Abstract
Background and aim:
Rheumatic Heart Disease (RHD) often evolves in congestive heart failure with development of pulmonary edema after a asymptomatic, latent phase. In the last years, Lung Ultrasound (LUS) has gained a primary role in the diagnosis and management of pleuropulmonary disorders, also in pediatric practice, and in the diagnosis and follow-up of pulmonary edema through qualitative analysis of ultrasound B-lines. Aim of this case report is to keep high clinicians’ attention to the diagnosis of Rheumatic Heart Disease also in high-income countries and to deepen the role and importance of lung ultrasound in clinical practice, in diagnosis and follow-up of pediatric lung diseases, especially in emergency settings as happened in our case.
Methods:
We present the case of a 14-year-old Italian boy from a medium-low socio-economic and cultural class Italian family, who was diagnosed with severe and advanced stage RHD, which had remained undiagnosed until then.
Results and Conclusions:
In the diagnostic process of our case, LUS played a fundamental role because it quickly directed us, contextually to the clinical and anamnestic evaluation, towards the right diagnosis, in a Pediatric Emergency Department. In clinical practice, the only LUS findings and the only qualitative analysis of the B-lines, have note made clinicians able to make a clear characterization yet. Thus, the study of cardiovascular function, laboratory parameters, anamnestic and clinical data continue to be useful tools in order to assist LUS in the diagnostic processes of lung diseases, as in our case. (www.actabiomedica.it)
Keywords: lung ultrasound, ultrasound imaging, critically ill, focused assessment sonography, cardiogenic pulmonary edema, children
Introduction
Rheumatic Heart Disease (RHD), a sequela of Rheumatic Fever (RF) affecting the heart valve system, often evolves in congestive heart failure after a asymptomatic, latent phase (silent) (1,2).
Almost eradicated in high-income countries (HICs) (1), the disease persists in the middle and low-income countries (MICs and LICs) (1). Incidence rates in these countries still reach epidemic levels with a disproportion of the disease burden even within the same country (3).
The current enormous refugee crisis worldwide and especially in Europe represents a paradigmatic translational shift (1). Many migrants experience lack of access and continuity to health care. This makes particularly challenging to detect and manage asymptomatic, early stage RHD, which could prevent excessive RH-related morbidity and mortality. The few reports in literature describing the ‘RHD and migrants’ topic refer to advanced-stage, severe, symptomatic RHD in need of cardiac surgery (1).
Although the greatest burden is currently found in MICs and LICs and in migrant and refugee populations in HICs [1], sporadic cases of ARF and especially RHD continue to be observed in wealthy nations even in populations that do not belong to the migrants and / or refugees (4).
The 2015 Jones Criteria identified the ARF incidence cut-off <2 of 100 000 school-aged children per year (3). Although Italy is classified as a high-income country, the incidence of ARF is above this threshold value (4).
We present the case of a 14-year-old Italian boy, who was diagnosed with severe and advanced stage Rheumatic Heart Disease, which had remained undiagnosed until then.
In particular, an aspect we want to investigate with this report is the fundamental role played by lung ultrasound (LUS) quickly directing us, contextually to the clinical and anamnestic evaluation, towards the right diagnosis, in a Pediatric Emergency Department (PED).
Case Report
The recent clinical history of our patient began on 15th August 2020 with the appearance of vomiting and coughing, therefore he was conducted by the treating pediatrician who found a right basal hypophonesis on chest auscultation. The physician prescribed a chest radiograph (Fig. 1). The suspicion was that of a right basal pleuropneumonia, so that antibiotic therapy was started. However, due to radiographic evidence of increased cardiac shadow, the boy was transferred to the PED of another hospital where the following tests were performed: - blood tests showing a slight increase in inflammation indices, - COVID19 nasopharyngeal swab and serological test for COVID19 resulted negative; - echocardiogram which showed a severe aortic and mitral steno-insufficiency with a Pulmonary Artery Pressure (PAP) of at least 75 mmHg with considerable dilation of the left cardiac cavities.
Figure 1.
