Abstract
Introduction
Previous studies have revealed that individuals with low birth weight (LBW) have higher risk of chronic kidney disease (CKD) and that LBW and CKD cluster in families. This study investigates how familial factors affect the association between birth-related risk markers and risk of CKD.
Methods
The Medical Birth Registry (MBR) of Norway has registered all births in Norway since 1967. Sibling data were available through the Norwegian Population Registry. The Norwegian Patient Registry has registered diagnostic codes for all admissions and outpatient visits to Norwegian hospitals since 2008. Data from these registries were linked. Risk of CKD according to whether the individual himself or at least one of his siblings had LBW was analyzed using logistic regression statistics.
Results
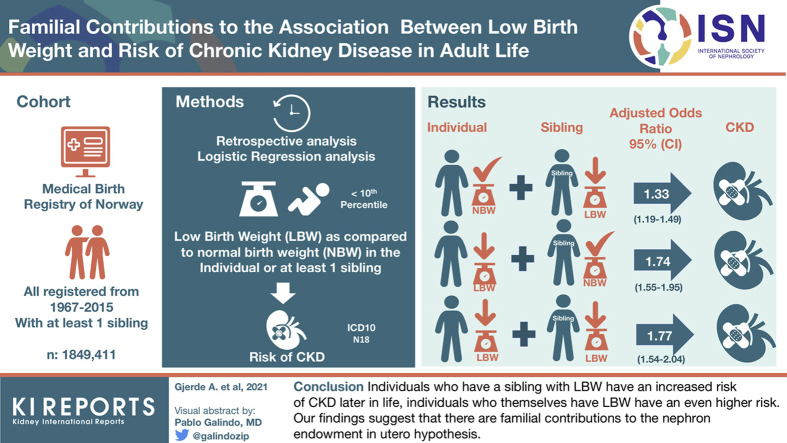
Of 1,847,565 individuals, 3336 had been diagnosed with CKD. Compared with individuals without LBW and no siblings with LBW, individuals without LBW but who had a sibling with LBW had adjusted odds ratio (aOR) of 1.33 (1.19–1.49), those with LBW but no siblings with LBW had aOR of 1.74 (1.55–1.95), and those with LBW and a sibling with LBW had aOR of 1.77 (1.54–2.04) for CKD. Similar results were found for LBW for gestational age, but preterm birth revealed weaker associations.
Conclusion
Individuals who have a sibling with LBW have an increased risk of CKD later in life, and individuals who themselves have LBW have an even higher risk. Our findings suggest that there are familial contributions to the nephron endowment in utero hypothesis.
Keywords: chronic kidney disease (CKD), genetic factors, intrauterine growth restriction, low birth weight (LBW), sibling, small for gestational age (SGA)
Graphical abstract
Several hypotheses have been proposed during the past decades suggesting that birth weight is inversely associated with risk of cardiovascular and other metabolic diseases in adult life through mechanisms of malnutrition in utero,1 unmeasured socioeconomic confounding,2 and genetic or other intergenerational factors influencing both birth weight and adult disease risk.3 Several studies have also revealed that both maternal and paternal genetic factors are associated with offspring birth weight.4, 5, 6 At the same time, there is a strong association between family history of end-stage kidney disease and increased risk of end-stage kidney disease.10, 11, 7, 8, 9 Despite a large number of studies during the past decades, the environmental versus familial contribution to the increased familial risk of kidney disease is still unclear.
The nephron endowment in utero hypothesis stated that as kidneys are developed in utero, risk of kidney disease in later life could be partially determined at birth.12 Several studies have supported this, and individuals with LBW seem to have a 70% to 80% increased risk of kidney disease in adult life.13,14 To investigate whether this association was confounded by familial factors, our research group previously published a study that investigated whether having a sibling with LBW was associated with an increased risk of end-stage kidney disease; the study revealed that only individuals who themselves had LBW had an increased risk and not individuals who had a sibling with LBW.15 No studies have however investigated the possible confounding role of familial factors in the association between birth-related risk markers and later CKD in general.
The MBR of Norway has registered data on all births in Norway since 1967, and sibling data have been registered in the Norwegian Population Registry. All patients who had been diagnosed with kidney disease during admissions or outpatient visits to Norwegian hospitals during the period 2008 to 2016 had been registered in the NPR. We linked these registries and studied how risk of CKD was affected by whether the individual himself or at least one of his/her siblings had LBW or small for gestational age (SGA). We hypothesized that family factors might modify effects of adverse birth-related markers and risk of CKD.
Methods
The MBR of Norway has registered extensive medical data on all births in Norway since 1967. Maternal and paternal national identification numbers have been registered in the Norwegian Population Registry. The NPR has registered International Classification of Diseases Tenth Revision (ICD-10) diagnostic codes for all admissions and outpatient visits to Norwegian hospitals since 2008; in Norway, most nephrologists are hospital based, and the data are therefore almost complete for specialist care. ICD-10 codes were registered by the treating physicians. In this study, we obtained data from the NPR for the period 2008 to 2016. The Norwegian Populaton Registry had date of death for all participants who died, until the end of 2016. We linked these registries using the national identification number.
