Abstract
Background and Aim:
Diaphragmatic dysfunction is seen in up to 60% of critically ill patients with respiratory failure, and it is associated with worse outcomes. The functionality of the diaphragm can be studied with simple and codified bedside ultrasound evaluation. Diaphragm excursion is one of the most studied parameters. The aim of this study was to assess the prevalence of diaphragmatic dysfunction in critically ill non-intubated patients admitted to a general intensive care unit with acute respiratory failure.
Methods:
We collected data, including ultrasound diaphragm excursion, at 2 time points: at T0 (at the time of recruitment, just before starting NIV) and at T1 (after one hour of NIV).
Results:
A total of 47 patients were enrolled. The prevalence of diaphragm dysfunction was 42.5% (95% CI 28, 3 - 57,8). Surgical patients showed a higher incidence (relative risk of 1.97) than medical patients. Mean DE was not significantly different between NIV responders (1,35 ± 0.78 cm) and non-responders (1.21 ± 0.85 cm, p 0,6). Patients with diaphragmatic dysfunction responded positively to NIV in 60% (95% CI 36.0 - 80.9%) of cases, while patients without diaphragmatic dysfunction responded positively to the NIV trial in 70.4% (95% CI 49.8 - 86.2%) of cases (p = 0.54). Taking the use of ultrasound diaphragm excursion as a potential predictor of NIV response, the corresponding ROC curve had an area under the curve of 0.53; the best balance between sensitivity (58.1%) and specificity (62.5%) was obtained with a cut-off diaphragm excursion of 1.37 cm.
Conclusions:
Diaphragm dysfunction is particularly frequent in critically ill patients with respiratory failure. The functionality of the diaphragm can be effectively and easily tested by bedside ultrasound examination. Overall, our results point towards tentative evidence of a trend of a different response to NIV in patients with vs without diaphragmatic dysfunction. (www.actabiomedica.it)
Keywords: acute respiratory failure, diaphragm ultrasound, diaphragm excursion, non-invasive ventilation
Background
Diaphragmatic dysfunction (DD) in patients with respiratory failure has often been neglected, and only in recent years has it become a well-regarded topic in the literature (1,2). Its prevalence in critically ill patients requiring invasive mechanical ventilation has been proven to be up to 60%. DD is associated with failure of weaning from mechanical ventilation, prolonged length of intensive care unit (ICU) stay and increased mortality (1-5). Several conditions have been associated with diaphragmatic weakness, such as sepsis, shock, hypoxia and post-surgical settings, creating a “multiple-hit mechanism”, in which various factors are combined to induce changes in respiratory mechanics leading to respiratory failure (1,6,7).
Post-surgical patients seem to be at high risk of diaphragm dysfunction, especially after cardio-thoracic or upper abdominal surgery (6): up to 79% of liver transplant patients have shown DD (8,9).
Simple and fast diaphragmatic bedside ultrasound evaluation techniques have been codified, giving great impetus to the study of diaphragm function (10-15). One of the most studied parameters is the diaphragm excursion (DE, cm), which is the echographic measurement of the inspiratory downward displacement of the hemidiaphragm (16). Several studies have investigated the ability of DE, alone or in combination with other parameters, to predict successful weaning from mechanical ventilation, but its role is not fully understood (17).
Acute respiratory failure is a common cause of admission to the ICU, and DD may be a primary contributory cause. When patients do not require emergent intubation, a trial of non-invasive ventilation (NIV) is often considered (18). Because of positive inspiratory pressure, diaphragm excursion is expected to be increased, but its behaviour has not been fully studied during non-invasive ventilation (a situation combining spontaneous breathing effort and positive inspiratory pressure) in patients with and without DD (19).
To the best of our knowledge, the prevalence of DD in patients with acute respiratory failure eligible for an NIV trial has been investigated by only a few studies (20), and the role of ultrasound assessment of DE as a predictor of NIV failure in this type of patient has not been researched.
