Abstract
Nucleus pulposus cell (NPC) transplantation can be a potential therapeutic approach for intervertebral disc degeneration (IDD). However, low cell viability has restricted the therapeutic capacity of NPCs, and sources of natural NPCs are limited. Bone marrow-derived mesenchymal stem cells (BMSCs) and adipose-derived mesenchymal stem cells (ADSCs) can be differentiated toward NPC-like cells. However, it is unknown whether there are differences in the abilities of these two cell types to differentiate into NPC-like cells, or which cell type exhibits the best differentiation ability. The present study compared the abilities of BMSCs and ADSCs to differentiate toward NPC-like cells with or without a 3D culture system to lay a foundation for stem cell transplantation therapy for IDD. BMSCs were isolated from the rat whole bone marrow cell using the repeated adherent culture method. ADSCs were isolated from rat adipose tissues in the subcutaneous inguinal region using the enzyme digestion method. Cells were identified using flow cytometry. Cell viability was assessed via Cell Counting Kit-8 assays, and reverse transcription-quantitative PCR and western blotting were carried out to evaluate the expression of NPC markers and chondrocyte-specific genes. Glycosaminoglycans (GAGs) and proteoglycans were examined via Alcian blue and safranin O staining, respectively. ADSCs in 3D culture displayed the highest cell proliferative ability, compared with the 2D culture system and BMSC culture. In addition, ADSCs in 3D culture exhibited increased GAG and proteoglycan synthesis than BMSCs. Compared with BMSCs in 3D culture, ADSCs in 3D culture exhibited higher mRNA and protein expression of NPC marker genes (hypoxia-inducible factor 1-α, glucose transporter 1) and chondrocyte-specific genes (Sox-9, aggrecan and type II collagen). The present findings indicated that ADSCs exhibited a better ability to differentiate into NPC-like cells in 3D culture compared with BMSCs, which may be of value for the regeneration of intervertebral discs using cell transplantation therapy.
Keywords: stem cells, differentiation, three-dimensional culture, nucleus pulposus cells
Introduction
Low back pain caused by intervertebral disc degeneration (IDD) seriously affects the quality of life of patients with this condition (1). Therapy for IDD includes conservative treatment (including physical therapy and pain management) and surgical treatment, but neither can reverse the pathological status of IDD (2). IDD is caused by a decrease in nucleus pulposus cells (NPCs) and a subsequent decrease in proteoglycans in the extracellular matrix (ECM) (3). The cell density and cell regeneration rate in the nucleus pulposus are low; therefore, repair after degeneration is difficult (4). To alleviate and treat IDD, researchers have focused on maintaining and increasing the number of NPCs and enhancing ECM deposition (5). In a previous study, regenerative therapy with stem cells has provided a novel approach for the treatment of IDD. This approach can help repopulate intervertebral discs by promoting the production of ECM, restoration of damaged tissue and secretion of growth factors to regulate inflammation and strengthen tissue regeneration, without causing additional intervertebral disc injury (6).
The native intervertebral disc is typically composed of two distinct anatomic regions, the annulus fibrosus and the nucleus pulposus (7). NPCs can be obtained from intervertebral disc tissues and have been used to regenerate the disc tissue. Although NPCs have been used to regenerate disc tissue (8,9), access to healthy NPCs, especially autologous cells, is limited in clinical settings. NPC-like cells are not obtained from the intervertebral disc tissue, but they exhibit a nucleus pulposus-like phenotype and can potentially provide a suitable autologous cell source for nucleus pulposus tissue regeneration (10). Continuous progress has been made with in vitro and in vivo experiments of stem cell therapies for IDD, and biological scaffolds have been widely used (11). In stem cell therapy for disc degeneration, the seed cell selection is the first consideration (12). Stem cells used in the biological treatment of IDD should exhibit the following characteristics (13): i) Be abundant and easy to obtain; ii) be able to differentiate well into NPCs; iii) be able to adapt to the local microenvironment of IDD with low oxygen, low glucose and slight acidity; iv) not elicit a strong immune response after transplantation; and v) have a low potential for tumor growth. At present, commonly used stem cells include mesenchymal stem cells (MSCs), induced pluripotent stem cells (iPSCs), hematopoietic stem cells and embryonic stem cells. Commonly used MSCs include bone marrow-derived MSCs (BMSCs), adipose-derived MSCs (ADSCs) and umbilical cord MSCs (13).
BMSCs and ADSCs are two of the most widely studied types of MSCs, and they are widely available and easy to culture in vitro (14). It was indicated that TGF-β1(15) and bone morphogenetic protein 7(16) could effectively induce the differentiation of BMSCs into NPC-like cells. Elabd et al (17) injected autologous BMSCs cultured in a hypoxic environment into the intervertebral disc of 5 patients with intervertebral discogenic low back pain and observed that the waist activity in 4 patients improved to different degrees. Noriega et al (18) conducted allogeneic BMSC transplantation in 24 patients with discogenic low back pain and demonstrated that allogeneic BMSC transplantation was also effective. Xu et al (19) revealed that ADSCs could be induced to differentiate into NPC-like cells after co-culture with NPCs. Clarke et al (20) confirmed that TGF-β1, growth differentiation factor 5 or growth differentiation factor 6 could induce better differentiation of ADSCs into NPC-like cells compared with BMSCs, and could promote the expression of ECM molecules.
In tissue engineering, biological scaffolds can maintain cell function and provide mechanical protection for cells (10). The selection of stem cell scaffolds for the treatment of IDD is an important issue (21,22). Gelatinous scaffolds exhibit low viscosity during cell delivery and can be gelled in situ after injection, thereby filling micro- and macro-cracks and restoring the height of intervertebral discs (23,24). Therefore, gelatinous scaffolds are an ideal scaffold system. Feng et al (10) successfully established a biological scaffold containing glucan-gelatin hydrogel-TGF-β1 and used the scaffold to effectively induce mouse BMSCs to differentiate into NPCs and promote the expression of related ECM genes.
Under appropriate conditions, BMSCs (25) and ADSCs (26) can differentiate into NPCs and reduce the apoptosis of NPCs. However, a comparative study on the differentiation of these two types of cells into NPC-like cells in 3D culture has not yet been reported, to the best of our knowledge. The present study aimed to investigate the differences between BMSCs and ADSCs in their ability to differentiate into cells with an NPC-like phenotype in 3D culture to provide a reference for the selection of candidate cells for the biotherapy of IDD-associated diseases.
