See Clinical Research on Page 2134
Nephrotic syndrome is characterized by nephrotic-range proteinuria, hypoalbuminemia, edema, and hyperlipidemia. In the absence of systemic diseases such as diabetes mellitus, systemic lupus erythematosus, or amyloidosis, kidney biopsy samples predominantly show minimal change disease (MCD) or focal segmental glomerulosclerosis (FSGS). These primary glomerular disorders, which should more accurately be identified as histologic patterns of injury rather than as diagnoses, are themselves heterogeneous in etiology, with unclear underlying pathogenesis. A complex interplay among genetic susceptibility and environmental, hemodynamic, and immune-mediated influences ultimately culminate in podocyte injury and disruption of the glomerular filtration barrier, which can lead to progressive deterioration in kidney function.
The glomerular filtration barrier consists of fenestrated endothelial cells, the glomerular basement membrane, and podocytes. Although proteinuria can result from injury to any of these components, podocytes have been identified as the key target cell for injury across the spectrum of progressive glomerular disease. Seminal studies by Wiggins et al. have shown that the loss of more than 40% of podocytes in a rodent model led to sustained heavy proteinuria and decreased kidney function.1 However, the pathogenesis of podocyte injury in proteinuric conditions remains poorly understood. The high recurrence rate of FSGS posttransplantation as well as the elegant case report2 of resolution of posttransplantation FSGS when the allograft was re-transplanted in a diabetic kidney disease patient, strongly suggests a role for circulating factor(s) in disease pathogenesis. Putative causative factors such as the urokinase-type plasminogen activator receptor (uPAR) and its soluble form (suPAR) have been widely studied. Anti-CD40 antibody, apolipoprotein A-I, CLCF1, and active proteases have also been explored as candidate circulating factors.3
More than 50 genes have been associated with FSGS and idiopathic nephrotic syndrome, and they are more prevalent in the pediatric population than in adults. Secondary etiologies include those due to maladaptive changes, medications/toxins, and viral infections. Complicating this classification is the identification of overlapping contributors such as suPAR, HIV, and SARS-COV-2 infection influencing disease susceptibility in patients with high-risk APOL-1 alleles.4, 5
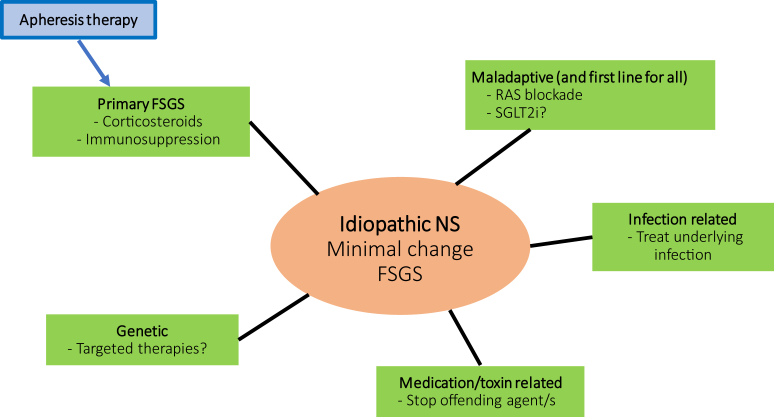
Ideally, the appropriate treatment of MCD, FSGS, and nephrotic syndrome would entail a precision-based approach, with tailored therapy directed at the underlying cause (Figure 1). Maladaptive disease and, some would argue, all etiologies should be treated with renin−angiotensin system blockade, with increasing justification for adding a sodium glucose co-transporter inhibitor. Disease due to circulating factors would be best treated with approaches that include corticosteroids, immunomodulatory agents, plasmapheresis, or immunoadsorption. Some diseases due to monogenic etiologies such as those implicated in coenzyme Q10 biosynthesis offer a path to personalized treatment and may respond to oral coenzyme Q10 supplementation.6 Patients with monogenic steroid-resistant nephrotic syndrome have a poor response to immunosuppressive therapy but also a lower risk of disease recurrence posttransplantation.
Figure 1.
Idiopathic nephrotic syndrome is typically represented histologically as minimal change disease or focal segmental glomerulosclerosis. As diagnostic and therapeutic capabilities expand, treatment strategies for maladaptive etiologies could be considered baseline across the spectrum. Apheresis therapy in combination with immunosuppression would most likely benefit patients with “primary” disease. Targeted therapeutics may be optimal for monogenetic disease, whereas cessation of offending agents and treatment of underlying infection would be the optimal approach for medication-related and infection-related disease, respectively. FSGS, focal segmental glomerulosclerosis; NS, nephrotic syndrome; RAS, renin−angiotensin system; SGLT2i, sodium-glucose cotransporter 2 inhibitors.
Although genetic testing panels are becoming more widely available, it is still difficult clinically to distinguish patients who have a genetic versus an immunologic basis for their disease. Assays to identify patients with disease due to circulating glomerular permeability factors are currently unavailable. Treatment algorithms to date still largely recommend a less-than-desirable generalized approach based on age, clinical presentation, histologic findings, and clinical course. Corticosteroids are first-line agents, initiated before kidney biopsy in children less than 12 years of age. In adults, steroids are recommended after kidney biopsy for MCD and FSGS, where complete and partial remission rates are typically less than 30%.7 Some patients are steroid dependent, requiring high doses for extended periods, whereas other patients are defined as steroid resistant. Patients who fail to respond to steroids are treated with other immunosuppressive agents such as calcineurin inhibitors, mycophenolate mofetil, and rituximab.
