Abstract
Background and aim:
To determine the efficacy of the synergistic use of High Power Laser Therapy (HPLT) with glucosamine sulfate (GS) in knee osteoarthritis.
Methods:
This 2-arm randomized controlled trial (RCT) enrolled 90 subjects (M=53, F=37, y= 55±11.2) and randomly allocated using a stratified sampling method in experimental group (A) with HPLT+GS 1500mg (GS - Dona®, Rottapharm, Monza, Italy) (n=45) or in a control group (B) with HPLT + placebo (n=45).
Results:
VAS score in Activities of day Living (ADL), Standardized stair climb test (SSCT), Zohlen’s sign (RASPING) and Rabot test were used, to evaluate patients at the beginning of the study (T0), at 2 months (T1) and at 6 months (T2). In the mean scores for VAS in ADL, SSCT, RABOT and RASPING at T1, no significant differences were found between the experimental and the control group with paired T and ANOVA test. But significant differences between groups (p<0.05) in all outcomes were observed at 6 months (T2).
Conclusions:
HPLT is useful in treating knee osteoarthritis, but when combined with Glucosamine Sulfate, thanks to the synergy of two interventions, can achieve a long-term effect up to 6 months after treatment. (www.actabiomedica.it)
Keywords: High Power Laser Therapy, glucosamine sulfate, knee osteoarthritis
Introduction
Knee osteoarthritis (OA) is typically defined by cartilage fissures and erosion, osteophytic genesis, and subchondral sclerosis (1). Defects of the articular cartilage, still today, represent an important clinical concern, particularly in young people, and are related to joint pain and disability (2). Osteoarthritis of the knee can be caused by mild and repeated trauma or noticeable injury (3). Mathematical modeling and clinical trials on animals confirm the shear forces as a critical component of the arthritic process (4). Chondrocyte turnover is essential to limit erosion of the matrix and stimulate cartilage regeneration, but shear stress can alter this process, damaging cartilage.
As a consequence of altered loads, the severity of the cartilage injury appears to be related to an expression of biological factors that influence matrix integrity and cellular metabolism (4). Glucosamine sulfate is one of the main components of the cartilage matrix and was used in the treatment of OA Glucosamine sulfate sodium chloride (Dona®, Viartril-S®, Arthryl®, Xicil®, Osaflexan®, Glusartel®, or other trademarks by Rottapharm, Italy) is also known as crystalline glucosamine (5). Glucosamine is a glycosaminoglycan component of the extracellular cartilage matrix. Oral administration of glucosamine is unlikely to be a key element in aggrecans or synovial fluid hyaluronate. Orally administered glucosamine has a direct cellular effect on reducing the production of inflammatory cytokines (6). Glucosamine inhibits the gene expression stimulated by the IL-1 of different markers of inflammation or degradation of the matrix in a cellular model similar to human chondrocytes, precisely at the concentrations of glucosamine detected in human plasma after the administration of oral therapeutic doses (7). Recently the use of high power laser therapy is widespread in physical medicine and develops continuously (8). By its high intensity power and a specific wavelength, it enables the treatment of different clinical conditions, p.e. OA (9). The proliferation of joint chondrocytes (photobiology effects) can be stimulated by laser irradiation (10,11). It has been shown that the average depth and width of the area to be treated are directly proportional to the laser power and exposure time, regardless of the irradiation method (12). Atik et al. stated that photobiomodulation is an option for the management of cartilage damage, for positive effects of biostimulation on cartilage tissue (13). But, so far, several studies have investigated the use of HPLT (14), few of its in combination. The present study aims to demonstrate the association efficacy of HPLT with glucosamine sulfate in osteoarthritis of the knee compared to HPLT alone.
Materials and Methods
The study, conducted according to the Helsinki Declaration, obtained ethical clearance from the local committee. Two physicians examined all participants with knee pain for eligibility for the study, a third author was involved, to clarify any disagreements. The inclusion criteria were: Age range 45-60 (y); Diagnosis of knee OA according to the criteria of the American College of Rheumatology (15); Grade 2 according to the radiographic scale of Kellgren- Lawrence; body mass index less than 30.
The exclusion criteria were: varus or valgus malalignment; neurological and cognitive disorders; recent knee surgery, physiotherapy or intra-articular infiltrations; oral corticosteroid or NSAIDs therapy in the last month; infectious arthritis; language/cognitive deficits; unable to walk unaided (Figure 1).