Materials and methods
Isolation of BMSCs and ADSCs
BMSCs were isolated and purified using the whole bone marrow cell repeated adherent culture method (27-30). All animal experiments were approved by the Animal Experiment and Ethics Committee of Kunming Medical University (Kunming, China; approval no. KM20190301). Three male Sprague-Dawley rats (6-8 weeks old; ~200 g in weight) were purchased from the Laboratory Animal Center of Kunming Medical University and housed in a humidity (50-65%) pathogen-free environment at 20-25˚C with free access to food and water under a 12/12 h light/dark cycle. Rats were euthanized with sodium pentobarbital overdose (160 mg/kg; intraperitoneal injection). Euthanasia was confirmed when the rats exhibited no heartbeat and breathing for 2-3 min and no blinking reflex. The femur and tibia of the rats were removed under aseptic conditions, and the bone marrow cavity was exposed after the two ends were cut off. The bone marrow cavity was washed with PBS three times. The wash solution was collected and centrifuged at 800 x g for 5 min at room temperature. Subsequently, the bone marrow cell pellet was suspended in 1 ml DMEM/F12 (Thermo Fisher Scientific, Inc.) medium containing 10% FBS (Thermo Fisher Scientific, Inc.) and plated into a 25 cm2 cell culture flask. Cells were then maintained at 37˚C with 5% CO2. The medium was changed every 3 days. When the cell culture reached 90% confluence, the cells were passaged at a ratio of 1:3.
Adipose tissues in the subcutaneous inguinal region were separated, and the fascial tissue and lymph nodes were eliminated with tweezers as much as possible. The adipose tissue was washed with PBS three times. After being cut into pieces, the tissue was centrifuged at 800 x g for 10 min at 4˚C, and the pelleted layer of adipose tissue was retained. After being resuspended in PBS, the tissue was centrifuged at 800 x g for 10 min at 4˚C. The pelleted layer of adipose tissue was mixed with 0.1% collagenase (Beijing Solarbio Science & Technology Co., Ltd.) solution (prepared in DMEM/F12 containing 10% FBS; each, Thermo Fisher Scientific, Inc.) and digested in a 5% CO2 incubator at 37˚C for 2 h. The solution was shaken every half hour. After the digested tissue solution was centrifuged at 600 x g for 5 min at 4˚C, the supernatant was discarded and the pellet was resuspended in PBS.
BMSCs and ADSCs were cultured in a 5% CO2 incubator at 37˚C with DMEM/F12 (Thermo Fisher Scientific, Inc.) containing 10% FBS, 1 IU/ml penicillin (Beijing Solarbio Science & Technology Co., Ltd.) and 1 µg/ml streptomycin (Beijing Solarbio Science & Technology Co., Ltd.). Cells from the third passage were preserved for further experiments.
Identification of BMSCs and ADSCs
BMSC and ADSC cell surface markers were examined via flow cytometry using the forward scatter/side scatter method. Cells were trypsinized and resuspended in PBS at 1x105 cells per 1.5 ml. BMSCs were incubated for 2 h at room temperature in the dark with the following FITC-conjugated antibodies: Anti-CD29 (1:50; cat. no. bs-20630R-FITC), anti-CD90 (1:200; cat. no. bs-0778R-FITC), anti-CD45 (1:100; cat. no. bs-10599R-FITC) and anti-CD34 (1:50; cat. no. bs-0646R-FITC; all from BIOSS). ADSCs were incubated for 2 h at room temperature with the following FITC-conjugated antibodies: Anti-rat stem cells antigen-1 (Sca-1; 1:100; cat. no. bs-3752R-FITC; BIOSS), anti-CD44 (1:50; cat. no. bs-0521R-FITC), anti-CD45 (1:100; cat. no. bs-10599R-FITC) and anti-CD11b (1:200; cat. no. bs-1014R-FITC; all from BIOSS). Subsequently, the cells were washed twice with PBS and analyzed by a CyFlow™ Space flow cytometer (Sysmex Partec GmbH) with FloMax 2.8 software (Sysmex Partec GmbH).
3D culture and grouping
The experiments were performed with four groups: i) BMSC control group; ii) ADSC control group; iii) BMSC 3D culture group; and iv) ADSC 3D culture group. In the control groups, cells were cultured with differentiating medium in 2% O2 and 5% CO2 at 37˚C. The 3D culture system was based on the bioactive hydrogel method (3D cell culture hydrogel kit; cat. no. FS0469; Shanghai Fushen Biotechnology Co., Ltd.) and was performed according to the manufacturer's protocol. In the 3D culture groups, gel solution and 1x106/ml BMSC or ADSC suspension were gently mixed in a centrifuge tube, which was gently and quickly inverted 1-2 times for 1 sec. After the cell culture plate was washed with 1X PBS, the gel-cell mixture was added and gently shaken to evenly spread the mixture in the plate (under different circumstances the mixture could quickly adhere to the walls of the plates). Subsequently, the cells in the 3D culture were incubated at 37˚C for 5-10 min to form a gel. After incubation, the culture medium was added by pipetting down towards the cell plate wall, and the cells were cultured at 37˚C in 5% CO2 for 24 h. After 24 h, half of the upper cell differentiation medium was gently removed with a pipette, and the same amount of fresh medium for NPC-like cells was added, after which the medium was regularly changed. To induce differentiation, cells in 3D culture were cultured with differentiating medium consisting of DMEM/F12 supplemented with 10 ng/ml TGF-β1 (cat. no. 96-100-21-2; PeproTech, Inc.), 100 nmol/l dexamethasone (cat. no. D8040; Beijing Solarbio Science & Technology Co., Ltd.), 50 mg/ml aseorbie acid (cat. no. A8100; Beijing Solarbio Science & Technology Co., Ltd.), 100 mg/ml sodium pyruvate (cat. no. P8380; Beijing Solarbio Science & Technology Co., Ltd.), 40 mg/ml proline (cat. no. P0011; Beijing Solarbio Science & Technology Co., Ltd.) and ITS-Plus Media Supplement (Collaborative Biomedical Products, Inc.) in 2% O2. After 7 days of culture, the cells were subjected to subsequent experiments.
