Abstract
Introduction.
There are no studies investigating populations of patients with both pulmonary embolism and chronic obstructive pulmonary disease (PE-COPD) with and without deep venous thrombosis (DVT).
Aim of the study.
To define the prevalence of DVT in COPD with PE and to compare the characteristics of COPD patients who develop PE, with and without DVT. Secondly, we aimed to assess differences in the localization of PE among study groups.
Methods.
116 patients with pulmonary embolism (PE) were enrolled in a retrospective study. Clinical data as well as echocardiographic and lower limb ultrasonography records were collected for all subjects. Subjects were divided into two groups according to the presence of COPD: Group 1, 54 patients with diagnosis of PE without COPD and Group 2, 66 patients diagnosed of PE with COPD. Then, individuals of Group 2 were subdivided in two subgroups according to the presence (n=21) or absence (n=45) of DVT.
Results.
33% of patients with COPD and PE showed DVT. These subjects had higher PaCO2 and ejection fraction (p<0.05 for all) and higher percentage of chronic renal failure and diabetes mellitus compared to those without DVT (p<0.05 for all). Moreover, in COPD-PE patients with DVT, the most frequent localization was proximal (54% of total), whereas COPD-PE patients without DVT showed a more frequent segmental localization (60% of total). No difference was found in clinical presentation and blood chemistry tests.
Conclusions.
DVT was non common in PE-COPD patients. Chronic renal failure, and type 2 diabetes mellitus are more frequent in PE-COPD patients with DVT, that showed a higher frequency of proximal localization, thereby indicating a greater risk of more severe clinical implications. Conversely, PE- COPD subjects without DVT showed a more frequent segmental localization and were less hypercapnic. PE should be taken into account in COPD with worsening of respiratory symptoms, also in absence of DVT. (www.actabiomedica.it).
Keywords: Venous thromboembolism, Chronic Obstructive Pulmonary Disease, Pulmonary embolism, In situ thrombosis
Introduction
Venous Thromboembolism (VTE) is a spectrum of diseases ranging from Pulmonary Embolism (PE) to deep venous thrombosis (DVT) and pulmonary infarction [1]. The most frequent clinical presentation of VTE in general settings is DVT, with two cases of DVT for one PE case [1].
Like other cardiovascular diseases, VTE is more prevalent among patients with Chronic Obstructive Pulmonary Disease (COPD) than those without COPD [2,3]. Indeed, compared to the general population, the risk of PE is greater than the risk of DVT in both stable and exacerbated COPD. Interestingly, PE is described in up to 25% of hospitalized patients with COPD [5]. Aleva et al found that the pooled prevalence of PE in unexplained acute exacerbation of COPD (AE-COPD) was 16.1% (95% CI, 8.3%-25.8%), whereas the pooled prevalence of DVT in AE-COPD was 10.5% (95% CI, 4.3%-19.0%) [6].
Furthermore, the diagnosis of PE in COPD is made difficult by the fact that the symptoms of these conditions can overlap [7]. The latter is a serious problem, considering that patients with COPD and PE have 70% higher rate of in-hospital death and twice the rate of dying within 30 days from diagnosis of VTE compared to those without COPD [8]. Similarly, patients with concomitant COPD and PE show an increased 1-year mortality compared to those without COPD (53.3%, relative risk 1.94) [6,9,10]. Based on the above, chronic respiratory diseases have been integrated in Pulmonary Embolism Severity Index [11].
Moreover, COPD may be an independent risk factor for PE, especially in subjects under 60 years old [12].
In light of these premises, the aim of our study was to define the prevalence of DVT in COPD with PE and to compare the characteristics of COPD patients who develop PE, with and without DVT. Secondly, we aimed to assess differences in the localization of PE among study groups.
Materials and Methods
Patients and Study design
The current retrospective study was performed in the units of respiratory diseases and internal medicine of the University hospital Policlinico of Bari, Italy.
We investigated all patients admitted to the above wards who were diagnosed with PE (n=149) according to the ESC ERS guidelines 2014 [11] from January 2014 to February 2016.