The primary aim of this study was to assess the prevalence of DD in non-intubated patients affected by acute respiratory failure admitted to a general ICU. Subsequently, we evaluated the diaphragm response to NIV and whether the ultrasound assessment of diaphragm excursion may be employed as a predictor of NIV failure.
Methods
Study Design
This is a single-centre observational prospective study approved by our Institutional Ethics Committee (no. 72/2015).
Our study was conducted in the Intensive Care Unit at Academic Hospital of Udine, Italy.
Patients were eligible for the study if they were admitted to the ICU with acute respiratory failure (defined as PaO2/FiO2 ratio < 300) and scheduled for an NIV trial.
The exclusion criteria were as follows:
lack or refusal of written informed consent
incompetent/non compos mentis patient
age < 18 years
severe hypoxia requiring immediate invasive ventilation, facial trauma, swallowing disorders and other NIV contraindications
haemodynamic instability
poor echographic transthoracic window, splenectomy, obesity (BMI > 30 kg/m2).
Experimental protocol
We collected data at 2 time points: at T0 (at the time of recruitment, just before starting NIV) and at T1 (after one hour of NIV).
At T0, we collected demographic data (age, sex, BMI), medical history (reason for ICU admission, associated diseases, SAPS II score) and pre-NIV respiratory and echographic data (PaO2/FiO2 ratio, respiratory rate, ultrasound diaphragmatic measurements) as described below.
After these measurements, patients were ventilated in NIV with BiPAP pressure support via the Dragër Evita 4® (Lübeck, Germany) ventilator and Respironics PerforMax® full face mask (Philips Respironics, Murrysville, PA, USA). A pressure support (PS) range of 5-7 cmH2O and a positive end-expiratory pressure (PEEP) of 5-10 cmH2O were used. In order to tolerate non-invasive ventilation, all patients were sedated if necessary with remifentanil up to a maximum dosage of 0.05 μg/kg/min continuous IV infusion to achieve a Ramsey score of 2, according to our internal ICU sedation protocol.
The following criteria were used to declare NIV failure at T1 and requirement of endotracheal intubation:
failure to increase PaO2 > 50% compared to the pre-NIV value,
increase of the PaCO2 > 15% compared to the pre-NIV value,
respiratory rate > 40 min-1.
Within 1 hour of NIV, we collected T1 intra-NIV ventilatory data (PaO2/FiO2 ratio, respiratory rate, PS, PEEP, peak pressure, echographic diaphragmatic measurements as described below).
We also collected outcome data at least 100 days after recruitment: subsequent need for tracheal intubation, duration (expressed in days) of intubation, length of intensive care stay, hospital LOS, and death.
Ultrasound diaphragmatic measurements
At both T0 and T1, we conducted a thoracic ultrasound exam to evaluate diaphragm motility. All US exams were conducted by an expert echographer using a Philips EN Visor® C 1.2 ultrasound system and a 3.5 MHz convex probe (Philips, Andover, MA, USA). We employed the measurement technique described by Boussuges, universally accepted in clinical practice and in the literature (15): the transhepatic and trans-splenic acoustic windows were studied with the probe positioned between the midclavicular and anterior axillary line in B-mode to detect optimal visualization of diaphragm excursion.
Three sets of ultrasonographic data were taken in M-mode on each side of the thorax in each patient at both T0 and T1. As a surrogate of diaphragmatic function, we measured inspiratory diaphragm excursion (DE, cm), defining diaphragmatic dysfunction (DD) as a DE < 1.00 cm. We recorded the following:
DE = diaphragm excursion (cm)
Slope = contraction speed (cm/sec)
Tins = inspiratory time, (sec)
Texp = expiratory time (sec)
Ttot = total time of respiration (sec)
Statistical analysis
All data were recorded in a Microsoft Excel 2010 spreadsheet, and statistical analysis was performed with MedCalc 18.2 (Ostend, Belgium). Mean, median, standard deviation and interquartile range are reported for quantitative variables, absolute and relative frequencies for qualitative variables. To test for outliers, the robust regression outlier removal method (ROUT) was used. Fisher’s exact test or the t/Kolmogorov-Smirnov test (for qualitative variables) were used to evaluate whether the observed differences between independent variables were not due to chance. The prevalence of DD was calculated, together with the respective 95% confidence interval. Sensitivity and specificity were used as indices of the accuracy of diaphragmatic dysfunction in predicting NIV success or failure. The ROC curve of DE compared to NIV outcome was used to identify the threshold of DE that guaranteed the best balance between different levels of sensitivity and specificity.
