Abstract
Cardiovascular diseases (CVDs) have been the most common cause of death worldwide for decades. Until recently the most affected patients were middle-aged and elderly, predominantly males, with more frequent ST elevation myocardial infarction (STEMI) caused by obstructive coronary artery disease (CAD). However, in the last two decades we have noticed an increased incidence of ischemia with non-obstructive coronary arteries (INOCA), which includes myocardial infarction with non-obstructive coronary arteries (MINOCA) and non-myocardial infarction syndromes, such as microvascular and vasospastic angina, conditions that have been particularly pronounced in women and young adults - the population we considered low-risky till than. Therefore, it has become apparent that for this group of patients conventional methods of assessing the risk of future cardiovascular (CV) events are no longer specific and sensitive enough. Heart failure with preserved ejection fraction (HFpEF) is another disease, the incidence of which has been rising rapidly during last two decades, and predominantly affects elderly population. Although the etiology and pathophysiology of INOCA and HFpEF are complex and not fully understood, there is no doubt that the underlying cause of both conditions is endothelial dysfunction (ED) which further promotes the development of left ventricular diastolic dysfunction (LVDD). Plasma biomarkers of ED, as well as natriuretic peptides (NPs), have been intensively investigated recently, and some of them have great potential for early detection and better assessment of CV risk in the future. (www.actabiomedica.it)
Keywords: amino terminal pro brain natriuretic peptide, biomarkers, coronary artery disease, heart failure, endothelium, microcirculation, risk assessment
Introduction
Approximately 85% cause of cardiovascular (CV) deaths are due to myocardial infarction (MI) and stroke (1), diseases the main cause of which is advanced atherosclerosis. Atherosclerotic vascular disease begins to develop early in life as a result of impaired nitric oxide (NO) production caused by a dysfunctional endothelium (2,3) and continues to progress for decades as a silent process. Until recently, coronary artery disease (CAD) was anatomically defined by obstructive atherosclerosis of the epicardial coronary arteries. It is now known that structural and functional disorders affect the entire coronary circulation, including microcirculation, and it is coronary microvascular disease (CMD) that is thought to be responsible for the sudden increase in the incidence of ischemia with non-obstructive coronary arteries (INOCA) (4,5) and burden of heart failure with preserved ejection fraction (HFpEF) (6,7). Because of the wide spectrum of clinical manifestation and low frequency of obstructive CAD, many patients with CMD have normal findings on physical examination that we routinely use nowadays (8,9). In addition, the CV risk assessment scales we most use today (10,11) are based on traditional risk factors that mainly include age, gender, systolic blood pressure, cholesterol, smoking status, prior documented CAD, moderate to severe chronic kidney disease, and diabetes mellitus - therefore a part of the patients remain underestimated.
So, it is not surprising that the association of CV risk factors with the incidence of endothelial disfunction (ED), left ventricular diastolic dysfunction (LVDD), CAD, and heart failure (HF) has been extensively investigated in recent decades (Fig. 1), and the main conclusion of the studies to date is that a comprehensive non-invasive bio signature, containing biomarkers and integrated non-invasive imaging can serve as a potential tool in early diagnosis and prognosis of CV risk (12,13).
Figure 1.
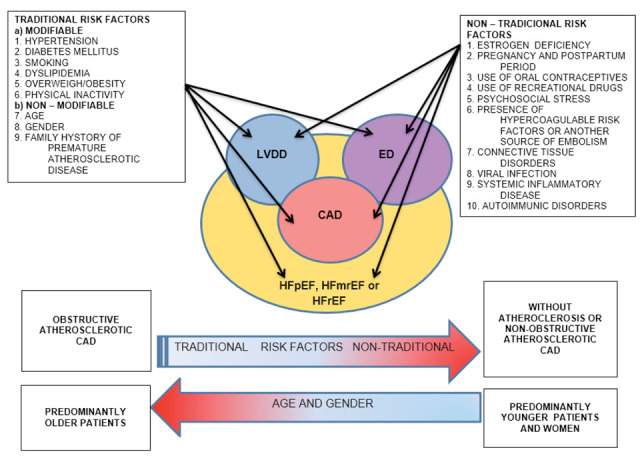
Connection among risk factors (traditional and non-traditional), left ventricular diastolic dysfunction, edothelial dysfunction, coronary artery disease and heart failure
Legend: It can be observed that traditional risk factors are more often related to the incidence of obstructive coronary artery disease in older people, whereas in younger people, especially women, non-traditional risk factors predominate with a higher frequency of non-obstructive coronary artery disease. LVDD= left ventricular diastolic dysfunction; ED=endothelial dysfunction; CAD=coronary artery disease, HFmrEF=heart failure with mid-range ejection fraction; HFpEF=heart failure with preserved ejection fraction; HFrEF=heart failure with reduced ejection fraction
Endothelial dysfunction
Pathophysiology
The endothelium is an unicellular layer that covers the inner surface of blood vessels, heart valves, and numerous body cavities. It is a complex organ with many autocrine, paracrine and endocrine properties which influence on vascular tone, fibrinolysis, cell growth and inflammation. Both, traditional and novel CV risk factors, including: smoking, aging, hypercholesterolemia, hypertension, hyperglycemia, a family history of premature atherosclerotic disease, obesity, elevated C-reactive protein, chronic systemic infection, menopause and stress are associated with ED, condition that is responsible for reducing NO bioavailability (Tab. 1) resulting to oxidative stress injury and disrupted blood rheology (14).