Patients were divided into two groups according to the presence of COPD, strictly following GOLD guidelines 2017 [13]: Group 1, consisting of 54 patients with diagnosis of PE without COPD and Group 2, made of 66 patients diagnosed of PE with concurrent COPD.
Subsequently, individuals of Group 2 were subdivided in two subgroups according to the presence or absence of DVT. (see Figure 1)
Figure 1.
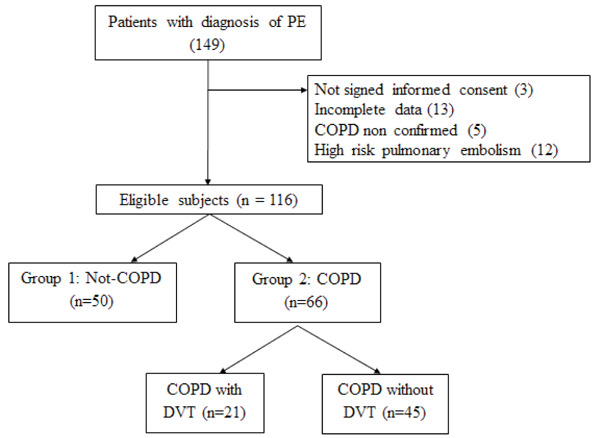
Design of the Study
PE: Pulmonary Embolismo; COPD: Chronic Obstructive Pulmonary Disease; DVT: Deep Vein Thrombosys
This study was conducted in accordance with International Conference on Harmonization guidelines and the Declaration of Helsinki. All procedures performed in the present study were approved by the ethical institutions at which the study was conducted and an informed consent was required to all participants.
Exclusion criteria were as follows: absence of informed consent, incomplete data, failure to confirm the diagnosis of COPD, high risk pulmonary embolism.
Diagnosis of PE was conducted at the Emergency Department (ED) of Policlinico, Bari with Computed tomographic pulmonary angiography (CTPA) or ventilation-perfusion scan according to ESC ERS guideline 2014 [11], with definition of localization (proximal, segmental or subsegmental).
Afterwards, if not performed at the ED, upon admission to the wards of respiratory medicine or internal medicine, each patient underwent the following investigations: collection of full medical history (including confirmation of COPD diagnosis and PE risk factor), clinical evaluation, arterial blood gas analysis, routine blood tests including D-dimer and NT-pro-BNP, echocardiography with estimation of ejection fraction and pulmonary artery systolic pressure (PASP) and lower limb ultrasonography for research of DVT. COPD was confirmed by medical data provided by patients or present in the ward archives.
We considered the following risk factors for PE: obesity, defined by BMI>30; recent trauma; immobility, defined as complete bed rest; use of contraceptive medications; current malignancy; active pneumonia; atrial fibrillation (AF); type 2 diabetes mellitus; current smoking. We have also evaluated presence of ischemic heart disease, systemic hypertension and chronic renal failure.
Lower limb ultrasonography
Complete lower limb compression ultrasonography was performed within 24h of the diagnosis of PE by the same operator with extensive experience in echo-colour Doppler (7,5 Mhz linear probe, Ascendus, Hitachi, Japan).
In detail, common iliac vein, common femoral vein, superficial femoral vein, saphenofemoral junction, popliteal vein and posterior tibial vein were investigated. Each vein was examined in both longitudinal and cross-section scansions. Ultrasonographic criteria for deep-vein thrombosis were non-compressibility or incomplete compressibility of the vein [14].
Statistical Analysis
Baseline characteristics of patients were reported as mean ± standard deviation (SD) for continuous variables and as a percentage (%) for categorical variables. Continuous variables were compared using Student’s t-test for independent samples and Chi-Square Test for dichotomous variables. Univariate analysis was used for significant parameters.
Data analyses were conducted using SPSS (version 16 for Windows; SPSS, Chicago, Ill., USA). Values of p <0.05 were considered as statistically significant.
Results
Baseline characteristics of our population are shown in Table 1.
Table 1.
