthodontic retainers)
The IHC examination performed in the late stage revealed the presence in the inflammatory infiltrate of B-lymphocytes (CD20), which are mature cells capable of immunoglobulin synthesis, their activation being an important step in the maturation of the antibody response. Thus, B-lymphocytes will divide in B-lymphocytes with memory and plasma cells. Also, they can act as APCs.
Figures 9,10,11,12,13,14,15 present the main findings consecutive to the immunohistochemistry investigation.
Figure 9.
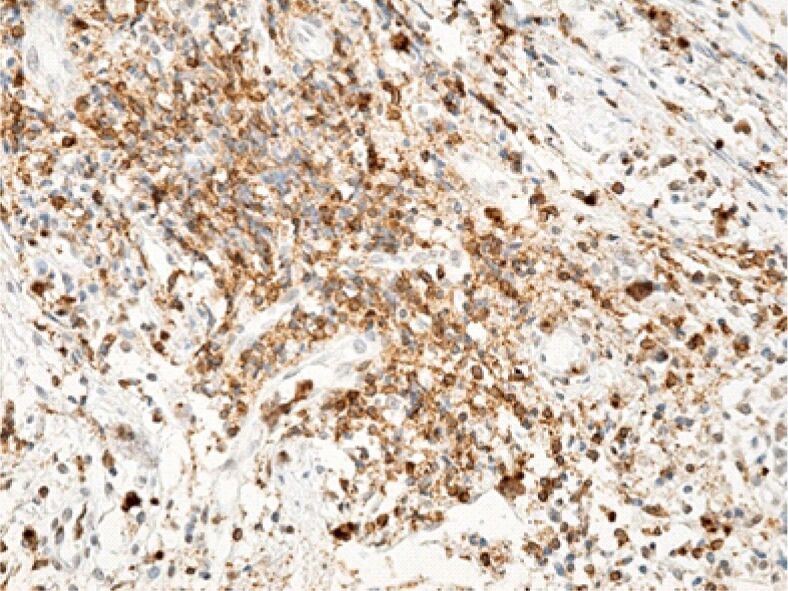
The area of the lamina propria is strongly infiltrated with lymphocytes. Immunolabeling with anti-CD45RO antibody, ×200
Figure 10.
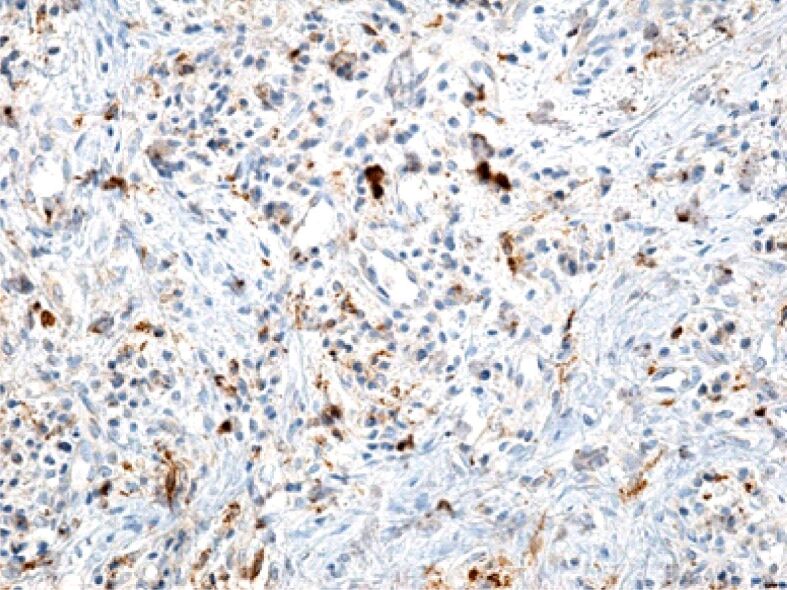
Gingival chorion infiltrated with numerous inflammatory cells, but with a relatively low macrophage. Immunolabeling with anti-CD68 antibody, ×200
Figure 11.