All individuals registered in the MBR between 1967 and 2015 who had at least 1 sibling registered in the MBR during the same period were selected (N = 2,016,267). Siblings were defined as individuals with the same mother and father. Individuals with no siblings and those with more than 7 siblings (N = 3789) were excluded to enable practical data handling and to avoid effect modification by very large number of siblings (with higher risks of at least 1 with LBW by chance). We also excluded individuals who had at least 1 sibling (including themselves) with multiple births (N = 69,751), those who died before age 1 year (N = 12,606), those who died before 2008 (N = 14,428), and those who had officially emigrated from Norway (N = 66,282). That left 1,849,411 individuals to be included in the analyses.
Birth-Related Variables
LBW was defined as birth weight less than the 10th percentile for gender (2970 g for men, 2880 g for women). From 1967 to 1998, gestational age was based on the last menstrual period and from 1999 onward on routine ultrasonographic examination in gestational weeks 17 to 20. For use in this study, gestational age was available for 95.6% of the participants and birth weight was available for 99.93% of the participants. On the basis of birth weight, gestational age, and gender, a z-score of birth weight for gestational age was calculated. We defined SGA as birth weight less than the 10th percentile for gestational age and gender (the 10% with the lowest z-score for each gender) (cutoff −1.30 for men and −1.28 for women). Preterm birth was defined as birth before 37 weeks of gestation. LBW, less than 2500 g, was also analyzed as an exposure variable.
In this study, pregestational maternal disease was defined as a diagnosis of maternal diabetes mellitus, kidney disease, rheumatic disease, or essential hypertension before pregnancy. Maternal marital status was dichotomized as either single (divorced or not living with partner) or not single (married or living with partner). Congenital malformations in the newborns were recorded as present if any malformation were observed before discharge from the hospital after birth; in the statistical analyses, a dichotomous variable was used.
Outcome Variables
The main outcome was defined as having been diagnosed with CKD (ICD-10 code N18) in at least one of the hospital contacts (admissions or outpatient visits). Both main and secondary diagnoses were included. The secondary outcomes were having been diagnosed with the following different groups of kidney disease: acute kidney disease (N17), glomerular disease (N00–N09), hereditary cystic renal disease (Q61), or kidney or urinary tract malformations (Q60, Q62–Q64).
The data file from NPR included ICD-10 codes for each hospital contact (admission or outpatient visit) with a kidney disease diagnosis (N01–N09, N17–N19, N25–N29, or Q60–Q64). Of the 1,849,411 individuals, 11,553 were registered with at least 1 episode of kidney disease (maximum 1370 episodes); 4464 had 1 episode, 1603 had 2 episodes, 925 had 3 episodes, 2475 had 4 to 9 episodes, 1146 had 10 to 19 episodes, and 940 had greater than or equal to 20 episodes. Patients were diagnosed with different combinations and sequences of ICD-10 codes, and for this study, we focused on whether or not a diagnosis or group of diagnoses had been recorded at least once.
Statistical Analysis
Data were analyzed in a cohort design with birth-related variables for the included individual and his/her sibling(s) as exposure variables and CKD and other kidney diagnoses as outcomes. Characteristics of different groups were compared using t tests for continuous variables and Pearson’s chi-square test for categorical variables. Risks were analyzed using logistic regression statistics. In adjusted analyses, gender, pregestational maternal disease, maternal marital status, and congenital malformations in the newborn were included in the logistic regression statistics and aORs were obtained. In separate analyses focusing on the associations in adulthood, only individuals born before 1990 were included.
In secondary analyses, we used Cox regression statistics to complement the logistic regression statistics. Exposure and outcome variables were the same as in the logistic regression analyses. Time until end point was calculated as number of months from birth to first occurrence of an exposure, and time until right censoring was calculated as number of months from birth to death or from birth until end of 2016. As we did not have data on outcomes until 2008, analyses were left truncated for the time period until 2008. Graphs of cumulative risk (hazard) were prepared using the Nelson–Aalen estimate of risk groups.
A two-tailed probability value of less than 0.05 was considered significant. Mean plus or minus SD is given for continuous variables, and estimate (95% confidence interval) is given for risk estimates. All analyses were performed using STATA version 15.1 (Stata Corp., College Station, TX).
Results
This study included a total of 1,849,411 individuals, of whom 1,006,859 individuals had 1 sibling, 633,746 had 2 siblings, and 208,806 had 3 or more. Mean number of siblings was 1.6 plus or minus 0.8, and mean number of years of follow-up was 28.4 plus or minus 12.7 years. Of the included individuals, 2413 were diagnosed with CKD, 2499 with acute kidney disease, 2666 with glomerulonephritis, 706 with hereditary cystic kidney disease, and 2065 individuals with kidney or urinary tract malformations.
Characteristics of included individuals for 4 subgroups based on whether the individuals or at least one of his or her siblings had LBW are found in Table 1. As compared with individuals who neither had LBW themselves or a sibling with LBW, adverse birth-related risk markers were as expected more common for individuals who themselves had LBW, but were also more common for individuals who had a sibling with LBW.
Table 1.