Baseline characteristics of study population. Abbreviations: DVT= Deep venous thrombosis; PE= Pulmonary embolism; PASP= Pulmonary artery systolic pressure.
| Parameter | COPD (n=66) | NON COPD (n=50) | p-value |
| Age (yrs) | 71 (14) | 65 (18) | <0,05 |
| Males (n) | 28 (42%) | 36 (72%) | <0.01 |
| DVT (n) | 21 (31.8%) | 24 (48%) | <0.05 |
| Non-smokers (n) | 13 (20%) | 42(83%) | <0.01 |
| Current smokers (n) | 26 (40%) | 5 (11%) | <0.01 |
| Ex-smokers (n) | 27 (40%) | 3 (6%) | <0.01 |
| PE-central (n) | 31 (47%) | 12 (25%) | <0.05 |
| PE-segmental (n) | 34 (51%) | 35 (69%) | ns |
| PE-subsegmental (n) | 1 (2%) | 3 (6%) | ns |
| PaO2 (mmHg) | 64 (16) | 74 (18) | <0,05 |
| PaCO2 (mmHg) | 43 (11) | 38 (9) | <0,05 |
| PASP (mmHg) | 44 (11) | 42 (11) | ns |
| NT-proBNP (pg/ml) | 4870 (11420) | 4070 (8023) | ns |
| D-Dimer (µg/mL) | 20 (109) | 5 (3,6) | ns |
| Ejection fraction (%) | 50 (9,5) | 53 (8) | ns |
| Obesity (n) | 27 (42%) | 15 (34%) | ns |
| Trauma (n) | 5 (8%) | 1 (2%) | ns |
| Immobility (n) | 17 (27%) | 5 (11%) | <0,05 |
| Anticonceptionals (n) | 0 (0%) | 0 (0%) | ns |
| Malignancy (n) | 12 (19%) | 10 (23%) | ns |
| Atrial Fibrillation (n) | 12 (19%) | 5 (11%) | ns |
| Pneumonia | 13 (20%) | 6 (13%) | ns |
| Pace Maker (n) | 3 (5%) | 5 (11%) | ns |
| Ischemic cardiomyopathy (n) | 10 (15,9%) | 9 (20%) | ns |
| Arterial hypertension (n) | 38 (60%) | 28 (63%) | ns |
| Diabetes mellitus (n) | 15 (24%) | 13 (29%) | ns |
| Chronic renal failure (n) | 16 (25%) | 10 (22%) | ns |
After ruling out 33 subjects which did not satisfy inclusion criteria, a total number of 116 patients with diagnosis of PE were enrolled in the study. COPD was documented in 66 patients (56% of the total), whereas DVT was described in 45 individuals (39% of the total), with higher prevalence in patients without COPD than those with COPD (48% vs 32%, p<0.05).
Main risk factors for PE in our population were: obesity, immobility, malignancies and type 2 diabetes mellitus. Furthermore, patients with COPD were older and had lower PaO2, higher PaCO2 and lower prevalence of DVT (p<0.05 for all).
Only 32% of patients with COPD and PE showed DVT. These subjects had higher PaCO2 and ejection fraction (p<0.05 for all) and higher percentage of chronic renal failure and type 2 diabetes mellitus if compared to those without DVT (p<0.05 for all, see Table 2).
Table 2.
Characteristics of COPD group, according to the presence or absence of DVT. Abbreviations: PE= Pulmonary embolism; PASP= Pulmonary artery systolic pressure; β-2: Beta-2 agonist; FEV1= Forced expiratory volume in 1 second; FVC= Forced vital capacity.