Table 1.
Differences between healthy and dysfunctional endothelium
| Healthy endothelium | Dysfunctional endothelium |
| 1. Vasodilatation | 1. Vasoconstriction |
| NO PGI-2 EDHF |
ET-1 AT-II TXA2 PGH2 ROS |
| 2. Anti-inflammation | 2. Inflammation |
| IL-10 TGF-β |
TNF-α IL -1β IL-6 MCP-1 MIP-1α ICAM-1 VCAM-1 ELAM-1 (E-selectin) BMP-4 |
| 3. Thrombolysis | 3. Thrombosis |
| NO PGI-2 |
vWF PAF |
| 4. Anti-coagulation | 4. Coagulation |
| GAGs/ATIII TFPI Thrombomodulin EPCR |
TF binding sites for coagulation factors and fibrin |
| 5. Fibrinolysis | 5. Anti-fibrinolysis |
| t-PA u-PA a plasminogen binding site PA receptors Annexin - II |
PAI TAFI |
| 6. Anti-proliferation | 6. Proliferation |
| under normal conditions proliferation is stopped | Heparan sulfate proteoglycans Integrins Components of the extracellular matrix Intracellular signaling molecules Growth factors: VEGF, FGF, PDGF, TGF Growth factor receptors |
Legend: NO=nitric oxide; PGI-2=prostacyclin; EDHF=endothelial hyperpolarizing factor; ET-1=endothelin; AT-II=angiotensin II; TXA-2=tromboxan A2; PGH2=prostaglandin H2; ROS=reactive oxygen species; IL-10=interleukin 10; TGF-β=transforming growth factor beta; TNF- α=tumor necrosis factor alpha; IL- 1β=interleukin 1 beta; IL-6=interleukin 6; MCP-1=monocyte chemoattractant protein 1; MIP-1α=macrophage inflammatory protein 1 alpha; ICAM-1=intracellular adhesion molecule 1; VCAM-1=vascular cell adhesion molecule 1; ELAM-1 (or E selectin)=endothelial-leukocyte adhesion molecule 1; BMP-4=bone morphogenetic protein 4; vWF=von Willebrand factor; PAF=platelet activating factor; GAGs/ATIII=glycosaminoglycans/ antithrombin III; TFPI=tissue factor pathway inhibitor; EPCR=endothelial cell protein C receptor; TF=tissue factor; t-PA=tissue type plasminogen activator; u-PA=urokinase type plasminogen activator; PA – receptors= plasminogen activator receptors; PAI=plasminogen activator inhibitor; ROS=reactive oxygen species; TAFI=thrombin activatable fibrinolysis inhibitor; VEGF=vascular endothelial growth factor; FGF=fibroblast growth factor; PDGF=platelet-derived growth factor; TGF=tissue growth factor
Today, in the era of the coronavirus disease 2019 pandemic (COVID-19), it is observed that new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) also affects the endothelium in two ways: directly through the co-factors necessary for the internalization of SARS-CoV-2 in human host cells which are expressed on endothelial cells (e.g. angiotensin 2 converting enzyme =ACE2, neuropilin-1 and C-type lectin transmembrane glycoprotein) and indirectly causing a cytokine storm. The result is ED, and the more severely affected the endothelium is, the more serious the clinical presentation of disease is. Moreover, serious altered endothelial function increases the risk of mortality, especially if affected individuals have comorbidities that are often accompanied by the preexistence of chronic endothelial dysfunction (e.g. old age, diabetes, hypertension and CVD) (15-19).
Impaired NO bioavailability can be the consequence of either a reduced production by endothelial nitric oxide synthesis (eNOS) or, more frequently, of an increased breakdown by reactive oxygen species (ROS) (20,21).
When endothelial cells lose their ability to maintain vascular homeostasis, it becomes susceptible to the invasion of lipids and leukocytes that are responsible for the formation of fatty streaks. If the situation persists, fatty streaks progress to atheromatous plaque that can rupture and set the condition for thrombogenesis and the vascular occlusion (22).
Diagnosis
Although there are several non-invasive techniques for evaluation of systematic ED, like venous occlusion pletysmography, peripheral artery tonometry (PAT) and laser Doppler-flowmetry, flow-mediated dilatation (FMD) of brachial artery is considered as gold standard today (23). This technique consists in causing forearm ischaemia by inflating a conventional cuff for measuring blood pressure 30 mmHg above systolic pressure for five minutes, and after that observes the amount of post ischaemic vasodilatation by measuring the increase of the basal values of the brachial artery by high-resolution ultrasonography, and it is expressed as a percentage. It can also be performed on the radial and femoral arteries (24-26). The systemic nature of atherosclerosis is reflected by the close correlation between ED in the forearm and coronary ED that could be induced with infusion of pharmacological stimuli (e.g. acetylcholine) during invasive coronary angiography (27).
