Abstract
Introduction
The externally validated Kidney Failure Risk Equation (KFRE) for predicting risk of end-stage renal disease (ESRD) has been developed, but its potential impact in a population on referrals for patients with chronic kidney disease (CKD) from primary to specialty nephrology care is not known.
Methods
A cross-sectional population-based study of individuals in United Kingdom primary care registered in The Health Improvement Network database was conducted. National Institute of Health and Care Excellence (NICE) 2014 CKD guidelines versus the 4-variable KFRE set at a >3% risk of ESRD at 5 years were applied to patients identified with CKD stage 3-5 between January 1, 2016, and March 31, 2017.
Results
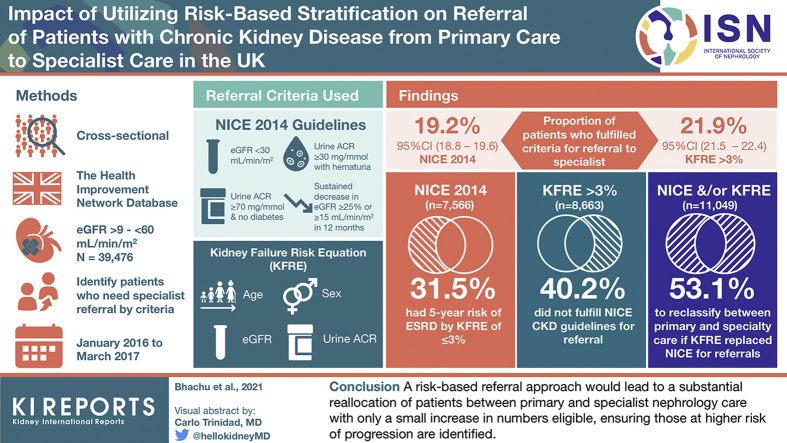
In all, 39,476 (36.6%) of 107,962 adults with CKD stage 3-5 had a urine albumin:creatinine ratio (ACR) available and entered into the primary analysis. Of that, 7566 (19.2%) patients fulfilled NICE criteria for referral, 2386 (31.5%) of whom had a ≤3% 5-year risk of ESRD. Also 8663 (21.9%) patients had a >3% 5-year risk of ESRD, 3483 (40.2%) of whom did not fulfill NICE criteria; this represents 8.8% of the primary population. By using the KFRE threshold rather than NICE criteria for referral, 5869 patients (14.9% of the primary analysis population) would have been reallocated between primary and specialist care. Imputational analysis was used for missing ACR measurements and showed similar results.
Conclusions
A risk-based referral approach would lead to a substantial reallocation of patients between primary care and specialist nephrology care with only a small increase in numbers eligible, ensuring those at higher risk of progression are identified.
Keywords: chronic kidney disease, cross-sectional study, disease progression, guidelines, kidney failure risk equation, patient referral
Graphical abstract
See Commentary on Page 2028
CKD stage 3-5 prevalence in adults was recently estimated as 7.3% in England.1 Individuals with CKD are at an increased risk of morbidity and mortality from cardiovascular disease.2, 3, 4 Progression to ESRD requiring treatment with renal replacement therapy (RRT) occurs in a small proportion of patients with CKD, with an incidence of 120 per million population in the United Kingdom in 2018.5 Guidelines for the referral of patients with CKD from primary care to specialist nephrology care are provided in the NICE 2014 CKD guideline.6 The indication for the large majority of these referrals is based on an increased risk of progression to ESRD and the need for preparation for management of ESRD.6 The guidance is based on expert opinion and a key measure incorporated within the referral criteria, the estimated glomerular filtration rate (eGFR), does not quantitatively adjust for confounders including age, sex, and urine ACR, all of which are independent variables for risk of progression to ESRD2; however, ACR is included in the guidance as a variable in its own right.
Many risk prediction models in kidney disease have been developed for kidney failure, but most are limited by their lack of external validation, poor methodology, and ease of use.7 A recent systematic review recommended the KFRE for use in predicting ESRD in the cohort of patients with CKD 3-5.7 The 4-variable KFRE estimates an individual’s risk of progression to ESRD at 2 years and 5 years with high precision8,9 based on age, sex, eGFR (calculated using the CKD Epidemiology Collaboration [CKD-EPI] equation) and urine ACR. CKD populations globally have contributed to this model’s extensive external validation,9 including CKD cohorts within the United Kingdom,9,10 and it is being evaluated in improvement programs in Canada.11, 12, 13 The equation can easily be imbedded into electronic medical records where patients’ clinical and laboratory data can be incorporated and it is also readily available online.14 There may be scope to improve the accuracy of the equation and studies have investigated the addition of other variables15, 16, 17, 18, 19, 20 or recalibrated to local populations,10 and have also explored broadening its use by validating certain populations such as renal transplant recipients21,22 and children.23,24 Several studies applying a risk of progression to ESRD of >3% at 5 years for triaging referrals from primary care to specialty care have found that it has reduced waiting times for high-risk patients13 and number of referrals.25 Using the KFRE in clinical pathways may lead to better access to appropriate care and system benefits.13
The objective of this study was to identify the proportion of individuals who fulfill current NICE guideline criteria for referral to specialist nephrology services compared to the proportion meeting a predefined KFRE risk threshold of >3% at 5 years for progression to ESRD and to assess the proportion of individuals who would be reclassified between primary care and specialist services according to this threshold.
Methods
This was a cross-sectional population-based observational study. Data were extracted from The Health Improvement Network (THIN) database, which comprises anonymized medical records of 3.6 million active patients from 787 general practices.26,27 The database is broadly representative of the UK population in terms of demographics, disease prevalence, and mortality rates.26
Patient data in THIN are derived from practices using Vision electronic medical record software, which stores information in a hierarchical system of clinical (Read) codes.28 The database includes information on patient demographics, diagnoses, prescriptions, and investigations. The baseline characteristics defined for analysis in this study are described in the protocol paper.29
The eGFR was calculated using the CKD-EPI equation (see Supplementary Methods S1) for all patients aged 18 years and older who had an index serum creatinine (sCr) reported between January 1, 2016, and March 31, 2017, and where there was a second sCr level >90 days before or after the index sCr level. Patients with two eGFRs <60 ml/min per 1.73 m2 more than 90 days apart were defined as having CKD stage 3-5. This was in accordance with the Kidney Disease Improving Global Outcomes criteria for eGFR-based classification of CKD.30 Proteinuria was quantified using urine ACR (mg/mmol) measured within 12 months of the index sCr record. Clinical (Read) codes were used to identify comorbidities.