| Parameter | COPD with DVT (n= 21) | COPD without DVT (n=45) | p-value |
| Age (yrs) | 67 (15) | 73 (12) | ns |
| Males (n) | 9 (42%) | 24 (54%) | ns |
| Non-smokers (n) | 5 (25%) | 17 (39%) | ns |
| Current smokers (n) | 4 (17%) | 8 (18%) | ns |
| Ex-smokers (n) | 12(58%) | 20 (43%) | ns |
| PE-central (n) | 12 (57%) | 17 (37.7%) | <0.05 |
| PE-segmental (n) | 9 (46%) | 27 (60%) | ns |
| PE-subsegmental (n) | 0 (0%) | 1 (4%) | ns |
| PaO2 (mmHg) | 58 (14) | 66 (20) | Ns |
| PaCO2 (mmHg) | 51 (12) | 40 (8) | <0,05 |
| PASP (mmHg) | 46 (12) | 43 (10) | ns |
| NT-proBNP (pg/ml) | 5019 (14900) | 4800 (9300) | ns |
| D-Dimer (µg/mL) | 438 (471) | 354(452) | ns |
| Ejection fraction (%) | 54 (7) | 49 (10) | <0,05 |
| Obesity (n) | 10 (47%) | 15 (35%) | ns |
| Trauma (n) | 0 (0%) | 1 (3%) | ns |
| Immobility (n) | 5 (24%) | 7 (17%) | ns |
| Anticonceptionals (n) | 0 (0%) | 0 (0%) | ns |
| Malignancy (n) | 7 (33%) | 6 (14%) | ns |
| Atrial Fibrillation (n) | 3 (14%) | 7 (17%) | ns |
| Pneumonia | 2 (9,5%) | 8 (19%) | ns |
| Pace Maker (n) | 0 (0%) | 6 (14%) | ns |
| Ischemic cardiomyopathy (n) | 3 (14%) | 9 (21%) | ns |
| Arterial hypertension (n) | 14 (66%) | 27 (64%) | ns |
| Diabetes mellitus (n) | 10 (47%) | 11 (26%) | <0.05 |
| Chronic renal failure (n) | 18 (47%) | 8 (13%) | <0,05 |
| Post β-2 FEV1 (%pred) | 79 (18) | 78 (29) | ns |
| Post β-2 FVC (%pred) | 86 (24) | 85 (22) | ns |
Moreover, in COPD-PE patients with DVT, the most frequent localization was proximal (57% of total), whereas COPD-PE patients without DVT showed a more frequent segmental localization (60% of total, see Table 2).
Discussion
To the best of our knowledge, this is the first study which investigates a population of PE-COPD cases with and without DVT.
PE is known to originate from embolization of a DVT, but in up to 50% of patients with PE no DVT is detected by ultrasonography or contrast venography studies [15-16].
We found a somewhat low prevalence of DVT (33%) in patients with both PE and COPD. This is in line with the lower prevalence of DVT compared to that of PE in COPD patients [1-2,4] and confirms that COPD may be an independent risk factor for PE. On the other hand, higher percentages of DVT (i.e. 60%) were described by Erel et al and Gunen et al (60% and 61%, respectively) [17-18].
Despite similar smoking status, our population of COPD-PE with DVT had higher values of PaCO2, possibly indicating a higher severity of their respiratory status or the presence of a concomitant COPD exacerbation. The latter condition may lead to patient immobility and promote DVT. Nonetheless, similarly to the findings of Akgun et al [19], no difference in terms of immobility was reported between COPD-PE with and without DVT. Moreover, it has to be highlighted the higher incidence of renal failure in COPD-PE patients with DVT; Wattanakit et al found that middle-aged and elderly patients with chronic kidney disease (stages 3 and 4) have an increased risk for incident DVT [20], but mechanisms linking chronic kidney disease and DVT are not well defined. Moreover, another study showed that patients with DVT and chronic renal disease were younger than those with only DVT [21], and these findings are in line with our data.
COPD-PE Patients with DVT had also higher prevalence of diabetes mellitus, a well-known risk factor for DVT [11,22-23]. Indeed, Diabetes mellitus is associated with several changes in coagulation and fibrinolysis that may lead to a thrombogenic susceptibility [11,22-23].
