Abstract
Healthy adipose tissue contains a wide variety of innate and adaptive immune cells, including macrophages, dendritic cells, mast cells, eosinophils, neutrophils, and lymphocytes. Numerous signaling molecules in the adipose microenvironment can positively or negatively modulate angiogenic processes, regulate the interaction between the vascular system and adipocytes, and participate in tumor progression. Mast cells are involved in the new formation or metabolism of fat, are present in abundant quantities in fatty tissue, among fat cells, and a number of mediators released from mast cells play a role in adipogenesis. Moreover, mast cells produce several pro-angiogenic factors and are involved in tumor angiogenesis. In this context, the angiogenic effect might be amplified when the adipocytes and mast cells act in concert, and treatment of adipose tissue- and mast cell-associated cancers with anti-angiogenic drugs may represent an alternative or adjuvant strategy for the treatment of these tumors.
Keywords: adipocytes, angiogenesis, anti-angiogenesis, mast cells, tumor growth
⧉ Introduction
Adipose tissue can be divided into two different types, white adipose tissue (WAT) acting as a lipid deposit and brown adipose tissue (BAT). WAT is composed of pre-adipocytes and adipocytes, fibroblasts, and macrophages. White adipocytes are large cells containing a unilocular lipid droplet that occupies 90% of the total cell area. Brown adipocytes are smaller than white adipocytes, their lipid droplets are dispersed in the cytoplasm, and hold numerous and large mitochondria, responsible for the darkened appearance of the tissue. They are also present in some deposits of WAT, thus giving them a beige color, which justifies the name “beige” adipose tissue.
Moreover, BAT is more vascularized than WAT [1]. Another adipocyte cell type, called “pink adipocyte”, has been described, present during lactation and gestation, due to a process wherein white adipocytes progressively transdifferentiate to acquire secretory, epithelial-like features [2].
Adipose tissue has been further categorized based on physiologic localization and function, as subcutaneous, visceral, marrow, breast, and intramuscular fat [3]. In humans, subcutaneous adipose tissue comprises ∼80% of total body fat and is contained primarily in the abdominal, gluteal, and femoral depots [4]. The number of adipocytes in humans increases during childhood and adolescence and remains constant during adulthood, and adipocytes in adult humans have an annual renewal rate of about 10% [5].
Healthy adipose tissue contains a wide variety of innate and adaptive immune cells, including macrophages, dendritic cells, mast cells, eosinophils, neutrophils, and lymphocytes, which collectively constitute ∼25% to 45% of stromal cells in humans [6].
⧉ Adipocytes and the vascular system
Endothelial cells and adipocytes have common progenitor cells that differentiate into adipocytes or endothelial cells, depending on exposure to different environments [7]. Immature adipocytes can be found near capillary showing the close association between adipose progenitors and vascularization of adipose tissue [8]. Numerous signaling molecules in the adipose microenvironment can positively or negatively modulate angiogenic processes and regulate the interaction between the vascular system and adipocytes.
⧉ Adipocytes and angiogenesis
Angiogenesis is essential for BAT hyperplasia [9], and the transition of WAT into BAT is accompanied by switching on an angiogenic phenotype [10]. Studies conducted in the mouse cornea and the chick embryo chorioallantoic membrane (CAM) have demonstrated that conditioned media obtained from pre-adipocytes and tissue homogenates from omentum or subcutaneous fat induces angiogenesis [11,12,13].
Expansion of adipose tissue during progression to obesity requires concomitant expansion of the adipose vascular bed through angiogenesis. In fact, the administration of anti-angiogenic agents in models of both genetic and diet-induced obesity either prevented weight gain [14] or induced dose-dependent, reversible weight reduction and adipose tissue loss [15].
Differentiation from pre-adipocytes to mature adipocytes is linked to high expression levels of angiogenic factors [11]. Adipose tissue produces a plethora of cytokines and growth factors involved in angiogenesis, including leptin, adiponectin, vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF-1), placental growth factor (PlGF), interleukin-6 (IL-6), angiogenin, tumor necrosis factor-alpha (TNF-α), and angiopoietins (Angs) [16]. Leptin induces angiogenesis, vascular fenestration, and vascular remodeling [17,18]. Adiponectin negatively affects angiogenesis [19]. VEGF-A overexpression in adipocytes hinders the expansion of adipose tissue [20], increases the number of beige adipocytes, and induces neovascularization of subcutaneous fat [21]. The blockade of the vascular endothelial growth factor receptor-2 (VEGFR-2) signaling pathway by a neutralizing antibody inhibits both angiogenesis and pre-adipocytes differentiation [22]. Adipose tissue produces matrix metalloproteinase-2 (MMP-2) and MMP-9, both involved in the regulation of angiogenesis [23]. Recruitment of inflammatory cells also significantly contributes to adipose neovascularization.