Patients were excluded from further analysis if they had an eGFR ≥60 ml/min per 1.73 m2, were coded as pregnant at the time of the recorded data, or were receiving RRT (defined as receiving dialysis or with a renal transplant). No defined clinical code is available for patients who are following a supportive care pathway for ESRD; therefore, patients were excluded from further analysis if they had an eGFR ≤9 ml/min per 1.73 m2, the mean eGFR at which patients with CKD commence RRT in the United Kingdom.31
The proportion of patients fulfilling criteria for referral to specialist nephrology care was calculated based on the NICE 2014 CKD guidelines. These comprised: (1) eGFR <30 ml/min per 1.73 m2; (2) urine ACR ≥30 mg/mmol with hematuria; (3) urine ACR ≥70 mg/mmol and no diabetes; (4) a sustained decrease in eGFR of ≥25% or a sustained decrease in eGFR of ≥15 ml/min per 1.73 m2 within 12 months. In addition, we calculated the proportion of patients with urine ACR >100 mg/mmol, including patients with diabetes; although this is currently not identified as a referral threshold for individuals with diabetes, these individuals are at particular risk of progression to ESRD.
The non–North American 4-variable KFRE9 (see Supplementary Methods S1) required: age (years), sex, CKD-EPI eGFR (ml/min per 1.73 m2) and urine ACR (mg/mmol), with the predefined threshold for referral from primary to specialty care of >3% risk at 5 years for progression to ESRD.
Analysis
Stata 14 (College Station, Texas, USA: StataCorp, 2015) was used for statistical analyses. The proportion of patients meeting the current NICE criteria for referral was compared to the proportion meeting the KFRE threshold for risk of progression to ESRD of >3% at 5 years. The proportion of patients reclassified from requiring or not requiring referral based on the applied criteria was then calculated. The baseline characteristics were described with categorical variables as n (%) and continuous numerical data as median (interquartile range). The primary analysis included only patients who had an ACR recorded within 1 year of index sCr measurement.
In a sensitivity analysis, missing urine ACR values were imputed using the following method: firstly, ACR measurements recorded within 5 years of the index sCr were accepted as a proxy for recent ACR within 1 year of sCr measurement. Using ACR measurements outside of 1 year and up to 5 years from the index sCr may provide some information on risk assessment by KFRE and/or NICE criteria; however, an extended time gap between eGFR calculated from the index sCr and ACR quantification increases the potential for error because of progression of the natural history of disease and change of variables that can impact on ACR, including use and dose changing of antiproteinuric agents and change in cofactors for albuminuria such as blood pressure and glycemic control. Further, missing ACR measurements were imputed using multiple imputation. Multiple imputation was performed 10 times using chained equations with predictive mean matching conditioned upon age, sex, body mass index category, ethnicity, CKD stage, and relevant comorbidities and medications (baseline diagnosis of diabetes, hypertension, coronary heart disease, heart failure, peripheral vascular disease and cerebrovascular disease, systemic lupus erythematosus, hematuria, and baseline prescription of calcineurin inhibitors such as cyclosporine, tacrolimus, and lithium). Individuals fulfilling the conditions for referral to specialty care were identified and the proportions meeting NICE criteria and/or KFRE threshold for referral for each of the imputed dataset were combined using Rubin’s rule. Proportions across the groups (ACR within 1 year from sCr, ACR 1 to 5 years from sCr, and missing ACR) were then averaged to calculate the overall proportion that would be reclassified. Diagnostic checks were completed to compare observed and imputed data; results are presented in Supplementary Figure S1 and Supplementary Table S1. This study has been reported in accordance with STROBE guidelines (see Supplementary Table S2).
Ethical Approval
The THIN data collection scheme and research performed using THIN data were approved by the National Health Service South-East Multicentre Research Ethics Committee in 2003; under the terms of this approval, studies must undergo independent scientific review. Approval for this analysis was obtained from the Scientific Review Committee (for the use of THIN data) in 2018 (Reference number: 18THIN061).