Cell viability
The viability of BMSC and ADSC in 3D and 2D culture conditions was evaluated with the Cell Counting Kit-8 (CCK-8) assay (BIOSS). A total of 3x104 BMSCs or ADSCs were plated into 96-well-plates for 3D and 2D culture. After culture at 37˚C for 48 h, a CCK-8 assay was used to detect cell viability. For 3D culture, cells were released from the gel via treatment with pyrolysis liquid (cat. no. FS0469-B; Shanghai Fushen Biotechnology Co., Ltd.) for 10 min at room temperature. The cells were washed with PBS, and the CCK-8 solution (10 µl/well) was added to the plates. Subsequently, the cells were incubated at 37˚C for 2 h. The optical density (OD) at 450 nm was measured using a microplate reader (BioTek Instruments, Inc.).
Visualization of sulfated glycosaminoglycans (GAGs) and proteoglycans
BMSCs and ADSCs from the control and 3D culture groups were stained with Alcian blue (cat. no. G1563; Beijing Solarbio Science & Technology Co., Ltd.) and safranin O (cat. no. G2540; Beijing Solarbio Science & Technology Co., Ltd.), respectively, to visualize the formation of GAGs and proteoglycans. For Alcian blue staining, cells and gels were washed with PBS three times, fixed in 4% paraformaldehyde (cat. no. P1110; Beijing Solarbio Science & Technology Co., Ltd.) for 20 min at room temperature, then incubated with a 1% Alcian blue staining solution for 4 h at room temperature. For safranin O staining, cells and gels were washed with PBS three times, fixed in 4% paraformaldehyde for 30 min at room temperature, then incubated with safranin O for 30 min at room temperature. After removing the dye solution, the cells and gels were briefly washed with ddH2O three times, and the staining intensity was then observed via light microscopy (magnification, x200).
Reverse transcription-quantitative PCR (RT-qPCR) assay
BMSCs and ADSCs were released from the gel via treatment with pyrolysis liquid (cat. no. FS0469-B; Shanghai Fushen Biotechnology Co., Ltd.) for 10 min at room temperature in the 3D cell culture hydrogel kit. Total RNA was extracted from cells using Trizol reagent (Takara Biotechnology Co., Ltd.) according to the manufacturer's instructions. cDNA was synthesized using a PrimeScript RT Reagent kit (Takara Biotechnology Co., Ltd.) according to the manufacturer's protocol. mRNA levels were measured via qPCR (ABI 7900; Applied Biosystems; Thermo Fisher Scientific, Inc.) using a TB Green Premix ExTaq kit (Takara Biotechnology Co., Ltd.). Relative gene expression was analyzed with the 2-ΔΔCq method (31). GAPDH was used as the reference gene. The primers for the genes were as follows: GAPDH forward, 5'-AGAACATCATCCCTGCATCC-3' and reverse, 5'-TTACTCCTTGGAGGCCATGT-3'; collagen II forward, 5'-CACTCATCTGTTGTGATGAGTTCTCC-3' and reverse, 5'-CAACACACACCAGCGCAGTTT-3'; aggrecan forward, 5'-GGGTGAGGTCTTTTATGCCA-3' and reverse, 5'-GCTTTGCAGTGAGGATCACA-3'; Sox-9 forward, 5'-TTGCTCGGAACTGTCTGGAA-3' and reverse, 5'-CCTGCTCGTCGGTCATCTT-3'; hypoxia-inducible factor 1-α (HIF-1α) forward, 5'-ACTATGTCGCTTTCTTGG-3' and reverse, 5'-GTTTCTGCTGCCTTGTAT-3'; and glucose transporter 1 (GLUT1) forward, 5'-GCCCTGGATGTCCTATCTGA-3' and reverse, 5'-CCCACGATGAAGTTTGAGGT-3'.
Western blot assay
BMSCs and ADSCs were released from the gel via treatment with pyrolysis liquid as aforementioned. Cell proteins were extracted using RIPA lysis buffer (Beyotime Institute of Biotechnology) and quantified using a BCA Protein Assay Kit (Beyotime Institute of Biotechnology). A total of 30 µg protein lysate of each group was separated via 10% SDS-PAGE and transferred to a PVDF membrane (Merck KGaA). Membranes were blocked with 5% skimmed milk in TBS -0.05% Tween 20 (TBST) for 1.5 h at room temperature and then incubated with primary antibodies at 4˚C overnight. Primary antibodies included rabbit anti-Sox-9 antibody (1:1,000; cat. no. bs-4177R; BIOSS), rabbit anti-HIF-1α antibody (1:1,000; cat. no. bs-0737R; BIOSS), rabbit anti-GLUT1 antibody (1:1,000; cat. no. bs-20173R; BIOSS), rabbit anti-aggrecan antibody (1:1,000; cat. no. bs-1223R; BIOSS), mouse anti-collagen II antibody (1:1,000; cat. no. bsm-33409M; BIOSS) and rabbit anti-β-actin antibody (1:2,000; cat. no. AC038; ABclonal Biotech Co., Ltd.). The membranes were then washed with TBST three times and incubated with HRP-conjugated goat anti-rabbit IgG (H+L) (1:5,000; cat. no. AS014; ABclonal Biotech Co., Ltd.) or HRP-conjugated goat anti-mouse IgG (H+L) secondary antibody (1:5,000; cat. no. AS003; ABclonal Biotech Co., Ltd.) for 1 h at room temperature. The bands were detected using a bioimaging system (Bio-Rad Laboratories, Inc.) using ECL reagent (Merck KGaA). The bands were analyzed using ImageJ 2x software (National Institutes of Health). β-actin was used to normalize the relative expression of proteins.
Statistical analysis
Data are presented as the mean ± SEM. Statistical analysis was performed using one-way ANOVA followed by Tukey's multiple comparison test with GraphPad Prism 5.0 software (GraphPad Software Inc.). The number of biological replicates was three, and the experiments were performed at least three times. P<0.05 was considered to indicate a statistically significant difference.
Results
Culture and identification of BMSCs and ADSCs
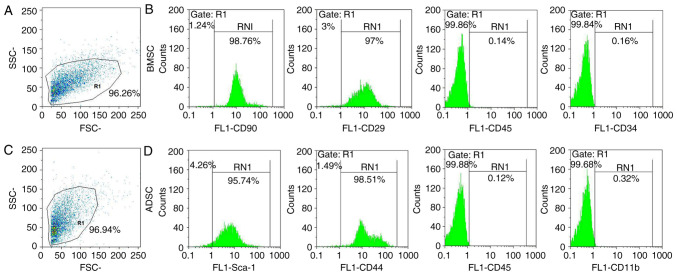
BMSCs and ADSCs were examined via flow cytometry. The present results demonstrated that rat BMSCs were positive for CD29 and CD90 and negative for CD45 and CD34(32) (Fig. 1A and B). Rat ADSCs expressed high levels of a number of stem cell-specific markers (Sca-1 and CD44) and were negative for CD45 and CD11b (26,33) (Fig. 1C and D). Therefore, the present results confirmed that rat ADSCs and BMSCs were successfully obtained and cultured.