Several of the pro-angiogenic factors listed above, including multiple VEGF isoforms, leptin, HGF, and Ang-2, are also elevated in the serum of obese subjects and are implicated in the systemic effects of obesity on cancer progression [24,25,26]. Both WAT and BAT contain dense microvascular networks, but microvascular density is higher in BAT as compared to WAT [27]. Obesity impairs the vasodilator response of the muscle microvasculature to insulin VEGF and reduces microvascular density [28].
Angiogenesis inhibitors reduce fat mass expansion in mice [15, 29,30]. Angiogenesis inhibitor TNP-470 prevents diet-induced obesity in mice, decreases appetite, fat mass, and expansion of adipose tissue by inhibiting aminopeptidase-2 [29]. Angiostatin and endostatin reduce fat mass [31]. A significant higher vessel density is present in the adipose tissue of tissue inhibitor of metalloproteinase-1 (TIMP-1) knockout mice compared with control mice [32,33].
⧉ Mast cells and adipocytes
The first reports on the measurement of mast cell numbers in different body sites date to 1950 [34]. Skin biopsy specimens of normal subjects contained 38.4±4 mast cells per square millimeter, while in the adipose tissue their number is 10.4±2 per square millimeter.
Studies on the effect of the thyrotrophic hormone on connective tissue showed that at the same time, as fat is mobilized from the normal depots, there is an accumulation of mast cells [35].
Mast cells are involved in the new formation or metabolism of fat. They are present in abundant quantities in fatty tissue, among fat cells (Figure 1). Visceral WAT of obese mice shows a higher number of mast cells compared with those of lean mice, while there is no significant difference in their number in subcutaneous WAT between obese and lean mice [36].
Figure 1.
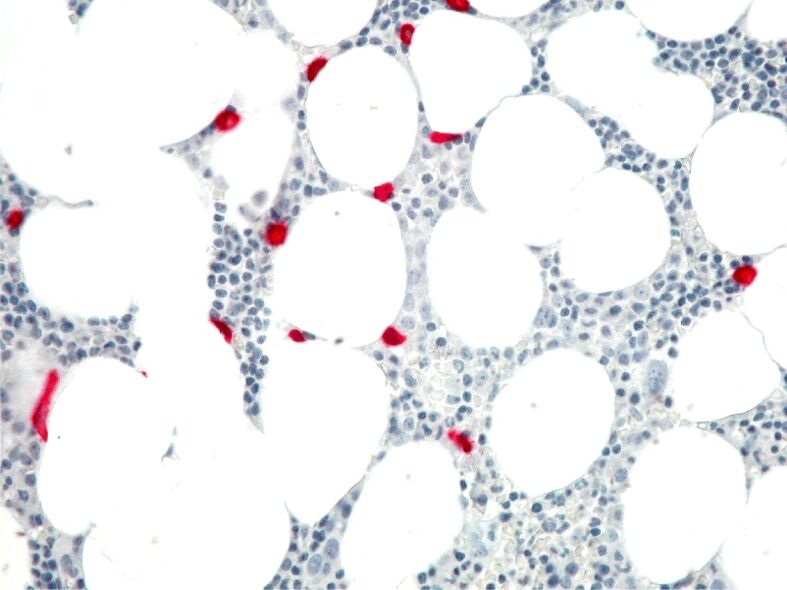
Close spatial relationship between tryptase-positive mast cells (in red) and adipocytes in a human bone marrow sample. A murine monoclonal antibody against tryptase (mAb AA1, Dako) was used. The immunodetection was performed with alkaline phosphatase anti-alkaline phosphatase (APAAP, Dako) and Fast Red as chromogen. Original magnification, ×200
Mast cells release several mediators, such as cytokines, chemokines, proteases, and prostaglandins, that play a role in adipogenesis [36]. As concerns the different role of tryptase and chymase stored in mast cell secretory granules, mast cells stimulate angiogenesis in adipose tissue by releasing chymase and inducing preadipocyte differentiation and also the proliferation of adipocytes [37]. Increased number of mast cells stained with tryptase has been reported in WAT of obese patients [38].
Mast cells from the rat peritoneal cavity express the receptors for both leptin and adiponectin, whose activation induces cytokines and reactive oxygen species production. Both adipokines induce migration of mast cells, leptin induces histamine and cysteinyl leukotriene secretion, and expression of C–C motif chemokine ligand 3 (CCL3) [39,40], while adiponectin induces the production of anti-inflammatory IL-10 [41].
Altintas et al. [42] found significantly more mast cells in visceral fat of obese mice compared with lean ones and found that subcutaneous fat behaved very differently as in the latter, obesity is accompanied only by a modest increase in mast cells density. Moreover, they found that a significant number of mast cells in the epididymal fat of obese mice were in the process of degranulation and secreted TNF-α, which contributes to local and systemic insulin resistance.