Regarding PE-COPD patients without DVT, also known as “de novo PE”, our data showed no differences in prevalence of trauma, active malignancies and right heart thrombi between the subgroups with and without DVT. Undeniably, well-known risk factors for de novo PE reported in literature are trauma [24], active cancer [25], inferior vena cava abnormalities, right heart thrombi and in situ thrombosis [26]. Therefore, based on our findings, we might speculate a role of COPD as risk factor for the development of PE. Several mechanisms may explain the link between COPD and VTE, such as thrombogenic potential within the pulmonary circulation [4-27], systemic inflammation [28]; elderly, reduced mobility [29], policytemia [30] smoking [31] and treatment with glucocorticoids for acute exacerbation of disease [32]. Concerning the old age of our cohort, it was previously reported that the diagnostic yield of lower limb compression ultrasonography was greater in the elderly, so the risk of wrong diagnosis on echography is very low in our cohort [33].
The localization of PE was formerly investigated by other authors. In detail, Aleva et al reported that in patients with acute exacerbations of COPD of unknown aetiology 68% of the detected emboli were located in the main pulmonary arteries, lobar arteries, or interlobar arteries [6]. Similar data were reported by Tillie Leblond et al [34]. Contrariwise, Akpinar et al documented a mainly subsegmental localization among hospitalized patients with COPD exacerbation [35].
Our study had several limitations. First, patients did not perform upper-extremity venous duplex scans (UED), even though cases of PE originating from upper limbs DVT are rare and usually linked to traumatic events or presence of central venous catheters [36]. Second, we did not define in our population the prevalence of exacerbated COPD and the severity of COPD. However, a number of studies did not report significant differences in prevalence of PE among COPD-GOLD stages [18-35]. Third, we did not evaluate blood inflammatory markers such as C-reactive protein or leucocytes and haemoglobin and haematocrit, very useful parameters for assessing differences in terms of systemic inflammation and polycytemia. Finally, we did not include in the study data about clinical manifestation and Wells score, since the diagnosis of EP was made at the emergency department. Undoubtedly, further studies with larger sample sizes must include all of the above parameters.
In conclusion, early recognition of PE is of vital importance but is may be challenging in patients with COPD, due to the overlap of clinical symptoms. Moreover, a negative result at venous ultrasonography is more frequent in cases of PE with COPD. Chronic renal failure, and type 2 diabetes mellitus are more frequent in PE-COPD patients with DVT showed a higher frequency of proximal localization, chronic renal failure and type 2 diabetes mellitus, thereby indicating a greater risk of more severe clinical implications. Conversely, PE- COPD subjects without DVT showed a more frequent segmental localization and were less hypercapnic.
Therefore, PE should be always taken into account in patients with COPD with worsening of symptoms and gas exchange parameters.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Bertoletti L, Quenet S, Mismetti P, et al. Clinical presentation and outcome of venous thromboembolism in COPD. European Respiratory Journal. 2012;39:862–868. doi: 10.1183/09031936.00058811. [DOI] [PubMed] [Google Scholar]
- Schneider C, Bothner U, Jick SS, Meier CR. Chronic obstructive pulmonary disease and the risk of cardiovascular diseases. European journal of epidemiology. 2010;25:253–260. doi: 10.1007/s10654-010-9435-7. [DOI] [PubMed] [Google Scholar]
- MacNee W. Systemic inflammatory biomarkers and co-morbidities of chronic obstructive pulmonary disease. Ann Med. 2013;45:291–300. doi: 10.3109/07853890.2012.732703. [DOI] [PubMed] [Google Scholar]
