Abstract
Background:
Priapism is defined as a penile erection that persists four or more hours and is unrelated to sexual stimulation. Priapism resulting from hematologic malignancy is most likely caused by venous obstruction from microemboli/thrombi and hyperviscosity caused by the increased number of circulating leukocytes in mature and immature forms. In patients with leukemia, 50% of cases of priapism are due to Chronic Myeloid Leukemia (CML). We present a systematic review of priapism in CML.
Acquisition of evidence:
An extensive literature research was carried out in PubMed, Google Scholar, SCOPUS, and Science Citation Index databases. The search included cases up to 4th August 2020.
Synthesis of evidence:
A total of 68 articles were found and included in our review, including 3 reviews from three different centers. We found 68 articles (102 patients; figure 1) and several case reports on priapism in CML. Priapism was noticed in some patients at the first presentation of CML. However, it was infrequently reported during the start of treatment, following the stop of medication and post-splenectomy. The mean age at presentation was 27.4 years, and the mean time from onset of priapism to the time to get medical attention (presentation) was 78.2 hours. The mean white blood cell count associated with priapism was 321.29x109/L, and the mean platelet count was 569 x10 9/L. The chronic phase of CML was the most common phase where priapism occurred. Most patients were Asian (>50%). Nearly a quarter of patients (27.4%) developed permanent erectile dysfunction.
Conclusions:
Priapism is a urological emergency requiring urgent multidisciplinary management to prevent erectile dysfunction. Because of the relatively rare occurrence of priapism in CML patients, there is no standard treatment protocol. (www.actabiomedica.it)
Keywords: Chronic myeloid leukemia, Chronic granulocytic leukemia, Erectile dysfunction, Priapism, Male fertility
Introduction
Hematological disorders are the leading cause of priapism, accounting for 20% of the cases and include SCD, hyperviscosity syndromes as seen with the myeloproliferative diseases, hypercoagulable states such as deficiencies of proteins C and S, antiphospholipid syndromes, And amyloidosis (1). Priapism is defined as a penile erection that persists four or more hours and is unrelated to sexual stimulation (2). The condition is classified into three subtypes: ischemic (low-flow), non-ischemic (high-flow), and stuttering (intermittent) priapism (3). Stuttering priapism is characterized by a recurrent and intermittent erection, frequently occurring in a specific patient population with SCD and less commonly with thalassemia (4). The clinical presentation of CML consists of lymphadenopathy (80%), asthenia, and fatigue (60%), spleen or liver enlargement (50%), weight loss (15–20%), and bleeding (10%), hyperleukocytosis about 80%, central nervous system affection (15%) kidney (5%) and priapism (≤ 3%) (5). Priapism due to hematological disorder is most likely due to venous obstruction from microemboli/thrombi as well as hyperviscosity due to an increased number of circulating leukocytes n mature and immature forms. Other accessory mechanisms are venous congestion of the corpora cavernosa secondary to mechanical pressure from the abdominal veins draining the spleen or infiltration of the sacral nerves or the central nervous system by leukemia cells (6). It is also seen that increased production of cytokines and adhesion molecules by leukemia cells result in endothelial cell activation and lead to increased sequestration of cells in the microvasculature (7).
Prolonged corporeal ischemia lasting more than 24 to 48 hours may result in varying extents Of irreversible fibrosis with endothelial and trabecula destruction of the erectile tissue and Subsequently in permanent erectile dysfunction and, therefore, is considered as a urologic Emergency. Reduced sperm count related to TKIS in patients with CML as well as priapism adversely affects the quality of life, particularly in populations where adolescent and young adults represent the majority of patients (8,9).
The objectives of this review were to: (a) assess the characteristics and risk factors of CML patients with priapism, (b) realize the common type of priapism in CML, (c) describe the management options adopted for priapism in CML, and (d) investigate the outcome and erectile dysfunction.
Acquisition of evidence
We searched the English literature (Google Scholar, PubMed, SCOPUS, and Science Citation Index databases) including original articles, reviews, case series, and case reports using the terms: “chronic myeloid leukemia,” “chronic myelogenous leukemia”, “chronic myelocytic leukemia” and “priapism”. A total of 68 articles were found and included in our review, among them 3 reviews were found from three different centers. The search included cases up to 4th August 2020.
Synthesis of evidence
We found 68 articles on priapism in CML (102 patients; figure 1) and several case reports (10-77). The youngest patient was 7 weeks old, and the oldest was 60 years old. The first case was reported in 1960 and the last in 2020. Most patients had priapism at their first presentation of CML. Three patients developed priapism after starting CML treatment, two after stopping treatment, and two were previously diagnosed. 80 /102 patients had splenomegaly (on clinical examination or by US abdomen), and 31/102 had hepatomegaly. The lowest white blood cell count (WBC) associated with priapism was 37 x10 9/L, and the highest was 782 x10 9/L.
Figure 1.
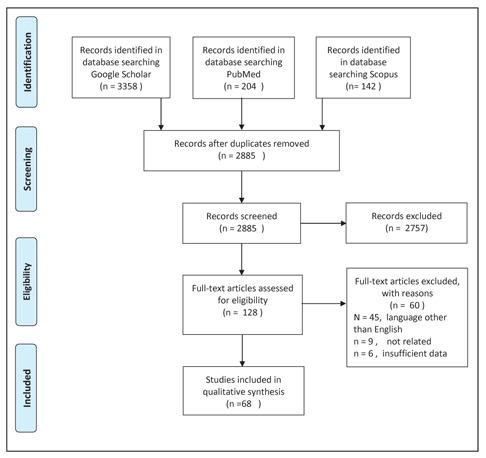
The PRISMA flow diagram detailing the cases of chronic myeloid leukemia (CML) presenting priapism.
Table 1 presents the characteristics of stuttering and ischemic priapism in patients with CML.
Table 1.