Results
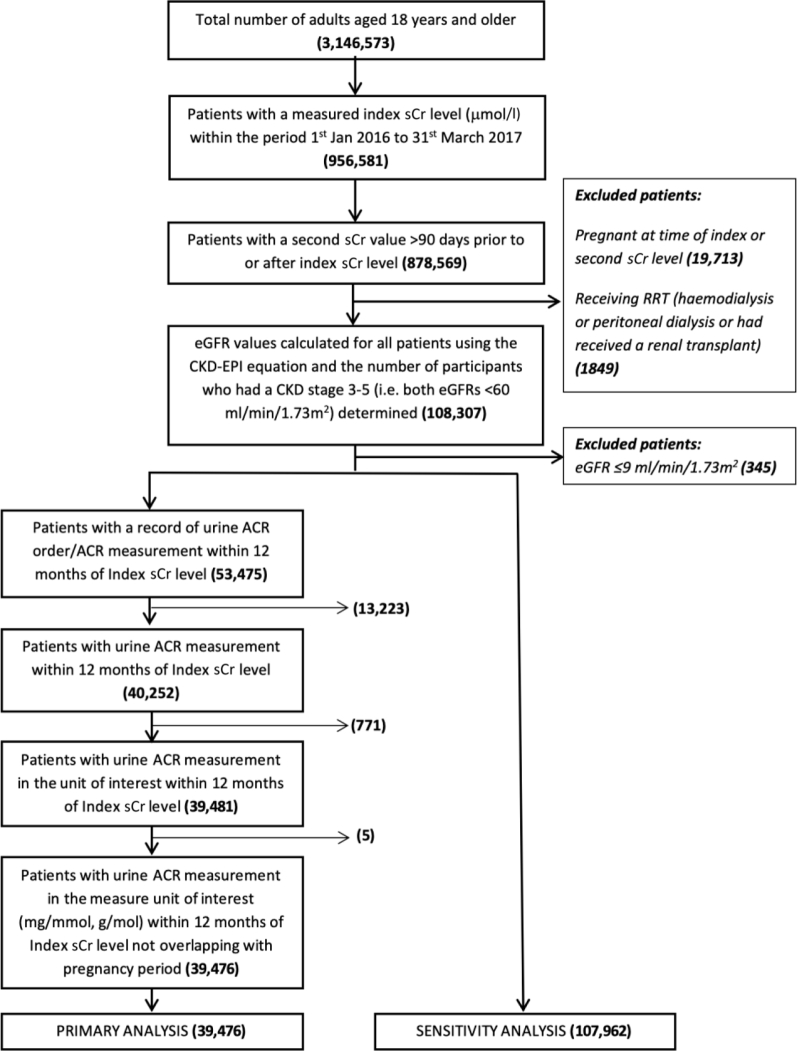
Between January 1, 2016, and March 31, 2017, 3,146,573 adults were available for follow-up in general practices in the THIN database; of these, 878,569 (27.9%, 95% confidence interval [CI]: 27.9% to 28.0%) had two sCr levels available within the study period. Exclusions comprised 19,713 (2.2%, 95% CI: 2.2% to 2.3%) patients who were pregnant within 365 days of a recorded sCr measurement and 1849 (0.21%, 95% CI: 0.20% to 0.22%) patients who were coded as receiving treatment with RRT. Of the remaining patients, 108,307 (3.4% of the adults in the THIN database, 95% CI: 3.4% to 3.5%) had confirmed CKD; 345 (0.3%, 95% CI: 0.3 to 0.4%) of these patients were excluded as they had an eGFR ≤9 ml/min per 1.73 m2. The final number of patients included in the analysis was 107,962 (Figure 1).
Figure 1.
Flow chart for inclusion in the study. ACR, albumin:creatinine ratio; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease – Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; RRT, renal replacement therapy; sCr, serum creatinine.
A urine ACR within 12 months of the index sCr not overlapping with a pregnancy period was available for 39,476 patients (36.6% of the patients in the analysis, 95% CI: 36.2% to 36.7%); these patients were included in the primary analysis.
Demographic and Laboratory Data
Table 1 shows the overall characteristics of the cohort grouped by the availability of ACR measurements. In patients who had an ACR available versus those who did not, there was a higher proportion of patients with diabetes, 51.1% versus 18.4% (odds ratio: 4.63, 95% CI: 4.50 to 4.76), and a lower eGFR (mean difference: 1.77 (95% CI: 1.64 to 1.90). Data on ethnicity were available for 56.1% of the study population. Calculation of eGFR using the CKD-EPI equation was made under the assumption that patients with missing ethnicity were of non-black origin.32
Table 1.
Study Population Characteristicsa
| Characteristic | Total (N = 107,962) | ACR Measurement Available Within 1 Year (n = 39,476) | ACR Measurement Unavailable Within 1 Year (n = 68,486) |
|---|---|---|---|
| Index eGFR (ml/min/1.73 m2), mean (SD) | 45.34 (10.41) | 44.22 (10.48) | 45.99 (10.31) |
| Age (yrs), median (IQR) | 79.52 (72.58-85.24) | 78.59 (71.94-84.09) | 79.93 (72.86-85.75) |
| Male | 46,310 (42.89) | 18,752 (47.50) | 27,558 (40.24) |
| BMI categories | |||
| Underweight (<18.5 kg/m2) | 1770 (1.64) | 435 (1.10) | 1335 (1.95) |
| Normal weight (18.5-25 kg/m2) | 27,387 (25.37) | 8601 (21.79) | 18,786 (27.43) |
| Overweight (25-30 kg/m2) | 39,452 (36.54) | 14,602 (36.99) | 24,850 (36.28) |
| Obese (>30 kg/m2) | 35,513 (32.89) | 15,077 (38.19) | 20,436 (29.84) |
| Missing | 3840 (3.56) | 761 (1.93) | 3079 (4.50) |
| Ethnicity | |||
| White | 45,472 (42.12) | 16,891 (42.79) | 28,581(41.73) |
| Black | 456 (0.42) | 167 (0.42) | 289 (0.42) |
| Chinese | 186 (0.17) | 73 (0.18) | 113 (0.16) |
| South Asian | 1145 (1.06) | 524 (1.33) | 621 (0.91) |
| Other | 150 (0.14) | 61 (0.15) | 89 (0.13) |
| Missing | 60,533 (56.09) | 21,760 (55.12) | 38,793 (56.64) |
| CKD stage (CKD-EPI eGFR, ml/min/1.73 m2) | |||
| 3a (45-59) | 62,251 (57.66) | 20,842 (52.80) | 41,409 (60.46) |
| 3b (30-44) | 35,312 (32.71) | 14,227 (36.04) | 21,085 (30.79) |
| 4 (15-29) | 9611 (8.90) | 4106 (10.40) | 5505 (8.04) |
| 5 (9-14) | 788 (0.73) | 301 (0.76) | 487 (0.71) |
| Comorbidities | |||
| Diabetes | 32,814 (30.39) | 20,187 (51.14) | 12,627 (18.44) |
| Hypertension | 77,075 (71.39) | 29,846 (75.61) | 47,229 (68.96) |
| Coronary heart disease | 28,382 (26.29) | 11,547 (29.25) | 16,835 (24.58) |
| Chronic heart failure | 11,946 (11.07) | 4621 (11.71) | 7325 (10.70) |
| Peripheral vascular disease | 8281 (7.67) | 3562 (9.02) | 4719 (6.89) |
| Cerebrovascular disease | 16,402 (15.19) | 5997 (15.19) | 10,405 (15.19) |
| Systemic lupus erythematosus | 349 (0.32) | 120 (0.30) | 229 (0.33) |
| Hematuria | 11,027 (10.21) | 4370 (11.07) | 6657 (9.72) |
| Renal stone/prostate disease | 8191 (7.59) | 3315 (8.40) | 4876 (7.12) |
| History of acute kidney injury | 4290 (3.97) | 1644 (4.16) | 2646 (3.86) |
| Medications | |||
| Calcineurin inhibitorb/lithium | 1462 (1.35) | 514 (1.30) | 948 (1.38) |
| ACR (mg/mmol), median (IQR) | 2.6 (1.0 to 9.2) |
ACR, albumin:creatinine ratio; BMI, body mass index; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease – Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
Values shown are n (%) unless otherwise stated.