In the scientific literature, there are currently no other studies that have analyzed the prevalence of diaphragmatic dysfunction in spontaneously breathing patients with respiratory failure admitted to intensive care; since there is no available estimate on which to base the calculation of the sample size required in our study, we assumed an a priori prevalence of diaphragmatic dysfunction of 50% to maximize the sample size.
To calculate the study prevalence with a 2-sided 95% confidence interval and a maximum accuracy error of 15% per queue, given an expected proportion of 50%, it was necessary to enroll 47 patients.
Results
A total of 47 patients were enrolled. Patient characteristics and demographics are shown in Table 1; no statistically significant differences were found between the two groups.
Table 1.
Demographic and clinical data.
| Overall n = 47 |
DD n = 20 |
noDD n = 27 |
p | |
| Age, years (mean ± SD) | 65.5±14.8 | 64.5±15.3 | 66.2±14.8 | 0.7 |
| Gender, male % | 57.4 | 55 | 59.3 | 1 |
| BMI, kg/m2 (mean ± SD) | 26.9±3.7 | 26.6±3.9 | 27.1±3.7 | 0.7 |
| Patient type (%): Surgical
|
38.3 61.7 |
55 45 |
25.9 74.1 |
0.068 |
| SAPS II (mean ± SD) | 44.0±12.3 | 40.9±8.1 | 46.5±14.6 | 0.14 |
| Comorbidities (%): HTNa DM type II Liver cirrhosis Kidney failure Oncologic Ischemic heart disease Atrial fibrillation Hematological COPD |
42.6 31.9 17.0 29.8 19.1 14.9 12.8 12.8 10.6 |
LEGEND: BMI, body mass index; OLTx, orthotopic liver transplantation; SAPS II, Simplified Acute Physiology Score II; HTNa, arterial hypertension; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; DD, patients with diaphragm dysfunction; noDD, patients without diaphragm dysfunction.
Post-surgical patients accounted for 38%, and the majority of them underwent hepatic surgery or orthotopic liver transplant (OLTx). The most common comorbidities were arterial hypertension (43%), type 2 diabetes mellitus (32%) and renal failure (30%).
Diaphragm dysfunction
The prevalence of DD in our patient population was 42.5% (95% CI 28.3 – 57.8). There were no differences in age, sex, BMI, SAPS II score (see Table 1), initial PaO2/FiO2 ratio (p = 0.98) or respiratory rate (p = 0.13) in patients with vs without DD.
Nearly 61% (95% CI 35.7 – 82.7) of post-surgical patients presented DD compared with 31% (CI 15.3 – 50.8) of medical patients. Post-surgical patients showed a higher prevalence of diaphragmatic dysfunction than medical patients, with a relative risk of 1.97 (CI 1.022 - 3.794, p = 0.0429).
Effect of non-invasive ventilation
NIV was generally well tolerated and efficacious, with a mean improvement in the PaO2/FiO2 ratio of 64±7 points (95% CI 42.97 – 85.20, p<0.001; Figure 1) and a decrease of 1.5±5.5 in respiratory rate (95% CI -3.153 – 0.08887, p = 0.06).
Figure 1.