The contribution of circulating factors to nephrotic syndrome pathogenesis has raised interest in extracorporeal therapies such as plasma exchange, immunoadsorption, and low-density lipoprotein apheresis.8 Plasma exchange has been the most widely studied, although randomized controlled study data are largely unavailable, and reported cases have invariably shown plasma exchange use in conjunction with other immunosuppressive therapy. Semi-selective immunoadsorption is a modified plasma exchange whereby high-affinity absorption columns containing specific ligands could facilitate selective circulating factor removal. Low-density lipoprotein apheresis has also been shown to induce a remission of proteinuria in some patients with FSGS, by an unclear mechanism, when used in conjunction with immunosuppressive therapy.
Published data regarding the efficacy and safety of extracorporeal therapy in native kidney disease have been lacking, with conflicting reports regarding efficacy, optimal treatment protocols, and concomitant medications. In this issue of KI Reports, Moret et al. examine the outcomes of adult patients who received apheresis treatment for biopsy-proven refractory idiopathic nephrotic syndrome (INS) identified retrospectively from several centers in France.9 Patients in this cohort had either MCD (9 of 21) or FSGS (12 of 21). Of the 21 patients identified between September 1997 and January 2020, 7 (33.3%) achieved remission, with 4 (57.1%) achieving complete remission and 3 (42.9%) achieving partial remission. Patients who were older and had lower levels of proteinuria and lower GFR at baseline were more likely to achieve remission. The authors also found several factors to be associated with a response after apheresis treatment, including dialysis for acute kidney injury before apheresis, age greater than 50 years, and a shorter time (<12 months) between diagnosis and apheresis, suggesting a potential early role of apheresis in the treatment of refractory INS.
This study has several limitations, including the small sample size that was predominantly male, a retrospective design, and limited genetic testing. Multiple extracorporeal therapy approaches were included whereby most patients received plasma exchange but others received immunoadsorption, double filtration plasmapheresis, low-density lipoprotein apheresis, or plasma exchange followed by immunoadsorption. There was significant disease heterogeneity, with 12 patients having FSGS and 9 patients MCD. Corticosteroid dosing was variable, as was the choice of co-administered immunosuppression. Nonetheless, the findings provide useful insight into the management of treatment resistant INS. The results show that apheresis may be an option in a subset of patients.
The findings also highlight the critical unmet need for better diagnostic capabilities to identify patients with a circulating factor or factors that increase glomerular permeability to albumin. In the absence of validated diagnostic assays, a clinicopathologic approach can be used. These patients typically have no family history of proteinuric kidney disease or detectible pathologic variants on advanced genetic testing with FSGS panels or whole exome sequencing. They are more likely to have a low serum albumin and kidney biopsy samples that show diffuse podocyte foot process effacement on electron microscopy.10 These individuals, in contrast to those with secondary or genetic causes for INS, would be most likely to benefit from apheresis, and have the highest risk of disease recurrence posttransplantation. Several questions remain unresolved, including which current apheresis approach is superior for patients with INS. The optimal timing, composition, and sequence of administration of concomitant immunosuppressive regimens will also need to be determined in the context of future studies.
Disclosure
KNC reports consulting fees from Travere, Goldfinch, Calliditas, and grants and personal fees from Mallinckrodt outside the submitted work. EA declares no competing interests.
References
- 1.Wharram B.L., Goyal M., Wiggins J.E. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 2.Gallon L., Leventhal J., Skaro A. Resolution of recurrent focal segmental glomerulosclerosis after retransplantation. N Engl J Med. 2012;366:1648–1649. doi: 10.1056/NEJMc1202500. [DOI] [PubMed] [Google Scholar]
- 3.Wen Y., Shah S., Campbell K.N. Molecular mechanisms of proteinuria in focal segmental glomerulosclerosis. Front Med (Lausanne) 2018;5:98. doi: 10.3389/fmed.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayek S.S., Koh K.H., Grams M.E. A tripartite complex of suPAR, APOL1 risk variants and alphavbeta3 integrin on podocytes mediates chronic kidney disease. Nat Med. 2017;23:945–953. doi: 10.1038/nm.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu H., Larsen C.P., Hernandez-Arroyo C.F. AKI and collapsing glomerulopathy associated with COVID-19 and APOL 1 high-risk genotype. J Am Soc Nephrol. 2020;31:1688–1695. doi: 10.1681/ASN.2020050558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atmaca M., Gulhan B., Korkmaz E. Follow-up results of patients with ADCK4 mutations and the efficacy of CoQ10 treatment. Pediatr Nephrol. 2017;32:1369–1375. doi: 10.1007/s00467-017-3634-3. [DOI] [PubMed] [Google Scholar]
- 7.Radhakrishnan J., Cattran D.C. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines—application to the individual patient. Kidney Int. 2012;82:840–856. doi: 10.1038/ki.2012.280. [DOI] [PubMed] [Google Scholar]
- 8.Raina R., Wang J., Sharma A. Extracorporeal therapies in the treatment of focal segmental glomerulosclerosis. Blood Purif. 2020;49:513–523. doi: 10.1159/000506277. [DOI] [PubMed] [Google Scholar]
- 9.Moret L.G.A., Dao M. Effect of apheresis therapy in adult patients with refractory idiopathic nephrotic syndrome in native kidneys. Kidney Int Rep. 2021;6:2134–2143. doi: 10.1016/j.ekir.2021.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vriese A.S., Wetzels J.F., Glassock R.J. Therapeutic trials in adult FSGS: lessons learned and the road forward. Nat Rev Nephrol. 2021:112. doi: 10.1038/s41581-021-00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]