shows the characteristics of CML patients with priapism
| Referenced | Age in Years | Priapism from onset to presentation to the hospital | First presentation or previously | Type of priapism | WBC x10^9/L | PLT x10^9/L | HB g/dL | Splenomegaly/ hepatomegally, below costal margin in cm or if presdnt | stage of CML |
| 10 | 18 | 72 hours | Yes | ischemic low flow | 100 | 1,002 | 6 | spleen 2-3 cm | chronic phase |
| 11 | 15 | 2 days | Yes | ischemic low flow | 135 | 197 | 9 | spleen to the umbilicus | chronic phase |
| 12 | 52 | 4 hours | Yes | ischemic low flow | 239 | 625 | 8.9 | spleen 8cm liver 3 cm | chronic phase |
| 13 | 19 | 18 hours | Yes | ischemic low flow | 513 | NA | NA | N/A | chronic phase |
| 14 | 38 | 30 hours | Yes | ischemic low flow | 378 | 155 | 10.5 | spleen 18 cm | chronic phase |
| 15 | 52 | 6 days | Yes | ischemic low flow | 282 | 368 | 10 | N/A | chronic phase |
| 16 | 21 | 72 hours | Yes | ischemic low flow | 619 | N/A | 7.4 | spienomegally | chronic phase |
| 17 | 30 | 9 days | Yes | ischemic low flow | 261 | 86 | 9.2 | spleen 10 cm | chronic phase |
| 18 | 29 | 3 days | Yes | ischemic low flow | 366.34 | 622 | 6.7 | spleen 5 cm, liver 2 cm | chronic phase |
| 19 | 20 | 4 hours | Yes | ischemic low flow | 158 | N/A | 10.9 | spleen 8 cm | chronic phase |
| 20 | 18 | 14 hours | Yes | ischemic low flow | 363 | 527 | 9.7 | spleen 9 cm | accerated |
| 21 | 55 | 2 days | Yes | ischemic low flow | 420 | 280 | 9.5 | spleen up to umbilicus , liver 2 cm | chronic phase |
| 22 | 17 | NA | Yes | ischemic low flow | 377,31 | 730 | 11.3 | Hepatosplenomegaly Blasts+ Promyelocytes-12% | chronic phase |
| 23 | 21 | 19 hours | Yes | ischemic low flow | 216 | 1746 | 8.3 | spleen 7 cm, liver 6 cm | chronic phase |
| 24 | 28 | 2 days | Yes | ischemic low flow | 294 | 94 | 6.6 | spleen 2 | chronic phase |
| 25 | 22 | 4 days | Yes | ischemic low flow | 218.6 | 324,2 | 8.2 | spleen 6 | chronic phase |
| 26 | 21 | 6 days | Yes | ischemic low flow | 410 | N/A | N/A | spleen 3 fingers | chronic phase |
| 27 | 18 | 75 hours | Yes | ischemic low flow | 323 | N/A | N/A | N/A | chronic phase |
| 28 | 18 | 7 days | Yes | ischemic low flow | 257 | 5450 | N/A | spleen 2-3 | chronic phase |
| 29 | 18 | 4 days | Yes | ischemic low flow | 144 | 350 | 9.6 | spleen 9-10 cm liver 2-3 cm | chronic phase |
| 30 | 30 | 16 hous | after stopping | ischemic low flow | 210 | 45 | 10.3 | huge splenomegaly | chronic phase |
| 31 | 18 | 5 days | Yes | ischemic low flow | 215 | 470 | 6.9 | spleen 10 cm | chronic phase |
| 32 | 18 | 10 days | after start of treatment | ischemic low flow | 320 | normal | 6.5 | hepato-splenomegaly. | blast crisi |
| 33 | 21 | 8 hours | Yes | ischemic low flow | 316 | 670 | 8.3 | liver palpable 6 cm , spleen 7 cm | chronic phase |
| 33 | 55 | 12 hours | Yes | ischemic low flow | 282 | 260 | 9 | spleen 7 cm | chronic phase |
| 34 | 36 | 5 days | diagnosed at 33 | ischemic low flow | N/A | N/A | N/A | N/A | chronic phase |
| 25 | 33 | 22 hours | Yes | ischemic low flow | 400 | 1 200 | 10.53 | spleen 4 cm | chronic phase |
| 36 | 16 | 11 days | Yes | ischemic low flow | 614.8 | 907 | 5.7 | liver 2cm spleen 4cm | blast crisis |
| 37 | 30 | 20 hours | Yes | ischemic low flow | 285 | 462 | 11.2 | spleen 2cm liver 3 cms | chronic phase |
| 37 | 25 | 2 days | Yes | ischemic low flow | 670 | 320 | 11.3 | spleen 1 cms | chronic phase |
| 37 | 28 | 7 hours | Yes | ischemic low flow | 441 | 422 | 7 | spleen 15 cms | chronic phase |
| 38 | 14 | 24 hours | Yes | ischemic low flow | 226.9 | 310 | 9.9 | spleen 6 cm | chronic phase |
| 39 | 16 | NA | Yes | ischemic low flow | 320 | 417 | 11 | spleen 4 cm | chronic phase |
| 40 | 25 | 16 hour | Yes | ischemic low flow | 501 570 | 269 | 10.3 | no organomegally | chronic phase |
| 41 | 9 | 9 hours | Yes | ischemic low flow | 274 | 1235 | 8.2 | spienomegally | blast crisis |
| 42 | 55 | 8 hours | Yes | ischemic low flow | 184 | 277 | 9 | spleen 3 cm liver 2 cm | chronic phase |
| 43 | 23 | 3 days | Yes | ischemic low flow | 660 | 321 | 8 | hepatosplenomegaly | chronic phase |
| 44 | 47 | 5 days | Yes | ischemic low flow | 297 | N/A | N/A | hepatosplenomegaly | chronic phase |
| 44 | 42 | 7 days | Yes | ischemic low flow | 390 | N/A | N/A | spleen 6 cm | chronic phase |
| 44 | 28 | 6 days | Yes | ischemic low flow | 206 | N/A | N/A | no hepatosplenomegally | chronic phase |
| 45 | 7 weeks | 5 days | Yes | ischemic low flow | 37 | 344 | 10 | liver and spleen were palpated below the costal margin. | blast crisis |
| 46 | 22 | 8 hours | Known CML, stopped medications | ischemic low flow | 157 | 670 | 5.4 | hepato-splenomegaly | chronic phase |
| 47 | 43 | 48 hours | NA? | ischemic low flow | N/A | N/A | N/A | N/A | blast crisis |
| 48 | 37 | 96 hours | Yes | ischemic low flow | 150 | 215 | 13.9 | N/A | chronic phase |
| 49 | 22 | N/A | after day 4 of treatment | ischemic low flow | 297 | N/A | N/A | hepatosplenomegaly | chronic phase |
| 50 | 29 | deveoped priapism after starting treatment busalfan and allopyranol in the 5th hospital admission | 5th day | ischemic low flow | 296 | 800 | N/A | spleen 4 cm | chronic phase |
| 51 | 46 | duration after presentation 22 days | Yes | ischemic low flow | 782 | 400 | N/A | spleen 14 cm | chronic phase |
| 51 | 42 | nonsustained | Yes | ischemic low flow | 367 | 179 | N/A | spleen 9 cm | chronic phase |
| 51 | 42 | 24 hr | Yes | N/A | 402 | 500 | N/A | spleen 10 cm | chronic phase |
| 51 | 18 | 4 dasys | Yes | N/A | 451 | 726 | N/A | spleen 18cm | chronic phase |
| 52 | 45 year | 18 hrs | Yes | N/A | N/A | N/A | N/A | N/A | chronic phase |
| 52 | 58 | 48 hrs | Yes | N/A | N/A | N/A | N/A | N/A | chronic phase |
| 52 | 40 | 12 hrs | Yes | N/A | N/A | N/A | N/A | N/A | chronic phase |
| 52 | 38 | 29 hr | Yes | N/A | N/A | N/A | N/A | N/A | chronic phase |
| 52 | 60 | 6 hrs | Previously diagnosed | N/A | N/A | N/A | N/A | N/A | chronic phase |
| 52 | 39 | 72 hrs | Yes | N/A | N/A | N/A | N/A | N/A | chronic phase |
| 53 | 9 | 3 Days | Yes | N/A | 155 | N/A | N/A | N/A | chronic phase |
| 53 | 11 | 5 days | Yes | N/A | 210 | N/A | N/A | N/A | chronic phase |
| 53 | 13 | 4 days | Yes | N/A | 120 | N/A | N/A | N/A | chronic phase |
| 53 | 10 | 3 days | Yes | N/A | 200 | N/A | N/A | N/A | chronic phase |
| 53 | 8 | 2 days | Yes | N/A | 170 | N/A | N/A | N/A | chronic phase |
| 54 | 22 | 6 days | Yes | N/A | 580 | N/A | 7.2 | spleen 22 cm, liver 10 cm | chronic phase |
| 54 | 30 | 8 days | Yes | N/A | 300 | N/A | 6 | hepatosplenomegally | chronic phase |
| 55 | 28 | 11 days | Yes | N/A | 400 | adequate | 11.75 | spleen 13 cm | chronic phase |
| 56 | 26 | N/A | Yes | ischemic low flow | 365 | 625 | N/A | spleen 17 cm liver 4 cm | chronic phase |
| 57 | 36 | N/A | Yes | ischemic low flow | 231 | 113 | 12 | hepatosplenomegaly by US | chronic phase |
| 58 | 8 | 1 day | Yes | ischemic low flow | 430 | 200 | 9 | liver 1 cm, spleen down to the left inguinal ligament | chronic phase |
| 59 | 12 | 3 days | Yes | ischemic low flow | 172 | N/A | N/A | spienomegally | chronic phase |
| 60 | 45 | 2 days | Yes | ischemic low flow | 410 | 350 | 6 | massive spleenomegally | chronic phase |