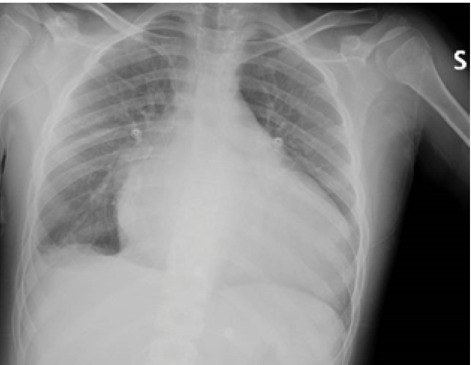
Chest digital radiography shows a non-specific HyperLucency area in the right basal lung consisting of consolidation associated with ipsilateral pleural effusion and an enlarged cardiac shadow.
In consideration of the complexity of the clinical situation, on 25th August 2020 he was then transferred to our PED with a diagnosis of “right basal pleuropneumonia with a new finding of heart disease characterized by aortic and mitral steno-insufficiency of severe degree”.
At the clinical evaluation in our PED, the patient was in good cardiovascular and oxygenation compensation conditions. LUS was performed at the same time as the clinical evaluation.
According to what was reported by the pediatrician colleagues of the other hospital and according to the chest X-ray, it was expected to find a pulmonary ultrasound picture of pleuropneumonia. Far from it, LUS findings (Fig. 2,3,4) did not show an inflammatory picture but a picture of pulmonary imbibition of moderate degree or an ultrasound septal pattern of early cardiogenic pulmonary edema (CPE) (5). The subpleural consolidation found on chest X-ray had the ultrasound characteristics of atelectasis and not of an inflammatory type consolidation and the pleural effusion could also be of cardiogenic nature (6).
Figure 2.
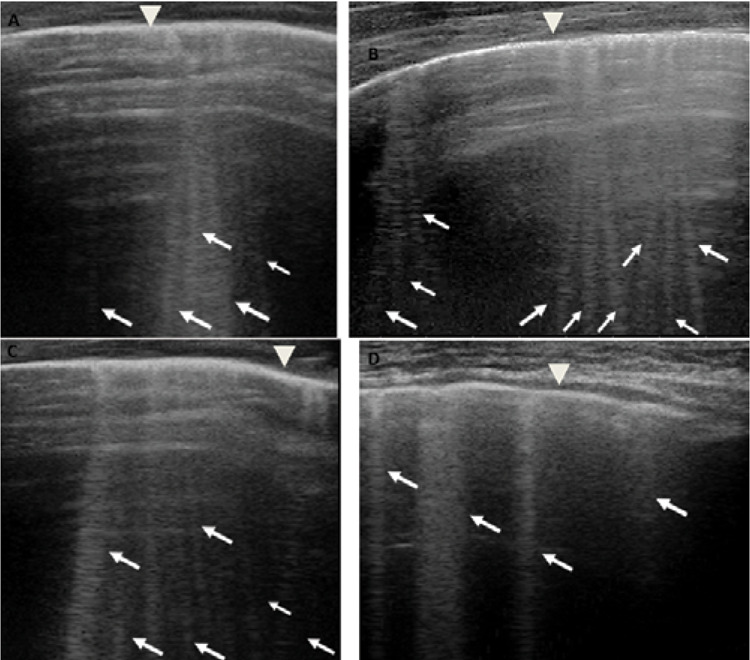
Grayscale lung ultrasound examination (linear probe, 12 MHz, and the small parts preset) shows the presence of separated vertical artifacts with a gravitational course (associated with a linear pleural line and a regular pleural sliding) diffuse on all the explored fields and present on each intercostal space explored and in particular in the basal fields bilaterally: Septal Pattern of early CPE [5]. Figures A and B show the SIS ultrasound pattern of the upper right and left fields respectively; those C and D show that of the right and left lower / basal fields respectively. The pleural line is regular (arrowhead). B-lines (arrows) are separated, laser like artefacts, with gravitational course spreading from the pleural line to the bottom of the screen and they show an internal sequence of alternating horizontal bands. CPE, cardiogenic pulmonary edema; SIS, sonographic interstitial syndrome.
Figure 3.