Ishijima et al. [43] demonstrated a key role for mast cells in the preadipocyte to adipocyte transition under both obese and non-obese conditions. By the mean of reverse transcription polymerase chain reaction (RT-PCR) and in vitro studies, they have shown that in the epididymal WAT and stromal vascular fraction (SVF) of mast cell-deficient (KitW-sh/W-sh) mice, the messenger ribonucleic acid (mRNA) amount of preadipocyte markers, such as preadipocyte factor-1 (Pref-1), adipocyte enhancer-binding protein 1 (AEBP1), and GATA binding protein 2 (GATA2), but not mature adipocyte ones, such as adipocyte protein 2 (aP2), peroxisome proliferator-activated receptor gamma (PPARγ), acyl‑coenzyme A synthetase 1 (Acsl1), and adipsin, increase compared to wild-type mice under both physiological and pathological conditions. Mast cells accumulate in the adipose tissue of obese individuals [44,45].
Mast cell-deficient mice present improved glucose tolerance and insulin sensitivity compared with wild-type mice [38]. Obesity and type 2 diabetes are inflammatory diseases, characterized by an excess of adipose tissue and chronic insulin exposition induces the formation of lipid bodies in mast cells [46]. Moreover, several animal models have demonstrated a pathogenetic role of mast cells also in human type 1 diabetes mellitus [36]. Intraperitoneal injection of Disodium Cromoglycate, an inhibitor of mast cell activation and degranulation, reduces diet-induced obesity and diabetes in mice [38].
⧉ Mast cells and angiogenesis
Mast cells produce several pro-angiogenic factors, including fibroblast growth factor-2 (FGF-2), VEGF, IL-8, TNF-α, transforming growth factor-beta (TGF-β), and nerve growth factor (NGF) [47,48,49,50,51,52,53,54,55,56]. As shown by in vivo and in vitro experiments, mast cells migrate in response to VEGF and PlGF-1 [57,58,59]. Granulated murine mast cells and their granules are able to stimulate an intense angiogenic reaction in the CAM assay, inhibited by anti-FGF-2 and -VEGF antibodies [60]. Intraperitoneal injection of the degranulating compound 48/80 stimulates angiogenesis in the rat and mouse mesentery window angiogenic assay [61,62]. Histamine and heparin induce the proliferation of endothelial cells in vitro and in vivo [63,64]. Tryptase, stored in mast cell secretory granules [65], stimulates the proliferation of endothelial cells, promotes vascular tube formation in vitro, degrades connective tissue matrix, and activates MMPs and plasminogen activator (PA), which in turn degrade the extracellular matrix with consequent release of VEGF or FGF-2 [66]. Mast cell-deficient W/Wv mice exhibit a decreased rate of tumor angiogenesis [67]. In human papillomavirus 16 (HPV16)-infected transgenic mouse model of epithelial carcinogenesis, mast cells infiltrated hyperplasia, dysplasias, and the invasive front of carcinomas, but not the core of tumors. Accumulation occurred proximal to developing capillaries and the stroma surrounding the advancing tumor mass [68]. Mast cells infiltrate and MMP-9 activation coincided with the angiogenic switch in premalignant lesions, and premalignant angiogenesis was abrogated in a mast cell-deficient HPV16 transgenic mouse [68,69]. In prostate tumors derived from both transgenic adenocarcinoma of the mouse prostate (TRAMP) mice and human patients, mast cells promote well-differentiated adenocarcinoma growth [70]. Mast cell infiltration around gastric cancer cells correlated with tumor angiogenesis and metastasis [71].
A high number of mast cells have been demonstrated in tumor angiogenesis, like hemangioma and hemangioblastoma [72], as well as several hematological and solid tumors, including lymphomas [73,74], multiple myeloma [75], myelodysplastic syndrome [76], B-cell chronic lymphocytic leukemia [77,78], breast cancer [79,80], squamous cell carcinoma of the esophagus [81], colon-rectal cancer [82], uterine cervix cancer [83,84,85], melanoma [86,87], pulmonary adenocarcinoma [88,89,90,91].
⧉ Cross-talk between adipocytes and mast cells in angiogenesis
Both adipocytes and mast cells are closely related to capillaries and secrete cytokines and growth factors involved in angiogenesis. Among them, VEGF and TNF-α are expressed by both cells, while other ones are expressed or by adipocytes or by mast cells. In this context, the angiogenic effect might be amplified when the adipocytes and mast cells act in concert. In fact, a close relationship exists between adipocytes and mast cells. Different mediators released from mast cells, such as cytokines, chemokines, proteases, and prostaglandins, are involved in adipogenesis [36]. Mast cells express the receptors for both leptin and adiponectin, both released by adipocytes and acting on angiogenesis. Leptin induces angiogenesis, vascular fenestration, and vascular remodeling [17,18], while adiponectin negatively affects angiogenesis [19].