| 61 | 22 | 5 days | Yes | iscemic low flow | 200 | increased | 7.5 | spleen 15 cm liver just palpable | chronic phase |
| 62 | 12 | 2 days | Yes | stuttering - ischemic | 460 | 350 | 8.2 | spleen 22 cm and hepatomegaly 4 cm. | chronic phase |
| 63 | 24 | 5 days | Yes | stuttering - ischemic | 207 | 545 | 10 | spleen 8 cm | chronic phase |
| 64 | 30 | 8 days | Yes | stuttering - ischemic | 240 | 186 | 7.5 | liver 1 cm spleen 6 cm | chronic phase |
| 64 | 18 | 18 hour | Yes | stuttering - ischemic | 288 | 388 | 8.7 | liver 3 spleen 10 | chronic phase |
| 64 | 32 | 4 days | Yes | stuttering - ischemic | 540 | 210 | 12.4 | liver 3 spleen 15 | chronic phase |
| 65 | 24 | 14 hours | Yes | stuttering - ischemic | 177.15 | N/A | 10.3 | N/A | chronic phase |
| 65 | 29 | 6 hours | Yes | stuttering - ischemic | 402.24 | N/A | 8.2 | N/A | chronic phase |
| 56 | 60 | 2 weeks | splenectomy 16 months before | stuttering - ischemic | 360 | 222 | N/A | had splenomegally, splenectomy | chronic phase |
| 66 | 13 | 3 days | Yes | stuttering - ischemic | 350 | 450 | 8.5 | spleen 4cm | chronic phase |
| 67 | 24 | prolonged | Yes | stuttering - ischemic | 540 | N/A | HCT 25% | spleen 7 cm | chronic phase |
| 51 | 33 | 3-5 days | Yes | stuttering - ischemic | 197 | 350 | N/A | spleen 14 cm | chronic phase |
| 51 | 27 | NA | Yes | stuttering - ischemic | 202 | 900 | N/A | spleen 12cm | chronic phase |
| 51 | 33 | 38 days | Yes | ischemic - stuttering | 240 | 1150 | N/A | spleen 19 cm | chronic phase |
| 51 | 28 | 70 days | Yes | stuttering - ischemic | 186 | 218 | N/A | spleen 7 cm | chronic phase |
| 51 | 28 | 24 hr | Yes | stuttering - ischemic | 500 | 345 | N/A | spleen 14 cm | chronic phase |
| 68 | 11 | 12 hours | Yes | stuttering - ischemic | 290 | 550 | he ma toe re te 22% | spleen 4 cm, mild hepatomegally | chronic phase |
| 51 | 23 | duration after presentation 36 days | Yes | ischemic - stuttering | 470 | 180 | N/A | spleen 14 | chronic phase |
| 50 | 17 | 24 hours | Yes | stuttering - ischemic | 290 | 230 | hematocrit: 28 per cent; | massively enlarged spleen, occupying the entire left half of the abdominal cavity | chronic phase |
| 69 | 7.5 | few hours | Yes | stuttering ischemic | 337 | N/A | 7 | splenomegally | chronic phase |
| 70 | 15 | 2 times resolved spontaneously | Yes | stuttering - ischemic | 480 | 130 | 9.4 | mildly enlarged on US | chronic phase |
| 41 | 9 | sveral days | Yes | stuttering - ischemic | 509 | 1200 | 10.1 | hepato-splenomegaly | chronic phase |
| 41 | 9 | 4 days | Yes | stuttering - ischemic | 169 | 663 | 10.3 | splenomegally | chronic phase |
| 71 | 36 | 34 hour | Yes | ischemic - stuttering | 65 | 800 | N/A | spleen 12cm | chronic phase |
| 71 | 30 | 4 day | Yes | ischemic - stuttering | 356.4 | 220 | PCV. 22 L/L | liver 6cm, spleen 10cm | chronic phase |
| 37 | 29 | 3 months duration | Yes | stuttering - ischemic | 284 | 370 | 10.5 | spleen 3 cm | chronic phase |
| 72 | 18 | 12 days | Yes | stuttering - ischemic | 199 | 504 | HC 17 | spleen 18 cm | chronic phase |
| 37 | 26 | 3 days | Yes | stuttering - ischemic | 292 | 490 | 8.9 | spleen 15 cm | chronic phase |
| 73 | 12 | 2 dyas | Yes | ischemic - stuttering | 346 | 924 | 9 | liver and spleen were enlarged, 5 cm | chronic phase |
| 74 | 22 | one month intermittent | Yes | stuttering-ischem ic | 185 | N/A | 10.7 | splenomegaly on US | chronic phase |
| 75 | 19 | over 24 hours | Yes | stuttering ischemic | 296 | 936 | 9.2 | spleen 3 cm , liver 1-2 | chronic phase |
| 76 | 27 | 9 hours | diagnosed 19 y | stuttering - ischemic | 450.01 | 509 | 11.4 | splenomegally on US | chronic phase |
| 77 | 18 | 6hours | Yes | ischemic stuttering | 588 | 109 | 7.3 | N/A | chronic phase |
Most patients had lower hemoglobin (Hb) levels, and in two reviews, the CML patients with priapism had lower Hb levels than their matched CML patients who didn’t have priapism.
Treatment modalities (Table 2) included medications, aspiration, and irrigation to the corpora cavernosa, radiotherapy, leukoreduction, and surgical shunts. Medications were used in 59 CML patients, aspiration of the corpora cavernosa in 49 patients, leukapheresis in 19 patients, radiotherapy in 9 patients, and shunt in 40 patients.
Table 2.
Treament modalites of priapism and the outcome of erectile dysfunction
| patient refernce | medications for priapism | Aspiration and irrigation | leukophresis | irradiation | shunt | Best responded to | Erectile dysfunction |
| 10 | Imatinib | No | Yes | No | No | leukapheresis | No |
| 11 | No | Yes | No | No | glanocorposal shunt, corporospongiosal shunt | shunt | N/A |
| 12 | No | Yes | No | No | Winter’s shunt | Winter’s shunt | No |
| 13 | No | Yes | Yes | No | No | leukapheresis | No |
| 14 | Yes | Yes | Yes | No | No | after leukophresis and medication | N/A |
| 15 | No | Yes | No | No | surgery penis shunts | surgery penis shunts | N/A |
| 16 | No | Yes | No | No | Winter’s shunt | Winter’s shunt partial response, hydroxyurea after combined complete response | Yes |
| 17 | Yes | Yes | No | No | bilateral T-shunts | afte shunt | Yes |
| 18 | No | No | No | No | Winter shunt | Winter shunt | N/A |
| 19 | No | Yes | No | No | No | cavernosa aspiration, epinephrine irrigation | No |
| 20 | No | Yes | No | No | No | cavernosa aspiration and irrigation with epinephrine | No |
| 21 | No | No | No | No | corpus cavernosa-glans shunt | corpus cavernosa-glans shunt | N/A |
| 22 | No | Yes | No | No | No | Aspiration following intra- cavernosal injection of phenylephrine. | N/A |
| 23 | No | Yes | No | No | No | cavernosa aspiration and epinephrine irrigation | No |
| 24 | hydroxyurea, allopurinol, Cytarabine | Yes | No | No | corporoglandular shunting | corporoglandular shunting | N/A |
| 25 | No | Yes | No | No | No | aspiration and irrigation of the corpora cavernosa | N/A |
| 26 | imitinib | Yes | Yes | No | No | Failed aspiration, Leukapheresis ended in, penile prosthesis, | Yes |
| 27 | allopurinol hydroxyurea | Yes | Yes | No | transglandular cavemosum- spongiosum shunt | transglandular cavemosum- spongiosum shunt | N/A |
| 28 | No | Yes | No | No | proximal corpora cavernosa-corpus spongiosum shunt | surgical proximal corpora cavernosa-corpus spongiosum shunt | No |
| 29 | No | No | Yes | Yes | No | improved after 48 hr leukapheresis procedure | N/A |
| 30 | No | Yes | No | No | No | aspiration and irrigation with ephedrine | Yes |
| 31 | hydroxyurea | No | Yes | No | No | hydroxyurea ,leukopheresis 5 sessions | N/A |
| 32 | hydroxyurea allopurinol | No | No | No | Winter’s shunt | shunt, hydroxyurea, allopurinol failed | Yes |
| 33 | oral pentazocaine, Allopurinol Hydroxyurea, busulpha n | No | No | No | No | Allopurinol Hydroxyurea | N/A |
| 33 | No | Yes | No | No | No | cavernosa aspiration and epinephrine irrigation | No |
| 34 | Yes | No | No | No | aspiration of the corpora cavernosa | N/A | |
| 25 | No | No | No | No | Proximal surgical shunt | Proximal surgical shunt was performed | Yes |
| 36 | No | Yes | No | No | No | cavernosa aspiration and epinephrine | N/A |
| 37 | Yes | No | No | winter shunt | Winter shunting | No | |
| 37 | hydroxyurea | Yes | No | No | Al-Ghorab shunt | Aspiration Shunt | No |
| 37 | hydroxyurea | Yes | No | No | Al-Ghorab shunt | Aspiration Shunt | EHS2. Grade 1 developed ED |