Characteristics of included subjects and maternal health according to whether the subject or at least 1 sibling had LBW. Norway, 1967 to 2016
| Characteristic | Neither individual nor sibling with LBW | Individual without LBW, sibling with LBW | Individual LBW, sibling not LBW | Individual and sibling with LBW |
|---|---|---|---|---|
| N total | 1,483,071 | 181,507 | 111,387 | 71,600 |
| Mean number of siblings | 1.6 ± 0.8 | 1.9 ± 1.0a | 1.5 ± 0.7a | 1.7 ± 0.9a |
| Mean number of years of follow-up | 28.4 ± 12.4 | 29.0 ± 12.2a | 29.2 ± 12.6a | 29.2 ± 12.4a |
| Proportion with preterm birth | 1.5% | 2.6%a | 28.0%a | 28.1%a |
| Proportion with SGA | 3.5% | 9.4%a | 60%a | 65%a |
| Proportion with maternal preeclampsia | 2.1% | 2.7%a | 8.5%a | 8.1%a |
| Proportion with 5-min Apgar score <7 | 0.7% | 0.6% | 2.6%a | 2.4%a |
| Proportion with maternal diseaseb | 2.2% | 2.5%a | 2.7%a | 3.4%a |
| Form of kidney disease | ||||
| N with chronic kidney disease | 2413 | 388a | 326a | 209a |
| N with acute kidney disease | 2499 | 368a | 275a | 185a |
| N with glomerulonephritis | 2666 | 375c | 245c | 177a |
| N with hereditary cystic kidney disease | 706 | 109c | 70c | 49c |
| N with kidney or urinary tract malformations | 2065 | 23 | 193b | 152a |
LBW, low birth weight; SGA, small for gestational age.
P < 0.001.
Maternal disease was defined as maternal diabetes mellitus, kidney disease, rheumatic disease, or essential hypertension diagnosed before pregnancy.
P < 0.01 as compared with the group with neither individual nor sibling with LBW.
Risk of CKD
Compared with individuals who did not have siblings with LBW, having at least 1 sibling with LBW was associated with an OR of 1.38 (1.25–1.50) for development of CKD. Similar associations were observed for SGA and preterm birth with ORs of 1.36 (1.24–50) and 1.35 (1.25–1.46) respectively. As stated previously, adverse birth-related risk markers were more common in individuals who had a sibling with LBW, and this could confound the analyses. In further analyses, we therefore analyzed the combined effects.
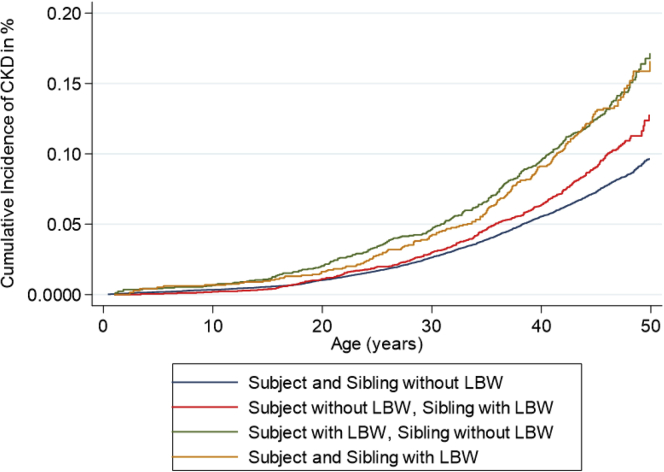
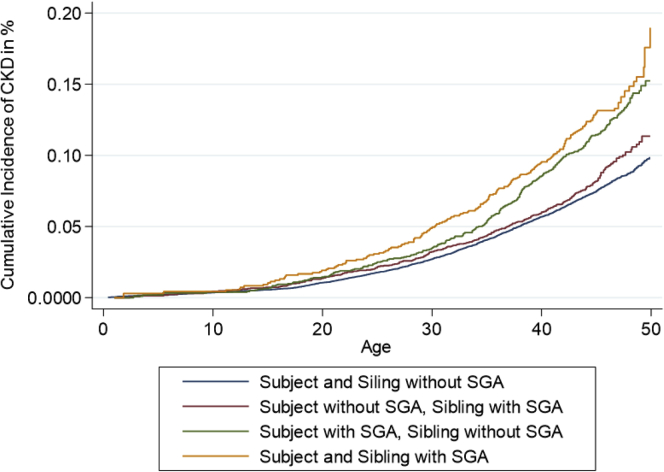
Compared with individuals without LBW and with no siblings with LBW, individuals without LBW but a sibling with LBW had an OR of 1.31 (1.18–1.46), individuals with LBW but no siblings with LBW had an OR of 1.80 (1.60–2.02), and individuals with LBW and a sibling with LBW had an OR of 1.80 (1.56–2.08) (Table 2 and Figure 1). After adjustments for sex, maternal disease, and number of recorded siblings, results were almost identical. The analyses were repeated for SGA, preterm birth, and having at least 1 of the 3 risk markers. SGA analyses revealed almost identical results, whereas preterm birth seemed to have a less pronounced association (Table 2 and Figure 2). Being born with LBW, SGA, or before 37 weeks of gestation was associated with higher risks than having a sibling with one of these risk markers, but the latter was consistently associated with ORs of 1.2 to 1.3. Unexpectedly, no considerable risk increase was observed when both the individual himself and his sibling had the risk factor. In a separate analysis in which a cutoff for birth weight of 2.5 kg was used to define LBW, we found that as compared with individuals without LBW and with no siblings with LBW, individuals without LBW but a sibling with LBW had an OR of 1.23 (1.07–1.40), those with LBW but no siblings with LBW had an OR of 2.08 (1.77–2.46), and those with LBW and a sibling with LBW had an OR of 1.32 (0.90–1.93) (notably, only 27 individuals developed CKD in this latter group). After adjustments, almost identical results were found (Table 2). These analyses were repeated separately for men and women. The results revealed that having a sibling with LBW or SGA tended to be associated with higher risks for men than women (Supplementary Table S1).