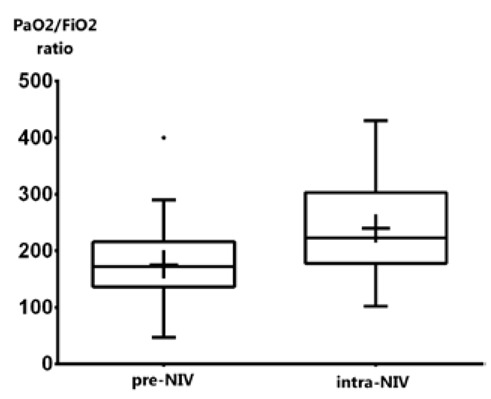
PaO2/FiO2 mean values before and after one hour of NIV. Box and whisker plot showing PaO2/FiO2 ratio before and after one hour of NIV. The box extend from the 25th to the 75th percentiles; whiskers indicate the minimum and maximum values; plus sign indicate the mean value.
Tins did not significantly change from before to during NIV, while Texp increased by 0.15 sec (95% CI 0.04 – 0.26, p = 0.007), together with total respiration time, which increased by 0.23 sec (95% CI 0.08 to 0.37, p = 0.002) - see Table 2. In our study, diaphragm excursion proved to be significantly increased during NIV (+ 0.2 cm, p = 0.001) due to mechanical pressure support, as expected (Table 2).
Table 2.
Oxygenation and ultrasonographic assessment of the diaphragmatic function before and after one hour of NIV.
| T0 pre-NIV | T1 intra-NIV | mean of differences (95% CI) | p | |
| PaO2/FiO2, | 175±65 | 239±78 | 64.1 42.9 – 85.2 |
< 0.001 |
| DE (cm) | 1.511±0.746 | 1.714±0.945 | 0.203 0.080 – 0.327 |
0.001 |
| RR (per minute) | 21.3±6.4 | 19.7±6.4 | -1.5 -3.15 – 0.01 |
0.06 |
| Tins (sec) | 0.836±0.258 | 0.879±0.323 | 0.033 -0.029 – 0.096 |
0.29 |
| Texp (sec) | 0.820±0.473 | 1.027±0.602 | 0.151 0.041 – 0.262 |
0.007 |
| Ttot (sec) | 1.64±0.61 | 1.906±0.820 | 0.227 0.083 – 0.370 |
0.002 |
LEGEND: DE, diaphragm excursion; RR, respiratory rate; Tins, inspiratory time; Texp, expiration time; Ttot, respiratory cycle total time. All values expressed as mean ± SD.
NIV treatment failed in 34% of patients (NIV non-responder, 95% CI 20.8 – 49.3). NIV-responder patients started with lower initial PaO2/FiO2 values and, on US diaphragm examination, showed longer respiratory times (both Tins and Texp) before NIV. There was no significant difference in age, sex, BMI, respiratory frequency, or peak pressures during NIV (Table 3).
Table 3.
NIV non-responder and NIV responder data before starting NIV trial.
| T0 (pre-NIV) | NIV non-responder | NIV responder | p |
| PaO2/FiO2 | 215.4±69.38 | 155.1±53.65 | 0.002 |
| RR (per minute) | 21.44±5.85 | 21.19±6.77 | 0.903 |
| Tins (sec) | 0.751±0.2086 | 0.8657±0.2557 | 0.039 |
| Texp (sec) | 0.6382±0.2889 | 0.912±0.5216 | 0.01 |
| Ttot (sec) | 1.389±0.4382 | 1.778±0.6408 | 0.004 |
| Pmax (cmH2O) | 11.75±2.295 | 13.35±2.727 | 0.0502 |
| Age (ys) | 64.63±14.38 | 65.9±15.34 | 0.783 |
| BMI (kg/m2) | 26.4±4.545 | 27.12±3.12 | 0.54 |
LEGEND: RR, respiratory rate; Tins, inspiratory time; Texp, expiration time; Ttot, respiratory cycle total time; Pmax, maximal inspiratory pressure; BMI, body mass index. All values expressed as mean ± SD.
Post-surgical patients responded to NIV in 55% of cases, while medical patients had a benefit in 72% of cases, but this difference did not reach statistical significance (p = 0.34).