- Park SH. Pulmonary embolism is more prevalent than deep vein thrombosis in cases of chronic obstructive pulmonary disease and interstitial lung diseases. Springer Plus. 2016;5:1777. doi: 10.1186/s40064-016-3475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizkallah J, Man SFP, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009;135:786–793. doi: 10.1378/chest.08-1516. [DOI] [PubMed] [Google Scholar]
- Aleva FE, Voets LW, Simons SO, de Mast Q, van der Ven AJMA, Heijdraet YF. Prevalence and localization of pulmonary embolism in unexplained acute exacerbations of COPD: a systematic review and meta-analysis. Chest. 2017;151:544–554. doi: 10.1016/j.chest.2016.07.034. [DOI] [PubMed] [Google Scholar]
- Monreal M, Munoz-Torreiro JF, Naraine VS, Jiménez D, Soler S, Rabuñal R, Gallego P. Pulmonary embolism in patients with chronic obstructive pulmonary disease or congestive heart failure. The American journal of medicine. 2006;119:851–858. doi: 10.1016/j.amjmed.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Piazza G, Goldhaber SZ, Kroll A, Goldberg RJ, Emery C, Spencer FA. Venous thromboembolism in patients with chronic obstructive pulmonary disease. The American journal of medicine. 2012;125:1010–1018. doi: 10.1016/j.amjmed.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson JL, Terrin ML, Duff A, Kelley MA. Pulmonary embolism and mortality in patients with COPD. Chest. 1996;110:1212–1219. doi: 10.1378/chest.110.5.1212. [DOI] [PubMed] [Google Scholar]
- Zielinski J, Mac Nee N, Wedzicha J, et al. Causes of death in patients with COPD and chronic respiratory failure. Monaldi Arch Chest Dis. 1997;52:43–47. [PubMed] [Google Scholar]
- Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs SJR, Huisman MV, Humbert M, et al. ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3069. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- Stein PD, Beemath A, Meyers FA, Olson RE. Pulmonary embolism and deep venous thrombosis in hospitalized adults with chronic obstructive pulmonary disease. Journal of Cardiovascular Medicine. 2007;8:253–257. doi: 10.2459/01.JCM.0000263509.35573.2c. [DOI] [PubMed] [Google Scholar]
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 Available from: http://goldcopd.org. [Google Scholar]
- Lensing AW, Prandoni P, Brandjes D, et al. Detection of deep-vein thrombosis by real-time b-mode ultrasonography. N Engl J Med. 1989;320:342–345. doi: 10.1056/NEJM198902093200602. [DOI] [PubMed] [Google Scholar]
- Yamaki T, Nozaki M, Sakurai H, Takeuchi M, Soejima K, Kono T. Presence of lower limb deep vein thrombosis and prognosis in patients with symptomatic pulmonary embolism: preliminary report. Eur J Vasc Endovasc Surg. 2009;37:225–231. doi: 10.1016/j.ejvs.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Girard P, Sanchez O, Leroyer C, Musset D, Meyer G, Stern JB, Parent F. Deep venous thrombosis in patients with acute pulmonary embolism: prevalence, risk factors, and clinical significance. Chest. 2005;128:1593–600. doi: 10.1378/chest.128.3.1593. [DOI] [PubMed] [Google Scholar]
- Erelel M, Ece T, Arseven O. The frequency of deep venous thrombosis and pulmonary embolus in acute exacerbation of chronic obstructive pulmonary disease. Respiratory Medicine. 2002;96:515–518. doi: 10.1053/rmed.2002.1313. [DOI] [PubMed] [Google Scholar]
- Gunen H, Gulbas G, In E, Yetkin O, Hacievliyagil SS. Venous thromboemboli and exacerbations of COPD. Eur Respir J. 2010;35:1243–1248. doi: 10.1183/09031936.00120909. [DOI] [PubMed] [Google Scholar]
- Akgun M, Meral M, Onbas O, Araz O, Koplay M, Aslan S, Mirici A. Comparison of clinical characteristics and outcomes of patients with COPD exacerbation with or without venous thromboembolism. Respiration. 2006;73:428–433. doi: 10.1159/000092952. [DOI] [PubMed] [Google Scholar]