Table 2.
Risk of CKD according to whether the individual or at least 1 of his/her siblings had LBW, SGA, or preterm birth. Norway, 1967 to 2016
| Subject | Sibling | N (total) | n (CKD) | Unadjusted model |
Adjusted modela |
||
|---|---|---|---|---|---|---|---|
| OR | P value | OR | P value | ||||
| Not LBW | Not LBW | 1,480,658 | 2413 | 1.0 (ref) | 1.0 (ref) | ||
| LBW | 181,119 | 388 | 1.31 (1.18–1.46) | <0.001 | 1.33 (1.19–1.49) | <0.001 | |
| LBW | Not LBW | 111,061 | 326 | 1.80 (1.60–2.02) | <0.001 | 1.74 (1.55–1.95) | <0.001 |
| LBW | 71,391 | 209 | 1.80 (1.56–2.08) | <0.001 | 1.77 (1.54–2.04) | <0.001 | |
| Not SGA | Not SGA | 1,425,817 | 2337 | 1.0 (ref) | 1.0 (ref) | ||
| SGA | 160,734 | 323 | 1.23 (1.10–1.39) | <0.001 | 1.25 (1.11–1.41) | <0.001 | |
| SGA | Not SGA | 109,379 | 320 | 1.79 (1.59–2.00) | <0.001 | 1.74 (1.55–1.95) | <0.001 |
| SGA | 62,188 | 207 | 2.03 (1.76–2.34) | <0.001 | 2.03 (1.76–2.34) | <0.001 | |
| Not preterm birth | Not preterm birth | 1,665,238 | 2926 | 1.0 (ref) | 1.0 (ref) | ||
| Preterm birth | 102,736 | 219 | 1.21 (1.06–1.39) | 0.006 | 1.23 (1.07–1.41) | 0.003 | |
| Preterm birth | Not preterm birth | 60,597 | 161 | 1.51 (1.29–1.77) | <0.001 | 1.44 (1.27–1.69) | <0.001 |
| Preterm birth | 17,494 | 40 | 1.30 (0.95–1.78) | 0.098 | 1.24 (0.91–1.70) | 0.2 | |
| Not LBW 2.5 kg | Not LBW 2.5 kg | 1,684,907 | 2938 | 1.0 (ref) | 1.0 (ref) | ||
| LBW 2.5 kg | 108,507 | 232 | 1.23 (1.07–1.40) | 0.003 | 1.24 (1.08–1.42) | 0.002 | |
| LBW 2.5 kg | Not LBW 2.5 kg | 40,928 | 149 | 2.09 (1.77–2.46) | <0.001 | 2.08 (1.80–2.45) | <0.001 |
| LBW 2.5 kg | 11,723 | 27 | 1.32 (0.90–1.93) | 0.151 | 1.30 (0.90–1.91) | 0.166 | |
| Not LBW, SGA, or preterm birth | Not LBW, SGA, or preterm birth | 1,319,347 | 2088 | 1.0 (ref) | 1.0 (ref) | ||
| LBW, SGA, or preterm birth | 252,227 | 492 | 1.23 (1.17–1.36) | <0.001 | 1.20 (1.08–1.33) | 0.001 | |
| LBW, SGA, or preterm birth | Not LBW, SGA, or preterm birth | 154,815 | 407 | 1.66 (1.50–1.85) | <0.001 | 1.50 (1.50–1.88) | <0.001 |
| LBW, SGA, or preterm birth | 119,676 | 359 | 1.90 (1.70–2.12) | <0.001 | 1.70 (1.50–1.90) | <0.001 | |
CKD, chronic kidney disease; LBW, low birth weight; OR, odds ratio; ref, reference; SGA, small for gestational age.
Adjusted for gender, maternal disease (defined as maternal diabetes mellitus, kidney disease, rheumatic disease, or essential hypertension diagnosed before pregnancy), and number of recorded siblings categorized as 1, 2, or greater than or equal to 3.
Figure 1.
Cumulative risk of CKD according to whether the individual or at least one of his or her siblings had LBW. Risk of CKD was increased both for individuals who had LBW themselves and for those who themselves did not have LBW but who had a sibling with LBW. The graphs separate most strongly in adult age. CKD, chronic kidney disease; LBW, low birth weight.
Figure 2.
Cumulative risk of CKD according to whether the individual or at least one of his or her siblings was born SGA. Risk of CKD was increased both for individuals who had SGA themselves and for individuals who themselves did not have SGA but who had a sibling with LBW. The graphs separate most strongly in adult age. CKD, chronic kidney disease; LBW, low birth weight; SGA, small for gestational age.