Cyclosporine or tacrolimus.
Primary Analysis
Table 2 shows the number and proportion of patients who fulfilled referral criteria by NICE and by KFRE risk threshold; the proportion of patients are broadly similar: 19.2% (95% CI: 18.8% to 19.6%) versus 21.9% (95% CI: 21.5% to 22.4%), respectively. Some patients had two or more indications for referral based on NICE criteria, but referral would be predominantly based on eGFR <30 ml/min per 1.73 m2, followed by a sustained decline in eGFR.
Table 2.
Proportion and Number of Patients Fulfilling Referral Criteria From Primary to Specialty Care
| Criteria | ACR Available Within 1 Year of Index sCr % (95% CI) (n = 39,476) |
Any ACR Statusa % (95% CI) (n = 107,962) |
|---|---|---|
| NICE guideline | ||
| All criteria | 19.17 (18.78 – 19.56) (7566) b |
17.02 (16.79 – 17.24) (18,369) b |
| eGFR <30 ml/min/1.73 m2 | 11.16 (10.85 – 11.48) (4407) |
9.63 (9.46 – 9.81) (10,399) |
| ACR >30 mg/mmol with hematuria | 1.49 (1.37 – 1.61) (587) |
1.13 (1.07 – 1.20) (1221) |
| ACR >70 mg/mmol without diabetes | 1.64 (1.53 – 1.77) (647) |
1.87 (1.79 – 1.95) (2015) |
| eGFR sustained decrease | 8.83 (8.55 – 9.11) (3486) |
7.77 (7.62 – 7.94) (8394) |
| KFRE | ||
| 5-year risk >3% | 21.94 (21.54 – 22.36) (8663) |
18.20 (17.97 – 18.43) (19,649) |
ACR, albumin:creatinine ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate; KFRE, kidney failure risk equation; NICE, National Institute of Health and Care Excellence; sCr, serum creatinine.
ACR available within 5 years of index sCr considered, further missing ACRs imputed using multiple imputation using chained equations.
Patients to be referred to specialist nephrology care based on individual NICE criteria do not add up to the total patients to be referred to specialist nephrology care based on the combination of individual NICE criteria. This is because some patients have two or more indications for referral based on the NICE 2014 guideline.
Table 3 shows the numbers of patients with CKD stage 3-5 who would have been reclassified between primary and specialty care, in either direction, based on the replacement of NICE criteria by the KFRE threshold (>3% ESRD risk at 5-years). For patients who fulfilled criteria for NICE referral (n = 7566), 31.5% (2386, 95% CI: 30.5% to 32.6%) had a 5-year risk of ESRD by KFRE of ≤3%, meaning 6.0% (95% CI: 5.8% to 6.3%) of the patients in the primary analysis who met NICE referral criteria would have been low risk by the KFRE threshold. For patients with a KFRE of >3% (n = 8663), 40.2% (3483, 95% CI: 39.2% to 41.2%) did not fulfil the current NICE CKD guideline for referral, meaning 8.8% (95% CI: 8.5% to 9.1%) of patients in the primary analysis who were high risk by the KFRE threshold did not fulfil NICE criteria. Of 11,049 (28.0%, 95% CI: 22.6% to 27.5%) patients who fulfilled NICE and/or KFRE criteria, 5869 (53.1%, 95% CI: 52.2% to 54.0%) would have been reclassified between primary and specialty care, in either direction. Therefore, 14.9% (95% CI: 14.5% to 15.2%) of patients with CKD stage 3-5 in the primary analysis would have been reclassified between primary and specialty care if the KFRE criteria replaced NICE criteria for referral.
Table 3.
Classification of Patients for Referral From Primary to Specialty Care Based on NICE Criteria or a KFRE>3% at 5 Years — Primary Analysis
| KFRE Threshold, n (% of Total Analysis) |
Total, n (%) | ||
|---|---|---|---|
| No Referral ≤3% 5-Year Risk (Low Risk) |
Referral >3% 5-Year Risk (High Risk) |
||
| NICE criteria | |||
| No referral | 28,427 (72.0) | 3483 (8.8) | 31,910 (80.8) |
| Referral | 2386 (6.0) | 5180 (13.1) | 7566 (19.2) |
| Total n (%) | 30,813 (78.1) | 8663 (21.9) | 39,476 (100) |
KFRE, kidney failure risk equation; NICE, National Institute of Health and Care Excellence.
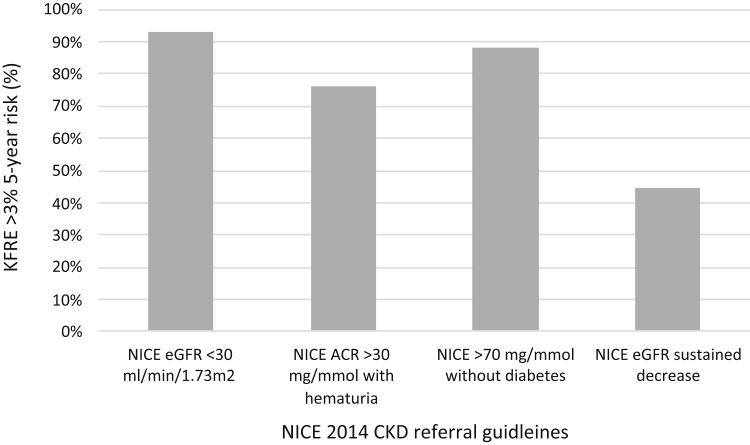
For the individual NICE 2014 CKD guidelines for referral considered in this study, the proportion with a 5-year KFRE >3% are presented in Figure 2: 92.8% (4090 of 4407, 95% CI: 92% to 93.6%) of those with eGFR <30 ml/min per 1.73 m2, 76.1% (447 of 587, 95% CI: 72.7% to 79.6%) of those with ACR >30 mg/mmol and hematuria, 88.1% (570 of 647, 95% CI: 85.6% to 90.6%) of those with ACR > 70 mg/mmol and no diabetes and 44.6% (1555 of 3486, 95% CI: 43.0 to 46.3%) of those with sustained decrease in eGFR.