| 38 | hydroxyurea | Yes | No | No | Al-Ghorab shunt | Aspiration Shunt | No |
| 39 | No | Yes | No | No | No | cavernosal aspiration, irrigation and phenylepinephrin partial response after 5 days improved | N/A |
| 40 | Hydroxiurea ARA-C | No | Yes | No | No | Hydroxiurea ARA-C / LeukapheresisOne session per day for 3d /LMWH | N/A |
| 41 | No | Yes | No | No | No | corporal aspiration | N/A |
| 42 | Cyclophosphamide LMWH SC | No | Yes | No | No | N/A | N/A |
| 43 | No | Yes | No | No | transglanular to corpus cavernosal shunt | transglanular to corpus cavernos-al shunt | N/A |
| 44 | hydroxyurea | No | yes | No | transglandular cavernospongiosum shunt | transglandular cavernospongiosum shunt | surgical curettage of the penis. |
| 44 | NA | Yes | No | No | winter’s T shunt, | N/A | N/A |
| 44 | NA | Yes | No | No | No | N/A | N/A |
| 45 | NA | Yes | No | No | winter’s T shunt | N/A | N/A |
| 46 | Vincristine sulfate and prednisone | No | No | Yes | No | radiation,complete resolution after 6 d | N/A |
| 47 | imatinib | Yes | No | No | No | cavernosaaspiration was unsuccessful. Imatinib | N/A |
| 48 | NA | Yes | No | No | Distal corporoglanular shunt | N/A | N/A |
| 49 | analgesics, anxiolytics and steroids | Yes | No | No | No | bilateral aspiration and irrigation | N/A |
| 50 | hydroxyurea, allopurinol and intravenous fluids imatinib | No | Yes | No | No | leukapheresis | N/A |
| 51 | busulfan | No | No | No | No | subsided gradually over a two to three week period | Yes |
| 51 | hyaluronidase | No | No | Yes | No | NA | N/A |
| 51 | Busulfan | No | Yes | No | No | NA | No |
| 51 | Busulfan, hydroxurea | No | Yes | No | sapheno-cavernous bypass. | NA | Yes |
| 52 | Busulfan, hydroxurea | No | Yes | No | sapheno-cavernous bypass. | NA | N/A |
| 52 | pain killers | NA | No | No | Proximal shunt | N/A | N/A |
| 52 | Cavernosal Pseudo- Ephedrine Inj, pain killers | NA | No | No | No | N/A | N/A |
| 52 | pain killers | NA | No | No | Proximal shunt | N/A | N/A |
| 52 | Cavernosal Pseudo- Ephedrine Inj, pain killers | NA | No | No | No | N/A | N/A |
| 52 | pain killers | NA | No | No | No | N/A | N/A |
| 53 | N/A | Yes | No | No | No | N/A | N/A |
| 53 | NA | NA | No | No | Winter shunt | NA | Yes |
| 53 | NA | NA | No | No | Winter shunt | NA | Yes |
| 53 | NA | NA | No | No | Winter shunt | NA | Yes |
| 53 | NA | NA | No | No | Winter shunt | NA | Yes |
| 54 | NA | NA | No | No | Winter shunt | NA | Yes |
| 54 | allopyranol hydroxyurea | NA | No | No | cavernosoum- spongiuosum shunt | allopyranol hydroxyurea, cavernosoum- spongiuosum shunt | No |
| 55 | allopyranol hydroxyurea | NA | No | No | cavernosoum- spongiuosum shunt | allopyranol hydroxyurea, cavernosoum- spongiuosum shunt | No |
| 56 | myeleran endoxan | No | No | Yes | No | radiation to the penis | N/A |
| 57 | NSAIDS and Diethyl Stilbesteol | No | No | No | glandulo-cavernosal shunt | improved after the shunt | Yes |
| 58 | No | Yes | No | No | No | N/A | N/A |
| 59 | cold compressision, priscoline hydrochloride, diethylstilbestrol | No | No | Yes | No | radiation therapy the penis | N/A |
| 60 | benzyle penicilin busulfan, trioxyphenbutazone | Yes | No | No | No | initial aspiration little response improved after irrigation (lowselys operation ) | N/A |
| 61 | N/A | NA | No | No | NA | immediate surgical decompression | N/A |
| 62 | NO | NO | NO | NO | saphenocavernous anastomosis | N/A | N/A |
| 63 | Yes | Yes | Yes | no | corpus cavernosa-glans shunt | Aspiration and irrigation | Yes |
| 64 | Busulfan | Yes | No | No | No | with bothe busulfan, irrigation and aspirartion of the corpora cavernosa | Yes |
| 64 | busulfan | Yes | No | Yes | No | improved after aspiration and irrigation | Yes |
| 64 | No | Yes | No | Yes | No | after aspiration and irrigation of corpora cavernosa | Yes |
| 65 | Yes | No | No | No | No | N/A | N/A |
| 65 | Yes | No | No | No | No | N/A | N/A |
| 56 | hydroxyurea allopurinol Imatinib | Yes | No | No | No | improved after aspiration | Yes |
| 66 | allopyranol | Yes | Yes | No | Distal shunt | r distal distal shunt procedure | N/A |
| 67 | Myleran intravenous A-139 1, demerol | No | No | No | No | flaccid after two weeks of medical therapy | N/A |
| 51 | hydroxyurea | No | Yes | No | sapheno-cavernous bypass. | after sapheno-cavernous bypass. | Yes |
| 51 | N/A | Yes | No | No | No | N/A | N/A |
| 51 | Busulfan | Yes | No | No | No | N/A | Yes |
| 51 | Busulfan Steroids. Anticoagulants. | No | No | No | No | N/A | Yes |
| 51 | busulfan | No | Yes | No | No | N/A | Yes |
| 68 | allopyranol hydroxyurea | No | No | No | No | the priapism subsided after 24 hr of starting medicalk treatment no aspiration was done | No |
| 51 | Busulfan Anticoagulants | NA | No | Yes | No | no benefit | Yes |
| 50 | busulfan | Yes | No | No | shunt between the right saphenous vein and corpus cavernosus | shunt between the right saphenous vein and corpus cavernosus | N/A |
| 69 | busulfan then, 6- mercaptopurine, busulfan again | No | No | Yes | No | improbed after radiotheray on the 2 weeks period | N/A |
| 70 | No | No | No | No | No | on presentation no priapism was there , two episodes of priapism that resolved, and he was treated three times with metronidazole for presumed balanitis. | N/A |
| 41 | 1 LMWH SC BID for one month , Hydroxyurea Cyclophosphamide | No | Yes | No | No | N/A | N/A |
| 41 | 1 LMWH SC / hydroxyurea | No | No | No | No | Hydroxyurea | N/A |
| 71 | hydroxycarbamide | aspiration done | No | No | No | hydroxycarbamid aspirin 4 units of PRBC | No |
| 71 | cyclophosphamide 1g stat, hydroxycarbamide 1g 12 hourly | No | No | No | No | 20th day of admission with significant healing of the penile shaft ulcers, significant detumescence | N/A |
| 37 | No | No | No | No | No | Imatinib | developed ED EHS2. Grade 1 |
| 72 | Yes | No | No | No | No | By the 4th week of cytoreduction | Yes |
| 37 | hydroxyurea | Yes | No | No | Al-Ghorab shunt | Aspiration Shunt | developed ED EHS2. Grade 2 |
| 73 | Yes | No | No | No | No | terbutaline 0.125 mg subcutaneously | N/A |
| 74 | Paracetamol Morphine Diazepam, prednisone Terbutaline terbutaline 5.0mg, hydroxyurea | No | No | No | Winter shunt | Corpora cavernosa aspiration (winter), prednisone 2, Terbutaline terbutaline 5.0mg, tab hydroxyurea | N/A |
| 75 | No | Yes | No | No | No | corporal aspiration and phenylephrine irrigation | N/A |
| 76 | No | Yes | No | No | No | aspiration followed by intracavernosal injection of 1 dose of phenylephrine | No |
| 77 | No | Yes | No | No | No | penile aspiration and irrigation | N/A |
Discussion
CML is a myeloproliferative neoplasm (MPN) characterized by the uncontrolled production of mature granulocytes. The three stages of the CML are the blast phase, accelerated phase, and chronic phase. Most of the patients are diagnosed incidentally with an elevated white blood cell count on the CBC during their chronic asymptomatic period. Unfortunately, in most cases, the diagnosis of CML is reached late, as it has a large variety of vague clinical manifestations that may include lymphadenopathy, fatigue, hepatosplenomegaly, weight loss, bleeding tendency, and thromboembolic phenomena due to hyperleukostasis (78).