We compared the unadjusted results in Table 2 during the first 40 years of life with those from the previous study by Ruggajo et al.15 of risk of ESKD. These analyses revealed much similar results, but there was a trend toward higher risk estimates in this study of CKD (Supplementary Table S2). The analyses from Table 2 were also repeated using Cox regression statistics, revealing nearly identical results (results not found). As hereditary or congenital kidney disease could cause LBW or SGA, we performed a separate analysis excluding all individuals who on at least 1 occasion had been diagnosed with hereditary cystic disease or congenital kidney disease, revealing nearly identical results.
In the analyses in Table 3, we analyzed whether OR of CKD increased with higher number of birth-related risk markers in the included individuals (LBW, SGA, or preterm birth). Analyses were stratified based on whether at least one of the siblings had at least one of the risk markers. As can be found, individuals who had a higher number of risk markers had higher risks. This was evident in both individuals who had siblings without risk markers and those who had siblings with risk markers. Individuals who had a sibling with LBW, SGA, or preterm birth, and who themselves had 3 risk factors, had the highest risk with an aOR of 2.6 (1.8–3.9). Individuals with 0 risk markers but who had a sibling with LBW, SGA, or preterm birth had a statistically significantly increased risk with aOR of 1.3 (1.1–1.4). Similar findings were found for individuals born before 1990 and thus revealed to be the same in the adult population. Similar results were found using Cox regression statistics (results not found).
Table 3.
Risk of CKD according to number of risk markers in the individuals (LBW, SGA, or preterm birth) and siblings
| Siblings | No. of risk markers | Total, N | Unadjusted model |
Adjusted modela |
Cohort born before 1990 Adjusted modela |
||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | aOR (95% CI) | P value | Total, N | aOR (95% CI) | P value | |||
| No sibling with LBW, SGA, or preterm birth | 0 | 1,257,320 | 1.0 (ref) | 1.0 (ref) | 628,773 | 1.0 (ref) | |||
| 1 | 74,756 | 1.35 (1.15–1.60) | <0.001 | 1.31 (1.14–1.54) | <0.001 | 42,229 | 1.21 (1.02–1.45) | 0.029 | |
| 2 | 71,081 | 1.90 (1.63–2.20) | <0.001 | 1.81 (1.56–2.10) | <0.001 | 38,154 | 1.70 (1.45–2.00) | <0.001 | |
| 3 | 4176 | 2.59 (1.60–4.20) | <0.001 | 2.41(1.50–4.00) | 0.001 | 1765 | 3.00 (1.72–4.94) | <0.001 | |
| At least 1 sibling with LBW, SGA, or preterm birth | 0 | 237,385 | 1.24 (1.17–1.38) | <0.001 | 1.26 (1.14–1.40) | <0.001 | 129,396 | 1.18 (1.05–1.32) | <0.003 |
| 1 | 41,761 | 1.83 (1.52–2.20) | <0.001 | 1.81 (1.50–2.16) | <0.001 | 24,427 | 1.60 (1.31–1.96) | <0.001 | |
| 2 | 67,277 | 1.92 (1.66–2.23) | <0.001 | 1.90 (1.64–2.20) | <0.001 | 38,228 | 1.82 (1.56–2.12) | <0.001 | |
| 3 | 5703 | 2.80 (1.90–4.14) | <0.001 | 2.62 (1.76–3.90) | <0.001 | 2600 | 2.91 (1.87–4.52) | <0.001 | |
aOR, adjusted odds ratio; CI, confidence interval; CKD, chronic kidney disease; LBW, low birth weight; No., number; OR, odds ratio; ref, reference; SGA, small for gestational age.
Adjusted for gender, maternal disease (defined as maternal diabetes mellitus, kidney disease, rheumatic disease, or essential hypertension diagnosed before pregnancy), and number of recorded siblings categorized as 1, 2, or greater than or equal to 3.
Risk of Various Forms of Kidney Disease
In further analyses, we analyzed risk of various forms of kidney disease based on whether the individual or at least one of his/her siblings had LBW, SGA, or preterm birth. These analyses were performed on much the same data set as in our previous study, and age differences at first diagnosis and combinations of diagnoses were the same14; patients diagnosed with kidney or urinary tract malformations were diagnosed at a younger age and CKD was diagnosed in older patients. Importantly, patients could be included in several disease groups. Analyses revealed stronger associations for CKD than for the other forms of kidney disease (acute kidney disease, glomerulonephritis, hereditary cystic kidney disease, and kidney or urinary tract malformations) (Table 4).
Table 4.