The microenvironment during the accumulation of adipose tissue resembles the tumor microenvironment during tumor vascularization. Human cancers, including breast cancer, prostate cancer, colorectal cancer, and pancreatic cancer are all originating from the adipose environment, and adipose vasculature predetermines the tumor microenvironment that supports tumor growth. Implantation of tumor cells in highly vascularized WAT and BAT tissues accelerates tumor growth, as it has been demonstrated in breast cancer, melanoma, and fibrosarcoma. Inoculation of tumor cells in the subcutaneous tissue, WAT and BAT resulted in markedly differential tumor growth rates and angiogenesis, which correlated with the degree of pre-existing vascularization in these tissues [92]. Metastatic cancers grow in a highly vascularized mesentery environment where the adipose tissue is a major component at an accelerated rate [93]. Peritoneal adipose tissue is a metastatic site for ovarian cancer [94,95].
In this context, the treatment of adipose tissue- and mast cell-associated cancers with anti-angiogenic drugs may represent an alternative or adjuvant strategy for the treatment of these tumors. Finally, mast cells thus appear to be new cellular actors of adipose tissue, inflammation, contributing to the complex paracrine interplay between the various immune cells that accumulate in adipose tissue in different pathological conditions, deserving further mechanistic evaluation to determine the potential causal role of mast cells in the physiopathology of these diseases.
Conflict of interest
The authors declare that they have no conflict of interests.
References
- 1.Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne) 2016;7:30–30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cinti S. Pink adipocytes. Trends Endocrinol Metab. 2018;29(9):651–666. doi: 10.1016/j.tem.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Wald D, Teucher B, Dinkel J, Kaaks R, Delorme S, Boeing H, Seidensaal K, Meinzer HP, Heimann T. Automatic quantification of subcutaneous and visceral adipose tissue from whole-body magnetic resonance images suitable for large cohort studies. J Magn Reson Imaging. 2012;36(6):1421–1434. doi: 10.1002/jmri.23775. [DOI] [PubMed] [Google Scholar]
- 4.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34(1):1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, Concha H, Hassan M, Rydén M, Frisén J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 6.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15(6):641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Pénicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109(5):656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 8.Napolitano L. The differentiation of white adipose cells. An electron microscope study. J Cell Biol. 1963;18(3):663–679. doi: 10.1083/jcb.18.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukowiecki L, Lupien J, Follea N, Paradis A, Richard D, LeBlanc J. Mechanism of enhanced lipolysis in adipose tissue of exercise-trained rats. Am J Physiol. 1980;239(6):E422–E429. doi: 10.1152/ajpendo.1980.239.6.E422. [DOI] [PubMed] [Google Scholar]
- 10.Cannon B, Jacobsson A, Rehnmark S, Nedergaard J. Signal transduction in brown adipose tissue recruitment: noradrenaline and beyond. Int J Obes Relat Metab Disord. 1996;20(Suppl 3):S36–S42. [PubMed] [Google Scholar]
- 11.Castellot JJ, Karnovsky MJ, Spiegelman BM. Differentiation-dependent stimulation of neovascularization and endothelial cell chemotaxis by 3T3 adipocytes. Proc Natl Acad Sci U S A. 1982;79(18):5597–5601. doi: 10.1073/pnas.79.18.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldsmith HS, Griffith AL, Kupferman A, Catsimpoolas N. Lipid angiogenic factor from omentum. JAMA. 1984;252(15):2034–2036. [PubMed] [Google Scholar]
- 13.Silverman KJ, Lund DP, Zetter BR, Lainey LL, Shahood JA, Freiman DG, Folkman J, Barger AC. Angiogenic activity of adipose tissue. Biochem Biophys Res Commun. 1988;153(1):347–352. doi: 10.1016/s0006-291x(88)81229-4. [DOI] [PubMed] [Google Scholar]
