Abstract
G protein-coupled receptors (GPCRs) transmit the signals of a variety of hormones and neurotransmitters and are targets of more than 30% of all FDA-approved drugs. We developed an approach for identifying the endogenous ligands for a family of orphan GPCRs that enables the development of novel therapeutics for the potential treatment of a wide variety of disorders including pain, diabetes, appetitive behaviors, infertility and obesity. With this approach, we have deorphanized five previously orphaned GPCRs.
G Protein Coupled Receptors as Potential Therapeutic Targets
It is estimated that 30–50% of all FDA-approved medications target G protein-coupled receptors (GPCRs).1,2 There are three major classes of GPCRs in humans: Class A (rhodopsin-like), Class B (secretin-like), and Class C (metabotropic/glutamate-like). These are large proteins that reside in the cell membrane with a characteristic 7-transmembrane spanning domain and an extracellular terminus that may be glycosylated.3 GPCRs also contain intracellular domains that interact with downstream signaling cascades that communicate the message imparted by a ligand (e.g. hormone) binding primarily to extracellular epitopes on the receptor. The selectivity of ligand-receptor binding makes them attractive candidates for drug development. Examples of existing therapeutics based upon knowledge of ligand structure/function relationships and receptor recognition motifs are numerous and include, for example, Class A receptor therapeutics targeting the angiotensin receptors, Class B receptor therapeutics targeting the GLP-1 receptor, and Class C receptor therapeutics targeting neurotransmitter receptors.
There may be as many as one thousand genes encoding GPCRs, but by far the largest number of these encode receptors in the Class A category. Remarkably, even with the development of the most modern molecular approaches, it is estimated that 130 or more of those identified genes encode GPCRs for which an endogenous ligand has yet to be identified. Think of these receptors as door locks, which protect entry into new knowledge of cell function and potential avenues for the treatment of human diseases. This is exactly the reason why pharmaceutical companies and independent laboratories have expended such effort and capital in the search for the keys to those locks, the ligands that interact with those so-called “orphan receptors.”
How do researchers identify endogenous ligands for these orphan receptors? The classic approach is to search tissue extracts for substances that interact with the receptor protein. This approach is handicapped by the need for large amounts of tissue, extensive protein purification steps and laborious ligand-receptor binding assays. The development of modern, robotic-based screening assays provided hope for increased success in the search for these endogenous ligands, but in fact this approach has been disappointing. All the same, those robotic screening assays have become critically important only once a candidate, endogenous ligand has been identified.4 These may be proteins recently discovered or hormones that have been known to exist and have significant biologic activity for years, for which a cognate receptor has not been identified.
Our laboratory group recently discovered two previously unrecognized endogenous peptide hormones, neuronostatin, and phoenixin.5,6 After validating the gene sequences and proteins encoded, we identified tissue sites where they are produced, physiologically relevant changes in their production, and finally tissues where they exert actions both in vitro and in vivo. Those foundational studies identified multiple sites of action, suggesting important contributions of the two hormones in such diverse functions as glucose homeostasis, growth, autonomic function, appetite/thirst, fluid and electrolyte homeostasis, and reproduction. The challenge then was to determine which of the myriad pharmacologic actions of these two hormones were physiologically relevant. To accomplish this task, we either had to create an animal model in which the gene expressing the hormone was ablated or find a population of animals or humans in which the gene product was missing or improperly produced. The first approach was impossible for several reasons. For neuronostatin, creation of a knockout animal by embryonic gene deletion would not be instructive because encoded in the same gene as neuronostatin (NST) is the potent growth restricting hormone somatostatin (SRIF). Gene compromise would negate production of both hormones. We had to develop a unique, alternative approach.