Figure 2.
Graph to show proportion of patients meeting current NICE 2014 CKD criteria for referral to nephrology care with a KFRE risk score >3% at 5 years. ACR, albumin:creatinine ratio; CKD, Chronic kidney disease; eGFR, estimated glomerular filtration rate; KFRE, Kidney Failure Risk Equation; NICE, National Institute of Health and Care Excellence.
Sensitivity Analysis
Urine ACR was imputed for the 68,486 (63.4%, 95% CI: 63.1% to 63.7%) of patients who had no ACR measurement within 12 months of the index sCr. From this group, 21,431 patients had a urine ACR value within 5 years from the index sCr date; this was accepted as a proxy measurement. For the remaining 47,055 patients, missing ACR data were replaced using multiple imputation. This analysis yielded similar results to the primary analysis (Table 2): 17.0% (95% CI: 16.8% to 17.2%) of patients fulfilled NICE criteria for specialty care referral; 9.6% (95% CI: 9.5% to 9.8%) with an eGFR <30 ml/min per 1.73 m2, 1.1% (95% CI: 1.1% to 1.2%) with ACR >30 mg/mmol and hematuria, 1.9% (95% CI: 1.8% to 1.9%) with an ACR >70 mg/mmol and no diabetes, and 7.8% (95% CI: 7.6% to 7.9%) with a decline in eGFR. Table 4 shows that for patients who fulfilled criteria for NICE referral (n = 18,369), 35.1% (6450, 95% CI: 34.4% to 35.8%) had a 5-year risk of ESRD by KFRE of ≤3%, which is 6.0% (95% CI: 5.8% to 6.1%) of the total CKD population. For patients with a KFRE of >3% (n = 19,649), 39.4% (7730, 95% CI: 38.7% to 40.0%) did not fulfil the current NICE CKD guideline for referral from primary to specialty care, which is 7.2% (95% CI: 7.0% to 7.3%) of the total population. Of 26,099 patients who fulfilled NICE and/or KFRE criteria, 14,180 (54.3%, 95% CI: 53.7% to 54.9%) would have been reclassified between primary care and specialty care, in either direction. Therefore, 13.1% (95% CI: 12.9% to 13.3%) of the total population would have been reclassified between primary care and specialty care, in either direction if the KFRE criteria replaced NICE criteria for referral.
Table 4.
Classification of Patients for Referral From Primary to Specialty Care Based on NICE Criteria or a KFRE>3% at 5 years — Sensitivity Analysis
| KFRE Threshold, n (% of Total Analysis) |
Total, n (%) | ||
|---|---|---|---|
| No Referral ≤3% 5-Year Risk (Low Risk) |
Referral >3% 5-Year Risk (High Risk) |
||
| NICE criteria | |||
| No referral | 81,863 (75.8) | 7730 (7.2) | 89,593 (83.0) |
| Referral | 6450 (6.0) | 11,919 (11.0) | 18,369 (17.0) |
| Total n (%) | 88,313 (81.8) | 19,649 (18.2) | 107,962 (100) |
KFRE, kidney failure risk equation; NICE, National Institute of Health and Care Excellence.
Adjustments in the Primary Analysis
Two additional analyses were performed to assess how different criteria could be used to stratify patients at risk of progression to ESRD. For example, adding a criterion of ACR >100 mg/mmol as a threshold for referral from primary to specialty care led to an absolute increase of 1.0% (95% CI: 0.09% to 0.29%) in patients eligible for referral. The NICE CKD guideline currently indicates that patients with diabetes and with an ACR >70 mg/mmol should be referred if there is a possible cause other than diabetes. Second, an analysis was performed for a 2-year risk of progression threshold which showed that 2.5% (95% CI: 2.38% to 2.69%) of patients had a KFRE risk of progression to ESRD of >10% at 2 years. This may be helpful within specialist services as a threshold at which patients move between clinics, for example, from a clinic focused on slowing progression of CKD to one that also prepares the patient for management of ESRD.
Discussion
This study found that using a risk-based threshold for patients with CKD rather than the current NICE CKD guideline criteria would lead to a major change in referral patterns of individuals from primary to specialist care. Both the primary and sensitivity analyses showed approximately 40% of patients with a >3% risk of ESRD by 5 years are missed by the current NICE referral criteria. Approximately one-third of patients who fulfill the current NICE criteria are at low risk of ESRD (≤3% at 5-years) including more than half of those with a sustained decrease in eGFR as defined in the NICE guidance. Therefore, some patients at low risk of progression to ESRD are accessing limited specialist nephrology resources, whereas others with a higher risk of progression do not meet the NICE criteria and are not identified as requiring referral. If KFRE rather than NICE CKD criteria was used for referral of patients, just under 15% of patients with CKD would be reclassified between primary and specialty care.
A strength of this study is that it used a major primary care database which includes all adult patients in the participating general practices. To our knowledge, this is the first time that a study of this size has reported the implication of using a risk equation on a population basis for CKD. A recent study has modelled the impact of the KFRE in one geographical area of the United Kingdom.10 This confirmed high discrimination for the KFRE for prediction of progression to ESRD by 5 years (C-statistic 0.926, 95% CI: 0.911 to 0.942). In patients in that catchment area, the use of the non–North American calibrated KFRE at a >3% 5-year risk led to an increase in the numbers fulfilling criteria for referral by 84.3%, based on numbers of patients meeting NICE CKD criteria (eGFR < 30 ml/min per 1.73 m2 and/or ACR ≥ 70 mg/mmol). This differs from the current study where additional NICE criteria (ACR > 30 mg/mmol with hematuria and sustained decrease in eGFR) were incorporated. This may have increased the number of patients eligible for referral based on NICE criteria and may explain the smaller increase in individuals of 11.1% eligible for referral on a change in criteria to a KFRE >3%. Of interest, further modelling in that study indicated that referral criteria of a ≥5% risk of ESRD or a urine ACR ≥70 mg/mmol increased the proportion of patients referred who are likely to develop ESRD while reducing the number of patients eligible for referral compared to the numbers that had been seen in specialty care in the geographical area studied.