- 14.Tam J, Duda DG, Perentes JY, Quadri RS, Fukumura D, Jain RK. Blockade of VEGFR2 and not VEGFR1 can limit diet-induced fat tissue expansion: role of local versus bone marrow-derived endothelial cells. PLoS One. 2009;4(3):e4974–e4974. doi: 10.1371/journal.pone.0004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99(16):10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117(9):2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouloumié A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83(10):1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 18.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci U S A. 2001;98(11):6390–6395. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bråkenhielm E, Veitonmäki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101(8):2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang B, Fu X, Liang X, Deavila JM, Wang Z, Zhao L, Tian Q, Zhao J, Gomez NA, Trombetta SC, Zhu MJ, Du M. Retinoic acid induces white adipose tissue browning by increasing adipose vascularity and inducing beige adipogenesis of PDGFRα+ adipose progenitors. Cell Discov. 2017;3:17036–17036. doi: 10.1038/celldisc.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J, Kim M, Sun K, An YA, Gu X, Scherer PE. VEGF-A-expressing adipose tissue shows rapid beiging and enhanced survival after transplantation and confers IL-4-independent metabolic improvements. Diabetes. 2017;66(6):1479–1490. doi: 10.2337/db16-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93(9):e88–e97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouloumié A, Sengenès C, Portolan G, Galitzky J, Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes. 2001;50(9):2080–2086. doi: 10.2337/diabetes.50.9.2080. [DOI] [PubMed] [Google Scholar]
- 24.Bell LN, Ward JL, Degawa-Yamauchi M, Bovenkerk JE, Jones R, Cacucci BM, Gupta CE, Sheridan C, Sheridan K, Shankar SS, Steinberg HO, March KL, Considine RV. Adipose tissue production of hepatocyte growth factor contributes to elevated serum HGF in obesity. Am J Physiol Endocrinol Metab. 2006;291(4):E843–E848. doi: 10.1152/ajpendo.00174.2006. [DOI] [PubMed] [Google Scholar]
- 25.Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Saito Y. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia. 2003;46(11):1483–1488. doi: 10.1007/s00125-003-1221-6. [DOI] [PubMed] [Google Scholar]
- 26.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 2005;29(11):1308–1314. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 27.Pellegrinelli V, Carobbio S, Vidal-Puig A. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia. 2016;59(6):1075–1088. doi: 10.1007/s00125-016-3933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagenmakers AJM, Strauss JA, Shepherd SO, Keske MA, Cocks M. Increased muscle blood supply and transendothelial nutrient and insulin transport induced by food intake and exercise: effect of obesity and ageing. J Physiol. 2016;594(8):2207–2222. doi: 10.1113/jphysiol.2014.284513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bråkenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, Cao Y. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res. 2004;94(12):1579–1588. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- 30.Neels JG, Thinnes T, Loskutoff DJ. Angiogenesis in an in vivo model of adipose tissue development. FASEB J. 2004;18(9):983–985. doi: 10.1096/fj.03-1101fje. [DOI] [PubMed] [Google Scholar]
- 31.Kim YM, An JJ, Jin YJ, Rhee Y, Cha BS, Lee HC, Lim SK. Assessment of the anti-obesity effects of the TNP-470 analog, CKD-732. J Mol Endocrinol. 2007;38(4):455–465. doi: 10.1677/jme.1.02165. [DOI] [PubMed] [Google Scholar]
- 32.Christiaens V, Lijnen HR. Role of the fibrinolytic and matrix metalloproteinase systems in development of adipose tissue. Arch Physiol Biochem. 2006;112(4-5):254–259. doi: 10.1080/13813450601093567. [DOI] [PubMed] [Google Scholar]
- 33.Lijnen HR, Demeulemeester D, Van Hoef B, Collen D, Maquoi E. Deficiency of tissue inhibitor of matrix metallo-proteinase-1 (TIMP-1) impairs nutritionally induced obesity in mice. Thromb Haemost. 2003;89(2):249–255. [PubMed] [Google Scholar]
- 34.Hellstrom B, Holmgren H. Numerical distribution of mast cells in the human skin and heart. Acta Anat (Basel) 1950;10(1–2):81–107. doi: 10.1159/000140456. [DOI] [PubMed] [Google Scholar]
- 35.Asboe-Hansen G. The mast cell. Cortisone action on connective tissue. Proc Soc Exp Biol Med. 1952;80(4):677–679. doi: 10.3181/00379727-80-19729. [DOI] [PubMed] [Google Scholar]