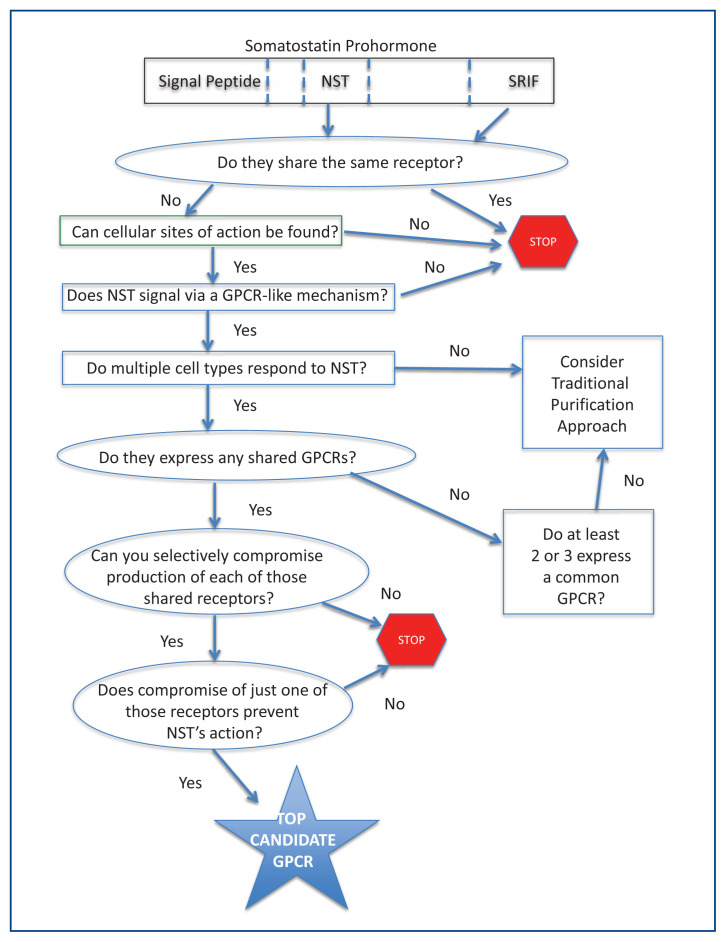
We knew that neuronostatin exerted pharmacologic effects unique from those of somatostatin and that it did not bind to the known somatostatin receptors.5 Thus, if we could identify the cognate receptor for neuronostatin, we might be able to compromise the production of that receptor and determine the importance of the peptide’s pharmacologic actions. We established in cell culture-based experiments that neuronostatin activates cell-signaling cascades typical of other GPCRs and thus made what proved to be a “correct guess.” We hypothesized that neuronostatin signals via one of the 130 orphan GPCRs and then developed a novel strategy to address that hypothesis, “The Deductive Reasoning Strategy.”7 (Figure 1)
Figure 1.
The Deductive Reasoning Strategy for the G protein-coupled receptor (GPCR) De-Orphanization. Demonstrated here is the identification of a top receptor candidate for neuronostatin (NST) a recently discovered, endogenous hormone,5 that is encoded in the same gene product as somatostatin (SRIF).
The Strategy, which was patented by Dr. Yosten, basically assumes that all cell types responding to neuronostatin will share at least one (and hopefully not too many) GPCR. Multiple cell types, in this case cardiac myocytes, pancreatic alpha cells, hypothalamic neurons and KATO III cells (a gastric tumor cell line), were analyzed by reverse transcriptase-polymerase chain reaction (RT-PCR) for the presence of messenger RNA encoding all the orphan GPCRs.7 Commonly expressed receptors were then compromised in vitro by the use of small interfering RNA molecules that blocked expression each of those candidate GPCRs, one at a time, and then those compromised cells tested for their response to neuronostatin. Initially, we had four candidate shared receptors, and we used the KATO III cells for the siRNA (small interfering RNA molecules that block translation selectively of the mRNA encoding the targeted receptor protein) compromise study because those cells are readily transfected with the siRNA constructs and their response to neuronostatin (increased early gene, c-fos, expression) was readily detectable. Using this approach, we identified GPR107 as the cognate receptor for neuronostatin, at least in cells in culture.7 Then, our task was to demonstrate the consequence of compromising GPR107 expression in vivo on the pharmacologic actions of the peptide and on basic physiologic mechanisms thought to require neuronostatin, such as autonomic function and glucose metabolism.
When approaching the in vivo experiments, it was important to choose a tissue site of action of neuronostatin that was well validated and approachable in vivo. We previously identified several central nervous system actions of the peptide and felt that intracerebroventricular delivery of the compromising siRNA would have the advantage over peripheral administration of sequestrating the siRNA construct within the targeted tissue,with less dilution by the general circulation. Indeed, we showed that neuronostatin acts pharmacologically in brain to raise blood pressure by actions on vasopressin release and increases sympathetic nerve trafficking. Could this action be blocked by prior compromise of GPR107 expression? Indeed it was, not only the pharmacologic effects of the peptide, but also normal baroreflex function in response to lowering and raising peripheral mean arterial pressure with a vasodilator (sodium nitroprusside) and a vasoconstrictor (phenylephrine), respectively. Non-GPR107-mediated central control of autonomic function, in this case the role of angiotensin II, was not compromised, demonstrating specificity.