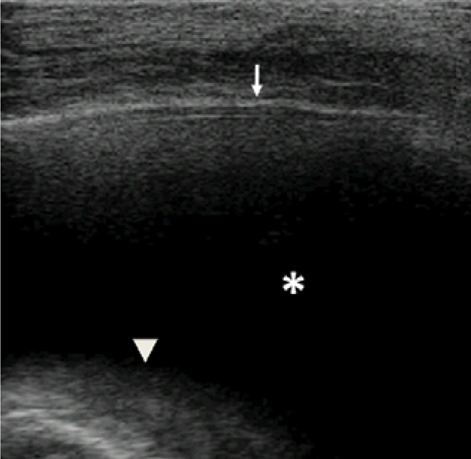
Grayscale lung ultrasound examination (linear probe, 12 MHz, and the small parts preset) shows, on the basal posterior-lateral fields on the right, an anechoic pleural effusion (asterisk) that extends to the lower apex of the clavicle and is about 3.5 cm in maximum depth. It is appeared like an anechoic space between the parietal (arrow) and visceral pleura (arrowhead) below which lung appears atelectatic.
Figure 4.
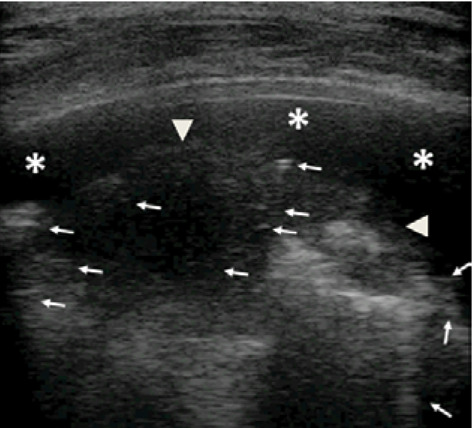
Grayscale lung ultrasound examination (linear probe, 12 MHz, and the small parts preset) shows, on the basal posterior-lateral fields on the right, within the pleural effusion (asterisks), a consolidation (arrowheads) with static air broncograms (punctate) and parallels between di them (arrows) as per atelectasis [6,8,10].
While performing LUS, the patient’s clinical history was deepened. It was reported that four years earlier the patient had presented an episode of arthritis of the ankle and knee joints with edema and pain during active mobilization. Following this episode, which was treated with betamethasone and in correspondence with which no other diagnostic tests were performed, a reduced exercise capacity remained.
At this point, therefore, on the basis of the current and remote clinical history and the result of the lung ultrasound, the diagnostic hypothesis was completely changed. We were presumably facing a boy with undiagnosed and untreated Rheumatic Heart Disease which had gone into worsening causing severe valvulopathy and initial cardiac decompensation with appearance pulmonary sub edema.
Additionally, we found -a prolonged PR interval; -a high pro-BNP value (12335pg/mL) that confirmed the volumetric overload of the left sections, which was also compatible with pulmonary imbibition (7) and -a high Antistreptolysin O (ASO) titer.
Therefore, the finding of all these results made it possible to make the diagnosis of rheumatic disease in accordance with the Jones criteria (3).
Discussion
In pediatric, the role of LUS has been debated for years. LUS has been underutilized, until the recent past, for lung evaluation. The bony thorax and the presence of air within the lungs were thought to interfere with the transmission of ultrasound waves. It now has been know that ultrasonography is well-suited to the pediatric chest because most children do not have much subcutaneous fat. The pediatric chest wall is only partially ossified, providing additional acoustic windows that are not available in older children or adults. The relatively non-ossified pediatric thorax and the thymus provide adequate acoustic windows for the evaluation of the anterio chest and mediastinum. Nonossified sternal cartilage and nonossified costal cartilage appear relatively hypoechoic on US. These structures gradually ossify with aging, decreasing acoustic access (6,8).
Over the years, the applications of lung ultrasound have changed and the main indications of LUS in children have expanded, even if the use of LUS limits must always be kept in mind: the need for contact between the affected lung and the pleural surface; the need to find an adequate acoustic window; acoustic phenomena are not always directly convertible into images of the human body as direct biomarkers; the inability of the LUS to assess the relationship of the lungs with the rib cage and other organs of the mediastinum; the need to have clinical and laboratory data available as an aid to diagnosis (6,8-10).
Despite this, in the last years, LUS has gained a primary role in the diagnosis and management of pleuropulmonary disorders, also in pediatric practice (8-10) when used by experienced hands.
LUS is a non-invasive, non-ionizing radiation tool and a rapid, affordable, point-of-care imaging modality that allow both real-time diagnosis and follow-up of respiratory diseases (8-10). LUS results are immediately available to the clinician, especially in emergency conditions who can therefore immediately orient himself towards a diagnosis so that a quick therapeutic decision can be made, as our case also demonstrates.