- 36.Shi MA, Shi GP. Different roles of mast cells in obesity and diabetes: lessons from experimental animals and humans. Front Immunol. 2012;3:7–7. doi: 10.3389/fimmu.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elieh Ali Komi D, Shafaghat F, Christian M. Crosstalk between mast cells and adipocytes in physiologic and pathologic conditions. Clin Rev Allergy Immunol. 2020;58(3):388–400. doi: 10.1007/s12016-020-08785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15(8):940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Żelechowska P, Agier J, Różalska S, Wiktorska M, Brzezińska-Błaszczyk E. Leptin stimulates tissue rat mast cell proinflammatory activity and migratory response. Inflamm Res. 2018;67(9):789–799. doi: 10.1007/s00011-018-1171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Żelechowska P, Wiktorska M, Różalska S, Stasikowska-Kanicka O, Wągrowska-Danilewicz M, Agier J, Brzezińska-Błaszczyk E. Leptin receptor is expressed by tissue mast cells. Immunol Res. 2018;66(5):557–566. doi: 10.1007/s12026-018-9029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milling S. Adipokines and the control of mast cell functions: from obesity to inflammation. Immunology. 2019;158(1):1–2. doi: 10.1111/imm.13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altintas MM, Azad A, Nayer B, Contreras G, Zaias J, Faul C, Reiser J, Nayer A. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J Lipid Res. 2011;52(3):480–488. doi: 10.1194/jlr.M011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishijima Y, Ohmori S, Ohneda K. Mast cell deficiency results in the accumulation of preadipocytes in adipose tissue in both obese and non-obese mice. FEBS Open Bio. 2013;4:18–24. doi: 10.1016/j.fob.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Divoux A, Moutel S, Poitou C, Lacasa D, Veyrie N, Aissat A, Arock M, Guerre-Millo M, Clément K. Mast cells in human adipose tissue: link with morbid obesity, inflammatory status, and diabetes. J Clin Endocrinol Metab. 2012;97(9):E1677–E1685. doi: 10.1210/jc.2012-1532. [DOI] [PubMed] [Google Scholar]
- 45.Finlin BS, Confides AL, Zhu B, Boulanger MC, Memetimin H, Taylor KW, Johnson ZR, Westgate PM, Dupont-Versteegden EE, Kern PA. Adipose tissue mast cells promote human adipose beiging in response to cold. Sci Rep. 2019;9(1):8658–8658. doi: 10.1038/s41598-019-45136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weller PF. Leukocyte lipid bodies – structure and function as "eicosasomes". Trans Am Clin Climatol Assoc. 2016;127:328–340. [PMC free article] [PubMed] [Google Scholar]
- 47.Abdel-Majid RM, Marshall JS. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J Immunol. 2004;172(2):1227–1236. doi: 10.4049/jimmunol.172.2.1227. [DOI] [PubMed] [Google Scholar]
- 48.Boesiger J, Tsai M, Maurer M, Yamaguchi M, Brown LF, Claffey KP, Dvorak HF, Galli SJ. Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of Fcε receptor I expression. J Exp Med. 1998;188(6):1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grützkau A, Krüger-Krasagakes S, Baumeister H, Schwarz C, Kögel H, Welker P, Lippert U, Henz BM, Möller A. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol Biol Cell. 1998;9(4):875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanbe N, Kurosawa M, Nagata H, Yamashita T, Kurimoto F, Miyachi Y. Production of fibrogenic cytokines by cord blood-derived cultured human mast cells. J Allergy Clin Immunol. 2000;106(1 Pt 2):S85–S90. doi: 10.1067/mai.2000.106777. [DOI] [PubMed] [Google Scholar]
- 51.Möller A, Lippert U, Lessmann D, Kolde G, Hamann K, Welker P, Schadendorf D, Rosenbach T, Luger T, Czarnetzki BM. Human mast cells produce IL-8. J Immunol. 1993;151(6):3261–3266. [PubMed] [Google Scholar]
- 52.Nilsson G, Forsberg-Nilsson K, Xiang Z, Hallböök F, Nilsson K, Metcalfe DD. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur J Immunol. 1997;27(9):2295–2301. doi: 10.1002/eji.1830270925. [DOI] [PubMed] [Google Scholar]
- 53.Qu Z, Huang X, Ahmadi P, Stenberg P, Liebler JM, Le AC, Planck SR, Rosenbaum JT. Synthesis of basic fibroblast growth factor by murine mast cells. Regulation by transforming growth factor beta, tumor necrosis factor alpha, and stem cell factor. Int Arch Allergy Immunol. 1998;115(1):47–54. doi: 10.1159/000023829. [DOI] [PubMed] [Google Scholar]
- 54.Qu Z, Kayton RJ, Ahmadi P, Liebler JM, Powers MR, Planck SR, Rosenbaum JT. Ultrastructural immunolocalization of basic fibroblast growth factor in mast cell secretory granules. Morphological evidence for bFGF release through degranulation. J Histochem Cytochem. 1998;46(10):1119–1128. doi: 10.1177/002215549804601004. [DOI] [PubMed] [Google Scholar]
- 55.Qu Z, Liebler JM, Powers MR, Galey T, Ahmadi P, Huang XN, Ansel JC, Butterfield JH, Planck SR, Rosenbaum JT. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol. 1995;147(3):564–573. [PMC free article] [PubMed] [Google Scholar]