Risk of various forms of kidney disease according to whether the individual or at least 1 of its siblings had adverse birth-related risk markers. Norway, 1967–2016
| Risk marker |
Sibling | Chronic kidney disease |
Acute kidney disease |
Glomerulonephritis |
Hereditary cystic kidney disease |
Kidney or urinary tract malformations |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | n | aOR (95% CI)a | n | aOR (95% CI)a | n | aOR (95% CI)a | n | aOR (95% CI)a | n | aOR (95% CI)a | |
| Not LBW | Not LBW | 2413 | 1.0 (ref) | 2499 | 1.0 (ref) | 2666 | 1.0 (ref) | 706 | 1.0 (ref) | 2065 | 1.0 (ref) |
| LBW | 388 | 1.33 (1.19–1.48) | 368 | 1.23 (1.10–1.36) | 375 | 1.16 (1.04–1.30) | 109 | 1.31 (1.06–1.60) | 236 | 0.96 (0.83–1.10) | |
| LBW | Not LBW | 326 | 1.75 (1.56–2.00) | 275 | 1.45 (1.28–1.64) | 245 | 1.21 (1.06–1.38) | 70 | 1.20 (0.99–1.60) | 193 | 1.10 (0.94–1.28) |
| LBW | 209 | 1.78 (1.54–2.05) | 185 | 1.54 (1.33–1.80) | 177 | 1.37 (1.18–1.60) | 49 | 1.40 (1.04–1.81) | 152 | 1.43 (1.21–1.70) | |
| Not SGA | Not SGA | 2337 | 1.0 (ref) | 2390 | 1.0 (ref) | 2568 | 1.0 (ref) | 715 | 1.0 (ref) | 2107 | 1.0 (ref) |
| SGA | 323 | 1.25 (1.11–1.40) | 302 | 1.13 (1.00–1.29) | 337 | 1.16 (1.04–1.31) | 79 | 1.02 (0.82–1.30) | 221 | 0.96 (0.77–1.10) | |
| SGA | Not SGA | 320 | 1.74 (1.55–1.96) | 316 | 1.71 (1.52–1.92) | 236 | 1.20 (1.04–1.36) | 66 | 1.13 (0.88–1.46) | 156 | 0.91 (0.78–1.08) |
| SGA | 207 | 2.03 (1.76–2.34) | 164 | 1.60 (1.37–1.88) | 160 | 1.42 (1.21–1.67) | 47 | 1.52 (1.13–2.04) | 111 | 1.21 (1.00–1.47) | |
| Not preterm | Not preterm | 2926 | 1.0 (ref) | 2956 | 1.0 (ref) | 3082 | 1.0 (ref) | 823 | 1.0 (ref) | 2352 | 1.0 (ref) |
| Preterm | 219 | 1.23 (1.07–1.41) | 210 | 1.18 (1.02–1.35) | 218 | 1.15 (1.0–1.32) | 47 | 0.92 (0.70–1.24) | 154 | 1.07 (0.90–1.26) | |
| Preterm | Not preterm | 161 | 1.44 (1.23–1.70) | 124 | 1.12 (0.67–1.05) | 126 | 1.11 (0.93–1.33) | 43 | 1.22 (0.90–1.70) | 119 | 1.20 (1.0–1.44) |
| Preterm | 40 | 1.24 (0.91–1.70) | 46 | 1.47 (1.10–1.97) | 40 | 1.22 (0.90–1.70) | 21 | 1.91 (1.22–3.0) | 30 | 1.01 (0.70–1.45) | |
aOR, adjusted odds ratio; CI, confidence interval; LBW, low birth weight; ref, reference; SGA, small for gestational age.
Adjusted for gender, maternal disease (defined as maternal diabetes mellitus, kidney disease, rheumatic disease, or essential hypertension diagnosed before pregnancy), and number of recorded siblings categorized as 1, 2, or greater than or equal to 3.
Discussion
This study confirms previous studies revealing LBW and SGA to be associated with a 70% to 80% higher risk of CKD in adult age. This study is to the best of our knowledge the first to show that siblings of individuals with LBW, SGA, or preterm birth also have a statistically significant 20% to 30% higher risk of CKD. Interestingly, if the individual him/herself had LBW, SGA, or preterm birth, the risk did not increase further if a sibling also had one of these risk markers. Of note, the higher risk in siblings was statistically significant also for other forms of kidney disease, such as acute kidney disease, glomerulonephritis, and hereditary kidney disease. Most previous studies have investigated risk of end-stage kidney disease, which is a rare end point; our study reveals an increased risk for most stages of CKD.
In the 1980s, Barker et al.1 revealed that birth weight was inversely associated with risk of cardiovascular and other metabolic diseases in adult life. The associations between size at birth and disease in adulthood have been explained by alterations in fetal nutrition and endocrine status caused by intrauterine malnutrition, resulting in a permanent change in the structure, physiology, and metabolism of affected individuals, predisposing them to disease in adulthood.16 Nevertheless, LBW may also be explained by the genes of the fetus and maternal genes affecting the quality of the intrauterine environment.4 A study from the MBR of Norway suggested that both maternal and fetal genetic factors may influence the duration of pregnancy.5 At the same time, gestational age and birth weight may also be explained by environmental factors that often are shared among relatives, such as smoking, diet, and socioeconomic status. A study by Lunde et al.4 investigated the genetic contributions to birth weight and found that approximately 50% of the variation in birth weight could be explained by genetic factors and that 9% to 15% of the variation could be explained by shared environmental factors. As kidney disease also has clear genetic contributions,7, 11,17 it has therefore been suggested that the same genetic factors could explain both LBW and risk of kidney disease. This study has revealed that individuals with LBW have a significantly higher risk than those who have a sibling with LBW, thus strengthening the argument that intrauterine programming itself is an important risk marker for adult kidney disease.