Figure 1.
Flow cytometry analysis for the identification of rat BMSCs and ADSCs. BMSC (A) gating strategy and (B) gene markers using flow cytometry. ADSC (C) gating strategy and (D) gene markers using flow cytometry. BMSC, bone marrow-derived mesenchymal stem cell; ADSC, adipose-derived mesenchymal stem cell; Sca-1, stem cells antigen-1.
Viability of BMSCs and ADSCs differentiated toward NPC-like cells
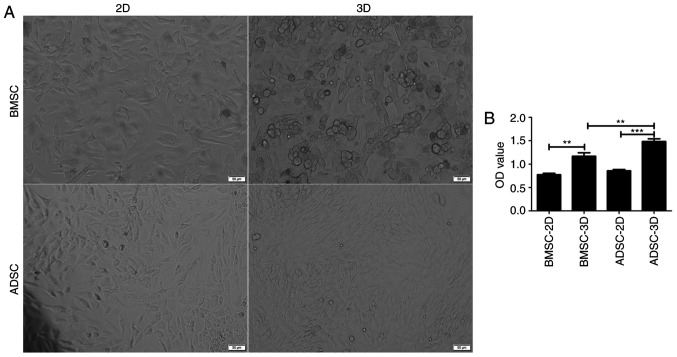
After 7 days of induction, the shape of BMSCs and ADSCs cultured in 3D gradually changed from a long spindle-like morphology to a round or triangular morphology. In addition, the cells became shorter, indicating an alteration toward NPC-like cells (10,34) (Fig. 2A). It was subsequently assessed whether these two cell types also exhibited differences in cell viability. CCK-8 assay results indicated that the OD values were higher in 3D culture (Fig. 2B) and that ADSCs in 3D culture presented higher OD values compared with BMSCs. The current results suggested that BMSCs and ADSCs exhibited an increased growth potential in 3D than in 2D culture. Moreover, ADSCs were indicated to present a greater potential for differentiation into NPC-like cells compared with BMSCs.
Figure 2.
Viability of BMSCs and ADSCs under different culture conditions. (A) Morphology of BMSCs and ADSCs cultured in a monolayer and in 3D gels. Scale bar, 50 µm. (B) Viability of BMSCs and ADSCs analyzed using Cell Counting Kit-8 assay (n=3). Data were analyzed via one-way ANOVA with Tukey's multiple comparison test. **P<0.01; ***P<0.001. BMSC, bone marrow-derived mesenchymal stem cell; ADSC, adipose-derived mesenchymal stem cell; OD, optical density.
NPC-like differentiation of BMSCs and ADSCs
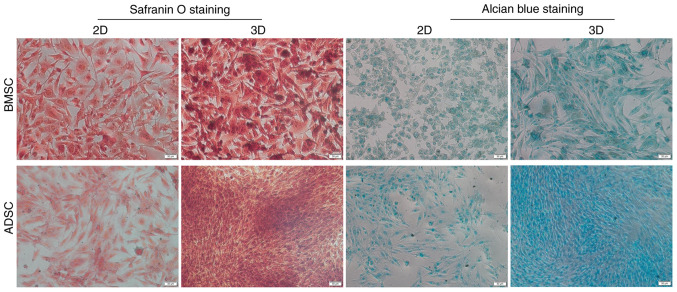
To further examine the potential of BMSCs and ADSCs to differentiate toward an NPC-like phenotype in 3D and 2D culture under hypoxia, the secretion of GAGs was examined. GAGs are the main components of ECM, and can be visualized via Alcian blue staining. Proteoglycan formation was also examined in all groups using safranin O staining. The present results indicated that BMSCs and ADSCs in 3D culture exhibited higher levels of GAGs and proteoglycans than those in the control groups; moreover, ADSCs in 3D culture exhibited higher levels of GAGs and proteoglycans than BMSCs in 3D culture, but there was no notable difference between the two groups in 2D culture (Fig. 3).
Figure 3.
Formation of proteoglycans and GAGs. Proteoglycans and GAG formation by BMSCs and ADSCs was visualized by safranin O and Alcian blue staining, respectively. Scale bar, 50 µm. BMSC, bone marrow-derived mesenchymal stem cell; ADSC, adipose-derived mesenchymal stem cell; GAG, glycosaminoglycan.
Gene expression analysis of BMSCs and ADSCs differentiated toward NPC-like cells
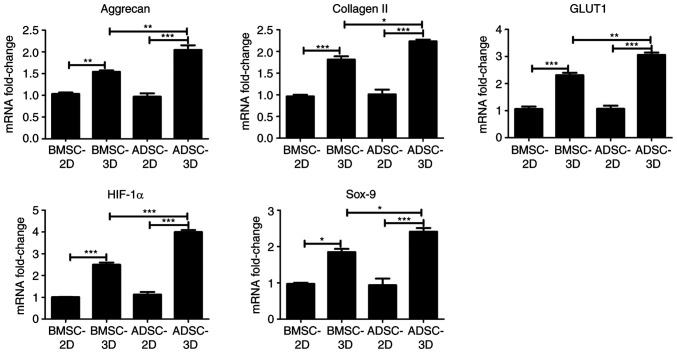
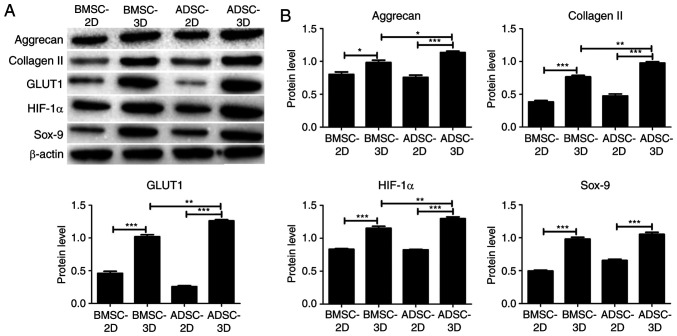
To further investigate the difference between BMSCs and ADSCs in their ability to differentiate toward NPC-like cells, RT-qPCR and western blot assays were performed to examine differences in the expression of NPC marker genes (HIF-1α and GLUT1) and chondrocyte-specific genes (Sox-9, aggrecan and type II collagen) among the groups. As presented in Fig. 4, there was a significant increase in the mRNA expression of HIF-1α, GLUT1, Sox-9, aggrecan and collagen II in BMSCs and ADSCs in 3D culture compared with BMSCs and ADSCs in 2D culture. In addition, the mRNA expression of HIF-1α, GLUT1, Sox-9, aggrecan and collagen II was significantly higher in the ADSC compared with the BMSC 3D culture group (Fig. 4). Similar results were obtained at the protein expression level via western blotting, as presented in Fig. 5. However, Sox-9 protein expression was not significantly higher in the ADSC 3D culture group when compared with the BMSC 3D culture group. The present data indicated that ADSCs were exhibited a higher tendency to differentiate into NPC-like cells compared with BMSCs upon differentiation induction.