Diaphragmatic dysfunction and NIV failure
The mean T0 diaphragm excursion was slightly larger in NIV-responder patients (mean DE 1.35 ± 0.78 cm) compared to non-responders (mean DE 1.21 ± 0.85 cm), but this difference was not statistically significant (p = 0.6).
Patients without DD responded positively to the NIV trial in 70.4% (95% CI 49.8 - 86.2%), while patients with DD responded positively to NIV in 60% (95% CI 36.0 - 80.9%) of cases (p = 0.54). The degree of respiratory support provided by the ventilator was similar in the two groups: the mean pressure support was 6.59 ± 2.02 cmH2O in patients without DD and 7.70 ± 2.20 cmH2O in patients with DD (p = 0.08), while the mean PEEP was 5.85 ± 1.10 cmH2O and 5.60 ± 1.39 cmH2O, respectively (p = 0.49).
Given the above differences, assuming the use of ultrasound diaphragm excursion as a potential predictor of NIV response, the corresponding ROC curve (Figure 2) had an area under the curve (AUC-ROC) of 0.53 (95% CI 0.382 – 0.680) (p = 0.7227). The best balance between sensitivity (58.1%) and specificity (62.5%) was obtained with a DE cut-off of 1.37 cm (Youden index J of 0.206).
Figure 2.
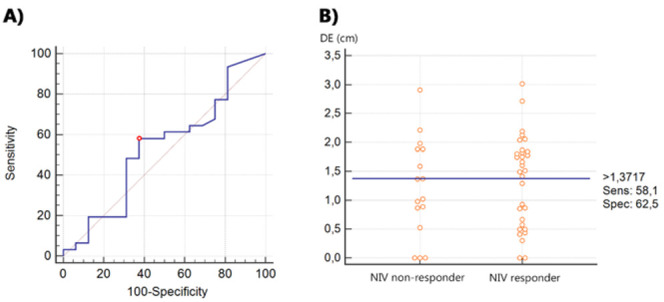
A) ROC Curve of the proficiency of diaphragm excursion as a predictor of NIV response. B) Dot plot separating NIV-responder and NIV non-responder patients according to their diaphragm excursion. The horizontal line indicates the cut-off point with the best separation (minimal false negative and false positive results) between the two groups. LEGEND: DE, diaphragm excursion;
We then evaluated the predictive capacity of the slope of the curve (cm/s), as its measurement should correspond to the speed (strength) of the diaphragm contraction. There were no significant differences between responders and non-responders (1.919 ± 0.9139 vs 2.154 ± 1.511, respectively, p = 0.695). The AUC-ROC was 0.505 (95% CI 0.395 to 0.614, p = 0.947). With a cut-off of 1.64 cm/s, the sensitivity was 56.2%, and the specificity was 29.6% (Youden Index J 0.18).
Outcome
The mean length of stay in the ICU was shorter in patients with normal diaphragm function (11 ± 9 days), compared to patients with DD (14 ± 13 days), but this difference did not reach statistical significance (p = 0.297). See Figure 3A.
Figure 3.
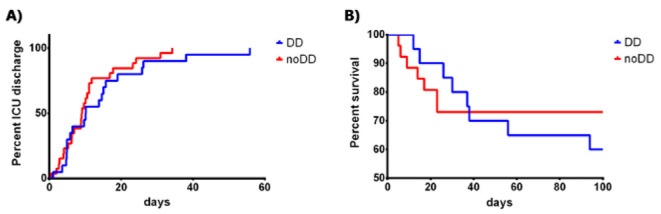
A) Kaplan-Meier Curve for ICU-LOS according to diaphragm dysfunction (Mantel-Cox test: p = 0,2959). B) Kaplan-Meier Curve for mortality according to diaphragm dysfunction (Mantel-Cox test: p = 0,6649). LEGEND: DD, patients with diaphragm dysfunction; noDD: patients without diaphragm dysfunction.