- Wattanakit K, Cushman M, Stehman-Breen, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. Journal of the American society of Nephrology. 2008;19:135–140. doi: 10.1681/ASN.2007030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneschvar HL, Seddighzadeh A, Piazza G, Goldhaber SZ. Deep vein thrombosis in patients with chronic kidney disease. Thrombosis and haemostasis. 2008;99:1035–1039. doi: 10.1160/TH08-02-0107. [DOI] [PubMed] [Google Scholar]
- Petrauskiene V, Falk M, Waernbaum I, Norberg M, Eriksson JW. The risk of venous thromboembolism is markedly elevated in patients with diabetes. Diabetologia. 2005;48:1017–1021. doi: 10.1007/s00125-005-1715-5. [DOI] [PubMed] [Google Scholar]
- Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- Van Gent JM, Zander AL, Olson EJ, Shackford SR, Dunne CE, Sise CB, Badiee J, Schechter MS, Sise MJ. Pulmonary embolism without deep venous thrombosis: De novo or missed deep venous thrombosis? Journal of Trauma and Acute Care Surgery. 2014;76:1270–1274. doi: 10.1097/TA.0000000000000233. [DOI] [PubMed] [Google Scholar]
- Schwartz T. Pulmonary embolism without deep venous thrombosis. Annals of vascular surgery. 2012;26:973–976. doi: 10.1016/j.avsg.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Barrios D, Rosa-Salazar V, Jiménez D, Morillo R, Muriel A, Del Toro J, López-Jiménez L, Farge-Bancel D, Yusen R, Monreal M. Right heart thrombi in pulmonary embolism. European Respiratory Journal. 2016; Nov;48:1377–1385. doi: 10.1183/13993003.01044-2016. [DOI] [PubMed] [Google Scholar]
- Voelkel NF, Cool CD. Pulmonary vascular involvement in chronic obstructive pulmonary disease. Eur Respir J. 2003;22:28s–32s. doi: 10.1183/09031936.03.00000503. [DOI] [PubMed] [Google Scholar]
- Levi M, van der Poll T, Schultz M. New insights into pathways that determine the link between infection and thrombosis. Neth J Med. 2012;70:114–20. [PubMed] [Google Scholar]
- Shetty R, Seddighzadeh A, Piazza G, Goldhaber SZ. Chronic obstructive pulmonary disease and deep vein thrombosis: a prevalent combination. Journal of thrombosis and thrombolysis. 2008;26:35–40. doi: 10.1007/s11239-007-0157-y. [DOI] [PubMed] [Google Scholar]
- Biswas M, Prakash PK, Cossburn M, Myers K, Hanna F. Life threatening thrombotic complications of relative polycythaemia. J Intern Med. 2003;253:481–483. doi: 10.1046/j.1365-2796.2003.01126.x. [DOI] [PubMed] [Google Scholar]
- Kim V, Goel N, Gangar J, Zhao H, Ciccolella DE, Silverman EK, Crapo JD, Criner GJ. Risk factors for venous thromboembolism in chronic obstructive pulmonary disease. Chronic obstructive pulmonary diseases. 2014;1:239. doi: 10.15326/jcopdf.1.2.2014.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesdottir SA, Horvath-Puho E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013;173:743–752. doi: 10.1001/jamainternmed.2013.122. [DOI] [PubMed] [Google Scholar]
- Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med. 2000;109:357–361. doi: 10.1016/s0002-9343(00)00493-9. [DOI] [PubMed] [Google Scholar]
- illie-Leblond I, Marquette CH, Perez T, Scherpereel A, Zanetti C, Tonnel AB, Remy-Jardin M. Pulmonary embolism in patients with unexplained exacerbation of chronic obstructive pulmonary disease: prevalence and risk factors. Annals of internal medicine. 2006;144:390–396. doi: 10.7326/0003-4819-144-6-200603210-00005. [DOI] [PubMed] [Google Scholar]
- Akpinar EE, Hoşgün D, Akpinar S, Ataç GK, Doğanay B, Gülhan M. Incidence of pulmonary embolism during COPD exacerbation. Jornal Brasileiro de Pneumologia. 2014;40:38–45. doi: 10.1590/S1806-37132014000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaijpoel N, van Es N, Porreca E, Büller HR, Di Nisio M. The diagnostic management of upper extremity deep vein thrombosis: A review of the literature. Thromb Res. 2017;156:54–59. doi: 10.1016/j.thromres.2017.05.035. [DOI] [PubMed] [Google Scholar]