Figure 4.
Gene expression of BMSCs and ADSCs under different culture conditions. The mRNA levels of HIF-1α, GLUT1, Sox-9, aggrecan and type II collagen were determined by reverse transcription-quantitative PCR (n=3). Data were analyzed via one-way ANOVA with Tukey's multiple comparison test. *P<0.05; **P<0.01; ***P<0.001. HIF-1α, hypoxia-inducible factor 1-alpha; GLUT1, glucose transporter 1; BMSC, bone marrow-derived mesenchymal stem cell; ADSCs, adipose-derived mesenchymal stem cell.
Figure 5.
Protein expression of BMSCs and ADSCs under different culture conditions. (A) The protein levels of HIF-1α, GLUT1, Sox-9, aggrecan and type II collagen were determined via western blot assays. (B) Quantitative results of the western blot assays (n=3). Data were analyzed via one-way ANOVA with Tukey's multiple comparison test. *P<0.05; **P<0.01; ***P<0.001. HIF-1α, hypoxia-inducible factor 1-α; GLUT1, glucose transporter 1; BMSC, bone marrow-derived mesenchymal stem cell; ADSC, adipose-derived mesenchymal stem cell.
Discussion
IDD is one of the main clinical causes of neck pain and low back pain (35). From a physiological structure perspective, the intervertebral disc is in a microenvironment with high mechanical strength, high osmotic pressure and low nutrition and oxygen levels, and is prone to degeneration with increasing age (36,37). During the degenerative process, intervertebral disc cell apoptosis increases and the matrix composition changes; in particular, there is gradual loss of collagen and proteoglycans, resulting in a deterioration of the local environment, which in turn accelerates cell apoptosis (38). At the same time, the number of intervertebral disc cells is limited, and the regenerative ability of these cells is weak (36). Once degeneration occurs, it is usually irreversible in the natural state (36). Although a number of surgical procedures have been applied in an attempt to relieve clinical symptoms, they all damage the structure of the intervertebral disc and accelerate IDD to a certain extent (39,40).
The past decade has witnessed an increase in regenerative medicine research and tissue engineering, with exciting results for disc regeneration, such as new biomaterials and improved cellular and molecular solutions (41,42). Previous studies have addressed IDD with biological solutions, including growth factors and cytokines, gene therapy, tissue engineering and cell therapy based on the transplantation technology (41,42). Cell therapy is a novel therapeutic approach that aims to delay or even reverse IDD by replenishing the body with stroma-rich cells to compensate for the lack of stromal components (especially collagen and proteoglycans) (43,44). Several types of cell transplantation therapies have entered the rapid research stage (43,44).
Chen et al (45) and Liu et al (46) uncovered through in vitro culture and iPSC identification that iPSCs exhibited the potential to differentiate into myeloid nucleoid cells. Ni et al (47) successfully isolated embryonic-derived MSCs and revealed that the expression of nucleoid cell markers increased significantly when they were cultured under hypoxia. Jin et al (33) induced adipogenic MSCs with TGF-b3 treatment and observed that the expression of intervertebral disc-like cell markers and ECM components was increased compared with adipogenic MSCs alone. Cao et al (48) demonstrated that BMSC can increase TGF-b and decrease the NF-κB pathway activity to promote proteoglycan, predominately type II collagen and Sox-9 gene expression, delaying the degeneration of intervertebral discs. Therefore, BMSCs and ADSCs can be differentiated into NPC-like cells, which may then be used for the treatment of IDD.
The aim of the present investigation was to compare the potential of these two types of cells to be induced to differentiate into NPC-like cells and serve as seed cells for cell therapy in the treatment of IDD. CCK-8 assay results indicated that cell viability was increased in the 3D compared with the 2D culture, and that ADSCs exhibited a higher viability than BMSCs, which suggested that the two stem cell types were suitable for growth in 3D culture, while ADSCs exhibited a greater growth potential in 3D culture. The same number of cells were inoculated into the plates, the final cell density in 3D culture was observed to be higher compared with the 2D culture. Collagen II, Sox-9 and aggrecan are chondrocyte-specific (49), while HIF-1α and GLUT1 are two NPC markers (50,51); however, NPCs can also be regarded as chondrocyte-like cells because of their expression of chondrocyte-specific genes (52). Risbud et al (53) suggested that under hypoxic conditions (2% O2), rat BMSCs can be differentiated toward an NPC-like phenotype in chondrogenic medium within alginate beads. In the present study, BMSCs and ADSCs treated with differentiating medium exhibited a significant increase in the expression of NPC marker genes (HIF-1α, GLUT1) and chondrocyte-specific genes (Sox-9, aggrecan and type II collagen) in 3D culture compared with 2D culture. Furthermore, ADSCs exhibited higher expression of these genes compared with BMSCs in 3D culture. In NPCs and chondrocytes, characteristic markers, including type II collagen, aggrecan and Sox-9 are expressed (33). HIF-1α is a key transcription factor, which can be used as a phenotypic marker to distinguish NPCs from chondrocytes (54). The present results demonstrated ADSCs had a greater ability to differentiate into NPC-like cells than BMSCs when 3D cultured.