After 100 days of follow-up, mortality in patients with diaphragm dysfunction was 40% (95% CI 19.1 - 63.9), while in patients with normal diaphragmatic function, it was 27% (95% CI 11.6 - 47.8), p=0.527 (see Figure 3B).
Discussion
The main result of the present study is the finding of a high prevalence of diaphragmatic dysfunction among patients admitted to intensive care (42.5%), according to the literature. The clinicians must pay attention to the presence of this organ failure, especially in surgical patients who, as expected, demonstrate the higher prevalence, as it is known that surgical manipulation (especially heart, thorax and upper abdominal surgery) can impair diaphragmatic muscle function (6). We should not overlook, however, that even in the non-surgical population, acute respiratory failure was accompanied by DD in almost 1 in 3 patients (31%) in this study.
We used DE, or diaphragm displacement, as the ultrasonographic parameter of diaphragmatic function, as it is the simplest and fastest method in spontaneous breathing patients at the admission of the critically ill patient to ICU. DE is associated with lung volume during the inspiratory phase (21) but does not correlate with inspiratory muscular effort (22) in patients undergoing assisted mechanical ventilation, and it is influenced by several factors (1,23). Since DE may be the result of the sum of the patient’s inspiratory activity and mechanical ventilatory support, DE has true value only when assessed during spontaneous breathing. In our study, DE proved to be significantly increased during NIV (p = 0.001) due to mechanical pressure support, as expected.
NIV employment correlates with benefits on respiratory mechanics as decreased respiratory rate, unloading of respiratory muscles and increased tidal volume and minute ventilation (24). These benefits are echographically represented by a decreased respiratory rate (-1.54 min-1), a prolonged Ttot (+0.23”) and Texp (+0.15”) and an increase in diaphragm excursion (+0.2 cm), all statistically significant measures. The lack of variation in inspiratory time is due, in our opinion, only to the benefit that NIV has on the muscular effort, respiratory rate and alveolar recruitment (through CPAP/PEEP) without altering the inspiratory time determined by the respiratory centers.
We believe that Tins and Texp may be considered surrogates of respiratory muscle functional reserve: patients with greater muscle fatigue (shorter Tins and Texp despite same initial mean respiratory rate) before NIV treatment may have a worse response to NIV than patients with a greater muscle functional reserve. In our study NIV responder patients demonstrated longer respiratory times at T0 (Tins 0.86” vs 0.75”, Texp 0.91” vs 0.64”, Ttot 1.78 vs 1.39”) than NIV non-responders, despite having the same initial mean respiratory rate as non-responder patients (21.2 vs 21.4 min-1) – see Table 3.
We observed that ventilator settings during NIV were not significantly different in patients with vs without DD. However, despite having identical PEEP values, patients with DD required higher pressure support than patients without DD (mean PS 7.70 vs 6.59 cmH2O, p = 0.08). This difference (at the limits of statistical significance) in the need for inspiratory support may be due to the lack of a certain amount of inspiratory capacity and muscle strength attributable to the impaired diaphragm in patients with DD, which must be compensated for externally with an increase in inspiratory support provided by the ventilator.
Confirming the hypothesis that initiated this study, we found that patients with DD were less responsive to NIV (60%) than patients without DD (70.4%), although this difference was not statistically significant (p = 0.54). Furthermore, the mean DE was slightly higher in NIV-responder patients (mean DE 1.35 ± 0.78 cm) than non-responders (mean DE 1.21 ± 0.85 cm, p=0.6). A hypothetical cut-off value of 1.37 cm of DE reached a decent sensitivity (58.1%) and specificity (62.5%) to identify a NIV responder subject (AUC-ROC 0.53).
We also evaluated the same predictive capacity of the slope of the DE curve (pend, cm/s), which corresponds to the speed of diaphragm contraction, and found no significant differences between responders and non-responders (p = 0.37). A cut-off value of 1.64 cm/s of the slope of the DE curve had a sensitivity of 56.2% and specificity of 29.6% to identify a NIV responder subject (AUC-ROC 0.50).