Figure 1.
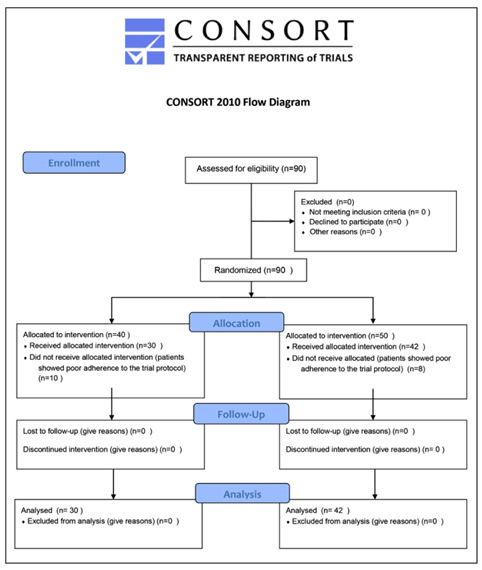
Study flow chart
All participants signed written informed consent and were randomized into two groups using the minimization, a stratified sampling method (16). A total of 90 subjects (M=53, F=37, y= 55±11.2) were allocated in group A with HPLT + Glucosamine sulfate 1500mg (GS - Dona®, Rottapharm, Monza, Italy) (n=45) or in group B (control group) with HPLT + placebo (n=45) (Table 1).
Table 1.
Characteristics of the patients
| Characteristics | HPLT+Placebo (n = 42) | HPLT+Glucosamine (n = 30) |
| Mean±SD age (y) | 54 ±11 | 56±9 |
| Mean±SD duration of arthritis (y) | 14±11 | 12±11 |
| Mean±SD weight (kg) | 80 ±19 | 81±11 |
Glucosamine was administered as a sachet of powder in a 1500 mg oral solution (5), once daily without food. Patients were instructed to avoid taking an analgesic, corticosteroids, or other NSAIDs for the duration of the study. GS treatment lasted 2 months.
Each group performed 12 sessions, 3 per week, with diode laser (Laserix© ph10, GNmed, Italy) applied in the perirotulum area, at the level of the articular hemirimes for a duration of 20 minutes with a wavelength of 905 nanometer, power of 4.5 Watt, dose of 70 J/cm2, pulse duration of 100 nanoseconds (17).
The visual analogue scale (VAS) was utilized to evaluate the intensity of pain perceived by the patient (a 10 cm line, from 0 or “no pain” to 10 “severe pain”). We assessed the pain experienced as the outcome: (1) during the activities of daily living (ADL), (2) during Standardized Stair Climbing Task (SSCT), (3) during the patellar grind evaluation (Rabot test) (18) and the Zohlen’s sign or Rasping test (4). Evaluations were performed at the first visit (T0), after the end of treatment (T1) and at 6 months (T2).
Statistical analysis
The R software (R version 3.6.0, R Core Team, USA) was used to perform the statistical analysis. Gaussian distributions were analyzed using the Shapiro-Wilk test. Using the paired T test and ANOVA, the experimental group was compared to the control group.
Results
All results were represented as Mean±SD (Table 2).
Table 2.
Outcome measures
| Group | T0 | T1 | T2 |
| VAS - ADL | |||
| experimental | 7.5±0.8 | 2.6±1.7 | 2.0±1.6 |
| control | 7.5±0.9 | 3.4±2.1 | 3.6±2.0 |
| VAS - SSCT | |||
| experimental | 8.8±0.8 | 2.2±2.0 | 2.7±2.0 |
| control | 8.8±0.7 | 3.5±2.6 | 4.3±2.1 |
| VAS - RABOT | |||
| experimental | 2.7±0.5 | 1.5±0.6 | 0.9±0.6 |
| control | 2.2±0.4 | 1.7±0.7 | 1.7±0.5 |
| VAS - RASPING | |||
| experimental | 3.4±0.5 | 1.8±0.7 | 1.4±0.6 |
| control | 3.6±0.5 | 2.5±0.6 | 2.6±0.8 |
Significant improvement was reported in both groups at T1 compared to T0 (p<0.05). No significant differences were found between the experimental group and control group in mean scores for VAS in ADL, SSCT, RABOT and RASPING at T1. A significant reduction in VAS-ADL, VAS-RABOT and VAS-RASPING was observed in the experimental group at T2 compared to T1. In the control group no improvement in all VAS scores were assessed. At T2, in both groups there was an evident reduction of VAS scores (p<0.05) (Figure 2).