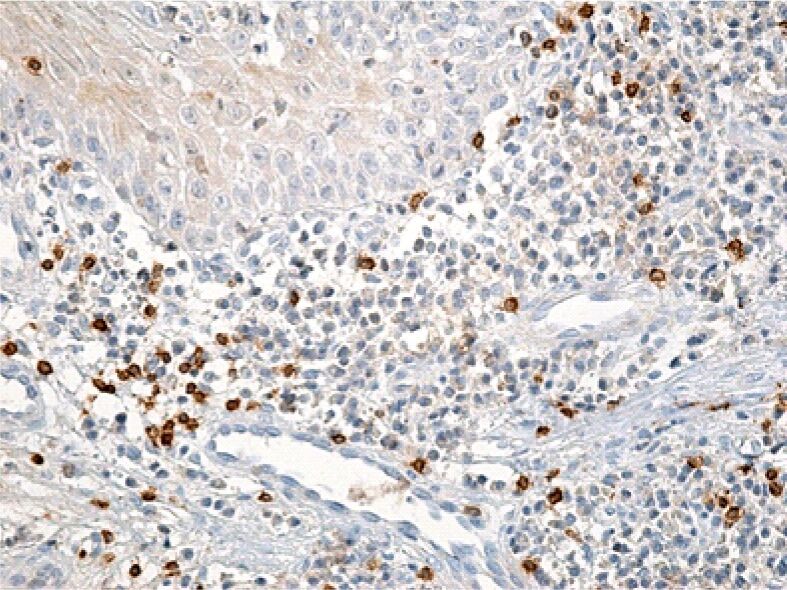
Superficial gingival chorion image with a rich inflammatory infiltrate but a relatively small number of T-lymphocytes. Immunolabeling with anti-CD3 antibody, ×200
Figure 12.
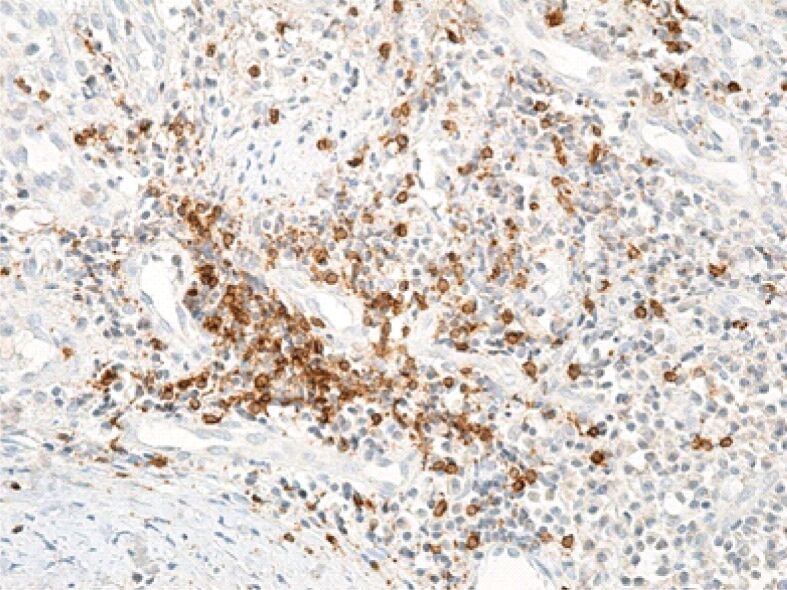
Gingival chorion area rich in T-lymphocytes. Immunolabeling with anti-CD3 antibody, ×200
Figure 13.