The mechanism of priapism in leukemia is believed to be related to blood sludging with white blood cells (6, 79). Severe anemia implies tissue hypoxia, which may interfere with the NO cGMP balance and precipitate the occlusion. Repeated priapism (stuttering) episodes can lead to prolonged ischemia and tissue damage (80). Most patients with priapism had lower Hb levels compared to their matched CML patients without priapism (78,81).
Although anemia may be an essential factor in priapism’s pathogenesis, it is not clear if blood transfusion is useful to alleviate an acute priapic attack, or it may worsen the condition. Blood exchange transfusion for treating patients with SCD and major priapism has been shown to be efficacious and safe.
WHO defines CML’s blast phase as more than 20% blasts (large cells) in bone marrow or peripheral smear (82). Although this could lead to more stasis, unexpectedly, priapism is not more common in the blast crisis phase and accelerated phase (only 5/102 patients). The majority of priapism cases (n = 97/102) occurred during the chronic phase.
In the era before the introduction of tyrosine kinase inhibitors, the chronic phase of CML accounted for 85% of CML presentation at the time of diagnosis (5). Most patients were below the age of 40 years, with a mean of 27.4 years. This means that it is more common in younger patients with CML patients. The peak age of priapism in adults (without CML) is between 20-50 years (83).
Thrombocytosis, with platelets (PLT) count above 600 x10 9/L is seen up to 30% of CML patients (5,84). The high mean PLT count may have some impact on the occurrence of priapism in CML and/or may influence the type of priapism (stuttering versus ischemic) rather than its occurrence. In CML patients with stuttering type priapism, the PLT count was lower (506.8 x10 9/L) compared to those with the ischemic type (609 x109/L). Moreover, there was no significant difference in the PLT count between CML patients with and without priapism (78).
In essential thrombocythemia (ET), another form of MPN with extremely high PLT counts, priapism was much less reported compared to CML (85). The few reports might reflect the minimal role of PLT in occluding the penile circulation compared to WBC.
The enlarged spleen indicates an advanced CML stage, which supports the late presentation. Moreover, splenomegaly is of prognostic importance; massive splenomegaly indicates poor prognosis and increased risk of dying due to CML (86). In this review, splenomegaly was reported in 80/102 (78.4%) patients and hepatomegaly in 31 patients, but organomegaly was not addressed in 20 patients (Table 3). Splenomegaly was seen in 28/31 (90%) with the stuttering type and in 50/70 (73.2%) with the ischemic type.
Table 3.
Characteristics of stuttering and ischemic priapism in patients with chronic myeloid leukemia (CML)
| Priapism type | Stuttering (n=32) | Ischemic (n=70) |
| Mean time to presentation | 220.9 hours (n. 20 ) | 77.8 hours |
| Mean Age (year) | 23.78 | 27.93 |
| Mean WBC | 314.9 x109/L | 320.49 x109/L |
| Mean PLT | 506.8 x109 /L | 609 x109/L |
| Mean Hb | 9.6 g/dl | 8.78 g/dl |
| CML phases | All chronic phase | 1 accelerated phase 4 blast phase 67 chronic phase |
| Erectile dysfunction | 16 not addressed 13 had erectile dysfunction 3 no erectile dysfunction |
41 not addressed 16 had erectile dysfunction 14 no erectile dysfunction |
| Splenomegaly (n) | 29 | 51 |
| Hepatomegaly (n) | 9 | 22 |
Ethnicity might have a role in the predisposition to priapism as 57% of the CML patients with priapism were Asian. Most of these reports came from India. This could be due to genetic susceptibility or difficulty accessing health care for the nonspecific symptoms of CML until WBC reached high levels, causing vascular stasis and priapism. Also, priapism occurred in patients with a problem with compliance or stopped medications (30, 46,76). Surprisingly, priapism developed after starting cytoreductive therapy in 3 patients (32, 49, 50). It is hard to conclude that initiating cytoreductive therapy will raise the risk of priapism.
Priapism is a urological emergency, which must be treated early to prevent erectile dysfunction. It is predicted that if priapism lasts more than 24 hours, the risk of permanent erectile dysfunction is more than 90% (87). Therefore, a rapid and expert reversal of the priapism is highly required. The mean time that a CML patient with priapism sought medical advice was 78.28 hours (n=76 ), which carried a high risk for developing permanent erectile dysfunction (88). However, despite this delayed presentation to medical attention, the reported erectile dysfunction was not high. The erectile dysfunction after the episode(s) of priapism in CML patients was reported in 29/102 (28.15%) and did not occur in 17/28 (60.7%). Probably, the lack of information and methods used to assess the erectile function in CML patients may explain the low reported percentage of erectile dysfunction in CML patients.
Over the past decade, we have witnessed significant advances in knowledge of CML’s biology and treatment. Imatinib is a first-line tyrosine kinase inhibitor for treating CML and has dramatically improved the prognosis of this disease. Chang et al. (89) have shown that Imatinib crosses the blood-testis barrier and reduces sperm density, sperm count, sperm survival rates, and sperm activity in CML patients in the chronic phase. But did not affect the structure of reproductive organs or sex hormone levels.
Forty patients needed a surgical shunt to relieve priapism, 5 of them had a partial response and continued chemotherapy to control the priapism. Forty-nine patients responded to aspiration and irrigation, and 2 of them required chemotherapy to control priapism due to incomplete response. Irradiation to the penis and spleen was used in 9 patients; leukapheresis was used in 19 patients, 6 of them required surgical shunts ( 43, 51, 63, 66).
Medications alone were used to treat priapism in 14 patients. However, reversing priapism using medications required longer duration compared to other modalities (mean duration in 7 patients was 14.4 days). Medications used included: hydroxyurea (n= 48), cyclophosphamide (n=3), busulfan (n=15), terbutalin (n=1), prednisolone (n=2), vincristine (n=1), priscoline hydrochloride (n=1), hyaluronidase injection (n=1), imatinib (n=5), cytarabine (n=1), diethylstilbestrol (n=2), cytarabine (n=2), low-molecular-weight heparin (n=3), anticoagulation (n=2), opioids (n= 1), blood transfusions (n=2).
The response to systemic therapy alone (medication) is usually prolonged and may represent the natural history of ischemic priapism rather than the effect of the medications (90). The American Urology Association (90) recommended an early treatment in a step-wise fashion starting with therapeutic aspiration (with or without irrigation) or intra-cavernous injection of sympathomimetics.