This study emphasizes that the majority of patients meeting current NICE CKD referral guidelines are based on eGFR criteria. However, eGFR alone lacks sensitivity for progression to ESRD.8 Most patients with eGFR <30 ml/min per 1.73 m2 met the threshold set for referral of KFRE >3% at 5-years; however, this was not the case for those with a sustained decline in eGFR where more than half were identified as low risk of progression.
In this study, 3.4% of the study population met the clinical definition of CKD stage 3-5 consistent with established Kidney Disease Improving Global Outcomes criteria for diagnosis using two measurements of kidney function >90 days apart. Patients on RRT and who were pregnant were not included in this calculated value. This figure is lower than estimates of CKD prevalence in other studies. Estimates of prevalence vary widely depending on the methodology used and each has its own limitations. In the United Kingdom, the Quality Outcome Framework, a system whereby primary care practices are financially incentivized for implementing good quality of care, includes maintaining a register of patients aged 18 years or older with CKD stage 3-5. In 2019–2020, 4.1% of patients were coded as having CKD.33 With this method there is a reliance on accurate coding and a study has shown there to be major miscoding of patients with CKD: 72.1% of patients with biochemically confirmed CKD appropriately coded, and 43.6% of patients coded as CKD with no biochemical evidence of stage 3-5 CKD.34 Some studies have used a single measure for sCr or eGFR which is likely to overestimate CKD as this will not reflect variation over time such as recovery from acute kidney injury. Data from a sample of nationally representative individuals in the Health Survey for England 2016 had CKD staging based on a single sCr value used to calculate CKD-EPI eGFR; estimated prevalence of stage 3-5 CKD was 7.3%.1 A further study, The New Opportunities for Early Renal Intervention by Computerised Assessment (NEOERICA) study reported a prevalence of 8.2% and also used single sCr values.35 Lastly, the Quality Improvement in Chronic Kidney Disease (QICKD) study described and compared the CKD prevalence values using different methodologies in a study population of 930,997 patients from 129 primary care practices in England. Crude prevalence of CKD stages 3-5 was 5.4% using two laboratory readings at least 3 months apart to report a Modification of Diet in Renal Disease eGFR. Using the single latest eGFR value changed the prevalence to 6.4% and paired sCr values to calculate eGFR by CKD-EPI formula and gave a prevalence of 4.8%. The limitation in THIN and other primary care records is that sCr values are derived from individuals accessing health care who go on to have a kidney function test. For individuals who are well, if the kidney function is not indicated or if patients poorly engage with health care services, they may not necessarily receive testing for kidney function. Some patients may not have had a kidney function blood test in the period under study. The lack of consistent prevalence estimates in the literature may make interpretation of the findings slightly challenging.
In the current study, the use of real-life data confirmed a lack of ACR testing in patients with CKD with 63.4% (68,486) missing this measurement in the specified period. As a consequence, the prognostic information provided by ACR, including risk of adverse outcomes such as cardiovascular disease, ESRD, and death, would not be available for these patients.36 Furthermore, albuminuria is used to direct care for patients with CKD, determining, for example, blood pressure targets, choice of antihypertensive treatments,6 and management with sodium glucose transport protein 2 inhibitors.37 Our results are consistent with a recent national CKD audit which identified major shortfalls in the measurement of ACR in clinical practice in the United Kingdom, an area that represents a major opportunity for quality improvement.38 To account for 21,431 (31.3%) of the missing ACR values, urine ACR measurements outside of 1 year and up to 5 years from the index sCr were used as a proxy in our sensitivity analysis. Although this allowed for risk assessment by KFRE and/or NICE criteria, there may be an increased potential for error given the prolonged time gap between renal function measurement (eGFR) and ACR quantification. Natural progression of disease and other variables such as use of antiproteinuric agents and change in blood pressure and glycemic control may impact on ACR. Multiple imputation was used for the remainder of patients with no urine ACR measurements (n = 47,055, 68.7%).
The appropriate risk threshold for referral from primary to specialty nephrology care is not known, and this is a weakness of this study. In some Canadian provinces, a risk threshold of >3% is being used in current clinical practice and this is being evaluated in improvement programs.11, 12, 13 A further weakness of the study included the exclusion of patients with an eGFR of ≥60 ml/min per 1.73 m2 and abnormal urinalysis. However, only young patients with nephrotic range proteinuria (ACR >300 mg/mmol) and an eGFR ≥60 ml/min per 1.73 m2 will have a risk of progression to ESRD of >3% at 5 years. This is a rare finding in clinical practice, and nephrotic syndrome with a normal eGFR is an independent indication for referral to specialty care. The study did not include the other recommendations from NICE for a referral to specialty care. These comprise patients with CKD who remain hypertensive on four or more blood pressure agents and patients who have rare or genetic causes of CKD. These groups are separate from the expert opinion progression thresholds defined by NICE or the KFRE calculation; therefore, they have no material impact on the modelling reported in this study.
The current NICE guideline recommends referral when there is a likelihood of progression to ESRD.6 The basis for these referral guideline recommendations to specialty care is that patients who present late to specialty care have worse outcomes compared to patients who have a timely referral. However, most studies on the impact of late referral have focused on patients at a very high risk of ESRD, where the late presenters have been known to the kidney service for <3 months before starting RRT.39 This study shows the risk of progression to ESRD is low for the majority of patients with CKD who meet current referral criteria. This is consistent with the published literature in this area and is because of the slow progression of CKD in most of those affected, and the competing risk of death.40
Whether referral (identified by the NICE CKD guideline or by a KFRE level of >3%) has an impact on clinical outcomes or patient-reported outcomes is not known. Most patients with CKD require careful and supportive management of risk factors for early cardiovascular disease, accurate management of which will also lower the risk of progression of CKD. Improving albuminuria testing to ensure that all patients can access treatment where there is a clear evidence base for outcomes is also a priority. Using the KFRE to ensure patients are supported to have an appropriate understanding of their risk of ESRD, and to support health care professionals in primary and specialty care in understanding the importance of accurate integration of ACR into clinical management and referral pathways may provide a further opportunity to improve clinical care.