Rat ADSCs can be differentiated toward an NPC-like phenotype in 3D alginate hydrogels and cultured in an induction medium containing TGF-β1 under hypoxic conditions (26). GAGs are the main components of ECM and were produced by the differentiated BMSCs and ADSCs cultured in differentiation medium, as detected by Alcian blue staining. Feng et al (55) also indicated that hypoxia markedly enhanced NPC phenotypes, resulting in a greater production of collagen type II and GAGs in nanofibrous scaffolds. Safranin O is a cationic basic dye (56) that can bind to 6-chondroitin sulfate or keratinic sulfate, but not to collagen (57). Proteoglycans in NPCs mainly include chondroitin sulfate or keratinic sulfate, and the content and distribution of chondroitin sulfate or keratinic sulfate in NPCs can be indirectly measured by safranin O staining (56,57). In the present study, higher GAG and proteoglycan levels were detected in 3D culture than in 2D culture; moreover, in 3D culture, ADSCs produced higher levels of GAGs and proteoglycans compared with BMSCs. The expression of NPC marker genes and chondrocyte-specific genes was detected using RT-qPCR and western blot assays, confirming that BMSCs and ADSCs produced higher proteoglycan levels under the 3D compared with the 2D culture condition, suggesting that the differentiation into NPC-like cells under the 3D culture condition was enhanced. The present results indicated that BMSCs and ADSCs differentiated toward an NPC-like phenotype, and that ADSCs had a greater ability to differentiate into NPC-like cells than BMSCs. Previous studies demonstrated that both BMSCs (53,58) and ADSCs (27) could differentiate toward NPC-like cells under the same conditions with a differentiating medium.
Although NPCs have been used to regenerate the disc tissue in previous studies (8,9), access to healthy NPCs, especially autologous cells, is very limited in clinical settings. BMSCs and ADSCs are multi-potential stem cells that can differentiate along several lineages, potentially providing a suitable autologous stem cell source for nucleus pulposus tissue regeneration (14). BMSCs or ADSCs are two of the most widely studied types of MSCs and can be derived from a wide range of sources, including bone and fatty tissue (13). Moreover, ADSCs are easier to obtain than BMSCs as adipose tissue is more readily available and widely distributed than bone in animals. Thus, these cell sources could be candidate cells for the treatment of IDD-related diseases.
The current study presents a number of limitations. Animal implantation experiments should be conducted to confirm that hypoxic induction can contribute to maintaining the NPC phenotype, and that BMSCs or ADSCs can be used to treat IDD-related diseases in vivo. In future studies, the therapeutic function of BMSC or ADSC-3D scaffold implantation will be explored on a mouse IDD model.
It has been reported that BMSCs and ADSCs exhibited an equal potential to be differentiated into osteocytes, adipocytes and chondrocytes (59-61). However, research comparing the differentiation of these two types of stem cells into NPC-like cells in the same study has not been previously reported. The present results are the first to indicate that ADSCs have a greater ability to differentiate into NPC-like cells than BMSCs when cultured in 3D hydrogels with differentiation medium under hypoxic conditions, to the best of our knowledge. Furthermore, while obtaining BMSCs represents a difficult procedure, ADSCs can be easily harvested from patients via a simple and minimally invasive approach. Thus, the present findings provided a reference for the selection of candidate cells for the treatment of IDD-related diseases.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by Yunnan Provincial Science and Technology Department-Kunming Medical University applied basic research joint special fund project (grant no. 201501UH00216).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XD and BL conceived and designed the study. XD, YG, ZZ, YX and CL performed the experiments. XD, YG and HL analyzed the data. XD and BL wrote the manuscript. XD and BL reviewed and edited the manuscript. XD and BL confirm the authenticity of all the raw data. All authors read and approved the manuscript.
Ethics approval and consent to participate
All animal experiments were approved by the Animal Experiment and Ethics Committee of Kunming Medical University (Kunming, China; approval no. KM20190301).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet. 2017;389:736–747. doi: 10.1016/S0140-6736(16)30970-9. [DOI] [PubMed] [Google Scholar]
- 2.Wang MY, Vasudevan R, Mindea SA. Minimally invasive lateral interbody fusion for the treatment of rostral adjacent-segment lumbar degenerative stenosis without supplemental pedicle screw fixation. J Neurosurg Spine. 2014;21:861–866. doi: 10.3171/2014.8.SPINE13841. [DOI] [PubMed] [Google Scholar]
- 3.Colombini A, Lombardi G, Corsi MM, Banfi G. Pathophysiology of the human intervertebral disc. Int J Biochem Cell Biol. 2008;40:837–842. doi: 10.1016/j.biocel.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Ghannam M, Jumah F, Mansour S, Samara A, Alkhdour S, Alzuabi MA, Aker L, Adeeb N, Massengale J, Oskouian RJ, Tubbs RS. Surgical anatomy, radiological features, and molecular biology of the lumbar intervertebral discs. Clin Anat. 2017;30:251–266. doi: 10.1002/ca.22822. [DOI] [PubMed] [Google Scholar]
- 5.Sakai D, Grad S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv Drug Deliv Rev. 2015;84:159–171. doi: 10.1016/j.addr.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Luoma K, Riihimaki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25:487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 7.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura K, Mochida J. Percutaneous reinsertion of the nucleus pulposus. An experimental study. Spine (Phila Pa 1976) 1998;23:1531–1538. doi: 10.1097/00007632-199807150-00006. discussion 1539. [DOI] [PubMed] [Google Scholar]
- 9.Okuma M, Mochida J, Nishimura K, Sakabe K, Seiki K. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: An in vitro and in vivo experimental study. J Orthop Res. 2000;18:988–997. doi: 10.1002/jor.1100180620. [DOI] [PubMed] [Google Scholar]