For CML patients, two points shall be considered. First, the late presentation to medical attention, which means that the slowly acting medications are less likely to be effective alone. In contrast, urological aspiration and irrigation within the first 24 hours decrease the risk of erectile dysfunction (91). Therefore, it is reasonable to start with aspiration and irrigation not to rely on oral medication for the treatment of CML alone. Secondly, treating the underlying mechanism of increased WBC count is needed to control and prevent priapism recurrence. Leukapheresis was used as a modality to treat both the high WBC of CML and priapism. The pitfalls of leukapheresis are that it is not available everywhere, is costly, and may require several sessions before a significant reduction in WBC count, which may take days. Besides, there is no clear-cut WBC value below which priapism is anticipated to be controlled. The mean WBC count after which priapism was controlled, is 22 x10 9/L. However, other patients were controlled only with WBC count between 3-10 x10 9/L. It remains an option for patients who failed aspiration and refused surgical shunt.
Similarly, the effects of irradiation were not rapid (6 days, 2 weeks, and 19 days), as documented in a few patients (45,58,69).
Four major types of surgical shunts are used for the treatment of priapism. These include percutaneous distal shunts, open distal shunts, open proximal shunts, and vein anastomoses/shunts (92). The goal of surgery is to create a channel or fistula that allows the deoxygenated blood to drain from the corpora cavernosa. For all shunt procedures, the patient should receive preventive perioperative antibiotics.
Guidelines advocate for an aggressive approach in treating patients with refractory priapism by proceeding in a serial fashion from distal to proximal shunts to vein shunting as quickly and safely as possible to achieve penile flaccidity (92).
A delayed penile implant was used in one patient. It is generally used for patients who developed erectile dysfunction; however, it can be used acutely to control priapism and prevent fibrous tissue formation (93). The erectile dysfunction occurs more frequently following proximal or vein shunts compared to the distal shunts. However, it is difficult to attribute the erectile dysfunction to the shunt operation as patients had received different modalities of treatment and had different duration before seeking medical attention (89).
Conclusion
Priapism is a rare complication of CML. It is mostly seen at the first presentation of the disease and much less during the start of treatment. It may occur after stopping the medication or post-splenectomy. Late presentation negatively affects the response to treatment as well as erectile function. Therefore, physicians must interfere early and follow a timely plan and shall follow the patients closely for developing erectile dysfunction. Conservative and medical therapy without urological intervention is less likely to be sufficient. Starting treatment of CML to decrease the high WBC count might accelerate the resolution of the priapism and sometimes is needed for a complete resolution.
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Rodgers R, Latif Z, Copland M. How I manage priapism in CML patients. Br J Haematol. 2012;158:155–64. doi: 10.1111/j.1365-2141.2012.09151.x. [DOI] [PubMed] [Google Scholar]
- Salonia A, Eardley I, Giuliano F, Hatzichristou D, Moncada I, Vardi Y, Wespes E, Hatzimouratidis K European Association of Urology. European Association of Urology guidelines on priapism. Eur Urol. 2014;65:480–9. doi: 10.1016/j.eururo.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Broderick GA, Kadioglu A, Bivalacqua TJ, Ghanem H, Nehra A, Shamloul R. Priapism: pathogenesis, epidemiology, and management. J Sex Med. 2010;7:476–500. doi: 10.1111/j.1743-6109.2009.01625.x. [DOI] [PubMed] [Google Scholar]
- Sardar S, Ali EA, Yassin MA. Thalassemia and Priapism: A Literature Review of a Rare Association. Cureus. 2021 Apr;13(4) doi: 10.7759/cureus.14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkina A, Wang J, Mathews V, Saydam G, Jung CW, Al Hashmi HH, et al. TARGET: a survey of real-world management of chronic myeloid leukaemia across 33 countries. Br J Haematol. 2020;190:869–76. doi: 10.1111/bjh.16599. [DOI] [PubMed] [Google Scholar]
- Mulhall JP, Honig SC. Priapism: etiology and management. Acade Emerg Med. 1996;3:810–6. doi: 10.1111/j.1553-2712.1996.tb03520.x. [DOI] [PubMed] [Google Scholar]
- Hashmat AI, Rehman JU. Priapism. In: Hashmat AI, Das S, editors. The Penis. Philadelphia Lea & Febiger; 1993. pp. 219–43. [Google Scholar]
- Yassin MA, Soliman AT, Sanctis V. Effects of tyrosine kinase inhibitors on spermatogenesis and pituitary gonadal axis in males with chronic myeloid leukemia. J Cancer Res Ther. 2014;2:116–21. [Google Scholar]
- Yassin MA, Abdulla MA-J, Chandra P, et al. Chronic Myeloid Leukemia in Adolescents and Young Adults: A Single Institute Experience. Blood. 2019;(134 Supplement 1):5915. [Google Scholar]
- Ergenc H, Varım C, Karacaer C, Çekdemir D. Chronic myeloid leukemia presented with priapism: Effective management with prompt leukapheresis. Niger J Clin Pract. 2015;18:828–30. doi: 10.4103/1119-3077.163282. [DOI] [PubMed] [Google Scholar]
- Thakur P, Verma V, Fotedar V, Singh K. Priapism in a Pediatric Chronic Myeloid Leukaemia Patient: Unusual Presentation of a Rare Disease in Children Case Report. Clin Cancer Investig J. 2019;8:76. [Google Scholar]
- Dhar J, Chhabra G, Khandelwal L, Batra A, Gupta N. Priapism as a debut presentation of chronic myeloid leukemia. J Coll Physicians Surg Pakistan. 2019;29:78–80. doi: 10.29271/jcpsp.2019.01.78. [DOI] [PubMed] [Google Scholar]
- Ponniah A, Brown CT, Taylor P. Priapism secondary to leukemia: Effective management with prompt leukapheresis. Int J Urol. 2004;11:809–10. doi: 10.1111/j.1442-2042.2004.00872.x. [DOI] [PubMed] [Google Scholar]
- Farhan S, Anjum F, Al-Qahtani FS, Al-Anazi KA. Chronic Myeloid Leukemia Presenting with Priapism. J Leuk. 2014;3:171. [Google Scholar]
- Becerra-Pedraza LC, Jiménez-Martínez LE, Peña-Morfin I, Nava-Esquivel R, Villegas-Martínez JA. Priapism as the initial sign in hematologic disease: Case report and literature review. Int J Surg Case Rep. 2018;43:13–7. doi: 10.1016/j.ijscr.2017.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htun TH, Dublin N, Parameswaran M, Razack AH, Chua CB. Chronic myeloid leukaemia presenting as Priapism - how should We treat these? J Heal Transl Med. 2008;11:27–9. [Google Scholar]
- Ocheni S, Ibegbulam O, Olusina D, Oyekunle A, Durosinmi M. Chronic myeloid leukaemia presenting as priapism: a report of 2 cases and review of literature. Int J Med Health Dev. 2010;15:76–81. [Google Scholar]
- Afrose R, Nebhnani D, Wadhwa N. Cutaneous myeloid sarcoma of the penile foreskin. Turk Patoloji Derg. 2015;31:131–5. doi: 10.5146/tjpath.2014.01258. [DOI] [PubMed] [Google Scholar]
- Jana K, Aggarwal R, Gawande A, Lal M. Priapism: A chronic myeloid leukemia harbinger in exigency. Ann Trop Med Public Health. 2013;6:583–5. [Google Scholar]