Thus, we demonstrated that neuronostatin is the key that unlocks the door guarded by GPR107. But where does this lead us in terms of developing therapeutics? That promise lies in another action of neuronostatin, one related to glucose homeostasis. Neuronostatin is produced, along with somatostatin, in delta cells of the pancreas. We demonstrated the ability of neuronostatin to inhibit glucose-stimulated insulin secretion, at least in part by an initial action on the neighboring alpha cells. Importantly, neuronostatin is able to stimulate glucagon expression and secretion from an alpha cell line and isolated whole islets. We are currently extending those initial cell culture experiments into whole animal studies, both rodent and human protocols, with the hypothesis that neuronostatin or a stable analog might be of value as a prophylactic agent to prevent insulin overdose-induced hypoglycemia or as an adjunctive therapeutic in diabetics suffering from hypoglycemia unawareness. In fact, those studies are now underway, both here in St. Louis and in collaborations with clinical investigators in Copenhagen.
Expanding Discovery with New Molecular Tools
How applicable is the Deductive Reasoning Strategy for the identification of other ligand-receptor pairs? The answer is quite simple: very. Table 1 demonstrates the ligand-receptor pairs we have de-orphanized to date using this approach.
Table 1.
Ligand-receptor pairs that have been de-orphanized to date using this approach.
| LIGAND/HORMONE | COGNATE RECEPTOR | POSSIBLE APPLICATIONS |
|---|---|---|
| Pro-insulin Connecting Peptide (C-Peptide)8 | GPR146 | Diabetes, vascular diseases |
| Phoenixin6,9,10 | GPR173 | Reproductive assist, fluid and electrolyte homeostasis |
| Adropin11 | GRP19 | Metabolic disorders, obesity |
| Neuronostatin5 | GPR107 | Metabolic disorders, diabetes |
| Cocaine- and amphetamine regulated transcript peptide (CARTp)16,17 | GPR160 | Pain management, appetite regulation, obesity |
A second problem encountered when using traditional gene compromise strategies for testing the importance of either a ligand or receptor is one of embryonic lethality. If either the ligand or the receptor is essential for placentation or embryonic development, disruption of that protein by traditional means (global embryonic deletion) will not work. This has led to the development of conditional gene compromise strategies, knockouts that can be induced during the postnatal period or adulthood. The approach has two additional advantages: tissue/site-specific gene compromise can be affected, and the compromise is permanent. For instance, in the case of neuronostatin, if the goal is to compromise peptide signaling just on alpha cells of the pancreas, in order to determine the physiological relevance of the glucagon-releasing action of the peptide, a transgenic rodent line could be created in which the gene encoding GPR107 is targeted for deletion only in that cell type. This would then allow for the interaction of neuronostatin and GPR107 in other tissues, or even in other cell types in the pancreatic islet, without any interruption. Genetic “tricks” also have been developed for “on/off ” switching of select gene deletion and re-expression in specific tissue.
Taking Discovery to Therapeutic Development
The goal in developing these state-of-the-art genetic manipulations is to not only determine whether a ligand is essential for normal physiologic function, but also the culprit in pathologic conditions resulting from loss of function of the ligand/receptor pair. However, these approaches require the initial identification of the receptor that transmits the ligand/hormone signal. This brings us back to the discovery process itself. Even after our Deductive Reasoning Strategy has identified a ligand for one of the orphan GPCRS, much characterization work is required. Physical association of the ligand with the receptor needs to be proven and, if possible, the epitopes (amino acid signatures) important for binding of ligand to receptor need to be identified. This would facilitate the development of stable, efficacious analogs as agonists or antagonists. Stability of the endogenous ligand, and any analogs developed, would have to be established and the pharmacokinetic properties of these molecules established. Development of effective analogs can be approached in two ways. First, knowledge of the amino acid sequence of protein ligands and the epitope essential for receptor binding would allow modifications to the amino acid backbone to be made to enhance binding affinity and avidity, and to prolong biologic half-life. An example of this would be the exendins, analogs of the mammalian hormone GLP-1, which are prescribed widely now for appetite and glucose metabolism regulation (e.g. liraglutide and dulaglutide).
A second approach, once a ligand/receptor pair has been identified and proven to be important in the regulation of distinct tissue functions, is the development of small molecules, usually organic in composition, with optimal binding characteristics (e.g. losartan). Numerous extensive libraries of small molecules have been assembled over the years in attempts to develop better therapeutics. Many of these molecules failed to meet the essential criteria in the projects for which they were developed, only later to be found to target a completely different class of receptors. These molecules have the advantage of ready synthesis and easily modified carbon backbones. Thus, a wide variety of modifications can be made following principles of intelligent design that result in enhanced efficacy with less off-target effects (e.g. the V2 antagonists, including tolvaptan). Often these small molecules display the added advantage of being orally active.