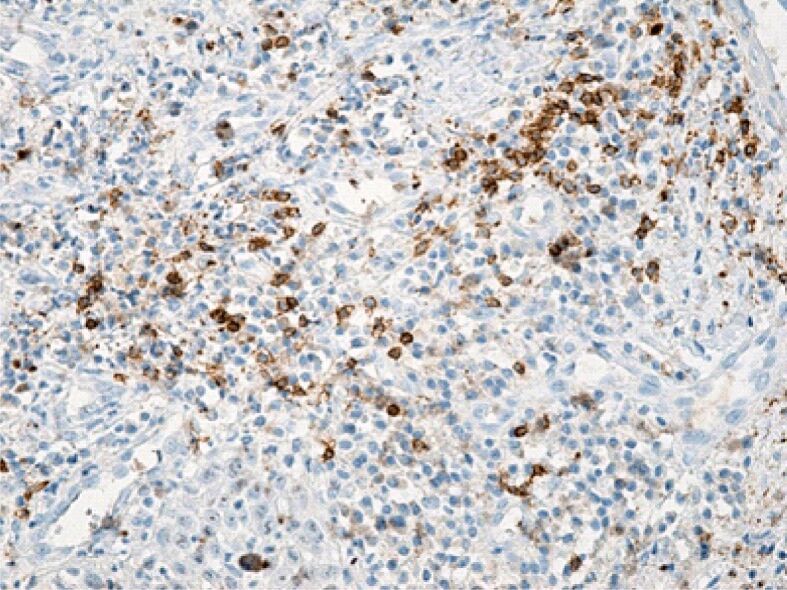
Deep gingival chorion infiltrated with a moderate amount of B-lymphocytes. Immunolabeling with anti-CD20 antibody, ×200
Figure 14.
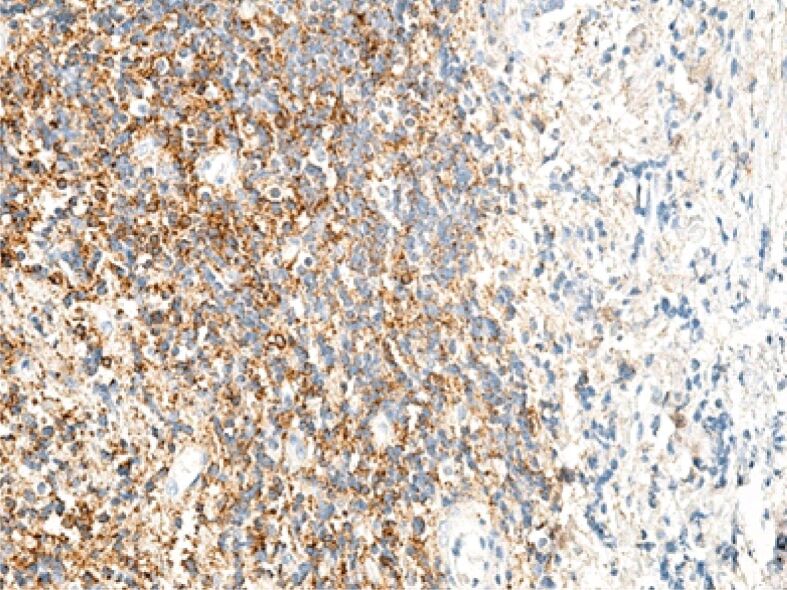
Superficial chorion area infiltrated with lymphocytes, especially B-lymphocytes. Immunolabeling with anti-CD20 antibody, ×100
Figure 15.