As discussed previously, an alternate explanation could be that much of the increased risk in individuals with LBW is because of genetic factors and shared environmental factors, and not because of intrauterine nephron endowment. Twin studies can provide an opportunity to test some of these hypotheses as twins share their maternal and early family environment factors and some or all of their genes.18,19 These have however revealed different results, and whereas a meta-analysis from 2004 concluded that there was an important contribution from shared genetic and environmental factors,18 others have revealed that the actual birth weight itself and fetoplacental factors seem to be more important.19,20 In our study, we revealed a contribution from familial factors but cannot discern whether this is because of genetic or shared environmental factors. It is however important to note that the 20% to 30% higher risk for individuals who had a sibling with LBW or SGA is much smaller than the 70% to 80% increased risk that was found if the individual himself had LBW or SGA. In line with previous studies of heredity in kidney disease,10, 11, 9 the risk seems to be increased for most types of kidney disease and not specific to particular diseases, although some diseases have clear mendelian inheritance. A study by Hoy et al.21 revealed that APOL1 risk allele status was strongly associated with age-related nephron loss in African Americans. Other genetic factors may have similar effects in other populations.
It has been discussed earlier whether LBW, SGA, or preterm birth is the most important risk marker. This study confirms previous findings from our group that LBW and SGA seem to be equally important and that preterm birth seems to be a weaker risk marker.14,22 This contrasts other studies which have revealed that preterm birth is also an important risk marker.23,24
In this study, similar to our previous studies, we did not observe significant sex differences in the association between LBW and SGA and later kidney disease. It was however interesting to find that having a sibling with LBW was only statistically significantly associated with CKD for men and not for women. The same trend could be found also for SGA and preterm birth with weaker effects in women than men.
The major strengths of our study are the opportunity to use the national registries to include a large number of participants with sibling data and prospective registration of birth-related variables of high quality. The long follow-up period of 50 years and the fact that most kidney disease diagnoses are assessed and treated in hospitals and thus included in this study are also important. The study population is mostly Caucasian, which is both a strength as the population is quite homogenous and a weakness as our findings may not have similar strength for other ethnic groups. Another strength of our study is that the Norwegian health care system is founded on the principles of universal access and individuals with a low socioeconomic status have the same access and right as the rest of the population.
The main weakness is that we could not record end points until 2008. Our data thus reflect prevalence of CKD during the years 2008 to 2015. Given the wide age range of 0 to 50 years, we believe that our data also could reflect incidence of CKD. Based on these reflections, we decided to perform the main statistics as logistic regression statistics, but also performed left-truncated survival statistics to investigate the age-associated risk of CKD. These 2 approaches revealed mainly identical results. It is also possible that there was under-reporting of CKD in our study, as only diagnosed kidney disease that is relevant for the patient care is recorded, not all patients who fulfill the criteria for CKD according to reduced estimated glomerular filtration rate or albuminuria. The treating physicians decide which ICD-10 diagnostic codes to use, and although we believe that these mostly are correct, diagnostic codes of kidney disease have to the best of our knowledge not been validated in Norway. Other limitations include lack of data on other important risk factors, such as diabetes, hypertension, smoking, dyslipidemia, and other exposures of kidney disease. It would also be interesting to have data on birth weight of individual’s mothers and fathers, but these data were not available.
In conclusion, our findings strengthen the hypothesis that intrauterine environment is important for risk of kidney disease in adult life. There is however a significant contribution from familial factors that could be related to either genetic or environmental factor. Future studies should attempt to identify which genes or environmental factors could explain the association between intrauterine growth restriction, nephron number, and risk of kidney disease in adult life.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study is supported by grants from Helse-Fonna and the Western Norway Regional Health authority funds. These supporters played no part in the development or approval of the manuscript. The study protocol was approved by the regional ethics committee with approval number 2017/627.
Footnotes
Supplementary File (PDF)
Table S1. Sex-stratified analysis of risk of CKD according to whether the individual or at least one of his/her siblings had LBW, SGA, or preterm birth. Norway, 1967–2016.
Table S2. Risk for ESKD in Ruggajo et al.15 and risk of CKD during the first 40 years of age in this study according to whether included individual or at least 1 sibling had LBW, SGA, or preterm birth. Unadjusted analyses.
STROBE Statement (PDF).
Supplementary Material
Table S1. Sex-stratified analysis of risk of CKD according to whether the individual or at least one of his/her siblings had LBW, SGA, or preterm birth. Norway, 1967–2016.
Table S2. Risk for ESKD in Ruggajo et al.15 and risk of CKD during the first 40 years of age in this study according to whether included individual or at least 1 sibling had LBW, SGA, or preterm birth. Unadjusted analyses.