- Dhanju AS, Tyagi P, Dhaliwal SS, et al. Priapism: a rare presentation in chronic myeloid leukemia. Int J Adv Med. 2019;6:1937–9. [Google Scholar]
- Aggarwal V, Himanshu, Sathi S, Gupta A, Agrawal P. Priapism in CML. Indian J Med Paediatr Oncol. 2008;29:30–1. [Google Scholar]
- Sareen R, Kapil M, Malpani BK. Priapism: A Rare Presentation of CML. J Hematol Oncol Forecast. 2018;1:1–3. [Google Scholar]
- Chang M-W, Tang C-C, Chang SS. Priapism--a rare presentation in chronic myeloid leukemia: case report and review of the literature. Chang Gung Med J. 2003;26:288–92. [PubMed] [Google Scholar]
- Huei TJ, Lip HTC, Omar S. A rare presentation of chronic myeloid leukaemia with priapism treated with corporoglandular shunting. Med J Malaysia. 2018;73:420–2. [PubMed] [Google Scholar]
- Wajih Ullah M, Rehman A, Cheeti A, et al. Priapism and chronic myelogenous leukemia. Int J Adv Res. 2018;6:144–6. [Google Scholar]
- Shaeer OK, Shaeer KZ, AbdelRahman IF, El-Haddad MS, Selim OM. Priapism as a result of chronic myeloid leukemia: Case report, pathology, and review of the literature. J Sex Med. 2015;12:827–34. doi: 10.1111/jsm.12812. [DOI] [PubMed] [Google Scholar]
- Becker HC, Pralle H, Weidner W. Therapy of priapism in high counting myeloid leukemia - A combined oncological-urological approach. Two case reports. Urol Int. 1985;40:284–6. doi: 10.1159/000281101. [DOI] [PubMed] [Google Scholar]
- Qu M, Lu X, Wang L, Liu Z, Sun Y, Gao X. Priapism secondary to chronic myeloid leukemia treated by a surgical cavernosa-corpus spongiosum shunt: Case report. Asian J Urol. 2019;6:373–6. doi: 10.1016/j.ajur.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swapna Y, Narmada N. Emergency leukapheresis in chronic myeloid leukemia presenting with priapism. Asian J Pharm Heal Sci. 2017;7:1701–4. [Google Scholar]
- Abd El Salam M, Ibrahim NH, Hassan S. Discontinuation of treatment in a chronic myeloid leukemia patient caused priapism: A case report. Hum Androl. 2019;9:21–3. [Google Scholar]
- Atas U, Meydanal YE, Iltar U, Ulas T, Salim O, Undar L. Priapism-A Rare Presentation of Chronic Myeloid Leukaemia. J Clin Diagnostic Res. 2019:13. [Google Scholar]
- Dogra PN, Kumar P, Goel R, Dash SC. Long duration priapism in blast crisis of chronic myeloid leukemia. J Ass Phys India. 2004;52:170. [PubMed] [Google Scholar]
- Jameel T, Mehmood K. Priapism – an unusual presentation in chronic myeloid leukaemia: case report and review of the literature. Biomedica. 2009;25:197–9. [Google Scholar]
- Irzi M, Mhanna T, El Houmaidi A, et al. Delayed penile prosthesis implantation in the delayed presentation of ischemic priapism. Arch Case Reports. 2020;4:4–6. [Google Scholar]
- Tazi L. Priapism as the first manifestation of chronic myeloid leukemia. Ann Saudi Med. 2009;29:412. doi: 10.4103/0256-4947.55176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Shafiq I, Shah MH, Khan S, Shahid G, Arabdin M. Chronic myeloid leukaemia presenting as priapism: A case report from Khyber Pakhtunkhwa. J Pak Med Assoc. 2018;68:942–4. [PubMed] [Google Scholar]
- Patil RB, Wasekar N, Bamborde S, et al. Priapism: Rare presenting manifestation of chronic myeloid leukemia and its management-Case series of 5 patients. Int J Sci Res. 2019;8:35–7. [Google Scholar]
- Hazra SP, Priyadarshi V, Gogoi D, Sharma PK, Pal DK, Chakraborty SC. Pediatric priapism: a rare first manifestation of leukemia. APSP J Case Rep. 2013;4:39. [PMC free article] [PubMed] [Google Scholar]
- Veljković D, Kuzmanović M, Mićić D, Šerbić-Nonković O. Leukapheresis in management hyperleucocytosis induced complications in two pediatric patients with chronic myelogenous leukemia. Transfus Apher Sci. 2012;46:263–7. doi: 10.1016/j.transci.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Ammouri Z, Mouhaoui M. Priapism a rare and unusual presentation in chronic myeloid leukemia [a case report] AMMUR. 2019;3:36. [Google Scholar]
- Castagnetti M, Sainati L, Giona F, Varotto S, Carli M, Rigamonti W. Conservative management of priapism secondary to leukemia. Pediatr Blood Cancer. 2008;51:420–3. doi: 10.1002/pbc.21628. [DOI] [PubMed] [Google Scholar]
- Avci AE, Kurtulus F, Fazlioglu A, Keskin S, Güçtaş Ö, Cek M. Priapism as an initial presentation of chronic myelogenous leukemia: A case report. UHOD. 2005;15:153–5. [Google Scholar]
- Morano SG, Latagliata R, Carmosino I, Girmenia C, Dal Forno S, Alimena G. Treatment of long-lasting priapism in chronic myeloid leukemia at onset. Ann Hematol. 2000;79:644–5. doi: 10.1007/s002770000199. [DOI] [PubMed] [Google Scholar]
- Kumar P, Rahman K, Kumari S, Singh MK, Gupta R, Nityanand S. Priapism as a rare presentation of chronic myeloid leukemia. J Can Res Ther. 2018;14:1442–3. doi: 10.4103/0973-1482.199388. [DOI] [PubMed] [Google Scholar]
- Graivier L, Gran G, Rhoades RB, Reynolds RC, Windmiller J. Priapism in a 7-week-old infant with chronic granulocytic leukemia. J Urol. 1971;105:137–9. doi: 10.1016/s0022-5347(17)61479-4. [DOI] [PubMed] [Google Scholar]
- Patil P L, Somkuwar K, Katariya P S, Gaikwad N. Priapism - A Rare Presentation in Chronic Myeloid Leukemia. Vidarbha J Intern Med. 2016;21:50–1. [Google Scholar]
- Agarwal A, Lavania P. Priapism in a patient of chronic myeloid leukemia: A case report. Indian J Urol. 2020;24:S53. [Google Scholar]
- Chaudhary R, Rai BK, Bhandari R, Yadav A. Unusual Case of Priapism in Emergency Department of Tertiary Care Hospital of Eastern Nepal. Int J Clin Urol. 2017;4:39–40. [Google Scholar]
- Shafique S, Bona R, Kaplan AA. A case report of therapeutic leukapheresis in an adult with chronic myelogenous leukemia presenting with hyperleukocytosis and leukostasis. Ther Apher Dial. 2007;11:146–9. doi: 10.1111/j.1744-9987.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- Stutz FH, Bergin JJ. Priapism in Leukemia: A report of two cases. Mil Med. 1970;135:44–8. [PubMed] [Google Scholar]
- Suri R, Goldman JM, Catovsky D, Johnson SA, Wiltshaw E, Galton DAG. Priapism complicating chronic granulocytic leukemia. Am J Hematol. 1980;9:295–9. doi: 10.1002/ajh.2830090308. [DOI] [PubMed] [Google Scholar]
- Chowdhury ZZ, Al-Asad H, Rahman MH, et al. Management of Priapism with Chronic Myeloid Lukaemia-A Rare Presentation and Our Experiences. Haematol J Bangladesh. 2020;3:39–41. [Google Scholar]
- Nabi G, Dogra PN. Chronic myeloid leukaemia presenting as priapism in children: need for multidisciplinary approach. East Afr Med J. 2000;77:576. [PubMed] [Google Scholar]
- Gupta A, Bambrey P, Varma S, Vaidyanathan S, Deodhar SD. Priapism in chronic myeloid leukaemia: combined medical and surgical treatment. A report on two patients. Indian J Cancer. 1987;24:176–9. [PubMed] [Google Scholar]
- Dutta TK, Purohit OP, Vaidyanathan V, Gupta BD, Rao MS. Radiation therapy of priaprism complicating chronic myeloid leukaemia--review and report of a case. Indian J Cancer. 1979;16:90–3. [PubMed] [Google Scholar]
- Ekeke O, Omunakwe H, Nwauche C. Chronic myeloid leukaemia presenting as priapism. Int Surg. 2015;100:552–7. doi: 10.9738/INTSURG-D-13-00223.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaeena W, Azzuz S. Undiagnosed Chronic Myelogenous Leukemia Presented By Priapism. Int J Acad Sci Res. 2020;4:20–1. [Google Scholar]