Left ventricular diastolic dysfunction
Pathophysiology
Left ventricular (LV) cardiac function depends mainly on the two large myocardial layers – the fibers of the midwall, which are circumferentially oriented, and fibers that are localized at subendocardial and epicardial position, which are longitudinally aligned from tip to base like a double helix configuration. This distribution allows the LV twist-untwist mechanism during systole and diastole (28).
Diastole is the phase of cardiac cycle during which the myocardium relaxes and ventricle dilates, allowing filling with blood with adequate pressure - normally 12 mmHg at rest and 15 mmHg during exercise. While mechanical process of diastole can be divided into only two phases: active called relaxation and passive called stiffness (29), physiologically diastole can be divided into four phases: isovolumic, rapid ventricular filling, slow ventricular filling and atrial systole. The first phase consists of isovolumic relaxation, which begins with the aortic valve closure and ends with the mitral valve opening when the second diastolic phase begins. The second phase is characterized by rapid ventricular filling, which is more pronounced in young healthy individuals, and this is correlated to the more elastic expansion of the ventricle. The third phase (diastasis) extends between the passive and the active filling of the ventricle, just before atrial contraction. During this period, filling decreases due to elevation in ventricular pressure, and therefore the flow from the atria into the ventricle is significantly reduced or even absent. The fourth phase is represented by active ventricular filling during atrial systole, which is more pronounced in elderly (30,31). Left ventricular diastolic dysfunction (LVDD) occurs in many different patterns – from simple slowing of ventricular relaxation, without significant hemodynamic consequences (grade 1), to elevation of LV filling pressures (grade 2) and development of pulmonary venous congestion (grade 3) (30).
Myocardial ischemia is the main cause of the slowing of relaxation by reducing adenosine triphosphate (ATP) production and therefore reducing removal of calcium from the cytosol (32). Changes in the cytoskeletal protein (titin), extracellular matrix structure, imbalance of proteolytic enzymes (such as matrix metalloproteinase), myocyte hypertrophy, interstitial fibrosis, renin-angiotensin aldosterone system hyperactivation, arterial vascular abnormalities (associated with aging and inflammation) promote increased LV stiffness (30,33).
Therefore, LVDD is the earliest change common to many CV diseases and risk factors. Any type of heart disease leading to structural myocardial disorders, pericardial effusion (34-36) or abnormalities at the cellular level that subsequently cause impaired relaxation and /or increased ventricular stiffness (30,37) will result in LVDD. Other diseases and conditions that have been reported in the literature to lead to LVDD are: age, arterial hypertension, diabetes, obesity, metabolic syndrome, increased sympathetic tone, renal failure and hyperinsulinemia (30,37,38). The prevalence of LVDD in the general population is estimated to be around 25% to 27% (33,38) and tends to worsen with aging, even in extremely healthy individuals, predisposing as a risk factor for HFpEF (12).
Diagnosis
LV catheterization is the gold standard for the diagnosis of LVDD, but it is not in routine use due to its invasiveness. Therefore the most commonly used method for assessing left ventricular diastolic function (LVDF) in clinical practice is transthoracic echocardiography (TTE). Two dimensional mode (2D), pulsed-wave Doppler (PWD) and tissue Doppler imaging (TDI) should be used for comprehensive assessment of LVDF. The differences between LVDF and LVDD are complicated because some of parameters are affected by aging as well as some of them are preload-dependent. In 2016, the American Society of Echocardiography (ASE) and the European Society of Cardiovascular Imaging (EACVI) published updated recommendations proposing the main parameters for evaluation of LVDF (depending on the estimated left ventricular ejection fraction =LVEF) and their cutoff values. (Tab. 2).
Table 2.
Cutoff values of main parameters for evaluation of left ventricular diastolic function depending on the estimated left ventricular ejection fraction
| Cutoff values | |
| LVEF ≥ 50% | |
| septal e or lateral e (cm/s) |
< 7 < 10 |
| average E/e | >14 |
| LAVI (mL/m2) | >34 |
| TRV (m/s) | >2.8 |
| LVEF < 50% | |
| E/A | > 0.8 and < 2 (indeterminate LAP) ≥ 2 (↑↑ LAP) |
| E (cm/s) | > 50 (indeterminate LAP) |
Legend: E= early diastolic transmitral flow velocity; E´= early diastolic myocardial tissue velocity; E/A= ratio of early to late diastolic filling velocity, LAVI= left atrium volume index; TRV= tricuspid regurgitation velocity
For patients with preserved LVEF (≥ 50%) LVDD is confirmed if three of four parameters listed in Tab.2 meet cutoff values. Conversely, if the three of four parameters do not meet the cutoff values, LVDF is considered to be normal. In a situation when only two parameters meet the cutoff values, LVDF is indeterminate and additional parameters (like isovolumic relaxation time = IVRT, deceleration time =DT, changes in mitral inflow velocities with Valsalva maneuver = Valsalva ∆ E/A, color M-mode flow propagation velocity = CMM Vp, time difference between atrial reversed flow wave duration and mitral A duration = a dur – A dur and time interval between peak R wave in QRS complex and onset of mitral E velocity subtracted from time interval between QRS complex and onset of E´ velocity = TE-E´) are needed for its evaluation. Patients with reduced LVEF (<50%) always have LVDD, at least grade 1, so the two main parameters listed in Tab. 2 help to distinguish LVDD stage 1 from LVDD stage 2 and 3, in which left atrium pressure (LAP) is usually elevated. LAP is considered highly elevated if E/A ≥ 2, and then LVDD grade 3 is confirmed. If both parameters do not meet cutoff values for indeterminate LAP, it is considered that LAP is normal and LVDD grade 1 is confirmed. Otherwise, additional parameters (average E/E´, LAVI and TRV) are required to assess the LAP and distinguish the LVDD grade 1 from 2 (31,35).