- 56.Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991;88(10):4220–4224. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Detmar M, Brown LF, Schön MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;111(1):1–6. doi: 10.1046/j.1523-1747.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- 58.Detoraki A, Staiano RI, Granata F, Giannattasio G, Prevete N, de Paulis A, Ribatti D, Genovese A, Triggiani M, Marone G. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J Allergy Clin Immunol. 2009;123(5):1142–1149. doi: 10.1016/j.jaci.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 59.Gruber BL, Marchese MJ, Kew R. Angiogenic factors stimulate mast-cell migration. Blood. 1995;86(7):2488–2493. [PubMed] [Google Scholar]
- 60.Ribatti D, Crivellato E, Candussio L, Nico B, Vacca A, Roncali L, Dammacco F. Mast cells and their secretory granules are angiogenic in the chick embryo chorioallantoic membrane. Clin Exp Allergy. 2001;31(4):602–608. doi: 10.1046/j.1365-2222.2001.00986.x. [DOI] [PubMed] [Google Scholar]
- 61.Norrby K, Jakobsson A, Sörbo J. Mast-cell-mediated angiogenesis: a novel experimental model using the rat mesentery. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52(3):195–206. doi: 10.1007/BF02889963. [DOI] [PubMed] [Google Scholar]
- 62.Norrby K, Jakobsson A, Sörbo J. Mast-cell secretion and angiogenesis, a quantitative study in rats and mice. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57(4):251–256. doi: 10.1007/BF02899089. [DOI] [PubMed] [Google Scholar]
- 63.Ribatti D, Roncali L, Nico B, Bertossi M. Effects of exogenous heparin on the vasculogenesis of the chorioallantoic membrane. Acta Anat (Basel) 1987;130(3):257–263. doi: 10.1159/000146454. [DOI] [PubMed] [Google Scholar]
- 64.Sörbo J, Jakobsson A, Norrby K. Mast-cell histamine is angiogenic through receptors for histamine1 and histamine2. Int J Exp Pathol. 1994;75(1):43–50. [PMC free article] [PubMed] [Google Scholar]
- 65.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77(4):1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 66.Blair RJ, Meng H, Marchese MJ, Ren S, Schwartz LB, Tonnesen MG, Gruber BL. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997;99(11):2691–2700. doi: 10.1172/JCI119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Starkey JR, Crowle PK, Taubenberger S. Mast-cell-deficient W/Wv mice exhibit a decreased rate of tumor angiogenesis. Int J Cancer. 1988;42(1):48–52. doi: 10.1002/ijc.2910420110. [DOI] [PubMed] [Google Scholar]
- 68.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13(11):1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103(3):481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pittoni P, Tripodo C, Piconese S, Mauri G, Parenza M, Rigoni A, Sangaletti S, Colombo MP. Mast cell targeting hampers prostate adenocarcinoma development but promotes the occurrence of highly malignant neuroendocrine cancers. Cancer Res. 2011;71(18):5987–5997. doi: 10.1158/0008-5472.CAN-11-1637. [DOI] [PubMed] [Google Scholar]
- 71.Yano H, Kinuta M, Tateishi H, Nakano Y, Matsui S, Monden T, Okamura J, Sakai M, Okamoto S. Mast cell infiltration around gastric cancer cells correlates with tumor angiogenesis and metastasis. Gastric Cancer. 1999;2(1):26–32. doi: 10.1007/s101200050017. [DOI] [PubMed] [Google Scholar]
- 72.Glowacki J, Mulliken JB. Mast cells in hemangiomas and vascular malformations. Pediatrics. 1982;70(1):48–51. [PubMed] [Google Scholar]
- 73.Fukushima N, Satoh T, Sano M, Tokunaga O. Angiogenesis and mast cells in non-Hodgkin’s lymphoma: a strong correlation in angioimmunoblastic T-cell lymphoma. Leuk Lymphoma. 2001;42(4):709–720. doi: 10.3109/10428190109099333. [DOI] [PubMed] [Google Scholar]
- 74.Ribatti D, Nico B, Vacca A, Marzullo A, Calvi N, Roncali L, Dammacco F. Do mast cells help to induce angiogenesis in B-cell non-Hodgkin’s lymphomas. Br J Cancer. 1998;77(11):1900–1906. doi: 10.1038/bjc.1998.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ribatti D, Vacca A, Nico B, Quondamatteo F, Ria R, Minischetti M, Marzullo A, Herken R, Roncali L, Dammacco F. Bone marrow angiogenesis and mast cell density increase simultaneously with progression of human multiple myeloma. Br J Cancer. 1999;79(3–4):451–455. doi: 10.1038/sj.bjc.6690070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ribatti D, Polimeno G, Vacca A, Marzullo A, Crivellato E, Nico B, Lucarelli G, Dammacco F. Correlation of bone marrow angiogenesis and mast cells with tryptase activity in myelodysplastic syndromes. Leukemia. 2002;16(9):1680–1684. doi: 10.1038/sj.leu.2402586. [DOI] [PubMed] [Google Scholar]
- 77.Molica S, Vacca A, Crivellato E, Cuneo A, Ribatti D. Tryptase-positive mast cells predict clinical outcome of patients with early B-cell chronic lymphocytic leukemia. Eur J Haematol. 2003;71(2):137–139. doi: 10.1034/j.1600-0609.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- 78.Ribatti D, Molica S, Vacca A, Nico B, Crivellato E, Roccaro AM, Dammacco F. Tryptase-positive mast cells correlate positively with bone marrow angiogenesis in B-cell chronic lymphocytic leukemia. Leukemia. 2003;17(7):1428–1430. doi: 10.1038/sj.leu.2402970. [DOI] [PubMed] [Google Scholar]