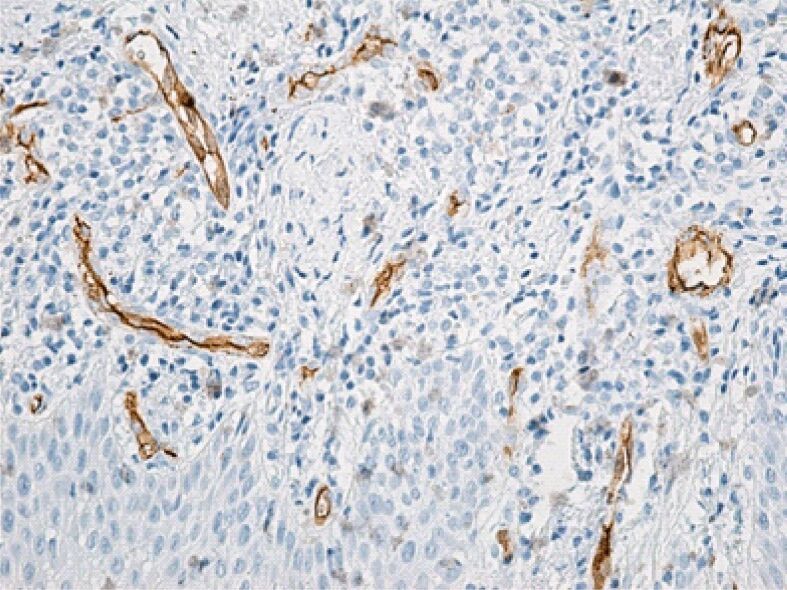
Superficial chorion with numerous angiogenesis vessels. Immunolabeling with anti-CD34 antibody, ×200
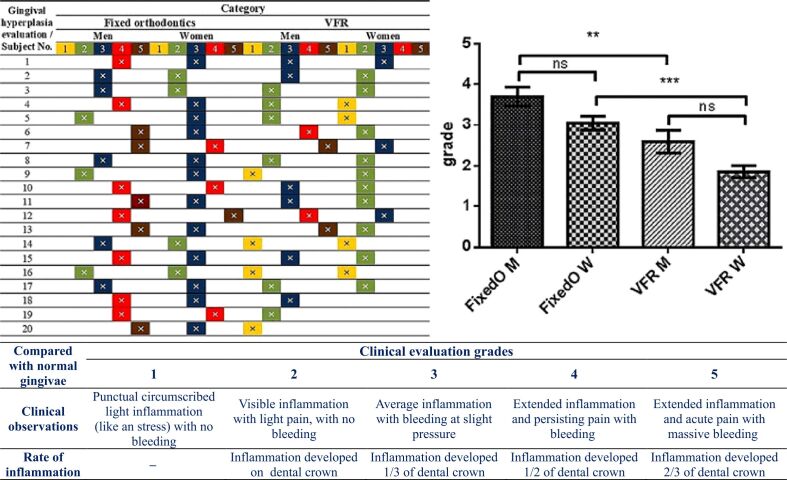
Our observations/evaluations analyzed statistically using ANOVA with the Bonferroni’s correction tests (considered by us to be the most suited in these kinds of clinical evaluations), confirmed with certainty what we clinically observed.
Also, with statistical significance, the comparative obtained values (p=0.01) for men and (p=0.001) for women illustrate our clinical observations, allowing us to affirm that, in our case, the men were more stressed in bearing AWD and VFR, in comparison with women, these obtaining in majority of cases inferior clinical evaluation grades compared with men.
The scale of the gingival hyperplasia clinical evaluation and ANOVA statistics for observed patients is revealed in Figure 16.
Figure 16.
The scale of gingival hyperplasia clinical evaluation and ANOVA statistics for observed patients (where: **p=0.01 is statistically significant and ***p=0.001 is highly statistically significant). ANOVA: Analysis of variance; FixedO: Fixed orthodontics; M: Men; ns: Not significant; VFR: Vacuum-formed retainers; W: Women
⧉ Discussions
The gingival fissure is a location prone to heavy bacterial exposure due to its fine epithelium and its proximity to a complex biofilm structure present on the tooth surface. The gingival environment is also of particular interest because it is highly susceptible to common inflammatory diseases such as periodontitis, as well because of orthodontic maneuvers [22,23].
GO induced by orthodontic treatment has been associated with severe epithelial proliferation response. This process is reinforced by increased packets of collagen fibers, similar to several types of GO, such as in orthodontic and gingival maneuvers, confirming data from the literature [9,10, 24,25].
Some differences were observed in the analysis of GO, two stages of gingival cytoarchitecture having been identified, associated with the use of AWD and VFR orthodontic appliances [26]. To this end, we agree that the increase in gingival volume associated with fixed orthodontic treatment becomes visible shortly after the brackets have been attached to the teeth, due to high mechanical stress and periodontal remodeling during orthodontic maneuvers, which initially did not exhibit any signs of inflammation.