STROBE Statement
References
- 1.Barker D.J., Osmond C., Golding J., Kuh D., Wadsworth M.E. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer M.S., Joseph K.S. Enigma of fetal/infant-origins hypothesis. Lancet. 1996;348:1254–1255. doi: 10.1016/s0140-6736(05)65750-9. [DOI] [PubMed] [Google Scholar]
- 3.Smith G.D., Harding S., Rosato M. Relation between infants’ birth weight and mothers’ mortality: prospective observational study. BMJ. 2000;320:839–840. doi: 10.1136/bmj.320.7238.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lunde A., Melve K.K., Gjessing H.K., Skjaerven R., Irgens L.M. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. Am J Epidemiol. 2007;165:734–741. doi: 10.1093/aje/kwk107. [DOI] [PubMed] [Google Scholar]
- 5.Lie R.T., Wilcox A.J., Skjaerven R. Maternal and paternal influences on length of pregnancy. Obstet Gynecol. 2006;107:880–885. doi: 10.1097/01.AOG.0000206797.52832.36. [DOI] [PubMed] [Google Scholar]
- 6.Samuelsen S.O., Stene L.C., Bakketeig L.S. Association of head circumference at birth among sibling pairs. Paediatr Perinat Epidemiol. 2004;18:26–32. doi: 10.1111/j.1365-3016.2004.00532.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson R., Grim C.E., Opgenorth T.J. A familial risk of chronic renal failure among blacks on dialysis? J Clin Epidemiol. 1988;41:1189–1196. doi: 10.1016/0895-4356(88)90023-6. [DOI] [PubMed] [Google Scholar]
- 8.Freedman B.I., Spray B.J., Tuttle A.B., Buckalew V.M., Jr. The familial risk of end-stage renal disease in African Americans. Am J Kidney Dis. 1993;21:387–393. doi: 10.1016/s0272-6386(12)80266-6. [DOI] [PubMed] [Google Scholar]
- 9.Skrunes R., Svarstad E., Reisaeter A.V., Vikse B.E. Familial clustering of ESRD in the Norwegian population. Clin J Am Soc Nephrol. 2014;9:1692–1700. doi: 10.2215/CJN.01680214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei H.H., Perneger T.V., Klag M.J., Whelton P.K., Coresh J. Familial aggregation of renal disease in a population-based case-control study. J Am Soc Nephrol. 1998;9:1270–1276. doi: 10.1681/ASN.V971270. [DOI] [PubMed] [Google Scholar]
- 11.Satko S.G., Sedor J.R., Iyengar S.K., Freedman B.I. Familial clustering of chronic kidney disease. Semin Dial. 2007;20:229–236. doi: 10.1111/j.1525-139X.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- 12.Brenner B.M., Garcia D.L., Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 13.White S.L., Perkovic V., Cass A. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009;54:248–261. doi: 10.1053/j.ajkd.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 14.Gjerde A., Reisaeter A.V., Skrunes R., Marti H.P., Vikse B.E. Intrauterine growth restriction and risk of diverse forms of kidney disease during the first 50 years of life. Clin J Am Soc Nephrol. 2020;15:1413–1423. doi: 10.2215/CJN.04080320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruggajo P., Skrunes R., Svarstad E., Skjaerven R., Reisæther A.V., Vikse B.E. Familial factors, low birth weight, and development of ESRD: a nationwide registry study. Am J Kidney Dis. 2016;67:601–608. doi: 10.1053/j.ajkd.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Barker D.J., Eriksson J.G., Forsén T., Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 17.Cañadas-Garre M., Anderson K., Cappa R. Genetic susceptibility to chronic kidney disease - some more pieces for the heritability puzzle. Front Genet. 2019;10:453. doi: 10.3389/fgene.2019.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeill G., Tuya C., Smith W.C. The role of genetic and environmental factors in the association between birthweight and blood pressure: evidence from meta-analysis of twin studies. Int J Epidemiol. 2004;33:995–1001. doi: 10.1093/ije/dyh260. [DOI] [PubMed] [Google Scholar]
- 19.Gielen M., Pinto-Sietsma S.J., Zeegers M.P. Birth weight and creatinine clearance in young adult twins: influence of genetic, prenatal, and maternal factors. J Am Soc Nephrol. 2005;16:2471–2476. doi: 10.1681/ASN.2004030210. [DOI] [PubMed] [Google Scholar]
- 20.Bergvall N., Iliadou A., Johansson S. Genetic and shared environmental factors do not confound the association between birth weight and hypertension: a study among Swedish twins. Circulation. 2007;115:2931–2938. doi: 10.1161/CIRCULATIONAHA.106.674812. [DOI] [PubMed] [Google Scholar]
- 21.Hoy W.E., Hughson M.D., Kopp J.B. APOL1 risk alleles are associated with exaggerated age-related changes in glomerular number and volume in African-American adults: an autopsy study. J Am Soc Nephrol. 2015;26:3179–3189. doi: 10.1681/ASN.2014080768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gjerde A., Lillas B.S., Marti H.P. Intrauterine growth restriction, preterm birth and risk of end-stage renal disease during the first 50 years of life. Nephrol Dial Transplant. 2020;35:1157–1163. doi: 10.1093/ndt/gfaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svensson A.C., Sandin S., Cnattingius S. Maternal effects for preterm birth: a genetic epidemiologic study of 630,000 families. Am J Epidemiol. 2009;170:1365–1372. doi: 10.1093/aje/kwp328. [DOI] [PubMed] [Google Scholar]
- 24.Crump C., Sundquist J., Winkleby M.A., Sundquist K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ. 2019;365:l1346. doi: 10.1136/bmj.l1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.