Although the current recommendations are focused on TTE, it should be noted that both nuclear scans and cardiac magnetic resonance (CMR) can be used to evaluate LV filling rates and volumes also (35).
When resting TTE does not explain the symptoms of HF or dyspnea, especially with exertion, stress echocardiography (SE) is indicated. It is best performed using supine bike protocol, or as a part of exercise treadmill testing using 2D and Doppler data at baseline and early recovery, and not using dobutamine, as the administration of the drug does not simulate the day-to-day physiologic stress. When the indications for SE are chest pain or dyspnea, considered as equivalent of angina, in patients with known or suspected CAD, it is important to prioritize the collection of 2D images for wall motion abnormalities (WMAs). As to the results, the test is considered definitely abnormal indicating LVDD when all of the following three conditions are met: average E/E ratio > 14 or septal E/E ratio > 15 with exercise, TRV > 2,8 m/sec with exercise and septal E velocity < 7 cm/sec or, if only lateral velocity is acquired, lateral E < 10 cm/sec at baseline. It has been demonstrated that increased LV filling pressure (demonstrated by E/E ratio) with exercise has incremental prognostic power according to clinical parameters, as well as 2D findings of myocardial ischemia (35).
Furthermore, there are some novel indices for better estimation of LVDF like LV global longitudinal strain (GLS), LV GLS rate, LV untwisting rate, left atrial (LA) systolic strain and mean wedge, which appears to be a promising variable, especially in distinguishing patients with HFpEF from those without HF (35).
Asymptomatic versus symptomatic LVDD and its progression to HF
Taking into account the symptoms and signs, LVDD in clinical practice can be asymptomatic or symptomatic. Asymptomatic LVDD (ALVDD), also called pre-clinical diastolic dysfunction, is characterized by presence of LV hypertrophy (LVH) identified by electrocardiography and TTE abnormalities, mentioned before, with LVEF ≥ 50%, and without HF symptoms and signs. Patients with ALVDD become symptomatic when LV end diastolic pressure increases. Symptomatic LVDD involves symptoms and signs of HF such as breathlessness, orthopnoea, paroxysmal nocturnal dyspnea, reduced exercise tolerance, fatigue, tiredness, increased time to recovery, ankle swelling, elevated jugular venous pressure, hepatojugular reflux, third heart sound called gallop rhythm and laterally displaced apical impulse.
Based on the measurement of LVEF, HF can be divided into: 1. HF with preserved (LVEF ≥50%) = HFpEF , 2. HF with reduced (LVEF <40%) = HFrEF and 3. HF with mid-range LVEF (in the range of 40–49%) = HFmrEF. Although the diagnosis of HFrEF can be made in the presence of specific symptoms and signs of HF with a calculated LVEF <40%, the diagnosis of HFmrEF and HFpEF is more challenging and, beside presence of LVDD, it requires also the increased plasma levels of natriuretic peptides (NPs): 1. B - type NP (BNP) > 35 pg/mL or 2. N terminal- proBNP (NT-proBNP) >125 pg/mL, with or without LVH and LA enlargement as sign of increased filling pressures (39). Although significant proportion of those with ALVDD would develop HFpEF, some of them will progress to HFmrEF or HFrEF, while the rest will not progress at all, and the risk of progression to different types of symptomatic HF depends of underlying etiology, cardiovascular comorbidities and noncardiac risk factors such as renal impairment, pulmonary airflow limitation and anemia (36).
Microcirculation and cell signaling pathways as a novel bridge in understanding association between ED and LVDD in development of CAD and HF
Although many studies supported evidence of association between ED (diagnosed by FMD) and CV events in patients without and with prior CAD (40-42), some of them reported impressive overall net correct risk re-classification with addition of FMD to Framingham risk score (FRS) also (40), which was particularly pronounced in low-risk populations (41).
In the last two decades there were also several studies which investigate relationship between ED (based on FMD), HFrEF and HFpEF. Although there was no doubt that HFrEF caused by obstructive CAD (determined by coronary angiography) was associated with ED, the association of HFrEF caused by non-obstructive CAD and ED was confusing (43,44). Contrary, in the studies which examined the association between HFpEF and ED (45,46), the main conclusion was that ED may contribute to the pathogenesis and maintenance of HFpEF, and, what was more important, that HFpEF patients had high prevalence of CMD (determined by coronary flow reserve =CFR) - even 75% (46).
Recently it has become known that LVDD occurs early in the ischemic cascade among patients with CMD (47) and that plasma levels of NPs, in addition to diagnosis of HF, play an important role in the prevention of both HF and adverse CV events (48). Moreover, it has become clear that microcirculation and CMD are novel bridge in understanding association between LVDD and ED in development of HF, predominantly with preserved ejection fraction (47,49).