We are also following the affirmation that comparatively, the VFR after three months of treatment is less stressful (and the noticed grades for inflammation were net inferior) to the arch wires. Also, men felt greater pain compared to women in both stages (AWD and VFR).
GO has no exudative and proliferative characteristics of chronic inflammation [27].
GO related to orthodontic therapy has been characterized by fibrous, thick, pink tissues without bleeding tendency, versus clinically observed bright red, soft, brittle, prone to bleeding lesions, which characterize allergic or inflammatory GO [2, 25, 27].
Orthodontic treatments may have, in the background, oral clinical manifestations which cannot be seen on radiographs, such as labial desquamation [28], gingival enlargement [29,30,31], erythema multiforme [32] and gingivitis [33].
These effects are usually associated with the inflammatory response of the gum triggered by the actuating force of orthodontic appliances, as well as with the release of nickel from the springs [34].
The inflammatory response of the gingival tissue to the nickel in the arches is considered by specialists as a type IV hypersensitivity and the clinical manifestation is in the form of allergic contact stomatitis to nickel [35,36,37].
Increased interdental gingival tissue is a side effect that does not develop in all patients but occurs more frequently during orthodontic treatment than as a side effect during other manifestations in the oral cavity [27, 30]. The advantage in these situations indicates that the fibrous gingival enlargements associated with fixed orthodontic appliances appear to be transient [25].
We observed that in these types of orthodontic treatment-induced progressive damage the relative number of B-cells (CD20), T-cells and dendritic cells (CD8) was expressed both in the early and late stages of gingival lesions. Our results showed that the proportion of T-lymphocytes and dendritic cells was higher in the early stages than in the late stages, and B-cells showed a higher number in the late stages [18].
Another study following the increased exposure to microbial attack, revealed a major T-cells rich inflammatory infiltrate, with negligible B-cells, a great quantity of neutrophils and a distinct APC network primed to control local immunity. In this study, and similar to our observations, comparisons of the immune cell network between the gingival and oral mucosa showed a higher abundance of inflammatory cells in the gingival environment, but, unlike this study, the most notable cell difference was the significantly lower presence of neutrophils in gingival tissue after orthodontic therapy than in the case of bacterial plaque [38].
⧉ Conclusions
We noticed that in the early stages of orthodontic treatment-induced progressive damage, T-cells and dendritic cells were predominant, while in the late stages B-cells predominated. At the same time, the level of lesion extension was much more elevated and included evident cytoarchitecture features in the case of arch wires compared to VFR cyto-immune aspects. Also, we observed that men are more subjected to the stress/pain generated by gingival proliferative growth stress related with fixed orthodontic therapy compared to women dismantling the myth of “stone” men resistance.
Conflict of interest
The authors declare no conflict of interests.
Acknowledgments
Acknowledgments
This research was in the frame of Doctoral School Network Timişoara – Victor Babeş University of Medicine and Pharmacy, Timişoara, Romania.