- 79.Bowrey PF, King J, Magarey C, Schwartz P, Marr P, Bolton E, Morris DL. Histamine, mast cells and tumour cell proliferation in breast cancer: does preoperative cimetidine administration have an effect. Br J Cancer. 2000;82(1):167–170. doi: 10.1054/bjoc.1999.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hartveit F. Mast cells and metachromasia in human breast cancer: their occurrence, significance and consequence: a preliminary report. J Pathol. 1981;134(1):7–11. doi: 10.1002/path.1711340103. [DOI] [PubMed] [Google Scholar]
- 81.Elpek GO, Gelen T, Aksoy NH, Erdoğan A, Dertsiz L, Demircan A, Keleş N. The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagus. J Clin Pathol. 2001;54(12):940–944. doi: 10.1136/jcp.54.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lachter J, Stein M, Lichtig C, Eidelman S, Munichor M. Mast cells in colorectal neoplasias and premalignant disorders. Dis Colon Rectum. 1995;38(3):290–293. doi: 10.1007/BF02055605. [DOI] [PubMed] [Google Scholar]
- 83.Graham RM, Graham JB. Mast cells and cancer of the cervix. Surg Gynecol Obstet. 1966;123(1):3–9. [PubMed] [Google Scholar]
- 84.Ribatti D, Finato N, Crivellato E, Marzullo A, Mangieri D, Nico B, Vacca A, Beltrami CA. Neovascularization and mast cells with tryptase activity increase simultaneously with pathologic progression in human endometrial cancer. Am J Obstet Gynecol. 2005;193(6):1961–1965. doi: 10.1016/j.ajog.2005.04.055. [DOI] [PubMed] [Google Scholar]
- 85.Benítez-Bribiesca L, Wong A, Utrera D, Castellanos E. The role of mast cell tryptase in neoangiogenesis of premalignant and malignant lesions of the uterine cervix. J Histochem Cytochem. 2001;49(8):1061–1062. doi: 10.1177/002215540104900816. [DOI] [PubMed] [Google Scholar]
- 86.Reed JA, McNutt NS, Bogdany JK, Albino AP. Expression of the mast cell growth factor interleukin-3 in melanocytic lesions correlates with an increased number of mast cells in the perilesional stroma: implications for melanoma progression. J Cutan Pathol. 1996;23(6):495–505. doi: 10.1111/j.1600-0560.1996.tb01441.x. [DOI] [PubMed] [Google Scholar]
- 87.Tóth-Jakatics R, Jimi S, Takebayashi S, Kawamoto N. Cutaneous malignant melanoma: correlation between neovascularization and peritumor accumulation of mast cells overexpressing vascular endothelial growth factor. Hum Pathol. 2000;31(8):955–960. doi: 10.1053/hupa.2000.16658. [DOI] [PubMed] [Google Scholar]
- 88.Imada A, Shijubo N, Kojima H, Abe S. Mast cells correlate with angiogenesis and poor outcome in stage I lung adenocarcinoma. Eur Respir J. 2000;15(6):1087–1093. doi: 10.1034/j.1399-3003.2000.01517.x. [DOI] [PubMed] [Google Scholar]
- 89.Takanami I, Takeuchi K, Naruke M. Mast cell density is associated with angiogenesis and poor prognosis in pulmonary adenocarcinoma. Cancer. 2000;88(12):2686–2692. [PubMed] [Google Scholar]
- 90.Tomita M, Matsuzaki Y, Onitsuka T. Effect of mast cells on tumor angiogenesis in lung cancer. Ann Thorac Surg. 2000;69(6):1686–1690. doi: 10.1016/s0003-4975(00)01160-7. [DOI] [PubMed] [Google Scholar]
- 91.Ullah E, Nagi AH, Lail RA. Angiogenesis and mast cell density in invasive pulmonary adenocarcinoma. J Cancer Res Ther. 2012;8(4):537–541. doi: 10.4103/0973-1482.106530. [DOI] [PubMed] [Google Scholar]
- 92.Lim S, Hosaka K, Nakamura M, Cao Y. Co-option of pre-existing vascular beds in adipose tissue controls tumor growth rates and angiogenesis. Oncotarget. 2016;7(25):38282–38291. doi: 10.18632/oncotarget.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen C, Parangi S, Tolentino MJ, Folkman J. A strategy to discover circulating angiogenesis inhibitors generated by human tumors. Cancer Res. 1995;55(19):4230–4233. [PubMed] [Google Scholar]
- 94.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177(3):1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Y, Metzinger MN, Lewellen KA, Cripps SN, Carey KD, Harper EI, Shi Z, Tarwater L, Grisoli A, Lee E, Slusarz A, Yang J, Loughran EA, Conley K, Johnson JJ, Klymenko Y, Bruney L, Liang Z, Dovichi NJ, Cheatham B, Leevy WM, Stack MS. Obesity contributes to ovarian cancer metastatic success through increased lipogenesis, enhanced vascularity, and decreased infiltration of M1 macrophages. Cancer Res. 2015;75(23):5046–5057. doi: 10.1158/0008-5472.CAN-15-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]