In 2013 Paulus et Tschope described a Novel Paradigm for HFpEF (50) which presumes the following sequence of events: a high prevalence of CV risk factors induce a systemic proinflammatory state which leads to development of ED in coronary microcirculation. Impairment of NO bioavailability reduces cyclic guanosine monophosphate (cGMP) content and protein kinase G (PKG) activity in adjacent cardiomyocytes. Low PKG activity favors hypertrophy development and increases resting tension because of hypophosphorylation of titin. Both stiff cardiomyocytes and interstitial fibrosis contribute to high diastolic LV stiffness and HF development (Fig. 2).
Figure 2.
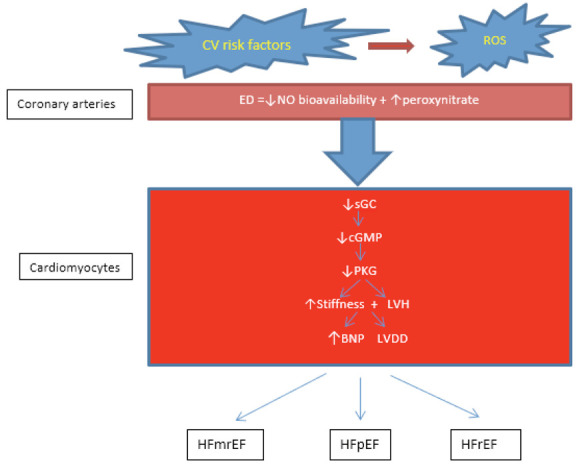
Pathophysiology of HF
Legend: CV risk factors stimulate ROS production and reduce the bioavailability of NO in the coronary arteries (both macro- and microcirculation). Impaired NO bioavailability decreases cGMP content and PKG activity in adjacent cardiomyocytes, leading to increased resting tension, development of LVH and LVDD, and BNP release. BNP= brain natriuretic peptide; cGMP= cyclic guanosine monophosphate; CV= cardiovascular; ED=endothelial dysfunction; HFmrEF=heart failure with mid-range ejection fraction; HFpEF=heart failure with preserve ejection fraction; HFrEF=heart failure with reduce ejection fraction; LVDD=left ventricular diastolic dysfunction; LVH=left ventricular hypertrophy; NO=nitric oxide; PGK=protein kinase G; ROS= reactive oxygen species; sGC= soluble guanilil cyclase.
So, it became clear that myocardial remodeling in HFpEF differs from HFrEF, in which remodeling is driven by loss of cardiomyocytes due to cell death as a results from ischemia, infection, or toxicity (50-53).
In 2019 Giannitsi et al (23) summarize knowledge of ED role in the pathogenesis and progression of HF. They discribed that in chronic HF patients culminates endothelial shear stress which stimulates eNOS expression. When eNOS expression is down-regulated, less NO is produced and vasoconstriction appears which furthermore reduces peripheral tissue and myocardial perfusion, reduces coronary flow and worsens ventricular function. Moreover, neurohormonal activation, release of inflammatory messengers such as prostaglandins, catecholamines and altered local shear stress due to low cardiac output, modulate gene expression and promote atherogenesis, increasing oxidative stress. The result is a decrease in NO bioavailability and a consequent progression of HF from the asymptomatic to the symptomatic phase, characterized by fluid retention, blood centralization, and clinical decompensation.
Because function of microcirculation wasn´t avaible to be determined by standard coronary angiography, there are several new non-invasive and invasive approaches for it´s evaluation. Non-invasive techniques include dynamic myocardial perfusion CT, positron emission tomography (PET), cardiac magnetic resonance (CMR) and Doppler echocardiography of the left anterior descending (LAD) coronary artery (which is difficult to be performed and give limited information). These diagnostic tests rely on interrogation of coronary vasomotor function by measuring regional and global myocardial blood flow (MBF) at rest and during stress, microvascular resistance, and CFR. Invasive techniques include invasive coronary flow reserve (iCFR), measuring index of microvascular resistance (IMR), fractional flow reserve (FFR) and instantaneous wave-free ratio (iFR) (54,55).
The role of biomarkers
Although there are many biomarkers of HF which were investigated in the last decades (56), only NPs are established in guidelines as a biomarkers of HF, till now (36). In recent studies values of NPs have been confirmed in screening and prevention of HF and CAD also (48,57, 58). Gallagher et al (48) reported that NPs are effective in refining risk prediction for HF and CAD and add predictive power to conventional risk factors. They based their conclusion mainly on two randomized clinical trials (57,58) that have shown NP-based screening and targeted prevention can reduce HF, LV dysfunction and other major adverse cardiovascular events (MACE). Another large meta analysis (59) which included 40 prospective cohorts with 95,617 participants without known CAD at the time of NT-proBNP measurement, shown that NT-proBNP strongly predicted first-onset HF, CAD and stroke, suggesting that NT-proBNP concentration assessment could be used to integrate HF into CAD primary prevention. Their ability to be measured rapidly through blood tests makes their widespread use more practical. They may also aid in the detection of a disease at an earlier stage before structural and functional changes become apparent on imaging, which allows time for intervention.