References
- 1.Redlich M, Shoshan S, Palmon A. Gingival response to orthodontic force. Am J Orthod Dentofacial Orthop. 1999;116(2):152–158. doi: 10.1016/s0889-5406(99)70212-x. [DOI] [PubMed] [Google Scholar]
- 2.Ramadan AAF. Effect of nickel and chromium on gingival tissues during orthodontic treatment: a longitudinal study. World J Orthod. 2004;5(3):230–234; discussion 235. [PubMed] [Google Scholar]
- 3.Joo HS, Otto M. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem Biol. 2012;19(12):1503–1513. doi: 10.1016/j.chembiol.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paster BJ, Dewhirst FE. Molecular microbial diagnosis. Periodontology 2000. 2009;51(1):38–44. doi: 10.1111/j.1600-0757.2009.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Øilo M, Bakken V. Biofilm and dental biomaterials. Materials (Basel) 2015;8(6):2887–2900. [Google Scholar]
- 6.de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MAWP, de Gruijl T, Piguet V, van Kooyk Y, Geijtenbeek TBH. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13(3):367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 7.Corbacho de Melo MM, Cardoso MG, Faber J, Sobral A. Risk factors for periodontal changes in adult patients with banded second molars during orthodontic treatment. Angle Orthod. 2012;82(2):224–228. doi: 10.2319/030911-172.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faccioni F, Franceschetti P, Cerpelloni M, Fracasso ME. In vivo study on metal release from fixed orthodontic appliances and DNA damage in oral mucosa cells. Am J Orthod Dentofacial Orthop. 2003;124(6):687–693; discussion 693–694. doi: 10.1016/j.ajodo.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Bharti V, Bansal C. Drug-induced gingival overgrowth: the Nemesis of gingiva unraveled. J Indian Soc Periodontol. 2013;17(2):182–187. doi: 10.4103/0972-124X.113066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roi CI, Gaje PN, Ceausu RA, Popovici RA, Raica M. Immunohistochemical analysis of S100-positive Langerhans cells in the healthy gingiva and periodontal disease. Rev Chim (Bucharest) 2018;69(1):232–235. [Google Scholar]
- 11.Cutler CW, Jotwani R. Dendritic cells at the oral mucosal interface. J Dent Res. 2006;85(8):678–689. doi: 10.1177/154405910608500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Awady AR, Arce RM, Cutler CW. Dendritic cells: microbial clearance via autophagy and potential immunobiological consequences for periodontal disease. Periodontol 2000. 2015;69(1):160–180. doi: 10.1111/prd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Kooyk Y, Engering A, Lekkerkerker AN, Ludwig IS, Geijtenbeek TBH. Pathogens use carbohydrates to escape immunity induced by dendritic cells. Curr Opin Immunol. 2004;16(4):488–493. doi: 10.1016/j.coi.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Pop SI, Dudescu M, Bratu DC, Merie VV, Pacurar M. Effect of esthetic coating on the load deflection and surface characteristics of the NiTi orthodontic archwires. Rev Chim (Bucharest) 2015;66(3):364–367. https://revistadechimie.ro/Articles.asp?ID=4431 [Google Scholar]
- 15.Mei L, Chieng J, Wong C, Benic G, Farella M. Factors affecting dental biofilm in patients wearing fixed orthodontic appliances. Progr Orthod. 2017;18(1):4–4. doi: 10.1186/s40510-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freitas AOA, Marquezan M, Nojima MCG, Alviano DS, Maia LC. The influence of orthodontic fixed appliances on the oral microbiota: a systematic review. Dental Press J Orthod. 2014;19(2):46–55. doi: 10.1590/2176-9451.19.2.046-055.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alfuriji S, Alhazmi N, Alhamlan N, Al-Ehaideb A, Alruwaithi M, Alkatheeri N, Geevarghese A. The effect of orthodontic therapy on periodontal health: a review of the literature. Int J Dent. 2014;2014:585048–585048. doi: 10.1155/2014/585048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon CP, Bratu DC, Motoc AGM, Popa G, Pop IS, Mederle OA. Immunohistochemical analysis of gingival proliferative processes associated with fixed orthodontic therapy. Rev Chim (Bucharest) 2020;71(2):302–306. [Google Scholar]
- 19.Podshadley AG, Haley JV. A method for evaluating oral hygiene performance. Public Health Rep. 1968;83(3):259–264. [PMC free article] [PubMed] [Google Scholar]