Overall, our results point towards the only tentative evidence of a non-statistically significant trend of a different response to NIV among respiratory patients with and without DD. Further studies with larger sample sizes are necessary to confirm or refute this hypothesis definitively.
In the current state of the research, DE seems not to be an appropriate a priori predictor of NIV failure.
The same was true of the outcome measures: both the ICU length of stay and the 100-day mortality showed a non-statistically significant trend towards a worse outcome for patients with DD, in accordance with what is reported in the literature (4). This also leads to an increase in patient management costs.
The main limitations of our study consist of the small sample size and the fact that the diaphragm thickening fraction was not acquired. In addition, no obese patients were recruited in the present study.
Conclusions
In conclusion, DD is a frequent occurrence in critically ill patients with respiratory failure, whether they are surgical or medical patients. DD is associated with various comorbidities and disease severity, but it is not only a condition that identifies the most serious patients; it may represent another form of organ failure. The functionality of the diaphragm can be effectively and easily tested by bedside ultrasound examination, and its measurement should be considered in every patient with respiratory failure.
Further studies with larger sample sizes are necessary to confirm or refute the hypothesis that the presence of DD can modify the patient’s response to NIV.
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Vetrugno L, Guadagnin GM, Barbariol F, et al. Ultrasound Imaging for Diaphragm Dysfunction: A Narrative Literature Review. J Cardiothorac Vasc Anesth. 2019;33:2525–2536. doi: 10.1053/j.jvca.2019.01.003. https://doi.org/10.1053/j.jvca.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Garofalo E, Bruni A, Pelaia C, et al. Comparisons of two diaphragm ultrasound-teaching programs: a multicenter randomized controlled educational study. Ultrasound J. 2019;11:21. doi: 10.1186/s13089-019-0137-4. https://doi.org10.1186/s13089-019-0137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung B, Moury PH, Mahul M, et al. Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med. 2016;42:853–861. doi: 10.1007/s00134-015-4125-2. https://doi.org/10.1007/s00134-015-4125-2. [DOI] [PubMed] [Google Scholar]
- Demoule A, Jung B, Prodanovic H, et al. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am J Respir Crit Care Med. 2013;188:213–219. doi: 10.1164/rccm.201209-1668OC. https://doi.org/10.1164/rccm.201209-1668OC. [DOI] [PubMed] [Google Scholar]
- Jiang JR, Tsai TH, Jerng JS, Yu CJ, Wu HD, Yang PC. Ultrasonographic evaluation of liver/spleen movements and extubation outcome. Chest. 2004;126:179–185. doi: 10.1378/chest.126.1.179. [DOI] [PubMed] [Google Scholar]
- Siafakas N, Mitrouska I, Bouros D, Georgopoulos D. Surgery and the respiratory muscles. Thorax. 1999;54:458–465. doi: 10.1136/thx.54.5.458. https://doi.org/10.1136/thx.54.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan J, Harrison TB, Rich MM, Moss M. Early development of critical illness myopathy and neuropathy in patients with severe sepsis. Neurology. 2006;67:1421–1425. doi: 10.1212/01.wnl.0000239826.63523.8e. [DOI] [PubMed] [Google Scholar]
- McAlister VC, Grant DR, Roy A, et al. Right phrenic nerve injury in orthotopic liver transplantation. Transplantation. 1993;55:826–830. doi: 10.1097/00007890-199304000-00027. [DOI] [PubMed] [Google Scholar]