- 10.Feng G, Jin X, Hu J, Ma H, Gupte MJ, Liu H, Ma PX. Effects of hypoxias and scaffold architecture on rabbit mesenchymal stem cell differentiation towards a nucleus pulposus-like phenotype. Biomaterials. 2011;32:8182–8189. doi: 10.1016/j.biomaterials.2011.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan D, Chen Z, Xiang X, Deng S, Liu K, Xiao D, Deng L, Feng G. The establishment and biological assessment of a whole tissue-engineered intervertebral disc with PBST fibers and a chitosan hydrogel in vitro and in vivo. J Biomed Mater Res B Appl Biomater. 2019;107:2305–2316. doi: 10.1002/jbm.b.34323. [DOI] [PubMed] [Google Scholar]
- 12.Binch ALA, Fitzgerald JC, Growney EA, Barry F. Cell-based strategies for IVD repair: Clinical progress and translational obstacles. Nat Rev Rheumatol. 2021;17:158–175. doi: 10.1038/s41584-020-00568-w. [DOI] [PubMed] [Google Scholar]
- 13.Nan L, FX , Zhang L, Liu Y, Wang F, Zhou SF. Research progresses of stem cell in the treatment of intervertebral disc degenerative disease. Chinese Journal of Injury Repair and Wound Healing (Electronic Edition) 2018;13:134–138. (in Chinese) [Google Scholar]
- 14.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 15.Han C, Jiang C, Yu C, Shen H. Differentiation of transforming growth factor β1-induced mesenchymal stem cells into nucleus pulposus-like cells under simulated microgravity conditions. Cell Mol Biol (Noisy-le-grand) 2015;61:50–55. [PubMed] [Google Scholar]
- 16.Xu J, E XQ, Wang NX, Wang MN, Xie HX, Cao YH, Sun LH, Tian J, Chen HJ, Yan JL. BMP7 enhances the effect of BMSCs on extracellular matrix remodeling in a rabbit model of intervertebral disc degeneration. FEBS J. 2016;283:1689–1700. doi: 10.1111/febs.13695. [DOI] [PubMed] [Google Scholar]
- 17.Elabd C, Centeno CJ, Schultz JR, Lutz G, Ichim T, Silva FJ. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: A long-term safety and feasibility study. J Transl Med. 2016;14(253) doi: 10.1186/s12967-016-1015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noriega DC, Ardura F, Hernández-Ramajo R, Martín-Ferrero MÁ, Sánchez-Lite I, Toribio B, Alberca M, García V, Moraleda JM, Sánchez A, García-Sancho J. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: A randomized controlled trial. Transplantation. 2017;101:1945–1951. doi: 10.1097/TP.0000000000001484. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Qi DL, Pang XJ, Jing CW. Rabbit nucleus pulposus cells facilitate differentiation of adipose-derived stem cells into nucleus pulposus-like cells. Indian J Cancer. 2015;52 (Suppl 1):e17–e21. doi: 10.4103/0019-509X.168950. [DOI] [PubMed] [Google Scholar]
- 20.Clarke LE, McConnell JC, Sherratt MJ, Derby B, Richardson SM, Hoyland JA. Growth differentiation factor 6 and transforming growth factor-beta differentially mediate mesenchymal stem cell differentiation, composition, and micromechanical properties of nucleus pulposus constructs. Arthritis Res Ther. 2014;16(R67) doi: 10.1186/ar4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roughley P, Hoemann C, DesRosiers E, Mwale F, Antoniou J, Alini M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials. 2006;27:388–396. doi: 10.1016/j.biomaterials.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 22.Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, Nakai T, Ando K, Hotta T. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: A potential therapeutic model for disc degeneration. Biomaterials. 2003;24:3531–3541. doi: 10.1016/s0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 23.Simona BR, Hirt L, Demkó L, Zambelli T, Vörös J, Ehrbar M, Milleret V. Density gradients at hydrogel interfaces for enhanced cell penetration. Biomater Sci. 2015;3:586–591. doi: 10.1039/c4bm00416g. [DOI] [PubMed] [Google Scholar]
- 24.Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA, Rizzi SC. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials. 2010;31:8494–8506. doi: 10.1016/j.biomaterials.2010.07.064. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Wu A, Han C, Chen C, Zhou T, Zhang K, Yang X, Chen Z, Qin A, Tian H, Zhao J. Bone marrow-derived mesenchymal stem cells in three-dimensional co-culture attenuate degeneration of nucleus pulposus cells. Aging (Albany NY) 2019;11:9167–9187. doi: 10.18632/aging.102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie LW, Fang H, Chen AM, Li F. Differentiation of rat adipose tissue-derived mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro. Chin J Traumatol. 2009;12:98–103. [PubMed] [Google Scholar]
- 27.Hanson K, Isder C, Shogren K, Mikula AL, Lu L, Yaszemski MJ, Elder BD. The inhibitory effects of vancomycin on rat bone marrow-derived mesenchymal stem cell differentiation. J Neurosurg Spine. 2021:1–5. doi: 10.3171/2020.10.SPINE201511. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 28.Pieróg J, Tamo L, Fakin R, Kocher G, Gugger M, Grodzki T, Geiser T, Gazdhar A, Schmid RA. Bone marrow stem cells modified with human interleukin 10 attenuate acute rejection in rat lung allotransplantation. Eur J Cardiothorac Surg. 2018;53:194–200. doi: 10.1093/ejcts/ezx257. [DOI] [PubMed] [Google Scholar]
- 29.Kinebuchi Y, Aizawa N, Imamura T, Ishizuka O, Igawa Y, Nishizawa O. Autologous bone-marrow-derived mesenchymal stem cell transplantation into injured rat urethral sphincter. Int J Urol. 2010;17:359–368. doi: 10.1111/j.1442-2042.2010.02471.x. [DOI] [PubMed] [Google Scholar]
- 30.Riahi M, Parivar K, Baharara J, Zandi R. Evaluation of the repair of diaphyseal fracture of femoral bone using bone marrow mesenchymal stem cells in nicotine-bearing rat. Bratisl Lek Listy. 2019;120:434–442. doi: 10.4149/BLL_2019_070. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Hu J, Deng G, Tian Y, Pu Y, Cao P, Yuan W. An in vitro investigation into the role of bone marrow-derived mesenchymal stem cells in the control of disc degeneration. Mol Med Rep. 2015;12:5701–5708. doi: 10.3892/mmr.2015.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin ES, Min J, Jeon SR, Choi KH, Jeong JH. Analysis of molecular expression in adipose tissue-derived mesenchymal stem cells: Prospects for use in the treatment of intervertebral disc degeneration. J Korean Neurosurg Soc. 2013;53:207–212. doi: 10.3340/jkns.2013.53.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei G HY, Qin W, Liao C, Lin Z. Differentiation of bone marrow mesenchymal stem cells into nucleus pulposus-like cells after co-culture with nucleus pulposus cells. Chin J Tissue Engineering Res. 2013;17:7834–7839. [Google Scholar]