This study emphasizes for clinicians and policymakers the inaccuracy of the current NICE CKD guideline for risk of progression to ESRD. Applying the KFRE with a risk threshold for referral has scope for carrying out clinical studies focused on assessing how KFRE is used to communicate with patients with CKD and defining a threshold for referral from primary to specialty care. This may represent an opportunity to improve quality of care with no additional cost, and to contribute to a core aspiration of the National Health Service long-term plan for the management of patients in primary care, including through novel service developments such as virtual clinics. NICE is currently revising the CKD guideline and the information provided in this paper provides further evidence for the use of risk equations to inform this.41
Conclusions
This study shows that moving from expert opinion to a risk-based threshold of ESRD of >3% for referral for patients with CKD from primary to specialty care would lead to a major redistribution of patients. This finding has implications for patients, clinicians, guideline groups, and resource allocation, and forms the basis of a major opportunity for improving care for patients with CKD.
Disclosure
PC reports grants from Kidney Research UK, National Institute for Health Research (NIHR), MRC, and EU Horizon 20/20 programme, and personal fees from NAPP Pharmaceuticals outside the submitted work. MC reports grants from the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, the NIHR Surgical Reconstruction and Microbiology Research Centre, the NIHR ARC West Midlands at the at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Health Data Research UK, Innovate UK (part of UK Research and Innovation) and Macmillan Cancer Support, and personal fees from Astellas, Merck, Daiichi Sankyo, Glaukos, GSK and the Patient-Centered Outcomes Research Institute (PCORI) outside the submitted work. DK reports grants from Macmillan Cancer Support, Innovate UK, the NIHR, NIHR Birmingham Biomedical Research Centre, and NIHR SRMRC at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, and personal fees from Merck and GSK outside the submitted work. KN reports grants from NIHR, MRC, Diabetes UK, personal fees from Astra Zeneca, Sanofi, MSD, and Boehringer Ingelheim outside the submitted work. HKB, AS, NJA, AF and KG have nothing to declare. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Acknowledgments
This project received funding from Renal Research–University Hospitals Birmingham NHS Trust; they had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
HKB, PC, MC and DK conceived the study. HKB developed the methodology with guidance provided by PC, AS, KG and KN. The process of data acquisition was completed by HKB, PC, AS, KG, and KN with AS and KN having full access to the data and who were responsible for the integrity and accuracy of the data collection in the study. Analysis performed by HKB, PC, AS, NJA, AF, and KN. PC drafted the manuscript. All authors revised it critically for intellectually important content and approved the final manuscript. All authors agree to be accountable for accuracy and integrity of the work. HKB attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Data Sharing
No data available, however, read code lists are available upon request via email to the corresponding author.
Footnotes
Supplementary Methods.
Figure S1. A graphical comparison of the observed and imputed data for albumin:creatinine ratio within 5 years.
Table S1. Summary statistics of the observed and imputed data for albumin:creatinine ratio within 5 years.
Table S2. Modified STROBE statement.
Supplementary Material
Supplementary Methods.
Figure S1. A graphical comparison of the observed and imputed data for albumin:creatinine ratio within 5 years.
Table S1. Summary statistics of the observed and imputed data for albumin:creatinine ratio within 5 years.
Table S2. Modified STROBE statement.
References
- 1.Hounkpatin H.O., Harris S., Fraser S.D.S. Prevalence of chronic kidney disease in adults in England: comparison of nationally representative cross-sectional surveys from 2003 to 2016. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-038423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nitsch D., Grams M., Sang Y. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 2013;346:f324. doi: 10.1136/bmj.f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsushita K., Coresh J., Sang Y. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–525. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herzog C.A., Asinger R.W., Berger A.K. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80:572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 5.UK Renal Registry (2020) UK Renal Registry 22nd Annual Report — data to 31/12/2018, Bristol, UK. https://renal.org/audit-research/annual-report/22nd-annual-report-data-31122018 Accessed November 26, 2020.
- 6.National Clinical Guideline Centre (UK) National Institute for Health and Care Excellence (UK); London, United Kingdom: 2014. Chronic Kidney Disease (Partial Update): Early Identification and Management of Chronic Kidney Disease in Adults in Primary and Secondary Care. Clinical Guidelines. [PubMed] [Google Scholar]
- 7.Ramspek C.L., de Jong Y., Dekker F.W. Towards the best kidney failure prediction tool: a systematic review and selection aid. Nephrol Dial Transplant. 2020;35:1527–1538. doi: 10.1093/ndt/gfz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tangri N., Stevens L.A., Griffith J. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305:1553–1559. doi: 10.1001/jama.2011.451. [DOI] [PubMed] [Google Scholar]
- 9.Tangri N., Grams M.E., Levey A.S. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315:164–174. doi: 10.1001/jama.2015.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Major R.W., Shepherd D., Medcalf J.F. The kidney failure risk equation for prediction of end stage renal disease in UK primary care: an external validation and clinical impact projection cohort study. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmelgarn B.R., Smekal M.D., Weaver R.G. Implementation and evaluation of a risk-based approach to guide chronic kidney disease care: protocol for a multiphase mixed-methods study. Can J Kidney Health Dis. 2018;5 doi: 10.1177/2054358117753618. 2054358117753618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harasemiw O., Drummond N., Singer A. Integrating risk-based care for patients with chronic kidney disease in the community: study protocol for a cluster randomized trial. Can J Kidney Health Dis. 2019;6 doi: 10.1177/2054358119841611. 2054358119841611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hingwala J., Wojciechowski P., Hiebert B. Risk-based triage for nephrology referrals using the kidney failure risk equation. Can J Kidney Health Dis. 2017;4 doi: 10.1177/2054358117722782. 2054358117722782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Kidney Failure Risk Equation. http://kidneyfailurerisk.com/ Accessed October 30, 2017.