- Ritz ND, Purfar M. Chronic myeloid leukemia with priapism in eight-year-old child. N Y State J Med. 1964;64:553–6. [PubMed] [Google Scholar]
- Babel CS, Jain KC, Mathur A, Bhu N. Priapism in child with chronic granulocytic leukemia. Indian Pediatr. 1976;13:961. [PubMed] [Google Scholar]
- Agrawal DK, Jha S, Verma A, Tripathi AK, Singh BN. Priapism, complicting chronic myeloid leukemia. A case report. Indian J Cancer. 1991;28:51–2. [PubMed] [Google Scholar]
- Ghalaut PS, Kalra GS, Gupta S. Priapism - A rare presentation in chronic myeloid leukaemia. J Assoc Physicians India. 1996;44:354–5. [PubMed] [Google Scholar]
- Bhatia P, Arya LS, Chinnappan D, Choudhry VP, Pati H. Priapism in chronic myelogenous leukemia. Indian J Pediatr. 1992;59:130–2. doi: 10.1007/BF02760917. [DOI] [PubMed] [Google Scholar]
- Mishra K, Jandial A, Singh V, Radotra B, Malhotra P. Priapism in chronic myeloid leukemia: Meeting at the crossroads and heading in different directions. Indian J Med Paediatr Oncol. 2020;41:418. [Google Scholar]
- Saikia T, Advani SH, Dinshaw KA, Gopal R, Nair CN, Chandwani IM. Priapism complicating chronic myeloid leukaemia and its management. J Indian Med Assoc. 1984;82:294–6. [PubMed] [Google Scholar]
- Villegas Osorio JF, Corchuelo Maíllo C, Cuevas Palomino A, Medina López RA. Ischaemic priapism as a presentation of chronic myeloid leukaemia. Arch Esp Urol. 2014;67:708–11. [PubMed] [Google Scholar]
- Clark AJ, Hsu P, Darves-Bornoz A, Tanaka ST, Mason EF, Katzenstein HM. Priapism in a 13-year-old boy. Pediatr Rev. 2018;39:617–9. doi: 10.1542/pir.2017-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haar H, Shanbrom E, Miller S. The treatment of leukemic priapism with A-139. J Urol. 1960;83:429–32. doi: 10.1016/S0022-5347(17)65732-X. [DOI] [PubMed] [Google Scholar]
- Shankar J. Priapism in Teenager Chronic Myelogenous Leukemia: a Rare Occurrence. Asian J Pharm Heal Sci. 2011;1:226. [Google Scholar]
- Graw RG, Skeel RT, Carbone PP. Priapism in a child with chronic granulocytic leukemia. J Pediatr. 1969;74:788–90. doi: 10.1016/s0022-3476(69)80144-7. [DOI] [PubMed] [Google Scholar]
- Abbott LS, Moineau G, Johnston DL. Case 1: An unusual cause of headaches and priapism in a teenager. Paediatr Child Health. 2008;13:299–301. doi: 10.1093/pch/13.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocheni S, Ibegbulam O, Olusina D, Oyekunle A, Durosinmi M. Chronic myeloid leukaemia presenting as priapism: a report of 2 cases and review of literature. J Coll Med. 2012;15:76–81. [Google Scholar]
- Musa A, Ndakotsu M, Abubakar S, Agwu P. Chronic myeloid leukemia with an initial presentation as ischemic priapism: A case report and review of literature. Arch Int Surg. 2017;7:68. [Google Scholar]
- Gupta A, Seth T, Gupta A. Successful use of terbutaline in persistent priapism in a 12-year-old boy with chronic myeloid leukemia. Pediatr Hematol Oncol. 2009;26:70–3. doi: 10.1080/08880010802435146. [DOI] [PubMed] [Google Scholar]
- Ervie M, Boongaling DC, Rose S, Mortel C, Deala RP. Priapism as a Rare Presentation of Chronic Myelogenous Leukemia. Philippine J Inter Med. 2015;53:1–5. [Google Scholar]
- Nerli RB, Magdum PV, Hiremath SC, et al. Priapism - A Rare Presentation in Chronic Myeloid Leukemia: Case Report. Urology Case Reports. 2016;4:8–10. doi: 10.1016/j.eucr.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HH, Zhang JH, DeWitt-Foy M, Waldron M, Mukherjee S, Montague DK. Urologic Management of Priapism Secondary to Chronic Myeloid Leukemia. Urology. 2019;125:24–8. doi: 10.1016/j.urology.2018.11.021. [DOI] [PubMed] [Google Scholar]
- Minckler MR, Conser E, Figueroa JJ, Scott AJ, Gaither J, Amini R. The Semantics of Priapism and the First Sign of Chronic Myeloid Leukemia. Case Rep Emerg Med. 2017;2017:2656203. doi: 10.1155/2017/2656203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DG, Szydlo RM, Goldman JM. Clinical features at diagnosis in 430 patients with chronic myeloid leukaemia seen at a referral centre over a 16-year period. Br J Haematol. 1997;96:111–6. doi: 10.1046/j.1365-2141.1997.d01-1982.x. [DOI] [PubMed] [Google Scholar]
- Steinhardt GF, Steinhardt E. Priapism in children with leukemia. Urology. 1981;18:604–6. doi: 10.1016/0090-4295(81)90467-2. [DOI] [PubMed] [Google Scholar]
- Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and Impotence in Homozygous Sickle Cell Disease. Arch Intern Med. 1980;140:1434–7. [PubMed] [Google Scholar]
- Jandial A, Mishra K, Sandal R, Lad D, Prakash G, Khadwal A, et al. CML patients presenting with priapism: Is there any disparity in outcome? J Clin Oncol. 2019;37(15 Suppl):e18545. [Google Scholar]
- Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- Cherian J, Rao AR, Thwaini A, Kapasi F, Shergill IS, Samman R. Medical and surgical management of priapism. Postgrad Med J. 2006;82:89–94. doi: 10.1136/pgmj.2005.037291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faderl S, Talpaz M, Estrov Z, O’Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. New Engl J Med. 1999;341:164–72. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- Ali EA, Nashwan AJ, Yassin MA. Essential thrombocythemia with (type2) calreticulin presented as stuttering priapism case report and review of literature. Clinical Case Reports. 2021 Jan;9(1):399–404. doi: 10.1002/ccr3.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfirrmann M, Baccarani M, Saussele S, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30:48–56. doi: 10.1038/leu.2015.261. [DOI] [PubMed] [Google Scholar]
- Pryor JP, Hehir M. The management of priapism. Br J Urol. 1982;54:751–4. doi: 10.1111/j.1464-410x.1982.tb13641.x. [DOI] [PubMed] [Google Scholar]
- Kumar M, Garg G, Sharma A, Pandey S, Singh M, Sankhwar SN. Comparison of outcomes in malignant vs. non-malignant ischemic priapism: 12-year experience from a tertiary center. Turkish J Urol. 2019;45:340–4. doi: 10.5152/tud.2019.75044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Zhou L, Chen X, et al. Impact of Imatinib on the Fertility of Male Patients with Chronic Myelogenous Leukaemia in the Chronic Phase. Target Oncol. 2017;12:827–32. doi: 10.1007/s11523-017-0521-6. [DOI] [PubMed] [Google Scholar]
- Montague DK, Jarow J, Broderick GA, et al. Members of the Erectile Dysfunction Guideline Update Panel; Americal Urological Association. American Urological Association guideline on the management of priapism. J Urol. 2003;170:1318–24. doi: 10.1097/01.ju.0000087608.07371.ca. [DOI] [PubMed] [Google Scholar]
- Kulmala RV, Tamella TL. Effects of priapism lasting 24 hours or longer caused by intracavernosal injection of vasoactive drugs. Int J Impot Res. 1995;7:131–6. [PubMed] [Google Scholar]
- Burnett AL, Sharlip ID. Standard Operating Procedures for Priapism. J Sex Med. 2013;10:180–94. doi: 10.1111/j.1743-6109.2012.02707.x. [DOI] [PubMed] [Google Scholar]
- Ralph DJ, Garaffa G, Muneer A, et al. The Immediate Insertion of a Penile Prosthesis for Acute Ischaemic Priapism. Eur Urol. 2009;56:1033–8. doi: 10.1016/j.eururo.2008.09.044. [DOI] [PubMed] [Google Scholar]