- 35.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vergroesen PP, Kingma I, Emanuel KS, Hoogendoorn RJ, Welting TJ, van Royen BJ, van Dieën JH, Smit TH. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthritis Cartilage. 2015;23:1057–1070. doi: 10.1016/j.joca.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 37.Chan S, Walser J, Käppeli P, Shamsollahi M, Ferguson S, Gantenbein-Ritter B. Region specific response of intervertebral disc cells to complex dynamic loading: An organ culture study using a dynamic torsion-compression bioreactor. PLoS One. 2013;8(e72489) doi: 10.1371/journal.pone.0072489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson DG, Tannoury C. Molecular pathogenic factors in symptomatic disc degeneration. Spine J. 2005;5 (Suppl 6):260S–266S. doi: 10.1016/j.spinee.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 39.van den Eerenbeemt KD, Ostelo RW, van Royen BJ, Peul WC, van Tulder MW. Total disc replacement surgery for symptomatic degenerative lumbar disc disease: A systematic review of the literature. Eur Spine J. 2010;19:1262–1280. doi: 10.1007/s00586-010-1445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller LE, Block JE. Safety and effectiveness of bone allografts in anterior cervical discectomy and fusion surgery. Spine (Phila Pa 1976) 2011;36:2045–2050. doi: 10.1097/BRS.0b013e3181ff37eb. [DOI] [PubMed] [Google Scholar]
- 41.Alini M, Roughley P, Antoniou J, Stoll T, Aebi M. A biological approach to treating disc degeneration: Not for today, but maybe for tomorrow. Eur Spine J 11 Suppl. 2002;2 (Suppl 2):S215–S220. doi: 10.1007/s00586-002-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.An HS, Thonar EJ, Masuda K. Biological repair of intervertebral disc. Spine (Phila Pa 1976) 2003;28 (Suppl 15):S86–S92. doi: 10.1097/01.BRS.0000076904.99434.40. [DOI] [PubMed] [Google Scholar]
- 43.Hohaus C, Ganey TM, Minkus Y, Meisel HJ. Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J 17 Suppl. 2008;4 (Suppl 4):492–503. doi: 10.1007/s00586-008-0750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, Alasevic OJ. Clinical experience in cell-based therapeutics: Disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24:5–21. doi: 10.1016/j.bioeng.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Lee EJ, Jing L, Christoforou N, Leong KW, Setton LA. Differentiation of mouse induced pluripotent stem cells (iPSCs) into nucleus pulposus-like cells in vitro. PLoS One. 2013;8(e75548) doi: 10.1371/journal.pone.0075548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu K, Chen Z, Luo XW, Song GQ, Wang P, Li XD, Zhao M, Han XW, Bai YG, Yang ZL, Feng G. Determination of the potential of induced pluripotent stem cells to differentiate into mouse nucleus pulposus cells in vitro. Genet Mol Res. 2015;14:12394–12405. doi: 10.4238/2015.October.16.6. [DOI] [PubMed] [Google Scholar]
- 47.Ni L, Liu X, Sochacki KR, Ebraheim M, Fahrenkopf M, Shi Q, Liu J, Yang H. Effects of hypoxia on differentiation from human placenta-derived mesenchymal stem cells to nucleus pulposus-like cells. Spine J. 2014;14:2451–2458. doi: 10.1016/j.spinee.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 48.Cao C, Zou J, Liu X, Shapiro A, Moral M, Luo Z, Shi Q, Liu J, Yang H, Ebraheim N. Bone marrow mesenchymal stem cells slow intervertebral disc degeneration through the NF-κB pathway. Spine J. 2015;15:530–538. doi: 10.1016/j.spinee.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 49.Sive JI, Baird P, Jeziorsk M, Watkins A, Hoyland JA, Freemont AJ. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol. 2002;55:91–97. doi: 10.1136/mp.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajpurohit R, Risbud MV, Ducheyne P, Vresilovic EJ, Shapiro IM. Phenotypic characteristics of the nucleus pulposus: Expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002;308:401–407. doi: 10.1007/s00441-002-0563-6. [DOI] [PubMed] [Google Scholar]
- 51.Richardson SM, Knowles R, Tyler J, Mobasheri A, Hoyland JA. Expression of glucose transporters GLUT-1, GLUT-3, GLUT-9 and HIF-1alpha in normal and degenerate human intervertebral disc. Histochem Cell Biol. 2008;129:503–511. doi: 10.1007/s00418-007-0372-9. [DOI] [PubMed] [Google Scholar]
- 52.Cui X, Liu M, Wang J, Zhou Y, Xiang Q. Electrospun scaffold containing TGF-β1 promotes human mesenchymal stem cell differentiation towards a nucleus pulposus-like phenotype under hypoxia. IET Nanobiotechnol. 2015;9:76–84. doi: 10.1049/iet-nbt.2014.0006. [DOI] [PubMed] [Google Scholar]
- 53.Risbud MV, Albert TJ, Guttapalli A, Vresilovic EJ, Hillibrand AS, Vaccaro AR, Shapiro IM. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: Implications for cell-based transplantation therapy. Spine (Phila Pa 1976) 2004;29:2627–2632. doi: 10.1097/01.brs.0000146462.92171.7f. [DOI] [PubMed] [Google Scholar]
- 54.Risbud MV, Guttapalli A, Stokes DG, Hawkins D, Danielson KG, Schaer TP, Albert TJ, Shapiro IM. Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: A metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–159. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- 55.Feng G, Li L, Liu H, Song Y, Huang F, Tu C, Shen B, Gong Q, Li T, Liu L, et al. Hypoxia differentially regulates human nucleus pulposus and annulus fibrosus cell extracellular matrix production in 3D scaffolds. Osteoarthritis Cartilage. 2013;21:582–588. doi: 10.1016/j.joca.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Aaron RK, Jolly G, Ciombor DM, Barrach HJ. A histochemical method for the demonstration of calcifying cartilage. Calcif Tissue Int. 1988;43:244–249. doi: 10.1007/BF02555141. [DOI] [PubMed] [Google Scholar]
- 57.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53:69–82. [PubMed] [Google Scholar]
- 58.Fang Z, Yang Q, Luo W, Li GH, Xiao J, Li F, Xiong W. Differentiation of GFP-Bcl-2-engineered mesenchymal stem cells towards a nucleus pulposus-like phenotype under hypoxia in vitro. Biochem Biophys Res Commun. 2013;432:444–450. doi: 10.1016/j.bbrc.2013.01.127. [DOI] [PubMed] [Google Scholar]
- 59.Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 60.Anokhina EB, Buravkova LB. Heterogeneity of stromal precursor cells isolated from rat bone marrow. Tsitologiia. 2007;49:40–47. [PubMed] [Google Scholar]
- 61.Keyser KA, Beagles KE, Kiem HP. Comparison of mesenchymal stem cells from different tissues to suppress T-cell activation. Cell Transplant. 2007;16:555–562. doi: 10.3727/000000007783464939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.