So far, many ED associated biomarkers have been identified (60), but the most popular of them (23) like asymmetrical dimethylarginine (ADMA), oxidized low density lipoprotein (oxLDL), endothelial microparticles (EMPs), endothelial progenitor cells (EPCs) and endothelial glycocalyx , were also indentified in CVD and HF (61-67). More interestingly plasma values of ADMA were compared to the plasma values of NT-proBNP (68), as well as to LVDD (69) and positive correlation was confirmed. The explanation is that ADMA and NT-proBNP have common mechanism of action via the cyclic guanosine monophosphate (cGMP) signaling pathway (70).
Discussion
Development of CAD and HF are multifactorial, partly genetically predetermined and largely acquired. In the last decades it has become clear that traditional risk factors and conventional methods of assessing the risk of future CV events have no longer been specific and sensitive enough, especially in the group considered to be low-risk so far. LVDD is the earliest change common to CAD, HF, as well as to CV risk factors, and it tends to worsen with aging, even in extremely healthy individuals. Therefore, it is difficult to distinguish asymptomatic from symptomatic LVDD, and even more difficult to determine the underlying cause of it in otherwise structurally normal heart. By including ED and CMD, as a new diagnosis which refers to cardiac microcirculation, in solving this problem, we got the answer to a part of the question. Indeed, it is the acute event of CMD that explains the sudden increase in the prevalence of INOCA, with more frequent atypical clinical presentation and worse prognosis. The chronic manifestation of CMD could be partly responsible for the increased incidence of HF, predominantly HFpEF. Only with the introduction of new diagnostic methods such as CFR, the function of coronary microcirculation becomes measurable.
Conclusion
New researches in this area are oriented to plasma biomarkers of HF and ED that could better predict progress of LVDD and ED in CAD and HF over time. Although there are several plasma biomarkers of HF and ED, some of which have great potential in elucidating this issue, a further research is needed, not only for diagnosis and risk assessment, but also because microvascular dysfunction may be a promising therapeutic target, especially in HFpEF. As we live in a COVID-19 pandemic, the biomarkers of ED and HF, as well as non-invasive imaging, are likely to be of interest to researchers in the coming years to monitor recovered patients and determine possible lasting effects of COVID-19-induced ED on the CV system.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- World Health Organization (WHO) Report of Cardiovascular diseases (CVDs) 17 May 2017. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) [Google Scholar]
- Garcia MM, Rodrigues MG, Reis Neto JA, Correia LC. Influence of subclinical atherosclerosis on diastolic function in individuals free of cardiovascular disease. Arq Bras Cardiol. 2010;95:473–8. doi: 10.1590/s0066-782x2010005000114. [DOI] [PubMed] [Google Scholar]
- Zeiher AM, Drexler H, Wollschläger H, Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–65. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA). Developing evidence – based therapies and research agenda for the next decade. Circulation. 2010;135:1075–92. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owan TE, Hodge DO, Herges RM, Jaconsen SJ, Roger VJ, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Eng J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- Taqueti VR, Solomon SD, Shah AM, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840–9. doi: 10.1093/eurheartj/ehx721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanski A, Gransar H, Hayes SW, et al. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing:1991 to 2009. J Am Coll Cardiol. 2013;61:1054–65. doi: 10.1016/j.jacc.2012.11.056. [DOI] [PubMed] [Google Scholar]
- Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- D'Agostino RB, Sr Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- Kane GC, Karon BL, Mahoney DW, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306(8):856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntmann VO, Taylor PC, Mayr M. Coupling vascular and myocardial inflammatory injury into a common phenotype of cardiovascular dysfunction: systematic inflammation and aging – A mini review. Gerontology. 2011;57:295–303. doi: 10.1159/000316577. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Evans PC, Rainger GE, Mason JC, et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. ESC. 2020 doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Ji W, Yang H, Chen S, Zhang W, Duan G. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduction and Targeted Therapy. 2020;5:293. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordo R, Paliogiannis P, Mangoni AA, Pintus G. SARS-CoV-2 and endothelial cell interaction in COVID-19: molecular perspectives. VascularBiology. 2021;3(1):R15–R23. doi: 10.1530/VB-20-0017. https://doi.org/10.1530/VB-20-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambardella J, Santulli G. What is linking COVID-19 and endothelial dysfunction? Updates on nanomedicine and bioengineering from the 2020 AHA Scientific Sessions. European Heart Journal - Cardiovascular Pharmacotherapy. 2020 doi: 10.1093/ehjcvp/pvaa145. doi:10.1093/ehjcvp/pvaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nägele MP, Haubner B, Tanner FC, Ruschitzka F, Flammer AJ. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis. 2020;314:58–62. doi: 10.1016/j.atherosclerosis.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher TF, Vanhoutte PM. The Endothelium: modulator of cardiovascular function. Boca Raton, FL: CRC Press; 1990. [Google Scholar]