LUS may be better than chest radiography in the diagnosis of community-acquired pneumonia [8-10], can define the etiology of pneumonia (10) and many studies have described and validated LUS scores (based mainly on vertical artifacts and subpleural consolidations) in neonatal respiratory disorders (7-9) and bronchiolitis (11).
Observing the ultrasound images of our patient (Figure 2), it is clear that we are dealing with a picture of a sonographic interstitial syndrome (SIS): the presence of multiple focal, patched or diffuse vertical artifacts (B-lines) fanning out from the lung wall interface (5). Ultrasound picture common to various respiratory diseases (5,9,11). To define its nature and possible origin, having always in mind the knowledge of the patient’s clinical background, not having many pediatric studies on interpretation of LUS vertical artifacts available, especially as regards the differentiation between edema / fibrosis diseases of origin pulmonary and cardiogenic edema, it is necessary to refer to the studies performed on adults (5,7,9).
In the presence of edema, ARDS, interstitial lung diseases, non-consolidative pneumonia and contusions, part of the lung volume which was originally occupied by air, may be replaced with water, connective, cells, hyaline membrane or edematous tissue, ultimately creating acoustic traps for the US beam containing a medium that is physically (in terms of acoustic impedance) very different to its surroundings (air) (5,7,12).
Therefore, the ultrasound imaging of SIS is an artefactual piece of information, whereas consolidations produce real anatomical images when the superficial lung is nearly completely (or completely) free of air (5, 12).
Therefore, B-lines, in their variable appearances, indicate a loss of peripheral lung aeration (without tissue consolidation) due to interstitial disease or simply to lung deflation without histologic changes (5,7,12).
However, B-lines, in absence of an analysis of their appearance, cannot easily differentiate the cause.
Soldati et al (5,7,12) demonstrated on physical models that B-lines are heterogeneous entities in terms of aggregation and visual structure, whose nature is linked to the superficial histologic characteristics of the lung.
Therefore, the vertical artifacts generated from a fibrotic or inflammatory lung (ARDS) have a different look from those generated by cardiogenic edema (CPE) (5,7,12).
Thickened (but anatomically intact) secondary interlobular septa may act as acoustic traps where specific frequencies give rise to separated, uniform, bright and long B-lines, without spared areas (septal pattern, see Fig. 2), especially in early CPE (5,12).
In ARDS subpleural random peri and intralobular distortion may explain the appearance of acoustically permissive irregular channels, different arrangements of B-lines, pleural irregularities, consolidations, inhomogeneous isolated air spaces and inhomogeneous edema (5,12).
In practical terms, these knowledges allowed us to identify a septal-type ultrasound pattern of SIS, distinguishing it from the inflammatory type: SIS with bright, long, separated, modulated B-lines arising from a normal pleural line is strongly predictive of early CPE (Fig. 2).
Finally, we want to discuss the role of chest X-ray played in our case diagnostic path. It has been certainly essential to show us “a red flag” that, otherwise, we would not have identified with the LUS: the increased cardiac shadow (Fig. 1).
As for the detection and characterization of lung disease, in the first place, with the chest X-ray, it was not able to identify cardiogenic pulmonary edema. The findings of several studies performed on adult patients, suggest that LUS is as specific and more sensitive than CXR in the identification of CPE (7).
Secondly, the radiograph showed a consolidation associated with pleural effusion without characterizing their nature but assuming that it was overall a picture of inflammatory / infectious origin. As demonstrated by several studies (8-11) and as understood by our experience with this case, LUS was able to differentiate between a consolidation of an inflammatory nature (which normally presents dynamic and / or fluid bronchograms) from a consolidation of an atelectasis nature (6,8,10) (Fig. 4). Furthermore, thanks to LUS it was possible to define not only the extent of the pleural effusion, but also its transudative and non-inflammatory/exudative nature (6,8,10) (Fig. 3).
Conclusion
In conclusion, we want to report this case – to keep high attention to a diagnosis whose rarity is perhaps overestimated in our high-income countries (4) and – to underline once again the importance of ultrasound in the diagnostic path and follow-up of pediatric pulmonary pathologies, especially in emergency settings as in our case.
Pending the development of specific studies on pediatric patients and of new software that, with the help of artificial intelligence can obtain qualitative information from US echo, we must rely on knowledge from studies performed on adult patients. Therefore, at the moment, in clinical practice, the only LUS findings and the only qualitative analysis of the B-lines, have not made clinicians able to make a clear characterization yet. Thus, the study of cardiovascular function, laboratory parameters, anamnestic and clinical data continue to be useful tools to assist the LUS in the diagnostic processes of lung diseases, as was the case in our case.