- 15.Lennartz C.S., Pickering J.W., Seiler-Mussler S. External validation of the kidney failure risk equation and re-calibration with addition of ultrasound parameters. Clin J Am Soc Nephrol. 2016;11:609–615. doi: 10.2215/CJN.08110715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenton A., Jesky M.D., Webster R. Association between urinary free light chains and progression to end stage renal disease in chronic kidney disease. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawhney S., Beaulieu M., Black C. Predicting kidney failure risk after acute kidney injury among people receiving nephrology clinic care. Nephrol Dial Transplant. 2020;35:836–845. doi: 10.1093/ndt/gfy294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamanouchi M., Hoshino J., Ubara Y. Value of adding the renal pathological score to the kidney failure risk equation in advanced diabetic nephropathy. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zacharias H.U., Altenbuchinger M., Schultheiss U.T. A novel metabolic signature to predict the requirement of dialysis or renal transplantation in patients with chronic kidney disease. J Proteome Res. 2019;18:1796–1805. doi: 10.1021/acs.jproteome.8b00983. [DOI] [PubMed] [Google Scholar]
- 20.Akbari A., Tangri N., Brown P.A. Prediction of progression in polycystic kidney disease using the kidney failure risk equation and ultrasound parameters. Can J Kidney Health Dis. 2020;7 doi: 10.1177/2054358120911274. 2054358120911274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tangri N., Ferguson T.W., Wiebe C. Validation of the kidney failure risk equation in kidney transplant recipients. Can J Kidney Health Dis. 2020;7 doi: 10.1177/2054358120922627. 2054358120922627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akbari S., Knoll G., White C.A. Accuracy of kidney failure risk equation in transplant recipients. Kidney Int Rep. 2019;4:1334–1337. doi: 10.1016/j.ekir.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winnicki E., McCulloch C.E., Mitsnefes M.M. Use of the kidney failure risk equation to determine the risk of progression to end-stage renal disease in children with chronic kidney disease. JAMA Pediatr. 2018;172:174–180. doi: 10.1001/jamapediatrics.2017.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sebastiao Y.V., Cooper J.N., Becknell B. Prediction of kidney failure in children with chronic kidney disease and obstructive uropathy. Pediatr Nephrol. 2021;36:111–118. doi: 10.1007/s00467-020-04661-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong R., Pirabhahar S., Turner K. Triage system for nephrology referrals using the kidney failure risk equation (KFRE) score. Nephrology. 2020;25(suppl 3):53. [Google Scholar]
- 26.Blak B.T., Thompson M., Dattani H. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19:251–255. doi: 10.14236/jhi.v19i4.820. [DOI] [PubMed] [Google Scholar]
- 27.Maguire A., Blak B.T., Thompson M. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf. 2009;18:76–83. doi: 10.1002/pds.1688. [DOI] [PubMed] [Google Scholar]
- 28.Booth N. What are the read codes? Health Libr Rev. 1994;11:177–182. doi: 10.1046/j.1365-2532.1994.1130177.x. [DOI] [PubMed] [Google Scholar]
- 29.Bhachu H.K., Cockwell P., Subramanian A. Cross-sectional observation study to investigate the impact of risk-based stratification on care pathways for patients with chronic kidney disease: protocol paper. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-027315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens P.E., Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 31.Gilg J., Methven S., Casula A. UK Renal Registry 19th annual report: chapter 1 UK RRT adult incidence in 2015: national and centre-specific analyses. Nephron. 2017;137(suppl 1):11–44. doi: 10.1159/000481363. [DOI] [PubMed] [Google Scholar]
- 32.Mathur R., Bhaskaran K., Chaturvedi N. Completeness and usability of ethnicity data in UK-based primary care and hospital databases. J Public Health (Oxf) 2014;36:684–692. doi: 10.1093/pubmed/fdt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quality and Outcomes Framework, 2019-20. NHS Digital. Published 2020. https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data/2019-20 Accessed March 27, 2021.
- 34.Jain P., Calvert M., Cockwell P. The need for improved identification and accurate classification of stages 3-5 chronic kidney disease in primary care: retrospective cohort study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevens P.E., O'Donoghue D.J., de Lusignan S. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int. 2007;72:92–99. doi: 10.1038/sj.ki.5002273. [DOI] [PubMed] [Google Scholar]
- 36.Tangri N., Kitsios G.D., Inker L.A. Risk prediction models for patients with chronic kidney disease: a systematic review. Ann Intern Med. 2013;158:596–603. doi: 10.7326/0003-4819-158-8-201304160-00004. [DOI] [PubMed] [Google Scholar]
- 37.Perkovic V., Jardine M.J., Neal B. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 38.Kim L.G., Cleary F., Wheeler D.C. How do primary care doctors in England and Wales code and manage people with chronic kidney disease? Results from the National Chronic Kidney Disease Audit. Nephrol Dial Transplant. 2018;33:1373–1379. doi: 10.1093/ndt/gfx280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smart N.A., Dieberg G., Ladhani M. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev. 2014;6:CD007333. doi: 10.1002/14651858.CD007333.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Hallan S.I., Matsushita K., Sang Y. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308:2349–2360. doi: 10.1001/jama.2012.16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Institute for Health and Clinical Excellence Surveillance report 2017— chronic kidney disease (stage 4 or 5): management of hyperphosphataemia (2013) NICE guideline CG157, chronic kidney disease in adults: assessment and management (2014) NICE guideline CG182 and chronic kidney disease: managing anaemia (2015) NICE guideline NG8. https://www.nice.org.uk/guidance/cg182/resources/surveillance-report-2017-chronic-kidney-disease-stage-4-or-5-management-of-hyperphosphataemia-2013-nice-guideline-cg157-chronic-kidney-disease-in-adults-assessment-and-management-2014-nice-guideline--4429248445/chapter/Surveillance-decision Accessed November 11, 2020. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.