- Taddei S, Ghiadoni L, Virdis A, Versari D, Salvetti A. Mechanisms of endothelial dysfunction: clinical significance and preventive non-pharmacological therapeutic strategies. Curr Pharm Des. 2003;9:2385–402. doi: 10.2174/1381612033453866. [DOI] [PubMed] [Google Scholar]
- Esper RJ, Nordaby RA, Vilariňo JO, Paragano A, Cacharrón JL, Machado RA. Endothelial dysfunction: a comprehensive appraisal. Cardiovascular Diabetology. 2006;5(4):P1–18. doi: 10.1186/1475-2840-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannitsi S, Maria B, Bechlioulis A, Naka K. Endothelial dysfunction and heart failure: A review of the existing bibliography with emphasis on flow mediated dilation. JRSM Cardiovascular Disease. 2019;8:1–7. doi: 10.1177/2048004019843047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias RG, Negrão CE, Krieger MH. Ơxido nítrico y sistema cardiovascular: activación celular, reactividad vascular y variante genética. Arq Bras Cardiol. 2011;96(1):68–75. [PubMed] [Google Scholar]
- Corretti MC, Todd J, Anderson AJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilatation of the brachial artery. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- Corretti MC, Plotnik GD, Vogel RA. Technical aspects of evaluating brachial artery vasodilatation using high-frequency ultrasound. Am J Physiol. 1995;268:H1397–H1404. doi: 10.1152/ajpheart.1995.268.4.H1397. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Gerhard MD, Meredith IT, et al. Systemic nature of endothelial dysfunction in atherosclerosis. Am J Cardiol. 1995;75:71B–74B. doi: 10.1016/0002-9149(95)80017-m. [DOI] [PubMed] [Google Scholar]
- Sengupta PP, Khandheria BK, Narula J. Twist and untwist mechanics of the left ventricle. Heart Fail Clin. 2008;4(3):315–24. doi: 10.1016/j.hfc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Mesquita ET, Jorge AJ. Insuficiêntia cardíaca com fraçăo de ejaçăo normal – novos critérios diagnósticos e avanços fisipatológicos. Arq Bras Cardiol. 2009;93(2):180–7. doi: 10.1590/s0066-782x2009000800018. [DOI] [PubMed] [Google Scholar]
- Palmiero P, Zito A, Maiello M, et al. Left ventricle diastolic function in hypertension: methodological considerations and clinical implications. J Clin Med Res. 2015;7(3):137–44. doi: 10.14740/jocmr2050w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossaify A, Nasr M. Diastolic dysfunction and the new recommendations for echocardiographic assessment of left ventricular diastolic function: summary of guidelines and novelties in diagnosis and grading. Journal of Diagnostic Medical Sonography. 2019;35(4):317–25. [Google Scholar]
- Tsujino T, Kawasaki D, Masuyama T. Left ventricular diastolic dysfunction in diabetic patients: pathophysiology and therapeutic implications. Am J Cardiovasc Drugs. 2006;6(4):219–30. doi: 10.2165/00129784-200606040-00002. [DOI] [PubMed] [Google Scholar]
- Kloch-Badelek M, Kuznetsova T, Sakiewicz W, et al. Prevalence of left ventricular diastolic dysfunction in European populations based on cross-validated diagnostic thresholds. Cardiovascular Ultrasound. 2012;10:10. doi: 10.1186/1476-7120-10-10. http://www.cardiovascularultrasound.com/content/10/1/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegers SE. Diastolic echo parameters: meaningless numbers or crucial information? Circ Cardiovasc Imaging. 2011;4(5):460–462. doi: 10.1161/CIRCIMAGING.111.968131. [DOI] [PubMed] [Google Scholar]
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Wan SH, Vogel MW, Chen HH. Pre-clinical diastolic dysfunction. J Am Coll Cardiol. 2014;63:407–16. doi: 10.1016/j.jacc.2013.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita ET, Lagoeiro Jorge AJ. Understanding asymptomatic diastolic dysfunction in clinical practice. Arq Bras Cardiol. 2013;100(1):94–101. doi: 10.1590/s0066-782x2013000100015. [DOI] [PubMed] [Google Scholar]
- Kuznetsova T, Herbots L, López B, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal 2016. 2016;37:2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The Multi-Ethnic Study of Atherosclerosis. Circulation. 2009;120(6):502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte DR, Westerink J, de Koning E, van dr Graaf Y, Grobbee DE, Bots ML. Is the association between flow-mediated dilatation and cardiovascular risk limited to low-risk population? J Am Coll Cardiol. 2005;45:1987–93. doi: 10.1016/j.jacc.2005.02.073. [DOI] [PubMed] [Google Scholar]
- Gökce N, Keaney JF Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–72. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- Meyer B, Mörtl D, Strecker K, et al. Flow-mediated vasodilation predicts outcome in patients with chronic heart failure comparison with B-type natriuretic peptide. J Am Coll Cardiol. 2005;46:1011–8. doi: 10.1016/j.jacc.2005.04.060. [DOI] [PubMed] [Google Scholar]