What’s next? As our discovery work has demonstrated, there are probably numerous, endogenously expressed peptides/proteins yet to be discovered that have important, physiologically relevant actions. Once identified, their cognate receptors will have to be identified. Similarly, peptides that are produced in a variety of human tissues and whose pharmacologic profiles have long been known still hold promise for the development of novel, efficacious therapeutics. Perhaps the best, recent of example of this is the peptide encoded by the cocaine- and amphetamine-regulated transcript, CARTp. First identified by classical peptide chemistry approaches in the 1980s and later re-discovered by more modern gene cloning approaches, this peptide is a potent inhibitor of food intake and reward-based behaviors, and more recently was found to act pharmacologically to induce pain.12,13,14,15,16 Progress in developing agonists for treating eating disorders/obesity and antagonists for treating peripheral neuropathies had been blocked by fact that, even after extensive efforts using conventional receptor identification approaches, no CARTp receptor had been identified. Because of the peptide’s interesting pharmacology, we teamed with Dr. Daniela Salvemini to identify the CARTp receptor using our Deductive Receptor Matching Strategy, identifying GPR160 as the CARTp receptor.16 Using a variety of biochemical and cellular approaches, we defined the signaling cascade activated by CARTp-GPR160 interactions, demonstrated the requirement of GPR160 for CARTp-induced signaling in vitro and in vivo. Even more importantly, we demonstrated that compromise of GPR160 expression in laminae 1–3 of the dorsal horn of the lumbar spinal cord blocks the development of spinal injury-induced neuropathy and reverses already-established neuropathy.16 More recently we demonstrated that compromise of GPR160 signaling in brainstem structures important for gut-brain feedback and termination of meals results in exaggerated eating and drinking under physiologically relevant conditions and that the anorexigenic action of CARTp in that tissue is prevented by GPR160 compromise.17
This work has opened new avenues for the development of novel therapeutics to treat peripheral neuropathies, if antagonistic analogs or small molecules can be developed that can be applied regionally over the lumbar spinal column. Those goals are currently being pursued in collaboration with Dr. Salvemini in the Department of Pharmacology and Physiology at SLU. In terms of the anorexigenic action of CARTp, we envision structurefunction relationships to reveal avenues for the development of CARTp-like, GPR160 agonists devoid of peripheral actions that can be used to control eating disorders and compulsive behaviors.
It doesn’t stop there. Our Deductive Receptor Matching Strategy is being applied to identify the cognate receptors for several other known peptide hormones. An excellent example of our current studies is a search for the cognate receptor for nesfatin, the most potent anorexigenic peptide yet described, for which, like CARTp, even after extensive efforts a unique receptor has yet to be identified.18,19,20 And there are more hormone targets to be identified, promising not only novel insight into the mechanisms of health and disease, but also providing a great training environment for the next generation of researchers who will carry our vision forward.
Footnotes
Gina L. C. Yosten, PhD, Daniela Salvemini, PhD, and Willis K. Samson, PhD, (above), are in the Department of Pharmacology and Physiology; Grant R. Kolar, MD, PhD, is in the Department of Pathology; all are in the Henry and Amelia Nasrallah Center for Neuroscience, Saint Louis University School of Medicine, St. Louis, Missouri.
Disclosure
Work described in this review was supported by Start-up Funds provided by the Saint Louis University School of Medicine (GLCY) and NIH Grants: DK118340 (GLCY), NS113257 (DS and GLCY) and HL121456 (WS).