Figure 2.
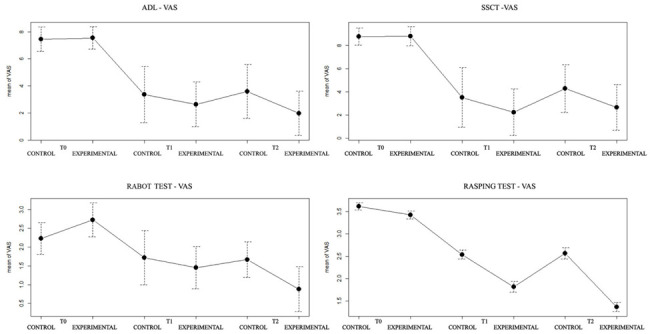
VAS scores in ADL, SSCT, RABOT and RASPING
In the experimental group 2 patients presented side effects (nausea, heartburn, headache) mostly mild and self-limiting. No side effects were recorded in the placebo group. No patient dropped out of the study due to side effects.
Discussion
In the treatment of symptomatic OA glucosamine has proven to be superior to control (19) and in chronic OA HPLT has proven effective in both small and large joints (20, 21,22), but so far there is no study on the association between HPLT and glucosamine sulfate in knee osteoarthritis. Glucosamine sulfate is one of the main components of the cartilage matrix, such as glycosaminoglycan. Synthesized from chitin of marine origin, it is a pure substance (MW = 573.31) (5). Glucosamine, sulphate, chloride and sodium ions are present in stoichiometric ratios of 2: 1: 2: 2 7. The crystalline form includes 384 mg of sodium chloride, in addition to the standard dose of 1500 mg (5). Glucosamine sulphate has the following therapeutic effects: anti-inflammatory, pro-anabolic, osteo-anabolic and anti-catabolic (23,24). Lippiello has shown that, in vitro, chondrocytes are more sensitive to chondro-protective agents in conditions that simulate joint stress in vivo (25).
Largo et al. described glucosamine sulfate as a drug able to modify symptoms and showed that glucosamine sulfate inhibits NFκB activation and COX-2 expression induced by IL-1β (7). Successively Chan et al. indicated that physiologically relevant concentrations of glucosamine decline gene expression and formation of NO and PGE2, decreasing the proteoglycan loosing and increase proteoglycan synthesis (26). Hochberg et al demonstrated that glucosamine and chondroitin sulfate are not inferior to celecoxib in improving pain and disability in knee OA at 6 months (27). But the role of glucosamine sulfate in the treatment of osteoarthritis of the knee is still controversial in the literature, in particular regarding pain relief or improved function (28). So, in the present study the synergy among the HPLT and glucosamine sulfate administration in patients affected by knee osteoarthritis was assessed to have in same time the anti-inflammatory action of the glucosamine sulfate and the analgesic effect of the HPLT (8). Lin et al. reported that laser treatment improved stress protein levels in experimental arthritic chondrocytes. Their synthesis is linked to the action of the laser in the preservation of chondrocytes and in the repair of cartilage (29). Thus, the ability to stimulate a similar cytokine pattern explains the significant differences found between groups not at the end of treatment (T1), but at 6 months (T2). At the end of the treatment, the anti-inflammatory and analgesic effect is guaranteed in both groups by laser therapy (17). The short-term effects of glucosamine sulfate depend on the inhibition of IL-1 and inducible NO production and the suppression of the COX-2 pathway on cartilage (7,26). Furthermore, since glucosamine is incorporated in the chondrocytes, it stimulates the production of physiological proteoglycans and inhibits catabolic exchange. These long-term effects explain the significant results at 6 months in the experimental group compared to control group. No side effects was observed about the use of HPLT, but 2 patients in a group treated with glucosamine sulfate referred some self-limited side effects, under literature data (30,31).
The limits of the study are the number of participants and the methods for micro and macro structural evaluation in the knee joint following treatment with HPLT and glucosamine sulfate, which could highlight the exact mechanism of its effect in the OA.