- Barbariol F, Vetrugno L, Pompei L, De Flaviis A, Rocca GD. Point-of-care ultrasound of the diaphragm in a liver transplant patient with acute respiratory failure. Crit Ultrasound J. 2015;7:3. doi: 10.1186/s13089-015-0021-9. https://doi.org/10.1186/s13089-015-0021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé BP, Dres M. Diaphragm dysfunction: diagnostic approaches and management strategies. J Clin Med. 2016;5:E113. doi: 10.3390/jcm5120113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dres M, Demoule A. Monitoring diaphragm function in the ICU. Curr Opin Crit Care. 2020;26:18–25. doi: 10.1097/MCC.0000000000000682. https://doi.org/10.1097/MCC.0000000000000682. [DOI] [PubMed] [Google Scholar]
- Matamis D, Soilemezi E, Tsagourias M, et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med. 2013;39:801–810. doi: 10.1007/s00134-013-2823-1. https://doi.org/10.1007/s00134-013-2823-1. [DOI] [PubMed] [Google Scholar]
- Pasero D, Koeltz A, Placido R, et al. Improving ultrasonic measurement of diaphragmatic excursion after cardiac surgery using the anatomical M-mode: A randomized crossover study. Intensive Care Med. 2015;41:650–656. doi: 10.1007/s00134-014-3625-9. https://doi.org/10.1007/s00134-014-3625-9. [DOI] [PubMed] [Google Scholar]
- Khurana J, Gartner SC, Naik L, Tsui BCH. Ultrasound identification of diaphragm by novices using ABCDE technique. Reg Anesth Pain Med. 2018;43:161–165. doi: 10.1097/AAP.0000000000000718. https://doi.org/10.1097/AAP.0000000000000718. [DOI] [PubMed] [Google Scholar]
- Umbrello M, Formenti P. Ultrasonographic assessment of diaphragm function in critically ill subjects. Respiratory Care. 2016;61:542–555. doi: 10.4187/respcare.04412. https://doi.org/10.4187/respcare.04412. [DOI] [PubMed] [Google Scholar]
- Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by M-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135:391–400. doi: 10.1378/chest.08-1541. https://doi.org/10.1378/chest.08-1541. [DOI] [PubMed] [Google Scholar]
- Vivier E, Muller M, Putegnat JB, et al. Inability of Diaphragm Ultrasound to Predict Extubation Failure: A Multicenter Study. Chest. 2019;155:1131–1139. doi: 10.1016/j.chest.2019.03.004. https://doi.org/10.1016/j.chest.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50:1602426. doi: 10.1183/13993003.02426-2016. https://doi.org/10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- Vivier E, Mekontso Dessap A, Dimassi S, et al. Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med. 2012;38:796–803. doi: 10.1007/s00134-012-2547-7. https://doi.org/10.1007/s00134-012-2547-7. [DOI] [PubMed] [Google Scholar]
- Cammarota G, Sguazzotti I, Zanoni M, et al. Diaphragmatic Ultrasound Assessment in Subjects With Acute Hypercapnic Respiratory Failure Admitted to the Emergency Department. Respir Care. 2019;64:1469–1477. doi: 10.4187/respcare.06803. https://doi.org/10.4187/respcare.06803. [DOI] [PubMed] [Google Scholar]
- Cohen E, Mier A, Heywood P, Murphy K, Boultbee J, Guz A. Excursion-volume relation of the right hemidiaphragm measured by ultrasonography and respiratory airflow measurements. Thorax. 1994;49:885–889. doi: 10.1136/thx.49.9.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbrello M, Formenti P, Longhi D, et al. Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study. Crit Care. 2015;19:161. doi: 10.1186/s13054-015-0894-9. https://doi.org/10.1186/s13054-015-0894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerhill EM, El-Sameed YA, Glidden TJ, McCool FD. Monitoring recovery from diaphragm paralysis with ultrasound. Chest. 2008;133:737–743. doi: 10.1378/chest.07-2200. https://doi.org/ 10.1378/chest.07-2200. [DOI] [PubMed] [Google Scholar]
- Diaz O, Iglesia R, Ferrer M, et al. Effects of noninvasive ventilation on pulmonary gas exchange and hemodynamics during acute hypercapnic exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156:1840–1845. doi: 10.1164/ajrccm.156.6.9701027. [DOI] [PubMed] [Google Scholar]