- Shah A, Gkaliagkousi E, Ritter JM, Ferro A. Endothelial function and arterial compliance are not impaired in subjects with heart failure of non-ischemic origin. J Cardiac Fail. 2010;16:114–20. doi: 10.1016/j.cardfail.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Kishimoto S, Kajikawa M, Maruhashi T, et al. Endothelial dysfunction and abnormal vascular structure are simultaneously present in patients with heart failure with preserved ejection fraction. Int J Cardiol. 2017;231:181–7. doi: 10.1016/j.ijcard.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Shah SJ, Lam CSP, Svedlund S, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. European Heart Journal. 2018;39:3439–50. doi: 10.1093/eurheartj/ehy531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF, Torales JM, Garcia LB, Centurion OA. Myocardial infarction and non-obstructive coronary arteries (MINOCA) associated to diastolic dysfunction of the left ventricle. J Cardiol Curr Resi. 2018;11(4):161–4. [Google Scholar]
- Gallagher J, Watson C, Campbell P, Ledwidge M, McDonald K. Natriuretic peptide-based screening and prevention of heart failure. Cardiac Failure Review. 2017;3(2):83–5. doi: 10.15420/cfr.2017:20:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–11. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- Gurusamy N, Das DK. Autophagy, redox signalling, and ventricular remodeling. Antioxid Redox Signal. 2009;11:1975–88. doi: 10.1089/ars.2009.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare JM. Oxidative stress and apoptosis in heart failure progression. Circ Res. 2001;89:198–200. [PubMed] [Google Scholar]
- Penn MS. The role of leukocyte-generated oxidants in left ventricular remodeling. Am J Cardiol. 2008;101:30D–3D. doi: 10.1016/j.amjcard.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options. JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:2625–41. doi: 10.1016/j.jacc.2018.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068. [DOI] [PubMed] [Google Scholar]
- Sato Y, Fujiwara H, Takatsu Y. Biochemical markers in heart failure. Journal of Cardiology. 2012;59:1–7. doi: 10.1016/j.jjcc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide–based screening and collaborative care for heart failure The STOP-HF Randomized Trial. JAMA. 2013;310(1):66–74. doi: 10.1001/jama.2013.7588. [DOI] [PubMed] [Google Scholar]
- Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected PreventiOn of cardiac eveNts in a populaTion of dIabetic patients without A history of Cardiac disease) A prospective randomized controlled trial. J Am Coll Cardiol. 2013;62:1365–72. doi: 10.1016/j.jacc.2013.05.069. [DOI] [PubMed] [Google Scholar]
- Natriuretic Peptides Studies Collaboration. Natriuretic peptides and integrated risk assessment for cardiovascular disease: an individual-participant-data meta-analysis. Lancet Diabetes Endocrinol. 2016;4:840–49. doi: 10.1016/S2213-8587(16)30196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves JA, Neves JA, de Cássia Meneses Oliveira R. Biomarkers of endothelial function in cardiovascular diseases: hypertension. J Vasc Bras. 2016;15(3):224–33. [Google Scholar]
- Willeit P, Freitag DF, Laukkanen JA, et al. Asymmetric dimethylarginine and cardiovascular risk: systematic review and meta-analysis of 22 prospective studies. J Am Heart Assoc. 2015;4(6):e001833. doi: 10.1161/JAHA.115.001833. doi/10.1161/JAHA.115.001833. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4599532/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Lian B, Lu H, Liao P, Guo L, Zhang M. Prognostic value of asymmetric dimethylarginine in patients with heart failure: A systematic review and meta-analysis. Hindawi BioMed Research International. 2020 doi: 10.1155/2020/6960107. Article ID 6960107, 9 pages. https://doi.org/10.1155/2020/6960107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Liu J. Association between circulating oxidized low-density lipoprotein and atherosclerotic cardiovascular disease. Chronic Diseases and Translational Medicine. 2017;3:89–94. doi: 10.1016/j.cdtm.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezin AE. Endothelial derived micro particles: biomarkers for heart failure diagnosis and management. J Clin Trial Cardiol. 2015;2(3):1–3. [Google Scholar]
- Tushuizen ME, Diamant M, Sturk A, Nieuwland R. Cell-derived microparticles in the pathogenesis of cardiovascular disease. Friend or foe? Arterioscler Thromb Vasc Biol. 2011;31:4–9. doi: 10.1161/ATVBAHA.109.200998. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Zhang C, Zhang G, Tao J. Endothelial progenitor cells in cardiovascular diseases. Aging Med. 2018;1:204–8. doi: 10.1002/agm2.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Nijst P, Kiefer K, Tang WHW. Endothelial glycocalyx as biomarker for cardiovascular diseases: mechanistic and clinical implications. Curr Heart Fail Rep. 2017;14(2):117–26. doi: 10.1007/s11897-017-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poreba R, Gac P, Poreba M, et al. Left ventricle diastolic dysfunction and plasma assymetric dimethylarginine concentration in persons with essential hypertension. Arch Med Sci. 2015;11(3):521–9. doi: 10.5114/aoms.2015.52354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razaei SS, Weisshaar S, Litschauer B, Gouya G, Ohrenberger G, Woltz M. ADMA and NT pro BNP are associated with overall mortality in elderly. Eur J Clin Invest. 2018 doi: 10.1111/eci.13041. 0:e 13041. https://doi.org/10.1111/eci.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork NI, Nikolaev VO. Signaling in the cardiovascular system – the role of compartmentation and its live cell imaging. Int J Mol Sci. 2018:19,801. doi: 10.3390/ijms19030801. doi: 10.3390/ijms 19030801. [DOI] [PMC free article] [PubMed] [Google Scholar]