Conclusion
The results report a significant reduction of all VAS assessments in the experimental group compared to the control group not at T1 but at T2. Thus, at T1 both groups achieved similar improvements, while at 6 months the experimental group maintained significant pain relief improvements over the control group. Our data suggest that HPLT is effective and safe, but its effectiveness in treating the symptoms of osteoarthritis of the knee improves when combined with the administration of glucosamine sulfate up to 6 months after the end of treatment.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- Altman R D. Overview of osteoarthritis. Am J Med. 1987; Oct 30;83(4B):65–9. doi: 10.1016/0002-9343(87)90597-3. [DOI] [PubMed] [Google Scholar]
- Johnson-Nurse C, Dandy D J. Fracture-separation of articular cartilage in the adult knee. J Bone Jt Surg - Ser B. 1985;67(1):42–43. doi: 10.1302/0301-620X.67B1.3968141. [DOI] [PubMed] [Google Scholar]
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- Lane Smith R, Trindade MCD, Ikenoue TT, Mawatari M, Das P, Carter DR, Goodman SB, Schurman DJ. Effects of shear stress on articular chondrocyte metabolism. Biorheology. 2000;37(1–2):95–107. [PubMed] [Google Scholar]
- Rovati LC, Girolami F, Persiani S. Crystalline glucosamine sulfate in the management of knee osteoarthritis: Efficacy, safety, and pharmacokinetic properties. Ther Adv Musculoskelet Dis. 2012;4(3):167–180. doi: 10.1177/1759720X12437753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen P, Bartels EM, Altman RD, Bliddal H, Juhl C, Christensen R. Risk of bias and brand explain the observed inconsistency in trials on glucosamine for symptomatic relief of osteoarthritis: A meta-analysis of placebo-controlled trials. Arthritis Care and Research. 2014;66(12):1844–1855. doi: 10.1002/acr.22376. [DOI] [PubMed] [Google Scholar]
- Largo R, Alvarez-Soria MA, IDíez-Ortego BS, Calvo BSE, Sánchez-Pernaute O, Egido J, Herrero-Beaumont G. Glucosamine inhibits IL-1β-induced NFκB activation in human osteoarthritic chondrocytes. Osteoarthr Cartil. 2003;11(4):290–298. doi: 10.1016/s1063-4584(03)00028-1. [DOI] [PubMed] [Google Scholar]
- Nouri F, Raeissadat SA, Eliaspour D, Rayegani SM, Rahimi MS, Movahedi B. Efficacy of high-power laser in alleviating pain and improving function of patients with patellofemoral pain syndrome: A single-blind randomized controlled trial. J Lasers Med Sci. 2019;10(1):37–43. doi: 10.15171/jlms.2019.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolillo AR, Paolillo FR, João JP, João HA, Bagnato VS. Synergic effects of ultrasound and laser on the pain relief in women with hand osteoarthritis. Lasers Med Sci. 2014;30(1):279–286. doi: 10.1007/s10103-014-1659-4. [DOI] [PubMed] [Google Scholar]
- Karaca B. Effectiveness of High-Intensity Laser Therapy in Subacromial Impingement Syndrome. Photomed Laser Surg. 2016;34(6):223–228. doi: 10.1089/pho.2015.4005. [DOI] [PubMed] [Google Scholar]
- Jia YL, Guo ZY. Effect of Low-Power He-Ne Laser Irradiation on Rabbit Articular Chondrocytes in Vitro. Lasers Surg Med. 2004;34(4):323–328. doi: 10.1002/lsm.20017. [DOI] [PubMed] [Google Scholar]
- Mo JH, Kim JS, Lee JW, Chung PS, Chung YJ. Viability and regeneration of chondrocytes after laser cartilage reshaping using 1,460 nm diode laser, Clin. Exp Otorhinolaryngol. 2013;6(2):82–89. doi: 10.3342/ceo.2013.6.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atik SO, Sezgin EA. Is There a Role for Photobiomodulation in Treating Damaged Articular Cartilage Due to Injury or Degeneration?, Photobiomodulation, Photomedicine. and Laser Surgery. 2020;38(1):1–2. doi: 10.1089/photob.2019.4666. [DOI] [PubMed] [Google Scholar]
- Wyszyńska J, Bal-Bocheńska M. Efficacy of High-Intensity Laser Therapy in Treating Knee Osteoarthritis: A First Systematic Review. Photomedicine and Laser Surgery. 2018;36(7):343–353. doi: 10.1089/pho.2017.4425. [DOI] [PubMed] [Google Scholar]
- Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, et al. Development of criteria for the classification and reporting of osteoarthritis: Classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Pocock SJ, Simon R. “Sequential Treatment Assignment with Balancing for Prognostic Factors in the Controlled Clinical Trial, Biometrics. 1975;31(1):103. [PubMed] [Google Scholar]
- Angelova A, Ilieva EM. Effectiveness of high intensity laser therapy for reduction of pain in knee osteoarthritis. Pain Res Manag. 2016 doi: 10.1155/2016/9163618. Epub Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legré-Boyer V, Boyer T. Examen clinique d’un genou douloureux. Revue du Rhumatisme Monographies. 2016;83(3):133–137. [Google Scholar]
- Towheed T, Maxwell L, Anastassiades TP, Shea BJ, Houpt JB, Robinson V, Hochberg MC, Wells G. Glucosamine therapy for treating osteoarthritis. Cochrane Database of Systematic Reviews. 2009;8(2):CD002946. doi: 10.1002/14651858.CD002946.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiglić-Rogoznica N, Stamenković D, Frlan-Vrgoc L, Avancini-Dobrović V, Vrbanić TSL. Analgesic effect of high intensity laser therapy in knee osteoarthritis, Coll. Antropol. 2011;35(S2):183–5. [PubMed] [Google Scholar]
- Kheshie AR, Alayat MSM, Ali MME. High-intensity versus low-level laser therapy in the treatment of patients with knee osteoarthritis: A randomized controlled trial. Lasers Med Sci. 2014;29(4):1371–1376. doi: 10.1007/s10103-014-1529-0. [DOI] [PubMed] [Google Scholar]
- Ammendolia A, Cespites M, Iocco M. Topical use of aloe gel and low-level laser therapy in overuse tendinitis of elite volleyball players: a randomized controlled trial. Sport Sci Health. 2016;12(2) Online. [Google Scholar]
- Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: A meta-analysis. Rheumatol Int. 2010;30(3):357–63. doi: 10.1007/s00296-009-0969-5. [DOI] [PubMed] [Google Scholar]
- Henrotin Y, Mobasheri A, Marty M. Is there any scientific evidence for the use of glucosamine in the management of human osteoarthritis? Arthritis Research and Therapy. 2012;14(1):201. doi: 10.1186/ar3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippiello L. Glucosamine and chondroitin sulfate: Biological response modifiers of chondrocytes under simulated conditions of joint stress. Osteoarthr Cartil. 2003;11(5):335–342. doi: 10.1016/s1063-4584(03)00026-8. [DOI] [PubMed] [Google Scholar]
- Chan PS, Caron JP, Rosa GJM, Orth MW. Glucosamine and chondroitin sulfate regulate gene expression and synthesis of nitric oxide and prostaglandin E2 in articular cartilage explants. Osteoarthr Cartil. 2005;13(5):387–394. doi: 10.1016/j.joca.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Hochberg MC, Martel-Pelletier J, Monfort J, Möller I, Castillo JR, Arden N, Berenbaum F, Blanc FJ, et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: A multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis. 2016;75(1):37–44. doi: 10.1136/annrheumdis-2014-206792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison-Muñoz S, Rojas-Briones V, Irarrázaval S. Is glucosamine effective for osteoarthritis? Medwave. 2017;17(S1):e6867. doi: 10.5867/medwave.2017.6867. [DOI] [PubMed] [Google Scholar]
- Lin YS, Huang MH, Chai CY, Yang RC. Effects of helium-neon laser on levels of stress protein and arthritic histopathology in experimental osteoarthritis. Am J Phys Med Rehabil. 2004;83(10):758–765. doi: 10.1097/01.phm.0000137310.15943.19. [DOI] [PubMed] [Google Scholar]
- Herrero-Beaumont G, Ivorra JAR, Del Carmen Trabado M, Blanco FJ, Benito P, Martín-Mola E, Paulino J, Marenco JL, et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms: A randomized, double-blind, placebo-controlled study using acetaminophen as a side comparator. Arthritis Rheum. 2007;56(2):555–567. doi: 10.1002/art.22371. [DOI] [PubMed] [Google Scholar]
- Sobol E, Shekhter A, Guller A, Baum O, Baskov A. Laser-induced regeneration of cartilage. J Biomed Opt. 2011;16(8):080902. doi: 10.1117/1.3614565. [DOI] [PubMed] [Google Scholar]