- 20.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(6):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 21.Barendregt DS, Timmerman MF, van der Velden U, van der Weijden GA. Comparison of the bleeding on marginal probing index and the Eastman interdental bleeding index as indicators of gingivitis. J Clin Periodontol. 2002;29(3):195–200. doi: 10.1034/j.1600-051x.2002.290302.x. [DOI] [PubMed] [Google Scholar]
- 22.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83(12):1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ancuta C, Ancuta E, Chirieac R, Anton C, Surlari Z, Iordache C. TNF inhibitors and periodontal inflammation in psoriatic arthritis. Rev Chim (Bucharest) 2017;68(8):1914–1918. [Google Scholar]
- 25.Carranza FA, Hogan EL, Saunders E, Newman MG, Takei HH, Klokkevold PR. Newman and Carranza’s clinical periodontology. 11. Philadelphia: Elsevier–Saunders; 2006. pp. 84–96. [Google Scholar]
- 26.Drăghici EC, Crăiţoiu Ş, Mercuţ V, Scrieciu M, Popescu SM, Diaconu OA, Oprea B, Pascu RM, Crăiţoiu MM. Local cause of gingival overgrowth. Clinical and histological study. Rom J Morphol Embryol. 2016;57(2):427–435. [PubMed] [Google Scholar]
- 27.Gursoy UK, Sokucu O, Uitto VJ, Aydin A, Demirer S, Toker H, Erdem O, Sayal A. The role of nickel accumulation and epithelial cell proliferation in orthodontic treatment-induced gingival overgrowth. Eur J Orthod. 2007;29(6):555–558. doi: 10.1093/ejo/cjm074. [DOI] [PubMed] [Google Scholar]
- 28.Vijayan V, Paul A, Babu K, Madhan B. Desquamative gingivitis as only presenting sign of mucous membrane pemphigoid. J Indian Soc Periodontol. 2016;20(3):340–343. doi: 10.4103/0972-124X.182602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponnam SR, Srivastava G, Jampani N, Kamath VV. A fatal case of rapid gingival enlargement: case report with brief review. J Oral Maxillofac Pathol. 2014;18(1):121–126. doi: 10.4103/0973-029X.131938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genelhu MCLS, Marigo M, Alves-Oliveira LF, Malaquias LCC, Gomez RS. Characterization of nickel-induced allergic contact stomatitis associated with fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 2005;128(3):378–381. doi: 10.1016/j.ajodo.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Kouraki E, Bissada NF, Palomo JM, Ficara AJ. Gingival enlargement and resolution during and after orthodontic treatment. N Y State Dent J. 2005;71(4):34–37. [PubMed] [Google Scholar]
- 32.Paulino L, Hamblin DJ, Osondu N, Amini R. Variants of erythema multiforme: a case report and literature review. Cureus. 2018;10(10):e3459–e3459. doi: 10.7759/cureus.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baygin O, Tuzuner T, Ozel MB, Bostanoglu O. Comparison of combined application treatment with one-visit varnish treatments in an orthodontic population. Med Oral Patol Oral Cir Bucal. 2013;18(2):e362–e370. doi: 10.4317/medoral.18261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eliades T, Trapalis C, Eliades G, Katsavrias E. Salivary metal levels of orthodontic patients: a novel methodological and analytical approach. Eur J Orthod. 2003;25(1):103–106. doi: 10.1093/ejo/25.1.103. [DOI] [PubMed] [Google Scholar]
- 35.Minciullo PL, Paolino G, Vacca M, Gangemi S, Nettis E. Unmet diagnostic needs in contact oral mucosal allergies. Clin Mol Allergy. 2016;14(1):10–10. doi: 10.1186/s12948-016-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotaru M, Iancu GM, Gheucă Solovăstru L, Glaja RF, Grosu F, Bold A, Costache A. A rare case of multiple clear cell acanthoma with a relatively rapid development of the lower legs. Rom J Morphol Embryol. 2014;55(3 Suppl):1171–1179. [PubMed] [Google Scholar]
- 37.Rai R, Dinakar D, Kurian SS, Bindoo YA. Investigation of contact allergy to dental materials by patch testing. Indian Dermatol Online J. 2014;5(3):282–286. doi: 10.4103/2229-5178.137778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dutzan N, Konkel JE, Greenwell-Wild T, Moutsopoulos NM. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 2016;9(5):1163–1172. doi: 10.1038/mi.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]