References
- 1.Tang XL, Wang Y, Li DL, Luo J, Liu MY. Orphan G protein-coupled receptors (GPCRs): biological functions and potential drug targets. Acta Pharmacol Sin. 2012;33(3):363–371. doi: 10.1038/aps.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang Y, Kenakin T, Liu C. Editorial: Orphan GPCRs As Emerging Drug Targets. Front Pharmacol. 2015;6:295. doi: 10.3389/fphar.2015.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signals of G protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- 4.Ngo T, Kufareva I, Coleman LJ, Graham RM, Abagyan R, Smith NJ. Identifying ligands at orphan GPCRs: current status using structure-based approaches. Br J Pharmacol. 2016;173(20):2934–2951. doi: 10.1111/bph.13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samson WK, Zhang JV, Avsian-Kretchmer O, Cui K, Yosten GLC, Klein C, Lyu R-M, Wang YX, Chen XQ, Yang J, Price CJ, Hoyda TD, Ferguson AV, Yuan XB, Chang JK, Hsueh AJ. Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions. J Biol Chem. 2008;283(46):31949–31959. doi: 10.1074/jbc.M804784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yosten GL, Lyu RM, Hsueh AJ, Avsian-Kretchmer O, Chang JK, Tullock CW, et al. A novel reproductive peptide, phoenixin. J Neuroendocrinol. 2013;25(2):206–215. doi: 10.1111/j.1365-2826.2012.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yosten GL, Redlinger LJ, Samson WK. Evidence for an interaction of neuronostatin with the orphan G protein-coupled receptor, GPR107. Am J Physiol Regul Integr Comp Physiol. 2012;303(9):R941–949. doi: 10.1152/ajpregu.00336.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yosten GLC, Kolar GR, Redlinger LJ, Samson WK. Evidence for an interaction between pro-insulin C-peptide and GPR146. Journal of Endocrinology. 2013;218:B1–B8. doi: 10.1530/JOE-13-0203. [DOI] [PubMed] [Google Scholar]
- 9.Gasparini SLM, Stein SP, Loewen CJ, Haddock J, Soo AV, Ferguson GR, Kolar GLC, Yosten WK. Samson. Novel regulator of vasopressin secretion: phoenixin. American Journal of Physiology Regul Integr Comp Physiol. 2017;314:R623–R628. doi: 10.1152/ajpregu.00426.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddock CJ, Almeida-Pereira G, Stein LM, Yosten GLC, Samson WK. A novel regulator of thirst behavior: phoenixin. American Journal of Physiology Regul Integr Comp Physiol. 2020;318(6):R1027–1035. doi: 10.1152/ajpregu.00023.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein LM, Yosten GLC, Samson WK. Adropin acts in brain to inhibit water drinking: possible interaction with the orphan G protein-coupled receptor, GPR19. American Journal of Physiology Regul Integr Comp Physiol. 2016;310:R476–480. doi: 10.1152/ajpregu.00511.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiess J, Villarreal J, Vale W. Isolation and sequence analysis of a somatostatin-like polypeptide from ovine hypothalamus. Biochemistry. 1981;20(7):1982–1988. doi: 10.1021/bi00510a038. [DOI] [PubMed] [Google Scholar]
- 13.Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15(3 Pt 2):2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aja S, Sahandy S, Ladenheim EE, Schwartz GJ, Moran TH. Intracerebroventricular CART peptide reduces food intake and alters motor behavior at a hindbrain site. Am J Physiol Regul Integr Comp Physiol. 2001;281(6):R1862–1867. doi: 10.1152/ajpregu.2001.281.6.R1862. [DOI] [PubMed] [Google Scholar]
- 15.Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci. 2008;9(10):747–758. doi: 10.1038/nrn2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yosten GL, Harada CM, Haddock CJ, Giancotti LA, Kolar GR, Patel R, Guo C, Chen Z, Zhang J, Doyle TM, Dickenson AH, Samson WK, Salvemini D. GPR160 de-orphanization reveals critical roles in neuropathic pain in rodents. J Clin Invest. 2020;130(5):2587–2592. doi: 10.1172/JCI133270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddock CJ, Almeida-Pereira G, Stein LM, Hayes MR, Kolar GK, Samson WK, Yosten GLC. Signaling in rat brainstem via Gpr160 is required for the anorexigenic and antidipsogenic actions of cocaine- and amphetamine-regulated transcript peptide. American Journal of Physiology Regul Integr Comp Physiol. 2020 doi: 10.1152/ajpregu.00096.2020. in press. doi.org:10.1152/ajpregu.00096.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yosten GLC, Samson WK. Nesfatin exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. American Journal of Physiology Regul Integr Comp Physiol. 2009;297:R330–R336. doi: 10.1152/ajpregu.90867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yosten GLC, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin antagonist. American Journal of Physiology Regul Integr Comp Physiol. 2010;298:R1642–R1647. doi: 10.1152/ajpregu.00804.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pate AT, Schnell AL, Ennis TA, Samson WK, Yosten GLC. Expression and function of nesfatin-1 are altered by stage of the estrous cycle. American Journal of Physiology Regul Integr Comp Physiol. 2019;317:R328–336. doi: 10.1152/ajpregu.00249.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]