Abbreviations:
- RHD:
Rheumatic Heart Disease
- RF:
Rheumatic Fever
- HICs:
high income countries
- MICs and LICs:
middle and low income countries
- LUS:
lung ultrasound
- PED:
Pediatric Emergency Department
- PAP:
Pulmonary Artery Pressure
- ASO:
Antistreptolysin O
- CPE:
cardiogenic pulmonary edema
- SIS:
sonographic interstitial syndrome
- CXR:
chest X-ray.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
Authors Contributions:
All authors have read and approved the manuscript for submission; have made a substantial contribution to the conception, design, gathering of data and a contribution to the writing and intellectual content of the article.
Ethics approval and Consent to participate:
Written informed consent was obtained from a boy’s parent. The study was approved by the Institutional Review Board and Ethic Committee (prot.36173/19 ID2729). All patients’ data were analyzed anonymously.
References
- Condemi F, Rossi G, Lupiz M, et al. Screening of asymptomatic rheumatic heart disease among refugee/migrant children and youths in Italy. Pediatr Rheumatol Online J. 2019;17(1):12. doi: 10.1186/s12969-019-0314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DA, Beaton AZ, Carapetis JR, et al. Rheumatic heart disease worldwide: JACC scientific expert panel. J Am Coll Cardiol. 2018;72:1397–416. doi: 10.1016/j.jacc.2018.06.063. [DOI] [PubMed] [Google Scholar]
- Gewitz MH, Baltimore RS, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131(20):1806–1818. doi: 10.1161/CIR.0000000000000205. [DOI] [PubMed] [Google Scholar]
- Fabi M, Calicchia M, Miniaci A, et al. Carditis in Acute Rheumatic Fever in a High-Income and Moderate-Risk Country. J Pediatr. 2019;215:187–191. doi: 10.1016/j.jpeds.2019.07.072. [DOI] [PubMed] [Google Scholar]
- Soldati G, Demi M, Demi L. Ultrasound patterns of pulmonary edema. Ann Transl Med. 2019;(7Suppl 1):S16. doi: 10.21037/atm.2019.01.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovrenski J. Pediatric lung ultrasound – pros and potentials. Pediatr Radiol. 2020;50(3):306–313. doi: 10.1007/s00247-019-04525-y. [DOI] [PubMed] [Google Scholar]
- Maw AM, Hassanin A, Ho PM, et al. Diagnostic Accuracy of Point-of-Care Lung Ultrasonography and Chest Radiography in Adults With Symptoms Suggestive of Acute Decompensated Heart Failure: A Systematic Review and Meta-analysis. JAMA Netw Open. 2019;2(3):e190703. doi: 10.1001/jamanetworkopen.2019.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomà P. Lung ultrasound in pediatric radiology – cons. Pediatr Radiol. 2020;50:314–320. doi: 10.1007/s00247-019-04524-z. [DOI] [PubMed] [Google Scholar]
- Buonsenso D, Soldati G, Curatola A, et al. Lung Ultrasound Pattern in Healthy Infants During the First 6 Months of Life. J Ultrasound Med. 2020;39(12):2379–2388. doi: 10.1002/jum.15347. [DOI] [PubMed] [Google Scholar]
- Berce V, Tomazin M, Gorenjak M, Berce T, Lovrenčič B. The Usefulness of Lung Ultrasound for the Aetiological Diagnosis of Community-Acquired Pneumonia in Children. Sci Rep. 2019;9(1):17957. doi: 10.1038/s41598-019-54499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supino MC, Buonsenso D, Scateni S, et al. Point-of-care lung ultrasound in infants with bronchiolitis in the pediatric emergency department: a prospective study. Eur J Pediatr. 2019;178:623–632. doi: 10.1007/s00431-019-03335-6. [DOI] [PubMed] [Google Scholar]
- Soldati G, Demi M, Smargiassi A, Inchingolo R, Demi L. The role of ultrasound lung artifacts in the diagnosis of respiratory diseases. Expert Rev Respir Med. 2019;13:163–172. doi: 10.1080/17476348.2019.1565997. [DOI] [PubMed] [Google Scholar]


